Abstract
Introduction
Interleukin (IL)-18 is involved in regulation of lipid and glucose metabolism. Mice lacking whole-body IL-18 signalling are prone to develop weight gain and insulin resistance, a phenotype which is associated with impaired fat oxidation and ectopic skeletal muscle lipid deposition. IL-18 mRNA is expressed in human skeletal muscle but a role for IL-18 in muscle has not been identified. Patients with HIV-infection and lipodystrophy (LD) are characterized by lipid and glucose disturbances and increased levels of circulating IL-18. We hypothesized that skeletal muscle IL-18 and IL-18 receptor (R) expression would be altered in patients with HIV-lipodystrophy.
Design and methods
Twenty-three HIV-infected patients with LD and 15 age-matched healthy controls were included in a cross-sectional study. Biopsies from the vastus lateralis muscle were obtained and IL-18 and IL-18R mRNA expression were measured by real-time PCR and sphingolipids (ceramides, sphingosine, sphingosine-1-Phosphate, sphinganine) were measured by HPLC. Insulin resistance was assessed by HOMA and the insulin response during an OGTT.
Results
Patients with HIV-LD had a 60% and 54% lower level of muscular IL-18 and IL-18R mRNA expression, respectively, compared to age-matched healthy controls. Patients with HIV-LD had a trend towards increased levels of ceramide (18.3±4.7 versus 14.8±3.0,p = 0.06) and sphingosine (0.41±0.13 versus 0.32±0.07, and lower level of sphinganine (p = 0.06). Low levels of muscle IL-18 mRNA correlated to high levels of ceramides (r = -0.31, p = 0.038) and sphingosine-1P (r = -0.29, p = 0.046) in skeletal muscle, whereas such a correlation was not found in healthy controls. Low expression of IL-18 mRNA in skeletal muscle correlated to elevated concentration of circulating triglycerides (Rp = -0.73, p<0.0001). Neither muscle expression of IL-18 mRNA or ceramide correlated to parameters of insulin resistance.
Conclusion
IL-18 (mRNA) in skeletal muscle appears to be involved in the regulation of intramuscular lipid metabolism and hypertriglyceridemia.
Introduction
The cytokine interleukin (IL)-18 is a member of the IL-1 family and has been identified as a cofactor that, together with IL-12, stimulates production of interferon gamma [1]. IL-18 is widely expressed in many mammalian cells/tissues including liver, adipose tissue and skeletal muscle [2–4] [5]. IL-18 is best known for its role in inflammation, whereby pro-inflammatory stimuli such as lipopolysaccharide, and tumor necrosis factor (TNF)-α leads to caspase-1 mediated cleavage of pro-IL-18 into mature IL-18. IL-18 is synthesized as an inactive precursor molecule (Pro-IL-18) that lacks a signal peptide and requires cleavage into a mature, active cytokine IL-18 that is then secreted from the cell [6].
IL-18 can then signal via a heterodimer of the transmembrane IL-18 receptors (α and β), and via a toll like receptor signaling cascade ultimately leading to the activation of nuclear factor κB (NFκB) and subsequent regulation of gene transcription [7]. Additional signaling pathways of IL-18 also exist, including activation of phosphatidylinositol-3 kinase (PI3K)/Akt [8,9], signal transducer and activator of transcription 3 (STAT 3) [10], mitogen-activated protein kinases (MAPK) [8,10], and c-Jun NH2-terminal kinase (JNK) [9,11] which are all implicated in energy metabolism.
As IL-18 plays a role in inflammation it is not surprisingly that circulating IL-18 levels are elevated in human obesity [12] and in patients with type 2 diabetes [13,14]. IL-18 is expressed in adipose tissue [15,16] especially in visceral adipose tissue [17,18].
Paradoxically, IL-18 has also been found to be directly involved in the regulation of lipid and glucose metabolism as mice lacking whole-body IL-18 signalling become obese and insulin resistant, [19,20]. In a previous study, we demonstrated that IL-18 receptor deficient mice display obesity, insulin resistance, impaired fat oxidation and ectopic lipid deposition in liver and skeletal muscle [21]. Moreover, administration of IL-18 to whole muscle strips ex vivo increased AMPK signaling and increases fat oxidation, and in vivo electroporation of IL-18 into skeletal muscle results in increased AMPK signaling and expression of mitochondrial genes in skeletal muscle and concomitantly inhibits high fat diet-induced-weight gain, suggesting that IL-18 is increasing skeletal muscle fat oxidation via AMPK and oppose ectopic lipid accumulation [21]. Taken together, in the progression toward obesity, there is continual production of IL-18 to oppose ectopic lipid accumulation [22]. However, the cellular origin of IL-18 remains enigmatic, although it has been shown that IL-18 released from adipose tissue is not produced by the adipocytes themselves [16,23]. Given that IL-18 is expressed in human skeletal muscle and exerts its effect on lipid metabolism in skeletal muscle ex vivo and in vivo in rodents, it suggest that IL-18 is regulated in skeletal muscle and associated to lipid metabolism. However, the role of IL-18 in skeletal muscle in humans have never been investigated.
A syndrome of lipodystrophy, characterised by subcutaneous fat loss, and a relative increase in central fat accumulation, was previously seen in patients with HIV, when treated with a combination of antiretroviral therapy, including thymidine-nucleoside analogues [24–26]. Lipodystrophy is mostly caused by antiretroviral induced adipose tissue dysfunction [27,28] and is associated with impaired fat oxidation [29,30], ectopic lipid deposition in muscle and liver [24,30–32], and mitochondrial dysfunction in skeletal muscle [33] leading to dyslipidemia and insulin resistance [24,25]. Elevated circulating levels of IL-18 are observed in HIV-infected patients and especially those with lipodystrophy [34,35]. The increase in circulating IL-18 is associated to the fat redistribution and in part derived from subcutaneous adipose tissue [36,37].
The ectopic accumulation of lipids in the skeletal muscle is closely linked with insulin resistance [38]. In the recent years it is found that it is not the total amount of lipid but the lipid intermediates causing insulin resistance. The lipid intermediates account for sphingolipids, e.g. ceramides, sphingosine, sphingoanine, sphingosine-1-P, and diacylglycerol (DAG) [39]. Especially, ceramides [40] and DAG have been supposed to induce insulin resistance [38]. Besides antagonize insulin signalling recent data also shows that ceramides impair mitochondrial functions [41].
In this study, we included material from a cohort of HIV patients with lipodystrophy [42] in order to obtain more information about the metabolic role of IL-18 in humans. Patients with HIV-lipodystrophy share some of the same metabolic disturbances as mice lacking IL-18 signalling but paradoxically the systemic levels of IL-18 are increased in those patients. We therefore aimed at determining whether muscle IL-18 mRNA and IL-18 receptor mRNA are altered, either increased or decreased, in these patients. Given the strong link between ceramides and insulin resistance [38] and mitochondrial function [41], we further studied the possible association of muscle IL-18 mRNA with ceramides and other sphingolipids.
Patients and methods
Patients and controls
A group of 23 HIV-infected men were recruited from the outpatient clinic of the Department of Infectious Disease, Rigshospitalet in Copenhagen. These subjects have been included in a former study and the inclusions of the subjects are described therein [42]. In short, LD was defined clinically by physical examination of peripheral lipoatrophy (defined by the presence of peripheral lipoatrophy with at least one moderate sign of fat loss in face, arms, buttocks, or legs based on a physical examination by a single investigator (BL) using a validated questionnaire developed by Carr et al [43]). All patients were on a stable and effective nucleoside analogue based antiretroviral therapy with no changes during the preceding 8 weeks.
Two groups of healthy controls were included: Fifteen age-matched HIV-negative healthy men served as controls for RT-PCR data (Group 1). These subjects have also been included in the before mentioned study [42]. But as muscle tissue for measurement of sphingolipids from healthy controls in group 1 were available for only two healthy subjects, 17 new healthy age-matched control subjects were included (Group 2). Demographic data were collected for each patient: age, duration of HIV infection, duration and types of all antiretroviral therapy, weight, height, CD4 count, HIV-RNA copies. Inclusion criteria: no signs of ongoing infections; fasting glucose < 7 mmol/L and 120 min glucose after an OGTT < 11.1 mmol/L, no dyslipidemia (triglycerides >1.7 mmol/L and/or HDL-cholesterol <0.9 mmol/L); suppressed viral load (<20 copies/mL). Exclusion criteria: Severe cardiovascular diseases; arthritis; severe neuropathy; hepatitis C; opportunistic infections that required hospitalisation within the last 6 weeks; diabetes (fasting glucose ≥7 mmol/L or 2-hrs. glucose >11 mmol/L after an OGTT); concurrent therapy with antidiabetic agents, anticoagulant or any hormones.
Written informed consent was obtained from all subjects according to the requirements from the local ethical committee and the Helsinki Declaration II, and the approval from the local ethical committee (KF 01269485): The Ethics Committee of Copenhagen and Frederiksberg) was obtained.
Biochemical measurements
Peripheral blood samples were obtained at 8 AM after an overnight fasting. Measurements of total cholesterol (mmol/L), HDL-cholesterol (mmol/L), LDL-cholesterol (mmol/L), triglycerides (mmol/L), plasma glucose (mmol/L) and insulin (pmol/L), were determined immediately using routine methods.
CD4 cell counts were calculated by flowcytometry and HIV-RNA copies were measured by the Amplicor HIV Monitor (Roche Molecular Systems, Branchburg, NJ) (lower limit of dectection: 20 copies/ml).
Body composition analysis
Fat and fat-free tissue masses for whole body, trunk and extremities were measured using dual-energy X-ray absorptiometry (DXA) scanner (Lunar Prodigy, GE Medical Systems Wisconsin, USA, version 8.8) [43]. Whole-body and regional fat measurements (trunk and extremities) were determined as previously described [44].
Maximal oxygen consumption (VO2max)
An incremental exercise to volitional fatigue was performed between 0800 h and 1000 h on a cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). Maximal oxygen consumption (VO2max) was measured with an indirect calorimetric system (Moxus modular VO2 system, AEI Technologies, Pittsburgh, PA) using a 2-way non-rebreathing valve (Hans Rudolph, Inc. Kansas City, Missouri) which recorded data every 15 seconds. Based on the pre-VO2max test a protocol was designed in order to reach VO2max within 8–12 min of exercise start [45]. Exhaustion was defined by two of the following: respiratory exchange ratios >1.10, VO2 reached a plateau and/or rpm <60 in more than 10 sec.
Insulin sensitivity
Insulin resistance was assessed from several measurements: fasting plasma insulin, homeostasis model (HOMA-IR) [46] and area under the curve (AUC) for the insulin concentration during a 75-g oral glucose tolerance test (OGTT)
Muscle tissue biopsies
Muscle tissue biopsies were obtained after an overnight fast by use of the percutaneous biopsy technique with suction from the quadriceps muscle under local anaesthesia with 2% lidocaine. Muscle tissue was immediately frozen in liquid nitrogen and stored at –80°C until analysed.
RNA extraction
RNA was extracted using Trizol™ (Life Technologies) according to manufacturer’s protocol. In short, 1 ml of Trizol™ was added to 20–30 mg of muscle tissue and homogenized using a Polytron (PT-MR2100, Kinematica) on setting 25–30 for 20–30 s and placed on ice. All samples were added 100 μl of chloroform, shaken vigorously and incubated for 5 min on ice. Samples were spun at 12000 g for 15 min at 4°C, and the upper aqueous phase was placed in a fresh eppendorph tube. The same volume of isopropanol was added and samples were placed at –20°C for 1 hour followed by centrifugation at 12000 g for 15 min at 4°C. The resulting RNA pellet was washed with 75% ethanol in DEPC-treated water and spun at 6000 g for 10 min at 4°C. The pellets were dissolved in DEPC-treated water.
Reverse transcription
One μg of total RNA was reverse transcribed in a 50-μl reaction according to manufacturer's protocol (Applied Biosystems, Taqman™ reverse transcription reagents) with the use of random hexamer primers. The reactions were run in a Perkin Elmer GeneAmp PCR system 9700 with conditions at 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min.
Analysis of gene expression levels in muscle tissue
Samples were analyzed for IL-18 and IL-18 receptor mRNA levels by real-time PCR using an ABI PRISM 7900 sequence detector (PE Biosystems). The gene expression levels were normalized to the housekeeping gene GAPDH (obtained from Applied Biosystems). Human IL-18 (Hs00155517_m1) and human IL-18 receptor (Hs00187256_m1) primers and Taqman® probe were obtained from Applied Biosystems. All reactions were run in triplicates.
Data were quantitated and normalized using the standard curve method.
Sphingolipid analysis
The content of S1P, SA1P, sphingosine, sphinganine, and ceramide was determined as described previously in detail (Knapp et al. 2013). Briefly, lipids were extracted from samples in the presence of internal standards (10 pmol of C17-sphingosine and 30 pmol of C17-S1P, Avanti Polar Lipids, Alabaster, AL). An aliquot of the lipid extract was transferred to a fresh tube with pre-added 40 pmol of N-palmitoyl-D-erythro-sphingosine (C17 base) (a kind gift of Dr Z. Szulc, Medical University of South Carolina) as an internal standard, and then subjected to alkaline hydrolysis to deacylate ceramide to sphingosine. The amount of S1P and SA1P was determined indirectly after dephosphorylation to sphingosine and sphinganine, respectively, with the use of alkaline phosphatase (bovine intestinal mucosa, Sigma). Free sphingosine and sphinganine, dephosphorylated sphingoid bases, and sphingosine released from ceramide were then converted to their o-phthalaldehyde derivatives and analyzed using a HPLC system (ProStar, Varian Inc., Palo Alto, CA) equipped with a fluorescence detector and C18 reversed-phase column (Varian Inc. OmniSpher 5, 4.6×150mm). The isocratic eluent composition of acetonitrile (Merck, Darmstadt, Germany): water (9:1, v/v) and a flow rate of 1 ml/min were used. Column temperature was maintained at 30°C [47].
Statistical analysis
Statistical calculations were performed using SAS 9.1 (USA). Data are presented as means +/- SD. P < 0.05 was considered significant in all analyses. Parameters between patients with HIV-lipodystrophy and healthy controls were compared with a Mann-Whitney test. Pearson's correlations were used to examine the relationship between mRNA expression in muscle, sphingolipid in muscle and markers of insulin sensitivity, as well as with anthropometric parameters.
Results
Baseline characteristics
Demographic data, blood biochemistry and body composition appear in Table 1.
Table 1. Baseline characteristics of patients and healthy controls.
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| Variab | Healthy controls (n = 15) | Patients with HIV-LD (n = 23) | Healthy controls (n = 17) | Patients with HIV-LD (n = 14)§ |
| Age (years) | 47.5 (6.1) | 47.9 (9.5) | 46.5 (6.0) | 48.3 (9.7) |
| Duration of HIV infection (years) | 15.6 (9.6) | 13.9 (7.0) | ||
| Duration of antiretroviral therapy (years) | 10.3 (4.3) | 8.9 (3.8) | ||
| CD4+ cell (cells/μl) | 558 (208) | 550 (250) | ||
| LogHIV-RNA (copies/ml) | 1.33 (0.12) | 1.32 (0.11) | ||
| Antiretroviral use | ||||
| Current Tymidine-NRTI use, No. (%) | 11 (47.8) | 7 (50.0) | ||
| Current PI use, No. (%) | 13 (56.7) | 8 (57.1) | ||
| Current NNRTI use, No. (%) | 11 (47.8) | 7 (50.0) | ||
| Physical activity parameters | ||||
| VO2max (LO2/min) | 2.5 (0.6) | 2.3 (0.5) | 3.4 (0.8) | 2.4 (0.5)*** |
| Body composition | ||||
| Body-mass index (kg/m2) | 23.7 (1.9) | 23.7 (2.9) | 23.3 (2.1) | 24.1 (3.0) |
| Weight (kg) | 76.9 (7.4) | 73.6 (11.2) | 79.4 (9.0) | 75.8 (11.1) |
| Waist (cm) | 90 (5.7) | 93.6 (6.4) | 91.1 (7.9) | 94.2 (7.2) |
| Waist-to.hip ratio | 0.94 (0.03) | 1.01 (0.04) | 0.91 (0.05) | 1.02 (0.04) |
| Fat mass (kg) | 15.7 (4.4) | 13.8 (5.3) | 16.2 (6.4) | 15.0 (5.3) |
| Trunk fat mass (kg) | 8.9 (3.0) | 9.8 (3.9) | 9.1 (4.0) | 10.9 (4.0) |
| Trunk fat percentage (%) | 56.1(5.2) | 71.2 (6.2) **** | 55.3 (5.1) | 72.5 (5.8)**** |
| Limb fat mass (kg) | 6.2 (1.5) | 3.5 (1.6)**** | 6.6 (2.5) | 3.6 (1.5)*** |
| Limb fat percentage (%) | 40.2 (4.9) | 25.1 (6.1) **** | 41.5 (4.2) | 24.0 (5.7)**** |
| Trunk-to-limb fat ratio | 1.4 (0.29) | 3.09 (1.17)* | 1.4 (0.25) | 3.3 (1.3)*** |
| Lean mass (kg) | 58.2 (5.2) | 57.0 (6.8) | 61.0 (5.4) | 57.9 (6.2) |
| Metabolic parameters | ||||
| Total-cholesterol (mmol/L) | 4.63 (0.64) | 5.5 (0.9)** | 4.81 (0.65) | 5.8 (0.7)*** |
| HDL-C (mmol/L) | 1.51 (0.32) | 1.23 (0.52)* | 1.41 (0.32) | 1.21 (0.36) |
| LDL-C (mmol/L) | 3.3 (0.6) | 3.7 (0.9) | 3.14 (0.74) | 3.94 (0.81)** |
| Triglycerides (mmol/L) | 0.76 (0.24) | 2.55(1.43)**** | 0.96 (0.26) | 2.88 (1..34)**** |
| Glucose (mmol/L) | 5.2 (0.3) | 5.4 (0.6) | 5.0 (0.1) | 5.4 (0.7) |
| Insulin (pmol/L) | 25 (8.9) | 52 (25)**** | 28.4 (9.9) | 56.5 (28.2)*** |
| HOMA-IR | 0.99 (0.37) | 2.2 (1.4)**** | 1.3 (0.14) | 2.5 (1.5) |
| Glucose area under the curve (mmol/Lmin) | 670 (126) | 826 (200)* | 654 (153) | 779 (176) |
| Insulin area under the curve (pmol/Lmin) | 23505 (10598) | 52360 (31017)** | 18436 (10510) | 45388 (37026)** |
Two groups of healthy men were included due to lack of muscle tissue. Group 1 served as controls for the RT-PCR data. Group 2 served as controls for the sphingolipid analysis.
§ Fourteen patients with HIV-LD are a part of the 23 patients with HIV-LD in group 1.
Data are presented as mean (SD). PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non- nucleoside reverse transcriptase inhibitor. HOMA-IR, homeostatic model assessment for insulin resistance.
*P < 0.05;
** P < 0.01;
***P < 0.001,
****P < 0.0001 by t-test comparing patients with HIV-LD and healthy controls within each cohort.
The healthy men and the patients with HIV-lipodysstrophy were age-matched. Two groups of healthy men were included due to lack of muscle tissue. Group 1 served as controls for the RT-PCR data. Group 2 served as controls for sphingolipid analysis.
In group 1, patients were matched based on their VO2 max. In group 2 we also tried to match HIV patients with their control based on physical activity, but at the end of the inclusion the HIV patients had lower levels of VO2 max compared with controls (Table 1).
Patients with HIV-lipodystrophy were characterised by reduced total limb fat mass, increased percentage of trunk fat mass, reduced percentage of limb fat mass and increased trunk-to-limb fat mass, indicating fat redistribution, compared to healthy controls. No differences were found regarding BMI, total fat mass, trunk fat mass or lean body mass.
Fasting triglycerides and total-cholesterol levels were higher in patients with HIV-lipodystrophy and so were fasting insulin, HOMA-IR and the insulin response during an OGTT when compared to control subjects from both group 1 and 2, indicating insulin resistance in patients with HIV-lipodystrophy (Table 1).
As previously demonstrated, plasma IL-18 was increased in patients with HIV-lipodystrophy compared to healthy controls (247 pg/ml (98) vs 199 pg/ml (102), p<0.05).
IL-18 and IL-18 receptor mRNA expression in skeletal muscle
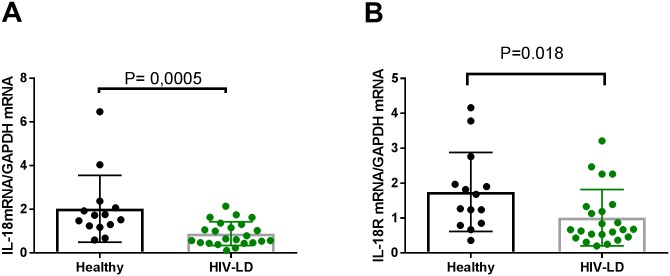
IL-18 mRNA (Fig 1A) and IL-18 receptor mRNA (Fig 1B) expression were reduced by 60% and 54%, respectively, in skeletal muscle in patients with HIV-lipodystrophy compared to healthy controls. (IL-18 mRNA: p = 0.0005 IL-18 receptor mRNA: p = 0.018). In the healthy control group, two subjects had very high levels of IL-18 mRNA. If these two subjects were excluded, IL-18 mRNA expression was still significantly lower in patients with HIV-lipodystrophy compared to healthy controls (p = 0.003) (data not shown).
Fig 1. Patients with HIV-lipodystrophy have reduced levels of IL-18 mRNA and IL-18 receptor mRNA in skeletal muscle.
(A) mRNA expression of IL-18 in skeletal muscle. In the healthy control group 2 subjects had very high levels of IL-18 mRNA levels. Even if those two subjects were deleted the difference between healthy controls and patients with HIV-Lipodystrophy were still high significant p = 0.003). (B) mRNA expression of IL-18 receptor in skeletal muscle. The levels of IL-18 mRNA and IL-18 receptor mRNA were calculated with GAPDH as a housekeeping gene. In the dot plots data for each subjects are given and the line represent means and SD. * P<0.05 and ***P<0.001 for healthy vs HIV-lipodystrophy patients.
Sphingolipid in skeletal muscle
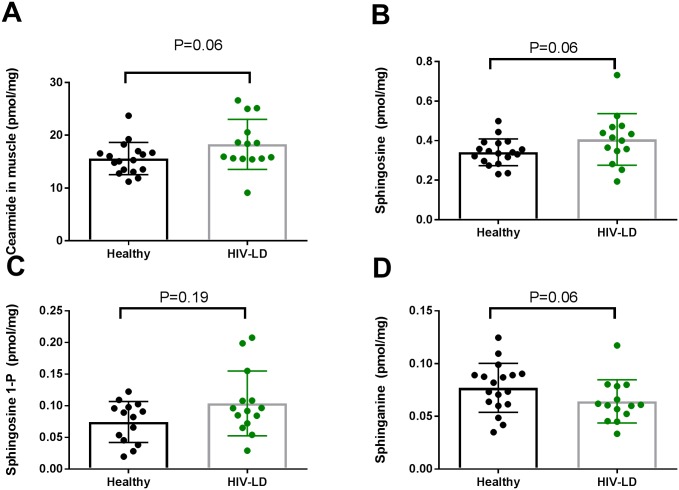
The sphingolipids ceramide (Fig 2A) and sphingosine (Fig 2B) content in muscle tended to be elevated in patients with HIV-lipodystrophy compared to healthy controls (P = 0.06).
Fig 2. The sphingolipids ceramide and sphingosine tended to be increased in vastus lateralis muscle from patients with HIV-lipodystrophy.
In the dot plots data for each subjects are given and the line represent means and SD.
The sphinganine content in muscle tended to be lower in patients with HIV-lipodystrophy compared to healthy controls (Fig 2D, p = 0.06)
Relationship between IL-18 mRNA and sphingolipid in skeletal muscle and circulating lipids
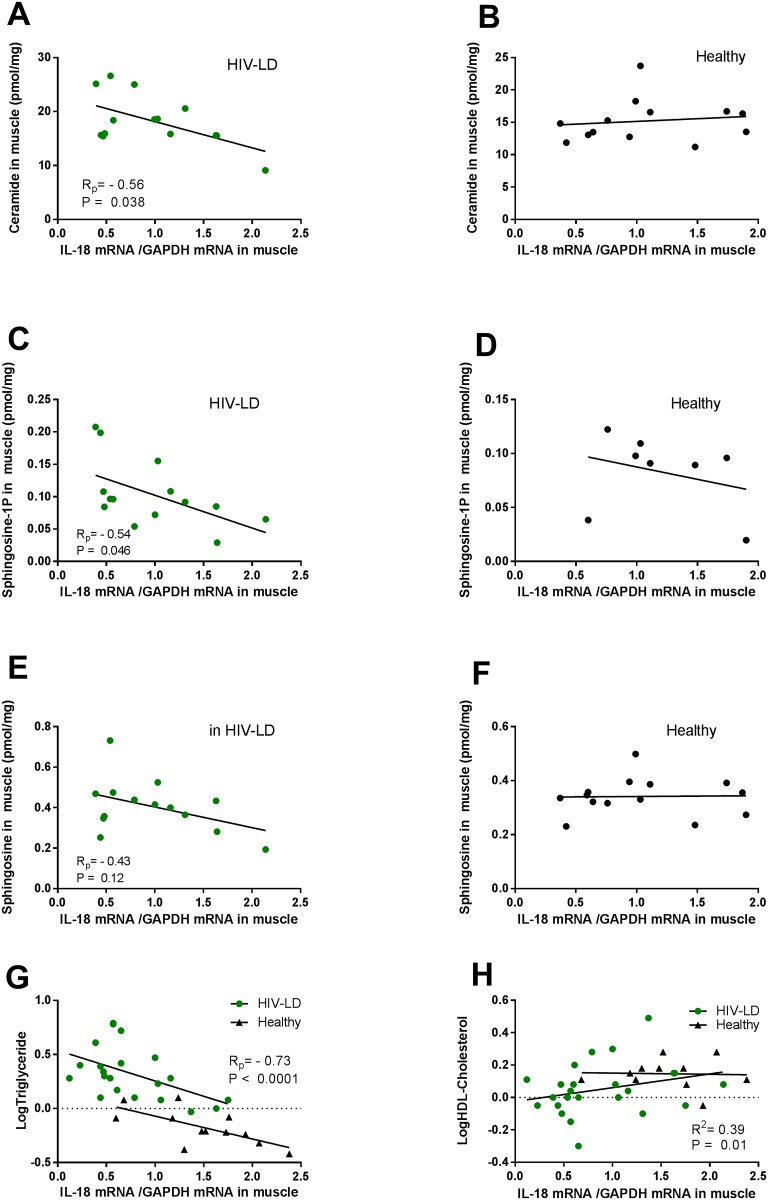
In patients with HIV-lipodystrophy, low expression of IL-18 mRNA in skeletal muscle correlated to high levels of ceramides (Rp = -0.56; p = 0.038) (Fig 3A) and high levels of sphingosine-1P (IL-18 mRNA Rp = -0.54, p = 0.046) (Fig 3C). The same trend was observed for sphingosine (Rp = -0.43, p = 0.12) (Fig 3E). In healthy subjects, IL-18 mRNA in skeletal muscle did not correlate to muscle sphingolipids (Fig 3B, 3D and 3F), although the correlation for sphingosine-1P only included 8 samples and a possible correlation may be lost due to low n-value.
Fig 3. The correlation relationship between muscle IL-18 mRNA and muscle sphingolipid content, circulating triglycerides and HDL-cholesterol in patients with HIV-lipodystrophy (to the right) and in healthy controls (to the left).
IL-18 mRNA in muscle is negatively correlated to ceramide (A) and sphingosine-1P (C) content in muscle in patients with HIV-Lipodystrophy, but not in healthy controls (B, D, F). Il-18 mRNA in muscle is negatively correlated to triglycerides in patients with HIV-Lipodystrophy and in healthy controls (G), and positively correlated to HDL-Cholesterol in patients with HIV-lipodystrophy. Regressions lines, correlations coefficient and significance levels are given for healthy controls and patients with HIV-Lipodystrophy separately.
Reduced expression of IL-18 mRNA in skeletal muscle was associated with increased levels of circulating triglycerides in all subjects pooled together (Rp = -0.73, p<0.0001) (Fig 3G). The same observation was found for IL-18R mRNA expression in skeletal muscle (Rp = -0.56, p = 0.0004, data not shown). When patients with HIV-lipodystrophy and healthy subjects were analysed individually, the same correlation between IL-18 mRNA and triglycerides remained (HIV-LD Rp = -0.51, p = 0.02; Healthy subjects Rp = -0.69, p = 0.014). Reduced expression of IL-18 mRNA and IL-18R mRNA in skeletal muscle was also associated with reduced levels of HDL-cholesterol in all subjects together (for IL-18 mRNA: Rp = 0.39, p = 0.01; for IL-18R mRNA: Rp = 0.48, p = 0.002) (Fig 3H) but not when the groups were analysed separately. No correlations were found between total-cholesterol or LDL-cholesterol and IL-18 mRNA (data not shown).
As patients with HIV-lipodystrophy had a lower VO2 max than the healthy controls in group 2 we examined if the increased levels of sphingolipids were related to low levels of VO2 max in patients with HIV-lipodystrophy. There was, however, no correlation between sphingolipids and VO2max/kg (ceramide Rp = 0.23, p = 0.42; Sphingosine-1P Rp = 0.20, p = 0.48; sphingosine Rp = 0.37, p = 0.19).
In addition, no correlation was found between muscle IL-18 mRNA expression and fat distribution (BMI, fat mass, limb or trunk fat mass) in healthy controls or in patients with HIV-lipodystrophy when analysed separately (data not shown).
No correlation was found between muscle IL-18 mRNA expression and glucose metabolism (plasma insulin, HOMA, glucose and insulin response during an OGTT) in healthy controls or in patients with HIV-lipodystrophy when analysed separately (data not shown).
Expression of mitochondrial genes and fatty acid transporters in skeletal muscles and correlation to ceramide content in skeletal muscle
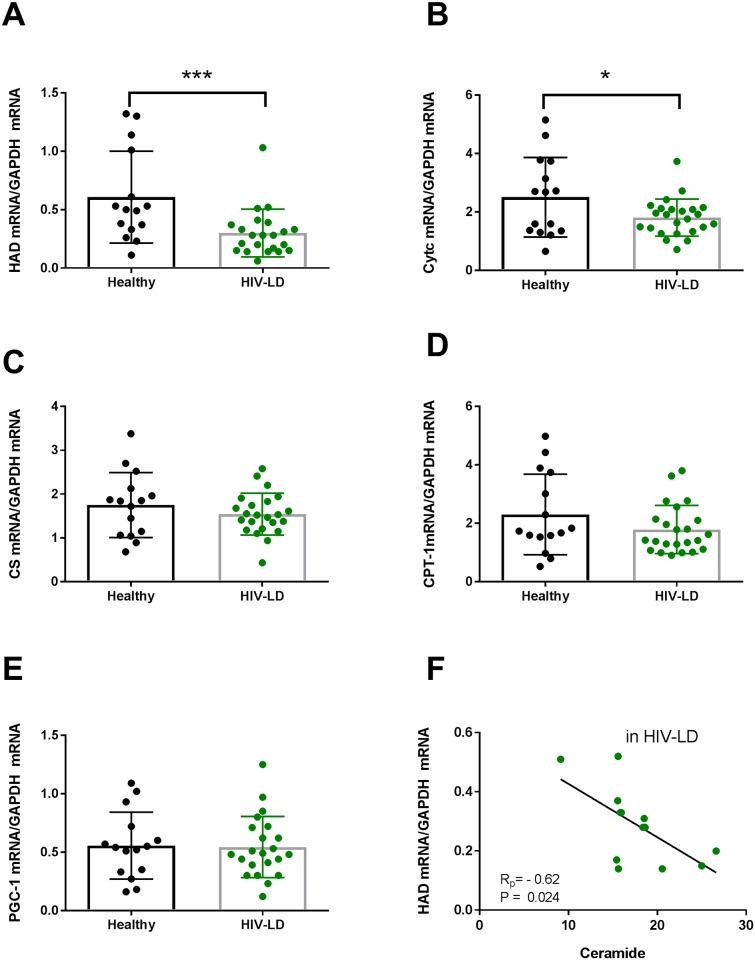
Patients with HIV-lipodystrophy displayed lower mRNA expressions of β-hydroxy acyl-CoA dehydrogenase (β-HAD) (p = 0.004) (Fig 4A) and cytochrome c oxidase (p = 0.03) (Fig 4B) compared to controls. No differences between groups were observed for citrate synthase, CPT-1, and PGC-1 alpha (Fig 4C, 4D and 4E).
Fig 4.
Patients with HIV-Lipodystrophy have reduced levels of HAD mRNA (A) and Cytochrome c mRNA (B) in skeletal muscle, but no difference in citrate synthase mRNA (C), CPT-1 mRNA (D) and PGC-1 mRNA (E). HAD mRNA correlated negatively to the ceramide content (F). The levels of genes were calculated with GAPDH as a housekeeping gene. In the dot plots data for each subjects are given and the line represent means and SD. * P<0.05 and ***P<0.001 for healthy vs HIV-lipodystrophy patients. Regressions lines are given HIV-patients separately.
In patients with HIV-Lipodystrophy the content of ceramide in muscle correlated negative to the levels of β-HAD mRNA expressions (R = -0.61; p = 0.02) (Fig 4F), but not to cytochrome c oxidase (R = -0.10, p = 0.72) (Data not shown).
Discussion
The major finding of this study is that the expression of IL-18 mRNA and IL-18 receptor mRNA is reduced in skeletal muscle in patients with HIV-lipodystrophy compared to healthy age-matched men. Furthermore, low expression of muscle IL-18 mRNA correlates to high levels of ceramides in skeletal muscle and to increased levels of circulating triglycerides and low levels of HDL-cholesterol in patients with HIV-lipodystrophy.
IL-18 and IL-18 receptor in skeletal muscle
IL-18 is expressed in skeletal muscle [5] and high expression of IL-18 has been reported in skeletal muscle in inflammatory diseases such as inflammatory myopathies [48] and COPD [49]. Macrophages and dendritic cells are suggested to be the main producers of IL-18 [48]. Furthermore, it has been suggested that inflammation e.g. TNF-α trigger the IL-18 expression in skeletal muscle [50].
In contrast to those studies, we found a reduced expression of IL-18 and IL-18 Receptor mRNA in skeletal muscles in patients with HIV-lipodystrophy compared to healthy controls, although circulating IL-18 was increased in those patients. Therefore, we conclude that IL-18 signaling pathway is impaired in skeletal muscle in patients with HIV-lipodystrophy and that the muscle cell is not a source of circulating IL-18. Instead, we suggest that in healthy humans IL-18 is working in a local manner in skeletal muscle. It has previously been demonstrated that IL-18 mRNA is induced e.g. by TNF without influence circulating IL-18 [50]. Furthermore, several proteins (e.g. IL-8 and BDNF) are produced by skeletal muscle but not released into the circulation, and those proteins work via autocrine or paracrine mechanisms, exerting their effects on signalling pathways within the muscle itself [51].
The explanation for reduced IL-18 and IL-18 receptor expression in skeletal muscle is intriguing as the underlying mechanisms for the IL-18 production and IL-18 receptor regulation are still poorly understood. IL-18 production is tightly regulated, and achieved in a caspase-1 dependent or in a caspase-1-independent way. The components for cleavage of caspase-1, the inflammasome complexes, such as nucleotide-binding oligomerization domain receptors 1 (NLRP)-1, NLRP-3 and NLRC-4, is present in adipose tissue [52], and in skeletal muscle [53]. In adipose tissue, NLRP1 is an innate immune sensor that functions in the context of metabolic stress to produce IL-18, preventing obesity and diet-induced metabolic dysfunction [22,23]. NLRP1 is also highly expressed in skeletal muscle in humans [54], but it is unknown how NLRP1 is regulated in skeletal muscle and if NLRP-1 stimulates production of IL-18 in skeletal muscle, in a similar way as in adipose tissue.
The reduced IL-18 and IL-18 receptor in skeletal muscle in patients with HIV-Lipodystrophy may also be link to IL-18 resistance. Obese subjects and person with type 2 diabetes demonstrate impaired IL-18 responsiveness in leucocyte despite increased circulating levels of IL-18 [55]. The IL-18 receptor is suggested to be the responsible molecular site for the observed IL-18 resistance, as leucocyte from obese and type 2 diabetics demonstrated a reduced IL-18 receptor expression on leucocyte. However, no mechanism for reduced IL-18 receptor expression was found and the expression of IL-18 on the leucocyte was not measured. Netea and colleagues [55] explains IL-18 resistance as an immunological phenomenon; and hypothesize that a similar resistance to the metabolic effect of IL-18 is also present [55]. It is possible that the reduced IL-18 receptor expression in skeletal muscle found in the current study play a role in a resistance to the metabolic effects of IL-18. This has been shown for IL-6. where skeletal muscle in obesity-associated type 2 diabetes develops a resistance to IL-6 [56]. However, this hypothesis has to be tested an in vitro study.
The role of IL-18 in lipid metabolism
In the last years, several animal studies have demonstrated that IL-18 play a role in metabolism, which is independent of its inflammatory role. Mice lacking IL-18 or the IL-18 receptor and therefore impaired IL-18 signalling become obese and display hyperinsulinemia, insulin resistance and dyslipidemia [19–21]. We found an inverse correlation between IL-18 expression in skeletal muscle and systemic triglycerides and HDL-cholesterol in both healthy subjects and in patients with HIV-lipodystrophy. Production of VLDL-TG is in part due to be the increased flux of FFA to the liver in combination with insulin resistance associated hyperinsulinemia [57,58] and this may be a way linking the role of IL-18 in skeletal muscle to dyslipidemia.
In addition, IL-18 and IL-18 receptor deficient mice have an excess of intramyocellular lipids (IMCL) which in part is explained by impaired beta-oxidation in muscle due to a defect in one of major pathway that regulates fatty acid oxidation AMP-activated protein kinase (AMPK) [21]. Treating myotubes or skeletal muscle strips with IL-18 activates AMPK and increases fat oxidation. Furthermore, overexpression of IL-18 in mice leads to reduced fat mass, increased activation of AMPK and increased mRNA abundance of β-hydroxyacyl-CoA-dehydrogenase (HAD), a key enzyme involved in mitochondrial function and hence increased fat oxidation [21] -, implicating IL-18 in metabolic homeostasis.
Having found that IL-18 mRNA expression was lower in skeletal muscle in patients with HIV-lipodystrophy, we investigated whether this was associated to an increased content of IMCL, as observed in mice lacking the IL-18 signalling pathway [21]. It is known that patients with HIV-lipodystrophy have increased level of IMCLs [24,30–32]. IMCLs are mainly composed by triacylglycerol but also include lipid intermediates such as diacylglycerol, sphingolipid, and phospholipid. Sphingolipids, including ceramide, sphingosine, sphingosine-1P and sphinganine have never been measured in skeletal muscle from patients with HIV-lipodystrophy, but are altered in other metabolic state such as obesity and type 2 diabetes. [59–61]. We found a trend towards increased contents of the sphingolipids ceramide and sphingosine, and that the reduced levels of IL-18 mRNA in muscle correlated with increased contents of ceramides and sphingosine-1P in patients with HIV-lipodystrophy, but not in healthy controls, demonstrating that skeletal muscle IL-18 and sphingolipids are linked. As this study is a cross-sectional study it is not possible to describe the causal relationship. Ceramide has been demonstrated to be involved in IL-18 production in macrophages and adipose tissue via increased production of reactive oxygen species, which acts as a secondary signal for NLRP3 activation leading to induction of caspase-1 cleavage and thereby increased IL-18 [62]. However, in the current study IL-18 expression was decreased and not increased in skeletal muscle in HIV-lipodystrophy, and ceramide can therefore not explain reduced expression of IL-18. In contrast, it is possible that low expression of IL-18 is causal involved in elevated content of ceramide, just like in the IL-18 deficient and IL-18 receptor deficient mice.
It is unknown whether increased level of ceramide is directly a cause of HAART, as no studies have demonstrated an effect of HAART per se on ceramide. Instead, hiv proteins (gp120 og TAT) can induces sphingolipids [63]. In our patients viral load was below the detection limits for all patients therefore it is unlikely that the increased levels of ceramide in muscle may be induced by hiv per se. Instead, ectopic lipid deposition is a part of the lipodystrophy syndrome seen in HAART treated HIV patients, and therefore the increased ceramide levels may be indirectly results of the antiretroviral therapy.
IL-18 and mitochondrial activity
As described in rodents, IL-18 increases fatty acid oxidation through activation of AMPK and mitochondrial oxidation [21]. Patients with HIV-lipodystrophy display impaired fat oxidation [30] [29] and two recent studies show impaired mitochondrial oxidative phosphorylation [64,65] and activity of enzymes involved in fat oxidation; e.g. β-HAD and citrate synthase [33] in skeletal muscle in HIV patients. Our data is in accordance with those findings as we found reduced expression of β-HAD and cytochrome c oxidase mRNA in skeletal muscle in HIV patients with lipodystrophy. Furthermore, we showed that the ceramide content is negatively correlated with the expression of β-HAD. It has previously been demonstrated that ceramides alter mitochondrial dynamics in skeletal muscle [41] and therefore, impaired mitochondrial function may be secondary to increased ceramide observed in skeletal muscle in patients with HIV-LD. However, impaired mitochondrial fatty acid oxidation is also known to increase IMCLs, diacylglycerols, and ceramides [66].
As specific mRNA measurements correlated to each due to the use of the same house keeping gene (GAPDH) it was not possible to make a correlational relationship analysis between IL-18 mRNA expression and mRNA expression of mitochondrial gene in our study. As a negative correlation between ceramide content and the expression of β-HAD mRNA as well as a negative correlation between ceramide content and the expression of IL-18 mRNA were present, we can only speculate that IL-18 mRNA may correlate to expression of mitochondrial genes as well.
In conclusion, our findings suggest that muscular IL-18 may be involved in the regulation of intramuscular lipid metabolism and hypertriglyceridemia.
Acknowledgments
We thank the subjects for their participation in this study. Ruth Rousing, Hanne Willumsen, and Flemming Jessen are thanked for excellent technical help. The Danish HIV-Cohort is thanked for providing us HIV-related data.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Danish National Research Foundation (DNRF55). The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. This study was further supported by grants from the Lundbeck foundation, the Danish AIDS Foundation, the Novo Nordisk Foundation, Direktør Emil Hertz og Hustru Inger Hertz´ Fond, Direktør Jacob Madsen og Hustru Olga Madsens Fond, Fonden for Lægevidenskabens Fremme. CIM/CFAS is a member of DD2 - the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). The funders had a no role in study design, data collection adn analysis, decision to publish, or prepation of the manuscript.
References
- 1.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T et al. (1995) Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378: 88–91. doi: 10.1038/378088a0 [DOI] [PubMed] [Google Scholar]
- 2.Novick D, Kim S, Kaplanski G, Dinarello CA (2013) Interleukin-18, more than a Th1 cytokine. Semin Immunol 25: 439–448. doi: 10.1016/j.smim.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 3.Fukami T, Miyazaki E, Matsumoto T, Kumamoto T, Tsuda T (2001) Elevated expression of interleukin-18 in the granulomatous lesions of muscular sarcoidosis. Clin Immunol 101: 12–20. doi: 10.1006/clim.2001.5080 [DOI] [PubMed] [Google Scholar]
- 4.Plomgaard P, Penkowa M, Pedersen BK (2005) Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev 11:53–63.: 53–63. [PubMed] [Google Scholar]
- 5.Bruun JM, Stallknecht B, Helge JW, Richelsen B (2007) Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 157: 465–471. doi: 10.1530/EJE-07-0206 [DOI] [PubMed] [Google Scholar]
- 6.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A et al. (1997) Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386: 619–623. doi: 10.1038/386619a0 [DOI] [PubMed] [Google Scholar]
- 7.Alboni S, Cervia D, Sugama S, Conti B (2010) Interleukin 18 in the CNS. J Neuroinflammation 7:9: 9. doi: 10.1186/1742-2094-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo JK, Kwon H, Khil LY, Zhang L, Jun HS, Yoon JW (2005) IL-18 induces monocyte chemotactic protein-1 production in macrophages through the phosphatidylinositol 3-kinase/Akt and MEK/ERK1/2 pathways. J Immunol 175: 8280–8286. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekar B, Mummidi S, Valente AJ, Patel DN, Bailey SR, Freeman G et al. (2005) The pro-atherogenic cytokine interleukin-18 induces CXCL16 expression in rat aortic smooth muscle cells via MyD88, interleukin-1 receptor-associated kinase, tumor necrosis factor receptor-associated factor 6, c-Src, phosphatidylinositol 3-kinase, Akt, c-Jun N-terminal kinase, and activator protein-1 signaling. J Biol Chem 280: 26263–26277. doi: 10.1074/jbc.M502586200 [DOI] [PubMed] [Google Scholar]
- 10.Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S et al. (2000) IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol 165: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y et al. (1999) Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol 162: 5041–5044. [PubMed] [Google Scholar]
- 12.Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G et al. (2002) Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab 87: 3864–3866. doi: 10.1210/jcem.87.8.8781 [DOI] [PubMed] [Google Scholar]
- 13.Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S et al. (2003) Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52: 605–608. doi: 10.1053/meta.2003.50096 [DOI] [PubMed] [Google Scholar]
- 14.Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T (2003) Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care 26: 2622–2627. [DOI] [PubMed] [Google Scholar]
- 15.Wood IS, Wang B, Jenkins JR, Trayhurn P (2005) The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun 337: 422–429. doi: 10.1016/j.bbrc.2005.09.068 [DOI] [PubMed] [Google Scholar]
- 16.Fain JN, Tichansky DS, Madan AK (2006) Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metabolism 55: 1113–1121. doi: 10.1016/j.metabol.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A et al. (2011) The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 152: 3769–3778. doi: 10.1210/en.2010-1480 [DOI] [PubMed] [Google Scholar]
- 18.Spoto B, Di BE, Mattace-Raso F, Sijbrands E, Vilardi A, Parlongo RM, Pizzini P et al. (2014) Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr Metab Cardiovasc Dis 24: 1137–1143. doi: 10.1016/j.numecd.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ et al. (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12: 650–656. doi: 10.1038/nm1415 [DOI] [PubMed] [Google Scholar]
- 20.Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T et al. (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A 104: 11097–11102. doi: 10.1073/pnas.0611523104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindegaard B, Matthews VB, Brandt C, Hojman P, Allen TL, Estevez E et al. (2013) Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes 62: 3064–3074. doi: 10.2337/db12-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MK, Yvan-Charvet L, Masters SL, Murphy AJ (2016) The modern interleukin-1 superfamily: Divergent roles in obesity. Semin Immunol 28: 441–449. doi: 10.1016/j.smim.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Murphy AJ, Kraakman MJ, Kammoun HL, Dragoljevic D, Lee MK, Lawlor KE et al. (2016) IL-18 Production from the NLRP1 Inflammasome Prevents Obesity and Metabolic Syndrome. Cell Metab 23: 155–164. doi: 10.1016/j.cmet.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 24.Villarroya F, Domingo P, Giralt M (2010) Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim Biophys Acta 1801: 392–399. doi: 10.1016/j.bbalip.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 25.Capeau J (2007) From lipodystrophy and insulin resistance to metabolic syndrome: HIV infection, treatment and aging. Curr Opin HIV AIDS 2: 247–252. doi: 10.1097/COH.0b013e3281e66919 [DOI] [PubMed] [Google Scholar]
- 26.de WR, Cohen K, Maartens G (2013) Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction. PLoS One 8: e63623 doi: 10.1371/journal.pone.0063623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers M, Walker UA, Sevastianova K, Setzer B, Wagsater D, Eriksson P et al. (2009) Gene expression and immunohistochemistry in adipose tissue of HIV type 1-infected patients with nucleoside analogue reverse-transcriptase inhibitor-associated lipoatrophy. J Infect Dis 200: 252–262. doi: 10.1086/599986 [DOI] [PubMed] [Google Scholar]
- 28.Villarroya F, Domingo P, Giralt M (2007) Mechanisms of antiretroviral-induced mitochondrial dysfunction in adipocytes and adipose tissue: in-vitro, animal and human adipose tissue studies. Curr Opin HIV AIDS 2: 261–267. doi: 10.1097/COH.0b013e32810fd785 [DOI] [PubMed] [Google Scholar]
- 29.Vassimon HS, de Paula FJ, Machado AA, Monteiro JP, Jordao AA Jr. (2012) Hypermetabolism and altered substrate oxidation in HIV-infected patients with lipodystrophy. Nutrition 28: 912–916. doi: 10.1016/j.nut.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 30.Luzi L, Perseghin G, Tambussi G, Meneghini E, Scifo P, Pagliato E et al. (2003) Intramyocellular lipid accumulation and reduced whole body lipid oxidation in HIV lipodystrophy. Am J Physiol Endocrinol Metab 284: E274–E280. doi: 10.1152/ajpendo.00391.2001 [DOI] [PubMed] [Google Scholar]
- 31.Torriani M, Hadigan C, Jensen ME, Grinspoon S (2003) Psoas muscle attenuation measurement with computed tomography indicates intra-muscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol (1985) 95(3):1005–10 [DOI] [PubMed] [Google Scholar]
- 32.Gan SK, Samaras K, Thompson CH, Kraegen EW, Carr A, Cooper DA et al. (2002) Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes 51: 3163–3169. [DOI] [PubMed] [Google Scholar]
- 33.Ortmeyer HK, Ryan AS, Hafer-Macko C, Oursler KK (2016) Skeletal muscle cellular metabolism in older HIV-infected men. Physiol Rep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindegaard B, Hansen AB, Gerstoft J, Pedersen BK (2004) High plasma level of interleukin-18 in HIV-infected subjects with lipodystrophy. J Acquir Immune Defic Syndr 36: 588–593. [DOI] [PubMed] [Google Scholar]
- 35.Falasca K, Ucciferri C, Manzoli L, Mancino P, Pizzigallo E, Conti P et al. (2007) Metabolic syndrome and cardiovascular risk in HIV-infected patients with lipodystrophy. Int J Immunopathol Pharmacol 20: 519–527. doi: 10.1177/039463200702000310 [DOI] [PubMed] [Google Scholar]
- 36.Lindegaard B, Hansen AB, Pilegaard H, Keller P, Gerstoft J, Pedersen BK (2004) Adipose tissue expression of IL-18 and HIV-associated lipodystrophy. AIDS 18: 1956–1958. [DOI] [PubMed] [Google Scholar]
- 37.Gallego-Escuredo JM, Villarroya J, Domingo P, Targarona EM, Alegre M, Domingo JC et al. (2013) Differentially altered molecular signature of visceral adipose tissue in HIV-1-associated lipodystrophy. J Acquir Immune Defic Syndr 64: 142–148. doi: 10.1097/QAI.0b013e31829bdb67 [DOI] [PubMed] [Google Scholar]
- 38.Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM et al. (2011) Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60: 2588–2597. doi: 10.2337/db10-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitessa SM, Abeywardena MY (2016) Lipid-Induced Insulin Resistance in Skeletal Muscle: The Chase for the Culprit Goes from Total Intramuscular Fat to Lipid Intermediates, and Finally to Species of Lipid Intermediates. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH et al. (2016) Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia 59: 785–798. doi: 10.1007/s00125-015-3850-y [DOI] [PubMed] [Google Scholar]
- 41.Park M, Kaddai V, Ching J, Fridianto KT, Sieli RJ, Sugii S et al. (2016) A Role for Ceramides, but Not Sphingomyelins, as Antagonists of Insulin Signaling and Mitochondrial Metabolism in C2C12 Myotubes. J Biol Chem 291: 23978–23988. doi: 10.1074/jbc.M116.737684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindegaard B, Hvid T, Grondahl T, Frosig C, Gerstoft J, Hojman P et al. (2013) Expression of fibroblast growth factor-21 in muscle is associated with lipodystrophy, insulin resistance and lipid disturbances in patients with HIV. PLoS One 8: e55632 doi: 10.1371/journal.pone.0055632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG (2003) An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet 361: 726–735. [DOI] [PubMed] [Google Scholar]
- 44.Lindegaard B, Hansen T, Hvid T, van HG, Plomgaard P, Ditlevsen S et al. (2008) The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. J Clin Endocrinol Metab 93: 3860–3869. doi: 10.1210/jc.2007-2733 [DOI] [PubMed] [Google Scholar]
- 45.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ (1983) Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol 55: 1558–1564. doi: 10.1152/jappl.1983.55.5.1558 [DOI] [PubMed] [Google Scholar]
- 46.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 47.Knapp M, Lisowska A, Zabielski P, Musial W, Baranowski M (2013) Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat 106: 53–61. doi: 10.1016/j.prostaglandins.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 48.Tucci M, Quatraro C, Dammacco F, Silvestris F (2006) Interleukin-18 overexpression as a hallmark of the activity of autoimmune inflammatory myopathies. Clin Exp Immunol 146: 21–31. doi: 10.1111/j.1365-2249.2006.03180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen AM, Penkowa M, Iversen M, Frydelund-Larsen L, Andersen JL, Mortensen J et al. (2007) Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease. Lung 185: 161–171. doi: 10.1007/s00408-007-9000-7 [DOI] [PubMed] [Google Scholar]
- 50.Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK (2006) Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab 291: E108–E114. doi: 10.1152/ajpendo.00471.2005 [DOI] [PubMed] [Google Scholar]
- 51.Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465. doi: 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 52.Smart MC, Dedoussis G, Yiannakouris N, Grisoni ML, Dror GK, Yannakoulia M et al. (2011) Genetic variation within IL18 is associated with insulin levels, insulin resistance and postprandial measures. Nutr Metab Cardiovasc Dis 21: 476–484. doi: 10.1016/j.numecd.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benetti E, Chiazza F, Patel NS, Collino M (2013) The NLRP3 Inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediators Inflamm 2013: 678627 doi: 10.1155/2013/678627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ (2010) Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol 22: 717–728. doi: 10.1093/intimm/dxq058 [DOI] [PubMed] [Google Scholar]
- 55.Zilverschoon GR, Tack CJ, Joosten LA, Kullberg BJ, Van Der Meer JW, Netea MG (2008) Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- 56.Scheele C, Nielsen S, Kelly M, Broholm C, Nielsen AR, Taudorf S et al. (2012) Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6. PLoS One 7: e39657 doi: 10.1371/journal.pone.0039657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boden G (2008) Obesity and free fatty acids. Endocrinol Metab Clin North Am 37: 635–6ix. doi: 10.1016/j.ecl.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebbert JO, Jensen MD (2013) Fat depots, free fatty acids, and dyslipidemia. Nutrients 5: 498–508. doi: 10.3390/nu5020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC et al. (2004) Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31. [DOI] [PubMed] [Google Scholar]
- 60.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P et al. (2007) Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50: 2366–2373. doi: 10.1007/s00125-007-0781-2 [DOI] [PubMed] [Google Scholar]
- 61.Coen PM, Dube JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG et al. (2010) Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59: 80–88. doi: 10.2337/db09-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma D, Kanneganti TD (2016) The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol 213: 617–629. doi: 10.1083/jcb.201602089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA et al. (2004) Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 55: 257–267. doi: 10.1002/ana.10828 [DOI] [PubMed] [Google Scholar]
- 64.Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC et al. (2011) Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 43: 806–810. doi: 10.1038/ng.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maagaard A, Holberg-Petersen M, Kollberg G, Oldfors A, Sandvik L, Bruun JN (2006) Mitochondrial (mt)DNA changes in tissue may not be reflected by depletion of mtDNA in peripheral blood mononuclear cells in HIV-infected patients. Antivir Ther 11: 601–608. [PubMed] [Google Scholar]
- 66.Wicks SE, Vandanmagsar B, Haynie KR, Fuller SE, Warfel JD, Stephens JM et al. (2015) Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc Natl Acad Sci U S A 112: E3300–E3309. doi: 10.1073/pnas.1418560112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.