Abstract
Objectives
Objectives were to 1) compare self-reported function, dexterity, activity performance, quality of life and community integration of the DEKA Arm to conventional prostheses; and 2) examine differences in outcomes by conventional prosthesis type, terminal device type and by DEKA Arm configuration level.
Methods
This was a two-part study; Part A consisted of in-laboratory training. Part B consisted of home use. Study participants were 23 prosthesis users (mean age = 45 ± 16; 87% male) who completed Part A, and 15 (mean age = 45 ± 18; 87% male) who completed Parts A and B. Outcomes including self-report and performance measures, were collected at Baseline using participants’ personal prostheses and at the End of Parts A and B. Scores were compared using paired t-tests. Wilcoxon signed-rank tests were used to compare outcomes for the full sample, and for the sample stratified by device and terminal device type. Analysis of outcomes by configuration level was performed graphically.
Results
At the End of Part A activity performance using the DEKA Arm and conventional prosthesis was equivalent, but slower with the DEKA Arm. After Part B, performance using the DEKA Arm surpassed conventional prosthesis scores, and speed of activity completion was equivalent. Participants reported using the DEKA Arm to perform more activities, had less perceived disability, and less difficulty in activities at the End of A and B as compared to Baseline. No differences were observed in dexterity, prosthetic skill, spontaneity, pain, community integration or quality of life. Comparisons stratified by device type revealed similar patterns. Graphic comparisons revealed variations by configuration level.
Conclusion
Participants using the DEKA Arm had less perceived disability and more engagement of the prosthesis in everyday tasks, although activity performance was slower. After home use experience, activity performance was improved and activity speed equivalent to using conventional prostheses.
Introduction
As the availability of and consumer demand for more advanced and expensive upper limb prosthetic devices increases, so does the need for studies that compare the effectiveness of these devices. The DEKA Arm is an example of a new technologically advanced upper limb prosthesis. It was developed under the Revolutionizing Prosthetics Program through DARPA funding,[1] and approved by U.S. Food and Drug Administration in 2014. It has recently become commercially available and is being called the Luke Arm. [2]
The device is available in 3 configurations or levels: radial configuration (RC) for persons with radial amputation; humeral configuration (HC) for persons with humeral amputation; and shoulder configuration (SC) for persons with shoulder disarticulation, forequarter amputation or very short transhumeral amputation. [1] Unique features of all configuration levels are a powered wrist which allows flexion and extension combined with radial and ulnar deviation, powered wrist pronation and supination and six programmable hand grip patterns. The HC and SC have powered elbow flexion and extension and powered humeral rotation. The SC has additional powered shoulder movements and is controlled by Endpoint control which allows simultaneous joint control. [3] The DEKA Arm is controlled primarily with inertial measurement units (IMUs) secured to top of the shoes. However this control method may be supplemented by electromyography (EMG) controls, pressure transducers, and conventional controls such as linear transducers, and rocker or other switches. [4] All control options are configurable using a wireless Prosthetist interface that allows customization of the prosthetic actions/functions assigned to each control as well as the thresholds and gains of each control. Selection of control type, and configuration of those controls are guided by user preference and prosthetist judgement. In all cases, subjects used some type of switch or transducer (pressure transducer, linear transducer, rocker switch for mode selection (HC level) and for going into and out of stand-by mode (all subjects). Experienced users of dual site EMG choose to retain dual site control for one DOF (typically hand open/close, and then use IMU controls for other DOFs and functions.
Although the technological capabilities of the DEKA Arm promise increase functionality, limited research has compared functional abilities with the DEKA Arm to function with the conventional prostheses [5] and no studies have compared outcomes such as quality of life and community integration. One earlier study directly compared performance based and self-reported outcomes from 26 subjects using both the DEKA Arm and conventional prostheses. Subjects using either the Generation 2 (Gen 2) (N = 17) or Generation 3 (Gen 3) (N = 9) prototypes of the DEKA Arm[5] found that use of the prosthesis to perform activities, spontaneity of prosthesis use and perceived difficulty performing self-selected tasks was greater with the DEKA Arm as compared to conventional prosthesis, but dexterity scores were worse in 2/7 tests. However, differences in outcomes varied by configuration level; for example, for SC users’ activity performance was rated better when using the DEKA Arm as compared to the conventional prosthesis.
Conclusions based on the prior analyses are limited for several reasons. First, all subjects were experienced prosthesis users (experience ranging from 3 months to decades), and their exposure to the DEKA Arm was limited to structured training. Therefore the amount of exposure to each type of device was not equivalent. Despite structured prosthetic training in the study, some subjects may not have fully acclimated to the DEKA Arm. It is conceivable that with additional experience gained from home use their perceived function and functional performance would continue to improve. Another limitation of the earlier study is that it compared outcomes of the existing prosthesis to outcomes of both the Generation 2 (Gen 2) and Generation 3 (Gen 3) prototypes of the DEKA Arm and did not perform any sub-group analysis by device prototype. Given that the Gen 3 prototype included significant design changes meant to improve usability [1] it is possible that the inclusion of Gen 2 users biased comparisons of the DEKA Arm to the conventional prostheses towards the null. Lastly, the earlier analysis compared outcomes of self-reported disability, prosthesis use, and dexterity and activity performance but did not compare other important outcomes such as quality of life and community integration that theoretically could be impacted by upper limb prosthesis use.
Additional research is needed to build upon prior work. Therefore, the purposes of this study were to: 1) compare self-reported function, dexterity, activity performance, quality of life and community integration of the Gen 3 DEKA Arm to conventional prostheses; and 2) examine differences in outcomes by conventional prosthesis type, terminal device type and by DEKA Arm configuration level.
Methods
Study design
The VA Home Study of an Advanced Upper Limb Prosthesis (Home Study) was a quasi-experimental study that used a time series design. The research was approved by the Institutional Review Boards of the Providence VA Medical Center, the James Haley VA (Tampa), the VA NY Health Harbor System, and the Center for the Intrepid at Brooke Army Medical Center. Written informed consent was obtained from all subjects. The study had 2 parts: in-laboratory training with the DEKA Arm (Part A) and home use with the DEKA Arm (Part B). All participants enrolled in Part A and then a subset continued to Part B. During Part A participants were fit with and trained to use the DEKA Arm. During Part B participants used the DEKA Arm at home for up to 12 weeks, and returned for in-person re-evaluations on-site every 4 weeks.
Subjects
Participants who reported that they regularly used a personal prosthesis (prosthesis users) and completed Part A of the study were included in this analysis. Participants eligible for Part A enrollment in the Home study were at least 18 years old and had an upper limb amputation at the transradial, transhumeral, shoulder disarticulation or scapulothoracic level. Persons with residual limb or skin conditions prohibiting socket fitting or with serious health conditions which the study staff believed would limit participation were excluded. At the End of Part A, the Principal Investigator in consultation with the study staff determined the eligibility for Part B based on observed behavior during Part A. Participants were eligible if they had at least fair functional use of the DEKA Arm (as gauged by the study occupational therapist), and demonstrated consistent safety awareness and sound judgement and the ability to troubleshoot minor technical issues.
Data collection
At Baseline, at the End of Parts A and B, the study occupational therapists (OTs) administered a set of standardized measures to participants (Table 1). Participants, who were prosthesis users, usually completed performance measures at Baseline wearing their own prosthesis. However, on occasion, a prosthesis user did not utilize his/her device during Baseline testing because it was unavailable or broken. At Baseline, questions in self-report measures referred only to the participant’s personal prosthesis. At the End of Parts A and B, participants answered questions pertaining to the DEKA Arm and completed performance tests using the DEKA Arm.
Table 1. Outcome measures.
| Measure | Construct | Brief description | Response | Higher scores indicate… |
|---|---|---|---|---|
| Dexterity | ||||
| Jebsen-Taylor Hand Function Test (JTHF) | Dexterity | 7 tests of hand function | Performance speed; items.sec | better performance |
| Activity | ||||
| Activities Measure for Upper-Limb Amputees (AM-ULA) | Activity performance | 18-everyday tasks | Task completion: speed, movement quality, skill and independence | better performance |
| University of New Brunswick Test of Prosthetic Function (UNB): Skill | Prosthetic skill | 10 components of daily tasks requiring bimanual engagement | Skillfulness of terminal device use. | better performance |
| University of New Brunswick Test of Prosthetic Function (UNB): Spontaneity | Prosthetic spontaneity | 10 components of daily tasks requiring bimanual engagement | Spontaneity of engaging the prosthesis in activities | better performance |
| Timed Measure of Activity Performance (T-MAP) | Activity performance | 5 activities of daily living | Task completion: speed | Worse performance |
| Brief Activity Measure for Upper Limb Amputees (BAM-ULA) | Activity performance | 10 items of functional task performance | Task completion: Unable to complete; Can complete | better performance |
| Self-Reported Function | ||||
| Disabilities of the Arm, Shoulder and Hand Score (QuickDASH) | Disability | Self-reported functional difficulty (8 items) 3 items about sleep, sensation and pain | Performance difficulty and impairment severity | greater disability |
| Upper-Extremity Functional Scale (UEFS) | Activity performance | Self-reported difficulty performing 23 everyday activities | Difficulty in performance | greater difficulty |
| Upper-Extremity Functional Scale (Use) | Use of prosthesis | Self-reported use of the prosthesis during everyday activities | Prosthesis use | more activities done with prosthesis |
| Patient-Specific Functional Scale (PSFS) | Difficulty performing activities | 5 self-selected activities difficult to do because of the amputation | Difficulty in performance | less difficulty |
| Other Measures | ||||
| Wong-Baker FACES Pain Rating Scale | Pain | Six faces showing levels of pain severity | Pain intensity | more pain |
| Quality of Life (QOL) | Quality of life | 16 question items about quality of life | Satisfaction with quality of life | better QOL |
| The Community Reintegration of Service Members Computer Adaptive test (CRIS-CAT) | Computer adaptive testing measuring participation in life roles | better community integration | ||
| CRIS-CAT Extent of Participation | Extent of participation | Frequency and amount | ||
| CRIS-CAT Perceived Limitations | Perceived difficulty | Perceived limitations | ||
| CRIS-CAT Satisfaction with Participation | Satisfaction | Satisfaction scale | ||
| Trinity Amputation and Prosthesis Experience Scales (TAPES) | Prosthetic satisfaction | 10 items satisfaction with prosthesis | Satisfaction | greater satisfaction |
We selected a broad range of validated measures to assess important constructs for upper limb amputees. Performance based measures included a dexterity measure, theJebsen-Taylor Hand Function Test (JTHFT),[6, 7] 4 and measures of activty performance: the Activities Measure for Upper Limb Amputees (AM-ULA) [8]; University of New Brunswick Test of Prosthetic Function for Unilateral Amputees (UNB);[9, 10] Timed Measure of Activity Performance (T-MAP), [11] and Brief Activity Measure for Upper Limb Amputees (BAM-ULA).[12] Although each of the activity measures assesses performance of daily activities, they differ considerably in their scoring criteria and item content. For instance, the T-MAP assesses the time it takes to perform an activity; while the AM-ULA assesses body compensation during activity performance. Given that there is no accepted gold standard activity measure, we believed that inclusion of multiple metrics would provide important information. Self-report Measures included: Disabilities of the Arm, Shoulder and Hand Score (QuickDASH),[13] Upper Extremity Functional Scale (UEFS),[14] Patient Specific Functional Scale (PSFS),[15] Wong-Baker FACES Pain Rating Scale (Wong-Baker),[16] Quality of Life (QOL) scale,[17] Community Reintegration of Service Members Computer Adaptive test (CRIS-CAT),[18] and; Trinity Amputation and Prosthesis Experience Satisfaction Scale (TAPES).[19] Each measure is described below.
Modified Jebsen-Taylor Hand Function (JTHFT)
The JJTHF is a measure of dexterity and simple functional activities[20]. The 7 JTHF subtasks are: writing; page turning; lifting small objects; feeding; lifting large, lightweight objects; lifting large, heavy objects; stacking checkers. The modified version used in this study caps maximal allowable time for each subtask at 2 minutes and scores the number of items completed per second. Thus, higher scores indicate better performance. The reliability and validity of the modified version was demonstrated in upper limb amputees[21], and the responsiveness of specific sub-tasks to prosthetic training with the DEKA Arm has been reported. [22]
The Activities Measure for Upper-Limb Amputees (AM-ULA)
The AM-ULA is measure of activity performance for prosthesis users.[8] The test has 18 items, each of which is scored from 0–4 (unable to excellent), with higher scores indicating better functional performance. The scoring rubric takes a variety of aspects of activity performance into consideration including: sub-task completion, skillfulness of prosthesis use, movement quality, independence, and overall time to perform that activity. Analysis of psychometric properties showed that it has excellent test-retest reliability, interrater reliability, and internal consistency and demonstrated known group validity[8]. The AM-ULA was been shown to be responsive to change after prosthetic training. [22]
University of New Brunswick test of prosthetic function (UNB)
The UNB test is a measure of prosthetic skill and spontaneity that is appropriate for unilateral amputees. [9]. The spontaneity scale measures the extent to which the amputee has incorporated the prosthesis into his or her body image and measures the tendency to use the prosthesis to assist with the task. The skill scale measures the dexterity with which the prosthesis is used. It includes the ability to open and close the terminal device to grasp and release objects with confidence, speed and consistency and maintain grasp without letting go accidentally, and apply correct amount of pressure. This study used one of the subtests of activities designed for ages 11–13 year olds which included 10 activities related to (1) wrapping a parcel, (2) sewing a button on cloth, (3) cutting meat, (4) drying dishes, and (5) sweeping floors. Each activity is rated a 5-point scale of 0–4 for dual functions: spontaneity of prosthetic function (Spontaneity) and skill of prosthetic function (Skill). Higher UNB scores indicate better performance. This subtest has been found to have acceptable internal consistency, test-retest, and interrater reliability and evidence of validity[23], and was responsive to change after prosthetic training. [22]
Timed Measure of Activity Performance (T-MAP)
The T-MAP is a timed based measure of common activity performance developed for upper limb amputees. [11]. It consists of 5 items that were adapted from the Rivermead Extended Activities Index [24] an Instrumental Activities of Daily Living (IADL) measure. The items in the T-MAP are: (1) drink, (2) wash face, (3) food preparation, (4) eating, and (5) dressing activities. The T-MAP revealed has very good internal consistency, excellent test-retest reliability, and evidence of construct validity [11].
Brief Measure of Activity performance (BAM-ULA)
The BAM-ULA is an observational measure of activity performance [12]. The 10 items included in the measure are: tuck a shirt in pants; lift a 20 lb. bag; open a water bottle; remove a wallet from back pocket; replace the wallet in back pocket; take a gallon of water from the refrigerator and place on the counter (lift gallon jug); pour water from a gallon jug; brush or comb hair; use a fork; and open a door with knob. Each item is rated 0 for ‘unable to complete’, or 1 for ‘did complete’. The scores for each item are summed to obtain the overall task completion score. Summary scores are calculated only when all 10 items are rated. Higher task completion scores indicated better performance. Analyses of psychometric properties in a sample of persons with upper limb amputation showed that the BAM-ULA has acceptable internal consistency, test-retest reliability, and displays evidence of construct and concurrent validity [12].
QuickDASH
The QuickDASH is an 11-item disability scale, a shorter version of the Disabilities of the Arm and Shoulder (DASH) measure, which has been validated for use in upper limb amputation. [13] It includes 8 items related to difficulty performing functional activities and 3 items level severity of impairments[25]. Respondents indicate the amount of difficulty performing activities, amount of limitation, or the extent of interference with activities (using 1–5 Likert scales with 1 indicating the least impairment and 5 indicating the most). Items asking about extent of arm, shoulder and hand pain and tingling are rated from 1 (none) to 5 (extreme). The QuickDASH has strong internal consistency and test-retest reliability in upper limb amputees, and demonstrates evidence of known group and construct validity. Additionally, the QuickDASH was shown to be moderately responsive to prosthetic training. [13]
The Upper-Extremity Functional Scale (UEFS)
Upper Extremity Functional Scale (UEFS) is one of the scales of the Orthotics and Prosthetics Users Survey (OPUS). [10, 14] The OPUS UEFS is the only self-report measure of activity performance developed specifically for adults with upper limb amputation.[26] It is a self-report measure of difficulty performing and ease of performing 23 every day activities self-care and IADL tasks [10, 14]. The tasks are rated on a 1–5 point scale (very easy to cannot perform activity), regardless of how the activities are performed (with or without a prosthesis). Total score are calculated using IRT methods. Higher scores indicate more difficulty in performing activities. However, respondents also indicate which of the items were performed using a prosthesis. These responses are used in the UEFS Use scale which is the proportion of items performed using the prosthesis. Higher scores of the UEFS Use scale indicate that more activities are done with a prosthesis. This study used 22 of the 23 UEFS items from the original measure. [5], eliminating the item “washing face”. The modified UEFS and UEF use scales have been shown to be reliable[8]. However, the UEFS, did not differentiate amputees users by level of amputation[21] and was not responsive to change after prosthesis training.,[27]
The Patient Specific Functional Scale (PSFS)
The PSFS is a patient-specific outcome measure that assesses functional status. The PSFS asks patients to identify up to five activities that they have difficulty performing due to their condition and then rate the amount of limitation they have in performing these activities on a scale of 0 to 10, with 0 being unable to perform the activity and 10 being able to perform the activity with no problem. Individual items are scored separately. Validity of the PSFS in a sample with upper-limb amputation was supported. [8] The PSFS was reported to be responsive to change for patients with arm impairments,[28] and for those with upper limb amputation who participate in prosthetic training with the DEKA Arm. [22]
The Wong-Baker FACES Pain Rating Scale (FACES)
The Wong-Baker FACES is a self-report measure of pain[29]. This measure has a 6-point pain scale that utilizes faces to indicate different levels of pain intensity. Patients are asked to choose the face that best describes how he/she is feeling. Higher pain scores indicate more severe pain
Quality of Life Scale (QOL)
The QOL consists of 16 questions that assess satisfaction with a variety domains that diverse patient groups with chronic illness define as quality of life [17]. Its items address: material comforts, health, relationships with family, intimate relationships, friendships, childrearing, helping others, participating in organizations, learning, self-knowledge, working, self-expression, socializing, being entertained, participating in active recreations and being independent. Patients are asked to describe how satisfied they are using a 1–7 scale (Terrible to Delighted). Higher scores indicate better quality of life. Reliability of the QOL scale in patients with a variety of chronic illnesses is supported. [30]
The Community Reintegration of Service Members Computer Adaptive test (CRIS-CAT)
The CRIS-CAT is a computer adaptive test version of the CRIS measure. [18,31] Like the CRIS, the CRIS-CAT has three sub-scales, each is comprised of items drawn from the 9 activity and participation content domains (or chapters), defined by the ICF. The Perceived Limitations to Participation subscale assesses Veterans’ perceived limitations in participation. The Extent of Participation subscale assesses how often Veterans experience a challenge in participation. The Satisfaction with Participation subscale assesses Veterans’ level of satisfaction with participation. Higher scores indicate better community integration. Reliability, structural, concurrent, construct and predictive validity of the CRIS-CAT scales have been reported. [32, 33]. Higher scores indicate better community integration
Trinity Amputations and Prosthetics Experience Scale (TAPES) Satisfaction Scale
The TAPES is a condition-specific instrument that assesses the psychosocial processes involved in adjusting to a prosthesis, the specific demands of wearing a prosthesis and the potential sources of maladjustment. The TAPES contains individually scored subscales, divided into 3 sections (psychosocial scales, activity restriction, and satisfaction with prosthesis).[34, 35] For device satisfaction, respondents to the TAPES indicate their level of satisfaction on a 5-point scale (very dissatisfied to very satisfied) regarding 10 items: color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction. Higher scores indicate greater satisfaction. The prosthetic satisfaction scale has been shown to have excellent internal consistency for upper limb amputees[19].
Data analysis
Participant demographics were examined for all prosthesis users who completed Part A as well as for the subgroup of prosthesis users who completed Part B. Descriptive statistics of all performance-based and self-report measures were examined at Baseline, End of Part A, and End of Part B. Scores for performance-based and self-report measures were compared between Baseline and End of A using paired t-tests. Wilcoxon signed-rank tests were used to compare Baseline and End of B outcomes for the full sample, and for the sample stratified by device type (body-powered or myoelectric) and terminal device type (single or multi-degree of freedom). Descriptive statistics of all measures by configuration level of the DEKA Arm were also compared graphically at Baseline, End of A and End of B. We also calculated Effect sizes (ES) differences for the full sample to quantify the magnitude of differences for those tests that were found to be statistically significantly different.
Multiple categories were identified to adjust for false discovery rates in “families” or categories of tests. The following categories were used: dexterity (7 measures), activity performance (5 measures), self-reported function (4 measures), pain, quality of life and community integration, satisfaction with prosthesis (6 measures). The Benjamini-Hochberg method was used to maintain a false discovery rate of 0.10 within each category of tests.
Results
Characteristics of 23 prosthesis users (mean age = 45 ± 16; 87% male) who completed Part A and the 15 prosthesis users (mean age = 45 ± 18; 87% male) who completed Parts A and B are shown in Table 2. Fifty two percent of Part A completers used an RC DEKA Arm, 30.4% used an HC Arm and 17.4% used an SC. Amongst Part B completers 53.3% used an RC, 33.3% an HC and 13.3% SC. The majority of participants used a myoelectric, single degree of freedom (DOF) prosthesis at Baseline (Table 2).
Table 2. Subject characteristics at each testing point (N = 23).
| Completed A | Completed B | |
|---|---|---|
| N = 23 | N = 15 | |
| Mn (sd) | Mn (sd) | |
| Age | 45.3 (16.0) | 44.6 (17.6) |
| Months of prosthesis Use | 182.9 (195.8) | 167.9 (196.6) |
| N(%) | N(%) | |
| Gender | ||
| Male | 20 (87.0) | 13 (86.7) |
| Female | 3 (13.0) | 2 (13.3) |
| Race | ||
| White only | 20 (87.0) | 13 (86.7) |
| Black only | 3 (13.0) | 2 (13.3) |
| Mixed/other | 0 (0.0) | 0 (0.0) |
| Veteran Status | ||
| Non-Veteran | 8 (34.8) | 5 (33.3) |
| Veteran | 11 (47.8) | 7 (46.7) |
| Active Duty | 4 (17.4) | 3 (20.0) |
| Amputation Level | ||
| Transradial | 12 (52.2) | 8 (53.3) |
| Transhumeral | 9 (39.1) | 6 (40.0) |
| Shoulder disarticulation/forequarter | 2 (8.7) | 1 (6.7) |
| DEKA Arm Configuration Level | ||
| RC | 12 (52.2) | 8 (53.3) |
| HC | 7 (30.4) | 5 (33.3) |
| SC | 4 (17.4) | 2 (13.3) |
| Control Scheme | ||
| IMU + other control* for mode/standby | 4(17.39) | 0 |
| IMU + EMG + other control* for mode/standby | 17(73.91) | 14 (93.33) |
| IMU + EMG | 1(4.35) | 1 (6.67) |
| EMG + other*control for mode/standby | 1(4.35) | 0 |
| Prosthesis Used at Baseline | ||
| Body Powered | 7 (30.4) | 5 (33.3) |
| Myoelectric | 15 (65.2) | 10 (66.7) |
| Hybrid | 1 (4.4) | 0 (0.0) |
| Terminal device type | ||
| Single degree of freedom | 17 (73.9) | 11 (73.3) |
| Multiple degree of freedom | 6 (26.1) | 4 (26.7) |
*Other controls may include pressure transducer, linear transducer, or rocker switch for mode selection. The majority used pressure transducer.
Table 3 shows scores for all measures by testing period. T-MAP time to completion was shorter at Baseline as compared to the End of A (P<0.001), but comparable between Baseline and End of B. AM-ULA scores improved (P<0.005) from Baseline to End of B. There were no significant changes between Baseline and End of A or End of B in any other measure of dexterity or activity performance by testing period.
Table 3. Outcomes across assessment time points.
| Baseline | End of A | T test | Baseline | End of B | W S-R | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mn (sd) | Mn (sd) | P | N | Mn (sd) | Mn (sd) | P | ||
| Dexterity | |||||||||
| Jebsen-Taylor Hand Function (JTHFT) | |||||||||
| JTHFT: Writing items/sec | 23 | 0.33 (0.23) | 0.34 (0.14) | 0.9284 | 15 | 0.35 (0.25) | 0.45 (0.20) | 0.1354 | |
| JTHFT: Page Turning items/sec | 23 | 0.07 (0.07) | 0.06 (0.04) | 0.2496 | 14 | 0.10 (0.07) | 0.11 (0.07) | 1.0000 | |
| JTHFT: Small items items/sec | 23 | 0.07 (0.08) | 0.08 (0.08) | 0.3730 | 14 | 0.08 (0.09) | 0.09 (0.07) | 0.6698 | |
| JTHFT: Feeding / Eating items/sec | 23 | 0.10 (0.08) | 0.07 (0.05) | 0.1164 | 14 | 0.12 (0.08) | 0.08 (0.08) | 0.0785 | |
| JTHFT: Checkers items/sec | 23 | 0.09 (0.08) | 0.08 (0.07) | 0.6318 | 14 | 0.09 (0.08) | 0.11 (0.08) | 0.1937 | |
| JTHFT: Light Cans items/sec | 23 | 0.20 (0.13) | 0.20 (0.16) | 0.7603 | 14 | 0.22 (0.14) | 0.26 (0.18) | 0.2958 | |
| JTHFT: Heavy Cans items/sec | 23 | 0.22 (0.14) | 0.21 (0.17) | 0.9105 | 14 | 0.24 (0.14) | 0.29 (0.16) | 0.3575 | |
| Activity | |||||||||
| AM-ULA | 23 | 16.7 (5.4) | 17.1 (4.8) | 0.5851 | 13 | 16.5 (4.8) | 19.8 (4.5) | *0.0024 | |
| UNB: Spontaneity | 22 | 3.1 (0.5) | 3.1 (0.5) | 0.9610 | 13 | 3.1 (0.4) | 3.3 (0.4) | 0.1943 | |
| UNB: Skill | 22 | 2.9 (0.5) | 2.9 (0.5) | 0.9038 | 13 | 3.0 (0.5) | 3.2 (0.4) | 0.1138 | |
| T-MAP | 20 | 533.8(228.5) | 786.6(413.1) | *0.0008 | 11 | 508.6 (264.2) | 676.6 (469.6) | 0.3203 | |
| BAM-ULA summary (new) | 16 | 6.9 (3.0) | 7.5 (1.7) | 0.3002 | 10 | 7.7 (2.2) | 8.3 (1.5) | 0.6563 | |
| Self-reported function | |||||||||
| QuickDASH | 23 | 28.3 (13.0) | 21.9 (10.3) | *0.0108 | 15 | 26.5 (11.3) | 20.8 (12.0) | *0.0313 | |
| Upper Extremity Functional Scale (UEFS) | 14 | 44.4 (6.0) | 44.2 (4.5) | 0.8810 | 10 | 43.0 (5.4) | 38.6 (9.3) | 0.2754 | |
| UEFS use | 23 | 0.4 (0.2) | 0.7 (0.3) | *0.0060 | 14 | 0.5 (0.2) | 0.7 (0.2) | *0.0105 | |
| Patient Specific Functional Scale (PSFS) | 23 | 2.6 (1.4) | 5.3 (1.8) | *0.0001 | 15 | 2.6 (1.3) | 6.2 (2.0) | *0.0001 | |
| Quality of life etc. | |||||||||
| Wong-Baker Pain Scale | 23 | 0.8 (1.0) | 0.9 (1.1) | 0.7040 | 15 | 0.5 (0.7) | 0.9 (1.0) | 0.0313 | |
| Quality of Life (QOL) Scale | 23 | 5.7 (0.6) | 5.7 (0.7) | 0.9201 | 15 | 5.7 (0.6) | 5.8 (0.8) | 0.5526 | |
| Community integration | |||||||||
| CRIS-CAT Extent of Limitations | 22 | 54.4(9.2) | 54.9 (8.5) | 0.7853 | 15 | 54.5 (9.2) | 57.6 (10.1) | 0.2378 | |
| CRIS-CAT Perceived Limitations | 22 | 55.9 (14.6) | 51.5 (9.4) | 0.2001 | 15 | 57.5 (17.0) | 60.3 (19.2) | 0.4810 | |
| CRIS-CAT Satisfaction with Participation | 22 | 53.4 (12.3) | 50.7 (5.9) | 0.2182 | 15 | 54.9 (14.0) | 56.0 (13.9) | 0.6721 | |
| TAPES Satisfaction Scale | 23 | 3.5 (0.6) | 3.5 (0.7) | 0.8222 | 15 | 3.6 (0.6) | 3.7 (0.9) | 0.5151 |
*significant after Benjamini-Hochberg adjustment with false discovery rate = 0.1.
Outcomes for three self-reported measures of function, the QuickDASH, UEFS use scale and PSFS, improved from Baseline to End of A and from Baseline to the End of B. Wong Baker Pain Scale ratings increased from Baseline to End of B but this finding was no longer statistically significant after correcting for multiple comparisons with the Benjamini-Hochberg procedure. All other statistically significant findings remained significant after correcting for multiple comparisons. Table 4 shows the ES for each of the statistically significant results.
Table 4. Effect size calculations for tests that were significantly different at Baseline vs. End of A and Baseline vs. End of B.
| End of A | End of B | |
|---|---|---|
| ES | ES | |
| Performance Measures | ||
| AM-ULA | NS* | 0.71 |
| T-MAP | 0.76 | NS |
| Self-reported function | ||
| QuickDASH | 0.55 | 0.49 |
| UEFS use | 1.18 | 1.00 |
| Patient Specific Functional Scale (PSFS) | 1.67 | 2.13 |
*NS not a statistically significantly difference.
Table 5 shows the outcomes across testing periods by type of conventional prosthesis used at Baseline. For the myoelectric/hybrid device users, the pattern of results comparing Baseline and End of A were similar to findings in the full sample (improved QuickDASH, UEFS use and PSFS scores and worse T-MAP). For body powered users, the JTHFT page turning test score was lower and T-MAP scores were worse (P < .05), but did not remain statistically significant after correcting for multiple comparisons. From Baseline to End of B the pattern of results were similar to the overall group for myoelectric prosthesis users; however, after correcting for multiple comparisons only the improvement in PSFS scores remained statistically significant. Among body-powered users, there were no statistically significant differences between Baseline and End of B.
Table 5. Outcomes across assessment time points by device type at Baseline and End of A / End of B.
| Baseline (BL) to End of A (EOA) | Baseline (BL) to End of B (EOB) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body Powered (N = 7) | Myoelectric/Hybrid (N = 16) | Body Powered (N = 5) | Myoelectric/Hybrid (N = 10) | |||||||||
| BL | EOA | W S-R | BL | EOA | W S-R | BL | EOB | W S-R | BL | EOB | W S-R | |
| Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | |
| Dexterity | ||||||||||||
| Jebsen-Taylor Hand Function (JTHFT) items/sec | ||||||||||||
| JTHFT: Writing | 0.35 (0.26) | 0.33 (0.17) | 0.938 | 0.32 (0.22) | 0.34 (0.14) | 0.860 | 0.36 (0.28) | 0.33 (0.19) | 1.000 | 0.34 (0.24) | 0.50 (0.19) | 0.049 |
| JTHFT: Page Turning | 0.11 (0.08) | 0.04 (0.02) | 0.031 | 0.06 (0.06) | 0.06 (0.04) | 0.413 | 0.12 (0.08) | 0.08 (0.05) | 0.313 | 0.08 (0.06) | 0.12 (0.08) | 0.426 |
| JTHFT: Small items | 0.07 (0.07) | 0.06 (0.03) | 0.688 | 0.06 (0.08) | 0.10 (0.09) | 0.309 | 0.07 (0.08) | 0.06 (0.03) | 1.000 | 0.08 (0.10) | 0.11 (0.08) | 0.652 |
| JTHFT: Feeding / Eating | 0.13 (0.10) | 0.09 (0.06) | 0.219 | 0.09 (0.06) | 0.07 (0.05) | 0.274 | 0.17 (0.10) | 0.07 (0.06) | 0.063 | 0.09 (0.05) | 0.08 (0.09) | 0.496 |
| JTHFT: Checkers | 0.05 (0.04) | 0.06 (0.05) | 0.297 | 0.11 (0.09) | 0.09 (0.07) | 0.433 | 0.06 (0.05) | 0.10 (0.09) | 0.125 | 0.10 (0.09) | 0.11 (0.07) | 0.652 |
| JTHFT: Light Cans | 0.10 (0.06) | 0.11 (0.08) | 0.813 | 0.24 (0.13) | 0.24 (0.17) | 0.706 | 0.11 (0.06) | 0.15 (0.07) | 0.438 | 0.28 (0.13) | 0.32 (0.19) | 0.652 |
| JTHFT: Heavy Cans | 0.12 (0.10) | 0.11 (0.07) | 0.938 | 0.26 (0.14) | 0.26 (0.18) | 0.940 | 0.13 (0.10) | 0.18 (0.09) | 0.625 | 0.31 (0.13) |
0.34 (0.16) | 0.496 |
| Activity | ||||||||||||
| AM-ULA | 14.5 (4.6) | 14.7 (4.9) | 0.938 | 17.6 (5.5) | 18.1 (4.5) | 0.678 | 15.4 (5.0) | 18.7 (3.1) | 0.063 | 17.2 (4.8) | 20.6 (5.3) | 0.047 |
| UNB: Spontaneity | 3.1 (0.4) | 3.0 (0.3) | 0.469 | 3.1 (0,5) | 3.1 (0.5) | 0.652 | 3.2 (0.3) | 3.3 (0.4) | 0.813 | 3.0 (0.5) | 3.3 (0.4) | 0.156 |
| UNB: Skill | 3.0 (0.5) | 2.7 (0.3) | 0.344 | 2.9 (0.5) | 3.0 (0.6) | 0.302 | 3.1 (0.5) | 3.2 (0.4) | 1.000 | 2.9 (0.5) | 3.2 (0.4) | 0.031 |
| T-MAP | 469.3 (94.0) | 827.0 (354.9) | 0.031 | 568.5 (272.9) | 764.9 (462.3) | *0.013 | 472.8 (105.1) | 880.5 (501.5) | 0.125 | 529.0 (330.9) | 560.1 (445.4) | 0.938 |
| BAM-ULA summary (new) | 4.8 (3.1) | 6.8 (0.8) | 0.250 | 7.8 (2.5) | 7.8 (1.9) | 1.000 | 6.7 (2.3) | 9.0 (1.0) | 0.500 | 8.1 (2.1) | 8.0 (1.6) | 1.000 |
| Self-reported function | ||||||||||||
| QuickDASH | 36.7 (15.6) | 26.3 (8.3) | 0.156 | 24.6 (10.1) | 20.0 (10.7) | *0.043 | 32.7 (13.7) | 25.0 (12.9) | 0.313 | 23.4 (9.0) | 18.6 (11.6) | 0.131 |
| Upper Extremity Functional Scale (UEFS) | 46.0 (6.9) | 43.2 (6.0) | 0.563 | 42.8 (5.0) | 45.1 (2.3) | 0.156 | 43.4 (5.6) | 42.2 (7.6) | 1.000 | 42.5 (5.8) | 35.1 (10.3) | 0.125 |
| UEFS use | 0.5 (0.2) | 0.7 (0.2) | 0.125 | 0.4 (0.2) | 0.6 (0.3) | *0.049 | 0.5 (0.2) | 0.6 (0.2) | 0.250 | 0.5 (0.2) | 0.7 (0.2) | 0.055 |
| Patient Specific Functional Scale (PSFS) | 2.5 (1.1) | 6.1 (1.3) | 0.031 | 2.7 (1.5) | 4.9 (1.8) | *0.001 | 3.0 (0.9) | 6.7 (1.7) | 0.063 | 2.4 (1.5) | 6.0 (2.2) | *0.002 |
| Quality of life etc. | ||||||||||||
| Wong-Baker Pain Scale | 1.6 (1.3) | 1.1 (0.7) | 0.438 | 0.5 (0.6) | 0.8 (1.2) | 0.258 | 1.0 (1.0) | 1.6 (1.3) | 0.250 | 0.3 (0.5) | 0.6 (0.7) | 0.250 |
| Quality of Life (QOL) Scale | 5.8 (0.7) | 6.1 (0.7) | 0.234 | 5.6 (0.5) | 5.5 (0.6) | 0.571 | 6.0 (0.8) | 6.1 (0.8) | 0.438 | 5.6 (0.4) | 5.6 (0.8) | 0.910 |
| Community integration CRIS-CAT | ||||||||||||
| Extent of Limitations | 55.5 (10.7) | 55.5 (4.3) | 0.875 | 54.0 (8.9) | 54.6 (9.7) | 0.845 | 54.0 (11.3) | 58.6 (7.2) | 0.250 | 54.8 (8.7) | 57.1 (11.6) | 0.633 |
| Perceived Limitations | 60.3 (17.8) | 51.7 (8.5) | 0.219 | 54.2 (13.6) | 51.4 (10.0) | 0.465 | 62.4 (18.8) | 62.4 (16.3) | 1.000 | 55.1 (16.5) | 59.3 (21.2) | 0.375 |
| Satisfaction with Participation | 58.7 (13.1) | 52.8 (6.9) | 0.156 | 51.4 (11.8) | 49.9 (5.5) | 0.688 | 60.0 (14.1) | 56.6 (11.7) | 0.250 | 52.4 (14.0) | 55.7 (15.4) | 0.152 |
| TAPES Satisfaction Scale | 3.4 (0.6) | 3.8 (0.8) | 0.375 | 3.6 (0.6) | 3.4 (0.7) | 0.131 | 3.5 (0.6) | 4.0 (0.9) | 0.438 | 3.5 (0.5) | 3.6 (0.9) | 0.922 |
*significant after Benjamini-Hochberg adjustment with false discovery rate = 0.1.
Table 6 shows scores for all outcomes across testing periods by degrees of freedom of the conventional prosthesis terminal device. Comparisons between Baseline and End of A showed that, users of a single DOF device had significantly worse JTHFT Feeding and T-MAP scores, but improved UEFS use and PSFS scores. In contrast, the only statistically significant difference in outcomes for multi-degree of freedom device users was in the JTHF feeding score which improved, however this was no longer statistically significant after controlling for multiple comparisons. Comparisons between Baseline and End of B showed that users of single DOF devices had worse JTHF feeding scores but improved AM-ULA, QuickDASH and PSFS scores, while no statistically significant differences were observed in users of multi DOF devices.
Table 6. Outcomes across assessment time points by device type at Baseline and End of A / End of B.
| Baseline (BL) to End of A (EOA) | Baseline (BL) to End of B (EOB) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single DOF (N = 17) | Multi DOF (N = 6) | Single DOF (N = 11) | Multi DOF (N = 4) | |||||||||
| BL | EOA | W S-R | BL | EOA | W S-R | BL | EOB | W S-R | BL | EOB | W S-R | |
| Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | Mn (sd) | Mn (sd) | P | |
| Dexterity | ||||||||||||
| Jebsen-Taylor Hand Function (JTHFT) items/sec | ||||||||||||
| JTHFT: Writing | 0.36 (0.24) | 0.32 (0.14) | 0.306 | 0.27 (0.20) | 0.38 (0.16) | 0.156 | 0.34 (0.28) | 0.39 (0.18) | 0.465 | 0.35 (0.17) | 0.60 (0.18) | 0.250 |
| JTHFT: Page Turning | 0.08 (0.07) | 0.05 (0.04) | 0.129 | 0.06 (0.06) | 0.08 (0.04) | 0.438 | 0.10 (0.07) | 0.08 (0.04) | 0.275 | 0.08 (0.06) | 0.18 (0.09) | 0.375 |
| JTHFT: Small items | 0.07 (0.08) | 0.09 (0.09) | 0.730 | 0.06 (0.08) | 0.08 (0.05) | 0.688 | 0.09 (0.09) | 0.08 (0.07) | 0.770 | 0.06 (0.09) | 0.11 (0.05) | 0.625 |
| JTHFT: Feeding / Eating | 0.13 (0.08) | 0.06 (0.05) | *0.001 | 0.04 (0.04) | 0.11 (0.05) | 0.031 | 0.14 (0.07) | 0.07 (0.06) | *0.004 | 0.06 (0.04) | 0.11 (0.12) | 0.625 |
| JTHFT: Checkers | 0.09 (0.08) | 0.07 (0.06) | 0.080 | 0.09 (0.10) | 0.12 (0.09) | 0.844 | 0.08 (0.08) | 0.11 (0.08) | 0.232 | 0.09 (0.10) | 0.11 (0.08) | 0.875 |
| JTHFT: Light Cans | 0.16 (0.10) | 0.18 (0.15) | 0.352 | 0.29 (0.17) | 0.26 (0.19) | 0.844 | 0.16 (0.10) | 0.19 (0.11) | 0.160 | 0.36 (0.12) | 0.41 (0.23) | 0.625 |
| JTHFT: Heavy Cans | 0.19 (0.13) | 0.17 (0.14) | 0.548 | 0.30 (0.15) | 0.32 (0.20) | 0.563 | 0.19 (0.13) | 0.22 (0.11) | 0.625 | 0.37 (0.11) | 0.46 (0.13) | 0.375 |
| Activity | ||||||||||||
| AM-ULA | 16.1 (5.2) | 16.1 (4.3) | 0.917 | 18.2 (6.0) | 19.9 (5.5) | 0.438 | 15.7 (5.0) | 18.8 (4.4) | *0.012 | 19.3 (3.3) | 23.3 (3.5) | 0.250 |
| UNB: Spontaneity | 3.1 (0.4) | 3.0 (0.4) | 0.295 | 2.9 (0.6) | 3.1 (0.5) | 0.563 | 3.2 (0.4) | 3.4 (0.4) | 0.367 | 3.0 (0.5) | 3.2 (0.1) | 0.500 |
| UNB: Skill | 3.0 (0.5) | 2.9 (0.5) | 0.511 | 2.7 (0.6) | 3.0 (0.6) | 0.313 | 3.1 (0.5) | 3.2 (0.4) | 0.367 | 2.7 (0.6) | 3.0 (0.2) | 0.250 |
| T-MAP | 547.1 (230.8) | 824.1 (433.3) | *0.001 | 480.8 (244.0) | 636.5 (369.5) | 0.375 | 544.4 (294.1) | 800.0 (494.9) | 0.055 | 413.0 (165.4) | 347.7 (149.5) | 0.250 |
| BAM-ULA summary (new) | 6.6 (2.9) | 7.3 (1.4) | 0.445 | 7.6 (3.3) | 8.0 (2.4) | 0.750 | 7.0 (2.2) | 8.0 (1.6) | 0.500 | 9.3 (1.2) | 9.0 (10) | 1.000 |
| Self-reported function | ||||||||||||
| QuickDASH | 28.6 (14.0) | 22.4 (10.4) | 0.124 | 27.3 (10.9) | 20.5 (10.8) | 0.094 | 26.9 (11.9) | 20.5 (13.3) | *0.043 | 25.6 (10.7) | 21.6 (8.8) | 0.625 |
| Upper Extremity Functional Scale (UEFS) | 44.7 (6.5) | 44.0 (4.7) | 0.966 | 42.6 (1.7) | 44.9 (4.0) | 0.500 | 42.9 (5.7) | 39.7 (9.3) | 0.496 | 43.8 (-) | 29.6 (-) | - |
| UEFS use | 0.5 (0.2) | 0.7 (0.3) | *0.046 | 0.3 (0.2) | 0.7 (0.4) | 0.156 | 0.5 (0.2) | 0.7 (0.2) | 0.098 | 0.4 (0.1) | 0.6 (0.1) | 0.125 |
| Patient Specific Functional Scale (PSFS) | 2.6 (1.2) | 5.1 (1.9) | *0.000 | 2.8 (1.8) | 5.7 (1.1) | 0.063 | 2.7 (1.1) | 5.9 (2.3) | *0.001 | 2.3 (1.8) | 7.0 (0.5) | 0.125 |
| Quality of life etc. | ||||||||||||
| Wong-Baker Pain Scale | 0.9 (1.1) | 1.1 (1.2) | 0.681 | 0.5 (0.5) | 0.3 (0.5) | 1.000 | 0.6 (0.8) | 1.1 (1.1) | 0.063 | 0.3 (0.5) | 0.5 (0.6) | 1.000 |
| Quality of Life (QOL) Scale | 5.6 (0.6) | 5.8 (0.7) | 0.199 | 5.8 (0.4) | 5.3 (0.5) | 0.063 | 5.7 (0.6) | 5.8 (5.9) | 0.534 | 5.7 (0.4) | 5.7 (0.6) | 1.000 |
| Community integration CRIS-CAT | ||||||||||||
| Extent of Limitations | 53.3 (10.0) | 54.1 (7.7) | 0.901 | 57.5 (6.1) | 56.8 (10.9) | 0.750 | 53.5 (10.2) | 57.6 (9.0) | 0.160 | 57.3 (6.2) | 57.5 (14.5) | 1.000 |
| Perceived Limitations | 57.1 (16.7) | 50.6 (7.2) | 0.157 | 52.5 (6.5) | 54.0 (14.5) | 0.875 | 59.3 (19.3) | 60.2 (17.8) | 0.504 | 52.8 (8.3) | 60.8 (25.7) | 0.750 |
| Satisfaction with Participation | 54.6 (13.9) | 50.5 (5.9) | 0.239 | 50.2 (6.2) | 51. 3 (6.5) | 0.563 | 57.0 (15.6) | 56.8 (14.3) | 0.871 | 49.3 (7.4) | 53.8 (14.4) | 0.625 |
| TAPES Satisfaction Scale | 3.6 (0.6) | 3.6 (0.8) | 0.738 | 3.4 (0.4) | 3.3 (0.4) | 0.875 | 3.6 (0.6) | 3.8 (1.0) | 0.621 | 3.4 (0.5) | 3.5 (0.5) | 0.875 |
*significant after Benjamini-Hochberg adjustment with false discovery rate = 0.1.
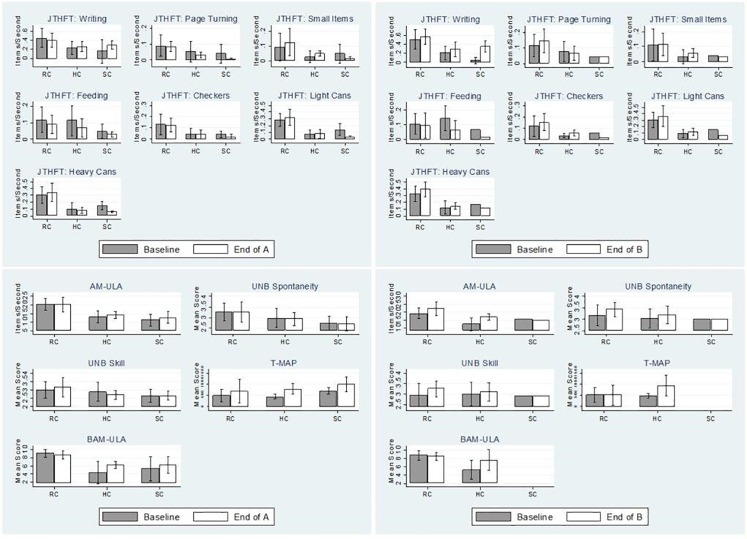
Fig 1A–1D shows performance outcomes from Baseline to End of A and Baseline to End of B by DEKA Arm configuration level. Change in dexterity scores varied by JTHF item and DEKA level. At End of A, subjects using the RC and HC devices had improved scores for three JTHF items while SC users only improved on the writing item. From Baseline to End of Part B, dexterity scores improved for RC users on 6 items and for HC users on 5 items. SC users scores at End of B had only improved for the JTHF writing task.
Fig 1. Performance-based measures at Baseline compared to End of A and End of B by configuration level.
AM-ULA scores were comparable for RC users at Baseline and End of A but better for HC and SC users. Comparisons of Baseline and End of B scores show AM-ULA scores better for RC and HC users, but equivalent for SC users. UNB Spontaneity scores were similar at Baseline and End of B, but improved at End of B for RC and HC users. UNB Skill scores improved for RC users at the End of A, declined for HC users and were equivalent for SC users. At the End of B UNB skill scores had improved slightly for HC users as well. From Baseline to End of A, T-MAP scores were greater (indicating slower performance) for all configuration levels. Whereas in the Baseline to End of B comparison T- MAP scores for the RC users were comparable, and scores for HC users still greater. From Baseline to End of A BAM-ULA scores were nearly equivalent for RC users, but improved for HC and SC users. This pattern persisted at End of B for RC and HC users (data not available for SC).
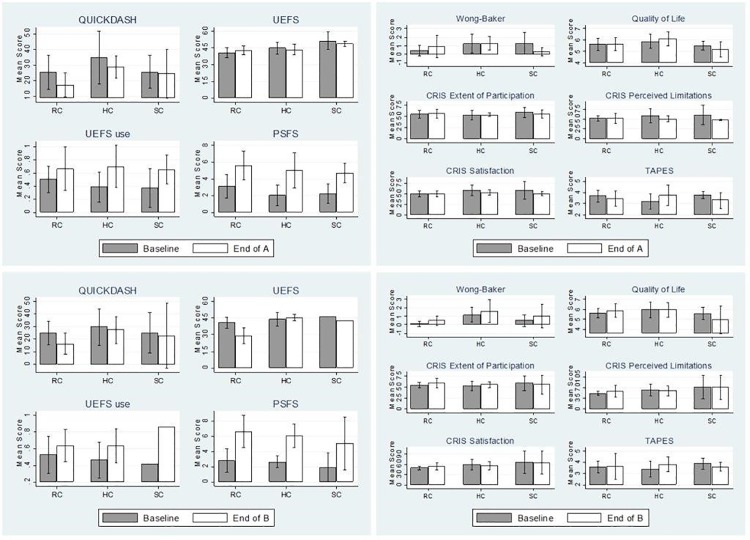
Fig 2A and 2B shows differences in self-report outcomes from Baseline to End of A and B by DEKA configuration level. QuickDASH scores declined (indicating less disability) for RC and HC users at the End of A, and for all 3 levels at the End of B. UEFS scores were very similar at Baseline and End of A, but clearly decreased (indicating less difficulty) for RC users at End of B. UEFS use scores improved for all configuration levels from Baseline to End of A and End of B. Finally, improvements of PSFS were consistent across configuration levels at both End of A and B.
Fig 2. Self-report measures at Baseline compared to End of A and End of B by configuration level.
Fig 2C and 2D show pain, quality of life, community integration and prosthesis satisfaction measures at each time point. Pain ratings increased for RC users and decreased for SC users at the End of A, but were elevated for all configuration levels at the End of B (differences not statistically significant after Bonferroni adjustment as mentioned above). Quality of Life improved slightly for HC users at End of A and for RC users at End of B, but was decreased for SC users. CRIS subscales were comparable or slightly from Baseline to End of A. However, from Baseline to End of B there was a small improvement in: RC users for all 3 subscales and in Extent of Participation for HC users. TAPES satisfaction scores indicated greater satisfaction with the prosthesis for HC users, but worse satisfaction for RC and SC users at the End of A. At the End of B, satisfaction of RC users was comparable to Baseline, satisfaction of HC users was higher than Baseline and satisfaction of SC users was slightly lower than Baseline.
Discussion
This study built on prior research by comparing perceived function and functional performance of users of both conventional prosthesis and Gen 3 DEKA Arm. Whereas prior studies involved comparisons between the DEKA Arm and conventional prostheses after in-laboratory training, our analyses also compared outcomes after several months of home use. In addition, we compared other important outcomes that have not previously been examined, including community integration, pain, quality of life and several new activity measures.
After in-laboratory training, activity performance of the DEKA Arm and conventional prosthesis was equivalent, however after home use AM-ULA scores using the DEKA Arm surpassed conventional prosthesis scores, suggesting that home use after completion of formal prosthetic training may lead to better function. Given an ES of 0.71, we can interpret the effect of the DEKA Arm on improvement AM-ULA scores was moderate. We also found that activity performance with DEKA Arm, as measured by the T-MAP was moderately slower than performance with conventional prostheses at the end of in-laboratory training (ES = 07.6) but was not significantly slower after home use. These findings are consistent with our recent analyses of all home study subjects (including users and non-users of a conventional prosthesis) that showed improvement in AM-ULA scores with several months of home use. [36]
We did not observe differences in dexterity measures between DEKA and conventional prosthesis. An earlier study found that some measures of dexterity using the DEKA Arm were worse than using when using conventional prostheses.[5] However, that study included subjects who were using an earlier prototype of the DEKA Arm, the Gen 2, as well as the prototype used in the current study, the a Gen 3, The changes to hand and finger shape and foot controls [1] in the Gen 3 may, in part, explain differences in findings. A study comparing usability and satisfaction of DEKA found that in overall satisfaction, the Gen 3 was rated more favorably than the Gen 2, as was satisfaction with switching grips and the usability of all 6 grips. [37]
The DEKA Arm’s impact on perceived difficulty in activity performance and disability, as measured by the PSFS and QuickDASH, was evident after in-laboratory training and after home use experience. The improvements in the PSFS were large (ES 1.67 and 2.13 for Parts A and B respectively), while the improvements in the QuickDASH were moderate (ES 0.55 and 0.49 for Parts A and B respectively). Participants reported using the DEKA Arm to perform more activities (UEFS use) as compared to their conventional prostheses. The impact of the DEKA Arm on the UEFS was large (ES 1.18 and 1.0 for Parts A and B respectively). We did not observe differences between the DEKA Arm and conventional prostheses in the full group in measures of dexterity, prosthetic skill, spontaneity, pain, community integration or quality of life.
Improved PSFS and UEFS use scores are consistent, in part, with our earlier study which found that users of the DEKA Arm reported less difficulty in activity performance (PSFS) and engagement of the DEKA Arm in a greater proportion of daily activities (UEFS use).[5] Previous studies reported that the majority of DEKA users listed new activities that they could perform using DEKA that they were unable to do with their existing prosthesis.[38, 39] [40] Further, 65% of DEKA users preferred using DEKA Arm for tasks that they could also perform with their existing prosthesis.
An earlier study reported more spontaneity of prosthesis use (UNB test) with the DEKA Arm as compared to conventional prosthesis, however we did not observe this relationship in the current study.[5] We did find that comparisons between the DEKA Arm and conventional prosthesis at Baseline and End of A and B varied by configuration level. Unfortunately, our sample size of SC users who completed Part B testing was very small and data were missing for some tests, making comparisons of scores after home use challenging for this level.
Comparisons stratified by device type revealed similar patterns to findings for the overall group, though our analyses for the smaller sub-groups (body-powered users and single DOF users) were underpowered. Nevertheless, some important trends in the data were observed. Thus, our results should be interpreted as preliminary, and could be useful to other research groups planning studies that compare outcomes across device types. Our post-hoc tests estimating the sample size needed to achieve 80% power at an alpha of .05 showed dramatically different sample sizes needed for each outcome. Our comparisons for body powered users were adequately powered to compare T-MAP scores at End of A, but not at the End of B. At the End of B, comparisons of the AM-ULA outcomes were adequately powered for body-powered users. A larger sample would be required to be adequately powered for other outcomes (e.g. at the End of A JTHFT page turning requires an N of 9, QuickDASH requires an N of 16, etc.). Our comparisons between the DEKA Arm and myoelectric device required far larger samples at both End of A and End of B (End of A sample estimates range from N = 20 to N = 700). Similar findings were observed in post-hoc calculation of power for comparisons between single and multi-DOF devices and the DEKA Arm. The Baseline to End of B comparisons of single DOF users were adequately powered for the JTHFT feeding and AM-ULA tests, but other comparisons were under-powered. Our study had several other limitations. Our sample size, while relatively large for a study of a new upper limb prosthesis, was still very small, limiting statistical comparisons. Finally, our analysis of outcomes by configuration level was performed graphically, and so needs to be interpreted cautiously.
Although the majority of participants had improvements in function attributable to gaining home use experience, in analyses shown in another manuscript, functional gains appeared to plateau after month 2. [41] However, we cannot be certain that all participants had fully acclimated to using the device and that greater gains in function would not have been achievable with more experience. Thus, it is possible that our analyses have underestimated the impact of the DEKA Arm. Future studies with longer periods of home use would be needed to examine longer term outcomes.
Conclusions
Participants using the DEKA Arm had less perceived disability and more engagement of the prosthesis in everyday tasks at the End of Part A, although their activity performance was slower. After home use experience, perceived disability was lower, prosthesis engagement higher, activity performance was improved and activity speed equivalent to using conventional prostheses. There were no differences between the DEKA Arm and conventional prostheses in measures of dexterity, prosthetic skill, spontaneity, community integration or quality of life.
While underpowered, comparisons stratified by device type and terminal device type revealed similar patterns to findings for the overall group. Comparisons between the DEKA Arm and conventional prosthesis by configuration level showed some variation by configuration level, but were limited by small sample sizes. The trends identified will be useful to other research groups planning studies to compare outcomes by device type.
Acknowledgments
Disclaimer: The information in this manuscript does not necessarily reflect the position or policy of the government; no official endorsement should be inferred. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of the U.S. Government.
This research was supported by United States Department of Veterans Affairs Rehabilitation Research & Development Service (RR&D), VA RR&D A9226-R and VA RR&D A9264A-S.
Data Availability
Data are available from the Providence VA Medical Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Individually Identifiable Data, excluding Veterans’ names and 38 USC §7332-protected information, will be shared pursuant to a written request and IRB approved waiver of HIPAA authorization, with the approval of the Under Secretary for Health, in accordance with VHA Handbook 1605.1 §13.b(1)(b) or §13.b(1)(c) or superseding versions of that Handbook. Please contact Val.Micucci@va.gov for more information.
Funding Statement
This research was supported by United States Department of Veterans Affairs Rehabilitation Research & Development Service (RR&D), VA RR&D A9226-R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Resnik L., Klinger S., Etter K The DEKA Arm: its features, functionality, and evolution during the Veterans Affairs Study to optimize the DEKA Arm. Prosthet Orthot Int, 2014. 38(6): p. 492–504. doi: 10.1177/0309364613506913 [DOI] [PubMed] [Google Scholar]
- 2.Mobius Bionics: Luke Arm. 2017; Available from: http://www.mobiusbionics.com/the-luke-arm.html.
- 3.Phillips S, Resnik L, Fantini C, Latlief G. Endpoint Control for a Powered Shoulder Prosthesis. Journal of Posthetics & Othotics. 2013;25(4):8. [Google Scholar]
- 4.Resnik L, Klinger S, Etter K, Fantini C. Controlling a multi-degree of freedom upper limb prosthesis using foot controls: user experience. Disabil Rehabil Assist Technol. July 31 2013. [DOI] [PubMed] [Google Scholar]
- 5.Resnik L, Borgia M, Latlief G, Sasson N, Smurr-Walters L. Self-reported and performance-based outcomes using DEKA Arm. J Rehabil Res Dev, 2014. 51(3): p. 351–62. doi: 10.1682/JRRD.2013.08.0180 [DOI] [PubMed] [Google Scholar]
- 6.Jebsen R, Taylor N, Trieschmann R, Trotter M, Howard L. An objective and standardized test of hand function. Arch Phys Med Rehabil. June 1969;50(6):311–319. [PubMed] [Google Scholar]
- 7.Resnik L. Borgia M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error. Phys Ther, 2011. 91(4): p. 555–65. doi: 10.2522/ptj.20100287 [DOI] [PubMed] [Google Scholar]
- 8.Resnik L., Adams L., Borgia M., Delikat J., Disla R., Ebner C., Walters L. S. Development and Evaluation of the Activities Measure for Upper Limb Amputees. Arch Phys Med Rehabil, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson E., Scott R, UNB Test of Prosthetics Function: A Test for Unilateral Upper Extremity Amputees, Ages 2–13. 1985, University of New Brunswick: Fredericton. [Google Scholar]
- 10.Burger H, Franchignoni F, Heinemann A, Kotnik S, Giordano A., Validation of the orthotics and prosthetics user survey upper extremity functional status module in people with unilateral upper limb amputation. J Rehabil Med, 2008. 40(5): p. 393–9. doi: 10.2340/16501977-0183 [DOI] [PubMed] [Google Scholar]
- 11.Resnik L., Borgia M.,Acluche F. Timed activity performance in persons with upper limb amputation: A preliminary study. J Hand Ther, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Resnik L,. Borgia M, Acluche F., Brief activity performance measure for upper limb amputees: BAM-ULA. Prosthet Orthot Int, 2017: p. 309364616684196. [DOI] [PubMed] [Google Scholar]
- 13.Resnik L. Borgia M Reliability, Validity and Responsiveness of the QuickDASH in Patients with Upper Limb Amputation. Arch Phys Med Rehabil, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann A. Bode R, O'Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users' Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int, 2003. 27(3): p. 191–206. doi: 10.1080/03093640308726682 [DOI] [PubMed] [Google Scholar]
- 15.Stratford P, Gill C, Westaway M, Binkley J, Assessing disability and change on individual patients: a report of a patient specific measure. Physiotherapy Canada, 1995. 47(4): p. 258–263. [Google Scholar]
- 16.Wong D. a, Baker C., Smiling faces as anchor for pain intensity scales. Pain, 2001. 89(2–3): p. 295–300. [DOI] [PubMed] [Google Scholar]
- 17.Burckhardt C. and Anderson K The Quality of Life Scale (QOLS): reliability, validity, and utilization. Health Qual Life Outcomes, 2003. 1: p. 60 doi: 10.1186/1477-7525-1-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resnik L., Plow M, Jette A Development of CRIS: measure of community reintegration of injured service members. J Rehabil Res Dev, 2009. 46(4): p. 469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmond D,MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil, 2005. 84(7): p. 506–13. [DOI] [PubMed] [Google Scholar]
- 20.Rider B. Linden C Comparison of standardized and non-standardized administration of the Jebsen Hand Function Test. Journal of Hand Therapy, 1988. 2: p. 121–123. [Google Scholar]
- 21.Resnik L. Borgia M. Reliability and Validity of Outcome Measures for Upper Limb Amputation. JPO: Journal of Prosthetics and Orthotics, 2012. 24(4): p. 192–212 [Google Scholar]
- 22.Resnik L, M. Borgia M. Responsiveness of outcome measures for upper limb prosthetic rehabilitation. Prosthet Orthot Int, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Resnik L, Baxter K, Borgia M, Mathewson K, Is the UNB test reliable and valid for use with adults with upper limb amputation? J Hand Ther, 2013. 26(4): p. 353–9; quiz 359. doi: 10.1016/j.jht.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 24.Rossier P., Wade D, Murphy M. An initial investigation of the reliability of the Rivermead Extended ADL index in patients presenting with neurological impairment. J Rehabil Med, 2001. 33(2): p. 61–70. [DOI] [PubMed] [Google Scholar]
- 25.Beaton D, Wright J,Katz J. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am, 2005. 87(5): p. 1038–46. doi: 10.2106/JBJS.D.02060 [DOI] [PubMed] [Google Scholar]
- 26.Resnik L, Borgia M, Silver B, Cancio J., Systematic Review of Measures of Impairment and Activity Limitation for Person with Upper Limb Trauma and Amputation. Arch Phys Med Rehabil, 2017. [DOI] [PubMed] [Google Scholar]
- 27.Resnik L. and Borgia M Responsiveness of outcome measures for upper limb prosthetic rehabilitation. Prosthet Orthot Int, 2016. 40(1): p. 96–108. doi: 10.1177/0309364614554032 [DOI] [PubMed] [Google Scholar]
- 28.Chatman A, Hyams S, Neel J, Binkley J., Stratford P., Schomberg A., et al. , The Patient-Specific Functional Scale: measurement properties in patients with knee dysfunction. Phys Ther. Aug 1997;77(8):820–829. [DOI] [PubMed] [Google Scholar]
- 29.Stuppy D., The Faces Pain Scale: reliability and validity with mature adults. Appl Nurs Res, 1998. 11(2): p. 84–9. [DOI] [PubMed] [Google Scholar]
- 30.Burckhardt C, Woods SL, Schultz A, Ziebarth D. Quality of life of adults with chronic illness: a psychometric study. Res Nurs Health. December 1989;12(6):347–354., Quality of life of adults with chronic illness: a psychometric study. Res Nurs Health, 1989. 12(6): p. 347–54. [DOI] [PubMed] [Google Scholar]
- 31.Resnik L., The CRIS: Measure of Community Reintegration of Service Members. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnik L, Borgia M, Ni P, Pirraglia P, Jette A, Reliability, validity and administrative burden of the community reintegration of injured service members computer adaptive test (CRIS-CAT)". BMC Med Res Methodol, 2012. 12(1): p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resnik L, Feng T, Pensheng N, Jette A, A Computer Adaptive Test to Measure Community Reintegration of Veterans. JRRD, 2012. 49 (4): p. 557–566. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher P., Maclachlan M. The Trinity Amputation and Prosthesis Experience Scales and quality of life in people with lower-limb amputation. Archives of Physical Medicine and Rehabilitation, 2004. 85(5): p. 730–736. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher P. MacLachlan M. Development and psychometric evaluation of the Trinity Amputation and Prosthesis Experience Scales (TAPES). Rehabilitation Psychology, 2000. 45(2): p. 130–54. [Google Scholar]
- 36.Resnik L., Acluche F., Borgia M. Function, quality of life and community integration of DEKA ARM users after discharge from prosthetic training. Under Review, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Resnik L. Borgia M. User ratings of prosthetic usability and satisfaction in VA study to optimize DEKA arm. J Rehabil Res Dev, 2014. 51(1): p. 15–26. doi: 10.1682/JRRD.2013.02.0056 [DOI] [PubMed] [Google Scholar]
- 38.Resnik, L. VA study to optimize the Gen 2 Deka Arm: qualitative findings. in Myoelectric Symposium. 2011.
- 39.Resnik L, Latlief G, Klinger L, Sasson N, Walters L, Do users want to receive a DEKA Arm and why? Overall findings from the Veterans Affairs Study to optimize the DEKA Arm. Prosthet Orthot Int, 2014. 38(6): p. 456–66. doi: 10.1177/0309364613506914 [DOI] [PubMed] [Google Scholar]
- 40.Resnik L., Fcluche F.,Borgia M., Does the DEKA Arm supplement or substitute for a conventional prosthesis? Under Review, 2017. [Google Scholar]
- 41.R Resnik L, Acluche F, Borgia M, Cancio J, Latlief G, Sasson N., Is function of upper limb prostheses users maintained, improved, or diminished after discharge from prosthetics Training? Findings from the VA Study of the DEKA Arm. Prosthetics and Orthotics International, In Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Providence VA Medical Center Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Individually Identifiable Data, excluding Veterans’ names and 38 USC §7332-protected information, will be shared pursuant to a written request and IRB approved waiver of HIPAA authorization, with the approval of the Under Secretary for Health, in accordance with VHA Handbook 1605.1 §13.b(1)(b) or §13.b(1)(c) or superseding versions of that Handbook. Please contact Val.Micucci@va.gov for more information.