Abstract
African American (AA) and European American (EA) women often exhibit differences in hemoglobin (Hb) and 25-hydroxyvitamin D [25(OH)D], both of which can be altered by calorie restriction leading to weight loss. Given these known differences it is of clinical interest to examine the potential for race-specific, adverse responses to weight loss. Sixty-four overweight (BMI 27–29.9 kg/m2), premenopausal women consumed a standardized, very-low calorie diet to reduce BMI < 25 kg/m2. Ancestry informative markers provided estimates of African admixture, an objective mean of expressing race. Blood sampling and anthropometric measures were performed at baseline and upon meeting target BMI. At baseline, in the overweight state, Hb (g/dL) (AA, 11.7 ± 0.9 vs. EA, 12.5 ± 0.8; p < 0.01) and 25(OH)D (nmol/L) (AA, 35.7 ± 12.9 vs. EA, 57.0 ± 20.0; p < 0.01) were lower in AAs. After weight loss, Hb decreased (AA, −0.5 ± 0.7 vs. EA, −0.4 ± 0.6; p = 0.48) to a similar extent among races. Conversely, 25(OH)D increased (AA, 43.4 ± 14.0 vs. EA, 68.2 ± 24.3; p < 0.01) though the magnitude of change (Δ) was not different (AA, +7.8 ± 13.5 vs. EA, +11.2 ± 16.7; p = 0.37) between races. Multiple linear regression revealed a positive association between ΔHb and Δ25(OH)D (r = 0.386; p < 0.01) adjusted for African admixture, Δtestosterone, and Δbody fat%. Path analyses revealed a significant indirect effect of Δbody fat% on ΔHb through Δ25(OH)D, β= −0.023, CI [−0.06, −0.004]. Following 15% weight loss, participants with the largest increase in serum 25(OH)D exhibited the smallest decrease in Hb. Future research should clarify the optimal degree of calorie restriction to stimulate weight loss while mitigating the potential risk of anemia associated with dieting efforts.
Keywords: Adiposity, Dieting, Hormones, Race Disparity
Introduction
Complications arising from adiposity-induced, low-grade inflammation are thought to contribute to iron dysregulation (Aeberli et al., 2009) and the consequent risk of developing anemia. Though weight loss can alleviate micronutrient absorption difficulties through a decrease in pro-inflammatory cytokines and hepcidin (Ausk & Ioannou, 2008; Tussing-Humphreys et al., 2009), severe calorie restriction can inadvertently decrease hemoglobin (Hb) (Gunga et al., 1996; Reljic et al., 2013). Testosterone (Leenen et al., 1994) and 25-hydroxyvitamin D [25(OH)D] (Rock et al., 2012), both of which are involved in erythropoiesis, can be readily influenced by weight loss. Complementing the known erythrogenic role of testosterone (Gordon et al., 1970), in vitro work has shown that 1,25-hydroxyvitamin D increases erythropoietin-receptor expression, and along with erythropoietin, up-regulates hematopoietic stem cell proliferation (Alon et al., 2002). Given that in vivo human studies have demonstrated an association between suboptimal 25(OH)D and prevalence of anemia (Yoo & Cho, 2015), it is of interest to evaluate how calorie restriction-induced changes in testosterone and 25(OH)D can influence Hb.
Despite having higher testosterone (Stolzenberg-Solomon et al., 2012) African American (AA) women frequently have lower Hb (Williams, 1981) and lower 25(OH)D (Smith et al., 2015) compared to European American (EA) women. Even when controlling for age, adiposity, and various parameters of iron status, AA women often exhibit mean Hb values ≈1.0 g/dL less that EA women (Williams, 1981) and thus may be at greater risk for anemia. This is especially important given that low Hb is an independent predictor of cardiovascular disease (Sarnak et al., 2002) and linked with reduced exercise tolerance and fatigue.
To this end, the current study sought to examine the potential for race-specific, adverse effects (i.e., reduced Hb) to a standardized, very-low calorie diet leading to weight loss. To appropriately investigate phenotypic differences among races, genetic admixture techniques were used to quantitatively evaluate participant ancestry. As such, the interrelationships of Hb, serum 25(OH)D (metabolite reflective of overall vitamin D status), total testosterone, and C-reactive protein (CRP; marker of inflammation) were evaluated before and after weight loss among premenopausal AA and EA women. The following hypotheses were made: 1) AA women would have lower Hb compared to EA women; 2) calorie restriction would decrease Hb; and 3) the magnitude of observed changes in Hb would be greater among AA women compared to EA women.
Methods
Overweight (BMI 27–29.9 kg/m2) AA (n = 33) and EA (n = 31) women aged 20–45 years reporting normal menstrual cycles and not taking oral contraceptives or any medications known to influence iron, glucose or lipid metabolism volunteered for this study. Further inclusion criteria were: i) nonsmoker; ii) normoglycemic as evaluated by 75-g oral glucose tolerance test; and iii) sedentary as defined by participating in exercise-related activities less than one time per week. All participants provided written informed consent prior to inclusion. Study procedures were approved by the local Institutional Review Board at University of Alabama at Birmingham.
Procedures
Participants included in this secondary analysis were part of a much larger study designed to evaluate the influence of exercise training on visceral fat regain 1 year after weight loss (Hunter et al., 2010). After initial screening, weight was stabilized (< 1% fluctuation) over a 4 week period via dietary control, during which body weight was measured 3–5×/week. At baseline, body composition analyses and blood sampling were performed after a 24-hour fast during the follicular phase of the menstrual cycle. Participants were randomly assigned to one of three weight loss interventions: i) diet only; ii) diet +aerobic exercise; or iii) diet +resistance exercise. A very-low, 800 kcal/day diet was prepared by the local Clinical Research Center (CRC) consisting of 20–22% energy as fat, 18–22% as protein, and 58–62% as carbohydrate. Posthoc dietary analyses of average iron intake was ≈10 mg/day and ≈24 mg/day during the weight loss and weight maintenance phases, respectively. Participants visited the CRC 2×/week for food pick-up and had their body weight measured. Upon reaching a BMI of < 25 kg/m2, participants were held in energy balance via dietary control to stabilize weight for an additional 4 weeks. Similar to baseline, body composition and blood sampling were performed in the same manner as previously described following an overnight fast during the follicular phase.
Body composition
Body composition was determined by dual-energy X-ray absorptiometry (DXA) (Prodigy; Lunar Radiation, WI). Scans were analyzed with ADULT software, LUNAR DPX-L version 1.33 (GE Medical Systems Lunar).
Serum assays
Whole blood was collected by venipuncture following an overnight fast. Serum was centrifuged and stored at −80 °C until analyses. Hb was measured by coulter method using the Beckman-Coulter hematology analyzer (Beckman Coulter, Inc., GA). Serum 25(OH)D was measured in duplicate by IDS ELISA assay (Immuno Diagnostic Systems, AZ). Mean intra- and inter-assay coefficient of variations (CV) were 6.2% and 4.9%, respectively. Total testosterone was measured via radioimmunoassay in duplicate by Coat-a-Count from Siemens (previously Diagnostic Products Corp, CA). Mean intra- and inter-assay CVs were 5.0% and 4.1%, respectively. CRP was measured in duplicate by high-sensitivity ELISA assay (ALPCO, NH). Mean intra- and inter-assay CVs were 5.6% and 7.8%, respectively.
Genetic admixture
Estimates of genetic admixture were used to account for the ancestral genetic components underlying racial classification, as previously described (Hunter et al., 2010). In short, a panel of 85 ancestry informative markers (AIMs) were genotyped and translated into estimates of African and European American ancestry using the maximum likelihood estimation techniques described by Hanis (Hanis et al., 1986) then implemented into the ADMIXMAP software (McKeigue, 2000). Admixture estimate ranged from 1–100 for each participant.
Aerobic exercise
Continuous walking/jogging was performed on a treadmill 3×/week, beginning with a 3–5 minute period for warm-up/stretching at each session. Through week 1, continuous exercise was maintained for 20 minutes at ≈67% of maximum heart rate (MHR). Gradually over an 8-week period, exercise intensity and duration increased so that by the final week, exercise was consistently maintained for 40 minutes at ≈80% MHR. Participants cooled down for 3–5 minutes, following cessation of exercise.
Resistance exercise
A brief, 3–5 minutes warm-up was performed on either a treadmill or cycle ergometer followed by light stretching before completing a whole-body resistance training circuit of upper-body and lower-body exercises 3×/week. During the initial 4 weeks of training, one set of 10 repetitions were performed for each exercise separated by a 2 minute rest period. Every 3 weeks strength was re-evaluated to ensure a progressive training load based on ≈80% of one-repetition max.
Statistical analyses
Means and standard deviations were calculated for each weight loss group. Exploratory analyses revealed the days needed to reach target BMI, total weight reduction, and total decrease in body fat% were not different among groups, therefore, group data was pooled then dichotomized by race. A 2×2 analysis of variance with repeated measures (pre- and post-) was used to compare differences for primary variables of interest at baseline and following weight loss. Comparisons of change (deltas; Δ) were made by determining the differences between weight loss and baseline values. Bivariate correlation analyses were used to examine the relationship among variables at baseline, following weight loss, and deltas (occurring with weight loss). Based on simple correlations, a multiple linear regression was used to evaluate the independent effects of Δ25(OH)D (from baseline to weight loss) on ΔHb, independent of African admixture, Δtestosterone, and Δbody fat%. Collinearity diagnostics for all variables were within acceptable limits and variable inflation factors for all models were < 1.14. Additionally, the direct and indirect effects of Δbody fat% on ΔHb through Δ25(OH)D were assessed using a path-analytic approach. The PROCESS macro for SPSS described by Hayes (Hayes, 2013) was selected as it utilizes a bootstrapping approach which is less influenced by sample size. In short, the bootstrapping procedure is accomplished by taking a large number of samples from the original dataset via random sampling with replacement. The result is an empirically derived sampling distribution of the indirect effect in which the upper and lower bounds of the 95% confidence interval (CI) match the 2.5% and 97.5% points of the sampling distribution. When the CIs do not contain zero, mediation is indicated. Data were analyzed with SPSS (v22; IBM Corporation, NY). Statistical significance was assumed if p-values were ≤ 0.05.
Results
Overview
By design, all participants lost body weight (p < 0.001) and body fat% (p < 0.001) (Table 1). No significant time by race interactions were observed nor were there differences in the number of days required to reach target BMI (< 25 kg/m2) among the weight loss groups: [1) diet only, 154 ± 43 days; 2) diet +aerobic exercise, 150 ± 71 days; 3) diet +resistance exercise, 149 ± 58 days; p = 0.97].
Table 1.
Descriptive characteristics and corresponding changes with weight loss.
| P-values | |||||
|---|---|---|---|---|---|
| Variables | African American (n = 33) | European American (n = 31) | Time | Race | T × R |
| African admixture (%) | 62 ± 15 | 16 ± 4 | < 0.001 | ||
| Age (yr) | 34 ± 7 | 34 ± 6 | 0.58 | ||
| Height (cm) | 166 ± 6 | 164 ± 7 | 0.25 | ||
| Body weight (kg) | |||||
| Overweight | 78.3 ± 4.7 | 76.5 ± 7.5 | < 0.001 | 0.24 | 0.61 |
| Post-weight loss | 66.6 ± 4.6 | 64.6 ± 7.7 | |||
| Body mass index (kg/m2) | |||||
| Overweight | 28.3 ± 1.2 | 28.2 ± 1.2 | < 0.001 | 0.48 | 0.56 |
| Post-weight loss | 24.0 ± 0.9 | 23.8 ± 1.2 | |||
| Body fat (%) | |||||
| Overweight | 44.3 ± 4.3 | 45.6 ± 3.1 | < 0.001 | 0.25 | 0.80 |
| Post-weight loss | 33.9 ± 5.0 | 34.9 ± 4.2 | |||
| Hemoglobin (g/dL) | |||||
| Overweight | 11.7 ± 0.9 | 12.5 ± 0.8 | < 0.001 | < 0.001 | 0.48 |
| Post-weight loss | 11.2 ± 1.1 | 12.1 ± 0.9 | |||
| 25(OH)D (nmol/L) | |||||
| Overweight | 35.7 ± 12.9 | 57.0 ± 20.0 | < 0.001 | < 0.001 | 0.37 |
| Post-weight loss | 43.4 ± 14.0 | 68.2 ± 24.3 | |||
| Testosterone (ng/dL) | |||||
| Overweight | 40.2 ± 29.9 | 38.2 ± 16.1 | 0.17 | 0.70 | 0.97 |
| Post-weight loss | 37.0 ± 24.3 | 34.9 ± 15.5 | |||
| C-reactive protein (mg/L) | |||||
| Overweight | 3.3 ± 4.3 | 2.1 ± 1.8 | <0.001 | 0.22 | 0.24 |
| Post-weight loss | 1.8 ± 2.2 | 1.4 ± 2.2 |
Based on reference ranges for non-pregnant, premenopausal women according to the World Health Organization: 15 (12 AA) of 64 (23%) participants had moderate-anemia (8.0–10.9 g/dL), 19 (12 AA) of 64 (30%) participants had iron-deficiency anemia (11.0–11.9 g/dL) while 30 (9 AA) of 64 (47%) participants had normal hemoglobin (>12.0 g/dL) after weight loss. Reference values follow: body mass index, 18.5–24.9 kg/m2 (normal); 25.0–29.9 kg/m2 (overweight); 25(OH)D >50 nmol/L (normal); total testosterone 15–60 ng/dL.
All values are presented as means ± SD; T × R, time by race interaction; AA, African Americans.
Overweight state
Prior to weight loss, AA women had lower 25(OH)D and lower Hb compared to EA women. Total testosterone and CRP were not different between races prior to weight loss. Negative associations were found between African admixture and 25(OH)D (r = −0.459; p < 0.01), as well as, African admixture and Hb (r = −0.389; p < 0.01). Positive associations were found between 25(OH)D and Hb (r = 0.376; p < 0.01) and 25(OH)D and body fat% (r = 0.254; p < 0.05).
Post-weight loss
Following weight loss, 25(OH)D increased while Hb decreased among both races. Mean values for total testosterone tended to decrease but the change was not significant. A significant time effect (p < 0.01) was observed for CRP following weight loss. Negative associations were found between African admixture and 25(OH)D (r = −0.456; p< 0.01) and Hb (r = −0.396; p < 0.01). A positive association was found between 25(OH)D and Hb (r = 0.319; p < 0.01).
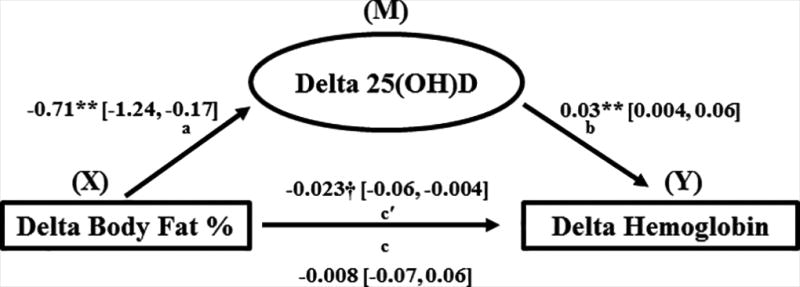
Among the changes (Δ), from pre- and post-weight loss, a positive association between Δ25(OH)D and ΔHb (r = 0.302; p < 0.05) was observed while Δ25(OH)D was negatively associated with Δbody fat% (r = −0.316; p < 0.01) (Table 2). A multiple linear regression was used to test the independent effects of Δ25(OH)D on ΔHb with weight loss. A positive association persisted (r = 0.386; p = 0.007) after adjusting for African admixture, Δtestosterone, and Δbody fat% (Table 3). Of note, adjusting for weight loss group did not affect the regression model suggesting the changes in Hb were not a product of group allocation/treatment. The PROCESS macro for SPSS (Hayes, 2013), tested the direct and indirect effects of Δbody fat% on ΔHb through Δ25(OH)D (Figure 2). Delta body fat% was negatively associated with Δ25(OH)D, β = −0.71, 95% CI [−1.24, −0.17]. Delta 25(OH)D was positively associated with ΔHb, β = 0.03, 95% CI [0.004, 0.06] such that a greater increase in 25(OH)D corresponded with an attenuated decrease in Hb, independent of Δbody fat%. Finally, a negative indirect effect of Δbody fat% on ΔHb through Δ25(OH)D was observed, β= −0.023, 95% CI [−0.06, −0.004]. Since the confidence interval did not include zero, mediation was confirmed (p ≤ 0.05).
Table 2.
Correlation matrix among the variable changes (Δ) from baseline with weight loss (n = 64).
| Variables | African admixture |
ΔHemoglobin | Δ25(OH)D | ΔTestosterone | ΔCRP | ΔPercent Fat |
|---|---|---|---|---|---|---|
| African admixture | -- | -- | -- | -- | -- | -- |
| ΔHemoglobin | −0.117 | -- | -- | -- | -- | -- |
| Δ25(OH)D | −0.093 | 0.302† | -- | -- | -- | -- |
| ΔTestosterone | 0.113 | 0.185 | 0.023 | -- | -- | -- |
| ΔCRP | −0.062 | −0.002 | 0.102 | −0.118 | -- | -- |
| ΔPercent Fat | 0.038 | −0.123 | −0.316* | −0.020 | −0.065 | -- |
Significance at p < 0.01;
Significance at p < 0.05. Δ, changes from baseline with weight loss; hemoglobin (g/dL); 25(OH)D, 25-hydroxyvitamin D (nmol/L); testosterone (ng/dL); CRP, C-reactive protein (mg/L).
Table 3.
Model estimation for changes (Δ) in hemoglobin with weight loss.
| Model R | R2 | Slope | Standardized β | Partial r | p-value | |
|---|---|---|---|---|---|---|
| Intercept | 0.43 | 0.18 | −0.223 | |||
| African admixture | −0.221 | −0.086 | −0.093 | 0.535 | ||
| Δ25(OH)D (nmol/L) | 0.017 | 0.403 | 0.386* | 0.007 | ||
| ΔTestosterone (ng/dL) | 0.006 | 0.156 | 0.169 | 0.257 | ||
| ΔBody Fat (%) | 0.030 | 0.120 | 0.124 | 0.407 |
To independently evaluate plausible factors influencing ΔHb the following variables were included for analyses: African admixture, Δ25(OH)D, Δtestosterone, and Δbody fat%
Significant at p < 0.01.
Figure 2.
Discussion
The current study revealed that calorie restriction (i.e., 800 kcal/day) leading to significant weight loss decreases Hb in both AA and EA women, and these changes (Δ) were independently associated with Δ25(OH)D. Simply put, participants with the largest increase in serum 25(OH)D, presumably from reduced adiposity, also exhibited the smallest decrease in Hb. Path analyses revealed a significant indirect effect of Δbody fat% on ΔHb through Δ25(OH)D. Since low Hb is an independent predictor of cardiovascular disease (Sarnak et al., 2002) and impairs aerobic capacity, further research should clarify optimal calorie restriction to stimulate weight loss while also mitigating the potential risk of anemia associated with dieting efforts.
Associations between obesity and low 25(OH)D have previously been reported (Aasheim et al., 2008; Lagunova et al., 2009; Snijder et al., 2005). While some debate exists about the exact nature of this association, it is generally believed that 25(OH)D is sequestered in adipose tissue which lowers bioavailability (Wortsman et al., 2000). However, weight loss with a concomitant loss in fat mass, has been shown to increase serum 25(OH)D in a dose-response manner (Rock et al., 2012). From that study, participants who lost >10% of their initial body weight expressed the largest increase in serum 25(OH)D (+12.5 nmol/L). Given the relatively homogenous cohort in the current study, all participants achieved a similar ≈15% reduction from their initial body weight which corresponded to a ≈23% reduction in body fat. Despite an average increase of 25(OH)D, a notable proportion of AA women from the present study had 25(OH)D levels below the recommended threshold (50 nmol/L) proposed by the Institute of Medicine (Dietary Reference Intakes for Vitamin D and Calcium, 2011). However, bioavailable, rather than total 25(OH)D is the clinically meaningful fraction (Ashraf et al., 2014), and as such, it is not clear whether our participants were actually vitamin D deficient or insufficient. Despite efforts to correct low 25(OH)D (Vieth et al., 2007), a degree of ambiguity exists regarding the appropriate 25(OH)D range (Sorkin et al., 2014). Nevertheless, participants from the current study exhibiting the largest increase in 25(OH)D also had the smallest decrease in Hb, suggesting a shared (albeit limited) role of 25(OH)D concerning erythrocyte regulation. In light of previous work showing 1,25(OH)2D administration stimulates erythropoietin receptor expression (Alon et al., 2002), the weight loss-induced increase in 25(OH)D, may have expanded bioavailability and thus worked synergistically with erythropoietin to preserve Hb during dieting.
Subclinical inflammation associated with excess adiposity is thought to upset iron availability through an upregulation of hepcidin (Yanoff et al., 2007). Hence, circulating Hb could be affected through a disruption in iron homeostasis. However, we did not find an association before or after weight loss between CRP and body fat%. Recently, Cheng and colleagues (Cheng et al., 2013) proposed a notable degree of comorbidities (e.g., obesity, age) may be obligatory before iron kinetics are adversely affected. Apart from being overweight upon enrollment, our participant sample were relatively young, free of comorbidities, with very few participants exceeding (>3 mg/L) the clinically relevant CRP threshold for cardiovascular disease. Thus, it is unlikely that perturbations in Hb following weight loss were due to elevated CRP.
Given the known erythrogenic properties of testosterone and previous work indicating that weight loss reduces testosterone in overweight women (Leenen et al., 1994), we initially expected that Hb would be affected through this mechanism. To our surprise, we did not find an association between testosterone and Hb before or after weight loss. Recently, testosterone administration has been shown to upregulate splenic iron release via hepcidin inhibition which increases iron availability for Hb synthesis (Guo et al., 2013). However, only modest changes in testosterone were observed in the present study and it is unlikely that altered testosterone profiles contributed to the observed changes in Hb.
Though the NIDDK has indicated very-low calorie dieting can permit weight loss up to 3–5 lbs./week, average time to reach target BMI (< 25 kg/m2) in the present work was ≈150 days. Subsequently, participants were held in energy balance for a further 4 weeks before post-weight loss blood samples were taken. It is possible that erythropoiesis may have affected, due in-part to length of calorie restriction, as previous work has shown calorie restriction combined with intense exercise directly suppresses erythropoietin (Gunga et al., 1996). Additionally, Caro et al. (Caro et al., 1981) have previously established that calorie restriction can readily effect erythropoietin production through a decrease in triiodothyronine (T3). Although erythropoietin and T3 were not measured in the present study, we have previously shown that energy restriction to 800 kcal/day decreases T3 (Weinsier et al., 2000). More recently, moderate weight loss (≈6.5%) over a 12-month period has been shown to lower T3 (Agnihothri et al., 2014). Due to the severity of caloric restriction in the present study, body mass (≈15%) and body fat% (≈23%) were reduced to a greater extent and at a faster rate, thus it seems plausible that Hb could have been affected due to changes in T3.
Several limitations arose during this investigation. The noted associations were based on single blood draws before and after weight loss. Causal relationships cannot be made nor can we account for the potential seasonal variations (i.e., sunlight exposure) in the observed outcomes. Given the notable time-frame needed to reach target BMI, we can only speculate that some cheat foods were being consumed during the dieting phase of the investigation. As such, potential variation in nutrient intake over time slightly restricts the extent our findings can be extrapolated to other very-low calorie dieting paradigms. Indeed low dietary iron intake could have contributed to the observed changes in Hb, however, recent work has indicated that depleted muscle glycogen can upregulate hepcidin thus interfering with iron absorption (Badenhorst et al., 2015). Since all participants consumed between ≈116–124 grams of carbohydrates per day, it is possible Hb could have been altered through this mechanism. However, during initial exploratory analyses there were no detectable differences found between exercising and non-exercising weight loss groups suggesting that changes in Hb were not a product of group allocation/treatment. Given the significant decrease Hb, we cannot definitively rule out the possibility of a ‘new-baseline’ following weight loss. Yet, it should be noted that a large proportion of women, particularly AA, were well-below the widely accepted threshold (< 12 g/dL) for anemia in non-pregnant women (WHO, 2011). Finally, only total 25(OH)D values were available, future work should examine the relationship between Hb and the bioavailable fraction of 25(OH)D following weight loss.
Novelty Statement
Similar to others, we found that reduced adiposity is related to increased serum 25(OH)D. Importantly though, participants who expressed the largest increase in 25(OH)D after weight loss also had the smallest decrease in Hb. As shown in Figure 1, 15% of the total variance in ΔHb was accounted for by Δ25(OH)D adjusted for African admixture, Δtestosterone, and Δbody fat%. Finally, path analyses revealed a significant indirect effect of Δbody fat% on ΔHb through Δ25(OH)D.
Figure 1.

Practical Application
Given the observed link between Hb and 25(OH)D before and after weight loss, additional research should determine if vitamin D supplementation can preserve Hb during dieting efforts. Our results indicate very-low calorie dieting may, in some, inadvertently lower Hb though it is unknown if such changes interfere with exercise tolerance and/or engagement in free-living physical activity.
Acknowledgments
We acknowledge Roland L. Weinsier, Paul A. Zuckerman, David R. Bryan and Brandon L. Kane for their respective contributions. We also wish to thank the participants for their willingness to complete this investigation. SJC completed data analyses, interpretation, and drafted the manuscript/tables/figures. EPP completed data analyses, interpretation and drafted manuscript. GF completed data analyses, interpretation and drafted manuscript. JRF completed data interpretation and drafted manuscript. BAG completed data interpretation and drafted manuscript. GRH conceived the study, oversaw data collection, analyses and interpretation, and drafted manuscript. All authors actively participated in preparation of the manuscript and approved the final version. This investigation was supported by the following grants from the National Institutes of Health: R25CA47888, R01AG027084-01, R01AG027084-S, P30DK056336, P60DK079626, and UL1RR025777.
Footnotes
The authors declare no conflict of interest.
References
- Aasheim ET, Hofso D, Hjelmesaeth J, Birkeland KI, Bohmer T. Vitamin status in morbidly obese patients: a cross-sectional study. The American Journal of Clinical Nutrition. 2008;87(2):362–369. doi: 10.1093/ajcn/87.2.362. doi:87/2/362 [pii] [DOI] [PubMed] [Google Scholar]
- Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. International Journal of Obesity (2005) 2009;33(10):1111–1117. doi: 10.1038/ijo.2009.146. [doi] [DOI] [PubMed] [Google Scholar]
- Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, Celi FS. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid: Official Journal of the American Thyroid Association. 2014;24(1):19–26. doi: 10.1089/thy.2013.0055. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, Nathan I. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Experimental Hematology. 2002;30(5):403–409. doi: 10.1016/s0301-472x(02)00789-0. doi:S0301472X02007890 [pii] [DOI] [PubMed] [Google Scholar]
- Ashraf AP, Huisingh C, Alvarez JA, Wang X, Gower BA. Insulin resistance indices are inversely associated with vitamin D binding protein concentrations. The Journal of Clinical Endocrinology and Metabolism. 2014;99(1):178–183. doi: 10.1210/jc.2013-2452. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring, Md.) 2008;16(10):2356–2361. doi: 10.1038/oby.2008.353. [doi] [DOI] [PubMed] [Google Scholar]
- Badenhorst CE, Dawson B, Cox GR, Laarakkers CM, Swinkels DW, Peeling P. Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. European Journal of Applied Physiology. 2015;115(12):2521–2530. doi: 10.1007/s00421-015-3252-3. [doi] [DOI] [PubMed] [Google Scholar]
- Caro J, Silver R, Erslev AJ, Miller OP, Birgegard G. Erythropoietin production in fasted rats. Effects of thyroid hormones and glucose supplementation. The Journal of Laboratory and Clinical Medicine. 1981;98(6):860–868. doi:0022-2143(81)90080-9 [pii] [PubMed] [Google Scholar]
- Cheng HL, Bryant CE, Rooney KB, Steinbeck KS, Griffin HJ, Petocz P, O'Connor HT. Iron, hepcidin and inflammatory status of young healthy overweight and obese women in Australia. PloS One. 2013;8(7):e68675. doi: 10.1371/journal.pone.0068675. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AS, Zanjani ED, Levere RD, Kappas A. Stimulation of mammalian erythropoiesis by 5beta-H steroid metabolites. Proceedings of the National Academy of Sciences of the United States of America. 1970;65(4):919–924. doi: 10.1073/pnas.65.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunga HC, Wittels P, Gunther T, Kanduth B, Vormann J, Rocker L, Kirsch K. Erythropoietin in 29 men during and after prolonged physical stress combined with food and fluid deprivation. European Journal of Applied Physiology and Occupational Physiology. 1996;73(1–2):11–16. doi: 10.1007/BF00262804. [DOI] [PubMed] [Google Scholar]
- Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, Bhasin S. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12(2):280–291. doi: 10.1111/acel.12052. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. American Journal of Physical Anthropology. 1986;70(4):433–441. doi: 10.1002/ajpa.1330700404. [doi] [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity (Silver Spring, Md.) 2010;18(4):690–695. doi: 10.1038/oby.2009.316. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GR, Chandler-Laney PC, Brock DW, Lara-Castro C, Fernandez JR, Gower BA. Fat distribution, aerobic fitness, blood lipids, and insulin sensitivity in African-American and European-American women. Obesity (Silver Spring, Md.) 2010;18(2):274–281. doi: 10.1038/oby.2009.229. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. 2011 doi:NBK56070 [bookaccession] [Google Scholar]
- Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Research. 2009;29(9):3713–3720. doi:29/9/3713 [pii] [PubMed] [Google Scholar]
- Leenen R, van der Kooy K, Seidell JC, Deurenberg P, Koppeschaar HP. Visceral fat accumulation in relation to sex hormones in obese men and women undergoing weight loss therapy. The Journal of Clinical Endocrinology and Metabolism. 1994;78(6):1515–1520. doi: 10.1210/jcem.78.6.8200956. [doi] [DOI] [PubMed] [Google Scholar]
- McKeigue PM. Multipoint admixture mapping. Genetic Epidemiology. 2000;19(4):464–467. doi: 10.1002/1098-2272(200012)19:4<464::AID-GEPI17>3.0.CO;2-M. [pii] [DOI] [PubMed] [Google Scholar]
- Reljic D, Hassler E, Jost J, Friedmann-Bette B. Rapid weight loss and the body fluid balance and hemoglobin mass of elite amateur boxers. Journal of Athletic Training. 2013;48(1):109–117. doi: 10.4085/1062-6050-48.1.05. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, Thomson CA. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring, Md.) 2012;20(11):2296–2301. doi: 10.1038/oby.2012.57. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. Journal of the American College of Cardiology. 2002;40(1):27–33. doi: 10.1016/s0735-1097(02)01938-1. doi:S0735109702019381 [pii] [DOI] [PubMed] [Google Scholar]
- Smith EM, Alvarez JA, Martin GS, Zughaier SM, Ziegler TR, Tangpricha V. Vitamin D deficiency is associated with anaemia among African Americans in a US cohort. The British Journal of Nutrition. 2015;113(11):1732–1740. doi: 10.1017/S0007114515000999. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. The Journal of Clinical Endocrinology and Metabolism. 2005;90(7):4119–4123. doi: 10.1210/jc.2005-0216. doi:jc.2005-0216 [pii] [DOI] [PubMed] [Google Scholar]
- Sorkin JD, Vasaitis TS, Streeten E, Ryan AS, Goldberg AP. Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese postmenopausal women. The Journal of Nutrition. 2014;144(5):734–742. doi: 10.3945/jn.114.190660. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Falk RT, Stanczyk F, Hoover RN, Appel LJ, Ard JD, Katki HA. Sex hormone changes during weight loss and maintenance in overweight and obese postmenopausal African-American and non-African-American women. Breast Cancer Research: BCR. 2012;14(5):R141. doi: 10.1186/bcr3346. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tussing-Humphreys LM, Liang H, Nemeth E, Freels S, Braunschweig CA. Excess adiposity, inflammation, and iron-deficiency in female adolescents. Journal of the American Dietetic Association. 2009;109(2):297–302. doi: 10.1016/j.jada.2008.10.044. [doi] [DOI] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. The American Journal of Clinical Nutrition. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. doi:85/3/649 [pii] [DOI] [PubMed] [Google Scholar]
- Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. The American Journal of Clinical Nutrition. 2000;72(5):1088–1094. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. [accessed [03/03/2016])]. (WHO/NMH/NHD/MNM/11.1) ( http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- Williams DM. Racial differences of hemoglobin concentration: measurements of iron, copper, and zinc. The American Journal of Clinical Nutrition. 1981;34(9):1694–1700. doi: 10.1093/ajcn/34.9.1694. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. The American Journal of Clinical Nutrition. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. International Journal of Obesity (2005) 2007;31(9):1412–1419. doi: 10.1038/sj.ijo.0803625. doi:0803625 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo EH, Cho HJ. Prevalence of 25-hydroxyvitamin D deficiency in Korean patients with anemia. Journal of Clinical Laboratory Analysis. 2015;29(2):129–134. doi: 10.1002/jcla.21740. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]


