Abstract
CD8+ T cells that recognize peptides presented by MHC class II molecules have been observed in a macaque SIV vaccine model. A new study by Ranasinghe et al. (2016) shows that virus-specific class-II-restricted CD8+ T cells can be found in some HIV-infected patients.
Antigen-specific CD8+ T cells classically detect intracellular pathogens by TCR-mediated recognition of short pathogen-derived peptides presented on the surface of infected cells by MHC class Ia molecules (reviewed in Neefjes et al., 2011). CD8+ T cells that recognize peptides presented by MHC class II molecules are not observed in most animal systems or in humans at a high prevalence except under some artificial circumstances. Such CD8+ T cells have been reported in mice transgenic for a class II-specific TCR or CD4-deficient mice (Matechak et al., 1996) and in some murine models of transplantation (Hirosawa et al., 2011). They have also been reported in humans to cross-recognize HLA class II and HLA class Ia in the setting of alloreactivity (Heemskerk et al., 2001; Rist et al., 2009). More recently, they have been observed in Rhesus macaques immunized with recombinant rhesus cytomegalovirus (RhCMV) strain 68-1/simian immunodeficiency virus (SIV) vectors as a model for an HIV vaccine (Hansen et al., 2013b). This vaccine strategy is the most efficacious thus far reported with over 50% of immunized animals able to stringently control and ultimately clear a highly pathogenic SIV challenge (Hansen et al., 2013a; Hansen et al., 2013b).
Given the extraordinary success of the recombinant RhCMV/SIV-vaccine approach, it was of particular interest to determine whether MHC class II-restricted CD8+ T cells can be found in natural human viral infections, particularly for HIV. In this issue, Ranasinghe et al. set out to determine whether HIV-specific, HLA class-II-restricted CD8+ T cell responses can be found in HIV-1-infected persons exhibiting naturally occurring control of virus replication without antiretroviral therapy, known as HIV controllers (Ranasinghe et al., 2016). To do this, they screened the blood of 101 HIV controllers and found 3 such examples. HLA class-II-restricted CD8+ T cells were found at a particularly high frequency in one individual, occurring in up to 12% of circulating CD8+ T cells. These were enumerated by both functional assays and MHC class II tetramer staining, indicating they are specific for HIV and restricted by MHC class II. This is surprising given such responses to our knowledge haven’t been found in the past in humans, suggesting that if they were to be found they would likely be present at a low frequency. The mapped epitope targeted by this response did not significantly overlap with epitopes targeted by other detectable class Ia-restricted CD8+ T cell responses in the same patient (Ranasinghe et al., 2016), indicating that the response was not due to class-Ia-restricted cells that can cross-recognize in the class II context. Although present in a small minority of patients, these data show that unconventional HLA class II-restricted CD8+ T cells can be found in a human chronic viral infection.
The RhCMV/SIV vector model is yielding some important clues regarding how HLA class II-restricted CD8+ T cells might be induced during a viral infection. The efficacy of this approach is associated with immunization with specific vectors lacking the Rh157.4-.6/UL128-131 genes (Hansen et al., 2013b). The CD8+ T cell response induced by these vectors is characterized by a lack of cells targeting MHC-Ia-restricted canonical epitopes. Instead they target a distinct set of highly diverse epitopes, including promiscuous “supertopes.” The response is extraordinarily broad, approximately three times greater than responses associated with SIV infection or conventional vaccination, and predominantly presented by MHC class II (63% of epitopes) and MHC-E (Hansen et al., 2016). Further examination revealed that individual MHC-II gene products could present multiple peptides and individual peptides could frequently be presented by multiple MHC-II gene products including those not carried by a given animal (Hansen et al., 2013b). Primate CMV gene expression appears to be responsible for these features, with canonical responses specifically suppressed by expression of the included Rh189/US11 gene and the promiscuous MHC-I and MHC-II-restricted CD8+ T cell responses occurring only in the absence of the UL128-131 genes (Hansen et al., 2013b).
How well this vaccination approach will translate to humans remains uncertain given questions regarding the mechanisms by which these unconventional responses arise in rhesus macaques, whether they would arise in humans, and whether they would be required for high-level control of HIV in humans. In the RhCMV/SIV model, CD8+ T cells specific for class II-presented peptides occur at a high prevalence, suggesting this is not the rare product of MHC-II cross-reactivity of an antigen-specific TCR selected on MHC-I molecules in the thymus, as has been suggested in cases of allo-reactivity in humans (Heemskerk et al., 2001). As suggested by Hansen et al., the ΔRh157.4-.6/UL128-131 RhCMV vectors, by modulation of vector cell tropism and the priming microenvironment, might bypass mechanisms that normally constrain the response in favor of conventionally targeted epitopes (Hansen et al., 2013b). Nonetheless, the rapid nature of this response and its breadth in adult macaques suggest that naive cells capable of this atypical recognition routinely exist in the periphery after thymic selection in macaques. Given that these cells are CD4−CD8+, they do not require the CD4 co-receptor for recognition and might not have required it for thymic selection or maturation. In mice transgenic for a class II-specific TCR, the majority of mature naive T cells are CD4+ T cells although a minor population express this TCR with CD8 (Matechak et al., 1996). It is possible that such a population exists in Rhesus macaques and is then expanded during infection with the UL128-131-deficient RhCMV vectors.
Although the CD8+ T cells described by Ranasinghe et al. were class II restricted, there are a few important differences between their findings and those reported in the RhCMV/SIV recombinant model. First, the responses were observed in a small fraction of patients and the cells had characteristics that suggest that they survived thymic selection through unique events rather than a more generalized pattern of selection. A single TCR V beta clonotype was expanded in the HLA class II-restricted CD8+ T cells of all three subjects; however, alpha TCR analysis revealed co-expression of two different alpha chains within the same CD8+ T cell, consistent with incomplete allelic exclusion (Ranasinghe et al., 2016). The authors speculate that the TRAV26-TRBV2 pair might have been responsible for positive selection of this clone in the thymus, which was followed by peripheral expansion via its TRAV6 TCR specificity that was able to bind DR11-Gag41 in the setting of subsequent HIV infection (Figure 1). However, it should be noted that CD8+ T cells specific for class II-presented peptides can infrequently be found in controller macaques (Hansen et al., 2016), similar in prevalence to those described by Ranasinghe et al. It is possible that low prevalence responses wherein cells are class I selected and then expanded by a class II presented peptide, and the high prevalence responses observed in the RhCMV/SIV setting, represent two separate pathways to the generation of these cells. The second feature that differentiates the re sponses observed in Ranasinghe et al. is that the class II restriction was more typical of a class II-restricted response and lacked the ability to recognize a given peptide bound to multiple class II molecules or the level of promiscuity observed in the RhCMV/SIV system (Hansen et al., 2013b).
Figure 1. Proposed Mechanisms of CD8+ T Cell Recognition of MHC-Presented Peptides.
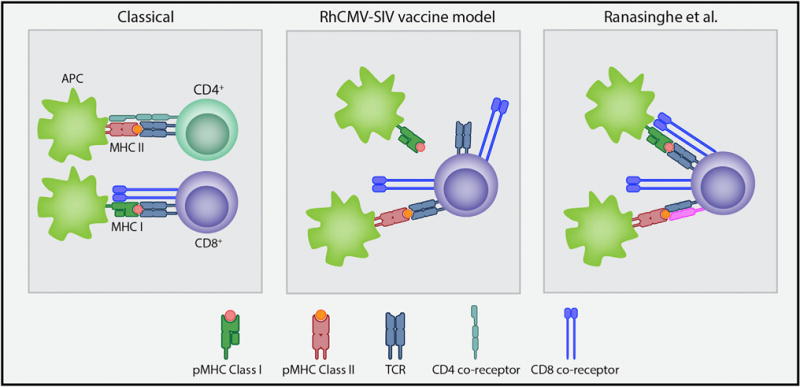
Classically, T cells recognize MHC-presented peptides in the context of class I with the aid of the CD8 co-receptor or class II with the aid of the CD4 co-receptor. In the RhCMV-SIV vaccine model, large frequencies of CD8+ T cells that break this paradigm recognize a diverse set of peptides presented in the binding groove of MHC class II proteins, which do not overlap with MHC-I-restricted canonical epitopes (Hansen et al., 2013b). Recognition by the T cell is not thought to involve binding by the CD8 co-receptor. Ranasinghe et al. describe CD8+ T cells with a single TCR V beta clonotype and 2 alpha chains, in which 1 alpha-beta chain pair (blue TCR) might be responsible for MHC-I-peptide binding during thymic selection and survival, and an alternative pair (blue and pink TCR) that preferentially recognizes peptides in the context of MHC-II proteins that might be expanded during subsequent HIV infection. pMHC, peptide MHC; TCR, T cell receptor; APC, antigen-presenting cell.
Although MHC restricted recognition by T cells is a field that is over 40 years old, the findings in Ranasinghe et al. and Hansen et al. underscore that there is still plenty to learn. Constructs similar to the RhCMV/SIV vectors described above are being prepared in human CMV expressing HIV gene products for use in clinical trials. Certainly this approach merits testing and the results are highly anticipated. Beyond learning the immunogenicity of this approach in humans, we will likely learn to what degree the class II-specific CD8+ T cell responses observed in the Rhesus system extend to humans. In addition, we will learn a considerable amount of basic immunology regarding the comparative differences between Rhesus and human MHC and the factors that govern selection, maturation, and expansion of virus-specific T cells. A better understanding of how the generation of class II-specific CD8+ T cells can be modulated potentially has important implications for vaccines or immunotherapies that may extend beyond viral infection to the fields of transplantation or cancer.
References
- Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Nature. 2013a;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, et al. Science. 2013b;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, et al. Science. 2016;351:714–720. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk MH, de Paus RA, Lurvink EG, Koning F, Mulder A, Willemze R, van Rood JJ, Falkenburg JH. Proc Natl Acad Sci USA. 2001;98:6806–6811. doi: 10.1073/pnas.111162298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosawa T, Torikai H, Yanagisawa M, Kamei M, Imahashi N, Demachi-Okamura A, Tanimoto M, Shiraishi K, Ito M, Miyamura K, et al. Cancer Sci. 2011;102:1281–1286. doi: 10.1111/j.1349-7006.2011.01949.x. [DOI] [PubMed] [Google Scholar]
- Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. Immunity. 1996;4:337–347. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- Neefjes J, Jongsma ML, Paul P, Bakke O. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- Ranasinghe S, Lamothe PA, Soghoian DZ, Kazer SW, Cole MB, Shalek AK, Yosef N, Jones RB, Donaghey F, Nwonu C, et al. Immunity. 2016;45:917–930. doi: 10.1016/j.immuni.2016.09.015. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rist M, Smith C, Bell MJ, Burrows SR, Khanna R. Blood. 2009;114:2244–2253. doi: 10.1182/blood-2009-05-222596. [DOI] [PubMed] [Google Scholar]


