Abstract
Screening for HIV-associated neurocognitive disorders (HAND) is important to improve clinical outcomes. We compared the diagnostic sensitivity and specificity of the mini-mental state examination, International HIV dementia scale (IHDS), Montreal cognitive assessment, Simioni symptom questionnaire and cognitive assessment tool-rapid version (CAT-rapid) to a gold standard neuropsychological battery. Antiretroviral-experienced participants from Cape Town, South Africa, and Baltimore, USA, were recruited. The sensitivity and specificity of the five tools, as well as those of the combined IHDS and CAT-rapid, were established using 2 × 2 contingency tables and ROC analysis. More than a third (65165) had symptomatic HAND. In detecting HIV-D, the CAT-Rapid had good sensitivity (94 %) and weak specificity (52 %) (cut-point ≤10), while the IHDS showed fair sensitivity (68 %) and good specificity (86 %) (cut-point ≤10). The combined IHDS and CAT-rapid showed excellent sensitivity and specificity for HIV-D at a cut-off score of ≤16 (out of 20; 89 and 82 %). No tool was adequate in screening for any HAND. The combination IHDS and CAT-rapid tool appears to be a good screener for HIV-D but is only fairly sensitive and poorly specific in screening for any HAND. Screening for milder forms of HAND continues to be a clinical challenge.
Keywords: HIV-dementia, HIV-associated neurocognitive disorders, Screening
Introduction
HIV-associated neurocognitive disorders (HAND) remain highly prevalent in clinic settings, despite the use of combination anti-retroviral therapy (CART) [1–4]. It is now established that HIV-dementia (HIV-D) is diminishing in incidence, but perhaps not prevalence [5, 6]. The less severe form of symptomatic HAND (mild neurocognitive disorder, or MND) and the asymptomatic form (asymptomatic neurocognitive impairment, or ANI) are estimated to occur at rates of 12 and 33 %, respectively, in CART-experienced individuals [4, 7].
These different degrees of HAND are separately diagnosed by the extent of neuropsychological impairment, as well as the presence or absence of functional impairment [8]. HIV-dementia (a “major neurocognitive disorder”) requires there to be impairment in at least two domains of neurocognitive function that are at least 2 standard deviations below normal performance; while for MND and ANI, impairment is of the order of at least one standard deviation worse than normal. When functional impairment is present to a marked degree, together with severe neurocognitive impairment, then HIV-D is diagnosed; when functional impairment is only mild, together with less severe neurocognitive impairment then MND is diagnosed; and when there is no overt functional impairment but mild neurocognitive impairment, then ANI is diagnosed. The diagnosis of a HAND is also one of exclusion. There are several important confounding conditions that may cause or contribute to neurocognitive impairment. These include hepatitis C co-infection, alcohol and substance abuse, and depression [9–11]. Any accurate assessment of HAND must probe for these factors. As common co-morbidities, the extent to which they cause impairment needs to be weighed up.
HIV-D is associated with high rates of mortality, poor medication adherence, and impairment in activities of daily living [12–14]. The effects of MND are, by definition, milder, but those of ANI have been debated [15, 16]. However, there is evidence that ANI may be associated with an increased risk for the development of HIV-D. In such cases, there is an option to intervene by offering CART (in untreated individuals) or neuro-targeted regimens (in those on existing treatment). This may alter the natural history of disease progression in HAND, although there are few longitudinal studies [5, 17, 18]. While there remains some debate on the actual benefit of diagnosing ANI, the argument for neuro-protection might outweigh the risks. In the absence of overt clinical symptomatology, active screening is required to detect cases.
While the gold standard for assessment of HAND is a detailed battery of neuropsychological tests, ideally tapping cognitive domains affected by HIV, these are seldom available to patients in busy settings [8, 19]. Hence, a number of screening tools for HIV-D have been developed and validated for use in these settings. These tools include the HIV dementia scale and the international HIV dementia scale [7, 20, 21]. Other screeners, such as the Montreal cognitive assessment (MOCA) and mini-mental state examination (MMSE) have been used in HIV settings, with varying results [17, 20, 22]. Any screener needs to be brief, easy to administer (by clinicians not trained in neuropsychological assessment), and ought to display adequate sensitivity and specificity.
Most screeners have been developed with HIV-D in mind. In the CART era, and with the growing acceptance that both MND and ANI are important to detect, there is more interest in screening for these milder forms of HAND. The MOCA has been used to detect mild cognitive impairment in geriatric populations, and shows variable sensitivity and specificity for mild HAND, depending on the cut-off score [23–25]. Similarly, the ability of the IHDS to detect MND and ANI is variable. In one study using a cut-off score of ≤11 to detect HIV-D plus MND, a sensitivity and specificity of 81 and 54 % respectively were reported [12, 26]. Others have reported lower sensitivity to mild HAND [1, 3, 27].
The use of CART may be associated with a change in the type of neuropsychological impairment [5]. Untreated neuro-HIV is thought to be a mainly sub-cortical disease, while treated disease alters this somewhat. Therefore, in populations where CART use is widespread, screeners should tap both cortical and sub-cortical impairment. In addition, the ability to detect milder forms of HAND and to distinguish between them involves high sensitivity not only to neuropsychological impairment, but also to the presence of functional impairment. In this study, we compare the use of five brief screening tools across two sites (one where HIV-B is prevalent, and the other where HIV-C is prevalent). Furthermore, we included a novel screener, the Cognitive Assessment Tool-rapid version (CAT-rapid) to establish its validity in these settings. Our intention was to compare the tools with respect to their ability to screen for HIV-D and for milder forms of HAND.
Methods
Participants
We recruited individuals established on ART in Cape Town, South Africa, and Baltimore, USA. These were participants previously recruited into larger parent cohort studies. In Cape Town, we invited participants who had completed research procedures on a cross-sectional neuro-cognitive and neuro-imaging study, and who had been established on ART for at least 6 months. The Human Research Ethics Committee of the Faculty of Health Sciences at the University of Cape Town approved the study. In Baltimore, HIV + individuals who were in a clinical research cohort developed specifically for HAND characterization (the clinical cohort of the NIMH Center for the therapeutics of HAND) were recruited. Sites from South Africa and the USA were selected and the data combined in order to demonstrate that a standard approach to diagnosis and screening might be applicable. A screener used in the USA should also be useful in Africa, India or South America. In addition to the procedures being the same, as well as cross continent case conferences (see below), most of the measures used have been previously reported across various settings.
Assessment and Instruments
The neuropsychological assessment battery was identical across the two sites, and included tests sensitive to the impact of HIV on cognitive function: Tests were grouped into domains of attention/concentration [Mental alternation test (MAT), WAIS digit symbol-coding subtest and digit span], learning/memory [Hopkins verbal learning test (HVLT) and Rey complex figure test (RCF)], psychomotor speed (Grooved pegboard test-dominant and non-dominant) and executive function (Color trails test, Stroop color-word test, and the Wisconsin card sorting test (WCST). For each test, a raw score was obtained, together with a standard deviation, based on control or normative data available for the site. We assigned a neuropsychological score of 2 (NP2) if the performance was poorer than 2 standard deviations (SD) from the mean on at least two domains; or more than 2 SD’s on one domain and at least 1 SD on two other domains. We assigned an NP score of 1, if the performance was poorer than 1 SD on at least 2 domains.
The five screening tools compared in this study were the:
International HIV dementia scale (IHDS) [7]. The IHDS has been validated in numerous settings, including the USA and South Africa [12]. It has established sensitivity for detecting HIV-dementia, and may be useful for milder forms of HAND [15]. The IHDS includes measures of psychomotor speed and processing, as well as short-term memory.
The Montreal cognitive assessment (MOCA). The MOCA has been used in HIV-infected individuals [17]. It does not measure psychomotor speed or processing, but includes measures of executive function, attention, language, and episodic memory.
The mini-mental state examination (MMSE). The MMSE has been extensively used and studied since its original description [19]. Its limitations in HAND detection have been noted [20].
The Simioni symptom questions (SSQ). These questions were derived from the approach described by Simioni et al., and which proved to be useful in a first-tier screening approach. Patients without complaints were not found to have HIV-dementia, although they might have milder HAND [22].
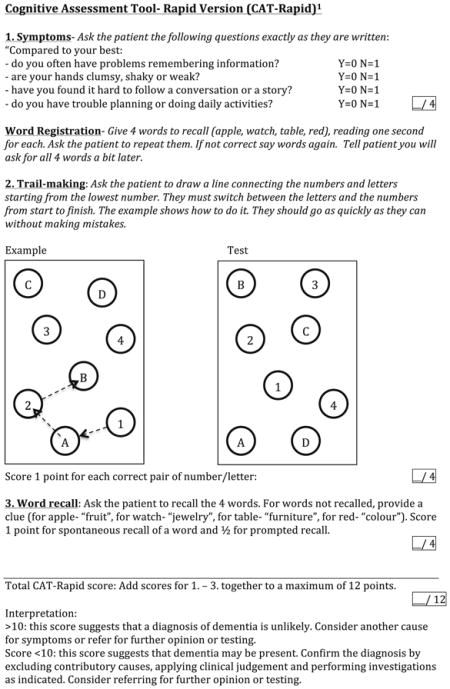
The cognitive assessment tool-rapid version (CAT-rapid). The CAT-rapid was developed by the first author (JJ), in response to the need to develop a brief HAND screening tool that includes functional symptom questions and a measure of executive function (see Appendix 1). The CAT-rapid includes four symptom questions, registration of four words, a mini-trail-making test of four letter/number pairs, and word recall.
In addition to these tools, we also used the combined performance of the IHDS (scored out of a possible 12 points) and the CAT-rapid (scored out of a 8 points discarding the 4-word recall), to arrive at a combined tool total score of 20. This “6th tool” was also included in the analysis.
We administered the centres for epidemiological studies-depression (CES-D) scale and the substance abuse and mental illness screener (SAMISS) to confirm the presence or absence of significant depressive symptomatology or alcohol abuse. Both have been extensively used and validated in international settings (see [23, 28]).
Functional impairment was ascertained in three ways: firstly, establishing self-reported ART adherence of >80 %; secondaly by administration of the modified Lawton Activities of daily living scale (LADL) [26]; and thirdly the presence of a positive response to functional symptoms reported on the SSQ and CAT-rapid. We assigned a functional assessment (FA) score of two (marked impairment), if the participant scored 14 or less on the LADL, OR 15 AND was noted to have an adherence problem. We scored FA1 if the LADL score was 15 OR there was either an adherence problem or a self-reported functional problem on the SSQ or CAT-rapid.
Procedures
Participants were administered the five screening tests, a detailed neuropsychological test battery, an assessment of activities of daily living and of subjective adherence, and a neuro-medical assessment. Participants in Cape Town did not have hepatitis C testing (as the population prevalence is approximately 6 %), while those in Baltimore did [29]. Cases were classified into HAND categories using American Academy of Neurology criteria. HAND diagnosis was confirmed by regular case conferences of all individuals from both sites by the PIs (JJ and NS) at each site. The order of testing was the same across both sites: We administered the IHDS, SSQ, CES-D, AUDIT and CAT-rapid, followed by the detailed battery. After the battery, we completed the assessment by administering the MMSE, functional assessment measures, and MOCA. The full battery including screeners took approximately 2 h and 30 min. The administration times of the screeners were: IHDS took 4 min, the SSQ took 3 min, the CAT-rapid took 7 min, the MOCA took 13 min, and the MMSE took 12 min.
Analysis
For each screener and AAN HAND category, participants were coded as “0” for normal and “1” for neurocognitive impairment. We used three groups of impairment as follows to establish sensitivity and specificity of each tool for various levels and types of impairment: [1] All three forms of HAND, [2] Symptomatic HAND: MND and HIV-D, and [3] HIV-Dementia. According to the established cut-off points for each tool, participants scoring above the cut-off point were coded as “0” (cognitively intact according to the screener), and those at or below the cut-off point were coded as “1” (cognitively impaired). We then generated a series of 2 × 2 contingency tables for the screeners versus the AAN HAND classification. The output displayed the number of true positives, true negatives, false positives and false negatives of the CAT-Rapid at the specified cut-off point. We then determined the tool’s sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at the specified cut-off point using http://www.medcalc.org/calc/diagnostic_test.php. The value of the PPV is that it is a measure of how many cases are detected by the tool, including those incorrectly included as cases, and therefore requiring further investigation to confirm; the PPV is of greater use where the proportion of “diseased” individuals in the study is similar to the general prevalence. The NPV is a measure of the proportion of cases that are discarded as non-cases, including those that were in fact cases, but missed. In principle for screening, high NPV’s are regarded as important. We report on sensitivity and specificity for each screener’s ability to detect HIV-D and any form of HAND. We regarded a value of >80 % as good sensitivity and >80 % as good specificity to maximize the utility of the respective screening tool.
Results
The sample included 156 participants (89 from SA, 67 from the USA). The sample median age was 40 years, and median education was 11 years. Nearly two-thirds (62.80 %) of the sample was female, and the median CD4 cell count was 460 cells/ml (see Table 1). Regarding confounders, we noted that in Baltimore, the average CES-D score was 10,13 (SD = 10.54); while in Cape Town it was 7,00 (SD = 9,90). Hazardous alcohol use in the preceding 3 months was reported in 5 participants in Baltimore and 4 in Cape Town. While hepatitis C infection status was not investigated in Cape Town, the prevalence in the Baltimore sub-sample was 38.8 %.
Table 1.
Characteristics of participants by HAND category
| Characteristics | N | Normal | ANI | MND | HIV-D | p value |
|---|---|---|---|---|---|---|
| Total | 156 | 50 | 41 | 46 | 19 | |
| SA | 89 | 39 | 24 | 22 | 4 | |
| USA | 67 | 11 | 17 | 24 | 15 | |
| Female, No. (%) | 98 | 36 (72.0) | 31 (75.6) | 25 (54.3) | 6 (31.4) | .003 |
| Black race (%) | 0 | 43(87.8) | 37(90.2) | 39 (84.8) | 14 (77.8) | .586 |
| Age, No. (years) | 154 | 49 | 40 | 46 | 19 | .001 |
| Median | 35.0 | 38.5 | 44.5 | 54.0 | ||
| Interquartile range | 13.5 | 24.3 | 26.5 | 16.0 | ||
| Education, No. (years) | 154 | 50 | 39 | 46 | 19 | .289 |
| Median | 11.0 | 11.0 | 11.0 | 12.0 | ||
| Interquartile range | 1.3 | 2.0 | 2.0 | 3.0 | ||
| Hazardous drinking, No. (%) | 0 | 2 (4.2) | 3 (7.7) | 3 (6.8) | 1 (5.6) | .923 |
| CES-D score | 154 | 49 | 41 | 46 | 18 | .000 |
| Median | 5 | 4 | 7 | 28 | ||
| Interquartile range | 11.0 | 11.0 | 16.0 | 31.5 | ||
| Viral load suppressed, No. (%) | 95 | 25 (89.3) | 19 (86.4 %) | 28 (96.6 %) | 14 (93.3 %) | .839 |
| CD4 Nadir/baseline, No, cells/ml | 144 | 46 | 38 | 44 | 16 | .691 |
| Median | 178.5 | 268.5 | 197 | 140.5 | ||
| Interquartile range | 84.5 | 74.25 | 76.0 | 60.5 | ||
| CD4 count, No., cells/ml | 124 | 43 | 33 | 32 | 16 | .006 |
| Median | 363.0 | 498.0 | 454.0 | 635.5 | ||
| Interquartile range | 300.0 | 229.0 | 329.5 | 460.8 |
SA South Africa, USA United States of America, ANI asymptomatic neurocognitive impairment, MND mild neurocognitive disorder, HIV-D HIV-dementia
Nearly half of the participants had symptomatic HAND: 46 (29 %) were classified as mild neurocognitive disorder (MND) and 19 (12 %) as having dementia (HIV-D)]. There were more participants with HIV-D in the USA (15, or 22 %) versus South Africa (4, or 5 %). There were significant differences between the HAND categories with respect to gender (there were more men with HIV-D); age (people with HIV-D were older); and CD4 cell count (participants with HIV-D had higher CD4 cell counts at the time of the assessment). There were no between-category differences with respect to level of education.
Table 2 presents the screening properties of the 5 tools.
Table 2.
Sensitivity and specificity of the five screening tools for HIV-D, any HAND and symptomatic HAND
| Cut-off point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| IHDS (≤10) | ||||
| Normal vs. HAND | 40.95 | 86.00 | 86.00 | 40.95 |
| Normal vs. Symptomatic | 51.56 | 86.00 | 82.50 | 58.11 |
| Normal vs. HIV-D | 68.42 | 86.00 | 65.00 | 87.76 |
| IHDS (≤11) | ||||
| Normal vs. HAND | 64.76 | 62.00 | 78.16 | 45.59 |
| Normal vs. Symptomatic | 68.75 | 62.00 | 69.84 | 60.78 |
| Normal vs. HIV-D | 73.68 | 62.00 | 42.42 | 86.11 |
| MoCA (≤26) | ||||
| Normal vs. HAND | 89.32 | 22.45 | 70.77 | 50.00 |
| Normal vs. Symptomatic | 90.48 | 22.45 | 60.00 | 64.71 |
| Normal vs. HIV-D | 100.00 | 22.45 | 32.14 | 100.00 |
| MMSE (≤24) | ||||
| Normal vs. HAND | 23.81 | 97.96 | 96.15 | 37.50 |
| Normal vs. Symptomatic | 24.62 | 97.96 | 94.12 | 49.48 |
| Normal vs. HIV-D | 26.32 | 97.96 | 83.33 | 77.42 |
| SSQ (≥1) | ||||
| Normal vs. HAND | 77.64 | 32.00 | 69.37 | 35.56 |
| Normal vs. Symptomatic | 75.38 | 32.00 | 59.04 | 50.00 |
| Normal vs. HIV-D | 78.95 | 32.00 | 30.61 | 80.00 |
| CAT-Rapid (≤10) | ||||
| Normal vs. HAND | 64.42 | 52.00 | 73.63 | 41.27 |
| Normal vs. Symptomatic | 78.12 | 52.00 | 67.57 | 65.00 |
| Normal vs. HIV-D | 94.44 | 52.00 | 41.46 | 96.30 |
IHDS International HIV-Dementia Scale, MoCA Montreal Cognitive Assessment, MMSE Mini-mental state examination, SSQ Simioni, Symptom Questionnaire, CAT-Rapid Cognitive Assessment Tool-Rapid, HAND HIV-associated neurocognitive disorder = Asymptomatic Neurocognitive Impairment or Mild Neurocognitive Disorder or HIV-Dementia, HIV-D HIV Dementia, PPV positive predictive value, NPV negative predictive value. Combined scores unavailable for 3 participants (N = 153)
Screening for HIV-D
The CAT-Rapid had good sensitivity and fair specificity while the IHDS showed fair sensitivity and good specificity. The MOCA showed excellent sensitivity but poor specificity.
Screening for Any HAND
No tool met the above criteria. The IHDS displayed poor sensitivity and good specificity, the MOCA again showed high sensitivity but weak specificity, while the SSQ and CAT-rapid showed fair sensitivities and fair-to-poor specificity.
Screening for Symptomatic HAND
While no tool met the criteria of good sensitivity and fair specificity, the CAT-rapid had good sensitivity and fair specificity, while the IHDS showed fair sensitivity and good specificity The MOCA and SSQ showed good to fair sensitivity, but poor specificity.
Table 3 presents the performance of the combined IHDS and CAT-rapid at several possible cut-off scores for HIV-D and any HAND. The combined tool showed excellent sensitivity and specificity for HIV-D at a cut-off score of ≤16 (89 and 82 %), while a score of ≤17 yielded results nearly as good (89 and 64 %). The ROC curve is presented in Fig. 1, with AUC of 0.89. The optimal cut-off for detecting any HAND may be regarded as either ≤17 or ≤18. Figure 2 presents the ROC curve supporting this interpretation. The IHDS/CAT-rapid combined tool performed the best out of all the screeners for HIV-D. In screening for any HAND, it was an improvement over the other screeners, but was still not optimal.
Table 3.
Sensitivity and specificity for cut-off points of the combined CAT-Rapid and IHDS (maximum score obtainable = 20)
| Cut-off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| ≤15 | ||||
| Normal vs. HAND | 31.00 | 90.00 | 84.49 | 38.79 |
| Normal vs. HIV-D | 72.22 | 90.00 | 72.22 | 90.00 |
| ≤16 | ||||
| Normal vs. HAND | 42.72 | 82.00 | 83.02 | 41.00 |
| Normal vs. HIV-D | 88.89 | 82.00 | 64.00 | 95.35 |
| ≤17 | ||||
| Normal vs. HAND | 59.22 | 64.00 | 77.22 | 43.24 |
| Normal vs. HIV-D | 88.89 | 64.00 | 47.06 | 94.12 |
| ≤18 | ||||
| Normal vs. HAND | 73.79 | 34.00 | 69.72 | 38.64 |
| Normal vs. HIV-D | 94.44 | 34.00 | 34.00 | 94.44 |
| ≤19 | ||||
| Normal vs. HAND | 88.35 | 18.00 | 68.94 | 42.86 |
| Normal vs. HIV-D | 100.00 | 18.00 | 30.51 | 100.00 |
CAT-Rapid Cognitive Assessment Tool-Rapid, IHDS International HIV-Dementia Scale, HAND HIV-associated neurocognitive disorder = Asymptomatic Neurocognitive Impairment or Mild Neurocognitive Disorder or HIV-Dementia, HIV-D HIV Dementia, PPV positive predictive value, NPV negative predictive value. Combined scores unavailable for 3 participants (N = 153)
Fig. 1.
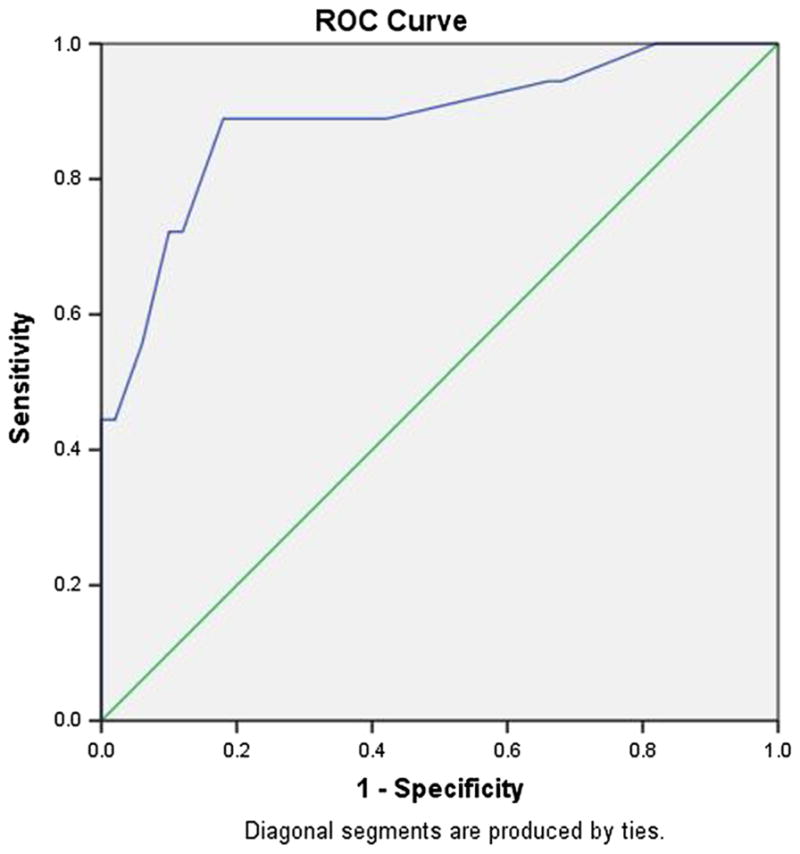
Receiver operating characteristic curve, Combined IHDS and CAT-Rapid. Normal versus HIV-Dementia. Area under the curve = .886
Fig. 2.
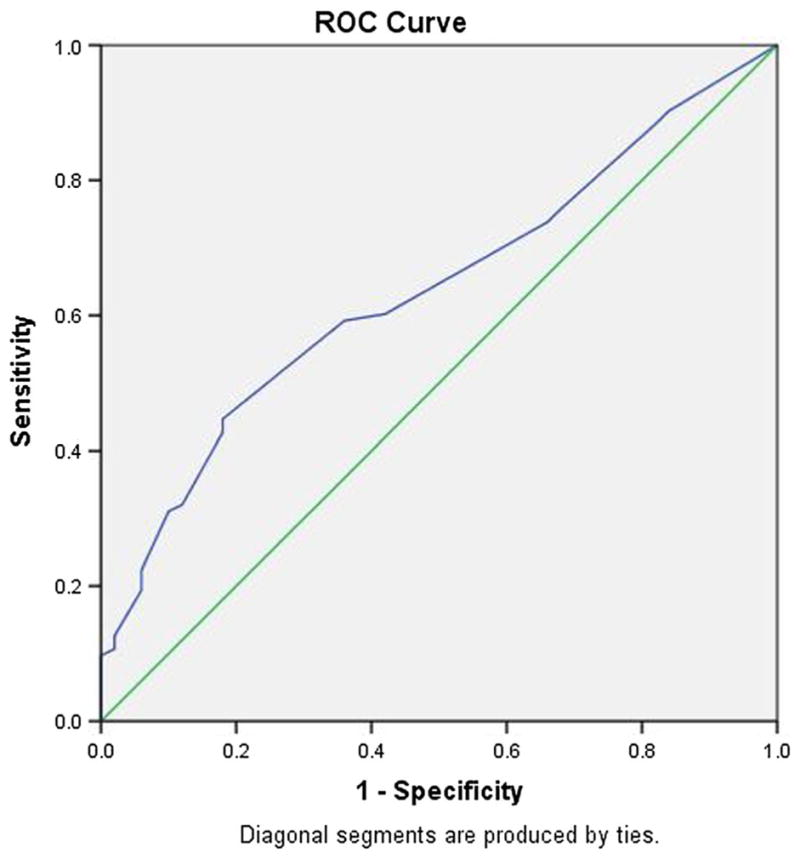
Receiver operating characteristic curve, Combined IHDS and CAT-Rapid. Normal versus HAND. Area under the curve = .638
Discussion
Few studies have compared several brief screening tools against a gold standard neuropsychological test battery in CART-experienced participants. We have demonstrated that both the IHDS and CAT-rapid are useful in screening for HIV-D. We confirm, as others have, that the MMSE has relatively poor sensitivity [20, 30]. No single tool proved to be ideal in screening for symptomatic HAND, although the CAT-rapid displayed fair sensitivity (78 %) with only weak specificity (54 %), while the IHDS showed weak sensitivity (52 %) and good specificity (86 %). The combined IHDS and CAT-rapid tool displayed improved sensitivity (89 %) and specificity (82 %) over all the other tools for HIV-D, but could not improve on the CAT-rapid or IHDS for any HAND screening.
The issue of screening for HAND has received support from some but not all groups [31, 32]. There seems to be little debate regarding screening for symptomatic forms of HAND (HIV-D and MND) [33]. Searching for ANI cases runs several risks, including the potential for over-diagnosis, distress to asymptomatic individuals receiving a diagnosis, uncertainty regarding treatment, and resources required for detecting these disorders [16]. In sub-Saharan Africa and other resource-limited settings (RLS), this is of critical concern, particularly as access to detailed neuropsychological testing is very limited. In addition, the ability of busy clinic staff to assess, treat and remove potential confounding conditions is also limited. We propose that screening for HIV-D be regarded as a priority. We confirm that the IHDS and the new tool (CAT-rapid) perform adequately for this purpose. Individuals in RLS who screen positive for HIV-D should receive additional assessment. The extent of this will depend on the clinical presentation and access to facilities. A pragmatic approach might be to (i) confirm the presence of severe neurocognitive impairment using additional screeners or tests (such as combined screeners, or additional tests where technicians are available), (ii) established the extent of functional impairment, (iii) assess for alcohol and substance abuse and depression (using tools such as the substance abuse and mental illness screener, or PHQ-2), and (iv) assess for biological factors depending on context (such as hepatitis C, syphilis or vitamin deficiency) [28, 34]. This type of screening program, if widespread, would require training and support. Some non-medical personnel may struggle in the administration of measures [27]. Another challenge is that patient report of functional impairment is often poor [4, 35].
Many screening tools are limited with respect to detecting mild HAND (either symptomatic or asymptomatic). We confirm this challenge. It may be that the signal from less severe disease is too weak for brief tests to measure. In this study, the MoCA displayed good sensitivity for any or symptomatic HAND, but poor specificity. One group has criticized the MoCA for its inability to provide a true quantitative measurement of cognition [13, 14, 33], while others note that certain items may be culturally insensitive [16, 36]. We have found similar limitations in detecting mild HAND with respect to the IHDS. Even using the cut-point of ≤11 did not improve sensitivity beyond 69 % for detecting any HAND (data not shown). The CAT-rapid at a cut off of ≤10 showed some utility for detecting symptomatic HAND but not any HAND, most probably due to the inclusion of the 4-symptom questions. In this study, the SSQ showed fair sensitivities across the range, but poor specificity, most likely reflecting the need for an objective measurement of cognition.
Screening tools by definition tap only some neurocognitive domains, and then only using one short test. It is possible that differences between individuals, as well as stages of infection may affect test performance. For example, differences in neuropsychological performance in CART-naïve versus experienced individuals may exist. Several authors have noted that, in untreated disease, sub-cortical features (such as psychomotor slowing and impaired retrieval of previously-learned information) are prominent, while in CART-experienced individuals, learning and executive functions may be impaired [5, 18, 37]. Therefore tools that tap psychomotor function (such as the IHDS) might be less useful for CART-experienced individuals, and more so when those features are mild.
In addition to potential differences between regions with respect to biologic factors, the issue of cultural and language differences may hinder screening and diagnostic efforts. Many of the screeners have been validated in low-resourced settings (for example the IHDS) (see [12, 38, 39]). Others have been researched but present issues of cultural fairness, for example the MOCA (see [40]). The use of control data may in part mitigate these effects, but further work to ensure that over-diagnosis does not occur should be done.
The combined IHDS/Cat-rapid displayed promise as a screener for HIV-D and was the best performer of all the tools in this regard. This may be a result of the effect of including measures of psychomotor functioning, memory, and executive function. The tool, however, was only marginally useful in detecting, at a cut-off of ≤18, any HAND. It had low sensitivity at this point.
So while the value of screening for mild and asymptomatic HAND is debated, an ideal approach to screening for them remains elusive. While tools such as the MoCA and combined IHDS/CAT-rapid display good-to-fair sensitivity, they display poor specificity. Using such tools is therefore likely to result in a number of false-positive tests and patients without any HAND referred for fuller neuropsychological assessment. An “intermediate level of assessment may improve these outcomes. This might entail an ultra-short battery of tests, or expanded set of screeners. While the screeners used in this study typically take 4–10 min to complete, an “intermediate” assessment test may take 10–30 min to complete (see for example [41]). In resource-limited settings, the absence of experienced technicians and neuropsychologists, these professionals may compound the problem. Improving the tools may improve the capacity, and mobile health tools using brief batteries are beginning to come to the fore [42].
This study was limited by the fact that there were some between-site differences-such as in the prevalence of HIV-D. Assumptions were made regarding the respective HAND prevalence across regions whether HIV-B and HIV-C respectively are prevalent. To some extent, it is now being accepted that rates of HAND in these settings are similar [43, 44]. In addition, at neither site did we have detailed objective measures of functional impairment. Instead, we relied on self-report and neurologic examination findings. We note also that the majority of the sample was female-approximately two-thirds. We confirm here, as in previous studies, that male gender confers a greater risk of HIV-D. In South Africa, the epidemic affects mainly women. While the reasons for male preponderance of impairment are unclear, it may reflect biological issues (such as higher rates of alcohol and substance abuse co-morbidity) and/or psychosocial issues (such a lower rates of health-seeking and therefore greater immuno-compromise).
While detecting HIV-D using screeners appears to be a tractable problem, albeit requiring training and supervision of staff, screening for milder forms of HAND continues to be a clinical challenge. An improved screener for these forms of HAND may require the inclusion of additional tests to enhance both sensitivity and specificity. Future studies may include sub-analysis of detailed neuropsychological batteries of impaired individuals to identify additional tests that tap mild to moderate impairment and that can be modified into brief and ecologically valid versions.
Acknowledgments
The authors would like to thank Mr Teboho Linda who conducted the neuropsychological assessments in Cape Town, South Africa. This work was supported in the USA in part by MH075673.
Appendix 1: Cognitive Assessment Tool-Rapid Version (CAT-Rapid)1
References
- 1.Rodrigues RA, Oliveira RL, Grinsztejn B, Silva MTT. Validity of the International HIV dementia scale in Brazil. Arq NeuroPsiquiatr. 2013;71(6):376–9. doi: 10.1590/0004-282X20130042. [DOI] [PubMed] [Google Scholar]
- 2.Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep. 2014;11(3):317–24. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- 3.Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, et al. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS. AIDS. 2013;27(15):2385–401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS. 2014;9(6):517–20. doi: 10.1097/COH.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ances BM, Clifford DB. HIV-associated neurocognitive disorders and the impact of combination antiretroviral therapies. Curr Neurol Neurosci Rep [Internet] 2008;8(6):455–61. doi: 10.1007/s11910-008-0073-3. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=18957181&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. The international HIV dementia scale: a new rapid screening test for HIV dementia. AIDS [Internet] 2005;19(13):1367–74. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16103767&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 8.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry W, Carlson MD, Barakat F, Hilsabeck RC, Schiehser DM, Mathews C, et al. Neuropsychological test performance in patients co-infected with hepatitis C virus and HIV. AIDS. 2005;19(Suppl 3):S79–84. doi: 10.1097/01.aids.0000192074.18691.31. [DOI] [PubMed] [Google Scholar]
- 10.Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, et al. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2010;16(2):185–8. doi: 10.3109/13550284.2010.481820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson JH, Heaton RK, Patterson TL, Wolfson T, Deutsch R, Brown SJ, et al. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108(3):225–34. doi: 10.1016/j.jad.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joska JA, Westgarth-Taylor J, Hoare J, Thomas KGF, Paul R, Myer L, et al. Validity of the International HIV Dementia Scale in South Africa. AIDS Patient Care STDS. 2011;25(2):95–101. doi: 10.1089/apc.2010.0292. [DOI] [PubMed] [Google Scholar]
- 13.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19(2):186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodkin K, Hardy DJ, Singh D, Lopez E. Diagnostic utility of the international HIV dementia scale for HIV-associated neurocognitive impairment and disorder in South Africa. J Neuropsychiatry Clin Neurosci. 2014;26(4):352–8. doi: 10.1176/appi.neuropsych.13080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139–51. doi: 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen MAM, Bosch M, Koopmans PP, Kessels RPC. Validity of the montreal cognitive assessment and the HIV dementia scale in the assessment of cognitive impairment in HIV-1 infected patients. J Neurovirol. 2015;21(4):383–90. doi: 10.1007/s13365-015-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–62. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 20.Skinner S, Adewale AJ, DeBlock L, Gill MJ, Power C. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10(4):246–52. doi: 10.1111/j.1468-1293.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 21.Bottiggi KA, Chang JJ, Schmitt FA, Avison MJ, Mootoor Y, Nath A, et al. The HIV dementia scale: predictive power in mild dementia and HAART. J Neurol Sci. 2007;260(1–2):11–5. doi: 10.1016/j.jns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Simioni S, Cavassini M, Annoni J-M, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2009;24:1243. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 23.Myer L, Smit J, Roux LL, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDS. 2008;22(2):147–58. doi: 10.1089/apc.2007.0102. [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian VR, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc [Internet] 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15817019&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 25.Overton ET, Azad TD, Parker N, Demarco Shaw D, Frain J, Spitz T, et al. The Alzheimer’s disease-8 and montreal cognitive assessment as screening tools for neurocognitive impairment in HIV-infected persons. J Neurovirol [Internet] 2013;19(1):109–16. doi: 10.1007/s13365-012-0147-5. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23345074&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koskas P, Henry-Feugeas MC, Feugeas JP, Poissonnet A, Pons-Peyneau C, Wolmark Y, et al. The lawton instrumental activities daily living/activities daily living scales: a sensitive test to alzheimer disease in community-dwelling elderly people? J Geriatr Psychiatry Neurol. 2014;27(2):85–93. doi: 10.1177/0891988714522694. [DOI] [PubMed] [Google Scholar]
- 27.Breuer E, Stoloff K, Myer L, Seedat S, Stein DJ, Joska J. Reliability of the lay adherence counsellor administered substance abuse and mental illness symptoms screener (SAMISS) and the International HIV Dementia Scale (IHDS) in a primary care HIV clinic in Cape Town, South Africa. AIDS Behav. 2012;16(6):1464–71. doi: 10.1007/s10461-011-0067-z. [DOI] [PubMed] [Google Scholar]
- 28.Breuer E, Stoloff K, Myer L, Seedat S, Stein DJ, Joska JA. The validity of the Substance Abuse and Mental Illness Symptom Screener (SAMISS) in people living with HIV/AIDS in primary HIV care in Cape Town, South Africa. AIDS Behav. 2014;18(6):1133–41. doi: 10.1007/s10461-014-0698-y. [DOI] [PubMed] [Google Scholar]
- 29.Parboosing R, Paruk I, Lalloo UG. Hepatitis C virus seropositivity in a South African Cohort of HIV co-infected, ARV naïve patients is associated with renal insufficiency and increased mortality. J Med Virol. 2008;80(9):1530–6. doi: 10.1002/jmv.21262. [DOI] [PubMed] [Google Scholar]
- 30.Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubadejo UN, Danesi AM. Comparison of the minimental state examination scale and the international HIV dementia scale in assessing cognitive function in Nigerian HIV patients on antiretroviral therapy. AIDS Res Treat. 2012;2012:581531. doi: 10.1155/2012/581531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mind Exchange Working Group. Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis. 2013;56:1004–17. doi: 10.1093/cid/cis975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryom L, Boesecke C, Gisler V, Manzardo C, Rockstroh JK, Puoti M, et al. Essentials from the 2015 European AIDS Clinical Society (EACS) guidelines for the treatment of adult HIV-positive persons. HIV Med. 2015;17:83–8. doi: 10.1111/hiv.12322. [DOI] [PubMed] [Google Scholar]
- 33.Brouillette M-J, Mayo N, Fellows LK, Lebedeva E, Higgins J, Overton ET, et al. A better screening tool for HIV-associated neurocognitive disorders. AIDS. 2015;29(8):895–902. doi: 10.1097/QAD.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akena D, Joska J, Obuku EA, Stein DJ. Sensitivity and specificity of clinician administered screening instruments in detecting depression among HIV-positive individuals in Uganda. AIDS Care. 2013;25(10):1245–52. doi: 10.1080/09540121.2013.764385. [DOI] [PubMed] [Google Scholar]
- 35.Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2011;18(01):79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins RN, Remien RH, Mellins CA, Joska JA, Stein DJ. Screening for HIV-associated dementia in South Africa: potentials and pitfalls of task-shifting. AIDS Patient Care STDS. 2011;25(10):587–93. doi: 10.1089/apc.2011.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.For the CHARTER and HNRC Groups. Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2010;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku NS, Lee Y, Ahn JY, Song JE, Kim MH, Kim SB, et al. HIV-associated neurocognitive disorder in HIV-infected Koreans: the Korean NeuroAIDS Project. HIV Med. 2014;15(8):470–7. doi: 10.1111/hiv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, et al. Neurocognitive impairment in HIV-1 clade C-versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19(6):550–6. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins RN, Joska JA, Thomas KGF, Stein DJ, Linda T, Mellins CA, et al. Exploring the utility of the Montreal Cognitive Assessment to detect HIV-associated neurocognitive disorder: the challenge and need for culturally valid screening tests in South Africa. Clin Neuropsychol. 2013;27(3):437–54. doi: 10.1080/13854046.2012.759627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh D, Joska JA, Goodkin K, Lopez E, Myer L, Paul RH, et al. Normative scores for a brief neuropsychological battery for the detection of HIV-associated neurocognitive disorder (HAND) among South Africans. BMC Res Notes. 2010;3:28. doi: 10.1186/1756-0500-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins RN, Brown H, Ehlers A, Joska JA, Thomas KGF, Burgess R, et al. A Smartphone App to Screen for HIV-Related Neurocognitive Impairment. J Mob Technol Med. 2014;3(1):23–6. doi: 10.7309/jmtm.3.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortega M, Heaps JM, Joska J, Vaida F, Seedat S, Stein DJ, et al. HIV clades B and C are associated with reduced brain volumetrics. J Neurovirol. 2013;19(5):479–87. doi: 10.1007/s13365-013-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul RH, Joska JA, Woods C, Seedat S, Engelbrecht S, Hoare J, et al. Impact of the HIV Tat C30C31S dicysteine substitution on neuropsychological function in patients with clade C disease. J Neurovirol. 2014;20(6):627–35. doi: 10.1007/s13365-014-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


