Abstract
OBJECTIVES
Non-invasive methods to objectively characterize overactive bladder (OAB) and other forms of voiding dysfunction using real-time ultrasound are currently under development but require accurate and precise serial measurements of bladder volumes during filling. This study’s objective was to determine the most accurate and precise ultrasound-based method of quantifying serial bladder volumes during urodynamics (UD).
METHODS
Twelve female participants with OAB completed an extended UD procedure with the addition of serial bladder ultrasound images captured once per minute. Bladder volume was measured using three ultrasound methods: (1) Vspheroid: two-dimensional (2D) method calculated assuming spheroid geometry; (2) Vbih: 2D correction method obtained by multiplying Vspheroid by a previously derived correction factor of 1.375; and (3) V3D: three-dimensional (3D) method obtained by manually tracing the bladder outline in six planes automatically reconstructed into a solid rendered volume. These volumes were compared to a control (Vcontrol) obtained by adding UD infused volume and the volume of estimated urine production.
RESULTS
Based on linear regression analysis, both Vbih and V3D were fairly accurate estimators of Vcontrol, but V3D was more precise. Vspheroid significantly underestimated Vcontrol.
CONCLUSIONS
Although the Vbih and V3D methods were more accurate than the more-commonly used Vspheroid method for measuring bladder volumes during UD, the V3D method was the most precise and could best account for non-uniform bladder geometries. Therefore, the V3D method may represent the best tool required for the continued development of non-invasive methods to diagnose OAB and other forms of voiding dysfunction.
Keywords: overactive bladder, transabdominal ultrasound imaging, urodynamics, volume calculations, volumetric ultrasound
INTRODUCTION
The ability to accurately and precisely measure the volume of the urinary bladder is an important tool in evaluating bladder function. For example, post-void residual (PVR) volume is utilized in the diagnosis of urinary retention, detrusor underactivity, and other bladder impairments [1-4]. In addition, measurements of both voided volume and PVR are needed for bladder training and other bladder assessments [5,6]. During urodynamics (UD), bladder volume during filling is assumed to be equal to the infused volume and does not typically include an estimation of urinary diuresis. The additional volume of urinary diuresis may be relatively small at a super-physiological fill rates which can be up to 100 ml/min [7]. However, considering the average diuresis rate of 10 ml/min in our accelerated hydration studies [8], it cannot always be considered negligible and may lead to inaccurate UD results.
Another important drawback to UD is its invasiveness which often causes anxiety and discomfort [9], has a risk of urinary tract infection [10], and can cause changes in the perception of bladder sensation [11]. The development of bladder sensation can be studied non-invasively in oral hydration studies that demonstrated significant differences between individuals with OAB and asymptomatic volunteers [8,12,13]. These studies mainly tracked volume by measuring voided volume at the end of a study and assuming a constant fill rate to linearly interpolate volume at specific time periods. However, the actual fill rate is unlikely to be constant [13], so a method to accurately and precisely measure serial, real-time bladder volumes in a non-invasive manner is essential.
Currently, techniques are under development where the addition of ultrasound during UD enables measurement of several new biomechanical properties of the bladder including wall tension, wall strain, wall stress, and dynamic elasticity [14]. Shearwave elastography and ultrasound lamb wave vibrometry have recently been used to estimate intravesical pressure [15,16], adding to the potential uses of non-invasive, ultrasound-based urodynamic methods. These novel metrics have the potential to improve the diagnosis and objective characterization of filling phase disorders, including OAB. More importantly, the use of serial ultrasound measurements during bladder filling may ultimately lead to the development of completely non-invasive “ultrasound urodynamics”. However, development of any non-invasive ultrasound-based UD methods requires utilization of the most accurate and precise measurement of serial bladder volumes during filling.
Therefore, a critical research objective is the development of non-invasive methods to more-accurately measure serial bladder volumes during filling. There is a long history of using two-dimensional (2D) ultrasound imaging to estimate bladder volume [17,18] and more recently, three-dimensional (3D) methods have become available [19-21]. Thus, the objective of this study was to compare three different ultrasound-based methods of calculating bladder volume to identify the most accurate and precise methodology and compare results to standard volume measurements obtained during UD.
METHODS
Experimental protocol
Women with bothersome symptoms of OAB were invited to participate in an Institutional Review Board approved prospective study using comparative-fill urodynamics [22,23] with ultrasound. Women clinically indicated for UD with high chronic urgency scoring 3–4 (“most of the time” or “all of the time”) on the International Consultation on Incontinence questionnaire (ICIq)-OAB question 5 “do you have to rush to the toilet to urinate?” were included in this study. After giving informed consent, urodynamic filling was administered with a 7 Fr catheter on a Laborie Aquarius TT system (Toronto, Canada). During an initial UD fill, bladder cystometric capacity was defined as the volume at which the participant reported 100% bladder sensation using a sensation meter [8]. During a subsequent fill at an infusion rate of 10% capacity per minute, transabdominal images of the bladder were obtained every 60 s using a GE Voluson E8 system (Madison, WI) with a 3D convex 4–8.5 MHz transducer. All images were obtained by a trained ultrasound technologist with supervision from an attending radiologist fellowship trained in abdominal imaging. Infusion was paused for 5–10 min at 40% and 70% of bladder capacity and infusion was stopped when the participant reported that they had reached 100% sensation. The pauses were part of a separate study to quantify any low amplitude rhythmic contractions during the bladder filling phase [24].
At the end of the fill, participants voided and any PVR was extracted by syringe aspiration through the filling catheter. Ultrasound was used to confirm that the bladder was empty. The total bladder volume at the end of the fill was calculated as the sum of the voided volume and the PVR. Any positive difference between the total volume (void + PVR) and the infused volume (VH2O) was considered to be due to urine production from the kidneys during the procedure. This urinary diuresis was assumed to have a constant rate. The control volume (Vcontrol) at the moment at which each image was obtained was calculated as the instantaneous VH2O plus the proportion of urinary diuresis up to that time point in the fill, as previously calculated by Byun et al. [25].
Image calculations
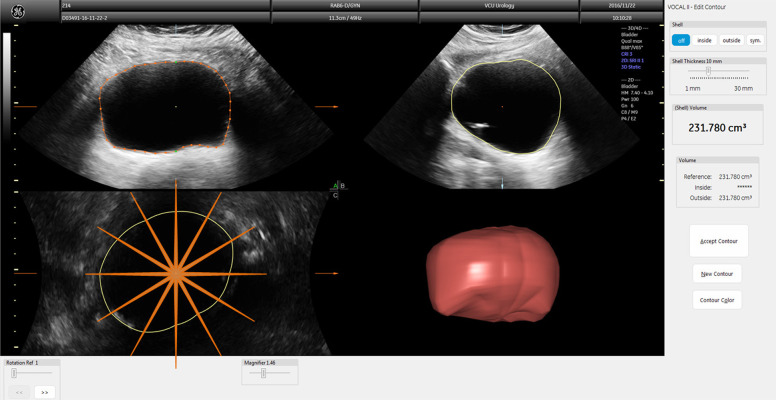
Ultrasound images were exported and analyzed offline using GE’s 4D View software (Version 14, GE Healthcare) by a trained individual who was blinded to the UD results. As previously described in Nagle et al. [8], 3D images were manually traced in six planes 30° apart using the virtual organ computer-aided analysis (VOCAL) software which automatically combined these cross-sections into a continuous rendered volume (Fig. 1). The 30° step size was chosen for increased speed of analysis and was found to yield similar volume measurements as compared to smaller step sizes in our initial analyses and by others [20,26,27]. The resulting computed volume (V3D) was recorded from the software’s display.
Figure 1.
Three orthogonal planes of a 3D bladder image showing how the bladder is traced in VOCAL to construct the 3D volume measurement. The top left is the transverse plane with orange perimeter indicating manual tracing; the top right is the sagittal plane with yellow perimeter made automatically; the bottom left is the coronal plane with yellow perimeter made automatically with orange lines overlaid indicating the transverse cross-sections traced; the bottom right is the reconstructed volume based on tracings from the six cross sections.
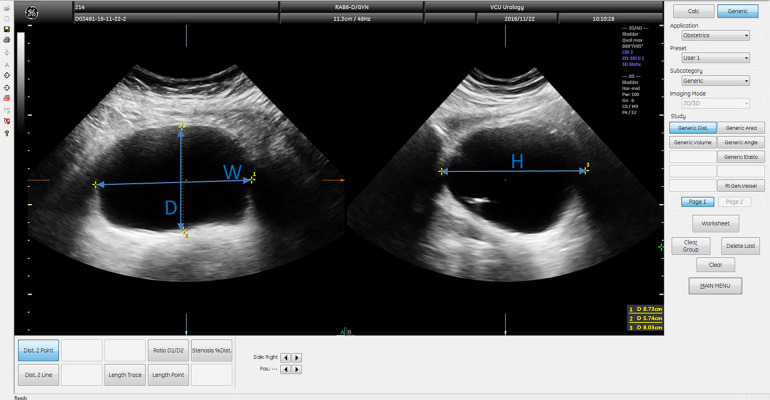
Two different 2D-image based calculations were used. Both utilized the height (H) in the transverse direction, the width (W) in the transverse direction, and the length (L) in the sagittal direction (Fig. 2). The first method assumed the bladder had a spheroid geometry and was calculated as
Figure 2.
Transverse (left) and sagittal (right) views of the same bladder image as Figure 1 showing diameters used to calculate 2D-image based volume measurements.
| (Eqn. 1) |
The second 2D method was based on research by Bih et al. [28] that calculated a correction factor for Vspheroid using linear regression on bladder images of healthy and spinal cord injured men and women as
| (Eqn. 2) |
Volume comparisons
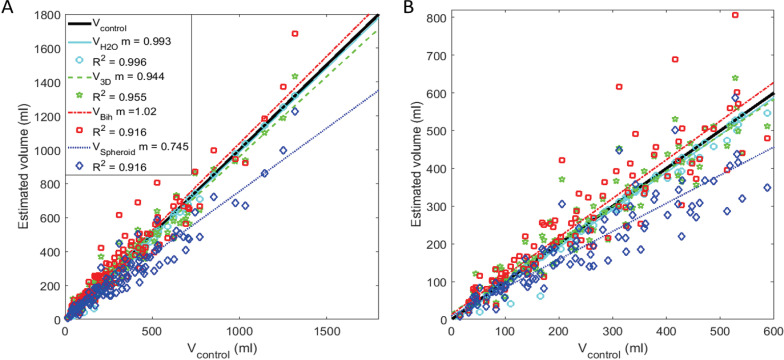
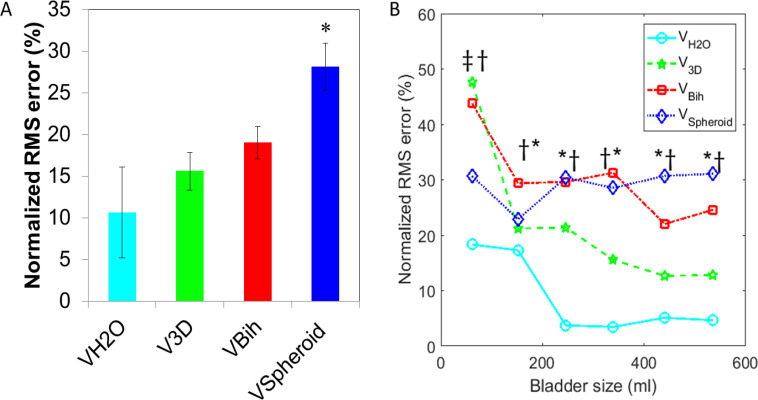
At each time point in which an image was obtained, VH2O, V3D, Vspheroid, and Vbih were plotted as a function of Vcontrol and a linear regression line was fit to the data from each volume calculation method (Fig. 3). The percent root-mean-squared (%RMS) error for each patient was calculated so that the average error among all of the patients could be calculated without being skewed by the individuals with more images (Fig. 4A). Finally, to measure error as a function of bladder size, all control volumes were grouped into increasing bins of 100 ml until data from less than half of the participants were still available (1–100, 101–200, 201–300, 301–400, 401–500, and 501–600) and %RMS error was calculated for all bladder volume data points within each interval (Fig. 4B). The errors from the ultrasound methods were compared to the baseline error of VH2O by multiple comparison ANOVA. All calculations were performed in MATLAB (2017a, MathWorks, Natick, MA).
Figure 3.
Plot of the four different volume estimators versus control volume (black 45° line). A. The full data set. B. Zoomed plot of (A) with bladder volumes up to 600 ml. Shown are VH2O data points in cyan stars with linear regression line in solid cyan, V3D data points in green pentagons and regression line in dashed green, Vbih data points in red squares and regression line in dotted red, and Vspheroid data points in blue diamonds and regression line in dashed dotted blue. Slope (m) and R-squared (R2) values of the linear regressions are shown in the legend in (A).
Figure 4.
Mean and standard error of the normalized root-mean-squared (%RMS) error of each volume estimator compaired to Vcontrol. A. Mean %RMS for each participant. Significant difference based on ANOVA with multiple comparisons to VH2O is denoted by a star. B. Normalized %RMS error as a function of bladder size. The ordinal location of each point represents the mean of all control bladder volumes of its 100 ml interval. Significant difference based on ANOVA with multiple comparisons to VH2O is denoted by a double dagger (V3D), dagger (Vbih), or star (Vspheriod). *P < 0.05.
RESULTS
A total of 14 women participated in the study. Data from two individuals could not be used due to incomplete data. Mean, standard error of the mean (SEM), and range of demographic and experimental information on the remaining 12 women is presented in Table 1. In the table, the ICIq total score is the total of the four symptom questions and can range from 0 to 16. The initial capacity is the voided volume plus extracted PVR from the initial cystometric fill. The capacity is the voided volume plus extracted PVR from the fill during which ultrasound imaging was performed.
Table 1.
Demographic and experimental data on the 12 women who completed the study.
| Variable | Mean ± SEM | Range |
|---|---|---|
| Age (year) | 53.7 ± 11.1 | 28 to 67 |
| BMI (kg/m2) | 33.5 ± 9.7 | 19 to 54 |
| ICIq question 5a score | 3.25 ± 0.13 | 3 to 4 |
| ICIq total score | 9.67 ± 2.93 | 5 to 15 |
| Initial capacity (ml) | 553.8 ± 89.6 | 132 to 1172 |
| Capacity (ml) | 523.5 ± 91.9 | 99 to 1225 |
| Urine production (ml) | 27.4 ± 9.52 | 0 to 109.1 |
| Number of images | 9.2 ± 0.9 | 3 to 13 |
In Figures 3–5, data is shown with VH2O in cyan stars, V3D in green pentagons, Vbih in red squares, and Vspheroid in blue diamonds. Figure 3 shows the data points and linear regression lines with the line of ideal case (45° line with slope of 1 and R2 of 1) in black. The slopes (m) and R-squared (R2) values of the regression lines are shown in the legend. The 95% confidence bounds of the regression line’s slope were 0.981 to 1.005 for VH2O, 0.905 to 0.983 for V3D, 0.965 to 1.084 for Vbih, and 0.702 to 0.789 for Vspheroid. Vspheroid was considered to be significantly different than VH2O because their 95% confidence intervals had no overlap while V3D and Vbih were considered to not be significantly less accurate than VH2O because there was overlap. Of the image-based methods, V3D was considered the most precise because its R2 value was the closest to unity. Figure 4A shows the %RMS error averaged for each method with data from all participants weighted equally. Again, Vspheroid was considered to have significantly higher error based on multiple comparison ANOVA comparing image based volumes to VH2O as denoted by the star in the figure. Figure 4B shows %RMS error as a function of bladder volume in 100 ml increments. At the smallest volume increment, V3D and Vbih had significantly more error than VH2O, and at all other volume increments, Vbih and Vspheroid had significantly more error than VH2O. Volume increments are only included up to 600 ml because less than half of the participants had bladder capacities higher than 600 ml.
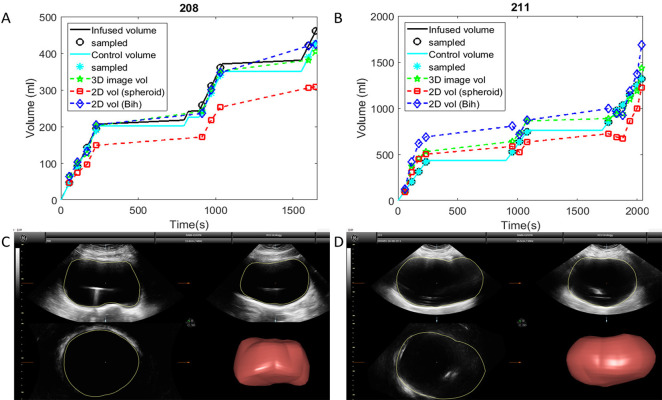
Figure 5.
Two examples of bladder volume measurements throughout filling. The bladder with volumes shown in (A) and final ultrasound image in (C) followed the most typical pattern. The bladder with volumes shown in (B) and final ultrasound image in (D) was more difficult to measure using only 2D methods because of its borders extended beyond the image width.
Two example patterns of bladder filling are shown in Figure 5. In the example in Figure 5A and 5C, V3D and Vbih were generally similar, which was the most common pattern. In the example in Figure 5B and 5D, V3D was more accurate, and Vbih overestimated bladder volume. Note that the shape of the bladder in Figure 5D was less regular and extended beyond the image boundaries making it difficult to determine the actual location of the bladder walls at large volumes.
DISCUSSION
Of the three ultrasound methods studied, V3D had the highest degree of precision based on the R2 value and had the highest accuracy based on %RMS error. However, Vbih had the highest overall accuracy based on the slope of its linear regression. Considering that both methods had slopes and R2 values close to unity and were not significantly different than the slope of VH2O, both methods could be considered acceptable ways measure bladder volume for many applications. However, when bladder volume is needed for fine calculations, such as to determine rate of physiologic bladder filling in hydration studies, the increased precision of V3D will be beneficial. In contrast, Vspheroid consistently underestimated bladder volume, demonstrating that the bladder should not be assumed to be a sphere or spheroid when calculating volume.
Individual differences in bladder size and shape contribute to Vbih having a lower precision than V3D. An advantage of the 3D method is the ability to make no assumptions about the bladder shape as is done in the 2D methods. Additionally, by examining the bladder borders in several planes simultaneously, the ultrasound technologist was able to better estimate where the bladder border must be located even when it was not easily visible or extended beyond the image borders. The main disadvantage of the 3D method is it that is more time consuming, requiring 3–6 min per image to complete a volume tracing as opposed to only 10–20 s to define the three diameters needed to calculate the 2D volumes. The time to obtain the 3D volume could be lowered by using the available automatic and semi-automatic options in 4D View such as SonoAVC as used by Sætherhaug et al. [21] to measure fetal bladder volume or by developing custom software to automate the process. The only advantage the spheroid method had was that it was built into the ultrasound system’s volume measurements making it the fastest volume estimation to obtain during clinical studies, but this number could easily be multiplied by a correction factor of 1.375 to obtain Vbih yielding a more accurate measure of volume.
The highest errors in V3D and Vbih were seen at the smallest bladder volumes (0–100 ml). There are two likely reasons for this. First, the bladder walls were more difficult to discern at very small volumes. Second, the difference between actual urinary diuresis rate and the constant rate assumption of filling is likely to be exaggerated in these small volumes. Interestingly, Vspheroid had little variation in error with bladder size.
Vcontrol was calculated assuming that the urinary diuresis was constant. However in hydration studies, the diuresis rate was seen to vary depending on the quantity of fluid consumed and the duration of time after fluid was consumed [8,13]. Thus Vcontrol is not a perfect reflection of the instantaneous bladder volume. In seven (50%) participants, the total volume was more than 15 ml larger than the volume infused, indicating the need to compute the control volume rather than assuming VH2O accurately reflected instantaneous bladder volume. Surprisingly, in two (16.7%) participants, the final voided volume plus PVR was more than 15 ml less than the volume infused. This may have been due to unusual bladder shapes preventing complete emptying of the bladder or the UD system may have needed recalibration. In these individuals, the control volume was considered to be the instantaneous infused volume as this was the best estimate possible.
The protocol used a fill rate of 10% initial bladder capacity per minute and took one ultrasound image each minute, which would ideally result in ten images from each individual. However, in this varied population of overactive bladder patients, some sensed that they reached their bladder capacity at a substantially different volumes during the fill analyzed in this study. Some felt they had reached capacity as early as 30% of their initial capacity while others reached 130% of their initial capacity. As a result, some participants were imaged at more bladder volumes than others.
While there is a wealth of literature on the use of ultrasound to measure bladder volume [17-21,28-30], this study is unique in that it specifically focused on women with overactive bladder symptoms. Additionally, this study compared volumes to a control volume that included both urodynamic infusion and urinary diuretic filling while most other studies have relied only on the infusion volume or voided volume. Finally, most other studies have only looked at bladder volumes at void and/or the PVR volume while this study examined the whole range of bladder filling and calculated accuracy over a large range of volumes.
An alternative to using conventional ultrasound would be to use an automatic portable device such as the BladderScan® [4,31-33]. These devices have the advantages of being very simple and quick to use. They work by creating a 3D model of the bladder based on detection of fluid tissue borders, and often have comparable accuracy to conventional ultrasound [34]. However, they may be less reliable and perform poorly in individuals that have other fluid filled structures near the bladder or bladders of irregular shape [4,31-33]. Also, in a setting such as an emergency room where conventional ultrasound is already used to image multiple organs, using a second device is impractical. It is expected that in most cases, bladder volume can be measured using either system, but research specifically comparing conventional 3D ultrasound to the results of portable devices would be necessary to determine this.
This study is limited by the small sample size and by the use only of women with relatively severe OAB symptoms. Further research will be required with larger numbers and on different patient populations both during UD and during non-invasive hydration studies to see if this methodology is more generalizable.
In conclusion, this study demonstrates that serial measurements of bladder volume during filling in women with OAB can be accurately computed during UD using both the 3D ultrasound and corrected 2D ultrasound (Vbih) methods. However, the 3D method was more precise and better accounted for bladders with irregular shapes. Thus, the 3D ultrasound method of real-time serial bladder volume measurements may represent the best available tool for the continued development of non-invasive “ultrasound urodynamics”.
Acknowledgments
Research funding for this study was provided by the Virginia Commonwealth University Presidential Research Quest Fund, the Dean’s Undergraduate Research Initiative, and NIH grant R01DK101719. The authors would like to thank Stefan Harris, Rachel Wilbur, Ronie-Rafael Altejar, Zachary Cullingsworth, Ashley Carroll, Kimberly Bradley, Sandy Smith, and Pam Harrell.
References
- 1.Taylor JA, Kuchel GA. (2006) Detrusor underactivity: Clinical features and pathogenesis of an underdiagnosed geriatric condition. J Am Geriatr Soc 54: 1920-1932. doi: 10.1111/j.1532-5415.2006.00917.x. PMID: [DOI] [PubMed] [Google Scholar]
- 2.Juma S. (2014) Urinary retention in women. Curr Opin Urol 24: 375-379. doi: 10.1097/MOU.0000000000000071. PMID: [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD. (2008) Urinary retention and post-void residual urine in men: separating truth from tradition. J Urol 180: 47-54. doi: 10.1016/j.juro.2008.03.027. PMID: [DOI] [PubMed] [Google Scholar]
- 4.Haylen BT, Lee J. (2008) The accuracy of post-void residual measurement in women. Int Urogynecol J Pelvic Floor Dysfunct 19: 603-606. doi: 10.1007/s00192-008-0568-0. PMID: [DOI] [PubMed] [Google Scholar]
- 5.Romanzi LJ, Groutz A, Heritz DM, Blaivas JG. (2001) Involuntary detrusor contractions: correlation of urodynamic data to clinical categories. Neurourol Urodyn 20: 249-257. doi: 10.1002/nau.1002. PMID: [DOI] [PubMed] [Google Scholar]
- 6.Cassadó J, Espuña-Pons M, Díaz-Cuervo H, Rebollo P. (2015) How can we measure bladder volumes in women with advanced pelvic organ prolapse? Ultrasound Obstet Gynecol 46: 233-238. doi: 10.1002/uog.14678. PMID: [DOI] [PubMed] [Google Scholar]
- 7.Gammie A, Clarkson B, Constantinou C, Damaser M, Drinnan M, et al. (2014) International Continence Society guidelines on urodynamic equipment performance. Neurourol Urodyn 33: 370-379. doi: 10.1002/nau.22546. PMID: [DOI] [PubMed] [Google Scholar]
- 8.Nagle AS, Speich JE, De Wachter SG, Ghamarian PP, Le DM, et al. (2016) Non-invasive characterization of real-time bladder sensation using accelerated hydration and a novel sensation meter: An initial experience. Neurourol Urodyn 36: 1417-1426. doi: 10.1002/nau.23137. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellerkmann RM, McBride AW, Dunn JS, Bent AE, Blomquist JL, et al. (2004) A comparison of anticipatory and postprocedure pain perception in patients who undergo urodynamic procedures. Obstet Gynecol 2004: 1034-1038. doi: 10.1016/j.ajog.2003.11.006. PMID: [DOI] [PubMed] [Google Scholar]
- 10.Ku JH, Kim SW, Kim HH, Paick J, Son H, et al. (2004) Patient experience with a urodynamic study: a prospective study in 208 patients. J Urol 171: 2307-2310. PMID: [DOI] [PubMed] [Google Scholar]
- 11.Erdem E, Tunçkiran A, Acar D, Kanik EA, Akbay E, et al. (2005) Is catheter cause of subjectivity in sensations perceived during filling cystometry? Urology 66: 1000-1003. doi: 10.1016/j.urology.2005.05.056. PMID: [DOI] [PubMed] [Google Scholar]
- 12.Heeringa R, de Wachter SGG, van Kerrebroeck PEV, van Koeveringe GA. (2011) Normal bladder sensations in healthy volunteers: a focus group investigation. Neurourol Urodyn 30: 1350-1355. doi: 10.1002/nau.21052. PMID: [DOI] [PubMed] [Google Scholar]
- 13.Haylen BT, Frazer MI, Sutherst JR, Ashby D. (1989) The accuracy of measurement of residual urine in women by urethral catheterisation. Br J Urol 63: 152-154. doi: 10.1111/j.1464-410X.1989.tb05153.x. PMID: [DOI] [PubMed] [Google Scholar]
- 14.Nagle AS, Klausner AP, Varghese J, Bernardo RJ, Colhoun AF, et al. (2017) Quantification of bladder wall biomechanics during urodynamics: A methodologic investigation using ultrasound. J Biomech 61: 232-241. doi: 10.1016/j.jbiomech.2017.07.028. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm RM, Yerkes EB, Nicholas JL, Snow-Lisy D, Diaz SD, et al. (2017) Ultrasound shear wave elastography: A novel method to evaluate bladder pressure. J Urol 198: 422-429. doi: 10.1016/j.juro.2017.03.127. PMID: [DOI] [PubMed] [Google Scholar]
- 16.Nenadic I, Mynderse L, Husmann D, Mehrmohammadi M, Bayat M, et al. (2016) Noninvasive evaluation of bladder wall mechanical properties as a function of filling volume: Potential application in bladder compliance assessment. PLoS One 11: doi: 10.1371/journal.pone.0157818. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartnell GG, Kiely EA, Williams G, Gibson RN. (1987) Real-time ultrasound measurement of bladder volume: a comparative study of three methods. Br J Radiol 60: 1063-1065. doi: 10.1259/0007-1285-60-719-1063. PMID: [DOI] [PubMed] [Google Scholar]
- 18.Poston GJ, Joseph AE, Riddle PR. (1983) The accuracy of ultrasound in the measurement of changes in bladder volume. Br J Urol 55: 361-363. PMID: [DOI] [PubMed] [Google Scholar]
- 19.Riccabona M, Nelson TR, Pretorius DH, Davidson TE. (1996) In vivo three-dimensional sonographic measurement of organ volume: validation in the urinary bladder. J Ultrasound Med 15: 627-632. doi: 10.7863/jum.1996.15.9.627. PMID: [DOI] [PubMed] [Google Scholar]
- 20.Kusanovic JP, Nien JK, Gonçalves LF, Espinoza J, Lee W, et al. (2008) The use of inversion mode and 3D manual segmentation in volume measurement of fetal fluid-filled structures: comparison with virtual organ computer-aided analysis (VOCAL). Ultrasound Obstet Gynecol 31: 177-186. doi: 10.1002/uog.5242. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sætherhaug T, Fasting MH, Røset MAH, Naylor M., Blaas HK, et al. (2006). Repeatability of volume calculations of the fetal urinary bladder. Bladder 3: e24. [Google Scholar]
- 22.Colhoun AF, Klausner AP, Nagle AS, Carroll AW, Barbee RW, et al. (2016) A pilot study to measure dynamic elasticity of the bladder during urodynamics. Neurourol Urodyn 2016: 1086-1090. doi: 10.1002/nau.23043. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habteyes FG, Komari SO, Nagle AS, Klausner AP, Heise RL, et al. (2017) Modeling the influence of acute changes in bladder elasticity on pressure and wall tension during filling. J Mech Behav Biomed Mater 71: 192-200. doi: 10.1016/j.jmbbm.2017.02.020. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colhoun AF, Speich JE, Cooley LF, Bell ED, Barbee RW, et al. (2016) Low amplitude rhythmic contraction frequency in human detrusor strips correlates with phasic intravesical pressure waves. World J Urol 35: 1255-1260. doi: 10.1007/s00345-016-1994-0. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byun SS, Kim HH, Lee E, Paick J, Kamg W, et al. (2003) Accuracy of bladder volume determinations by ultrasonography: are they accurate over entire bladder volume range? Urology 62: 656-660. doi: 10.1016/S0090-4295(03)00559-4. PMID: [DOI] [PubMed] [Google Scholar]
- 26.Peixoto-Filho FM, Sá R, Lopes L, Velarde L, Marchiori E, et al. (2007) Three-dimensional ultrasound fetal urinary bladder volume measurement: reliability of rotational (VOCAL™) technique using different steps of rotation. Arch Gynecol Obstet 2007: 345-349. doi: 10.1007/s00404-007-0360-2. [DOI] [PubMed] [Google Scholar]
- 27.Raine-Fenning NJ, Clewes JS, Kendall NR, Bunkheila AK, Campbell BK, et al. (2003) The interobserver reliability and validity of volume calculation from three-dimensional ultrasound datasets in the in vitro setting. Ultrasound Obstet Gynecol 21: 283-291. doi: 10.1002/uog.61. PMID: [DOI] [PubMed] [Google Scholar]
- 28.Bih LI, Ho CC, Tsai SJ, Lai YC, Chow W. (1998) Bladder shape impact on the accuracy of ultrasonic estimation of bladder volume. Arch Phys Med Rehabil 79: 1553-1556. doi: 10.1016/S0003-9993(98)90419-1. PMID: [DOI] [PubMed] [Google Scholar]
- 29.Hwang JY, Byun S, Oh S, Kim HC. (2004) Novel algorithm for improving accuracy of ultrasound measurement of residual urine volume according to bladder shape. Urology 64: 887-891. doi: 10.1016/j.urology.2004.06.054. PMID: [DOI] [PubMed] [Google Scholar]
- 30.Ghani KR, Pilcher J, Rowland D, Patel U, Nassiri D, et al. (2008) Portable ultrasonography and bladder volume accuracy--a comparative study using three-dimensional ultrasonography. Urology 72: 24-28. doi: 10.1016/j.urology.2008.02.033. PMID: [DOI] [PubMed] [Google Scholar]
- 31.Nusee Z, Ibrahim N, Rus RM, Ismail H. (2014) Is portable three-dimensional ultrasound a valid technique for measurement of postpartum urinary bladder volume? Taiwan J Obstet Gynecol 53: 12-16. doi: 10.1016/j.tjog.2013.01.028. PMID: [DOI] [PubMed] [Google Scholar]
- 32.Beckers GM, van der Horst HJ, Frantzen J, Heymans MW. (2013) The BladderScan BVI 6200® is not accurate enough for use in a bladder retraining program. J Pediatr Urol 9: 904-909. doi: 10.1016/j.jpurol.2012.12.013. PMID: [DOI] [PubMed] [Google Scholar]
- 33.Schnider P, Birner P, Gendo A, Ratheiser K, Auff E. (2000) Bladder volume determination: portable 3-D versus stationary 2-D ultrasound device. Arch Phys Med Rehabil 81: 18-21. doi: 10.1016/S0003-9993(00)90215-6. PMID: [DOI] [PubMed] [Google Scholar]
- 34.Ahmad R, Hoogeman MS, Quint S, Mens JW, de Pree I, et al. (2008) Inter-fraction bladder filling variations and time trends for cervical cancer patients assessed with a portable 3-dimensional ultrasound bladder scanner. Radiother Oncol 89: 172-179. doi: 10.1016/j.radonc.2008.07.005. PMID: [DOI] [PubMed] [Google Scholar]