Abstract
Pregnancy is associated with a variety of physiological changes that can alter the pharmacokinetics and pharmacodynamics of several drugs. However, limited data exists on the pharmacokinetics and pharmacodynamics of the majority of the medications used in pregnancy. In this article, we first describe basic concepts (drug absorption, bioavailability, distribution, metabolism, elimination, and transport) in pharmacokinetics. Then, we discuss several physiological changes that occur during pregnancy that theoretically affect absorption, distribution, metabolism, and elimination. Further, we provide a brief review of the literature on the clinical pharmacokinetic studies performed in pregnant women in recent years. In general, pregnancy increases the clearance of several drugs and correspondingly decreases drug exposure during pregnancy. Based on current drug exposure measurements during pregnancy, alterations in the dose or dosing regimen of certain drugs are essential during pregnancy. More pharmacological studies in pregnant women are needed to optimize drug therapy in pregnancy.
Keywords: Pregnancy, Pharmacology, Pharmacokinetics, placental transfer, clinical pharmacology
Introduction
Approximately 64% of women in the United States are prescribed one or more medications during pregnancy for acute or chronic conditions such as urinary tract infections, nausea, depression, hypertension, diabetes, preeclampsia, preterm labor, gestational diabetes mellitus, or asthma.1 The average number of medications used in pregnancy, not including vitamins and minerals, is reported to be 4.2.2 For a majority of these medications, there is no pharmacological data available in pregnant women.3 A systematic evaluation of the pharmacokinetics, concentration–effect relationships, placental transfer, fetal drug exposure, and concentrations in breast milk is generally lacking for these drugs. This problem results from a lack of initiative by the pharmaceutical companies to conduct research in pregnant women, probably related to the cost and medical–legal issues. Currently, the dosing regimen of most medications used by pregnant women is largely based on the data in men and non-pregnant women, which can lead to either overdosing with excessive side effects or under-dosing with an inadequate therapeutic response in pregnant mothers. In addition, the application of any available data from animal models of pregnancy is limited due to species-dependent differences in metabolism/pharmacokinetics of the drugs and due to our inability to extrapolate the results from animals to humans.1
To enhance our understanding of obstetric pharmacology and the rational use of medications during pregnancy, the Obstetric-Fetal Pharmacology Research Unit (OPRU) Network, which is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), has embarked on several projects (OPRU link, 〈https://www.nichd.nih.gov/research/supported/Pages/opru_network.aspx〉). The OPRU Network investigators have contributed to the improved pharmacological understanding of a number of commonly used drugs during pregnancy, including statin drugs, antidiabetic drugs, antihypertensives, antibiotics, antivirals, benzodiazepines, drugs used in preterm delivery, tocolytics, and immunosuppressants, and provided a rationale for optimizing the dosing regimen of these agents. Clinical studies4–14 have been performed to evaluate the disposition and efficacy of drugs during pregnancy, while nonclinical research15–24 has been conducted to investigate the mechanisms of drug disposition and response in pregnancy. We have searched English-language literatures through MEDLINE/PubMed using the keywords “pregnancy, human, pharmacokinetics, pharmacodynamics, clearance, concentrations” and identified publications based only on in vitro and in vivo human data. The objectives of this article are to address the basic pharmacokinetic and pharmacodynamic concepts useful in Obstetric Pharmacology, to summarize physiological changes that occur during pregnancy that can alter the pharmacokinetics of a drug, and to present a few examples of pharmacokinetic alterations during pregnancy based on studies published during the period 2005–2014.
Basic pharmacokinetics
Pharmacokinetics (PK) describes the time course of drug concentration in the body. The processes of drug absorption (A), distribution (D), metabolism (M), excretion (E), and transport (T) ultimately determine the concentration–time profile of a drug in various parts of the body. When a drug is administered extravascularly, it goes through a process of absorption from the site of administration, then distributes to various parts of the body, and finally gets out of the body either as the parent drug or as metabolites. Metabolites produced are usually more polar than the parent drug and are more readily excreted by the kidney or through the bile. Metabolism of drugs (phase I oxidation and phase II conjugation) occurs primarily in the liver but can also take place in other organs such as the small intestine and the kidney. While movement of drugs in and out of cells in general occurs through passive diffusion, certain drugs require membrane transporters to move in and out of cells. In particular, during pregnancy, placental transporters play an important role in regulating the access of xenobiotics from a mother to the fetus. When pharmacokinetic studies are performed, blood samples are collected over dosing intervals and the blood or plasma concentrations of a drug are typically measured using a specific and sensitive assay method. The concentration–time profile is then used to determine various pharmacokinetic parameters of the drug.
Clinical pharmacological study design in pregnancy
The primary objectives of most clinical pharmacological studies in pregnant women are to describe the ADMET properties of drugs and to optimize dosing regimen of drugs in this population. The most desirable study design is to compare the pharmacokinetic parameters (e.g., apparent oral clearance) during pregnancy with that observed either prior to pregnancy or during postpartum, as a non-pregnant control comparison within the same subject. In this longitudinal study design, a group of women are enrolled and individually followed up prior to pregnancy and throughout gestation or across gestation into the postpartum period for pharmacokinetic and pharmacodynamics evaluations. However, from a practical point of view, our ability to perform longitudinal studies is impacted by the inability to obtain the data before pregnancy. While data collected from postpartum are used as substitute for pre-pregnancy studies, this approach may be limited by recruitment issues postpartum and the lack of knowledge of the time taken to recover completely to pre-pregnancy status and the potential impact of breast-feeding on the pharmacokinetics of drugs. In the cross-sectional study design that is more often used in practice, but is a less desirable study design, the pharmacokinetic parameters in a group of pregnant women are compared with those obtained from a group of non-pregnant women, either healthy adult volunteers or those inflicted with the same illness for which the drug is approved. It is important to relate any changes in the pharmacokinetic parameters observed to the pathophysiological changes that occur in pregnancy. Compared to the longitudinal study design, the cross-sectional study design may mask any real impact of pregnancy on the pharmacokinetics and pharmacodynamics of drugs.
Once the concentration–time profile of a drug over dosing intervals is obtained, non-compartmental and compartmental pharmacokinetic analyses are performed to calculate pharmacokinetic parameters. Population pharmacokinetic modeling and simulation also represents a feasible approach when only sparse (limited) sampling is available25 from pregnant subjects. Nonlinear mixed-effects modeling (NON-MEM) with stepwise covariate modeling is used to build structural covariate models. The aim of covariate modeling is not only to find covariates that significantly influence the population pharmacokinetic parameters but also to provide dosing recommendations for certain drugs used in pregnancy. Using this approach, pregnancy has been identified as a significant covariate for pharmacokinetics for some drugs, including labetalol26 and azithromycin.27
Analysis of plasma or blood concentration vs time profile
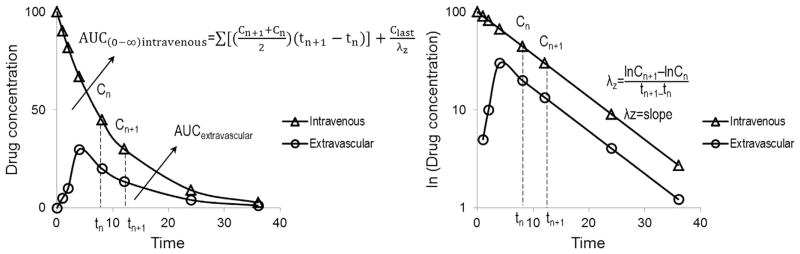
Various pharmacokinetic parameters that can be obtained from the plasma or blood concentration vs time curve are shown in Figure and are discussed below.
Fig.
Plasma concentrations (Y-axis) versus time (X-axis) after a single dose of intravenous or extravascular administration of a drug. Left panel is shown in normal scale and right panel is shown in semilog scale. The parameters include the area under the curve (AUC), drug concentrations (Cn+1 measured at time n+1 and Cn measured at time n), the last measured drug concentration (Clast), and the terminal disposition rate constant (λz) for a drug that exhibits linear pharmacokinetics). Bioavailability here is defined as AUC extravascular/AUC intravenous.
Area under the concentration–time curve (AUC, unit: μg/mL h) is a measure of overall drug exposure in a subject. Calculation of AUC is usually performed using the trapezoidal rule, as shown in Figure. The terminal disposition rate constant (λz, unit: 1/h) is calculated as the slope of the terminal linear portion of a semilog concentration–time curve.
Drug absorption and bioavailability
Bioavailability (F) is a measure of the rate and extent to which a drug is available to the systemic circulation. When a drug is administered extravascularly, the entire administered dose or absorbed amount may not be available to the systemic circulation. After oral administration, a drug may be incompletely absorbed or can be degraded in the gut. Even completely absorbed, a drug can undergo significant first-pass metabolism in the gut or the liver or can be effluxed by drug transporters, leading to a lower bioavailability. The bioavailability is usually determined by comparing dose-normalized AUC after an extravascular drug administration (e.g., oral or intramuscular or subcutaneous) to that after intravenous drug administration (Fig.). Absolute bioavailability is expressed as follows:
| (1) |
Drug distribution
Volume of distribution (L or L/kg body weight) is a parameter that relates the amount of the drug in the body to the concentration of the drug at the site of measurement. After intravenous administration of a drug with first-order elimination, assuming instantaneous distribution of the drug into the entire body, the apparent volume of drug distribution in the body is calculated as follows:
| (2) |
For a drug that exhibits multi-compartment behavior, the volume of the central compartment (Vc) describes the initial volume into which the drug distributes instantaneously:
| (3) |
Volume of the central compartment is important in the clinical setting as it determines the loading dose for an intravenous bolus injection in order to achieve a desired peak plasma concentration of a drug.
Another volume term, volume of distribution at terminal phase (Vβ), is used to estimate the amount of drug in the body during the terminal disposition phase, where λz is the terminal disposition rate constant (Fig.):
| (4) |
After extravascular administration, the apparent volume of distribution of a drug is estimated as Vd/F, since the actual fraction of the dose that is bioavailable (F) is not normally known.
In plasma, a drug will be in an unbound form or bound to plasma proteins. The ratio of unbound drug to total drug is known as the fraction unbound (fUP) in plasma. Physiologically, the volume of distribution is determined by plasma volume (VP), total body water—plasma volume (VT), the unbound fraction of the drug in plasma (fUP), and unbound fraction of the drug in tissues (fUT):
| (5) |
Drugs that predominantly stay within the vascular system will have a volume of distribution corresponding to plasma volume (VP). Drugs that are not bound to any proteins in blood or in the tissues will have a volume of distribution corresponding to the total body water (VP + VT). Drugs that are highly bound to tissues (small fUT) will have a very large volume of distribution, and only a small amount of the drug will be in the vascular system. Lipophilicity of a drug, often expressed as octanol/water partition-coefficient (log P), is an important physicochemical parameter influencing plasma/tissue protein binding and volume of distribution. Molecular size or molecular weight of a drug can affect its membrane permeation and therefore the distribution of a drug as well.
Drug metabolism and elimination
Quantitatively, liver normally accounts for the metabolism and clearance of a majority of drugs, although other organs such as the intestine, kidney, and placenta may also contribute to the clearance of a few drugs. Drug-metabolizing enzymes mainly localized to cellular endoplasmic reticulum catalyze the biotransformation of drugs to often pharmacologically inactive or sometimes active metabolites [e.g., codeine (prodrug) conversion to morphine (active) and morphine conversion to morphine-6-glucuronide (active)] in humans. The NADPH-cytochrome P450 reductase (P450R)/cytochrome P450s (P450s, namely CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4/5) are enzymes that mediate phase I oxidative reactions, whereas the UDP-glucuronosyltransferases (UGTs, namely UGT1A1, UGT1A4, UGT2B4, and UGT2B7), sulfotransferases (SULTs, namely SULT1A1, SULT1A2, SULT2A, and SULT2B), glutathione S-transferases, and N-acetyltransferases are enzymes that mediate phase II conjugative reactions of most drugs. In addition, there are several transporters that are expressed in organs such as the liver, kidney, and small intestine that are responsible for the movement of the drug and/or metabolites into or out of the organ (Table 1).
Table 1.
Transporters in the liver, kidney, small intestine, and placenta in humans.
| Organs in humans | Influx transporters | Eflux transporters |
|---|---|---|
| Liver | NTCP, OAT2, OATP1/2, and OCT1/3 (blood side) | BCRP, BSEP MATE1, MDR1/3, and MRP2 (bile side) |
| Kidney | MRP1/3/5/6, OAT1/2/3, OATP4C, OCT2/3, and OST (blood side) | ASBT, BCRP, MATE1/2, MDRI, MRP2/4, OCTN1/2, OAT4, OATP1, and PEPT (urine side) |
| Intestine | MRP1/3, OCT1, and OST (blood side) | ASBT, BCRP, MDRI, MRP2, OATP1/2, OCT3, and PEPT (luminal side) |
| Placenta | MRP5, OAT4, OATP2B, and OCT3 (fetal blood side) | BCRP, MDRI, MRP1/2/3, and OATP4A (maternal blood side) |
Note: ASBT, apical sodium-dependent bile acid transporter; BCRP, breast cancer resistance protein; BSEP, bile salt export pump; MATE, multidrug and toxic compound extrusion; MDR, multi-drug-resistant ABC transporter; MRP, multidrug resistance-associated protein; NTCP, sodium-taurocholate cotransporting polypeptide; OAT, organic anion transporters; OATP, organic anion-transporting polypeptide; OCT, organic cation transporters; OST, organic solute transporter; PEPT, peptide transporter.
Clearance (CL, unit: L/h or L/h/kg body weight) is the most important pharmacokinetic parameter of a drug, as it determines the drug exposure and measures the overall ability of the human body to eliminate a drug. Total body clearance is the volume of blood or plasma that is completely cleared of the drug in a unit of time. Blood and plasma clearances will be equal only when the drug concentrations in blood and in plasma are equal. Drugs can be cleared from the body by many organs. The total body clearance of a drug is the sum of all the clearances by various organs.
| (6) |
where CLt is total body clearance, CLh is hepatic clearance, CLr is renal clearance, and CLother is clearance by all other organs (gastrointestinal tract, pulmonary, etc.).
After intravenous administration, the clearance of the drug is given as follows:
| (7) |
When a drug is given via an extravascular route, absolute clearance cannot be calculated, instead the apparent clearance is estimated, which includes the bioavailability factor, such that the apparent drug clearance is as follows:
| (8) |
The clearance of a drug in the liver (CLh) is determined by hepatic blood flow (Qh, 90 L/h in an adult human being) and the extraction ratio of the drug in the liver (ER):
| (9) |
Extraction ratio is considered as an index of how efficiently the liver extracts drug from the blood that goes through it, and it ranges from 0 to 1. ER across an organ is dependent on the fraction of unbound drug (fUP) and intrinsic clearance of the organ (CLint, the inherent ability to remove the drug by the organ in the absence of any limitations):
| (10) |
Drugs with low ER (ER < 0.3) show a capacity-limited clearance (CL = fUP × CLint), where the clearance is primarily determined by the unbound fraction of the drug and the intrinsic clearance. Drugs with a hepatic clearance calculated based on blood concentration <18 L/h are considered low hepatic clearance drugs. Drugs with high ER (ER > 0.7) show organ blood flow-limited clearance (CL = Q), where the clearance is directly affected by changes in blood flow. Drugs with hepatic clearance calculated based on blood concentration >63 L/h are considered high hepatic clearance drugs. In case of high clearance drugs, most or all the drug delivered to that organ is completely cleared during its passage.
For drugs that are cleared by the kidney, renal clearance is determined from the amount of unchanged drug excreted in the urine (Au(0–∞)) divided by the area under the plasma concentration–time curve:
| (11) |
A drug’s overall renal clearance is a function of glomerular filtration (CLGF), tubular secretion (CLTS), and tubular reabsorption (TR). It can be expressed as follows:
| (12) |
Increased glomerular filtration rate and altered expression of drug transporters can alter the clearance of certain drugs during pregnancy.
The terminal half-life (t1/2, unit: h) of a drug is dependent on the volume of distribution and clearance of the drug:
| (13) |
The half-life gives an estimate of the time required to remove half of the drug out of the body or for drug concentrations to fall to 50% of the initial value. Half-life is also used to determine the time to reach a steady state after initiation of a new dosing regimen (it takes about 5–6 t1/2) as well as to determine the dosing frequency for a drug.
In general, if there is an increase in clearance or apparent clearance of a drug during pregnancy, it may necessitate an increase in the dose of the drug to maintain drug exposure that is comparable to what is observed in non-pregnant state. If there is a decrease in half-life, it would necessitate an increase in dosing frequency during pregnancy.
Basic pharmacodynamics
Pharmacodynamics (PD) describes the mechanism and magnitude of the observed pharmacological effects, that is, the toxic or therapeutic clinical response. Pharmacokinetic–pharmacodynamic models can be viewed as models for an input (e.g., drug concentration) in an observed site to the response (e.g., effect, E) of a system at a given time. Unlike drug plasma concentrations, drug concentration at the target site cannot be measured directly. Therefore, blood, plasma, or serum concentrations are used as surrogate markers for the concentrations at the site of action. Linking the drug concentrations to a pharmacological effect provides a more meaningful approach for determining safe and effective dosing regimens.
Drug exposure and toxicity in the fetus
It is important to consider potential toxicity of a drug to the fetus when a drug is administered to a pregnant woman. Unnecessary drug exposure (other than typically antiretroviral therapy) in a developing fetus can result in severe fetal toxicity, such as teratogenicity associated with antiepileptic drugs,28 and severe anhydramnios induced by angiotensin II receptor antagonists.29 In general, data on fetal drug exposures is limited to either case reports or pharmacoepidemiology studies.30 Case reports describe an unusual clinical outcome after drug exposure to a fetus during pregnancy and subsequent effects on the infant. Case reports might well raise suspicions, but follow-up evaluations are always necessary to assess the risk. Formal epidemiology studies, including prospective and retrospective studies, provide a better way of evaluating whether gestational exposure adversely affects the developing infant. So far, birth defects are known to occur with increased frequency in infants born to women with diabetes, independent of the drugs used to treat the disease.30 More research is needed to better understand the effects of drug exposure and maternal conditions on fetal development.
Maternal physiological changes during pregnancy that can impact the pharmacokinetics and pharmacodynamics of drugs
There are a number of physiological changes that occur during pregnancy that can impact the pharmacokinetics and pharmacodynamics of drugs (Table 2). The extent and rate of absorption of drugs can be altered by (1) nausea and vomiting during pregnancy; (2) prolongation of gastrointestinal transit times due to the increase in progesterone and estrogen concentrations during pregnancy31; (3) altered gastric volume and gastric pH as a result of pharmacological agents used in pregnancy to alleviate gastrointestinal disorders, such as antacids, histamine-2 receptor antagonists, and proton pump inhibitors; and (4) alteration in the expression and activity of drug-metabolizing enzymes and transporters in the gut.
Table 2.
Pathophysiological changes in pregnant women that can impact pharmacokinetics of certain drugs.
| Changes | Potential impact on pharmacokinetics |
|---|---|
| Physiologic changes | |
| Nausea and vomiting | ↓ Absorption (↓ peak conc. and ↓ oral bioavailability) |
| Delayed motility | Delayed absorption (↑ time to peak conc.) |
| Decreased gastric acid secretion | ↓ Oral bioavailability for anionic drugs |
| Body weight gain | ↑ Apparent volume of distribution |
| Body fat gain | ↑ Apparent volume of distribution for lipophilic drugs |
| Increased plasma volume | ↑ Apparent volume of distribution |
| Decreased plasma albumin and αl-acid glycoprotein | ↑ Unbound drug concentration for high clearance drugs; unbound drug concentration not expected to be altered for low clearance drugs. (It is important to base therapy on unbound drug, for drugs that are highly bound.) |
| Changes in hepatic drug-metabolizing enzymes | Altered hepatic clearance for low extraction ratio drugs administered intravenously or high extraction drugs administered orally |
| Increased hepatic blood flow | ↑Hepatic clearance for high extraction ratio drugs |
| Increased renal blood flow | ↑ Renal clearance for unchanged drug |
| Increased glomerular filtration | ↑ Renal clearance for unchanged drug |
| Other maternal factors | |
| Chronic disease or pregnancy complications | Changes in ADMET/PD |
| Maternal poly-pharmacy | PK/PD drug interactions |
| Maternal–Fetal factors | |
| Placenta | Placental drug metabolism, placental transporters |
| Fetus | Hepatic CYP3A7 does not contribute to clearance in mother, but it can influence concentration of drug and metabolites in the fetus and ↑ apparent volume of distribution |
| Amniotic fluid | Drug accumulation and ↑ apparent volume of distribution |
Note: ↑, increase; ↓, decrease; CYP, cytochrome P450; PK, pharmacokinetics; PD, pharmacodynamics; ADME drug, absorption, distribution, metabolism, and excretion.
The volume of distribution can be altered by the following factors (1) changes in maternal body weight (up to 50–70% increase in pregnant women at the time of delivery) and accumulation of body fat (3–4 kg) in pregnant women can alter the volume of distribution of certain drugs; (2) in addition, gain in extracellular and plasma volume (about 50–70%)32 in pregnant women, particularly during the third trimester; (3) lower levels of plasma albumin and α1-acid glycoprotein throughout pregnancy (reaching concentrations approximately 70–80% of non-pregnant values at the time of delivery) will decrease drug binding and alter the volume of distribution of certain drugs32; and (4) extent of drug transfer across the placenta. The volume of distribution will slowly return to its original value once the pregnant woman delivers the baby.
Changes in the activity of hepatic drug-metabolizing enzymes (increased activity of hepatic CYP2A6, CYP2C9, CYP2D6, CYP3A4, and UGT during pregnancy33 and decreased activity of CYP1A2 and CYP2C19 in pregnancy33,34), altered hepatic blood flow, and changes in the expression of drug transporters in the liver can lead to altered hepatic clearance of certain drugs. The enlarged maternal kidneys, increased urinary dead space35; ~50–80% increase in maternal renal blood flow35; ~50% increase in glomerular filtration rates in the first trimester32; and ~30–50% increase in creatinine clearance can lead to increased renal clearance of certain drugs.35
Other factors that can contribute to alterations in pharmacokinetics/pharmacodynamics during pregnancy include maternal age, race, ethnicity, body weight, singleton vs multiple gestations, gestational age, smoking history, alcohol usage, dietary habits, and illegal drug use by pregnant women and are associated with a range of maternal/fetal complications. Some comorbid medical conditions in pregnant women may serve as confounding factors or potential additional covariates, including cystic fibrosis, renal impairment, and liver disease.36
Role of placenta and fetus
In general, lipophilic drugs readily cross the placenta. The fetus and the amniotic fluid can act as additional compartments for drug distribution or drug accumulation. The human placenta also expresses a number of xenobiotic metabolic enzymes (phase I: CYP1, CYP2, and CYP3; phase II: GST alpha and pi, NAT, SULT1A1 and SULT1A3, and some UGT1A and UGT2B)37 and active membrane transporters. Placental drug metabolism has been evaluated using in vitro perfusion systems for several drugs including hydroxyprogesterone caproate,20,23,38 glyburide,39,40 and bupropion.41 P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) are mainly expressed in the syncytiotrophoblast and actively efflux a wide range of xenobiotics (Table 1).42 During pregnancy, the expression of drug transporter is regulated by a number of transcription factors and steroid hormones, such as progesterone, estrogen, and corticosteroids. The expression profile of the placental transporters varies with advancing gestation. In normal pregnancy, placental P-gp has been shown to decline near term, leaving the fetus potentially more exposed to certain drugs commonly administered to pregnant women than earlier in pregnancy (i.e., synthetic glucocorticoids, selective serotonin reuptake inhibitors, glyburide, and antiretrovirals). The levels of drug transporters have also been reported to be altered in pathological pregnancies (preterm, preeclampsia, growth restriction, and infection).42 In women with pre-term labor with inflammation, placental protein and mRNA expression of P-gp and BCRP measured at gestational age of 28–33 weeks increase.43 Increased expression of organic cation transporter (OCT3) in human placenta at later stages of gestation may play a role in modulating fetal drug exposure of OCT3 substrates.44
In general, human fetal liver has a lower capacity for metabolism of drugs compared to an adult. CYP3A7 (CYP3A4/5 in adult) accounts for up to 50% of total fetal hepatic cytochrome P450 content. Human fetal livers express moderate levels of SULT2A1 and SULT1E1, which are comparable with those in the adult human liver, but lower levels of CYP1A1, CYP2D6, CYP2C19, and UGT2B4.45–48 The human fetal liver is not expected to significantly contribute to the overall clearance of a drug but can metabolize drugs to some extent, potentially producing metabolites locally that may contribute to adverse effects in the fetus.
Clinical pharmacological data for certain drugs used in pregnancy
Even though there is a paucity of data on the effect of pregnancy on the pharmacokinetics of several drugs, studies have been performed for specific drugs that help in optimizing the dosing of these agents in pregnancy. Some of the available data on these drugs are summarized in the following section and in Table 3.
Table 3.
Clinical pharmacokinetic and pharmacodynamic data for selected drugs in pregnancy.
| Drugs | Metabolism/elimination path | Maternal PK in pregnancy | Fetal:maternal ratio | PK-PD |
|---|---|---|---|---|
| Analgesics | ||||
| Acetaminophen50 | Hepatic UGT1A, SULT1A, CYP2E1, and acetyltransferase | 50% ↑ Clearance, 140% ↑ glucuronide/parent, 80% ↑ oxidatives/parent, and ↔ clearance for acetaminophen sulfate | l.0 | Concentration–toxicity |
| Antiepileptic | ||||
| Lamotrigine52–54,58–65 | Hepatic UGT1A3 and 1A4 | 65–164% ↑ Clearance, ↑ metabolite/parent, and ↓ concentration | 1.2 | ↑ Seizure frequency |
| Phenytoin54 | Hepatic CYP2C9 | 117% ↑ Clearance, altered plasma protein binding, ↓ total concentration, and ↔ unbound concentration | NA | NA |
| Levetiracetam61,66,67 | 66% Unchanged via renal | ↑ Renal clearance and ↓ concentration | 1.1 | NA |
| Antihypertensive | ||||
| Clonidine68,69 | Hepatic CYP2D6 | 80% ↑ Total clearance, ↔ renal clearance | 1.0 | NA |
| Labetalol26,70 | Hepatic UGT1A1 | ↔ or ↑ clearance | 0.5 | Concentration–efficacy |
| Atenolol71 | Renal excretion | ↑ Renal clearance | NA | NA |
| Antidiabetes | ||||
| Glyburide55,56 | Hepatic CYP2C9 and 3A | ↑ Clearance and ↓ concentration | 0.7 | NA |
| Metformin72 | Renal excretion and minimal metabolism | ↔ or ↑ Clearance | 0.7 | NA |
| Antiretrovirals | ||||
| Indinavir73,74 | Hepatic CYP3A | 68% ↓ Exposure | 0.12 | No correlation |
| Lopinavir/ritonavir75–83 | CYP3A | 58% ↑ Clearance, ↓ exposure, and ↔ unbound concentration | 0.2 | Concentration–toxicity |
| Nelfinavir82–85 | Hepatic CYP3A and 2C19 | 100% ↑ Clearance, ↓ exposure, ↓ total concentration, and ↓ unbound concentration | 0.3–0.4 | Concentration–efficacy |
| Nevirapine83,86,87 | Hepatic CYP3A4 and 2B6 | ↔ Or ↑ clearance and 22% ↓ exposure | 0.8 | NA |
| Efavirenz75,88 | Hepatic CYP3A and 2B6 | ↔ Clearance and ↓ concentration | 0.5 | NA |
| Tenofovir89–90 | Renal filtration and active tubular secretion | ↑ Clearance | 0.6 | NA |
| Lamivudine91 | 71% Unchanged via renal | 22% ↑ Clearance and ↔ pk | 0.86–5 | NA |
| Emtricitabine92 | UGT | 21% ↑ Clearance | 1.0 | NA |
| Anti-influenza | ||||
| Oseltamivir93 | Hepatic esterases and renal filtration and secretion | ↔ pk For oseltamivir and 44% ↑ clearance for oseltamivir carboxylate | 0.4 | NA |
| Antifungal | ||||
| Metronidazole6 | Hepatic | ↔ pk | 0.3–2 | NA |
| Antimalarial | ||||
| Sulfadoxine-pyrimethamine94 | N-acetyltransferase | 67% ↑ Clearance | NA | NA |
| SSRIs | ||||
| Sertraline95 | Hepatic CYP2C9, 2C19, 2D6, 3A, and UGT1A1 | ↔ pk | 0.67 | NA |
| Fluoxetine | Hepatic CYP2D6 | 2 6 ↑ Metabolite/parent | 0.67 | NA |
| Immunosuppressants | ||||
| Tacrolimus13,14,96 | Hepatic CYP3A | 39% ↑ Clearance, ↓ whole-blood conc., and 1.9–2.0 ↑ unbound concentration | 0.71 | NA |
| Opioid substitute | ||||
| Methadone97 | Hepatic CYP2B6, 2C19, 2C9, 2D6, and 3A4 | 90% ↑ Clearance | NA | NA |
| Antineoplastic agents | ||||
| Doxorubicin98–100 | Hepatic CYP2D6, 3A4, and P-glycoprotein | ↑ or ↓ Clearance | NA | NA |
| Epirubicin98,99 | Hepatic UGT2B7 | 43% ↑ Clearance | NA | NA |
| Paclitaxel98,99 | Hepatic CYP2C8 and 3A4 | 21% ↑ Clearance | NA | NA |
Note: ↑, increase; ↓, decrease; ↔, no change; CYP, cytochrome P450; UGT, UDP-glucuronosyltransferase; SSRIs, selective serotonin reuptake inhibitors; TDM, therapeutic drug monitoring; PK, pharmacokinetics; PD, pharmacodynamics; NA, not available.
Acetaminophen
Acetaminophen, also known as paracetamol, is the drug of choice for the treatment of mild to moderate pain in pregnant women. It is also one of the most widely used over-the-counter medications and the most common overdosed medication during pregnancy. Over 90% of the therapeutic dose of acetaminophen is converted to inactive glucuronide and sulfate conjugates and then excreted by the kidneys. Less than 5% of acetaminophen is metabolized to highly reactive oxides, such as the active electrophilic metabolite N-acetyl-p-benzo-quinoneimine (NAPQI), through hepatic CYP2E1. Most of NAPQI is further bound by reduced glutathione and excreted in the urine as conjugates. In near-term pregnancy, the clearance of acetaminophen is increased by 50%, with clearance to acetaminophen glucuronide increasing by 140%, and clearance to oxidative metabolites of acetaminophen increasing by 80%.49 In contrast, the clearance to acetaminophen sulfate remains similar whether at delivery or postpartum. With maternal overdose of acetaminophen, the amount of toxic metabolite NAPQI formed exceeds the binding capacity of glutathione and is distributed to the fetal circulation (acetaminophen freely crosses the placenta), and its toxic intermediary metabolites can accumulate in fetal circulation, given that glucuronidation capacity of the fetal liver is low. Toxic intermediary metabolites produced can cause both maternal and fetal hepatic necrosis.50
Drug for prevention of preterm delivery
17-Alpha hydroxyprogesterone caproate is the only FDA-approved drug for the prevention of preterm birth in singleton pregnant women with a history of a prior spontaneous preterm birth. 17-Alpha hydroxyprogesterone caproate is administered intramuscularly once a week at a dose of 250 mg. It is predominantly cleared from the body by metabolism. CYP3A appears to be the enzyme primarily responsible for its metabolism.51 After intramuscular administration, its half-life ranges from 9 to 16 days in women with singleton gestation.8 Body mass index (BMI) has been found to contribute to the inter-individual differences in trough plasma concentrations and area under the curve of 17-alpha hydroxyprogesterone caproate, with women of higher BMI having a lower drug exposure as determined by AUC.7 Increased progesterone levels or co-administrated medications that are inducers or inhibitors of CYP3A (Table 4) during pregnancy may also account for some of the large variation in plasma 17-alpha hydroxyprogesterone caproate concentrations that is seen in pregnant patients.
Table 4.
Medications and dietary components used during pregnancy that are known cytochrome P450 3A4 inducers or inhibitors.
| Cytochrome P450 3A4 inducers | Cytochrome P450 3A4 inhibitors |
|---|---|
| Anticonvulsants (carbamazepine) | Azole antifungals (ketoconazole and itraconazole) |
| Bactericidals (rifampicin) | Antidepressants (nefazodone) |
| St. John’s wort | Protease inhibitors (ritonavir, indinavir, nelfinavir, and saquinavir) |
| Grapefruit juice (bergamottin) | |
| Macrolide antibiotics (clarithromycin and erythromycin) |
Antiepileptic drugs
Antiepileptic drugs are commonly used in pregnant women for the treatment of epilepsy.52 Lamotrigine is primarily metabolized in humans through glucuronidation to inactive lamotrigine-2N-glucuronide by hepatic UGT1A3 and 1A453 and is then excreted in the urine as conjugates. Pregnancy causes a substantial increase in the rate of glucuronidation,53 resulting in an increase in the apparent oral clearance of lamotrigine, necessitating an increase in dose during pregnancy (Table 3). Lamotrigine is known to cross the placenta. It is secreted in breast milk.54 Phenytoin is primarily metabolized by CYP2C9. Pregnancy causes a 117% increase in total phenytoin clearance and a 61% decrease in total phenytoin concentrations in the third trimester compared to baseline.54 However, free phenytoin concentrations decrease slightly in the third trimester. Phenytoin dosage should be adjusted based on free phenytoin concentrations. These observations emphasize the importance of monitoring antiepileptic drugs closely during pregnancy and making appropriate changes in the dosing regimen, as necessary.
Antihypertensive drugs
In general, multiple antihypertensive agents are widely used in pregnancy to treat women with chronic hypertension, gestational hypertension, and preeclampsia. Pathophysiology in pregnancy affects pharmacokinetics as well as pharmacodynamic of the antihypertensive drugs used (Table 3).
Antidiabetic drugs
Glyburide is predominantly metabolized by CYP2C9 and CYP3A. The apparent oral clearance of glyburide during pregnancy is significantly increased, resulting in decreased exposure in women with gestational diabetes mellitus (Table 3).55 This is due to the pregnancy-related up-regulation of both enzymes. More than twice the dose of glyburide in pregnancy is needed to achieve the same concentrations seen in the non-pregnant population.56
Antiretroviral drugs
Physiological changes during pregnancy can alter antiretroviral drug concentrations, and concerns have been raised that target concentrations are not maintained throughout pregnancy (Table 2). Suboptimal drug exposure can result in HIV RNA rebound, the selection of resistant virus, and an increased risk of HIV-1 transmission to the infant.57 In some cases, dose adjustments are necessary during pregnancy to achieve comparable antiretroviral exposure to non-pregnant adults.
Selective serotonin reuptake inhibitors (SSRIs)
SSRIs have become an important treatment option for perinatal women suffering from depression. There are no clear patterns for pharmacokinetic changes and dosing guidelines for SSRIs in pregnancy.
Immunosuppressant drugs
Tacrolimus and cyclosporine have been used successfully in pregnant women following solid organ transplantation and in those with autoimmune diseases. However, these pregnancies are considered to be at high risk for maternal, fetal, and neonatal complications. During pregnancy, there are multiple factors that can increase the fraction of unbound tacrolimus, including but not limited to low concentrations of albumin and α1-acid glycoprotein as well as decreased red blood cell counts. The clinical titration of dosage to maintain whole-blood tacrolimus trough concentrations in the usual therapeutic range can lead to elevated unbound concentrations and possibly toxicity in pregnant women with anemia and hypo-albuminemia. Measurement of unbound tacrolimus concentrations for pregnant women might better reflect the active form of the drug, although these are technically challenging (tacrolimus highly and saturably binds in red blood cells) and often unavailable in usual clinical practice. A small amount of tacrolimus is excreted in the breast milk, which is unlikely to elicit adverse effects in the nursing infant. Very limited recent information is available on the effects of pregnancy on pharmacokinetics and pharmacodynamics of cyclosporine.
Conclusion
In summary, additional clinical pharmacology studies are essential in pregnancy. Here, we propose some general guidelines for optimal use of medications during pregnancy.
Drug therapy during pregnancy should weigh the benefits of the therapeutic treatments against the risks of adverse events to the woman, fetus, and newborn. Certain drugs should be avoided in pregnant women due to the potential exposure to the developing fetus and the corresponding undesired consequences.
When it is safe to use a drug during pregnancy, dosing regimen should be designed to optimize drug exposure in mothers, taking into account the changes in the activity of metabolic enzymes and transport of drugs during pregnancy.
A change in the pharmacokinetics during pregnancy per se may not necessitate a change in the dosing regimen of a drug. The pharmacodynamics response to the drug should also be taken into account in making dosing changes.
In certain cases, it may be necessary to deliver drugs to the fetus as well (anti-HIV drugs). Appropriate selection of the drug should be based on data on the placental transfer of such drugs.
When it is difficult to predict dosing regimens, frequent therapeutic monitoring should be considered in pregnant women to guide therapy of drugs such as immunosuppressives, anticonvulsants, antidepressants, and antiretroviral drugs.
Decisions on dosage adjustment should be made whenever possible using pharmacologically active unbound drug plasma concentrations rather than total drug concentrations.
Based on clinical evidences, pregnancy increases the apparent clearance of glyburide, indinavir, emtricitabine, lamivudine, paclitaxel, sulfadoxine-pyrimethamine, lopinavir, lamotrigine, clonidine, nelfinavir, and methadone. Pharmacokinetics of metronidazole and fenoterol seems not to be altered during pregnancy. Controversial effects of pregnancy on drug clearance have been reported for metformin and nevirapine.
Gradual reduction in dose, if it has been increased, during pregnancy is often warranted to avoid overdosing within a few days to weeks after delivery.
Acknowledgments
Supported in part by the Obstetric-Fetal Pharmacology Research Unit Network, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant nos. U10HD047905 and U10HD047892.
References
- 1.Chambers CD, Polifka JE, Friedman JM. Drug safety in pregnant women and their babies: ignorance not bliss. Clin Pharmacol Ther. 2008;83(1):181–183. doi: 10.1038/sj.clpt.6100448. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51, e51–e58. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas SH, Yates LM. Prescribing without evidence—pregnancy. Br J Clin Pharmacol. 2012;74(4):691–697. doi: 10.1111/j.1365-2125.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833–840. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyal S, Kim JD, Anderson GD, et al. Atenolol pharmacokinetics and excretion in breast milk during the first 6 to 8 months postpartum. J Clin Pharmacol. 2010;50(11):1301–1309. doi: 10.1177/0091270009358708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Nanovskaya TN, Zhan Y, et al. Pharmacokinetics of metronidazole in pregnant patients with bacterial vaginosis. J Matern Fetal Neonatal Med. 2011;24(3):444–448. doi: 10.3109/14767058.2010.497573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol. 2011;205(1):40, e41–e48. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation. Am J Obstet Gynecol. 2012;207(5):398, e391–e398. doi: 10.1016/j.ajog.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caritis SN, Simhan HN, Zhao Y, et al. Relationship between 17-hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. Am J Obstet Gynecol. 2012 Nov;207(5):396, e391–e398. doi: 10.1016/j.ajog.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caritis SN, Venkataramanan R, Thom E, et al. Relationship between 17-alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2014;210(2):128, e1–e6. doi: 10.1016/j.ajog.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas DM, Quinney SK, McCormick CL, Jones DR, Renbarger JL. A pilot study of the impact of genotype on nifedipine pharmacokinetics when used as a tocolytic. J Matern Fetal Neonatal Med. 2012;25(4):419–423. doi: 10.3109/14767058.2011.583700. [DOI] [PubMed] [Google Scholar]
- 12.Haas DM, Quinney SK, Clay JM, et al. Nifedipine pharmacokinetics are influenced by CYP3A5 genotype when used as a preterm labor tocolytic. Am J Perinatol. 2013;30(4):275–281. doi: 10.1055/s-0032-1323590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng S, Easterling TR, Umans JG, et al. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34(6):660–670. doi: 10.1097/FTD.0b013e3182708edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S, Easterling TR, Hays K, et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol. 2013;76(6):988–996. doi: 10.1111/bcp.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195(4):1081–1085. doi: 10.1016/j.ajog.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Nanovskaya TN, Patrikeeva S, Zhan Y, Hankins GD, Ahmed MS. Transplacental transfer of oseltamivir carboxylate. J Matern Fetal Neonatal Med. 2012;25(11):2312–2315. doi: 10.3109/14767058.2012.693993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanovskaya T, Patrikeeva S, Zhan Y, Fokina V, Hankins GD, Ahmed MS. Transplacental transfer of vancomycin and telavancin. Am J Obstet Gynecol. 2012;207(4):331, e331–e336. doi: 10.1016/j.ajog.2012.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanovskaya TN, Patrikeeva SL, Paul J, Costantine MM, Hankins GD, Ahmed MS. Transplacental transfer and distribution of pravastatin. Am J Obstet Gynecol. 2013;209(4):373, e371–e375. doi: 10.1016/j.ajog.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuppett CD, Zhao Y, Caritis S, Zhang S, Zhao W, Venkataramanan R. Effect of endogenous steroid hormones on 17-alpha-hydroxyprogesterone caproate metabolism. Am J Obstet Gynecol. 2013;208(1):86, e81–e86. doi: 10.1016/j.ajog.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Hemauer SJ, Yan R, Patrikeeva SL, et al. Transplacental transfer and metabolism of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2008;199(2):169, e161–e165. doi: 10.1016/j.ajog.2007.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010;202(4):383, e381–e387. doi: 10.1016/j.ajog.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79(6):921–925. doi: 10.1016/j.bcp.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan R, Nanovskaya TN, Zharikova OL, Mattison DR, Hankins GD, Ahmed MS. Metabolism of 17alpha-hydroxyprogesterone caproate by hepatic and placental microsomes of human and baboons. Biochem Pharmacol. 2008;75(9):1848–1857. doi: 10.1016/j.bcp.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Naraharisetti SB, Liu L, et al. Contributions of human cytochrome P450 enzymes to glyburide metabolism. Bio-pharm Drug Dispos. 2010;31(4):228–242. doi: 10.1002/bdd.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood I, Duan J. Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact. 2009;24(2–4):259–274. doi: 10.1515/dmdi.2009.24.2-4.259. [DOI] [PubMed] [Google Scholar]
- 26.Fischer JH, Sarto GE, Hardman J, et al. Influence of gestational age and body weight on the pharmacokinetics of labetalol in pregnancy. Clin Pharmacokinet. 2014;53(4):373–383. doi: 10.1007/s40262-013-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer JH, Sarto GE, Habibi M, et al. Influence of body weight, ethnicity, oral contraceptives, and pregnancy on the pharmacokinetics of azithromycin in women of child-bearing age. Antimicrob Agents Chemother. 2012;56(2):715–724. doi: 10.1128/AAC.00717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wlodarczyk BJ, Palacios AM, George TM, Finnell RH. Anti-epileptic drugs and pregnancy outcomes. Am J Med Genet Part A. 2012;158a(8):2071–2090. doi: 10.1002/ajmg.a.35438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos-Thompson MA, Hillaire-Buys D, Muller F, et al. Fetal toxic effects of angiotensin II receptor antagonists: case report and follow-up after birth. Ann Pharmacother. 2005;39(1):157–161. doi: 10.1345/aph.1E250. [DOI] [PubMed] [Google Scholar]
- 30.FDA. Reviewer guidance evaluating the risks of drug exposure in human pregnancies. 2005 〈 http://www.fda.gov/downloads/scienceresearch/specialtopics/womenshealthresearch/ucm133359.pdf〉.
- 31.Ali RA, Egan LJ. Gastroesophageal reflux disease in pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):793–806. doi: 10.1016/j.bpg.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham FG, Leveno K, Bloom S, Hauth J, Rouse D, Spong C. Williams Obstetrics. 23. McGraw-Hill Professional; 2009. [Google Scholar]
- 33.Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs. Clin Pharmacokinet. 2009;48(3):159–168. doi: 10.2165/00003088-200948030-00002. [DOI] [PubMed] [Google Scholar]
- 34.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Moya J, Phillips L, Sanford J, Wooton M, Gregg A, Schuda L. A review of physiological and behavioral changes during pregnancy and lactation: Potential exposure factors and data gaps. J Expo Sci Environ Epidemiol. 2014;24(5):449–458. doi: 10.1038/jes.2013.92. [DOI] [PubMed] [Google Scholar]
- 36.Laurberg P, Cerqueira C, Ovesen L, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010;24(1):13–27. doi: 10.1016/j.beem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos. 2010;38(10):1623–1635. doi: 10.1124/dmd.110.033571. [DOI] [PubMed] [Google Scholar]
- 38.Fokina VM, Zharikova OL, Hankins GD, Ahmed MS, Nano-vskaya TN. Metabolism of 17-alpha-hydroxyprogesterone caproate by human placental mitochondria. Reprod Sci. 2012;19(3):290–297. doi: 10.1177/1933719111419248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravindran S, Zharikova OL, Hill RA, Nanovskaya TN, Hankins GD, Ahmed MS. Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons. Biochem Pharmacol. 2006;72(12):1730–1737. doi: 10.1016/j.bcp.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Zharikova OL, Fokina VM, Nanovskaya TN, et al. Identification of the major human hepatic and placental enzymes responsible for the biotransformation of glyburide. Biochem Pharmacol. 2009;78(12):1483–1490. doi: 10.1016/j.bcp.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earhart AD, Patrikeeva S, Wang X, et al. Transplacental transfer and metabolism of bupropion. J Matern Fetal Neonatal Med. 2010;23(5):409–416. doi: 10.1080/14767050903168424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. Placental drug transporters and their role in fetal protection. Placenta. 2012;33(3):137–142. doi: 10.1016/j.placenta.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW. ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos. 2011;39(6):1000–1007. doi: 10.1124/dmd.111.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee N, Hebert MF, Prasad B, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos. 2013;41(12):2225–2232. doi: 10.1124/dmd.113.054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardi G, Cetin I. Human fetal growth and organ development: 50 years of discoveries. Am J Obstet Gynecol. 2006;194(4):1088–1099. doi: 10.1016/j.ajog.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 46.O’Shaughnessy PJ, Monteiro A, Bhattacharya S, Fraser MJ, Fowler PA. Steroidogenic enzyme expression in the human fetal liver and potential role in the endocrinology of pregnancy. Mol Hum Reprod. 2013;19(3):177–187. doi: 10.1093/molehr/gas059. [DOI] [PubMed] [Google Scholar]
- 47.Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21(4):169–175. doi: 10.1002/jbt.20179. [DOI] [PubMed] [Google Scholar]
- 48.Duanmu Z, Weckle A, Koukouritaki SB, et al. Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther. 2006;316(3):1310–1317. doi: 10.1124/jpet.105.093633. [DOI] [PubMed] [Google Scholar]
- 49.Thiele K, Kessler T, Arck P, Erhardt A, Tiegs G. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. J Reprod Immunol. 2013;97(1):128–139. doi: 10.1016/j.jri.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Kulo A, Peeters MY, Allegaert K, et al. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br J Clin Pharmacol. 2013;75(3):850–860. doi: 10.1111/j.1365-2125.2012.04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S, Ou J, Strom S, Mattison D, Caritis S, Venkataramanan R. Identification of enzymes involved in the metabolism of 17alpha-hydroxyprogesterone caproate: an effective agent for prevention of preterm birth. Drug Metab Dispos. 2008;36(9):1896–1902. doi: 10.1124/dmd.108.021444. [DOI] [PubMed] [Google Scholar]
- 52.Clark CT, Klein AM, Perel JM, Helsel J, Wisner KL. Lamotrigine dosing for pregnant patients with bipolar disorder. Am J Psychiatry. 2013;170(11):1240–1247. doi: 10.1176/appi.ajp.2013.13010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17 beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37(9):1841–1847. doi: 10.1124/dmd.109.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73(2):142–149. doi: 10.1212/WNL.0b013e3181a6b325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85(6):607–614. doi: 10.1038/clpt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caritis SN, Hebert MF. A pharmacologic approach to the use of glyburide in pregnancy. Obstet Gynecol. 2013;121(6):1309–1312. doi: 10.1097/AOG.0b013e31829007f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckoreelall K, Cressey TR, King JR. Pharmacokinetic optimization of antiretroviral therapy in pregnancy. Clin Pharmacokinet. 2012;51(10):639–659. doi: 10.1007/s40262-012-0002-0. [DOI] [PubMed] [Google Scholar]
- 58.Sabers A. Algorithm for lamotrigine dose adjustment before, during, and after pregnancy. Acta Neurol Scand. 2012;126(1):e1–e4. doi: 10.1111/j.1600-0404.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 59.Wegner I, Edelbroek P, de Haan GJ, Lindhout D, Sander JW. Drug monitoring of lamotrigine and oxcarbazepine combination during pregnancy. Epilepsia. 2010;51(12):2500–2502. doi: 10.1111/j.1528-1167.2010.02771.x. [DOI] [PubMed] [Google Scholar]
- 60.Fotopoulou C, Kretz R, Bauer S, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85(1):60–64. doi: 10.1016/j.eplepsyres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol. 2008;83:227–240. doi: 10.1016/S0074-7742(08)00013-5. [DOI] [PubMed] [Google Scholar]
- 62.Franco V, Mazzucchelli I, Gatti G, et al. Changes in lamotrigine pharmacokinetics during pregnancy and the puerperium. Ther Drug Monit. 2008;30(4):544–547. doi: 10.1097/FTD.0b013e318178e2a9. [DOI] [PubMed] [Google Scholar]
- 63.Ohman I, Luef G, Tomson T. Effects of pregnancy and contraception on lamotrigine disposition: new insights through analysis of lamotrigine metabolites. Seizure. 2008;17(2):199–202. doi: 10.1016/j.seizure.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 64.Ohman I, Beck O, Vitols S, Tomson T. Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia. 2008;49(6):1075–1080. doi: 10.1111/j.1528-1167.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 65.Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70(22 Pt 2):2130–2136. doi: 10.1212/01.wnl.0000289511.20864.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Longo B, Forinash AB, Murphy JA. Levetiracetam use in pregnancy. Ann Pharmacother. 2009;43(10):1692–1695. doi: 10.1345/aph.1M231. [DOI] [PubMed] [Google Scholar]
- 67.Tomson T, Palm R, Kallen K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48(6):1111–1116. doi: 10.1111/j.1528-1167.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 68.Claessens AJ, Risler LJ, Eyal S, Shen DD, Easterling TR, Hebert MF. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010;38(9):1393–1396. doi: 10.1124/dmd.110.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buchanan ML, Easterling TR, Carr DB, et al. Clonidine pharmacokinetics in pregnancy. Drug Metab Dispos. 2009;37(4):702–705. doi: 10.1124/dmd.108.024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38(1):62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45(1):25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 72.de Oliveira Baraldi C, Lanchote VL, de Jesus Antunes N, et al. Metformin pharmacokinetics in nondiabetic pregnant women with polycystic ovary syndrome. Eur J Clin Pharmacol. 2011;67(10):1027–1033. doi: 10.1007/s00228-011-1053-0. [DOI] [PubMed] [Google Scholar]
- 73.Cressey TR, Best BM, Achalapong J, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013;76(3):475–483. doi: 10.1111/bcp.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Unadkat JD, Wara DW, Hughes MD, et al. Pharmacokinetics and safety of indinavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2007;51(2):783–786. doi: 10.1128/AAC.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartelink IH, Savic RM, Mwesigwa J, et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol. 2014;54(2):121–132. doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patterson KB, Dumond JB, Prince HA, et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013;63(1):51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fayet-Mello A, Buclin T, Guignard N, et al. Free and total plasma levels of lopinavir during pregnancy, at delivery and postpartum: implications for dosage adjustments in pregnant women. Antivir Ther. 2013;18(2):171–182. doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 78.Calza L, Manfredi R, Trapani F, et al. Lopinavir/ritonavir trough concentrations with the tablet formulation in HIV-1-infected women during the third trimester of pregnancy. Scand J Infect Dis. 2012;44(5):381–387. doi: 10.3109/00365548.2011.642306. [DOI] [PubMed] [Google Scholar]
- 79.Lambert JS, Else LJ, Jackson V, et al. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Med. 2011;12(3):166–173. doi: 10.1111/j.1468-1293.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 80.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54(4):381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouillon-Pichault M, Jullien V, Azria E, et al. Population analysis of the pregnancy-related modifications in lopinavir pharmacokinetics and their possible consequences for dose adjustment. J Antimicrob Chemother. 2009;63(6):1223–1232. doi: 10.1093/jac/dkp123. [DOI] [PubMed] [Google Scholar]
- 82.Fang A, Valluri SR, O’Sullivan MJ, et al. Safety and pharmacokinetics of nelfinavir during the second and third trimesters of pregnancy and postpartum. HIV Clin Trials. 2012;13(1):46–59. doi: 10.1310/hct1301-046. [DOI] [PubMed] [Google Scholar]
- 83.Ivanovic J, Nicastri E, Anceschi MM, et al. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr HIV Res. 2009;7(6):620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 84.Read JS, Best BM, Stek AM, et al. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and post-partum. HIV Med. 2008;9(10):875–882. doi: 10.1111/j.1468-1293.2008.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirt D, Urien S, Jullien V, et al. Pharmacokinetic modelling of the placental transfer of nelfinavir and its M8 metabolite: a population study using 75 maternal-cord plasma samples. Br J Clin Pharmacol. 2007;64(5):634–644. doi: 10.1111/j.1365-2125.2007.02885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benaboud S, Ekouevi DK, Urien S, et al. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55(1):331–337. doi: 10.1128/AAC.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamorde M, Byakika-Kibwika P, Okaba-Kayom V, et al. Suboptimal nevirapine steady-state pharmacokinetics during intrapartum compared with postpartum in HIV-1-seropositive Ugandan women. J Acquir Immune Defic Syndr. 2010;55(3):345–350. doi: 10.1097/QAI.0b013e3181e9871b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cressey TR, Stek A, Capparelli E, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and post-partum. J Acquir Immune Defic Syndr. 2012;59(3):245–252. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benaboud S, Hirt D, Launay O, et al. Pregnancy-related effects on tenofovir pharmacokinetics: a population study with 186 women. Antimicrob Agents Chemother. 2012;56(2):857–862. doi: 10.1128/AAC.05244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirt D, Urien S, Ekouevi DK, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109) Clin Pharmacol Ther. 2009;85(2):182–189. doi: 10.1038/clpt.2008.201. [DOI] [PubMed] [Google Scholar]
- 91.Benaboud S, Treluyer JM, Urien S, et al. Pregnancy-related effects on lamivudine pharmacokinetics in a population study with 228 women. Antimicrob Agents Chemother. 2012;56(2):776–782. doi: 10.1128/AAC.00370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stek AM, Best BM, Luo W, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–235. doi: 10.1111/j.1468-1293.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beigi RH, Han K, Venkataramanan R, et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204(6 suppl 1):S84–S88. doi: 10.1016/j.ajog.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karunajeewa HA, Salman S, Mueller I, et al. Pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women. Antimicrob Agents Chemother. 2009;53(10):4368–4376. doi: 10.1128/AAC.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Freeman MP, Nolan PE, Jr, Davis MF, et al. Pharmacokinetics of sertraline across pregnancy and postpartum. J Clin Psychopharmacol. 2008;28(6):646–653. doi: 10.1097/JCP.0b013e31818d2048. [DOI] [PubMed] [Google Scholar]
- 96.Hebert MF, Zheng S, Hays K, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95(7):908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolff K, Boys A, Rostami-Hodjegan A, Hay A, Raistrick D. Changes to methadone clearance during pregnancy. Eur J Clin Pharmacol. 2005;61(10):763–768. doi: 10.1007/s00228-005-0035-5. [DOI] [PubMed] [Google Scholar]
- 98.Van Calsteren K, Verbesselt R, Ottevanger N, et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: a preclinical and clinical study. Acta Obstet Gynecol Scand. 2010;89(10):1338–1345. doi: 10.3109/00016349.2010.512070. [DOI] [PubMed] [Google Scholar]
- 99.van Hasselt JG, van Calsteren K, Heyns L, et al. Optimizing anti-cancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel. Ann Oncol. 2014 doi: 10.1093/annonc/mdu140. (in press) [DOI] [PubMed] [Google Scholar]
- 100.Ryu RJ, Eyal S, Kaplan HG, et al. Pharmacokinetics of doxorubicin in pregnant women. Cancer Chemother Pharmacol. 2014;73(4):789–797. doi: 10.1007/s00280-014-2406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]