ABSTRACT
Objective:
To summarize the best available evidence regarding the effectiveness of interventions for preventing frailty progression in older adults.
Introduction:
Frailty is an age-related state of decreased physiological reserves characterized by an increased risk of poor clinical outcomes. Evidence supporting the malleability of frailty, its prevention and treatment, has been presented.
Inclusion criteria:
The review considered studies on older adults aged 65 and over, explicitly identified as pre-frail or frail, who had been undergoing interventions focusing on the prevention of frailty progression. Participants selected on the basis of specific illness or with a terminal diagnosis were excluded. The comparator was usual care, alternative therapeutic interventions or no intervention. The primary outcome was frailty. Secondary outcomes included: (i) cognition, quality of life, activities of daily living, caregiver burden, functional capacity, depression and other mental health-related outcomes, self-perceived health and social engagement; (ii) drugs and prescriptions, analytical parameters, adverse outcomes and comorbidities; (iii) costs, and/or costs relative to benefits and/or savings associated with implementing the interventions for frailty. Experimental study designs, cost effectiveness, cost benefit, cost minimization and cost utility studies were considered for inclusion.
Methods:
Databases for published and unpublished studies, available in English, Portuguese, Spanish, Italian and Dutch, from January 2001 to November 2015, were searched. Critical appraisal was conducted using standardized instruments from the Joanna Briggs Institute. Data was extracted using the standardized tools designed for quantitative and economic studies. Data was presented in a narrative form due to the heterogeneity of included studies.
Results:
Twenty-one studies, all randomized controlled trials, with a total of 5275 older adults and describing 33 interventions, met the criteria for inclusion. Economic analyses were conducted in two studies. Physical exercise programs were shown to be generally effective for reducing or postponing frailty but only when conducted in groups. Favorable effects on frailty indicators were also observed after the interventions, based on physical exercise with supplementation, supplementation alone, cognitive training and combined treatment. Group meetings and home visits were not found to be universally effective. Lack of efficacy was evidenced for physical exercise performed individually or delivered one-to-one, hormone supplementation and problem solving therapy. Individually tailored management programs for clinical conditions had inconsistent effects on frailty prevalence. Economic studies demonstrated that this type of intervention, as compared to usual care, provided better value for money, particularly for very frail community-dwelling participants, and had favorable effects in some of the frailty-related outcomes in inpatient and outpatient management, without increasing costs.
Conclusions:
This review found mixed results regarding the effectiveness of frailty interventions. However, there is clear evidence on the usefulness of such interventions in carefully chosen evidence-based circumstances, both for frailty itself and for secondary outcomes, supporting clinical investment of resources in frailty intervention. Further research is required to reinforce current evidence and examine the impact of the initial level of frailty on the benefits of different interventions. There is also a need for economic evaluation of frailty interventions.
Keywords: frail older adults, frailty, intervention, prevention, systematic review
GRADE Summary of Findings
1. Clinical/medical component
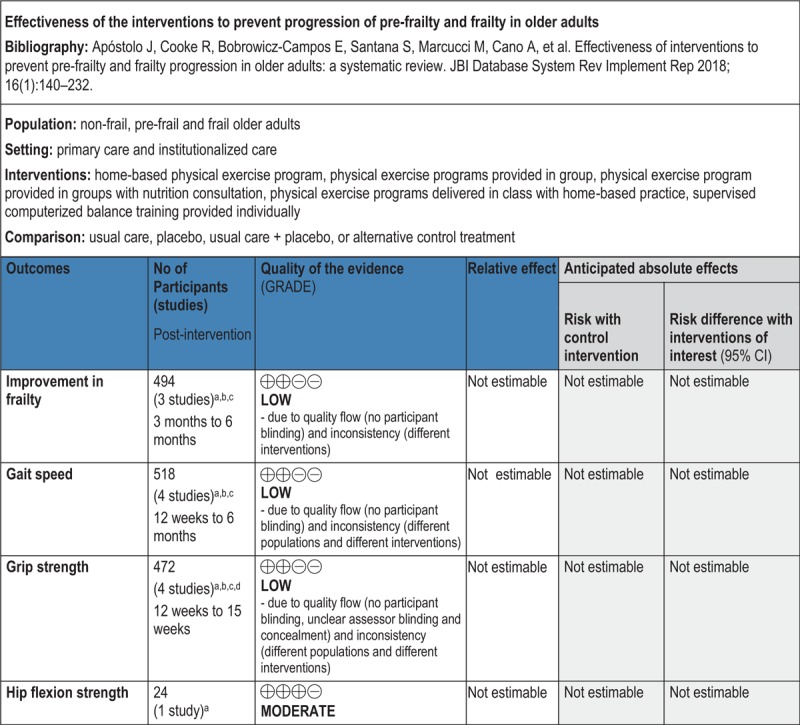
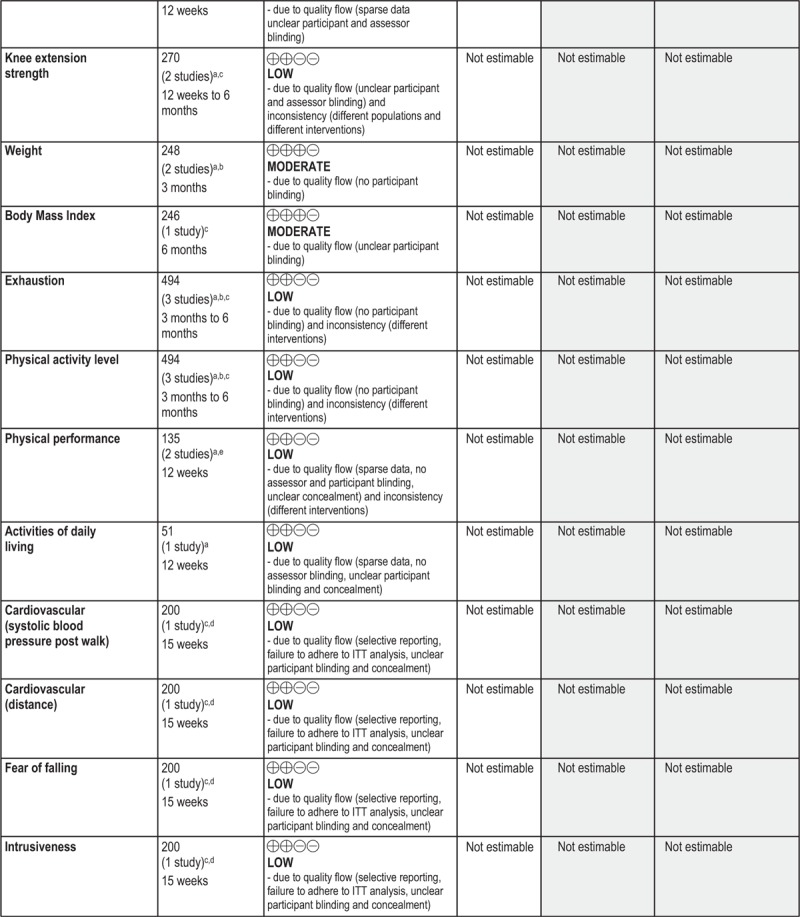
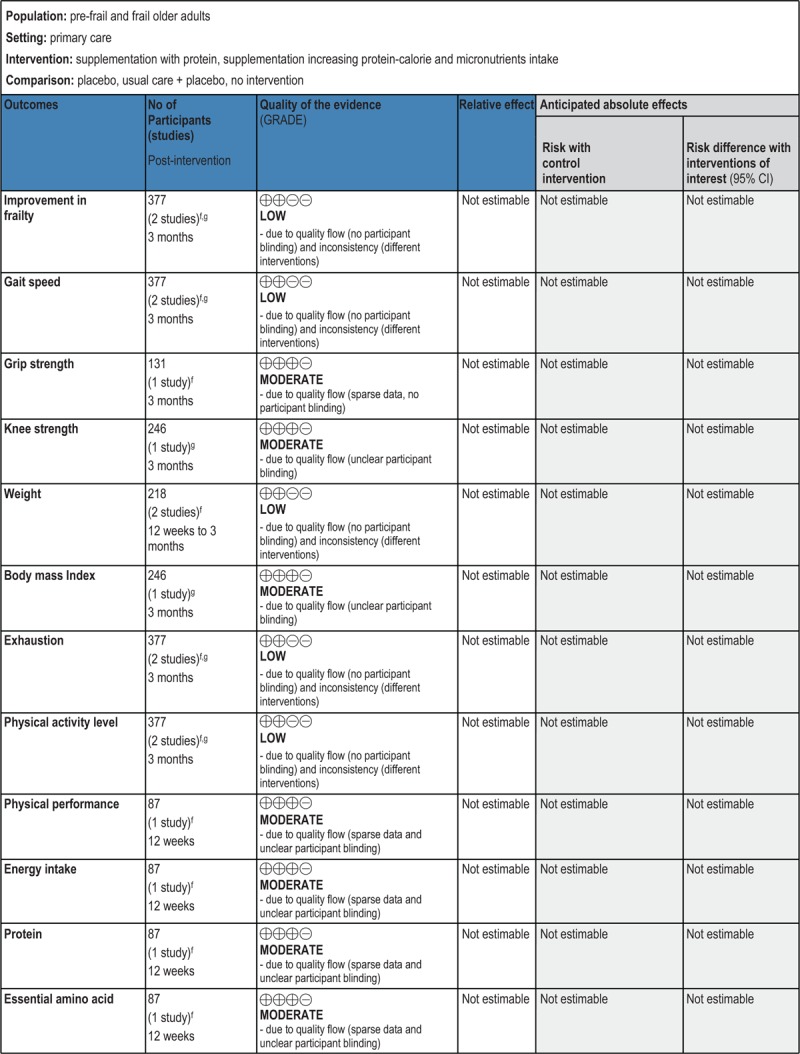
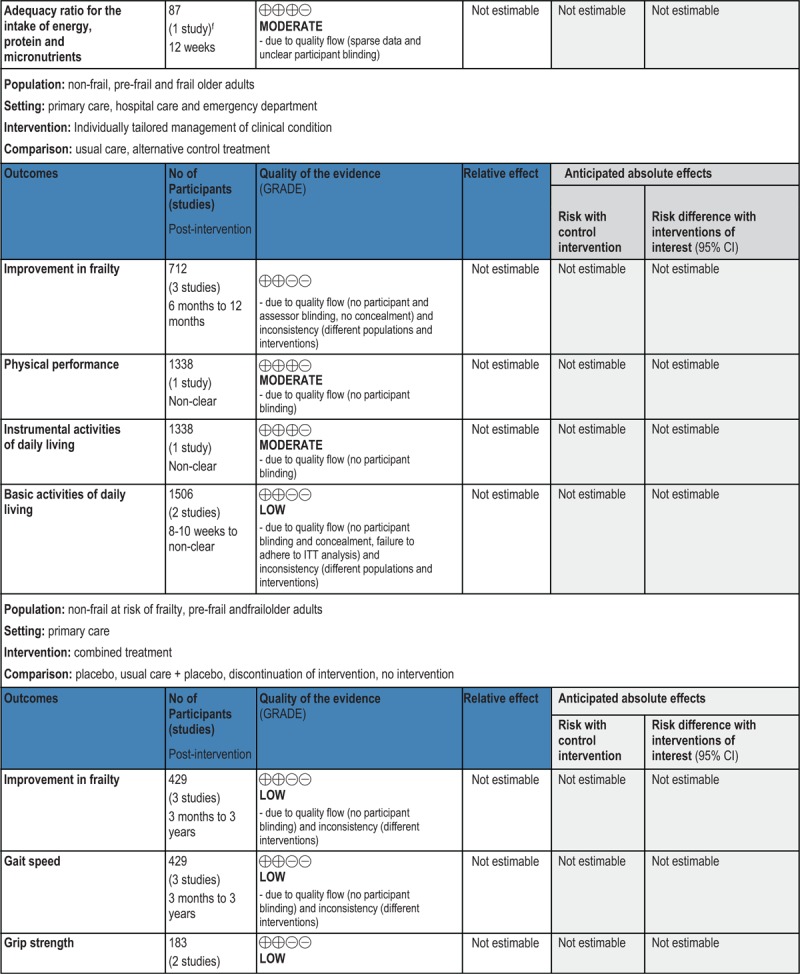
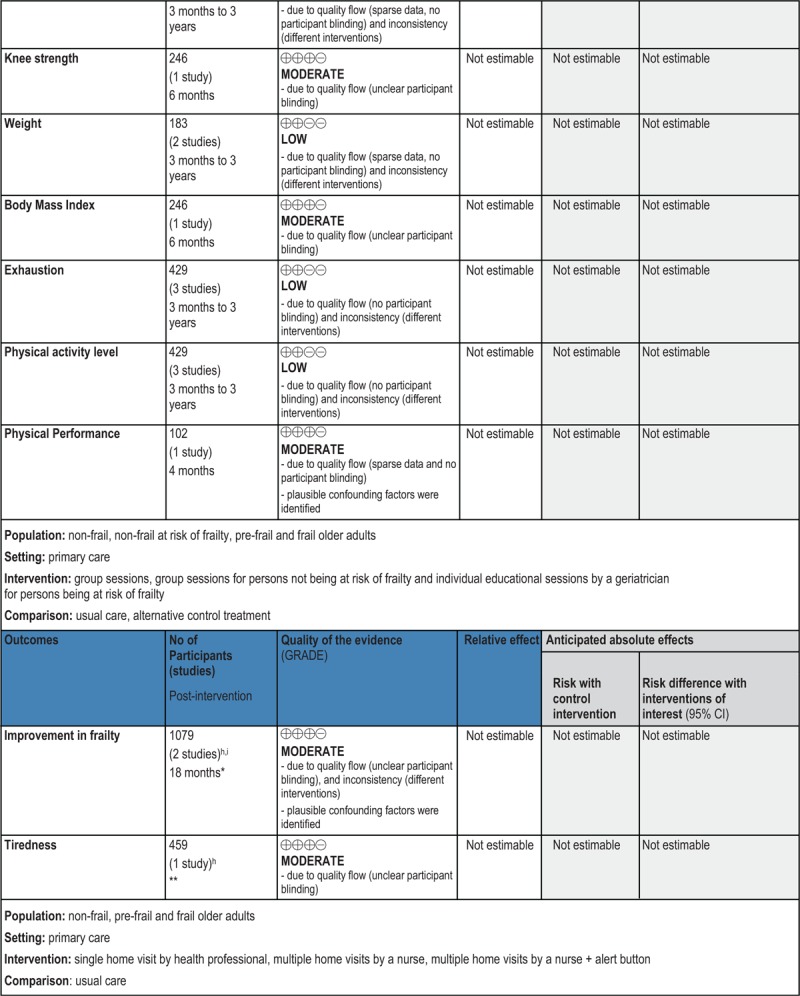
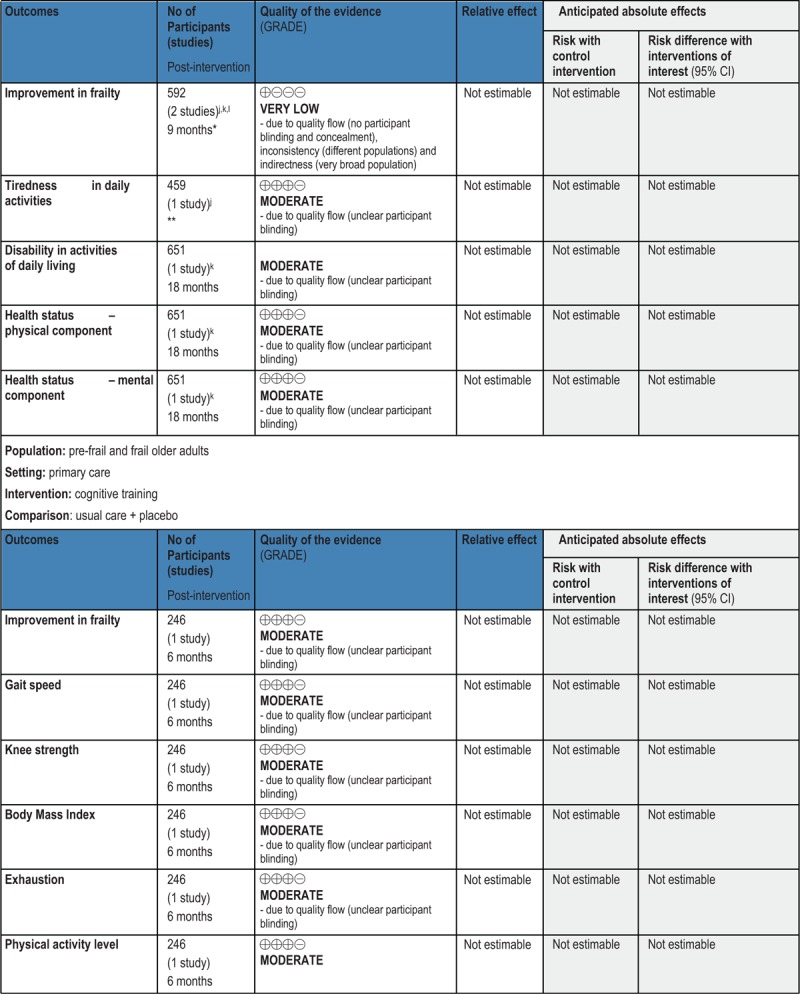
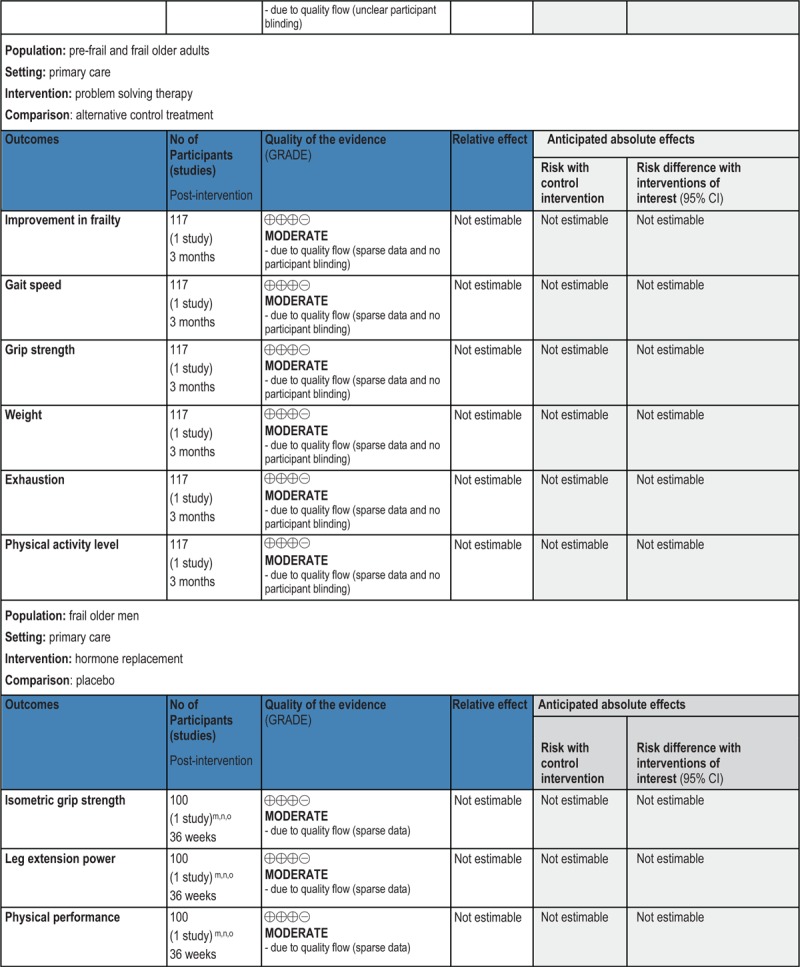

2. Economic component
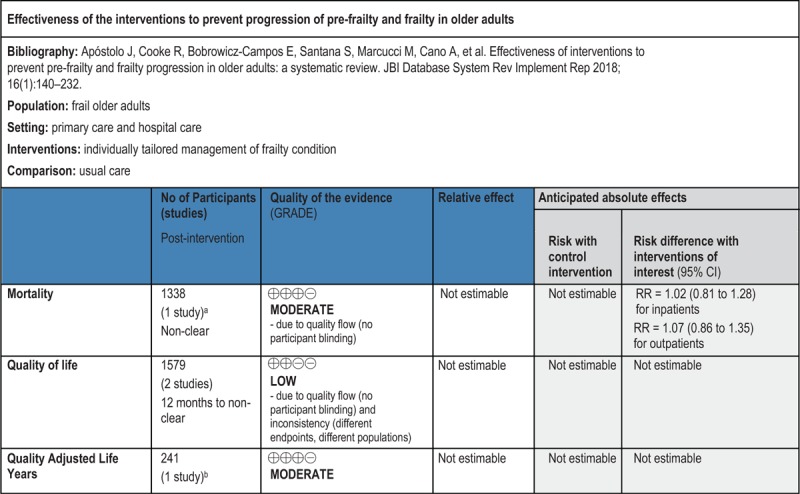
Introduction
Frailty is an age-related state of decreased physiological reserves characterized by a weakened response to stressors and an increased risk of poor clinical outcome.1 Frailty predisposes falls and fractures, disability, dependency, hospitalization and institutional placement, and ultimately leads to death.2 It can be preceded by, but also occurs in the absence of, chronic disease.3,4 According to some authors, this clinical condition results from decrease in reserves across multiple physiological systems that are normally responsible for healthy adaptation to stress.2,5,6 Alternatively, it is considered that frailty is due to critical accumulation of dysregulation in important signaling pathways and subsequent depletion of homeostatic reserve and resilience.2,7,8 Other authors describe this state of increased vulnerability as being associated with the reduced capacity to compensate ageing-related molecular and cellular damage.9 Independently of pathophysiological conceptualization, it is assumed that frailty is a dynamic process that leads to a spiral of decline in various functional domains and that exacerbates risk of geriatric syndromes.2,3,10
The phenotypic markers of frailty, operationalized based on data from the Cardiovascular Health Study (CHS),6 include global weakness with low muscle strength (e.g. poor grip strength), overall slowness (particularly of gait), decreased balance and mobility, fatigability or exhaustion, low physical activity and involuntary weight loss.2,3,6 For diagnostic purposes, at least three of these components must be observed.6 The presence of only one or two of them is considered an indicator of the state of pre-frailty. In a broader approach, it is assumed that frailty can also manifest through cognitive impairment,11-15 although, according to evidence, the decline in cognition is very selective, being limited to executive functions, attention, verbal fluency and processing speed. It is also well documented that frail elders manifest some impairment in activities of daily living and report significant reduction of quality of life.11,16 Furthermore, recent studies have shown that frailty may be related to mood change,15,17 or to social factors such as social support or living alone,11 although the nature of this association, as well as its relevance to the frailty construct, needs to be clarified.16 Based on the comprehensive approach to frailty, several screening and diagnostic instruments have been developed. One of these instruments is the Frailty Index, elaborated within the framework of the Canadian Study of Health and Aging (CSHA).4 This instrument defines frailty in terms of a multidimensional risk state that arises from the interaction of multiple interdependent factors linked to the physical, psychological and social domains of individual functioning, and measures the number of deficits of the person. Presently, several variants of this tool are available in clinical practice.18 The definition of frailty in terms of cumulative deficits is also presented in the Edmonton Frail Scale19 and The Tilburg Frailty Indicator.20 There are also studies that operationalize frailty as a limited set of indicators, such as impairment in activities of daily living (ADLs),21 low physical activity,22 low mobility with poor nutrition23 or others, using for assessment purposes indicator-related scales, measurements or indexes.
Regarding the prevalence of frailty, systematic comparison of numerous studies24 shows that frailty in community dwelling adults aged 65 and over varies from 4% to 17%. In case of pre-frailty, the frequency varies between 19% and 53% in different studies.24 These differences between estimates depend on the operational definition of frailty (based on physical markers or using a broader multidimensional approach) and the population studied (e.g. the results of epidemiological studies can be affected by demographic variables, such as age and gender, as well as the presence of chronic disease or other comorbid conditions).
Because of the frequency of its occurrence and the weight of its consequences, frailty is seen as a threatening condition for older adults, requiring attention from healthcare professionals, social care practitioners, researchers and policy-makers.10,24 The implications of the involvement of these agents can be observed at various levels, with issues related to improving prognosis and preventing deterioration from a pre-frail to frail status of greatest interest and relevance. In relation to interventions, attempts to manage adverse consequences of frailty are often focused on minimization of risk of disability and dependency or the treatment of underlying conditions and symptoms. In a complementary approach, frailty management involves the development of coping strategies, necessary to control potential stress factors or diminish the extent of their impact.25 So far, various types of interventions have been proposed, among them, physical activity, psychosocial intervention, health and social care provision, cognitive stimulation, nutrition, medication/medical maintenance adherence focused intervention, intervention based on information and communication technologies, and multifactorial intervention. The results of studies conducted in this area have indicated that treating frailty in older adults is a realistic therapeutic goal.26-29 However, it is still difficult to determine how effective these types of interventions are and how efficiency can be influenced by other factors, for example, severity of clinical condition, and importantly, which types of interventions are more likely to be effective. It is also unclear whether the interventions for frailty have an impact on clinical outcomes related to drug prescription and analytical parameters (such as results of laboratory analyses, blood tests, etc.).30,31 The focus of attention should also be on economic data, namely, the costs relative to benefits and/or savings associated with implementing interventions for pre-frailty and frailty,32 thus informing clinical decision makers on the likelihood of cost effectiveness.
A preliminary search33 of the JBI Database of Systematic Reviews and Implementation Reports, the Cochrane Database of Systematic Reviews, PROSPERO, CINAHL and MEDLINE, performed during the development of the protocol,34 revealed that there were systematic reviews reporting evidence on the effectiveness of intervention programs in frail older adults.35-38 However, to the best of our knowledge, these reviews have focused only on physical exercise programs and have identified as outcomes of interest physical frailty and/or functional capacity or mobility, without addressing wider domains (e.g. psychological, social) of individual functioning. Additionally, not all of these reviews provided the indication of a clear operational definition or measurement of frailty as a criterion for inclusion, and two of them35,38 were published before 2010. Neither have these reviews provided evidence on the economic effectiveness of the physical exercise-based treatment. Moreover, there is currently no systematic review (neither published nor in progress) on the clinical/medical and economic effectiveness of other types of interventions to prevent or reduce frailty in advanced age. In our opinion, presenting the full spectrum of different types of interventions available in clinical practice could be extremely useful to practitioners for choosing the treatment type. Therefore, it was considered necessary to examine the effectiveness of the interventions to prevent progression of pre-frailty and frailty in older adults, which involves a critical analysis based on scientific evidence. This review was conducted according to an a priori published protocol.34
Review question
The objective of this review was to identify the effectiveness of interventions to prevent progression of pre-frailty and frailty in older adults. More specifically, the review questions were:
What is the effectiveness of interventions in preventing or reducing frailty in older adults?
How does effectiveness vary with degree of frailty?
Are there factors that moderate the effectiveness of interventions?
What is the economic evidence of interventions for pre-frailty and frailty?
Inclusion criteria
Participants
This review considered studies that included older adults (female and male) aged 65 years and over, explicitly identified as pre-frail or frail by researchers or associated medical professionals according to a pre-specified scale or index, and who received health care and support services in any type of setting (primary care network, nursing homes, hospitals). This review excluded studies that included participants selected because of one specific illness or that only considered patients with a terminal diagnosis.
Interventions
The clinical/medical component of the review considered studies that evaluated any type of interventions to prevent progression of pre-frailty and frailty in older adults. These interventions included, but were not limited to, physical activity, multifactorial intervention, psychosocial intervention, health and social care provision, cognitive, nutrition or medication/medical maintenance adherence focused interventions.
The economic component of the review considered studies that performed any type of health economic analysis of interventions to prevent progression of pre-frailty and frailty in older adults.
Comparator
The effectiveness of interventions of interest was compared with usual care, alternative therapeutic interventions or no intervention.
Outcomes
The primary outcome of interest was frailty indicated by any validated scale or measurement or index (e.g. Frailty Index, Fried's frailty criteria based on phenotype model or Edmonton Frailty Scale). We also considered outcomes of frailty assessed by a limited set of indicators, since its operational definition was clearly stated by the authors.
Secondary outcomes included degree of change or no change, indicated by any validated scale or measurement or index, in domains of cognition (e.g. assessed by Mini-Mental State Examination), quality of life (e.g. assessed by EuroQol Group 5-Dimension Self-Report Questionnaire), quality-adjusted life year (QALY) (assessed by comparing length of life with commonly used indicators of quality of life), ADL (assessed by Barthel Index, Katz ADL Index or other), caregiver burden (e.g. Caregiver Burden Inventory), functional capacity (e.g. Physical Activity Scale for Elderly), depression and other mental health-related outcomes (e.g. Geriatric Depression Scale), self-perceived health (e.g. Self-Rated Health), and social engagement (e.g. Scale of Gijón). Secondary outcomes also included change or no change in analytical parameters (e.g. measured by clinical tests), drugs and prescriptions (e.g. indicated by medical records), and prevalence of adverse outcomes, such as falls and fractures, mortality, hospitalization, institutionalization, comorbidities (e.g. indicated by medical records or self-reported).
In addition, costs and/or costs relative to benefits and/or savings associated with implementing the interventions for pre-frailty and frailty were considered.
Types of studies
The clinical/medical component of this review considered for inclusion any experimental study designs that were related to the effectiveness of interventions for pre-frailty and frailty, including randomized controlled trials (RCTs), non-randomized trials and quasi-experimental studies. In the case of absence of RCTs, non-randomized trials and quasi-experimental studies, other research designs of quantitative nature, such as cohort studies, were considered for inclusion.
The economic component of this review considered the inclusion of cost effectiveness, cost benefit, cost minimization or cost utility studies. Any quantitative study measuring clinical effectiveness that incorporated economic data was considered. Studies where the effectiveness of the intervention on frailty levels was not measured were excluded.
Methods
Search strategy
The search strategy aimed to find both published and unpublished studies. A three-step search strategy was utilized in this review. An initial limited search of MEDLINE via EBSCOhost Web and CINAHL was undertaken followed by analysis of the text words contained in the title and abstract, and of the index terms used to describe the article. A second search using all identified keywords and index terms was then undertaken across all included databases. Thirdly, the reference lists of all identified reports and articles were searched for additional studies. Studies published in English, Portuguese, Spanish, Italian and Dutch, from January 2001 to November 25, 2015, were considered for inclusion in this review. The initial timeframe from 2001 was chosen because it is the year of publication of Fried's8 paper that is seen as seminal for research on frailty condition.
The search for published studies included the following electronic databases: CINAHL, MEDLINE, Scopus, Embase, Cochrane Central Register of Controlled Trials and SciELO.
The searched databases for unpublished studies included: ProQuest Theses and Dissertations, OpenGrey, Banco de teses da CAPES (www.capes.gov.br) and Dissertation Abstracts Online (e-Thos).
The initial keywords used in the exploratory stage of the search for studies in electronic databases were: frailty, elder∗, old∗, intervention∗. To capture all available evidence, in the stage of second search additional keywords, considering various terminology and spelling, were used. These final keywords were: frail∗, pre-frail∗, elder∗, old∗, intervention∗, therap∗, treatment∗, program∗, effect∗, efficacy. A detailed record of the search strategies used in the included databases can be found in Appendix I.
Assessment of methodological quality
Reviewers, in pairs, independently screened titles and abstracts prior to retrieving full texts. The full-texts were assessed for eligibility in respect of type of participants, study design and outcomes. At completion of the search process, each paper selected for retrieval was assessed independently by two reviewers for methodological validity prior to inclusion in this systematic review, as originally outlined in the review protocol.34 Any disagreements that arose between the reviewers were resolved through discussion, or with other reviewers. All authors contributed to paper assessments and critical appraisal.
For the purpose of critical appraisal of the studies focusing on the clinical/medical component of this review, the standardized instruments from the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI) were used.33 These instruments included the JBI Critical Appraisal Checklist for Randomized Control/Pseudo-randomized Trial, the JBI Critical Appraisal Checklist for Comparable Cohort/Case Control and the JBI Critical Appraisal Checklist for Descriptive/Case Series, and were chosen accordingly to the study design.
For the purpose of critical appraisal of the studies focusing on the economic component of this review, the standardized instrument from the Joanna Briggs Institute Analysis of Cost, Technology and Utilization Assessment and Review Instrument (JBI ACTUARI) was used,39 namely, the JBI Critical Checklist for Economic Evaluations.
In order to ensure quality of analyzed evidence, a cut-off point for inclusion of studies focusing on the clinical/medical component was applied. Experimental studies were considered as meeting a minimum of quality when they obtained at least five “Yes” ratings on the JBI Critical Appraisal Checklist for Randomized Control/Pseudo-randomized Trial, the JBI Critical Appraisal Checklist for Comparable Cohort/Case Control or the JBI Critical Appraisal Checklist for Descriptive/Case Series. In relation to studies that focused on the economic component of this review, it was decided to include only those in which the effect on clinical outcomes of the intervention was reported with sufficient methodological quality. Simultaneously, the anticipation of the reduced number of such studies resulted in the decision not to apply any additional cut-off point for the JBI Critical Appraisal Checklist for Economic Evaluations, and to analyze the implications of the possible methodological weaknesses related to the economic component in the discussion section.
Data extraction
Data from studies focusing on the clinical/medical component of this review were extracted using the standardized data extraction tool from JBI SUMARI.33 For data extraction from the studies focusing on the economic component of this review, the standardized data extraction tool from JBI ACTUARI39 was applied. In both cases the data extraction process was conducted by two independent reviewers. Disagreements were resolved by discussion to reach consensus.
The extracted data included specific details about the interventions, populations, study methods and outcomes of significance to the review questions and specific objectives. In case of missing or unclear information, the authors of the included studies were contacted.
Data synthesis
Differences in populations, interventions, comparators and outcomes of the included studies focusing on the clinical/medical component of this review did not allow for direct comparison, and therefore meta-analysis was not possible. Consequently, the results of these studies were synthesized in narrative and tabular form.
Significant variability in study methodology was also observed in the studies focusing on the economic component of this review. Due to this variability, it was not possible to combine the economic results in statistical meta-analysis. Results, therefore, have been presented in narrative and tabular form.
Deviation from the protocol
The secondary outcomes indicated in the protocol of this systematic review34 included the outcome of depression, however in the final report other mental health-related outcomes were additionally considered. This deviation is due to the fact that in some cases the depressive symptomatology was evaluated together with symptoms of anxiety or others.
Results
Study selection
The results of the search and study selection process are presented in Figure 1. A total of 4726 potentially relevant studies were identified in the literature search. Of those, 2227 were duplicates. From the remaining 2499 records, 2121 were excluded after title and abstract assessment, and then 346 were excluded after full-text analysis as they did not meet the inclusion criteria. The methodological quality of the remaining 32 studies was assessed. From those 32 studies, one was a pseudo-randomized control trial with two groups, two were before and after studies, two were pseudo-randomized control trials with one group, and 27 were RCTs. Four of those 32 studies (three RCTs and the pseudo-randomized control trial with two groups) provided data related to both clinical/medical and economic components of the interventions. The assessment of methodological quality focused on the clinical/medical component resulted in the exclusion of 11 studies and inclusion of 21 studies. The reasons for study exclusion are detailed in Appendix II.
Figure 1.
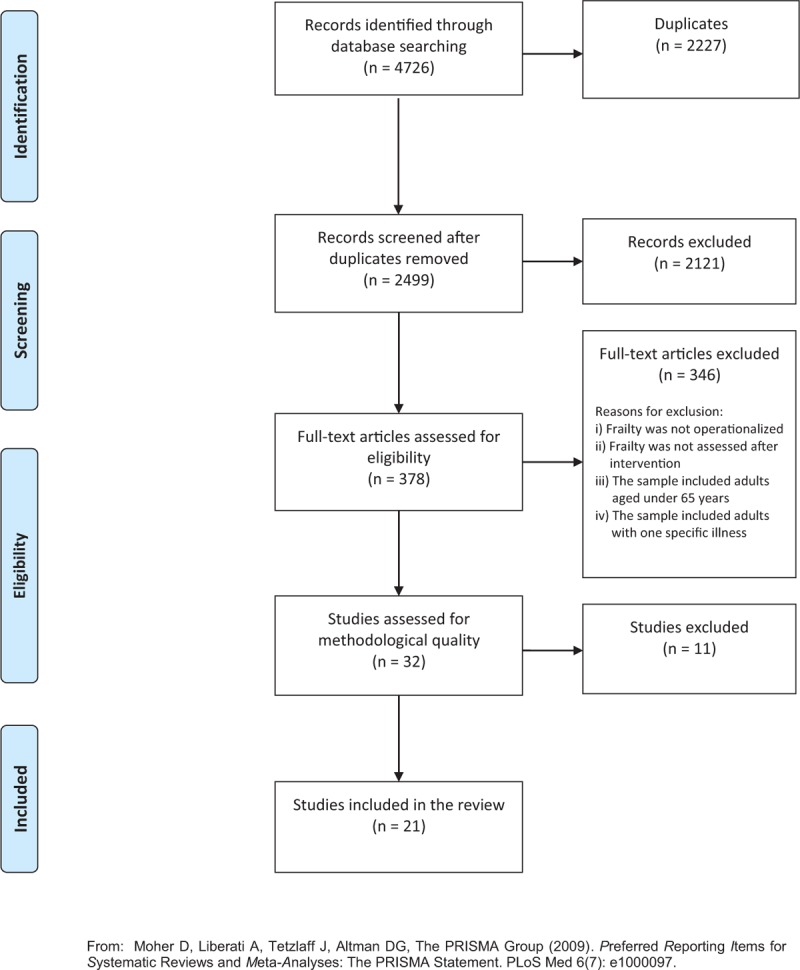
Flowchart of the study selection and inclusion process
All 21 studies21-23,29,32,40-55 included in this review were RCTs. They described a total of 33 interventions. Two of the included RCTs32,44 additionally provided economic data. In one of these studies32 the costs and cost-effectiveness of a multidisciplinary intervention versus usual care were compared. The second study44 analyzed the costs of health services providing geriatric assessment and management with comparison to usual inpatient and outpatient care.
Methodological quality
The reviewers, in teams of two, independently assessed the methodological quality of 32 studies. The authors of 23 studies were contacted to obtain more details in relation to missing or unclear data. Eleven authors replied. Based on the authors’ answers, eight studies were included for further analysis and three studies were excluded as they did not obtain the minimum of five “yes” answers in the critical appraisal checklist. Besides these three studies, eight other failed to reach the cut-off point for inclusion. Appendix II lists the studies that were excluded based on critical appraisal and indicates the reasons for the exclusion. Tables 1 and 2 outline the critical appraisal scores for the included studies.
Table 1.
Assessment of methodological quality of clinical/medical component of included studies
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
| Behm, et al., 201540 | U | U | Y | Y | Y | Y | U | Y | U | Y |
| Bonnefoy, et al., 201222 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Cadore et al., 201441 | Y | U | Y | U | U | Y | N | Y | U | Y |
| Chan et al., 201242 | Y | N | Y | Y | Y | N | U | Y | U | Y |
| Clegg, et al., 201443 | Y | N | Y | Y | N | Y | Y | Y | Y | Y |
| Cohen, et al., 200244 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Eklund, et al., 201329 | U | N | N | Y | N | Y | Y | Y | U | Y |
| Fairhall, et al., 201532 | Y | N | Y | Y | Y | Y | Y | Y | U | Y |
| Favela, et al., 201345 | Y | N | N | Y | Y | Y | Y | Y | U | Y |
| Giné-Garriga, et al., 201046 | U | U | U | Y | N | Y | Y | Y | Y | Y |
| Gustafsson, et al., 201247 | U | U | Y | Y | Y | Y | U | Y | Y | Y |
| Hars et al., 201448 | Y | U | Y | N | Y | N | U | Y | U | Y |
| Kim et al., 201549 | Y | N | Y | Y | Y | Y | U | Y | U | Y |
| Kim & Lee, 201323 | Y | U | Y | Y | Y | Y | U | Y | U | Y |
| Li et al., 201050 | U | U | U | Y | Y | Y | U | Y | U | Y |
| Monteserin et al., 201051 | Y | Y | Y | Y | Y | N | U | Y | N | Y |
| Muller et al., 200652 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Ng, et al., 201553 | Y | U | Y | Y | Y | Y | U | Y | Y | Y |
| Van Hout et al., 201054 | Y | U | Y | Y | Y | N | U | Y | U | Y |
| Vriendt et al., 201621 | U | N | N | N | Y | Y | Y | Y | Y | Y |
| Wolf et al., 200355 | Y | U | U | N | Y | Y | N | Y | Y | Y |
| % | 71 | 14 | 71 | 81 | 76 | 81 | 43 | 100 | 38 | 100 |
N, no; U, unclear; Y, yes.
Table 2.
Assessment of methodological quality of economic component of included studies
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
| Cohen, et al., 200244 | Y | U | N | Y | U | U | U | N | N | N | N |
| Fairhall, et al., 201532 | Y | Y | Y | Y | Y | Y | U | Y | N | N | U |
| % | 100 | 50 | 50 | 100 | 50 | 50 | 0 | 50 | 0 | 0 | 0 |
N, no; U, unclear; Y, yes.
On clinical/medical components, there was consensus among the reviewers to include 21 studies,21-23,29,32,40-55 all of them RCTs. None of these studies obtained 10 “yes” answers in the critical appraisal checklist, and the highest score of nine “yes” answers was obtained by only three studies22,44,52 (see Table 1). The methodological weakness most frequently identified was related to use of participant blinding procedures with regards to treatment assignment, namely, in eight studies21,29,32,42-45,49, the participants were not blind to treatment allocation (Q2), and in 10 studies23,40,41,46-48,50,53-55 the information provided about participant blinding was unclear. Due to the nature of the interventions, the practical difficulties of the blinding process were recognized. In relation to persons assessing outcomes, lack of their blinding with regard to treatment assignment (Q5) was pointed out in four studies,22,29,43,46 and in one study,41 information provided about this issue was unclear. In three studies21,29,45 the allocation to treatment groups was not concealed from the allocator (Q3). There were also three studies46,50,55 in which the information about allocation concealment procedure was insufficiently detailed. The lack of detailed description of randomization procedure (Q1) was detected in six studies.21,29,40,46,47,50 The authors of one study41 were not clear about the statistical treatment of the outcomes of people who withdrew (Q4), and in three studies21,48,55 the analysis of drop-outs was not conducted. In four studies42,48,51,54 the control and treatment groups were not comparable at entry and the baseline differences were not considered in statistical analysis (Q6). Non-identical group treatment other than the intervention of interest (Q7) was observed in two studies41,55; from the remaining 19 studies, ten23,40,42,47-51,53,54 did not provide a clear statement about this issue. In all studies the outcomes were measured in the same way for all groups (Q8) and appropriate statistical analyses were used (Q10). In relation to the reliability of outcome measurement (Q9), in 11 studies23,29,32,40-42,45,48-50,54 unclear or insufficient information to judge this issue was provided, and in two studies51,52 the measures used for the outcomes assessment were not culturally adapted or validated (information provided by the authors of these studies).
In relation to the two included studies with an economic component, both32,44 clearly stated the objective of the study and were placed in a particular decision making context (Q1). Both32,44 also reported solid evidence showing that the clinical effectiveness of the examined intervention had been established (Q4). Detailed description of the intervention and comparator (Q2), measures used for costs and outcomes (Q5), and sufficient explanation about how costs and outcomes were valued (Q6) were provided in only one study.32 The second of the included economic studies44 was unclear in relation to these three questions. Relevant costs and outcomes for each examined intervention, defined accordingly to the objective of the study (Q3), were identified in the study examining the multidisciplinary intervention,32 but not in the study focusing on treatment based on geriatric assessment and management.44 Additionally, there was no incremental analysis conducted of costs and consequences in this second study (Q8).44 Neither of these studies32,44 conducted sensitivity analysis to establish validity of economic results (Q9) or presented sufficient information to answer the questions that users/decision makers would want to know when making decisions about the implementation of the examined intervention (Q10). In addition, a clear report about the adjustment of costs and outcomes for differential timing was not provided in either study (Q7). In relation to the generalizability of the results to other settings with similar characteristics, the study analyzing the multidisciplinary intervention32 was unclear about this issue, and in the study examining the costs of geriatric assessment and management,44 transferability of findings was not discussed.
Characteristics of included studies
Date of publication of the included studies ranged from 2002 to 2016 (with the study from 2016 available online in November 2015) and all were published in English. In the sections below the main features of the included studies are summarized. Detailed information about the setting, methods, participants, interventions, outcomes, authors’ conclusions and limitations are provided in Appendix III.
Study settings
Summary information on the setting and geographical location of the included studies is presented in Table 3. Twelve of included studies were undertaken in Europe (three in Sweden,29,40,47 three in Spain,41,46,51 two in the Netherlands,52,54 one in Belgium,21 one in Switzerland,48 one in the United Kingdom43 and one in France22). From the remaining nine studies, five were undertaken in Asia (Taiwan,42,50 Singapore,53 Japan49 and South Korea23), two in the United States of America,44,55 one in Mexico45 and one in Australia.32 Participants were recruited from the community,21,40,42,47,48,50,52,53 through primary health care centers,46,51,54 medical inpatient and/or outpatient clinics or centers,43-45 an emergency department,29 a long-term care institution,41 rehabilitation facilities,32 an association involved in home assistance for the elderly,22 social security lists,45 national registers,23,49 and local advertisements.55 The interventions described in the included studies were undertaken in the community,21-23,32,40,43,45-48,52-55 primary care centers,51 medical centers,44 community hospitals,42,50 an institute of gerontology,49 the community and hospital,29 and elderly care institutions.41
Table 3.
Setting, geographical location and characteristics of participants of included studies
| Study | Geographical location and setting | Sample | Inclusion and exclusion criteria |
| Behm, et al., 201540Gustafsson, et al., 201247 | Two urban districts in Gothenburg, Sweden Community | N = 459 (64% female)Median age: 85–86 yearsRange of age: 80–97 years | Inclusion: community dwelling older adults aged 80 years or more, living in their ordinary housing, not dependent on the home help service or care arranged by the urban districts, independent in ADLs and cognitively intact (MMSE score ≥25)Exclusion: no exclusion criteria were provided |
| Bonnefoy, et al., 201222 | France Community | N = 102 (86% female)Median age: 84 yearsRange of age: not provided | Inclusion: community dwelling older adults aged 80 years or more*, able to walk without assistance at home, received assistance from a housing association for not more than two hours per week, being at risk of becoming frailExclusion: a cardiovascular event within last 3 months, history of bone fractures, hospitalization, uncontrolled hypertension, dementia or a rapidly evolving disease |
| Cadore et al., 201441 | Pamplona, SpainElderly care institutions | N = 24 (70% female)Mean age: 91.9 (± 4.1) yearsRange of age: not provided | Inclusion: institutionalized older adults aged 85 years or more, that met Fried's criteria for frailtyExclusion: absence of frailty or pre-frailty, dementia, disability (defined as a Barthel Index < 60 and inability to walk independently without help of another person), recent cardiac arrest, unstable coronary syndrome, active cardiac failure, cardiac block, or any unstable medical condition |
| Chan et al., 201242 | Toufen, Taiwan Community hospital | N = 117 (59% female)Mean age: 71.4 (± 3.7) yearsRange of age: 65–79 | Inclusion: community dwelling older adults aged 65 years or more and with frailty (CCSHA-CFS-TV score > 2 and <7)Exclusion: institutionalization; communication barriers; hearing/visual impairments affecting daily activity; cognitive impairment (MMSE score ≤ 16); functional impairment (Barthel Index ≤ 35); active alcohol-abuse problems, organic mental disorders; history of schizophrenia or a diagnosis of a bipolar disorder; any mental problems (other than depression) under psychiatric care; active suicidal ideation; absence of Fried's criteria for frailty |
| Clegg, et al., 201443 | Bradford, United Kingdom Community | N = 84 (71% female)Mean age: 79 (± 9.2) yearsRange of age: not provided | Inclusion: older adults living at home and under the care of a case manager or community matron; housebound; attending a day center or respite care; residing in assisted living sites; being at discharge from intermediate care hospitalsExclusion: being unable to stand and walk independently; currently participating in an alternative exercise program; being registered blind; having poorly controlled angina; having another household member already in the trial; having severe dementia or receiving palliative care |
| Cohen, et al., 200244 | age:United States of AmericaVeterans Affairs Medical Centers | N = 1338 (2% female)Mean age: 74.2 years (SD not provided)Range of age: not provided | Inclusion: older adults aged 65 years or more, hospitalized on a medical or surgical ward, with an expected length of stay of at least two days, considered as being frail and with stable clinical conditionExclusion: conditions of admission from nursing home, receiving care at an outpatient clinic for GEM, previous hospitalization in an inpatient unit for GEM, current enrollment in another clinical trial, severe disabling disease or terminal condition or severe dementia, not English language speaking, lack of access to a telephone (for follow-up), or being unwilling or unable to return for follow-up clinic visits |
| Eklund, et al., 201329 | Mölndal, SwedenSahlgrenska University Hospital and community | N = 161 (55% female)Mean age: not providedRange of age: not provided | Inclusion: older adults who sought care at the emergency department and who were discharged to their own homes, aged 80 years and older or 65 to 79 years, with at least one chronic disease and dependent in at least one ADLExclusion: acute severe illness with immediate need of assessment and treatment by a physician (within ten minutes), dementia (or severe cognitive impairment, clinically assessed by the nurse with geriatric competence at the emergency department), and palliative care |
| Fairhall, et al., 201532 | Australia Community | N = 241 (68% female)Mean age: calculated separately for each group varied from 83.2 (± 5.91) to 83.4 (± 5.81) yearsRange of age: 71–101 years | Inclusion: community dwelling older adults aged 70 years or more, meeting the CHS criteria for frailty, with a life expectancy exceeding 12 months (estimated by Implicit Illness Severity Scale score of 3 or less)Exclusion: residing in a residential aged care facility, having severe cognitive impairment (MMSE score ≤ 18) |
| Favela, et al., 201345 | Ensenada, Baja California, Mexico Community | N = 133 (55% female)Average: 75–76 yearsRange of age: 70–90 years | Inclusion: older adults aged 60 years or more with frailty as indicated by Frailty Index scoreExclusion: no exclusion criteria were provided |
| Giné-Garriga, et al., 201046 | Barcelona area, Spain Community | N = 51 (61% female)Mean age: 84 (± 2.9) yearsRange of age: not provided | Inclusion: older adults aged 80–90 years that meet criteria for frailtyExclusion: conditions of being unable to walk, undergoing an exercise program, a diagnosis of severe dementia (not able to understand or follow verbal commands), or having a stroke, hip fracture, myocardial infarction or hip- or knee- replacement surgery within the previous 6 months |
| Hars et al., 201448 | Geneva, Switzerland Community | N = 52 (98% female)Mean age: 74.6 (± 7.8 years)Range of age: not provided | Inclusion: community dwelling older adults aged 65 years or more, at increased risk of falling (indicated by self-reported falls, balance assessment and frailty phenotype)Exclusion: past experience of Jaques-Dalcroze eurhythmics, except during childhood; self-report of major orthopedic surgery or limb fracture less than 4 months prior to enrollment into the extension study |
| Kim, et al., 201549 | Itabashi ward of Tokyo, JapanTokyo Metropolitan Institute of Gerontology | N = 131 (100% female)Mean age: calculated separately for each group varied from 80.3 (± 3.3) to 81.1 (± 2.8) yearsRange of age: not provided | Inclusion: community dwelling women aged over 75 years, meeting criteria for frailty according to Fried definitionExclusion: severe knee or back pain; severely impaired mobility; impaired cognition (MMSE score < 24); missing baseline data; and unstable cardiac conditions such as ventricular dysrhythmias, pulmonary edema, or other musculoskeletal conditions |
| Kim & Lee, 201323 | Gangbuk-gu, Seoul, South Korea Community | N = 87 (79% female)Mean age calculated separately for each group varied from 78.4 (±6.0) to 78.9 (±5.5) yearsRange of age: not provided | Inclusion: older adults aged 65 years or more, frail, able to walk inside a room and with low socioeconomic statusExclusion: conditions of participation in any exercise program or clinical nutrition program, being ordered to restrict a high protein diet, and being unable to walk or functionally deteriorated |
| Li, et al., 201050 | Taipei, Taiwan Community Hospital | N = 310 (48% female)Mean age: 78.8 (± 8.4) yearsRange of age: 65–106 years | Inclusion: older adults aged 65 or more, categorized as frail or pre-frail according to Fried Frailty CriteriaExclusion: conditions such as being bedridden, receiving home care by visiting nurses, less than 6 months’ life expectancy (such as terminal cancer patients), and difficulty in verbal communication (such as severe cognitive or hearing impairments) |
| Monteserin et al., 201051 | Barcelona, SpainPrimary Health Care Center | N = 620 (60% female)Mean age: 79.9 years (SD not provided)Range of age: 75–94 | Inclusion: older adults aged 75 years or moreExclusion: concurrent inclusion in another study, diagnosis of a terminal disease, institutionalization, severe cognitive impairment, difficulties in accessing the primary health care center and inability or unwillingness to give informed consent |
| Muller et al., 200652 | Rotterdam area, the Netherlands Community | N = 100 (0% female)Mean age: calculated separately for each group varied from 78.2 (± 3.0) to 78.8 (± 3.5)Range of age: not provided | Inclusion: no hospitalized, no diseased, independently living men aged 70 years or more, with low scores on strength tests (isometric grip strength < 30 kg, leg extensor power < 100 Nm)Exclusion: severe arthropathic deformation of the knee joint; myocardial infarction within the last 6 months; history of stroke or transient ischemic attacks; high systolic/diastolic blood pressure; any active malignant disease with significant impact on the physical condition; history of prostatic cancer; diabetes mellitus treated with insulin; abnormal liver function with clinical significance; history of alcohol or drug abuse within the last 2 years; and/or participation in another clinical study |
| Ng, et al., 201553 | Southwest region of Singapore Community | N = 246 (61% female)Mean age: 70 (± 4.7) yearsRange of age: not provided | Inclusion: community dwelling older adults aged 65 years or more, meeting CHS criteria for frailty or pre-frailty, able to ambulate without personal assistance, and living at homeExclusion: significant cognitive impairment (MMSE score ≤ 23); major depression; severe audiovisual impairment; any progressive, degenerative neurologic disease; terminal illness with life expectancy <12 months; participation in other interventional studies; or being unavailable to participate for the full duration of the study |
| Van Hout et al., 201054 | The Netherlands Community | N = 651 (71% female)Mean age: calculated separately for each group varied from 81.3 (± 3.9) to 81.5 (± 4.3) yearsRange of age: not provided | Inclusion: community dwelling older adults aged 75 years or more, lived at home and frail (based on COOP-WONCA charts)Exclusion: terminal illness (as determined by primary care physicians); dementia (self-report of memory deterioration, MMSE < 24 or 7-min screen > 50%); living in residential homes; participating in other research projects |
| Vriendt et al., 201621 | East-Flanders, a province in Flanders region, the Dutch speaking part of Belgium Community | N = 168 (80% female)Mean age: calculated separately for each group varied from 79.9 (± 6.3) to 80.9 (± 7.3) yearsRange of age: not provided | Inclusion: community dwelling older adults aged 65 years or more, single, receiving healthcare support, Dutch speaking and having one or more functional problems in basic ADL, operationalized by the BEL-profile scaleExclusion: incontinence as the sole basic ADL problem, suffering dementia (based on the diagnosis of a physician) and already receiving community based occupational therapy prior to this study |
| Wolf et al., 200355 | Atlanta, United States of America Community | N = 200 (81% female)Mean age: calculated separately for each group varied from 75.4 (± 4.1) to 76.9 (± 4.8) yearsRange of age: not provided | Inclusion: community dwelling older adults aged 70 years or more, living in unsupervised environments and being ambulatoryExclusion: the presence of debilitating conditions such as severe cognitive impairments, metastatic cancer, crippling arthritis, Parkinson's disease or major stroke, or profound visual deficits that could compromise balance or ambulation |
*One participant was 78 years old.
ADL, activities of daily living; CCSHA-CFS-TV, Chinese Canadian Study of Health and Ageing – Clinical Frailty Scale (telephone version); CHS, Cardiovascular Health Study; GEM, geriatric evaluation and manage:ment; MMSE, Mini-Mental State Examination.
In relations to studies reporting economic evidence, one32 was conducted in Australia and included participants who sought care at the emergency department and were discharged to their own home. The other study44 was conducted in the United States of America and was set in Veterans Affairs Medical Centers with established inpatients and outpatients programs of evaluation and management.
Participants
The 21 studies analyzed in this review included a total of 5275 older adults. The number of study participants ranged from 24 (in the study comparing a multicomponent exercise program with mobility exercises)41 to 1338 (in the study comparing inpatient care in the evaluation and management unit with usual inpatient care, and outpatient care in the evaluation and management clinics with usual outpatient care).44 In two studies,40,47 the same sample was considered.
The age range of studied samples was reported in seven studies,32,40,42,45,47,50,51,55 and was 65 to 106 years. For the remaining 14 studies the verification of the age-related criterion for inclusion (age of 65 years and over) defined by the authors of this review was based on the analysis of inclusion criteria provided by the authors of primary studies. Sixteen studies21,23,32,41-44,46,48-55 reported the mean age of their samples; however in six of these studies21,23,32,49,52,54 only data calculated separately for each group was provided. A mean age of total samples varied from 70 (± 4.7)53 to 91.9 (± 4.1)41 years. From the remaining five studies, three22,40,47 provided median age that varied from 8422 to 85–8640,47 years, one study indicated an average age of 75–76 years, and in one study29 the information about mean, median or average age was missing. One study45 considered older adults aged 60 years or more as eligible for inclusion; however the age of the included participants ranged from 70 to 90 years, satisfying the inclusion criteria of this review.
Gender was reported in all studies. In a total review sample of 5275 older adults, approximately 49% were women. One study used only male participants52 and one study used only female participants.49 Two studies44,50 included more men than women. In one of these44 only two percent of participants were female. In the second50 the proportion of female and male participants was more balanced, being 48:52. The remaining studies21-23,29,32,40-43,45-48,51,53-55 included more women than men, with the proportion of female participants ranging from 55%29,45 to 98%.48
In ten studies23,32,41,44-46,49,52,54,55 the condition of being frail was mandatory for inclusion. Three studies42,50,53 included both pre-frail and frail older adults, and one study22 only included older adults at risk of frailty. In six studies29,40,43,47,51,55 the baseline level of frailty was assessed but inclusion in the study did not depend on the presence or absence of a frailty condition. However, in one of these studies51 the condition of being at risk of frailty (or not) influenced the treatment of participants who were allocated to the intervention group. There was also one study48 that included older adults at increased risk of falling, with frailty considered as one of the indicators of this risk. The characteristics of the participants and criteria for inclusion and exclusion used in the analyzed studies are described in Table 3.
The economic study examining the cost-effectiveness of a multifactorial interdisciplinary intervention with comparison to usual care32 was conducted with 241 community dwelling older adults, predominantly female (68%) and aged71 to 101 years. All the participants included in this study met the criteria for frailty. The study that analyzed the costs of health services providing geriatric assessment and management with comparison to usual inpatient and outpatient care44 was delivered to 1338 older adults, predominantly male (98%), with a mean age of 74.2 years, and who were hospitalized on a medical or surgical ward. In this study the condition of being frail was not mandatory for inclusion.
Frailty definition
The included studies used different operational definitions of frailty. The definition cited most frequently, in nine studies,32,41,42,45,47-50,53 was the one based on the CHS phenotypic indicators of frailty, including weakness, fatigue/exhaustion, weight loss, low physical activity and slowness. One study additionally considered poor balance.47 Importantly, the operationalization of frailty indicators differed from study to study. For example, in five studies, weakness was measured using grip strength,32,42,45,47,49 and in one study53 through knee extension in the dominant leg. In the remaining three studies,41,48,50 detailed information was not provided. The stratification of weakness measurement by gender was conducted in five studies,32,42,45,47,53 and by body mass index (BMI) in only two studies.42,53 Finally, the cut-off score for grip strength varied from 13 kg47 to 21 kg42 for women and from 21 kg47 to 32 kg42 for men.
One45 of the studies using CHS phenotypic indicators of frailty also used the Frailty Index of Rockwood et al.,56 integrating 34 variables, with a cut-off score of 0.14. Comprehensive assessment of frailty was also conducted by other authors.29,40,43,44,51,54,55 For example, in two studies44,51 frailty was assessed using information on functional and cognitive status, falls, dependence in ADL, depression, malnutrition, incontinence, polypharmacy and comorbidity, and in one study43 changes in functional and cognitive status were considered. Another study54 defined frailty in terms of self-reported scores in the worst quartile of at least two of six COOP-WONCA charts (an instrument used in primary care settings worldwide that allows quick identification of functional health status57), including overall health, physical fitness, changes in health, daily activities, mental health and social activities. In two studies,29,40 frailty measurements took into account eight indicators, such as weakness, fatigue/exhaustion, weight loss, low physical activity, slowness, poor balance, visual impairment and cognitive impairment, with one of these studies40 using complementary measurement of tiredness in daily activities. The last of the listed studies55 identified frailty by the presence of biomedical, functional and psychosocial indicators.
Some studies21-23,46,52 based frailty assessment on a very limited set of indicators. These indicators included: the presence of low gait speed and/or poor physical activity,22 the presence of poor physical activity and self-reported exhaustion,46 the presence of low mobility and poor nutrition,23 the presence of weakness and changes in physical performance (physical frailty),52 and impairment in basic ADL functioning.21Table 4 summarizes information about frailty assessment conducted in the analyzed studies.
Table 4.
Definitions of frailty used in the included studies, measured outcomes and assessment tools
| Study | Operational definition of frailty/pre-frailty used by authors of included studies | Measured outcomes* | Tools and time-points assessment |
| Behm, et al., 201540 | Excessive tiredness in daily activities assessed by Mob-T Scale (indicated by affirmation of being too tired to perform the activity) and presence of at least three of eight core frailty indicators: - weakness (< 13 kg for women and < 21 kg for males for the right hand, and < 10 kg for women and < 18 kg for males for the left hand) - fatigue (affirmation of suffering general fatigue over the last 3 months) - weight loss (affirmation of having weight loss over the last 3 months) - low physical activity (1–2 walks/week or less) - poor balance (Berg's balance scale score ≤ 47) - gait speed (walking four meters or less in 6.7 s) - visual impairment (visual acuity < 0.5 in both eyes using the KM chart) - cognition (MMSE score < 25) | FrailtyDeterioration in frailty from baseline based on sum of frailty indicators and tiredness in daily activities | Mob-T ScaleMaximal hand grip strengthGothenburg quality of life instrument (symptom scale)Gothenburg quality of life instrument (symptom scale)Number of walks for weekBerg's balance scaleGait speedKM chartMMSEAssessment at baseline, and one year and two years after intervention |
| Bonnefoy, et al., 201222 | Presence of low gait speed (< 0.8 m/second) and/or poor physical activity (Physical Activity Scale for the Elderly score < 64 for men and < 52 for women) | Physical activityMaximal weekly walking time and distanceFunctional outcomes - walking speed - ADLs - instrumental ADLs - mobilityNutritional outcomesSafety outcomes - injuries during exercise - emergency hospitalization - transfer to long-stay institutions | PASEGait speedTime up-and-go testOne-minute chair-rise countSix-step climb timeFat free Mass and Body Mass indexthe Mini Nutritional AssessmentDietary intakeAssessment at baseline and after four-month study |
| Cadore et al., 201441 | Presence of three or more frailty indicators, as defined by Fried et al. (2001): - weakness - exhaustion - weight loss - low physical activity - slowness | Functional statusDual task performanceIncidence of fallsMaximal isometric and dynamic strengthMuscle powerMuscle cross-sectional areaMuscle tissue attenuation | Time up-and-go testsDual tasksFICSIT-4 tests of static balanceBarthel IndexQuestionnaire of falls incidenceMaximal isometric and dynamic strength and muscle power using 1-repetion maximum testComputer tomography scans using 64-row CT scannerAssessment at baseline and after 12-week intervention |
| Chan et al., 201242 | Frailty indicated by score 3–6 on CCSHA-CFS-TV and then by score ≥ 1 on CHS-PCF. Frailty indicators defined as: - weakness (≤ 29–32 kg for men and ≤ 17–21 kg for women, depending on BMI) - exhaustion (affirmative response for statements “I felt that everything I did was an effort” and “I could not get going” and indication that this situation was present at least occasionally or more frequent) - weight loss (unintentional weigh loss of > 3 kg or > 5% of body weight in the previous year) - low physical activity (weekly energy expenditure for activities ≥ 2 metabolic equivalent tasks of fewer than 383 kcal for men and 270 for women) - gait speed (five-meter walking time ≥ 7 s for men with height ≤ 173 cm or ≥ 6 s for men with height > 173 cm; and walking time ≥ 7 s for women with height ≤ 159 cm or ≥ 6 s for women with height > 159 cm) | Changes in frailtyHealth-related outcomes - cognitive function - mental disorders - ADL - health care re source utilization - health-related quality of lifeComplex body composition and musculoskeletal system domain - body mass index - fat free mass (Inbody 3.0®, as a substitute of lean body mass) - lowest T score from spine and hip bone mineral density - left one-leg-stand time - dominant leg extension powerBlood chemistry - 25(OH) Vitamin D | Maximal hand grip strengthCES-D (two questions)Self-report of weight lossTaiwan International Physical Activity Questionnaire Short FormGait speedMMSEPrimary Care Evaluation of Mental DisordersBarthel IndexHealth care re source utilization questionnaireEQ-5DAssessment at baseline, at the end of intervention (three months after baseline assessments), and six and 12 months after baseline assessmentsFor MMSE, bone mineral density and 25(OH) Vitamin D, data was collected only at baseline and 12 months later. |
| Clegg, et al., 201443 | Score > 8 in the Edmonton Frailty Scale that samples 10 domains, including cognitive impairment, functional ability and mobility, measured using the Timed up-and-go test | Basic mobility and functional abilityADLsHealth-related quality of lifeDepression | Edmonton Frailty ScaleTimed up-and-go testEQ-5DGeriatric Depression Scale – Short Form 15Assessment at baseline and at 14 weeks post-randomizationEdmonton Frailty Scale was administrated only at baseline |
| Cohen, et al., 200244 | Presence of at least two of following criteria: - inability to perform one or more basic ADL - a stroke within the previous three months - a history of falls - difficulty walking - malnutrition - dementia - depression - one or more unplanned admissions in the previous three months - prolonged bed rest - incontinence | SurvivalHealth-related quality of life - dimensions of physical functioning, physical limitations, emotional limitations, bodily pain, energy, mental health, social activity, general healthFunctional status - ability to perform basic and instrumental ADLs - physical performance | SF-36Katz Index of ADLFillenbaum brief measure of instrumental ADLsPhysical Performance TestAssessment at baseline, immediately after intervention, and 12 months after randomization: |
| Eklund, et al., 201329 | Presence of more than two frailty indicators: - weakness (<13 kg for women and <21 kg for men for the dominant hand, and < 10 kg for women and <18 kg for men for the non-dominant hand) - fatigue (affirmation of suffering general fatigue or tiredness over the last 3 months) - weight loss (affirmation of having weight loss over the last 3 months) - low physical activity (1–2 walks/week or less) - poor balance (Berg's balance scale score ≤ 47) - gait speed (walking four meters or less in 6.7 s) - visual impairment (visual acuity < 0.5 in both eyes using the KM chart) - cognition (MMSE score < 25) | Changes in levels of frailtyADL | Maximal hand grip strengthGoteborg quality of life instrument (symptom scale)Goteborg quality of life instrument (symptom scale)Number of walks for weekBerg's balance scaleGait speedKM chartMMSEKatz ADL IndexAssessment at baseline, and at three-, six - and 12-month after discharge |
| Fairhall, et al., 201532 | Presence of three or more Fried's frailty criteria: - weakness (grip strength ≤18 kg for women and ≤ 30 kg for men) - fatigue (affirmative response for statements “I felt that everything I did was an effort” and “I could not get going” and indication that this situation was present a moderate amount of time or most of the time) - weight loss/shrinking (self-report of unintentional weight loss ≥ 4.5 kg in previous 12 months or loss of ≥ 5% of weight in prior year by direct measurement of weight) - low physical activity (in the past three months not performing weight-bearing physical activity, spending more than four hours per day sitting or going for a short walk once per month or less) - slowness (≥ 6 seconds to walk four meters, with or without a walking aid) | FrailtyHealth-related Quality of Life | Maximal hand grip strengthCES-D (two questions)Self-report of weight lossPhysical activity questionnaireGait speedEQ-5DAssessment at baseline, at three and 12 months |
| Favela, et al., 201345 | 1. Score ≥ 0.14 in Frailty Index integrating 34 variables (Rockwood et al., 2001)2. Presence of three or more Fried's frailty criteria: - weakness (grip strength <17 kg for women and < 30 kg for men) - fatigue (affirmative response for statements “I felt that everything I did was an effort” and “I could not get going” and indication that this situation was present a moderate amount of time or most of the time) - weight loss (unintentional weight loss of 4.5 kg in the prior year or after 9-month of follow-up) - low physical activity (evaluated by International Physical Activity Questionnaire and indicating the following pattern of activity: less than three days of vigorous-intensity activity of at least 20 minutes per day, less than five days of moderate-intensity activity and walking less than 30 minutes per day) - slowness (inability to walk 8 feet or taking more than 7 seconds to walk this distance) | Frailty | Frailty IndexMaximal hand grip strengthCES-D (two questions)Self-report of weight lossInternational Physical Activity QuestionnaireGait speedAssessment at baseline and in the final phase nine months laterFrailty Index was administrated only at baseline |
| Giné-Garriga, et al., 201046 | Presence of:1. poor physical ability - walking along a 3-m course and back at a quick comfortable pace/rapid gait test > 10 seconds - failing to stand up five times from a seated position in a hardback chair with arms folded2. and self-reported exhaustion - affirmative response for statements “I felt that everything I did was an effort” and “I could not get going”, and cumulative score ≥ 2 | Physical frailty - ADL - gait speed - balancePhysical performance - balance - speed - strength - mobility | Barthel IndexRapid-gait testStand-up testBalance tests - semitandem - tandem - single legLower Body Strength testModified Timed up-and-go testAssessment at baseline, week 12 and week 36 |
| Gustafsson, et al., 201247 | Sum of six core frailty indicators: - weakness (grip strength < 13 kg for women and < 21 kg for males for the right hand, and < 10 kg for women and < 18 kg for males for the left hand) - fatigue (affirmation of suffering general fatigue over the last 3 months) - weight loss (affirmation of having weight loss over the last 3 months) - low physical activity (1–2 walks/week or less) - poor balance (Berg's balance scale score ≤ 47) - slow gait speed (walking four meters or less in 6.7 s) | FrailtySelf-rated healthADL | Maximal hand grip strengthGothenburg quality of life instrument (symptom scale)Gothenburg quality of life instrument (symptom scale)Number of walks for weekBerg's balance scaleGait speedSelf-Related Health QuestionnaireADL cumulative scale focusing on nine personal and instrumental activitiesAssessment at baseline and at 3-month follow-up |
| Hars et al., 201448 | Presence of at least one of Fried's frailty criteria: - low grip strength - exhaustion - unintentional weight loss - low physical activity - slow walking speed | FrailtyPhysical activity level - Gait - Balance - StrengthFunctional performanceFall historyNutritional StatusQuality of lifeAnxietyDepressionCognitive functionSelf-rated health statusMedications | Stride length variabilityUsual gait speedOne-legged stanceTimed up-and-go testFive-Times-Sit-to-Stand-TestStructured face-to-face interviewsMini-Nutritional Assessment Short Form12-Item Short Form Health SurveyHospital Anxiety and Depression ScaleMMSEClock-drawing testSelf-rated healthOriginal assessment at baseline and after one year of interventionExtension study assessment: four years after original trial enrolment |
| Kim, et al., 201549 | Presence of three or more Fried's frailty criteria: - weakness (grip strength <19 kg) - fatigue (affirmative response for statements “I felt that everything I did was an effort” and “I could not get going”) - weight loss (unintentional weight loss > 2–3 kg in the last 6 months, or > 1–1.5 kg post-intervention, or > 1.3–2 kg at follow up) - low physical activity (affirmative response to at least 3 of the following 4 statements: “I regularly takes walks less than once a week”, “I do not exercise regularly”, “I do not actively participate in hobbies or lessons of any sort”, “I do not participate in any social groups for elderly people or volunteering”) - slowness (usual walking speed < 1.0 m/s) | Frailty statusBody composition (muscle mass, bone mineral density, body fat)Functional FitnessHematological Parameters (BDNF, IGF-I, IGFBP-3, serum myostatin) | Maximal hand grip strengthCES-D (two questions)Self-report of weight lossPhysical activity questionnaireGait speedInterview SurveyDual-energy X-ray absorptiometryKnee extension strengthWalking speedTimed up-and-go testHuman BDNF, IGF-I and IGFBP-3 Quantikine ELISA kitsHuman Myostatin ELISA kitAssessment at baseline, after three-month intervention and at four-month follow-up after intervention |
| Kim & Lee, 201323 | Presence of low mobility (usual gate speed < 0.6 m/second) and poor nutrition (Mini Nutritional Assessment score < 24) | Functional statusFunctional performancePhysical performanceWeakness (grip strength)Nutritional statusMobilityAdverse effects | Physical Functioning testsShort Physical Performance Battery testsTimed up-and-go test and one-legged stanceMaximal hand grip strengthDietary intake assessed by three non-consecutive 24-hour recallsGait speedSign or symptom that the participant complained about after initiation of nutritional supplementAssessment at baseline and after 12-week intervention |
| Li, et al., 201050 | Sum of five frailty indicators as defined by Fried (2001): - unintentional weigh loss of at least 4.5 kg in the previous year - self-reported exhaustion - weaknesses (grip strength) - slow walking speed - low physical activity | FrailtyFunctional Status including ADL | Frailty assessment as defined by Fried (2001) – without detailed descriptionBarthel IndexAssessment at baseline and six months later |
| Monteserin et al., 201051 | Presence of at least two of the following conditions: - age of 85 years or more - Gijón Social Scale score ≥ 9 - Pfeiffer Scale score ≥ 2 - Yesavage Depression Scale score ≥ 1 - Charlson Comorbidity Index score ≥ 2 - Barthel Index score ≥ 91 - Mininutritional Assessment Short Form score ≥ 12 - polimedication (higher than the mean number of drugs taken by the study population) - having fall history in the last six months (> one fall in the last six months) - having daily urinary incontinence in the last six months | Functional statusInstrumental ADLsCognitive statusDepressionNutritional statusSocial support evaluationComorbidityComposite outcome of all causes of death, admissions to nursing home facilities and admissions to a home care progra | Scale of GijónPfeiffer ScaleYesavage Depression scaleCharlson Comorbidity IndexBarthel IndexLawton IndexMininutritional Assessment Short FormFalls registerAssessment at baseline and at the end of the study (after 18-month intervention) |
| Muller et al., 200652 | Physical frailty measured by means of specific test battery, including isometric grip strength, leg extensor power and physical performance | FrailtyADLCognitive functionBone mineral densityBody compositionAtherosclerosisBlood pressureHormone levels | Grip strengthLeg extensor powerPhysical performance - standing balance - walking speed - chair riseModified Stanford Health Assessment QuestionnaireMMSE7.5-MHz linear array transducerx-ray absorptiometryBlood samples analyzed by RIA using commercial kitsAssessment at baseline and on the end point one to four times in 36 week. |
| Ng, et al., 201553 | Sum of five frailty indicators as defined by Fried (2001): - unintentional weigh loss (BMI: weight/height2 <18.5 kg/m2 or self-reported unintentional weight loss ≥ 10 pounds (4.5 kg) in the last 6 months) - self-reported exhaustion (composite score < 10 on 3 questions: “Did you feel worn out?,” “Did you feel tired?,” “Did you have a lot of energy?,” with appropriate reversed scorings). - weaknesses (muscle strength assessed by knee extension in the dominant leg; lowest quartile of values stratified for BMI and sex was used to denote weaknesses) - slowness (6-meter fast gait speed test; the lowest quintile of values stratified for height and age was used to denote slowness) - low activity (frequency and duration of six different activities in the past two weeks; the lowest quintile was used to classify participants with low activity)Presence of one or two symptoms indicates pre-frailty, presence of three symptoms or more indicates frailty | Changes in frailty statusChanges in frailty componentsChanges in frequency of hospitalizationsChanges in frequency of fallsDependency in instrumental ADLs | Self-reported weight loss or BMIGait speedPhysiological Profile Assessment (weakness component)Medical Outcomes Study SF-12 scale (vitality domain)Longitudinal Ageing Physical Activity QuestionnaireSelf-report of falls and hospitalizationsIndex of instrumental ADLsAssessment at baseline and at three-, six - and twelve months |
| Van Hout et al., 201054 | Frailty defined by self-reported score in the worst quartile of at least two of six COOP-WONCA charts: - overall health (score ≥ 4) - physical fitness (score ≥ 5) - changes in health (score ≥ 4) - daily activities (score ≥ 4) - mental health (score ≥ 3) - social activities (score ≥ 3)Scoring range varied from 1(excellent) to 5 (very bad). | Functional statusDisability in ADL and instrumental ADLHospital admittanceTime until placement in nursing homes or homes for disabled older personsTime until death | COOP-WONCA charts measuring functional healthSF-36Groningen Activity Restriction ScaleLocal hospital registry, supplemented with self-report dataPrimary care physicians medical records, hospital database and nursing homes registriesAssessment at baseline and at six and 18 monthsGroningen Activity Restriction Scale was administrated only at baseline and at 18 months |
| Vriendt, et al., 201621 | Impairment in the basic ADL functioning | Basic ADLsHealth-related Quality of Life - dimensions of physical functioning, physical role functioning, bodily pain, mental health, vitality | BEL-profile Scale/WHO – questionnaireSF-36Assessment at baseline and at the end point of the program (between 8 and 10 weeks after randomization) |
| Wolf et al., 200355 | Frailty identified by the presence of biomedical, functional and psychosocial indicators | Biomedical outcomes - strength - flexibility - cardiovascular endurance - body compositionFunctional outcomes - instrumental ADLPsychosocial well-being outcomes - depression - fear of falling - self-perception of present and future health - quality of sleep and intrusivenessFalls and injurious falls incidence | Functional performance testsHeart rate and blood pressure records after 12-minute walkSkinfold measuresLawton and Brody IADL scaleCES-DFear of falling questionnaireQuestionnaire related to psychosocial outcomesNicholas MMT 0116 muscle tester (Lafayette Instruments)The average force developed from three contractions using the Jamar Smedley-type hand dynamometer (Therapeutic Equipment Corporation)Assessment at baseline, after 15-week intervention, and at four-month follow upAssessment of heart rate, blood pressure and skinfold thickness only before and after intervention |
*The outcomes measured only at baseline were not included.
ADL, Activities of daily living; BDNF, Brain-derived neurotrophic factor; IGF-I, Insulin-like growth factor 1; IGFBP-3, insulin-like growth factor-binding protein 3; CCSHA-CFS-TV, Chinese Canadian Study of Health and Ageing – Clinical Frailty Scale (telephone version); CES-D, Center for Epidemiological Studies - Depression Scale; CHS-PCF, Cardiovascular Health Study Phenotypic Classification of Frailty; EQ-5D, EuroQol Group 5-Dimension Self-Report Questionnaire; IADL, Instrumental activities of daily living; KM chart, visual acuity chart; MMSE, Mini-Mental State Examination; PASE, Physical Activity Scale for the Elderly; SF-36, Medical Outcomes Study 36-Item Short-Form General Health Survey.
Interventions/comparators
The interventions examined in the included studies were categorized as physical exercise programs (n = 7),41-43,46,49,53,55 nutritional supplementation (n = 3),23,49,53 hormone replacement (n = 1),52 individually tailored management of clinical condition (n = 5),21,29,32,44,50 group sessions (n = 3),40,47,51 home visits (n = 4),40,45,47,54 psychological therapy (n = 1),42 cognitive training (n = 1),53 individual educational session by a geriatrician (n = 1),51 and combined treatment (n = 4).22,48,49,53 Two studies40,47 included the same sample and examined the same experimental interventions (multi-professional senior group meetings with one home visit and single preventive home visit), differing in the measured outcomes and in the time point of the outcomes assessment. Control conditions used for comparison purposes were as follows: usual care,29,32,43-45,51,54 usual care with education,46 education,42,55 usual care with placebo,53 placebo,49,52 screening evaluation without further management of individual needs,50 community services,21,40,47 and mobility exercises.41 In one study48 the control group included participants who discontinued experimental intervention. Finally, in two studies,22,23 detailed description of the control interventions was missing. In one of these studies,23 the preventive effect of protein-energy supplementation was examined and the control group did not receive nutritional supplement. The second study22 investigated the preventive effect of home-based exercise program with dietary protein supplementation, without providing any information about the control intervention. More detailed information regarding both interventions and comparators is provided in Table 5.
Table 5.
Characteristics of the interventions described in included studies
| Study | Experimental condition | Control condition | Duration of intervention |
| Behm, et al., 201540Gustafsson, et al., 201247 | Multi-professional senior group meetings with one home visit Meetings with no more than six participants in each group, conducted by an occupational therapist, a physiotherapist, a registered nurse and a qualified social worker, focused on information and discussion about the aging process and possible health consequences and providing strategies for solving various problems that may arise in the home environment. The content of the group discussions varied according to the attending participants’ individual experiences and needs. After group meetings one follow-up home visit was provided.Single preventive home visitVisit from a trained professional (occupational therapist, physiotherapist, registered nurse or qualified social worker) including verbal and written information and advice about (i) local meeting places, activities run by local associations, physical training for seniors, and other services; (ii) kinds of help and support offered by volunteers or municipal professionals; and (iii) availability of assistive devices and housing modifications. Environmental fall risks in the home were identified, and advice on how to prevent them was included. | Ordinary range of community servicesServices offered by the municipal agency for care for the aged and provided when requested. They may include meals on wheels, help with cleaning and shopping, assistance with personal care, safety alarms, transportation services, and health care. | Duration of intervention: four weeksMulti-professional senior group meetings included four weekly meetings with 2-hour duration and a follow-up home visit conducted 2–3 weeks after the meetings.Preventive home visit was held once and had duration of 1.5 - 2 hours. |
| Bonnefoy, et al., 201222 | Home based exercise program with dietary protein supplementationEvery dose of protein supplements contained 80% milk, soy and alfalfa protein, 10 g protein including 3.49 g of branched amino acids (2.41 g L-Leucin, 0.51 g L-isoleucin, 0.57 g L-valin), and 44.3 kcal.Exercises program included: (i) flexibility exercises (rotation of the neck to the right and left, flexion/extension, right and left turns of the trunk in a sitting position, and hip and shoulder movements); (ii) strength exercises (contraction of the back muscles, arm pushes while sitting, calf raises, elevation of the hips, and the get-up-and-go test); (iii) balance exercises (one-leg stands, sideways and tandem walking). For endurance, participants were also advised to walk for pleasure as often as possible.Before the intervention the physiotherapist prescribed the exercises and gave a booklet explaining how to perform these exercises and how to fill in compliance diaries. He/she also explained how to add protein supplements to regular food, and delivered the supplements for 1.5 months. During the intervention period home helpers encourage participants to exercise, verify that protein supplements were taken correctly, and make sure the diary was filled out. | No intervention | Period of intervention: four months.Each exercise session was supposed to last approximately 20 minutes and be performed once a day. |
| Cadore et al., 201441 | Multicomponent exercise programMulticomponent exercise intervention composed of lower and upper body resistance training with progressively increased loads that optimized the muscle power output, combined with balance and gait retraining exercises that progressed in difficulty and functional exercises. All training sessions were supervised by one experienced physical trainer. The training sessions included 5 min of warm-up, 10 min balance and gait retraining, 20 min of resistance training, and 5 min of stretching (cool-down). A minimum of 2 days elapsed between consecutive training sessions. To reduce the participant dropout, music was played during all sessions. | Mobility exercisesExercises consisted of small active and passive movements applied as a series of stretches in a rhythmic fashion to the individual joints. | Period of intervention in experimental group: 12 weeks.Multicomponent exercise sessions with duration approximately of 40 minutes, performed twice a week. A minimum of 2 days elapsed between consecutive training sessions.Period of intervention in control group: 12 weeks.Mobility exercise sessions with duration of 30 minutes per day, performed at four days per week. |
| Chan et al., 201242 | Exercise and nutrition consultationThe program included warm up exercise (15 minutes) with brisk walks followed by gentle stretching of major joints and muscles for 5 repetitions each (10 minutes). Resistance training (20–30 min) with rubber band and bottled water (0.6–1L) as weight for major muscles of upper and lower limbs with 10 to 15 repetitions for each. Postural control activities and balance training were also provided (10 minutes) by asking participants to perform tandem gaits and one leg standing with eyes open/close, step up and down stairs, toe walking and heel walking. Finally a cool down session (5 minutes) with gentle relaxation movements are done. During exercise sessions the participants were inquired about their dietary compliance and their dietary questions were answered.Problem solving therapyParticipants received therapy by trained case managers. This therapy focuses on how to solve the “here-and-now” problems contributing to participants’ mood-related conditions and helps increase their self-efficacy. | Educational bookletBooklet on frailty, healthy diets, exercise protocols, and self-coping strategies were given to participants. The participants were contacted monthly to check on how much they had read the booklet and how well they had complied with the suggested diet and exercise protocols. | Period of intervention: three monthsExercise and nutrition: thrice-weekly sessions with duration of one hourProblem solving therapy: 6 sessionsEducational booklet: once a month |
| Clegg, et al., 201443 | Home-based exercise programProgram was delivered by community-based physiotherapists. Its core components are strengthening exercises for the muscle groups required for basic mobility skills. These exercises do not require special equipment and that can be performed without professional supervision; however they are graded in three levels, being their prescription dependent on participants’ individual ability. The number of exercise repetition increases with improvement of performance.Participants receive weekly support from physiotherapists through five face-to-face home visits and seven telephone calls. | Usual careParticipants continued to receive usual care from the primary healthcare team and, other than baseline and follow-up assessments, had no contact with the research team. | Period of intervention: 12 weeks.Participants were requested to complete the routine exercise with duration < 15 minutes three times a day on five days of the week. |
| Cohen, et al., 200244 | Inpatient geriatric care in multidisciplinary evaluation and management unitsMultidisciplinary team consisted of a geriatrician, a social worker, and a nurse followed their standard protocols for geriatric evaluation and management, with specific instructions to complete the history taking and physical examination; develop a list of problems; assess the patient's functional, cognitive, affective, and nutritional status; evaluate the caregiver's capabilities; and assess the patient's social situation. The team met at least twice a week to discuss the plan of care. Preventive and management services (e.g., dietetics, physical and occupational therapy, and clinical pharmacy) were coordinated to address the problems identified, with a general emphasis on maintaining the patient's functional status.Usual inpatient care followed by care at outpatient geriatric clinicParticipants received all appropriate hospital services except for those provided by the team on the geriatric evaluation and management unit. | Usual inpatient care followed by usual outpatient careParticipants received all appropriate hospital services and after discharge were provided with at least one follow-up appointment in an appropriate clinic. | Not clear |
| Eklund, et al., 201329 | Continuum care by multi-professional teamMulti-professional team for care and rehabilitation included professionals in nursing with geriatric competence (emergency department), occupational therapy, physiotherapy and social work (municipality). Continuum care components were: (i) frailty screening and geriatric assessment at emergency department; (ii) case-management in the municipality; (iii) hospital care and/or rehabilitation at hospital if needed; (iv) tracking of the patients in hospital wards and/or in the municipality; (v) care planning; (vi) rehabilitation in the municipality if assessed as needed at care planning; (vii) follows-up other than research, within a week after care planning and then at least every month for a year.Continuum of care had a person-centered approach and was created for the older person from the emergency department, through the hospital ward and on to their own homes. | Usual careOrdinary care including hospital care and/or rehabilitation at hospital if needed, care planning by multidisciplinary team (only for participants with need of hospital care), rehabilitation in the municipality if assessed as needed at care planning, follows-up other than research. | Not clear |
| Fairhall, et al., 201532 | Multifactorial interdisciplinary intervention targeting identified frailty characteristicsThe intervention, delivered by an interdisciplinary team (two physiotherapists, a geriatrician, rehabilitation physician, dietician, and nurse), was individualized to each participant based on the frailty criteria present. It incorporated the principles of geriatric evaluation and management (including medication review and management of chronic health conditions). The participants also received visits from physiotherapists, and were prescribed a home program of lower limb balance and strength exercises. When needed, dietician assessment and management was provided. In addition, regular interdisciplinary case-conferences were conducted. | Usual careUsual care from community services and general practitioner, that may include assessment and delivery of care needs, and medical and allied health management. | Period of intervention: 12 months.Participants received 10 physiotherapy visits and were prescribed a home program of exercises to be undertaken for 20 to 30 minutes 3 to 5 times per week for 1 year. |
| Favela, et al., 201345 | Nurse home visits aloneDuring the intervention medical history was performed and areas of potential improvement were identified. Then, possible lifestyle changes were discussed with patients and their relatives or caregivers (whenever possible) and specific methods to achieve these changes were negotiated. In addition, subjects’ pharmacological treatment was reviewed and adherence was encouraged.Nurse home visits including alert buttonsThe same as above. In addition, patients could contact their nurses whenever they felt the need by pressing the alert button.In both conditions, patients continued to receive usual care from family physicians at the clinic. | Usual careUsual care at the Family Medicine Clinic | Period of intervention: nine months.Nurse home visits were held weekly. |
| Giné-Garriga, et al., 201046 | Functional circuit-training programSupervised intervention based on a combination of functional (static and dynamic) balance and strength-based exercises. Balance exercises were of increasing complexity, and when an easier step was achieved without assistance, the individual went on to perform the next more complex set of exercises. In case of strength exercises (rising from a chair, stair climbing, knee bends, floor transfer, lunges, leg squat, leg extension, leg flexion, calf raise, and abdominal curl using ankle weights), the number of repetitions and then the load were increased. Every session began with a warm-up, walking at usual pace for 10 min, and ended with cool-down, stretching for 5 min.During the exercise period, participants were instructed to continue their routine daily activities and not perform any new exercise except for the interventional program. | Health education meeting and usual careFour sessions including health topics relevant to older adults, such as nutrition, medication use, foot care, sleep hygiene, and other health-related areas.Usual care from the primary-care practice provided whenever needed.Participants were asked to continue their routine daily activities. | Period of intervention: 12 weeks.Functional circuit-training program: conducted twice a week/every session with duration of 45 minutes.Health education meeting: conducted once a week/every session with duration of 60 minutes. |
| Hars et al., 201448 | Continued intervention of music-based multitask training The original trial consisted of 6-month music-based multitask exercise program based on Jaques-Dalcroze eurhythmics (a music education through movement method). This program included varied multitask exercises involving multiple-task practice which highly challenged motor-, cognitive- and social-related abilities, and was performed to the rhythm of improvised piano music.Extension study was held in various community locations, under the supervision of certified instructors who were involved in the original trial. Each class consisted of a warm-up followed by varied multitask exercises of progressive difficulty, sometimes involving the handling of objects (e.g., percussion instruments), performed individually, in pairs or more. Basic exercises consisted of walking following the piano music, responding directly or oppositely to changes in music's rhythmic patterns, phrases, form or other aspects. | Discontinued intervention of music-based multitask trainingDiscontinued participation after the original trial completion. | Period of original trial: 6 months.Period of extension study: 4 years, over 45 weeks per year.Sessions with duration of one hour were conducted once a week. |
| Kim, et al., 201549 | Milk fat globule membrane (MFGM) supplementationThe supplement composition was 21.5% protein, 44.0% fat, 26.5% carbohydrate, 33.3% phospholipids, 6.4% ash, and 1.6% moisture. Each pill contained 167 mg of MFGM, and six pills (total 1 g) were ingested in the mornings, prior to activity. The pills were yogurt-flavored. In addition, participants were asked to fill out a daily diary on which they recorded whether or not they took the full amount of the supplement. These diary sheets were collected every two weeks.Exercise + placebo Training program of moderate intensity was conducted by one instructor and two assistant trainers in four small groups. The exercise session included a five minute warm-up, 30 minutes of strengthening exercises, 20 minutes of balance and gait training, followed by a five minute cool-down. The strengthening exercises were performed in a progressive sequence from the seated to standing positions, and progressive resistance was applied through the use of the Thera-bands, and increasing repetition of each time of exercise. Resistance or progression was only increased on a group basis, when no significant fatigue or loss of proper execution was observed.The placebo pills were of similar shape, taste, and texture of the MFGM pills, and they included whole milk powder (26.3% protein, 25.2% fat, 39.5% carbohydrate, 0.286% phospholipids, 5.7% ash, and 3.3% moisture) instead of MFGM.Exercise + milk fat globule membrane (MFGM) supplementationParticipants in this group underwent exercise program and take MFGM supplementation as described above. | PlaceboThe placebo group followed the same protocol as the MFGM supplementation group. The pills were of similar shape, taste, and texture of the MFGM pills, and they included whole milk powder (26.3% protein, 25.2% fat, 39.5% carbohydrate, 0.286% phospholipids, 5.7% ash, and 3.3% moisture) instead of MFGM. | Period of intervention: three months.Exercise program: conducted twice a week/every session with duration of 60 minutes.MFGM supplements and placebo: given daily. |
| Kim & Lee, 201323 | Protein-energy supplementationParticipants were provided two 200-mL cans of commercial liquid formula (additional 400 kcal of energy, 25g of protein, 56g of carbohydrate 9.4g of essential amino acids, 9 g of lipid, 400 mL of water, and micronutrients) per day. Compliance was measured every 2 weeks during a home visit by the research dietitian. The participants were clearly instructed not to replace their usual meal with the liquid supplement; rather, they were encouraged to use the supplement to increase overall food intake. | No interventionThe participants did not receive any treatment or counseling during the study period, and home healthcare services provided by National Home Healthcare Services workers were suspended. The participants were visited by research dietitian and received small gift every month. | Period of intervention: 12 weeks.Nutritional supplements were given daily. |
| Li, et al., 201050 | Screening evaluation and appropriate intervention based on screening resultsScreening evaluation was based on comprehensive geriatric evaluation. Two board-certified geriatricians independently reviewed the participants’ assessment results along with their present and past medical histories, current medication, and recent laboratory data. The intervention programs were conducted by medical professionals at the community hospital, as well as at appropriate community facilities. They included medication adjustment, exercise instruction, nutrition support, physical rehabilitation, social worker consultation, and/or specialty referrals. | Screening evaluationScreening evaluation was based on comprehensive geriatric evaluation. | Not clear |
| Monteserin et al., 201051 | Recommendation about healthy habits and adherence to treatment in group sessions After comprehensive geriatric assessment, patients at non-risk of frailty were provided with recommendations about health promotion, disease prevention and self-care through the group session led by a trained nurse. They patients were also given the booklet containing health recommendations.Individual sessions with geriatricianAfter comprehensive geriatric assessment, patients at risk of frailty received an individual educational session by a geriatrician. The geriatrician informed each patient about specific health areas that could be improved through lifestyle changes, developing a shared plan to emphasize the reduction of disability raising main aspects like drug therapy, sensory impairment, instability and falls, incontinence aids, dietary modifications, inclusion in physical exercise programs, participation in senior center activities and psychological counselling. The geriatrician included in the medical record a health report detailing specific recommendations for evaluation and management that could be of interest to the patient's General Practitioner and nurse. | Usual careStandard care from the General Practitioner. | Period of intervention: interventions consisted of individual sessions, the period between the assessment and the intervention is not clearGroup session with duration of 45 minutes.Individual session with duration of over 30 minutes. |
| Muller et al., 200652 | Atamestane + dehydroepiandrosterone (DHEA)Participants received a combination of atamestane (100 mg/d) and DHEA (50 mg/d). For each treatment period of 28 days, the volunteer received two glasses with 28 tablets each. Subjects were instructed to take the drugs during breakfast. To endure compliance, volunteers were required to return empty glasses and the remaining trial medication at each clinical visit. A pill count that indicated an overall compliance of less than 80% was registered as noncompliance.DHEAParticipants received DHEA (50 mg/d) and placebo. The protocol of the trial was the same as described for atamestane + DHEA group.AtamestaneParticipants received atamestane (100 mg/d) and placebo. The protocol of the trial was the same as described for atamestane + DHEA group. | PlaceboParticipants received two placebo tablets that had an outer appearance identical with that of either atamestane tablets or DHEA tablets. The protocol of the trial was the same as described for atamestane + DHEA group. | Period of intervention: 36 weeksTablets were taken on each day of the treatment period without a treatment-free interval during the 36 weeks. |
| Ng, et al., 201553 | Nutritional supplements A multi-fiber commercial formula, iron and folate supplement, vitamin B6 and vitamin B12 supplement, and calcium and Vitamin D supplement, designed to augment caloric intake by about 20% and provide about one third of the recommended daily allowances of vitamins and minerals, were administrated by interventional nurse. Given the variability in individual energy requirements, participants were encouraged to attain the maximal tolerable energy intake to gain 0.5 kg per week.Physical training The exercise program included resistance exercises integrated with functional tasks; and balance training exercises involving functional strength, sensory input, and added attentional demands. These exercises were of moderate, gradually increasing intensity, and tailored to participants’ individual abilities. They were conducted by a qualified trainer. After 12 weeks participants were encouraged to continue the exercise program at home.Cognitive training In the first 12 weeks participants participated in cognitive-enhancing activities designed to stimulate short-term memory (learning strategies), and enhance attention and information-processing skills (tasks such as “spot the differences,” categorical naming, and coding), and reasoning and problem solving abilities (matrix reasoning exercises, mazes, and tangram-like games). In the subsequent 12 weeks “booster” sessions, focusing on the revision of the cognitive skills learned in the first 12 weeks, were conducted.Combination treatmentParticipants in this group underwent all three aforementioned interventions | Standard care + placeboStandard care from health and aged care services, including primary and secondary level care from government or private clinics and hospitals, and community-based social, recreational, and daycare rehabilitation services.Placebo capsules contained nondairy creamer, liquid caramel, sugar, and water, and were identical in appearance to the active nutritional supplements. They were administrated by interventional nurses. Participants were instructed to not replace their meals with supplements. | Period of intervention: six months.Nutritional supplements and placebo: taken daily.Physical training: 90-minute sessions conducted on two days per week during first 12 weeks; and individual sessions at home, supposed to be performed daily, during subsequent 12 weeks.Cognitive training: two-hour weekly sessions during first 12 weeks; and two-hour fortnightly sessions during subsequent 12 weeks. |
| Van Hout et al., 201054 | Proactive home visits by trained community nursesThe home-visits program had a preventive function and consisted of (a) the assessment of the care needs with a multidimensional computerized geriatric instrument, which enabled direct identification of health risks; (b) identification of care priorities together with the person, with focus on home safety, fall prevention, medication adherence, and health promotion; (c) designing and execution of individually tailored care plans; (d) involvement of other visiting health professionals to add notes to the care plan; (e) execution and monitoring participants by telephone and on average three home visits, evaluation of changes in care needs and adaptation of the care plan when needed. | Usual careUsual care could involve visits from primary care physician, district nurse, physiotherapist and/or social worker, day care, meals on wheels or no care. | Period of intervention: 18 monthsNurse visit: (i) one assessment session with duration of 45–75 minutes, (ii) session(s) focused on designing of care plan, (iii) at least four visits dedicated to execution and monitoring of the care plan.After a year, the participants were reassessed and the protocol was repeated. |
| Vriendt, et al., 201621 | Activity oriented and community based programThe intervention was delivered by occupational therapist according to standardized protocol. It was based on a systematic therapy process and includes 4 phases: (1) client-centered goal-setting (the assessment of functional problems and their impact on health related quality of life plus comprehensive geriatric assessment); (2) negotiating a therapy plan (based on choices and preferences of the participants); (3) the actual intervention (training of functions and skills, education of the primary care giver or professional care giver, advise and instruction in the use of assistive devices or a comprehensive intervention including all aforementioned); (4) an evaluation of the outcome and finally reporting to relevant others (as general practitioner and the community care team). | Community care as usual Support in housekeeping and self-care, healthcare support from a nurse and social support from social worker. | Period of intervention: eight to ten weeks.Frequency: not clear |
| Wolf et al., 200355 | Tai ChiTai Chi classes emphasized all components of movement that typically become limited with aging. Specifically, the progression involved a gradual reduction of the base of standing support until single limb stance was achieved, increased body and trunk rotation, and reciprocal arm movements. Participants were encouraged to home practice, but this practice was not monitored.Computerized balance trainingTraining involving use of a Balance System, high technological approach, and being performed individually. During the task, the participant have to move the cursor seen on the screen at eye level into specific targets that can be placed anywhere on the screen. This task is successfully achieved by moving the center of mass with no foot displacement. The goal is to progressively increase sway to the limits of postural stability. Added to this paradigm is the capability of moving the floor upon which the pylons are placed at either linear or angular directions at varying velocities.The training period consisted of positioning progressively more difficult targets that required increased sway first in the absence of, and then with, concomitant floor movement. For each session, subjects were asked to practice these tasks with eyes open and then with eyes closed, thus demanding more dependence upon vestibular and somatosensory systems to maintain balance. | Education exercise-control conditionParticipants met with a gerontological nurse/researcher to discuss topics of interest, such as pharmacological management, sleep disorders, cognitive deficits coping with bereavement, and other. The participants were also instructed not to change their exercise level. | Period of intervention: 15 weeks.Tai Chi group: Participants were encouraged to practice at least 15 minutes twice a day.Computerized Balance Training group: frequency and duration of sessions not clear.Education group: weekly session with one-hour duration. |
Follow-up and measurement intervals
In ten studies21-23,41,43,45,47,50-52 the outcomes of interest were assessed twice, at baseline and at the end of the intervention or study, with measurement intervals varying from eight to 10 weeks21 to 18 months.51 In eight studies32,40,44,46,48,49,54,55 three assessment sessions were conducted. The largest measurement interval between the baseline and the last follow-up assessments was four years,48 and the smallest one seven months.49 Finally, three studies29,42,53 provided four assessment sessions. In one of these studies42 the outcomes were measured at baseline, after a three-month intervention, and at six and 12 months. The same measurement intervals (at baseline, and three, six and 12 months) were indicated by the authors of another study.53 However in this case the intervention lasted six months so that one of the assessment sessions (at three months) was conducted in the course of the intervention. In the third study29 the outcomes were measured at baseline and at three, six and twelve months after discharge, with the period of the intervention being unclear (for more detailed information see Table 4).
Methods of economic analysis
The aim of the study developed by Fairhall et al.32 was to compare the costs and cost-effectiveness of a multifactorial interdisciplinary intervention targeting identified frailty characteristics versus usual care from the community services and general practitioners. The effectiveness outcome measures included prevalence of frailty (assessed according to CHS criteria) and level of quality of life (assessed based on EuroQol questionnaire including items regarding mobility, self-care, usual activities, pain/discomfort and anxiety/depression), with data being collected at baseline, and at three and 12 months. In addition, quality adjusted life years (QALYs) were calculated based on data obtained from the quality of life measurements. For the calculation of the number of QALYs gained or lost over the 12 months of follow-up, trapezoidal integration (an approach used for measure of the area under a function plotted on a graph) was used. The study authors32 also evaluated health and community resource utilization, that included costs of primary care appointments with general practitioner and nurse or other health professional, costs of hospital-based care, costs of permanent and respite residential care, with high and low care, and costs of home help, transport and meal delivery. The resource use over 12 months was translated into monetary values using local or national prices or unit costs as appropriate. Monetary amounts were presented in 2011 Australian dollars. Finally, complete-case cost-utility and cost-effectiveness of multifactorial interdisciplinary intervention versus usual care were carried out, with the adopted perspective of health and community care funder. These economic evaluations included comparison of the difference in cases of transition out of frailty and in total costs between intervention and the control groups, as well as incremental cost-effectiveness ratios (ICERs) determined to assess the additional expenditure required to achieve additional benefits of the intervention.
Cohen et al.44 compared the costs of an intervention consisting of inpatient and outpatient geriatric evaluation and management versus inpatient and outpatient usual care. The effectiveness of these interventions was measured based on changes in basic and instrumental ADLs, physical performance and quality of life (assessed by Short Form 36 Health Survey). Data about these outcomes was collected at discharge and 12 months. In addition, probability of survival and relative risk of death at one year were calculated. The economic evaluations included utilization and costs of health care services, determined using a computer program at each center, centralized Veterans Affairs databases, and patients’ or caregivers’ reports of non-Veterans Affairs nursing home care. The costs considered in the analyses included overall costs of initial hospitalization and overall costs of health care after discharge (that is, costs of inpatient, outpatient, and long-term care provided by Veteran Affairs Medical Centers, as well as care in private nursing homes, without including the costs of inpatient and outpatient care at non-Veterans Affairs facilities). Detailed information about specific costs was not provided. Monetary amounts were presented in dollars. The authors did not provide information about the adopted perspective.44
Outcomes
The overview of the results on the outcome of frailty, indicated by any validated scale, measurement or index, or assessed by a limited set of indicators, is provided in Table 6. Table 7 summarizes the impact on the secondary outcomes.
Table 6.
Effectiveness of interventions described in the included studies for outcome of frailty
| Study | Intervention/control condition | Primary outcome – frailty | Significance |
| Behm, et al., 201540 | Multi-professional senior group meetings with one home visit (n = 171) | At baseline: - Sum of indicators: 14% non-frail, 70% pre-frail, 16% frail - Tiredness in daily activities: 6%One year after intervention - Deterioration on frailty: 49% - Frail ≤ 3 indicators: 34% - Tiredness in daily activities: 22%Two years after intervention - Deterioration on frailty: 60% - Frail ≤ 3 indicators: 47% - Tiredness in daily activities: 32% | Between group change on frailty status:Decrease on frailty status measures as tiredness in daily activities was higher in the control group than in the senior meetings group p = .029 or in the preventive visit group p = .006 |
| Single preventive home visit (n = 174) | At baseline: - Sum of indicators: 13% non-frail, 67% pre-frail, 20% frail - Tiredness in daily activities: 6%1 year after intervention - Deterioration on frailty: 44% - Frail ≤ 3 indicators: 34% - Tiredness in daily activities: 19%2 years after intervention - Deterioration on frailty: 58% - Frail ≤ 3 indicators: 52% - Tiredness in daily activities: 30% | ||
| Ordinary range of community services (n = 114) | At baseline: - Sum of indicators: 11% non-frail, 70% pre-frail, 19% frail - Tiredness in daily activities: 6%1 year after intervention - Deterioration on frailty: 38% - Frail ≤ 3 indicators: 39% - Tiredness in daily activities: 33%2 years after intervention - Deterioration on frailty: 68% - Frail ≤ 3 indicators: 59% - Tiredness in daily activities: 39% | ||
| Bonnefoy, et al., 201222 | Home based exercise program with dietary protein supplementation (n = 53) | At baseline (Median, 1st and 3rd quartiles): - Maximal walking distance (m): 1000 (450; 1750) - Maximal walking time (mn): 30 (15; 60) - PASE score: 33.6 (30.0; 65.5)After 4-month intervention (% from baseline, 1st and 3rd quartiles): - Variation in maximal walking distance (%): 0.00 (−55.0; 33.3) - Variation in maximal walking time (%): 0.00 (−33.3; 50.0) - Variation in PASE score (%): 0.00 (−20.9; 6.0) | Between group change:Maximum walking time kept stable in the intervention group and decreased in the control group p = .009 (for age and sex adjusted analysis p = .015)In subgroup of good compliers with intervention program (n = 23) also significant between group differences on maximum walking distance and maximum walking time were found (p = .007; p = .004, respectively) |
| No intervention (n = 49) | At baseline - Maximal walking distance (m): 1000 (300; 2000) - Maximal walking time (mn): 30 (15; 60) - PASE score: 33.6 (27.1; 55.0)After 4-month intervention (% from baseline, 1st and 3rd quartiles): - Variation in maximal walking distance (%): −16.7 (−40.0; 0.0) - Variation in maximal walking time (%): −25.0 (−50.0; 0.00) - Variation in PASE score (%): 0.00 (−16.7; 31.7) | ||
| Cadore et al., 201441 | Multicomponent exercise program(n = 11) | At baseline (mean ± SD): - Gait velocity (ms−1): 0.76 ± 0.07; - Hand grip (N): 165 ± 63 - Hip flexion strength (N):1.057 ± 262 - Knee extension strength (N): 1.451 ± 441After 12-week intervention (mean ± SD): - Gait velocity (ms−1): 0.80 ± 0.08 - Hand grip (N): 183 ± 52 - Hip flexion strength (N): 1.284 ± 203 - Knee extension strength (N): 1.745 ± 460 | Group x time interaction:Gait velocity p < .05Hip flexion strength p < .05 (intervention group > control group)Hand grip p < .01 (intervention group > control group)Knee extension strength p < .01 (intervention group > control group)Within group change in frailty components: In the intervention group improvement on hip flexion strength p < .01 and knee extension strength p < .05In the control group decrease in gait speed p < .05, hand grip p < .05 and knee extension strength p < .05 |
| Mobility exercises (n = 13) | At baseline (mean ± SD): - Gait velocity (ms−1): 0.68 ± 0.06 - Hand grip (N): 157 ± 64 - Hip flexion strength (N): 865 ± 268 - Knee extension strength (N): 1.206 ± 336After 12-week intervention (mean ± SD): - Gait velocity (ms−1): 0.60 ± 0.07 - Hand grip (N): 130 ± 58 - Hip flexion strength (N): 834 ± 382 - Knee extension strength (N): 1.042 ± 353 | ||
| Chan et al., 201242 | Exercise and nutrition consultation (n = 55) | At baseline: - Pre-frail: 84%; Frail: 16% - Weight loss: 33%; Exhaustion: 45%; Low activity level: 5%; Slowness: 18%; Weakness: 60%After 3-month intervention: - Improvement in frailty: 45% - Weight loss: 16%; Exhaustion: 29%; Low activity level: 4%; Slowness: 11%; Weakness: 20%6 months after baseline assessment: - Improvement in frailty: 42% - Weight loss: 15%; Exhaustion: 31%; Low activity level: 4%; Slowness: 7%; Weakness: 16%12 months after baseline assessment: - Improvement in frailty: 40% - Weight loss: 20%; Exhaustion: 35%; Low activity level: 4%; Slowness: 11%; Weakness: 13% | Between group change on frailty status:At 3 months the improvement in frailty status was higher in the exercise and nutrition consultation group than in the respective control group p = .008Between group change on frailty components: no significant difference |
| Problem solving therapy (n = 57) | At baseline: - Pre-frail: 84%; Frail: 16% - Weight loss: 21%; Exhaustion: 39%; Low activity level: 9%; Slowness: 26%; Weakness: 74%After 3-month intervention: - Improvement in frailty: 44% - Weight loss: 12%; Exhaustion: 28%; Low activity level: 5%; Slowness: 9%; Weakness: 30%6 months after baseline assessment: - Improvement in frailty: 35% - Weight loss: 11%; Exhaustion: 28%; Low activity level: 5%; Slowness: 11%; Weakness: 16%12 months after baseline assessment: - Improvement in frailty: 35% - Weight loss: 14%; Exhaustion: 28%; Low activity level: 5%; Slowness: 12%; Weakness: 21% | ||
| Educational booklet (control condition for exercise and nutrition consultation) (n = 62) | At baseline: - Pre-frail: 90%; Frail: 10% - Weight loss: 19%; Exhaustion: 37%; Low activity level: 10%; Slowness: 9%; Weakness: 81%After 3-month intervention: - Improvement in frailty: 27% - Weight loss: 10%; Exhaustion: 27%; Low activity level: 6%; Slowness: 3%; Weakness: 27%6 months after baseline assessment: - Improvement in frailty: 26% - Weight loss: 13%; Exhaustion: 29%; Low activity level: 6%; Slowness: 5%; Weakness: 26%12 months after baseline assessment: - Improvement in frailty: 31% - Weight loss: 15%; Exhaustion: 32%; Low activity level: 6%; Slowness: 5%; Weakness: 27% | ||
| Educational booklet (control condition for problem solving therapy) (n = 60) | At baseline: - Pre-frail: 90%; Frail: 10% - Weight loss: 3%; Exhaustion: 43%; Low activity level: 7%; Slowness: 12%; Weakness: 68%After 3-month intervention: - Improvement in frailty: 28% - Weight loss: 13%; Exhaustion: 28%; Low activity level: 5%; Slowness: 5%; Weakness: 18%6 months after baseline assessment: - Improvement in frailty: 32% - Weight loss: 17%; Exhaustion: 42%; Low activity level: 5%; Slowness: 2%; Weakness: 27%12 months after baseline assessment: - Improvement in frailty: 35% - Weight loss: 20%; Exhaustion: 38%; Low activity level: 5%; Slowness: 3%; Weakness: 20% | ||
| Clegg, et al., 201443 | Home-based exercise program (n = 40) | At baseline (mean ± SD): - TUGT Score: 52.0 ± 62.4After 12-week intervention (mean ± SD): - TUGT Score: 62.4 ± 77.7 | Between group change: no significant difference |
| Usual care (n = 30) | At baseline (mean ± SD): - TUGT Score: 57.9 ± 74.1After 12-week intervention (mean ± SD): - TUGT Score: 97.0 ± 116.7 | ||
| Cohen, et al., 200244 | Inpatient geriatric care in multidisciplinary evaluation and management units (not clear) | At baseline vs After intervention (mean change in score): - Basic ADL score: 0.23; Instrumental ADL score: −0.30 - Physical Performance Test score: 3.12 At baseline vs 12 months after randomization (mean change in score): - Basic ADL score: 0.27; Instrumental ADL score: −0.20 - Physical Performance Test score: 4.50 | Between group changeIn the inpatient groups - basic ADL at discharge p < .001 (evaluation and management group > usual care group) - physical performance at discharge p < .001 (evaluation and management group > usual care group)In the outpatient groups - physical performance at 12 months (adjusted for the length of stay) p = .003 (evaluation and management group > usual care group) |
| Outpatient care in geriatric evaluation and management clinics (not clear) | At baseline vs After intervention (mean change in score): - Basic ADL score: 0.20; Instrumental ADL score: −0.28 - Physical Performance Test score: 2.34 At baseline vs 12 months after randomization (mean change in score/values adjusted for the length of stay): - Basic ADL score: 0.27/0.05; Instrumental ADL score: −0.18/0.09 - Physical Performance Test score: 4.67/2.13 | ||
| Usual inpatient care (not clear) | At baseline vs After intervention (mean change in score): - Basic ADL score: 0.15; Instrumental ADL score: −0.30 - Physical Performance Test score: 1.75 At baseline vs 12 months after randomization (mean change in score): - Basic ADL score: 0.25; Instrumental ADL score: −0.20 - Physical Performance Test score: 4.24 | ||
| Usual outpatient care (not clear) | At baseline vs After intervention (mean change in score): - Basic ADL score: 0.20; Instrumental ADL score: −0.30 - Physical Performance Test score: 2.60 At baseline vs 12 months after randomization (mean change in score/values adjusted for the length of stay): - Instrumental ADL score: 0.25/0.03; Basic ADL score: −0.21/0.08 - Physical Performance Test score: 4.07/1.30 | ||
| Eklund, et al., 201329 | Continuum Care by multi-professional team(n = 85) | At baseline: - Non-frail: 5%; pre-frail: 26%; frail: 69%3 months after discharge: - Improvement: 8%; Maintained level: 78%; Decrease: 14%6 months after discharge: - Improvement: 12%; Maintained level: 74%; Decrease: 14%12 months after discharge: - Improvement: 12% Maintained level: 74%; Decrease: 14% | Between group change in frailty status: no significant difference |
| Usual care (n = 76) | At baseline: - Non-frail: 0%; pre-frail: 24%; frail: 76%3 months after discharge: - Improvement: 13%; Maintained level: 76%; Decrease: 11%6 months after discharge: - Improvement: 17%; Maintained level: 75%; Decrease: 8%12 months after discharge: - Improvement: 22%; Maintained level: 68%; Decrease: 9% | ||
| Fairhall, et al., 201532 | Multifactorial interdisciplinary intervention targeting identified frailty characteristics (n = 120) | At baseline: - Frailty prevalence: 100% (3 frailty criteria: 64%; 4 frailty criteria: 28%; 5 frailty criteria: 8%)At 3 months: - Frailty prevalence: 64%At 12 months: - Frailty prevalence: 62% | Between group change in frailty prevalence (adjusted for month 0):At 12 months frailty prevalence was lower in the intervention group than in the control group p = .02 |
| Usual care (n = 121) | At baseline: - Frailty prevalence: 100% (3 frailty criteria: 65%; 4 frailty criteria: 25%; 5 frailty criteria: 10%)At 3 months: - Frailty prevalence: 75%At 12 months: - Frailty prevalence: 77% | ||
| Favela, et al., 201345 | Nurse home visits alone (n = 44) | At baseline: - Frail: 43.2% - Weight loss: 18.2%; Exhaustion: 25.0%; Weakness: 50.0%; Slow walking speed: 95.5%; Low activity level: 52.3%After 9-month intervention: - Frail: more than 65% (exact data not provided) - Improvement in frailty: about 11% (exact data not provided) - Development of frailty: 24.3% | Between group change in frailty prevalence:In post-intervention assessment nurse home visits + alert buttons group less frail than control group p < .05 (either for observed in those followed up, or estimated including deaths and losses to follow-up) |
| Nurse home visits including alert buttons (n = 45) | At baseline: - Frail: 46.7% - Weight loss: 15.6%; Exhaustion: 33.3%; Weakness: 60.0%; Slow walking speed: 97.8%; Low activity level: 57.8%After 9-month intervention: - Frail: 23.3% - Improvement in frailty: 12.8% - Development of frailty: 5.1% | ||
| Usual care (n = 44) | At baseline: - Frail: 45.5% - Weight loss: 20.5%; Exhaustion: 18.2%; Weakness: 52.3%; Slow walking speed: 100%; Low activity level: 43.2%After 9-month intervention: - Frail: 58.3% - Improvement in frailty: about 10% (exact data not provided) - Development of frailty: 12.8% | ||
| Giné-Garriga, et al., 201046 | Functional circuit-training program (n = 22) | At baseline (mean ± SD; 95%CI): - Barthel Index Score: 73.41 ± 2.35 (68.67; 78.15) - Rapid Gait test (s): 11.73 ± 0.60 (10.52; 12.93) - Stand-up test (s): 19.55 ± 0.71 (18.12; 20.97)After 12-week intervention (mean ± SD; 95%CI): - Barthel Index Score: 79.32 ± 2.35 (74.58; 84.06) - Rapid Gait test (s): 9.20 ± 0.60 (7.99; 10.41) - Stand-up test (s): 15.55 ± 0.66 (14.21; 16.89)At 36 weeks (mean ± SD; 95%CI): - Barthel Index Score: 77.0 ± 2.38 (72.19; 81.80) - Rapid Gait test (s): 10.05 ± 0.62 (8.82; 11.29) - Stand-up test (s): 17.81 ± 0.68 (16.43; 19.18) | Group x time interaction:Barthel Index p < .001 (intervention group > control group)For week 0 – week 12 p < .001For week 0 – week 36 p = .001For week 12 – week 36 p = .049Rapid gait test p < .001 (intervention group > control group)For week 0 – week 12 p < .001For week 0 – week 36 p < .001For week 12 – week 36 p = .031Stand-up test p < .001 (intervention group > control group)For week 0 – week 12 p < .001For week 0 – week 36 p = .002For week 12 – week 36 p < .001Within group change: In the intervention group improvement from baseline to week 12 and from baseline to week 36 in Barthel Index, Rapid Gait test and Stand-up test p < .05 |
| Health education meeting and usual care (n = 19) | At baseline (mean ± SD; 95%CI): - Barthel Index Score: 70.79 ± 2.53 (65.69; 75.89) - Rapid Gait test (s): 11.87 ± .065 (10.57; 13.16) - Stand-up test (s): 17.05 ± 0.93 (15.16; 18.93)After 12-week intervention (mean ± SD; 95%CI): - Barthel Index Score: 67.90 ± 2.53 (62.79; 73.00) - Rapid Gait test (s): 12.39 ± 0.65 (11.10; 13.69) - Stand-up test (s): 17.93 ± 0.92 (16.07; 19.79)At 36 weeks (mean ± SD; 95%CI): - Barthel Index Score: 66.73 ± 2.73 (61.26; 72.21) - Rapid Gait test (s): 12.76 ± 0.74 (11.29; 14.23) - Stand-up test (s): 17.47 ± 1.08 (15.31; 19.63) | ||
| Gustafsson, et al., 201247 | Multi-professional senior group meetings with one home visit (n = 171) | At baseline vs 3 months after intervention:- No progression of frailty between baseline and follow-up: 64% | Between group change in frailty status: No significant difference |
| Single preventive home visit (n = 174) | At baseline vs 3 months after intervention:- No progression of frailty between baseline and follow-up: 70% | ||
| Ordinary range of community services (n = 114) | At baseline vs 3 months after intervention:- No progression of frailty between baseline and follow-up: 71% | ||
| Hars et al., 201448 | Continued intervention of music-based multitask training (n = 23) | At baseline: - Number of frailty components (mean ± SD): 0.5 ± 0.6 - Weight loss: 4%; Exhaustion: 26%; Low activity level: 0%; Slow walking speed: 9%; Weakness: 13%After 1-year intervention: - Number of frailty components (mean ± SD): 0.7 ± 0.6 - Weight loss: 0%; Exhaustion: 9%; Low activity level: 0%; Slow walking speed: 9%; Weakness: 48%3 years after intervention: - Number of frailty components (mean ± SD): 0.5 ± 0.8 - Weight loss: 0%; Exhaustion: 9%; Low activity level: 0%; Slow walking speed: 9%; Weakness: 35% | Within group change from baseline to 4-year follow-up:Gait speed and gait velocity in the continued intervention p < .05Gait velocity in the discontinued intervention p < .05Between group changeGait speed p = .006 (continued intervention > discontinued intervention)Handgrip strength p = .018 (continued intervention > discontinued intervention)Change on frailty status: Pre-frail participants from the continued intervention group were more likely to become robust at 4 years than participants from the discontinued intervention group (p = 0.004) |
| Discontinued intervention of music-based multitask training (n = 29) | At baseline: - Number of frailty components (mean ± SD): 1 ± 0.7 - Weight loss: 21%; Exhaustion: 17%; Low activity level: 0%; Slow walking speed: 14%; Weakness: 48%After 1-year intervention: - Number of frailty components (mean ± SD): 0.9 ± 0.6 - Weight loss: 0%; Exhaustion: 10%; Low activity level: 0%; Slow walking speed: 7%; Weakness: 69%3 years after intervention: - Number of frailty components (mean ± SD): 1.3 ± 0.8 - Weight loss: 3%; Exhaustion: 10%; Low activity level: 0%; Slow walking speed: 34%; Weakness: 79% | ||
| Kim, et al., 201549 | Milk fat globule membrane (MFGM) supplementation (n = 32) | At baseline: - Number of frailty components (mean ± SD): 3.7 ± 0.7 (3 frailty criteria: 43.8%; 4 frailty criteria: 40.6%; 5 frailty criteria: 15.6%) - Weight loss: 62.5%; Exhaustion: 62.5%; Low activity level: 93.8%; Weakness: 65.6%;Slow walking speed: 68.8%After 3-month intervention: - Reversal rate of frailty: 28.1% - Reversal rate from baseline to post-intervention for Weight loss: −18.7%; Exhaustion: 18.7%; Low activity level: 40.6%; Weakness: −12.5%;Slow walking speed: −12.5%4 months after intervention: - Reversal rate of frailty: 25.0% - Reversal rate from baseline to follow-up for Weight loss: 15.6%; Exhaustion: 25.0%; Low activity level: 9.4%; Weakness: −9.4%;Slow walking speed: 15.6% | Within group change from baseline to post-intervention:Weight loss in placebo group p < .05Exhaustion in exercise + placebo, exercise + MFGM, and placebo groups p < .05Physical activity in all intervention and placebo groups p < .05Within group change from baseline to follow-up:Weight loss in exercise + MFGM, and exercise + placebo groups p < .05Exhaustion in all intervention groups p < .05Physical activity in exercise + MFGM group p < .05Walking speed in exercise + MFGM group p < .05Between group change in post intervention assessment:Weight loss p = .007 (exercise + MFGM < MFGM, placebo)Exhaustion p < .001 (exercise + MFGM, MFGM, and placebo groups < exercise + placebo)Between group change in follow-up assessment:Weight loss p = .005 (exercise + MFGM > MFGM, placebo; exercise + placebo > placebo)Exhaustion p = .007 (exercise + MFGM, exercise + placebo, MFGM > placebo)Physical activity p = .004 (exercise + MFGM > exercise + placebo, MFGM, placebo)Walking speed p < .001 (exercise + MFGM > exercise + placebo; MFGM > placebo) |
| Exercise + placebo (n = 33) | At baseline: - Number of frailty components (mean ± SD): 3.6 ± 0.7 (3 frailty criteria: 54.4%; 4 frailty criteria: 30.3%; 5 frailty criteria: 15.2%) - Weight loss: 60.6%; Exhaustion: 84.8%; Low activity level: 75.8%; Weakness: 72.7%;Slow walking speed: 60.6%After 3-month intervention: - Reversal rate of frailty: 51.5% - Reversal rate from baseline to post-intervention for Weight loss: −12.1%; Exhaustion: 69.7%; Low activity level: 57.6%; Weakness: 3.0%;Slow walking speed: 9.1%4 months after intervention: - Reversal rate of frailty: 39.4% - Reversal rate from baseline to follow-up for Weight loss: 33.3%; Exhaustion: 42.4%; Low activity level: 9.1%; Weakness: −3.1%;Slow walking speed: 18.2% | ||
| Exercise + milk fat globule membrane (MFGM) supplementation (n = 33) | At baseline: - Number of frailty components (mean ± SD): 3.8 ± 0.7 (3 frailty criteria: 33.3%; 4 frailty criteria: 48.5%; 5 frailty criteria: 18.2%) - Weight loss: 72.7%; Exhaustion: 60.6%; Low activity level: 90.9%; Weakness: 69.7%;Slow walking speed: 66.7%After 3-month intervention: - Reversal rate of frailty: 57.6% - Reversal rate from baseline to post-intervention for Weight loss: 0.00%; Exhaustion: 30.3%; Low activity level: 54.5%; Weakness: 6.1%;Slow walking speed: 18.2%4 months after intervention: - Reversal rate of frailty: 45.5% - Reversal rate from baseline to follow-up for Weight loss: 39.4%; Exhaustion: 33.3%; Low activity level: 36.4%; Weakness: 3.0%;Slow walking speed: 42.4% | ||
| Placebo (n = 32) | At baseline: - Number of frailty components (mean ± SD): 3.5 ± 0.6 (3 frailty criteria: 51.5%; 4 frailty criteria: 45.5%; 5 frailty criteria: 3.0%) - Weight loss: 45.5%; Exhaustion: 60.6%; Low activity level: 90.9%; Weakness: 63.6%;Slow walking speed: 57.6%After 3-month intervention: - Reversal rate of frailty: 30.3% - Reversal rate from baseline to post-intervention for Weight loss: −30.3%; Exhaustion: 30.3%; Low activity level: 30.3%; Weakness: 6.1%;Slow walking speed: −3.0%4 months after intervention: - Reversal rate of frailty: 15.2% - Reversal rate from baseline to follow-up for Weight loss: −6.1%; Exhaustion: −6.1%; Low activity level: 9.1%; Weakness: −9.1%;Slow walking speed: 0.0% | ||
| Kim & Lee, 201323 | Protein-energy supplementation (n = 41) | At baseline (mean ± SD): - Physical Functioning score: 17.0 ± 5.3 - SPPB score: 5.5 ± 1.5 - Energy intake (kcal/day): 965 ± 309; Protein (g/day): 35.4 ± 15.9; Essential amino acid (g/day): 9.1 ± 4.1; Adequacy ratio for the intake of energy, protein and micronutrients: 55.4 ± 20.2; Body weight (kg): 47.4 ± 9.3After 12-week intervention (mean ± SD): - Physical Functioning score: 18.2 ± 4.9 - SPPB score: 5.8 ± 1.6 - Energy intake (kcal/day): 1124 ± 315; Protein (g/day): 54.7 ± 21.2; Essential amino acid (g/day): 16.9 ± 6.0; Adequacy ratio for the intake of energy, protein and micronutrients: 87.4 ± 24.2; Body weight (kg): 49.0 ± 9.4 | Between group change in frailty components:SPPB p = .039;Energy intake p = .008;Protein p < .001;Essential amino acid p < .001Adequacy ratio p < .001There was a modest correlation between relative change in physical functioning with relative change in protein intake (rs = .23, p = .037) and mean adequacy ratio (rs = .25, p = .023).Change in Short Physical Performance Battery correlated significantly with change in mid-arm circumference (rs = .31; p = .004). |
| No intervention (n = 43) | At baseline (mean ± SD): - Physical Functioning score: 18.4 ± 5.8 - SPPB score: 5.7 ± 1.8 - Energy intake (kcal/day): 951 ± 331; Protein (g/day): 35.9 ± 15.0; Essential amino acid (g/day): 10.4 ± 4.9; Adequacy ratio for the intake of energy, protein and micronutrients: 60.4 ± 23.6; Body weight (kg): 44.4 ± 7.7After 12-week intervention (mean ± SD): - Physical Functioning score: 18.4 ± 6.1 - SPPB score: 5.4 ± 2.2 - Energy intake (kcal/day): 896 ± 277; Protein (g/day): 32.7 ± 10.3; Essential amino acid (g/day): 9.0 ± 3.7; Adequacy ratio for the intake of energy, protein and micronutrients: 56.2 ± 18.8; Body weight (kg): 45.8 ± 8.0 | ||
| Li, et al., 201050 | Screening evaluation and intervention based on screening results (n = 129) | At baseline: - Non-frail: 0%; Pre-frail: 82.9%; Frail: 17.1%6 months after baseline assessment: - Non-frail: 3.9%; Pre-frail: 78.3%; Frail: 17.8% | Deterioration in frailty status: no significant differences |
| Screening evaluation (n = 140) | At baseline: - Non-frail: 0%; Pre-frail: 80.4%; Frail: 19.6%6 months after baseline assessment: - Non-frail: 2.1%; Pre-frail: 73.6%; Frail: 24.3% | ||
| Monteserin et al., 201051 | Group sessions (n = 157) | At baseline: - 49% at risk of frailtyAfter 18-month intervention: - Reversal rate of frailty: 27.9% - Rate of becoming at risk of frailty: 20.4% | Between group change in frailty status:From not at risk to at risk status p = .023 (control group > intervention group)From at risk to not at risk status p = .027 (control group < intervention group) |
| Individual sessions with geriatrician (n = 151) | |||
| Usual care (n = 312) | At baseline: - 42.9% at risk of frailtyAfter 18-month intervention: - Reversal rate of frailty: 13.5% - Rate of becoming at risk of frailty: 33.8% | ||
| Muller et al., 200652 | Atamestane + dehydroepiandrosterone (DHEA) (n = 26) | At baseline (mean ± SD): - Isometric grip strength (kg): 33.3 ± 6.3 - Leg extension power (Nm): 105.7 ± 17.6 - Physical performance score: 8.35 ± 2.35After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - Isometric grip strength (kg): 0.0 (−1.9; 1.9) - Leg extension power (Nm): −1.8 (−8.7; 5.0) - Physical performance score: 0.2 (−0.7; 1.2) | No differences in change of isometric grip strength and physical performance for the treatment groups, compared with placebo groupLeg extension power declined in all intervention groupsTime effect on frailty measures: no significant |
| DHEA(n = 25) | At baseline (mean ± SD): - Isometric grip strength (kg): 32.7 ± 5.2 - Leg extension power (Nm): 110.8 ± 14.7 - Physical performance score: 7.80 ± 2.35After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - Isometric grip strength (kg): 1.3 (−0.6; 3.2) - Leg extension power (Nm): −5.4 (−12.4; 1.6) - Physical performance score: 0.7 (−0.3; 1.7) | ||
| Atamestane (n = 25) | At baseline (mean ± SD): - Isometric grip strength (kg): 33.8 ± 6.3 - Leg extension power (Nm): 101.9 ± 13.4 - Physical performance score: 8.48 ± 2.14After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - Isometric grip strength (kg): 0.2 (−1.8; 2.1) - Leg extension power (Nm): −1.9 (−8.9; 5.0) - Physical performance score: 0.2 (−0.8; 1.2) | ||
| Placebo (n = 24) | At baseline (mean ± SD): - Isometric grip strength (kg): 34.0 ± 7.1 - Leg extension power (Nm): 103.0 ± 13.5 - Physical performance score: 8.58 ± 2.12After 36-week intervention (changes from baseline (placebo), mean, 95%CI): - Isometric grip strength (kg): −1.2 (−2.4; −0.0) - Leg extension power (Nm): 6.4 (−0.1; 12.7) - Physical performance score: −0.1 (−0.9; 0.7) | ||
| Ng, et al., 201553 | Nutritional supplements (n = 50) | At baseline (mean ± SD): - Number of frailty components: 2.1 ± 0.78; pre-frail: 67.4%; frail 32.7% - Weight loss: 4.1%; Slowness: 40.8%; Weakness: 53.1%;Exhaustion: 14.3%; Low activity level: 18.4% - BMI (kg/m2): 24.0 ± 4.31; knee strength (kg): 14.0 ± 5.27; physical activity: 165.7 ± 104.7; gait speed (s): 5.8 ± 1.81; energy: 10.7 ± 1.23At 3 months (mean ± SD): - Number of frailty components: 1.5 ± 1.06 - BMI (kg/m2): 24.3 ± 4.33; knee strength (kg): 15.8 ± 5.38; physical activity: 201.5 ± 119.2; gait speed (s): 4.8 ± 1.21; energy: 11.4 ± 1.79After 6-month intervention (mean ± SD): - Number of frailty components 1.4 ± 0.78 - BMI (kg/m2): 23.9 ± 4.47; knee strength (kg): 15.1 ± 4.77; physical activity: 264.5 ± 134.9; gait speed (s): 5.0 ± 1.02; energy: 11.2 ± 1.56At 12 months (mean ± SD): - Number of frailty components: 1.5 ± 0.91; Frailty reduction: 35.6% - BMI (kg/m2): 24.2 ± 4.23; knee strength (kg): 15.0 ± 4.34; physical activity: 279.1 ± 139.0; gait speed (s): 5.2 ± 1.21; energy: 11.6 ± 1.85 | Mean change from baseline for frailty score: Nutritional supplements at 12 months p < .05Cognitive training at 12 months p < .05Physical training at 3 months p < .05, at 6 and 12 months p < .01Combined treatment at 3 and 6 months p < .05, at 12 months p < .01Mean change from baseline for frailty prevalence:All intervention groups p < .01Time effect on frailty components: BMI p = .001;knee strength, physical activity, gait speed, energy p < .001Group effect on frailty components: no significantGroup x time interaction on frailty components: knee strength p = .009;physical activity p = .038 |
| Physical training (n = 48) | At baseline (mean ± SD): - Number of frailty components: 2.2 ± 0.85; pre-frail: 60.4%; frail 39.6% - Weight loss: 6.3%; Slowness: 47.9%; Weakness: 54.2%;Exhaustion: 14.6%; Low activity level: 22.9% - BMI (kg/m2): 23.5 ± 3.03; knee strength (kg): 14.1 ± 4.63; physical activity: 162.5 ± 117.2; gait speed (s): 6.1 ± 2.08; energy: 10.8 ± 1.10At 3 months (mean ± SD): - Number of frailty components: 1.2 ± 0.75 - BMI (kg/m2): 23.5 ± 2.92; knee strength (kg): 16.0 ± 4.00; physical activity: 185.8 ± 116.9; gait speed (s): 4.8 ± 0.89; energy: 11.7 ± 1.68After 6-month intervention (mean ± SD): - Number of frailty components: 1.3 ± 0.87 - BMI (kg/m2): 23.7 ± 3.06; knee strength (kg): 16.9 ± 5.47; physical activity: 220.1 ± 139.7; gait speed (s): 5.0 ± 1.04; energy: 11.5 ± 1.71At 12 months (mean ± SD): - Number of frailty components: 1.4 ± 0.80; Frailty reduction: 41.3% - BMI (kg/m2): 23.4 ± 3.23; knee strength (kg): 15.5 ± 5.19; physical activity: 202.0 ± 134.6; gait speed (s): 4.9 ± 0.99; energy: 11.4 ± 1.89 | ||
| Cognitive training (n = 50) | At baseline (mean ± SD): - Number of frailty components: 2.0 ± 0.91; pre-frail: 74.0%; frail 26.0% - Weight loss: 4.0%; Slowness: 26.0%; Weakness: 56.0%;Exhaustion: 20.0%; Low activity level: 24.0% - BMI (kg/m2): 23.1 ± 2.70; knee strength (kg): 12.9 ± 3.88; physical activity: 179.3 ± 113.3; gait speed (s): 5.4 ± 1.16; energy: 10.5 ± 1.20At 3 months (mean ± SD): - Number of frailty components: 1.3 ± 0.81 - BMI (kg/m2): 23.3 ± 3.01; knee strength (kg): 14.9 ± 4.41; physical activity: 194.8 ± 118.6; gait speed (s): 4.7 ± 0.97; energy: 11.7 ± 1.78After 6-month intervention (mean ± SD): - Number of frailty components: 1.4 ± 0.78 - BMI (kg/m2): 23.4 ± 2.97; knee strength (kg): 15.2 ± 5.20; physical activity: 194.8 ± 115.4; gait speed (s): 4.6 ± 0.80; energy: 11.3 ± 1.71At 12 months (mean ± SD): - Number of frailty components: 1.4 ± 0.94; Frailty reduction: 35.6% - BMI (kg/m2): 23.0 ± 3.52; knee strength (kg): 15.0 ± 4.35; physical activity: 227.1 ± 98.7; gait speed (s): 5.2 ± 1.05; energy: 11.5 ± 2.07 | ||
| Combination treatment (n = 49) | At baseline (mean ± SD): - Number of frailty components: 2.1 ± 0.81; pre-frail: 73.5%; frail 26.5% - Weight loss: 2.0%; Slowness: 34.7%; Weakness: 51.0%;Exhaustion: 16.3%; Low activity level: 32.7% - BMI (kg/m2): 24.4 ± 3.79; knee strength (kg): 14.9 ± 5.50; physical activity: 160.6 ± 115.9; gait speed (s): 5.4 ± 1.25; energy: 10.7 ± 1.38At 3 months (mean ± SD): - Number of frailty components: 1.3 ± 0.84 - BMI (kg/m2): 24.4 ± 3.78; knee strength (kg): 16.8 ± 5.82; physical activity: 201.6 ± 115.3; gait speed (s): 4.7 ± 1.20; energy: 11.9 ± 1.67After 6-month intervention (mean ± SD): - Number of frailty components: 1.4 ± 0.87 - BMI (kg/m2): 24.6 ± 3.64; knee strength (kg): 17.5 ± 6.40; physical activity: 197.2 ± 139.4; gait speed (s): 4.8 ± 1.13; energy: 11.8 ± 1.71At 12 months (mean ± SD): - Number of frailty components: 1.2 ± 1.07; Frailty reduction: 47.8% - BMI (kg/m2): 24.1 ± 3.83; knee strength (kg): 17.2 ± 6.59; physical activity: 201.0 ± 138.0; gait speed (s): 5.3 ± 2.17; energy: 12.0 ± 1.81 | ||
| Standard care + placebo (n = 50) | At baseline (mean ± SD): - Number of frailty components: 1.8 ± 0.80; pre-frail: 86.0%; frail 14.0% - Weight loss: 6.0%; Slowness: 30.0%; Weakness: 40.8%;Exhaustion: 12.0%; Low activity level: 10.0% - BMI (kg/m2): 23.6 ± 3.35; knee strength (kg): 15.5 ± 4.73; physical activity: 176.9 ± 111.0; gait speed (s): 5.6 ± 2.07; energy: 10.6 ± 1.55At 3 months (mean ± SD): - Number of frailty components: 1.3 ± 0.85 - BMI (kg/m2): 24.1 ± 3.33; knee strength (kg): 16.5 ± 4.68; physical activity: 183.5 ± 114.6; gait speed (s): 5.1 ± 2.09; energy: 11.2 ± 1.99After 6-month intervention (mean ± SD): - Number of frailty components: 1.4 ± 1.06 - BMI (kg/m2): 24.1 ± 3.61; knee strength (kg): 15.0 ± 4.53; physical activity: 195.0 ± 103.0; gait speed (s): 4.9 ± 1.47; energy: 11.3 ± 1.68At 12 months (mean ± SD): - Number of frailty components: 1.6 ± 0.97; Frailty reduction: 15.2% - BMI (kg/m2): 23.8 ± 3.58; knee strength (kg): 14.8 ± 4.47; physical activity: 209.7 ± 123.3; gait speed (s): 5.2 ± 1.72; energy: 10.9 ± 1.67 | ||
| Van Hout et al., 201054 | Proactive home visits by trained community nurses (n = 331) | At baseline (mean ± SD): - SF-36 physical component: 31.8 ± 10.0; SF-36 mental component: 44.2 ± 11.4; GARS: ± 55.5 (9.8)At 6 months (mean ± SD): - SF-36 physical component: 31.4 ± 9.3; SF-36 mental component: 44.5 ± 10.5After 18-month intervention (mean ± SD): - SF-36 physical component: 30.7 ± 9.2; SF-36 mental component: 43.9 ± 11.2; GARS: 51.8 ± 10.4 | Group x time interaction: no significant difference |
| Usual care (n = 320) | At baseline (mean ± SD): - SF-36 physical component: 31.9 ± 9.9; SF-36 mental component: 45.0 ± 11.3; GARS: 56.8 ± 9.8At 6 months (mean ± SD): - SF-36 physical component: 32.1 ± 9.4; SF-36 mental component: 45.4 ± 10.6After 18-month intervention (mean ± SD): - SF-36 physical component: 32.2 ± 9.3; SF-36 mental component: 45.2 ± 11.2; GARS: 53.0 ± 10.5 | ||
| Vriendt, et al., 201621 | Activity oriented and community based program (Baseline: n = 86Follow-up: n = 82) | At baseline (mean ± SD): - basic ADL: 66 ± 25After 8–10-week intervention (mean difference): - basic ADL: 3.6 | Between group difference: p = 0.013 |
| Community care as usual (Baseline: n = 82Follow-up: n = 80) | At baseline (mean ± SD): - basic ADL: 69 ± 23After 8–10-week intervention (mean difference): - basic ADL: -3.1 | ||
| Wolf et al., 200355 | Tai Chi (n = 72) | At baseline (mean ± SD): - Grip strength left: 23.2 ± 8.2; systolic blood pressure post walk (mmHg): 172.1 ± 27.7; distance (miles): 0.57 ± 0.09 - Fear of falling: 56%; Intrusiveness (agree): 79%After 15-week intervention (mean ± SD): - Grip strength left: 22.5 ± 8.5; systolic blood pressure post walk (mmHg): 158.9 ± 27.4; distance (miles): 0.55 ± 0.10 - Fear of falling: 48%; Intrusiveness (agree): 83%4 months after intervention (mean ± SD): - Grip strength left: 22.8 ± 8.1 - Fear of falling: 53%; Intrusiveness (agree): 85% | Group x time interaction:Grip strength left p = .025Tukey test:Tai Chi group less likely to decline over time than other groups Group x time interaction:Walk distance p = .040Tukey test:Tai Chi group < other groupsChanges in pre to post intervention scores for Tai Chi and Education groups:Fear of falling p = .046 |
| Computerized Balance Training (n = 64) | At baseline (mean ± SD): - Grip strength left: 24.8 ± 8.1; systolic blood pressure post walk (mmHg): 170.5 ± 33.0; distance (miles): 0.56 ± 0.09 - Fear of falling: 71%; Intrusiveness (agree): 82%After 15-week intervention (mean ± SD): - Grip strength left: 23.8 ± 8.0; systolic blood pressure post walk (mmHg): 165.5 ± 25.8; distance (miles): 0.57 ± 0.08 - Fear of falling: 73%; Intrusiveness (agree): 80%4 months after intervention (mean ± SD): - Grip strength left: 23.1 ± 8.0 - Fear of falling: 67%; Intrusiveness (agree): 82% | ||
| Education exercise-control condition (n = 64) | At baseline (mean ± SD): - Grip strength left: 23.8 ± 6.5; systolic blood pressure post walk (mmHg): 164.0 ± 26.8; distance (miles): 0.57 ± 0.08 - Fear of falling: 55%; Intrusiveness (agree): 85%After 15-week intervention (mean ± SD): - Grip strength left: 22.0 ± 6.2; systolic blood pressure post walk (mmHg): 162.3 ± 27.3; distance (miles): 0.58 ± 0.11 - Fear of falling: 64%; Intrusiveness (agree): 78%4 months after intervention (mean ± SD): - Grip strength left: 22.2 ± 6.6; - Fear of falling: 60%; Intrusiveness (agree): 84% |
ADL, Activities of daily living; BMI, body mass index; BMI, body mass index; GARS, Groningen Activity Restriction Scale; OR, odds ratio; PASE, Physical Activity Scale for the Elderly; SD, standard deviation; SF-36, Short Form 36 Health Survey; SPPB, Short Physical Performance Battery; TUGT, Timed Up and Go Test.
Table 7.
Effectiveness of interventions described in the included studies for secondary outcomes
| Study | Intervention/control condition | Secondary outcomes | Significance |
| Bonnefoy, et al., 201222 | Home based exercise program with dietary protein supplementation (n = 53) | At baseline (Median, 1st quartile; 3rd quartile):- BMI (kg/m2): 24.3 (21.0; 27.0); Fat free mass: 0.54 (0.52; 0.59); Mini nutritional assessment: 25.8 (23.3; 27.0) - ADL score: 6 (5.5; 6); instrumental ADL score: 7 (6; 8) - Get up and go test (mn): 21 (18; 27); Walking speed (m/s): 0.7 (0.5; 0.9)After 4-month intervention (% variation from baseline, 1st and 3rd quartiles): - BMI (kg/m2): 0.0 (−2.4; 2.2); Fat free mass: −1.50 (−5.9; 5.7); Fat free mass: 0.4 (−4.7; 6.8); Mini nutritional assessment: 0.0 (−5.9; 6.0) - ADL score: 9 (17.3% degradation); instrumental ADL score: 11 (22.9% degradation) - Get up and go test (mn): 5.45 (−17.2; 13.2); Walking speed (m/s): 0.0 (−13.7; 16.1); Time for 6-step raise: 0.0 (0.0; 16.7); Number chair rise in 1 min: 0.0 (−18.2; 18.2) | Between group difference:Body mass index remained stable in the intervention group and increased in the control group (for age and sex adjusted analysis p = .026)Mini nutritional assessment remained stable in the intervention group and decreased in the control group (for age and sex adjusted analysis p = .009)Control group also reduced significantly instrumental ADL (for age and sex adjusted analysis p = .05)Predictors of good compliance:Significant association between instrumental ADL and good compliance was found (p = .0011) |
| No intervention (n = 49) | At baseline (Median, 1st quartile; 3rd quartile): - BMI (kg/m2): 25.0 (23.5; 28.7); Fat free mass: 0.55 (0.51; 0.64); Mini nutritional assessment: 26.0 (24.0; 27.5) - ADL score: 6 (5.5; 6); instrumental ADL score: 7 (5; 8) - Get up and go test (mn): 23 (17; 36); Walking speed (m/s): 0.55 (0.4;0.9)After 4-month intervention (% variation from baseline, 1st and 3rd quartiles): - BMI (kg/m2): 0.8 (−1.3; 2.2); Fat free mass: 0.0 (−5.4; 3.9); Fat free mass: −0.4 (−6.2; 2.6); Mini nutritional assessment: −1.9 (−5.4; 5.8) - ADL score: 6 (13% degradation); instrumental ADL score: 15 (34.9% degradation) - Get up and go test (mn): 0.0 (−11.5; 15.5); Walking speed (m/s): 0.0 (−19.5; 15.9); Time for 6-step raise: 0.0 (−16.7; 25.0); Number chair rise in 1 min: 0.0 (−16.7; 15.4) | ||
| Cadore et al., 201441 | Multicomponent exercise program (n = 11) | At baseline (mean ± SD): - TUGT (s): 19.9 ± 8.0; Raise from a chair: 6.2 ± 4.1; Balance: 0.44 ± 0.5 - Gait velocity arithmetic task (m s−1):0.60 ± 0.08; Cognitive score (arithmetic): 2.1 ± 0.9; Gait velocity verbal task (m s−1): 0.53 ± 0.06; Cognitive score (verbal): 5.6 ± 1.7 - TUGT arithmetic task (m s−1):23.8 ± 11.4; Cognitive score (arithmetic): 2.3 ± 0.9; TUG verbal task (m s−1): 25.7 ± 11.5; Cognitive score (verbal): 6.2 ± 3.0 - Falls incidence: 0.77 ± 0.44 - Muscle cross sectional area quadriceps femoris total (mm2): 6.738 ± 1.609; Muscle cross sectional area knee flexor total (mm2): 2.256 ± 725After 12-week intervention (mean ± SD): - TUGT (s): 18.8 ± 7.9; Raise from a chair: 9.8 ± 6.0; Balance: 0.66 ± 0.5 - Gait velocity arithmetic task (m s−1):0.61 ± 0.07; Cognitive score (arithmetic): 2.6 ± 0.5; Gait velocity verbal task (m s−1): 0.59 ± 0.06; Cognitive score (verbal): 5.6 ± 1.0 - TUGT arithmetic task (m s−1):20.7 ± 7.0; Cognitive score (arithmetic): 2.4 ± 1.0; TUG verbal task (m s−1): 22.4 ± 8.5; Cognitive score (verbal): 6.5 ± 2.7 - Falls incidence: 0.0 ± 0.0 - Barthel Index deterioration 0.09 ± 0.30 - Muscle cross sectional area quadriceps femoris total (mm2): 7.004 ± 1.700; Muscle cross sectional area knee flexor total (mm2): 2.436 ± 685 | Group x time interaction:TUGT p < .01Rise from a chair p < .01Balance p < .05TUGT verbal task p < .01Incidence of falls p < .001Muscle cross sectional area quadriceps femoris total p < .05Muscle cross sectional area knee flexor total p < .01Between group difference:After training the control group was shown to be significantly more deteriorated on ADLs and to have significantly higher incidence of falls (p < 0.001).Within group change: In the intervention group improvement on TUGT e TUGT verbal task (both p < .05), raise from a chair (p < .01) and falls incidence (p < .001), and increase in muscle cross sectional area quadriceps femoris total (p < .05) and muscle cross sectional area knee flexor total (p < .01)In the control group decrease in gait velocity arithmetic and verbal tasks (both p < .05) |
| Mobility exercises (n = 13) | At baseline (mean ± SD): - TUGT (s): 18.4 ± 5.1; Raise from a chair: 6.3 ± 3.4; Balance: 0.36 ± 0.5 - Gait velocity arithmetic task (m s−1):0.56 ± 0.05; Cognitive score (arithmetic): 2.2 ± 0.8; Gait velocity verbal task (m s−1): 0.50 ± 0.05; Cognitive score (verbal): 5.5 ± 1.8 - TUGT arithmetic task (m s−1):22.7 ± 6.2; Cognitive score (arithmetic): 1.8 ± 1.0; TUG verbal task (m s−1): 22.8 ± 5.0; Cognitive score (verbal): 6.7 ± 2.7 - Falls incidence: 0.93 ± 0.3 - Muscle cross sectional area quadriceps femoris total (mm2): 6.879 ± 1.107; Muscle cross sectional area knee flexor total (mm2): 2.485 ± 679After 12-week intervention (mean ± SD): - TUGT (s): 21.8 ± 6.3; Raise from a chair: 5.4 ± 3.9; Balance: 0.3 ± 0.5 - Gait velocity arithmetic task (m s−1):0.49 ± 0.06; Cognitive score (arithmetic): 2.1 ± 0.9; Gait velocity verbal task (m s−1): 0.46 ± 0.06; Cognitive score (verbal): 5.6 ± 1.7 - TUGT arithmetic task (m s−1):23.5 ± 7.4; Cognitive score (arithmetic): 1.9 ± 0.8; TUG verbal task (m s−1): 26.1 ± 8.2; Cognitive score (verbal): 6.6 ± 1.0 - Falls incidence: 0.8 ± 0.4 - Barthel Index deterioration 0.60 ± 0.52 - Muscle cross sectional area quadriceps femoris total (mm2): 6.720 ± 1.071; Muscle cross sectional area knee flexor total (mm2): 2.375 ± 561 | ||
| Chan et al., 201242 | Exercise and nutrition consultation (n = 55) | At baseline (mean ± SD): - MMSE: 24.8 ± 3.9; PRIME-MD: 2.1 ± 3.2; Barthel Index: 98.8 ± 3.7; EQ-5D: 0.94 ± 0.08; Health resource utilization: 1.5 ± 1.7 - BMI (kg/m2): 25.0 ± 3.3; Fat free mass: 42.3 ± 7.0 - Bone mineral density > -1: 25%; Bone mineral density ≤ -1: 75% - Left one leg stand time (sec): 5.7 ± 6.9; Dominant leg extension power: 26.3 ± 5.1 - 25(OH) Vitamin D (ng/mL): 17.8 ± 5.3After 3-month intervention (change from baseline, mean ± SD): - PRIME-MD: −0.96 ± 2.92; Barthel Index: 1.09 ± 3.81; EQ-5D: 0.02 ± 0.08; Health resource utilization: 0.04 ± 1.36 - Left one leg stand time (sec): 2.86 ± 9.19; Dominant leg extension power: 3.06 ± 7.136 months after baseline assessment (change from baseline, mean ± SD): - PRIME-MD: −0.05 ± 2.84; Barthel Index: 0.36 ± 2.33; EQ-5D: −0.004 ± 0.12; Health resource utilization: 0.60 ± 1.81 - Left one leg stand time (sec): 2.57 ± 8.39; Dominant leg extension power: 3.69 ± 9.1512 months after baseline assessment (change from baseline, mean ± SD): - MMSE: −0.15 ± 2.53; PRIME-MD: −0.16 ± 3.17; Barthel Index: 0.55 ± 2.99; EQ-5D: 0.01 ± 0.09; Health resource utilization: 0.05 ± 1.8 - BMI (kg/m2): −0.31 ± 1.19; Fat free mass: −0.46 ± 1.36 - Bone mineral density > −1: 26%; Bone mineral density ≤ −1: 74% - Left one leg stand time (sec): 3.69 ± 9.15; Dominant leg extension power: −6.44 ± 10.08 - 25(OH) Vitamin D (ng/mL): 4.85 ± 7.69 | Between group change: Exercise and nutrition consultation vs educational booklet - Bone mineral density - 25(OH) Vitamin DProblem solving therapy vs educational booklet - Dominant leg extension power at 6 and 12 monthsWithin group change from baseline:Exercise and nutrition consultation group: - at 3 months Barthel Index, one leg stand time (both p < .05), dominant leg extension power (p < .01) - at 6 months one leg stand time (p < .05) - at 12 months BMI, fat free mass (both p < .05), one leg stand time (p < .01), dominant leg extension power and 25(OH) Vitamin D (both p < .001)Problem solving therapy group: - at 3 months PRIME-MD, Barthel Index (both p < .05), dominant leg extension power (p < .001) - at 6 months one leg stand time (p < .05), and dominant leg extension power (p < .01) - at 12 months BMI (p < .05), fat free mass and dominant leg extension power (both p < .01), one leg stand time and 25(OH) Vitamin D (p < .001)Educational booklet group (control condition for exercise and nutrition and consultation): - at 3 months EQ-5D and dominant leg extension power (both p < .05), PRIME-MD and Barthel Index (both p < .01) - at 12 months fat free mass and one leg stand time (both p < .01), dominant leg extension power (p < .001)Educational booklet group (control condition for problem solving therapy): - at 3 months EQ-5D and Barthel Index (both p < .01) - at 12 months fat free mass and one leg stand time (both p < .05), 25(OH) Vitamin D (p < .001), dominant leg extension power (p < .001) |
| Problem solving therapy (n = 57) | At baseline (mean ± SD): - MMSE: 24.7 ± 3.8; PRIME-MD: 2.7 ± 3.3; Barthel Index: 98.2 ± 5.4; EQ-5D: 0.95 ± 0.08; Health resource utilization: 1.6 ± 1.7 - BMI (kg/m2): 25.0 ± 3.8; Fat free mass: 42.2 ± 7.3 - Bone mineral density > −1: 23%; Bone mineral density ≤ −1: 77% - Left one leg stand time (sec): 5.3 ± 6.7; Dominant leg extension power: 23.9 ± 6.5 - 25(OH) Vitamin D (ng/mL): 17.9 ± 5.5After 3-month intervention (change from baseline, mean ± SD): - PRIME-MD: −1.32 ± 3.64; Barthel Index: 1.05 ± 3.98; EQ-5D: 0.01 ± 0.09; Health resource utilization: −0.07 ± 1.67 - Left one leg stand time (sec): 2.38 ± 8.91; Dominant leg extension power: 3.42 ± 7.366 months after baseline assessment (change from baseline, mean ± SD): - PRIME-MD: −0.42 ± 2.96; Barthel Index: 0.88 ± 4.13; EQ-5D: 0.0001 ± 0.09; Health resource utilization: 0.42 ± 1.74 - Left one leg stand time (sec): 3.10 ± 8.93; Dominant leg extension power: 2.71 ± 6.0812 months after baseline assessment (change from baseline, mean ± SD): - MMSE: −0.05 ± 2.35; PRIME-MD: −0.77 ± 3.27; Barthel Index: 0.88 ± 3.29; EQ-5D: 0.01 ± 0.07; Health resource utilization: 0.39 ± 2.05 - BMI (kg/m2): −0.36 ± 1.15; Fat free mass: −0.59 ± 1.30 - Bone mineral density > −1: 20%; Bone mineral density ≤ −1: 80% - Left one leg stand time (sec): 4.31 ± 10.23; Dominant leg extension power: −3.52 ± 9.65 - 25(OH) Vitamin D (ng/mL): 3.4 ± 7.80 | ||
| Educational booklet (control condition for exercise and nutrition consultation) (n = 62) | At baseline (mean ± SD): - MMSE: 24.1 ± 3.9; PRIME-MD: 2.8 ± 3.5; Barthel Index: 97.9 ± 5.4; EQ-5D: 0.94 ± 0.08; Health resource utilization: 1.7 ± 2.2 - BMI (kg/m2): 25.8 ± 3.9; Fat free mass: 43.6 ± 7.9 - Bone mineral density > −1: 16%; Bone mineral density ≤ −1: 84% - Left one leg stand time (sec): 5.8 ± 5.9; Dominant leg extension power: 25.2 ± 6.8 - 25(OH) Vitamin D (ng/mL): 17.2 ± 6.2After 3-month intervention (change from baseline, mean ± SD): - PRIME-MD: −1.29 ± 4.50; Barthel Index: 1.53 ± 4.11; EQ-5D: 0.03 ± 0.08; Health resource utilization: −0.35 ± 2.70 - Left one leg stand time (sec): 0.92 ± 9.01; Dominant leg extension power: 1.72 ± 6.66 months after baseline assessment (change from baseline, mean ± SD): - PRIME-MD: −0.65 ± 4.03; Barthel Index: 0.73 ± 4.78; EQ-5D: 0.004 ± 0.12; Health resource utilization: 0.03 ± 2.55 - Left one leg stand time (sec): 1.81 ± 8.47; Dominant leg extension power: 1.35 ± 7.0012 months after baseline assessment (change from baseline, mean ± SD): - MMSE: 0.06 ± 2.52; PRIME-MD: −0.77 ± 3.65; Barthel Index: 0.89 ± 3.68; EQ-5D: 0.02 ± 0.10; Health resource utilization: 0.03 ± 2.44 - BMI (kg/m2): −0.18 ± 1.05; Fat free mass: −0.62 ± 1.84 - Bone mineral density > −1: 11%; Bone mineral density ≤ −1: 89% - Left one leg stand time (sec): 3.43 ± 9.15; Dominant leg extension power: −4.44 ± 8.59 - 25(OH) Vitamin D (ng/mL): 1.19 ± 5.41 | ||
| Educational booklet (control condition for problem solving therapy) (n = 60) | At baseline (mean ± SD): - MMSE: 24.2 ± 4.0; PRIME-MD: 2.3 ± 3.4; Barthel Index: 98.4 ± 4.0; EQ-5D: 0.93 ± 0.08; Health resource utilization: 1.7 ± 2.2 - BMI (kg/m2): 25.8 ± 3.5; Fat free mass: 43.7 ± 7.6 - Bone mineral density > −1: 17%; Bone mineral density ≤ -1: 83% - Left one leg stand time (sec): 6.2 ± 6.0; Dominant leg extension power: 27.4 ± 5.1 - 25(OH) Vitamin D (ng/mL): 17.2 ± 6.0After 3-month intervention (change from baseline, mean ± SD): - PRIME-MD: −0.97 ± 4.03; Barthel Index: 1.58 ± 3.96; EQ-5D: 0.03 ± 0.08; Health resource utilization: −0.27 ± 2.58 - Left one leg stand time (sec): 1.34 ± 9.34; Dominant leg extension power: 1.33 ± 6.326 months after baseline assessment (change from baseline, mean ± SD): - PRIME-MD: −0.32 ± 4.00; Barthel Index: 0.25 ± 3.50; EQ-5D: 0.001 ± 0.14; Health resource utilization: 0.18 ± 2.65 - Left one leg stand time (sec): 1.30 ± 7.84; Dominant leg extension power: 0.18 ± 6.6812 months after baseline assessment (change from baseline, mean ± SD): - MMSE: −0.02 ± 2.69; PRIME-MD: −0.22 ± 3.58; Barthel Index: 0.58 ± 3.46; EQ-5D: 0.02 ± 0.11; Health resource utilization: −0.28 ± 2.25 - BMI (kg/m2): −0.13 ± 1.08; Fat free mass: −0.50 ± 1.90 - Bone mineral density > −1: 17%; Bone mineral density ≤ −1: 83% - Left one leg stand time (sec): 2.84 ± 7.92; Dominant leg extension power: −7.14 ± 8.74 - 25(OH) Vitamin D (ng/mL): 2.49 ± 5.85 | ||
| Clegg, et al., 201443 | Home-based exercise program (n = 40) | At baseline (mean ± SD): - Barthel Index: 15.9 ± 3.7; EQ-5D: 0.53 ± 0.30; GDS-15: 3.8 ± 2.7After 12-week intervention (mean ± SD): - Barthel Index: 15.6 ± 4.0; EQ-5D: 0.51 ± 0.34; GDS-15: 3.4 ± 3.3 | Between group change: no significant difference |
| Usual care (n = 30) | At baseline (mean ± SD): - Barthel Index: 15.8 ± 4.1; EQ-5D: 0.52 ± 0.25; GDS-15: 5.0 ± 3.2After 12-week intervention (mean ± SD): - Barthel Index: 15.0 ± 4.0; EQ-5D: 0.46 ± 0.26; GDS-15: 4.8 ± 3.0 | ||
| Cohen, et al., 200244 | Inpatient geriatric care in multidisciplinary evaluation and management units (not clear) | At baseline vs After intervention (mean change in score): - SF-36 subscales Physical Functioning: −1.5; Physical limitations: 4.5; Emotional limitations: 13.0; Bodily pain: 15.3; Energy: 0.8; Mental health: −0.3; Social activity: 2.7; General health: −0.02At baseline vs 12 months after randomization (mean change in score): - SF-36 subscales Physical Functioning: 6.7; Physical limitations: 34.0; Emotional limitations: 22.0; Bodily pain: 24.9; Energy: 4.5; Mental health: 4.5; Social activity: 18.3; General health: −5.5 - Probability of survival: 78.4% ± 1.6; - Number of days in the hospital: 35.3 ± 1.4; number of days of initial hospitalization: 23.2 ± 1; number of medical consultations: 2.8 (SD not provided); number of surgical consultations: 2.1 (SD not provided); number of days in long-term care: 15.0 ± 1.8 | Between group change:In the inpatient groups - at discharge: physical functioning (p = .006), bodily pain (p = .001), energy (p = .01), and general health (p = .006) - at 12 months: bodily pain (p = .01) - intervention group had greater number of days in the hospital and longer initial hospitalization (both p < .001), and also higher number of medical and surgical consultations (both p < .001), but lower number of days in long term care (p = 0.03)In the outpatient groups - at discharge: energy (p = .02) and general health (p = .04) - at 12 months: energy (p = .009), mental health (p = .001) and general health (p = .01) |
| Outpatient care in geriatric evaluation and management clinics (not clear) | At baseline vs after intervention (mean change in score): - SF-36 subscales Physical Functioning: −3.7; Physical limitations: 5.9; Emotional limitations: 13.4; Bodily pain: 11.9; Energy: 0.8; Mental health: −0.6; Social activity: 2.4; General health: −0.5At baseline vs 12 months after randomization (mean change in score): - SF-36 subscales Physical Functioning: 6.8; Physical limitations: 31.3; Emotional limitations: 22.1; Bodily pain: 21.9; Energy: 5.4; Mental health: 6.3; Social activity: 18.3; General health: −4.4 - Probability of survival: 78.0% ± 1.6 - Number of days in long-term care: 15.4 ± 1.8 | ||
| Usual inpatient care (not clear) | At baseline vs after intervention (mean change in score): - SF-36 subscales Physical Functioning: −5.4; Physical limitations: 4.7; Emotional limitations: 12.0; Bodily pain: 9.2; Energy: −2.6; Mental health: −1.5; Social activity: 1.0; General health: −3.4At baseline vs 12 months after randomization (mean change in score): - SF-36 subscales Physical Functioning: 4.5; Physical limitations: 29.8; Emotional limitations: 20.3; Bodily pain: 20.0; Energy: 1.8; Mental health: 2.5; Social activity: 16.4; General health: −7.1 - Probability of survival: 78.8% ± 1.6 - Number of days in the hospital: 28.3 ± 1.4; number of days of initial hospitalization: 15.0 ± 0.9; number of medical consultations: 1.3 (SD not provided); number of surgical consultations: 1.2 (SD not provided); number of days in long-term care: 17.1 ± 1.8 | ||
| Usual outpatient care (not clear) | At baseline vs after intervention (mean change in score): - SF-36 subscales Physical Functioning: −3.2; Physical limitations: 3.2; Emotional limitations: 11.6; Bodily pain: 12.8; Energy: −2.5; Mental health: −1.2; Social activity: 1.2; General health: −2.9At baseline vs 12 months after randomization (mean change in score): - SF-36 subscales Physical Functioning: 4.5; Physical limitations: 32.5; Emotional limitations: 20.2; Bodily pain: 22.9; Energy: 1.0; Mental health: 0.8; Social activity: 16.4; General health: −8.2 - Probability of survival: 79.2% ± 1.5 - Number of days in long-term care: 16.8 ± 1.8 | ||
| Eklund, et al., 201329 | Continuum Care by multi-professional team (n = 85) | At baseline: - ADL independent in all activities: 20%3 months after discharge: - ADL improvement: 42%; ADL maintained level: 38%; ADL decrease: 20%6 months after discharge: - ADL improvement: 36%; ADL maintained level: 32%; ADL decrease: 31%12 months after discharge: - ADL improvement: 39% ADL maintained level: 24%; ADL decrease: 38% | Between group change:Intervention group as compared to controls was more likely to improve degree of ADL independence at 3 and 12 months (OR = 2.37, 95% CI 1.20–4.68; OR = 2.04, 95% CI 1.03–4.06, respectively), and less likely to decrease degree of ADL independence at 3 and 6 months (OR = 0.51, 95% CI 0.25–1.04; OR = 0.52, 95% CI 0.27–0.98, respectively) |
| Usual care (n = 76) | At baseline: - ADL independent in all activities: 26%3 months after discharge: - ADL improvement: 24%; ADL maintained level: 43%; ADL decrease: 33%6 months after discharge: - ADL improvement: 28%; ADL maintained level: 26%; ADL decrease: 46%12 months after discharge: - ADL improvement: 24%; ADL maintained level: 29%; ADL decrease: 47% | ||
| Fairhall, et al., 201532 | Multifactorial interdisciplinary intervention (n = 120) | At baseline: (mean ± SD): - EQ-5D: 0.67 ± 0.23At 3 months: - EQ-5D: 0.56 ± 0.31At 12 months: - EQ-5D: 0.49 ± 0.32 | Between group change: no significant difference |
| Usual care (n = 121) | At baseline: (mean ± SD): - EQ-5D: 0.66 ± 0.23At 3 months: - EQ-5D: 0.47 ± 0.34At 12 months: - EQ-5D: 0.47 ± 0.34 | ||
| Giné-Garriga, et al., 201046 | Functional circuit-training program (n = 22) | At baseline (mean ± SD): - Balance - semitandem (s): 6.67 ± 0.63; tandem (s): 3.21 ± 0.67; single leg (s): 2.39 ± 0.57 - Gait – normal speed (m/s): 0.82 ± 0.04; fast speed (m/s): 1.11 ± 0.06 - Modified TUGT – assessment questionnaire: 9.32 ± 0.40; total time (s): 38.04 ± 1.32; stand up (s): 2.59 ± 0.17; ball kick time (s): 3.00 ± 0.28; kick 8 m (s): 14.76 ± 0.80; total time kick (s): 24.28 ± 0.83; left knee extensor maximal voluntary contraction (Nm/kg): 0.77 ± 0.06After 12-week intervention (mean ± SD): - Balance - semitandem (s): 9.27 ± 0.63; tandem (s): 6.60 ± 0.67; single leg (s): 6.60 ± 0.57 - Gait – normal speed (m/s): 0.94 ± 0.04; fast speed (m/s): 1.28 ± 0.06 - Modified TUGT – assessment questionnaire: 11.82 ± 0.40; total time (s): 35.04 ± 1.32; stand up (s): 2.02 ± 0.17; ball kick time (s): 2.80 ± 0.28; kick 8 m (s): 12.27 ± 0.80; total time kick (s): 22.77 ± 0.83; left knee extensor maximal voluntary contraction (Nm/kg): 0.92 ± 0.06At 36 weeks (mean ± SD): - Balance - semitandem (s): 8.49 ± 0.68; tandem (s): 5.40 ± 0.72; single leg (s): 3.10 ± 0.64 - Gait – normal speed (m/s): 0.88 ± 0.04; fast speed (m/s): 1.23 ± 0.06 - Modified TUGT – assessment questionnaire: 10.28 ± 0.42; total time (s): 37.50 ± 1.32; stand up (s): 2.55 ± 0.18; ball kick time (s): 2.97 ± 0.30; kick 8 m (s): 13.90 ± 0.81; total time kick (s): 23.40 ± 0.85; left knee extensor maximal voluntary contraction (Nm/kg): 0.82 ± 0.06 | Group x time interaction:Semitandem p < .01 For week 0 – week 12 p < .001 For week 0 – week 36 p = .021Tandem p = .003 For week 0 – week 12 p < .001 For week 0 – week 36 p = .001Single leg p < .001 For week 0 – week 12 p < .001 For week 12 – week 36 p < .001Gait normal speed p < .001 For week 0 – week 12 p < .001 For week 0 – week 36 p = .003 For week 12 – week 36 p = .002Gait fast speed p < .001 For week 0 – week 12 p < .001 For week 0 – week 36 p < .001TUGT assessment questionnaire p < .001 For week 0 – week 12 p < .001 0 – week 36 p = .009 For week 12 – week 36 p < .001TUGT total time p < .001 For week 0 – week 12 p < .001 For week 0 – week 36 p = .01 12 – week 36 p < .001TUGT stand up p = .001 For week 0 – week 12 p = .001 For week 12 – week 36 p = .005TUGT kick 8 m p < .001 For week 0 – week 12 p < .001 For week 12 – week 36 p < .001TUGT total time kick p < .001 For week 0 – week 12 p = .001Left knee extensor maximal voluntary contraction p < .001 For week 0 – week 12 p < .001 For week 12 – week 36 p = .031 |
| Health education meeting and usual care (n = 19) | At baseline (mean ± SD): - Balance - semitandem (s): 7.26 ± 0.68; tandem (s): 3.11 ± 0.73; single leg (s): 2.05 ± 0.62 - Gait – normal speed (m/s): 0.82 ± 0.04; fast speed (m/s): 1.13 ± 0.06 - Modified TUGT – assessment questionnaire: 8.63 ± 0.44; total time (s): 39.34 ± 1.42; stand up (s): 2.91 ± 0.18; ball kick time (s): 3.73 ± 0.31; kick 8 m (s): 14.22 ± 0.86; total time kick (s): 25.44 ± 0.89; left knee extensor maximal voluntary contraction (Nm/kg): 0.75 ± 0.06After 12-week intervention (mean ± SD): - Balance - semitandem (s): 6.23 ± 0.68; tandem (s): 1.98 ± 0.73; single leg (s): 1.98 ± 0.62 - Gait – normal speed (m/s): 0.80 ± 0.04; fast speed (m/s): 1.07 ± 0.06 - Modified TUGT – assessment questionnaire: 8.37 ± 0.44; total time (s): 41.34 ± 1.42; stand up (s): 3.18 ± 0.18; ball kick time (s): 3.82 ± 0.31; kick 8 m (s): 14.75 ± 0.86; total time kick (s): 26.59 ± 0.89; left knee extensor maximal voluntary contraction (Nm/kg): 0.71 ± 0.06At 36 weeks (mean ± SD): - Balance - semitandem (s): 4.34 ± 0.97; tandem (s): 1.64 ± 0.96; single leg (s): 0.38 ± 0.65 - Gait – normal speed (m/s): 0.81 ± 0.04; fast speed (m/s): 1.08 ± 0.07 - Modified TUGT – assessment questionnaire: 8.37 ± 0.55; total time (s): 41.95 ± 1.44; stand up (s): 3.44 ± 0.25; ball kick time (s): 3.86 ± 0.38; kick 8 m (s): 14.66 ± 0.95; total time kick (s): 26.27 ± 1.01; left knee extensor maximal voluntary contraction (Nm/kg): 0.61 ± 0.07 | ||
| Gustafsson, et al., 201247 | Multi-professional senior group (n = 171) | At baseline vs 3 months after intervention: - No deterioration in self-related health: 88% - No deterioration in ADLs: 79% | Between group change:Preventive visit group as compared to controls was more likely to no deteriorate in self-rated health (OR = 2.21, 95% CI 1.12–4.37, p = .02);Senior meetings group as compared to controls was more likely to no deteriorate in self-rated health (OR = 1.81, 95% CI 0.93–3.49, p = .08) and ADLs (OR = 1.95, 95% CI 1.14–3.33, p = .01)Senior meetings group as compared to preventive visit group was more likely to maintain independence in ADLs (OR = 1.92, 95% CI 1.19–3.12, p = .01) |
| Single preventive home visit (n = 174) | At baseline vs 3 months after intervention: - No deterioration in self-related health: 90%- No deterioration in ADLs: 66% | ||
| Ordinary range of community services (n = 114) | At baseline vs 3 months after intervention: - No deterioration in self-related health: 81% - No deterioration in ADLs: 66% | ||
| Hars et al., 201448 | Continued intervention of music-based multitask training (n = 23) | At baseline (mean ± SD): - BMI (kg/m2): 25.3 ± 4.0 - History of falls after 65 years: 96%; falls in the past 12 months: 57%; medication > 4: 39% - Physical activity level (kcal/week): 2304 ± 1010; SF-12: 31.4 ± 1.9; Mini Nutritional Assessment: 12.8 ± 1.3; HADS: 10.7 ± 5.8; MMSE: 27.1 ± 2.4; Clock-drawing test: 9.0 ± 1.7; self-rated health status: 2.6 ± 0.8 - Single Task Condition - Gait speed velocity (cm/s): 111.5 ± 19.4; Dynamic balance double support phase (%): 22.6 ± 3.2; Dynamic balance support base (cm): 7.1 ± 2.5 - Dual Task Condition - Gait speed velocity (cm/s): 93.2 ± 27.2; - One-legged stance task (s): 7.3 ± 3.3; TUGT (s): 9.7 ± 2.2; Simplified Tinetti test: 0.3 ± 0.5After 1-year intervention (mean ± SD): - BMI (kg/m2): 25.1 ± 3.8 - History of falls after 65 years: 96%; falls in the past 12 months: 52%; medication > 4: 43% - Physical activity level (kcal/week): 2312 ± 999; SF-12: 31.9 ± 2.3; Mini Nutritional Assessment: 12.5 ± 1.8; HADS: 9.4 ± 4.9; MMSE: 28.0 ± 2.2; Clock-drawing test: 8.4 ± 1.7; self-rated health status: 2.7 ± 0.9 - Single Task Condition - Gait speed velocity (cm/s): 113.3 ± 19.0; Dynamic balance double support phase (%): 20.5 ± 3.9; Dynamic balance support base (cm): 6.7 ± 2.3 - Dual Task Condition - Gait speed velocity (cm/s): 97.9 ± 19.5; - One-legged stance task (s): 8.8 ± 2.0; TUGT (s): 9.3 ± 1.9; Simplified Tinetti test: 0.0 ± 0.2; Five-times-sit-to-stand test (s): 11.8 ± 3.43 years after intervention (mean ± SD): - BMI (kg/m2): 24.2 ± 3.8 - History of falls after 65 years: 96%; falls in the past 12 months: 22%; participants with ≥ 1 falls: 70%; participants with ≥ 2 falls: 35%; medication > 4: 35% - Physical activity level (kcal/week): 2022 ± 789; SF-12: 31.8 ± 3.1; Mini Nutritional Assessment: 13.2 ± 1.2; HADS: 8.0 ± 4.8; MMSE: 27.4 ± 2.3; Clock-drawing test: 8.9 ± 1.4; self-rated health status: 2.7 ± 0.6 - Single Task Condition - Gait speed velocity (cm/s): 118.3 ± 21.1; Dynamic balance double support phase (%): 25.5 ± 3.0; Dynamic balance support base (cm): 7.4 ± 3.0 - Dual Task Condition - Gait speed velocity (cm/s): 102.7 ± 22.1; - One-legged stance task (s): 9.3 ± 2.0; TUGT (s): 10.0 ± 2.0; Simplified Tinetti test: 0.1 ± 0.5; Five-times-sit-to-stand test (s): 11.8 ± 2.6 | Between group change:Continued intervention group as compared to discontinued intervention group had lower relative risk of falls (= 0.69, 95%CI 0.53–0.91, p = .008) and multiple falls (= 0.53, 95%CI 0.29–0.99, p = .045)Within group change from baseline to 4-year follow-up:Continued intervention - gait velocity in single-task and dual-task conditions, dynamic balance - double support phase, Simplified Tinetti test (all p < .05)Discontinued intervention - dynamic balance - double support phase and support base, TUGT (all p < .05)Linear mixed-effect analysis on change from 1 year to 4 years: - Single-task condition gait speed p < .001 - Dynamic balance double support phase p = .022 - Dual-task condition gait speed p = .012 - One-legged stance task p = .013 - TUGT p = .001 - Five-times-sit-to-stand test p < .001 |
| Discontinued intervention of music-based multitask training (n = 29) | At baseline (mean ± SD): - BMI (kg/m2): 26.4 ± 3.5 - History of falls after 65 years: 83%; falls in the past 12 months: 59%; medication > 4: 34% - Physical activity level (kcal/week): 2501 ± 1191; SF-12: 30.8 ± 2.8; Mini Nutritional Assessment: 12.6 ± 1.5; HADS: 10.1 ± 5.3; MMSE: 25.9 ± 3.1; Clock-drawing test: 8.8 ± 1.0; self-rated health status: 2.7 ± 0.8 - Single Task Condition - Gait speed velocity (cm/s): 102.1 ± 16.5; Dynamic balance double support phase (%): 24.0 ± 3.4; Dynamic balance support base (cm): 8.4 ± 3.5 - Dual Task Condition - Gait speed velocity (cm/s): 78.5 ± 17.6; - One-legged stance task (s): 6.7 ± 3.4; TUGT (s): 10.5 ± 2.1; Simplified Tinetti test: 1.0 ± 1.1After 1-year intervention (mean ± SD): - BMI (kg/m2): 26.3 ± 4.1 - History of falls after 65 years: 90%; falls in the past 12 months: 66%; medication > 4: 45% - Physical activity level (kcal/week): 2586 ± 1216; SF-12: 31.9 ± 2.2; Mini Nutritional Assessment: 12.6 ± 1.7; HADS: 9.3 ± 3.8; MMSE: 27.6 ± 1.6; Clock-drawing test: 8.7 ± 1.5; self-rated health status: 2.8 ± 0.8 - Single Task Condition - Gait speed velocity (cm/s): 108.5 ± 17.4; Dynamic balance double support phase (%): 22.5 ± 3.0; Dynamic balance support base (cm): 8.9 ± 3.4 - Dual Task Condition - Gait speed velocity (cm/s): 88.1 ± 15.9; - One-legged stance task (s): 8.2 ± 2.6; TUGT (s): 9.8 ± 1.8; Simplified Tinetti test: 0.7 ± 1.1; Five-times-sit-to-stand test (s): 12.3 ± 2.63 years after intervention (mean ± SD): - BMI (kg/m2): 25.0 ± 4.9 - History of falls after 65 years: 93%; falls in the past 12 months: 48%; participants with ≥ 1 falls: 86%; participants with ≥ 2 falls: 66%; medication > 4: 17% - Physical activity level (kcal/week): 2379 ± 1138; SF-12: 31.1 ± 2.7; Mini Nutritional Assessment: 13.1 ± 1.0; HADS: 8.6 ± 3.4; MMSE: 26.9 ± 2.3; Clock-drawing test: 9.2 ± 1.2; self-rated health status: 2.8 ± 0.6 - Single Task Condition - Gait speed velocity (cm/s): 97.9 ± 17.4; Dynamic balance double support phase (%): 29.6 ± 4.2; Dynamic balance support base (cm): 9.4 ± 3.7 - Dual Task Condition - Gait speed velocity (cm/s): 81.5 ± 18.8; - One-legged stance task (s): 6.8 ± 3.4; TUGT (s): 12.7 ± 3.7; Simplified Tinetti test: 1.0 ± 1.0; Five-times-sit-to-stand test (s): 14.7 ± 4.1 | ||
| Kim, et al., 201549 | Milk fat globule membrane (MFGM) supplementation (n = 32) | At baseline (mean ± SD): - Skeletal muscle mass (kg): 13.4 ± 1.7; leg muscle mass (kg): 10.1 ± 1.3 - Grip strength (kg): 17.5 ± 2.7; knee extension strength (N): 185.2 ± 52.1; usual walking speed (s): 4.9 ± 1.2; TUGT (s): 8.8 ± 2.8 - BDNF (ng/ml): 6.97 ± 0.94; (IGFBP3/IGF1)x100: 3.97 ± 1.36After 3-month intervention: - Skeletal muscle mass (kg): 13.27 ± 1.63; leg muscle mass (kg): 9.23 ± 3.01 - Grip strength (kg): 18.37 ± 1.92; knee extension strength (N): 186.42 ± 60.47; usual walking speed (s): 1.08 ± 0.23; TUGT (s): 10.53 ± 2.77 - BDNF (ng/ml): 7.11 ± 1.05; (IGFBP3/IGF1)x100: 4.11 ± 1.624 months after intervention: - Skeletal muscle mass (kg): 13.54 ± 1.76; leg muscle mass (kg): 10.23 ± 1.37 - Grip strength (kg): 16.75 ± 2.24; knee extension strength (N): 181.26 ± 51.38; usual walking speed (s): 1.11 ± 0.20; TUGT (s): 7.76 ± 1.52 - BDNF (ng/ml): 7.39 ± 1.47; (IGFBP3/IGF1)x100: 4.24 ± 1.51 | Group x Time interaction:Usual walking speed p = .005 (exercise + MFGM > MFGM)TUGT p < .001 (exercise + MFGM, exercise + placebo > MFGM, placebo)(IGFBP3/IGF1)x100 p = .013 (Ex+MFGM > placebo) |
| Exercise + placebo (n = 33) | At baseline (mean ± SD): - Skeletal muscle mass (kg): 13.8 ± 1.7; leg muscle mass (kg): 10.5 ± 1.3 - Grip strength (kg): 17.8 ± 2.8; knee extension strength (N): 179.1 ± 40.9; usual walking speed (s): 4.6 ± 0.9; TUGT (s): 8.2 ± 2.0 - BDNF (ng/ml): 6.37 ± 1.44; (IGFBP3/IGF1)x100: 4.18 ± 1.46After 3-month intervention: - Skeletal muscle mass (kg): 14.04 ± 1.77; leg muscle mass (kg): 10.34 ± 2.40 - Grip strength (kg): 18.36 ± 3.28; knee extension strength (N): 188.45 ± 47.82; usual walking speed (s): 1.26 ± 0.27; TUGT (s): 7.87 ± 1.83 - BDNF (ng/ml): 7.07 ± 1.01; (IGFBP3/IGF1)x100: 4.90 ± 2.464 months after intervention: - Skeletal muscle mass (kg): 14.31 ± 2.08; leg muscle mass (kg): 10.93 ± 1.68 - Grip strength (kg): 17.75 ± 2.9; knee extension strength (N): 190.32 ± 46.2; usual walking speed (s): 1.21 ± 0.22; TUGT (s): 7.04 ± 1.45 - BDNF (ng/ml): 7.03 ± 1.66; (IGFBP3/IGF1)x100: 5.36 ± 1.73 | ||
| Exercise + milk fat globule membrane (MFGM) supplementation (n = 33) | At baseline (mean ± SD): - Skeletal muscle mass (kg): 13.2 ± 1.5; leg muscle mass (kg): 10.1 ± 1.1 - Grip strength (kg): 17.1 ± 3.9; knee extension strength (N): 178.8 ± 55.2; usual walking speed (s): 4.5 ± 0.9; TUGT (s): 7.7 ± 1.7 - BDNF (ng/ml): 6.60 ± 1.54; (IGFBP3/IGF1)x100: 5.50 ± 2.28After 3-month intervention: - Skeletal muscle mass (kg): 13.51 ± 1.61; leg muscle mass (kg): 10.30 ± 1.21 - Grip strength (kg): 17.83 ± 4.05; knee extension strength (N): 191.52 ± 54.81; usual walking speed (s): 1.25 ± 0.24; TUGT (s): 7.98 ± 1.44 - BDNF (ng/ml): 7.18 ± 1.09; (IGFBP3/IGF1)x100: 5.02 ± 1.964 months after intervention: - Skeletal muscle mass (kg): 13.64 ± 1.69; leg muscle mass (kg): 10.41 ± 1.36 - Grip strength (kg): 17.00 ± 3.88; knee extension strength (N): 178.72 ± 45.92; usual walking speed (s): 1.23 ± 0.21; TUGT (s): 6.93 ± 1.61 - BDNF (ng/ml): 7.68 ± 1.17; (IGFBP3/IGF1)x100: 4.63 ± 1.89 | ||
| Placebo (n = 32) | At baseline (mean ± SD): - Skeletal muscle mass (kg): 13.4 ± 1.6; leg muscle mass (kg): 10.1 ± 1.2 - Grip strength (kg): 18.7 ± 3.2; knee extension strength (N): 184.7 ± 50.1; usual walking speed (s): 4.7 ± 1.5; TUGT (s): 8.5 ± 3.5 - BDNF (ng/ml): 6.10 ± 1.47; (IGFBP3/IGF1)x100: 4.65 ± 1.72After 3-month intervention: - Skeletal muscle mass (kg): 13.55 ± 1.67; leg muscle mass (kg): 10.28 ± 1.30 - Grip strength (kg): 19.18 ± 3.50; knee extension strength (N): 194.32 ± 54.14; usual walking speed (s): 1.13 ± 0.22; TUGT (s): 10.0 ± 4.32 - BDNF (ng/ml): 6.36 ± 1.31; (IGFBP3/IGF1)x100: 5.38 ± 1.934 months after intervention: - Skeletal muscle mass (kg): 13.70 ± 1.75; leg muscle mass (kg): 10.39 ± 1.38 - Grip strength (kg): 18.08 ± 2.92; knee extension strength (N): 199.95 ± 52.65; usual walking speed (s): 1.18 ± 0.23; TUGT (s): 7.99 ± 3.79 - BDNF (ng/ml): 6.52 ± 1.33; (IGFBP3/IGF1)x100: 5.20 ± 1.91 | ||
| Kim & Lee, 201323 | Protein-energy supplementation (n = 41) | At baseline (mean ± SD): - Usual gait speed (m/s): 0.35 ± 0.13; TUGT (s): 22.2 ± 12.4; grip strength (kg): 15.3 ± 4.6; one-legged stance (s): 3.4 ± 2.8 - Mid-arm circumference: 24.7 ± 3.3 - Serum blood urea nitrogen (mgl/dL): 17.3 ± 8.4; creatinine clearance (mL/min): 36.2 ± 12.7After 12-week intervention (mean ± SD): - Usual gait speed (m/s): 0.35 ± 0.13; TUGT (s): 21.4 ± 12.2; grip strength (kg): 15.1 ± 4.8; one-legged stance (s): 2.6 ± 1.9 - Mid-arm circumference: 25.8 ± 2.7 - Serum blood urea nitrogen (mgl/dL): 19.3 ± 8.2; creatinine clearance (mL/min): 39.1 ± 15.5 | Between group change: Gait speed p = .039;TUGT p = .038 |
| No intervention (n = 43) | At baseline (mean ± SD): - Usual gait speed (m/s): 0.38 ± 0.13; TUGT (s): 21.5 ± 12.7; grip strength (kg): 16.3 ± 5.0; one-legged stance (s): 3.9 ± 3.5 - Mid-arm circumference: 23.2 ± 2.6 - Serum blood urea nitrogen (mgl/dL): 19.0 ± 12.1; creatinine clearance (mL/min): 34.9 ± 13.5After 12-week intervention (mean ± SD): - Usual gait speed (m/s): 0.32 ± 0.14; TUGT (s): 26.4 ± 25.3; grip strength (kg): 16.4 ± 5.3; one-legged stance (s): 3.5 ± 3.5 - Mid-arm circumference: 24.3 ± 2.7 - Serum blood urea nitrogen (mgl/dL): 19.9 ± 9.9; creatinine clearance (mL/min): 36.7 ± 13.8 | ||
| Li, et al., 201050 | Screening evaluation and intervention (n = 129) | At baseline (mean ± SD): - ADL: 95.7 ± 15.76 months after baseline assessment: - ADL maintained level: 86.3%; ADL improvement: 4.6%; ADL decrease: 9.2% | Deterioration in ADL: no significant differences |
| Screening evaluation (n = 140) | At baseline (mean ± SD): - ADL: 92.8 ± 19.46 months after baseline assessment: - ADL maintained level: 88.6%; ADL improvement: 1.4%; ADL decrease: 10.o% | ||
| Monteserin et al., 201051 | Group sessions (n = 157) | At baseline: - GDS-5 > 1: 21.8%; - comorbidity: without 47.1%, slight: 26.6%; moderate: 12.3%; severe: 14.0%After 18-month intervention: - GDS-5 > 1: 26.7%; - Death or admission to nursing home or admission to home care program: 15.9%, but for subgroup at risk of frailty: 16.3% | Between group change:For all participants - GDS-5 (p = .048), with control group > intervention groupFor participants at risk of frailty - death or admission to nursing home or admission to home care program (p = .028), with control group > intervention group |
| Individual sessions with geriatrician (n = 151) | |||
| Usual care (n = 312, from which 134 at risk of frailty and 178 at non-risk of frailty) | At baseline: - GDS-5 > 1: 22.1% - comorbidity: without 50.6%, slight: 24.7%; moderate: 16.7%; severe: 8.0%After 18-month intervention: - GDS-5 > 1: 35.8%; - Death or admission to nursing home or admission to home care program: 17.4%, but for subgroup at risk of frailty: 28.4% | ||
| Muller et al., 200652 | Atamestane + dehydroepiandrosterone (DHEA) (n = 26) | At baseline (mean ± SD): - ADL (HAQ score): 10.7 ± 4.4; MMSE: 27.7 ± 2.4 - Bone mineral density (total body): 1.16 ± 0.07 - Fat mass (kg): 20.4 ± 5.9; Lean mass (kg): 49.0 ± 46.1; BMI (kg/m2): 24.9 ± 3.1 - Atherosclerosis (mm) – common carotid: 1.01 ± 0.14; bifurcation: 1.36 ± 0.56 - Blood pressure (mm Hg) – systolic: 156.7 ± 19.3; diastolic: 84.2 ± 10.7 - DHEA (nmol/liter): 17.7 ± 14.5; total testosterone (nmol/liter): 12.8 ± 3.3; Estriadol (pmol/liter): 132 ± 36; IGF-I: 105 ± 28 (μg/liter); IGFBP-1: 46.9 ± 22 (μg/liter); IGFBP-3: 2.8 ± 0.6 (μg/liter)After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - ADL (HAQ score): 1.28 (−0.60; 3.15); MMSE: 0.10 (−0.68; 0.88) - Bone mineral density (total body): −0.004 (−0.014; 0.006) - Fat mass (kg): −0.56 (−1.69; 0.57); Lean mass (kg): 0.40 (−1.87; 2.68); BMI (kg/m2): 0.14 (−0.29; 0.57) - Atherosclerosis (mm) – common carotid: −0.006 (−0.060; 0.048); bifurcation: 0.038 (-−0.249; 0.324) - DHEA (nmol/liter): 11.6 (−4.1; 27.3); total testosterone (nmol/liter): 8.5 (5.9; 11.1); Estriadol (pmol/liter): 44 (13; 75); IGF-I (μg/liter): 9.3 (1.3; 17.3); IGFBP-1 (μg/liter): 3.6 (−4.7; 11.9); IGFBP-3 (μg/liter): −0.1 (−0.3; 0.1) | Between group difference: Placebo group showed significant decrease in BMI. Compared with the placebo group, both the atamestane and DHEA groups increased BMI (p value was not provided) |
| DHEA(n = 25) | At baseline (mean ± SD): - ADL (HAQ score): 10.4 ± 3.9; MMSE: 27.8 ± 2.2 - Bone mineral density (total body): 1.14 ± 0.10 - Fat mass (kg): 20.3 ± 4.8; Lean mass (kg): 49.4 ± 5.2; BMI (kg/m2): 24.3 ± 2.2 - Atherosclerosis (mm) – common carotid: 1.00 ± 0.14; bifurcation: 1.43 ± 0.57 - Blood pressure (mm Hg) – systolic: 154.4 ± 23.9; diastolic: 86.0 ± 9.6 - DHEA (nmol/liter): 14.9 ± 11.6; total testosterone (nmol/liter): 11.5 ± 2.8; Estriadol (pmol/liter): 124 ± 44; IGF-I: 106 ± 30 (μg/liter); IGFBP-1: 44.6 ± 17.4 (μg/liter); IGFBP-3: 2.7 ± 0.7 (μg/liter)After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - ADL (HAQ score): −0.17 (−2.10; 1.75); MMSE: −0.11 (−0.91; 0.69) - Bone mineral density (total body): −0.000 (−0.010; 0.011) - Fat mass (kg): −0.02 (−1.16; 1.13); Lean mass (kg): −0.76 (−3.05; 1.54); BMI (kg/m2): 0.81 (0.37; 1.25) - Atherosclerosis (mm) – common carotid: −0.006 (−0.061; 0.048); bifurcation: 0.113 (−0.204; 0.430) - DHEA (nmol/liter): 24.6 (8.5; 40.7); total testosterone (nmol/liter): 3.5 (0.8; 6.2); Estriadol (pmol/liter): 65 (34; 97); IGF-I (μg/liter): 0.4 (−7.7; 8.5); IGFBP-1 (μg/liter): 0.2 (−8.2; 8.6); IGFBP-3 (μg/liter): −0.1 (−0.3; 0.1) | ||
| Atamestane (n = 25) | At baseline (mean ± SD): - ADL (HAQ score): 11.1 ± 3.9; MMSE: 27.0 ± 2.2 - Bone mineral density (total body): 1.15 ± 0.12 - Fat mass (kg): 21.0 ± 6.6; Lean mass (kg): 48.2 ± 5.2; BMI (kg/m2): 25.0 ± 2.9 - Atherosclerosis (mm) – common carotid: 1.02 ± 0.13; bifurcation: 1.35 ± 0.39 - Blood pressure (mm Hg) – systolic: 154.6 ± 20.7; diastolic: 80.4 ± 12.9 - DHEA (nmol/liter): 13.4 ± 6.6; total testosterone (nmol/liter): 11.6 ± 3.7; Estriadol (pmol/liter): 131 ± 48; IGF-I: 104 ± 28 (μg/liter); IGFBP-1: 45.8 ± 14.8 (μg/liter); IGFBP-3: 2.9 ± 0.8 (μg/liter)After 36-week intervention (differences between placebo and study agent, mean, 95% CI): - ADL (HAQ score): −1.44 (−3.35; 0.48); MMSE: 0.51 (−0.31; 1.32) - Bone mineral density (total body): −0.005 (−0.015; 0.005) - Fat mass (kg): 0.34 (−0.79; 1.48); Lean mass (kg): 0.13 (−2.13; 2.39); BMI (kg/m2): 0.36 (−0.08; 0.80) - Atherosclerosis (mm) – common carotid: −0.013 (−0.068; 0.041); bifurcation: 0.176 (−0.121; 0.473) - DHEA (nmol/liter): 1.2 (−14.7; 17.1); total testosterone (nmol/liter): 4.9 (2.2; 7.5); Estriadol (pmol/liter): −18 (−50; 13); IGF-I (μg/liter): 0.8 (−7.4; 8.9); IGFBP-1 (μg/liter): 2.3 (−6.2; 10.8); IGFBP-3 (μg/liter): 0.0 (−0.2; 0.2) | ||
| Placebo (n = 24) | At baseline (mean ± SD): - ADL (HAQ score): 10.9 ± 4.3; MMSE: 28.3 ± 1.1 - Bone mineral density (total body): 1.14 ± 0.10 - Fat mass (kg): 21.0 ± 7.2; Lean mass (kg): 49.1 ± 6.4; BMI (kg/m2): 24.8 ± 3.7 - Atherosclerosis (mm) – common carotid: 0.96 ± 0.12; bifurcation: 1.09 ± 0.26 - Blood pressure (mm Hg) – systolic: 148.1 ± 21.1; diastolic: 82.2 ± 10.1 - DHEA (nmol/liter): 14.4 ± 10.3; total testosterone(nmol/liter): 12.2 ± 4.4; Estriadol (pmol/liter): 135 ± 58; IGF-I: 103 ± 32 (μg/liter); IGFBP-1: 45.6 ± 21.1 (μg/liter); IGFBP-3: 2.7 ± 0.7 (μg/liter)After 36-week intervention (changes from baseline (placebo), mean, 95%CI): - ADL (HAQ score): 0.54 (−0.46; 1.55); MMSE: 0.17 (−0.49; 0.83) - Bone mineral density (total body): −0.004 (−0.012; 0.004) - Fat mass (kg): −0.18 (−1.04; 0.69); Lean mass (kg): −0.57 (−1.58; 0.43); BMI (kg/m2): −0.62 (−0.99; −0.25) - Atherosclerosis (mm) – common carotid: −0.003 (−0.034; 0.029); bifurcation: 0.047 (−0.067; 0.160) - DHEA (nmol/liter): −0.3 (−3.7; 0.7); total testosterone (nmol/liter): −1.3 (−2.4; −0.2); Estriadol (pmol/liter): −26 (−39; −13); IGF-I (μg/liter): −0.6 (−6.6; 5.3); IGFBP-1 (μg/liter): −3.3 (−11.1; 4.4); IGFBP-3 (μg/liter): −0.3 (−0.4; −0.2) | ||
| Ng, et al., 201553 | Nutritional supplements (n = 49) | At baseline (mean ± SD): - Instrumental ADL – ADL dependency: 1 ± 2.0 - Hospitalization in past 12 months: 1 ± 2.0At 3 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 2.1% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 2.1%After 6-month intervention: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.6% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 4.3%At 12 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 6.7% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 8.3% | Between group difference: no significant difference |
| Physical training (n = 48) | At baseline (mean ± SD): - Instrumental ADL – ADL dependency: 0 ± 0.0 - Hospitalization in past 12 months: 6 ± 12.5At 3 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 2.1% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 4.2%After 6-month intervention: - Instrumental ADL – ADL dependency (point-prevalence frequency): 8.3% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 6.3%At 12 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 8.7% - Hospitalization in past 12 months (cumulated frequency): 6.3%; any fall (cumulated frequency): 6.3% | ||
| Cognitive training (n = 50) | At baseline (mean ± SD): - Instrumental ADL – ADL dependency: 1 ± 2.0 - Hospitalization in past 12 months: 3 ± 6.0At 3 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 2.1% - Hospitalization in past 12 months (cumulated frequency): 6.4%; any fall (cumulated frequency): 2.1%After 6-month intervention: - Instrumental ADL – ADL dependency (point-prevalence frequency): 2.2% - Hospitalization in past 12 months (cumulated frequency): 6.3%; any fall (cumulated frequency): 2.1%At 12 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.4% - Hospitalization in past 12 months (cumulated frequency): 10.2%; any fall (cumulated frequency): 4.1% | ||
| Combination treatment (n = 49) | At baseline (mean ± SD): - Instrumental ADL – ADL dependency: 1 ± 2.0 - Hospitalization in past 12 months: 3 ± 6.1At 3 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.2% - Hospitalization in past 12 months (cumulated frequency): 0.0%; any fall (cumulated frequency): 2.1%After 6-month intervention: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.3% - Hospitalization in past 12 months (cumulated frequency): 8.3%; any fall (cumulated frequency): 2.1%At 12 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.4% - Hospitalization in past 12 months (cumulated frequency): 12.2%; any fall (cumulated frequency): 4.1% | ||
| Standard care + placebo (n = 50) | At baseline (mean ± SD): - Instrumental ADL – ADL dependency: 4 ± 8.0 - Hospitalization in past 12 months: 1 ± 2.0At 3 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 10.4% - Hospitalization in past 12 months (cumulated frequency): 2.1%; any fall (cumulated frequency): 10.4%After 6-month intervention: - Instrumental ADL – ADL dependency (point-prevalence frequency): 4.3% - Hospitalization in past 12 months (cumulated frequency): 4.2%; any fall (cumulated frequency): 10.4%At 12 months: - Instrumental ADL – ADL dependency (point-prevalence frequency): 6.5% - Hospitalization in past 12 months (cumulated frequency): 4.2%; any fall (cumulated frequency): 10.4% | ||
| Van Hout et al., 201054 | Proactive home visits by trained community nurses (n = 331) | After 18-month intervention: - Hospital admittance ≥ 1: 49.2%; acute hospital visit ≥ 1: 32.3%; institutionalization: 13.4%; mortality: 6.4% | Between group difference: In subgroups of participants with poorest EQ-5D (<55): - intervention group had higher risk to be admitted to hospital (OR = 1.95, 95% CI 1.2–3.1, p = .005)In subgroup of participants with two or more chronic disease: - intervention group had higher risk on acute hospital visits (OR = 1.6, 95% CI 1.04–2.4, p = .03) |
| Usual care (n = 320) | After 18-month intervention: - Hospital admittance ≥ 1: 55.9%; acute hospital visit ≥ 1: 28.8%; institutionalization: 11.8%; mortality: 6.9% | ||
| Vriendt, et al., 201621 | Activity oriented and community based program (Baseline: n = 86Follow-up: n = 82) | At baseline (mean ± SD):-SF36 physical functioning: 23 ± 27; SF36 physical role functioning: 57 ± 44; SF36 bodily pain: 47 ± 27; SF36 mental health: 61 ± 24; SF36 vitality: 50 ± 21After 8–10-week intervention (mean difference): - SF36 physical functioning: 3.0; SF36 physical role functioning: 7.4; SF36 bodily pain: 4.5; SF36 mental health: −0.1; SF36 vitality: 1.2 | Between group difference: SF36 bodily pain subscale: p = 0.049 |
| Community care as usual (Baseline: n = 82Follow-up: n = 80) | At baseline (mean ± SD):-SF36 physical functioning: 19 ± 23; SF36 physical role functioning: 52 ± 45; SF36 bodily pain: 55 ± 29; SF36 mental health: 60 ± 24; SF36 vitality: 52 ± 22After 8–10-week intervention (mean difference): - SF36 physical functioning: 1.4; SF36 physical role functioning: 4.4; SF36 bodily pain: −5.1; SF36 mental health: −0.1; SF36 vitality: −1.3 | ||
| Wolf et al., 200355 | Tai Chi (n = 72) | Follow-up: - Distribution of falls according to FICSIT definition: 56; distribution of falls according to Atlanta Site Definition: 29; Average follow-up time: 171 days | Between group difference:Tai Chi group: - unadjusted risk ratio for time to one or more falls according to FICSIT definition (RR = 0.632, 95%CI 0.45–0.89, p = .009) - adjusted risk ratio for time to one or more falls according to FICSIT definition (RR = 0.511, 95%CI 0.361–0.725, p = .017) - adjusted risk ratio for time to one or more falls according to Atlanta Site definition (RR = 0.525, 95%CI 0.321–0.860, p = .010)Risk of falls associated with falls last year (FICSIT: p = .0006; Atlanta Site: p = .0003), fear of falling (FICSIT: p = .0004; Atlanta Site: p = .0002), and trouble falling asleep (FICSIT: p = .00006; Atlanta Site: p = .00003).Risk of injurious falls associated with falls last year (p = .003) and fear of falling (p = .029) |
| Computerized Balance Training (n = 64) | Follow-up: - Distribution of falls according to FICSIT definition: 76; distribution of falls according to Atlanta Site Definition: 44; Average follow-up time: 164 days | ||
| Education exercise-control condition (n = 64) | Follow-up: - Distribution of falls according to FICSIT definition: 77; distribution of falls according to Atlanta Site Definition: 37; Average follow-up time: 164 days |
ADL, Activities of daily living; BDNF, brain-derived neurotrophic factor; BMI, body mass index; DHEA, dehydroepiandrosterone; EQ-5D, EuroQol Quality of Life Scale; FICSIT, Frailty and Injuries, Cooperative Studies of Intervention Techniques; GDS, Geriatric Depression Scale; HADS, Hospital Anxiety and Depression Scale; HAQ, Stanford Health Assessment Questionnaire; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; MMSE, Mini-Mental State Examination; PRIME-MD, Primary Care Evaluation of Mental Disorders; SD, standard deviation; SF-36, Short Form 36 Health Survey; TUGT, Timed Up and Go Test.
Effectiveness of interventions to prevent progression of frailty
Physical exercise programs delivered in class41,42,46,49 or delivered in class and followed by home-based practice53,55 were shown to be effective for preventing the progression of pre-frailty and frailty, at least in some of the frailty indicators (sum of indicators,42,53 weakness,41,55 weight loss,49 slowness/gait speed,41,46,53 gait distance,55 balance,46 exhaustion,49 physical activity,49 activities of daily living,46 and fear of falls55). These positive effects were observed for different types of exercise programs (including Tai Chi,55 resistance training with42 and without41,53 nutrition consultation, and comprehensive multicomponent training46,49) and for different samples (community dwelling older adults,42,49,53,55 older adults aged between 80 years and 90 years contacted through the primary health care center,46 institutionalized older adults aged 85 or over41).
On the other hand, home-based exercise programs43 developed with housebound older adults showed only a non-significant trend to improve mobility. No effects on the prevention of frailty progression were observed for computerized balance training55 performed individually with supervision.
Providing nutritional supplements alone, including milk fat globule membrane (MFGM)49 and protein-energy formula,23 or increasing protein-calorie and micronutrients intake,53 was also shown to be favorable for prevention of frailty progress. Milk fat globule membrane49 provided to frail women from the community improved physical activity immediately after the intervention and reduced long-term exhaustion, but had no significant post-intervention effect on weight. In another study,23 a protein-energy formula provided to community dwelling older adults with low mobility and who were also malnourished (both indicators used for operational definition of frailty), had beneficial effects for energy intake. However, this improvement in energy intake was not associated with significant change in body weight. An intervention based on increase of protein-calorie and micronutrient intake53 provided to pre-frail and frail older adults from the community improved frailty scores from the baseline, with observed change being significant at 12 months, but not at three or six months. The positive effects of this intervention were observed through the increase in long-term physical activity. Regarding body mass index, the highest mean change was observed follow-up at three months; however, this change was not significant.
Hormone replacement with atamestane and/or dehydroepiandrosterone52 and conducted with independently living men without disease or recent hospitalization and with low scores on strength tests was shown to have no influence on frailty measurements.
Interventions based on individually tailored management of clinical condition by a multi-professional team and according to individual needs21,29,32,44,50 were shown to have inconsistent effects on frailty prevalence. A twelve month multifactorial interdisciplinary intervention provided to community dwelling older adults meeting with frailty showed a significant impact on frailty prevalence.32 Improvement in frailty (operationalized in terms of impairment in basic activities of daily living) was also observed in relation to client-centered, individually tailored activity oriented program conducted with community dwelling older adults receiving healthcare support.21 Regarding inpatient care in geriatric evaluation and management units44 that included screening geriatric evaluation and management according to individual care plan developed by a multi-professional team, positive effects were demonstrated on basic ADL and physical performance at discharge, but not at the follow up at 12 months. However, when the same intervention was developed in geriatric clinics with outpatients who had previously received all appropriate hospital services,44 the positive effects were only observed on physical performance at 12 months. On the other hand, a six month intervention based on screening evaluation results,50 developed with pre-frail and frail community dwelling older adults, as well as continuum care29 provided to older adults with at least one chronic disease and dependent in at least one ADL, discharged from emergency departments, were shown to have no effects on frailty status.
Group sessions focused on the ageing process and health,40 conducted with community dwelling older adults resulted in postponing progression in tiredness in daily activities (measured through Mob-T Scale) for up to one year. When tiredness was not included in the analysis and when the follow-up at three months was considered,47 group meetings focused on ageing process and health were shown to have no effect on frailty.
Educational sessions focused on health promotion, disease prevention and self-care,51 provided in groups for participants who were not at risk of frailty, and individually by a geriatrician for participants who were at risk of frailty, showed positive impact for a change of frailty status.
Regarding home visits by nurses provided to community dwelling older adults, positive effects were observed in relation to nine months of weekly nurse home visits focused on life-style changes and accompanied by an alert button,45 but not in relation to nine months of weekly nurse home visits focused on life-style changes without alert button45 or 18 months of nurse home visits focused on designing of care plan.54 The positive impact of the treatment including alert button was observed on the frailty prevalence, namely on the percentage of older adults who developed frailty during the follow up period. The authors45 suggested that a visiting nurse combined with use of technology could produce a sense of security in the patients diminishing the level of risk. They also considered the possibility that the technology could be a tool to make better clinical decisions and to achieve closer patient care.
Favorable effects were also verified for a single preventive home visit by a trained professional40 provided to community dwelling older adults. In this case, the intervention resulted in postponing progression in tiredness in daily activities for up to one year. The authors of the intervention also described the results obtained in the follow–up at three months,47 however the definition of frailty presented in this study did not include tiredness. In this three months follow-up study47 no changes in frailty prevalence were observed.
Cognitive training designed to stimulate short-term memory, and enhance attention, information-processing skills, and reasoning and problem-solving abilities53 reduced the frailty score from baseline at 12 months (six months after the intervention). Regarding frailty components, the significant improvement was observed in knee strength immediately after the intervention (at six months) and in follow-up assessment (at 12 months). Other frailty components remained unchanged.
Problem solving therapy42 was shown to have no effects on frailty.
Combined multidisciplinary treatment, including nutritional supplementation, physical training and cognitive training53 provided to community dwelling older adults, showed a significant positive impact on frailty prevalence in follow up at three-, six- and 12 months. Physical exercise programs with supplementation were also shown to be effective for preventing the progression of frailty in community dwelling older adults, independently of type of supplement used (protein supplement22 or MFGM49). Protein supplementation in combination with exercise prevented decrease in maximal walking time, but had no effects on maximal walking distance and physical activity. In addition, for good compliers, a significant increase in walking outcomes was observed.22 Exercise and MFGM supplementation had positive long-term effects on all frailty indicators, with the exception of muscle strength.49 Finally, long-term music-based multi task exercise48 (also conducted with community dwelling older adults) improved gait speed and handgrip strength. In addition, pre-frail participants from the continued intervention group were more likely to become robust at four years than participants from the discontinued intervention group.
Effectiveness of interventions to prevent deterioration in secondary outcomes
Data for secondary outcomes of interest was presented in 19 studies.21-23,29,32,41-44,46-55 These outcomes included quality of life,21,32,42-44 self-rated health,47,48 depression or other mental health-related outcomes,42,43,48,51 cognition,41,42,48,52 functional capacity/mobility,22,23,41,42,46,48,49 ADLs,22,29,41-43,47,50,52,53 analytical parameters including blood analyses,42,49,52 body composition parameters23,41,42,49,52 and nutrition-related outcomes (body weight, body mass index, fat mass and lean mass, score on nutritional tests, etc.),22,42,48,52 and adverse outcomes23,41,42,44,48,51-54,55 (see Table 7). The presentation of data regarding secondary outcomes followed the categories of interventions provided above.
All physical exercise programs delivered in classes focused on the outcomes of functional capacity/mobility. These were a multicomponent exercise program,41 resistance training with nutrition consultation,42 and a functional circuit-training program.46 All were shown to have beneficial effects on the functional capacity or mobility outcomes. More specifically, the multicomponent exercise program41 provided to institutionalized patients improved Timed Up and Go Test (TUGT)performance with single and dual tasks, as well as rise from chair and balance. This multicomponent exercise program also enhanced high-density muscle cross-sectional area, reduced incidence of falls and postponed deterioration in ADLs.41 The functional circuit-training program46 delivered to frail older adults improved balance and gait performance, lower body strength and physical functions assessed by Modified TUGT. The positive effects of a functional circuit-training program were observed either immediately after the intervention (at week 12) or in follow-up assessments (at week 36). The intervention combining resistance training with nutrition consultation,42 developed with frail older adults from the community, was revealed to be beneficial for balance and lower body strength. The improvement in balance was observed immediately after the intervention, and maintained up to nine months after the intervention. The improvement in lower body strength was observed only immediately after the intervention, showing significant decrease from baseline in follow-up assessment. The resistance training with nutrition consultation42 also had a positive impact on ADLs immediately after the intervention. Moreover, the follow-up assessments revealed that this intervention significantly increased levels of 25(OH) Vitamin D and decreased body mass index and fat free mass. On the other hand, resistance training with nutrition consultation had no influence on quality of life, mental health-related outcomes (assessed based on Primary Care Evaluation of Mental Disorders), cognitive performance (assessed based on Mini-Mental State Examination) or health care resource utilization.42 The physical exercise sessions with placebo supplementation, provided to community dwelling older women,49 were shown to have a positive impact on TUGT scores. In this study49 skeletal muscle mass or leg muscle mass were also measured, but changes observed on these outcomes were not significant.
Regarding two exercise programs delivered in classes and followed by home-based practice (resistance and balance training53 and Tai Chi55), both were examined from the perspective of adverse outcomes (falls incidence53,55 and number of hospitalizations53). The study on Tai Chi55 revealed that this intervention was effective for reducing the risk ratio for falls incidence. In comparison, the resistance and balance training53 was shown to have no impact (either short-term or long-term) on falls incidence or number of hospitalizations. In the study on resistance and balance training,53 outcomes of dependency in ADLs and in instrumental ADLs were also considered. The differences observed between intervention and the control groups in the course of the intervention, immediately after the intervention and at six month follow up, were shown to be non-significant.
The study on computerized balance training55 performed individually with supervision in the sample of community dwelling older adults focused on the outcome of falls incidence. Between-group analysis indicated no significant difference in changes in the fall-related outcomes between the intervention and control groups.
In the study describing a home-based exercise program43 developed with housebound older adults, the outcomes of quality of life, depression and ADLs were considered. Between-group analyses (performed immediately after 12-week intervention and 12 months after randomization) showed no difference on any of these outcomes.
Providing nutritional supplements alone, including MFGM49 and protein-energy formula,23 had significant positive effects on outcomes related to functional capacity/mobility, but not on outcomes related to body composition, including skeletal muscle mass,49 leg muscle mass49 and mid-arm circumference.23 Protein-energy supplementation also had no impact on the serum level of blood urea nitrogen and creatinine clearance. In relation to the intervention increasing protein-calorie and micronutrients intake,53 the secondary outcomes were dependency in ADLs and in instrumental ADLs, self-reported hospitalizations and self-reported falls. Between-group analyses conducted in the course of the intervention, immediately after the intervention and at 12 months showed that the frequency of occurrence of these outcomes in the intervention group was not significantly different to frequency observed in the control condition.
The secondary outcomes of interest examined for the intervention based on hormone replacement with atamestane and/or dehydroepiandrosterone52 included ADLs, cognition, nutrition-related outcomes (such as body mass index, lean body mass and fat body mass), bone mineral density, and serum concentration of total testosterone, estradiol and dehydroepiandrosterone. In addition, data regarding indicators of generalized atherosclerosis was collected. The analysis of differences between placebo and study agent indicated a post-intervention increase in body mass index in the atamestane and dehydroepiandrosterone (DHEA) groups. Comparatively, the body mass index of the placebo group significantly decreased from baseline. The changes on the other outcomes were not statistically significant.
Secondary outcomes examined in the studies describing interventions based on individually tailored management of clinical condition21,29,32,44,50 were: quality of life21,44 and health-related quality of life,32 independency in ADLs,29,50 probability of survival,44 and health care resource utilization.44 The study describing a six-month intervention developed with frail or pre-frail older adults50 examining the outcome of independency in ADLs was revealed to be not effective for this outcome of interest. On the other hand, continuum care by multi-professional team delivered to older adults discharged from emergency departments29 was shown to improve independency in ADLs up to one year and, simultaneously, to postpone dependency in ADLs up to six months. The study presenting data on effectiveness of the multifactorial interdisciplinary intervention for the health-related quality of life32 has shown that the post-intervention changes observed in the intervention group are not significantly different from those observed in the control group. In the study describing client-centered, individually tailored activity oriented program21 data related to different dimensions of quality of life was presented. The client-centered program, as compared to community care as usual, was shown to improve all analyzed dimensions of quality of life, with the exception of mental health for which a score decrease of 0.1 was observed. However, only the change on bodily pain dimension was statistically significant. Finally, the geriatric evaluation and management of clinical conditions developed with inpatients and outpatients from the Veterans Affairs Medical Centers44 were shown to have a significant impact on some dimensions of quality of life, but not on probability of survival, namely, at discharge the participants from the inpatient intervention group, as compared to inpatient usual care, were found to have lower decrease in dimensions of general function and general health, and improvement in dimensions of bodily pain and energy. Between-group differences in bodily pain were maintained 12 months after the intervention. Regarding groups of outpatients, those who received geriatric evaluation and management of clinical condition were shown to have a lower decrease in general health at discharge and at follow-up at 12 months, lower decrease in energy at discharge and improvement in energy at follow-up at 12 months, and improvement in mental health at follow-up at12 months. The study authors44 also evaluated health care resource utilization. The participants in the inpatient intervention group, as compared with the participants in the inpatient control group, spent more days in hospital, had longer initial hospitalization and higher number of medical and surgical consultations, but they spent less days in long-term care. Differences in health care resource utilization between outpatient groups were not significant.
The secondary outcomes included in the study examining the effectiveness of multi-professional senior meetings47 were self-rated health and ADLs. The multi-professional senior meetings, as compared to the ordinary range of community services, delayed deterioration on both self-rated health and ADLs. The significant positive effect on maintaining independency in ADLs was also found when multi-professional senior meetings were compared with a single preventive home visit. The study, describing a group session51 conducted with participants not at risk of frailty and an individual educational session51 by a geriatrician conducted with participants at risk of frailty, focused on the outcome of depression and adverse outcomes such as death, admission to nursing home and admission to home care program. The post-intervention assessment indicated that the proportion of participants at risk of depression was significantly higher in the control group receiving usual care than the intervention groups. Regarding adverse outcomes, the significant differences were found only for the subgroups of participants at risk of frailty, with participants from the control group showing higher risk of death, admission to nursing home or admission to home care program.
The study describing 18-month home visits by nurses54 provided data about healthcare resource utilization (including hospital admittance) and adverse outcomes (such as acute hospital visit, institutionalization and mortality). Between-group analyses revealed no statistically significant difference on any of the outcomes measures. However, when the subgroup analysis based on scores in self-rated health and number of comorbid chronic conditions were conducted, some relevant effects were observed. Namely, participants from the intervention group who achieved the poorest score in self-rated health had a higher risk of hospital admission, and participants from the intervention group that had two or more chronic conditions had a higher risk of acute hospital visits. The study describing single preventive home visit by a trained professional47 presented data on self-rated health and ADLs. The assessment conducted at three months indicated that a single preventive home visit, as compared to the ordinary range of community services, was more likely to postpone deterioration in self-rated health, but not in ADLs.
Six month cognitive training developed with community dwelling frail older adults53 was shown to have no beneficial effects on dependency in ADLs and in instrumental ADLs, self-reported hospitalizations and self-reported falls.
No significant differences on healthcare-resource utilization were also reported in relation to problem solving therapy provided to community dwelling frail older adults.42 On the other hand, in the problem solving therapy group, body mass index and fat free mass decreased significantly from baseline, and the levels of 25(OH) Vitamin D increased significantly (all at 12 months). Moreover, this therapy had significant positive effects on mental health-related outcomes, quality of life and ADLs (all at three months) and functional capacity/mobility (at three, six and 12 months). However, in comparison to the control condition consisting of educational booklets, only follow-up changes on outcomes related to functional capacity/mobility were shown to be significant.42
In relation to combined multidisciplinary interventions the secondary outcomes of interest were self-rated health status,48 cognitive performance,48 mental health-related outcomes,48 nutrition-related outcomes,48 dependency in ADLs and in instrumental ADLs,53 functional capacity/mobility,22,48,49 body composition parameters49 and adverse outcomes.48,53 In the study examining the effectiveness of treatment consisting of nutritional, physical and cognitive components,53 the changes on secondary outcomes (including dependency in ADLs and in instrumental ADLs, self-reported hospitalizations and self-reported falls) were assessed three times, in the course of the intervention (at three months), immediately after the intervention (at six months) and at the follow-up at six months. At no point were significant differences from the control condition (standard care plus placebo) observed. Both studies describing exercise programs with supplementation22,49 focused on the outcomes of functional capacity/mobility. The MFGM supplementation with exercise training provided to women from the community49 was shown to have a positive impact on functional capacity/mobility and some hematological parameters. However, this MFGM/exercise-based intervention49 had no influence on skeletal muscle mass or leg muscle mass. Regarding home-based exercise with dietary protein supplementation,22 there were no significant effects on functional capacity/mobility. On the other hand, home-based exercise with dietary protein supplementation stabilized body mass index and postponed deterioration on the mini nutritional assessment score, and in the instrumental ADLs. Finally, after continued intervention of music-based multitask training,48 the community dwelling older adults who had increased risk of falling had better performance on gait speed (in both single and dual tasks) and balance. They also did better on the TUGT and five-times-sit-to-stand test. The continued intervention of music-based multitask training also significantly reduced risk of falls and multiple falls. However, this intervention had no effect on self-rated health status, cognitive performance (assessed based on Mini-Mental State Examination and Clock-drawing test), levels of depression and anxiety, and nutrition-related outcomes such as body mass index or Mini Nutritional Assessment score.48
The influence of degree of frailty on the effectiveness of interventions
Three studies22,29,44 examined the influence of degree of frailty on the effectiveness of the applied intervention. Eklund et al.29 examined whether the baseline differences in the level of frailty (measured based on CHS criteria) had modifying effects on ADL outcome and found that this confounding variable had no significant impact for the continuum care results. In Cohen et al.,44 subgroup analyses were conducted aiming to show whether the main effects of inpatient and outpatient geriatric evaluation and management depend on the functional status of patients. Functional status included indicators of basic and instrumental ADLs, both used for frailty measurements. Low functional status was defined in terms of assistance required with at least three ADLs, and high functional status in terms of assistance required with less than three ADLs. This variable had no influence on the main effects observed in the study.44 Bonnefoy et al.22 conducted exploratory subgroup analysis comparing good compliers to poor compliers. The authors assessed the participants’ compliance using diaries, participants’ knowledge of exercise during the final evaluation, protein supplement bag counts and interviews of home helpers supervising delivery of the intervention, with a compliance rate of 50% considered as satisfactory. The groups of good and poor compliers differed before the intervention on the Physical Activity Scale for the Elderly score and on walking speed, both considered as frailty indicators. The good compliers had better baseline performance on the Physical Activity Scale for the Elderly and better baseline performance on walking speed. After the four-month home based exercise program with dietary protein supplementation, good compliers, as compared with poor compliers, improved maximum walking distance and maximum walking time in 29.15% (first quartile: 0.0; third quartile: 66.7) and 33.3% (first quartile: -20.0; third quartile: 50.0), respectively. The change in both variables was significantly different from that observed in the control group (maximum walking distance: p = .007; maximum walking time p = .004).
Other factors moderating the effectiveness of interventions
Other factors moderating the effectiveness of interventions were examined in eight studies.22,23,29,42,44,51,54,55 In one of these studies, Bonnefoy et al.22 analyzed the relevance of good compliance to the home based exercise with dietary protein supplementation intervention program. The subgroup of good compliers, identified by the study authors,22 had fewer falls within the six months before the intervention, higher baseline score on the Mini Nutritional Assessment test, higher baseline energy intake, better baseline physical performance and walking speed, and better baseline performance in ADLs and instrumental ADLs. Regarding post-intervention results, good compliers benefited significantly more on the maximum walking distance and maximum walking speed, with improvement about 30% higher than the subgroup of poor compliers. A logistic regression analysis showed that the best predictor of good compliance was the score on the test examining instrumental ADLs (OR for each 1-point increase in instrumental ADLs score = 2.84, 95% CI 1.52; 5.31, p = .0011).
Subgroup analyses examining the potential moderating effect of baseline characteristics were also performed by van Hout et al.54 These authors showed that among participants with the poorest self-rated health (EuroQol Quality of Life Scale score < 55), the participants who received an 18-month intervention consisting of nurse home visits had a significantly higher risk of being admitted to a hospital than those who received usual care (intervention subgroup: OR = 1.95, 95% CI 1.2; 3.1, p = .005). In addition, a higher risk of an acute hospital visit was found among participants included in the intervention group who had two or more chronic disease (OR = 1.6, 95% CI 1.04; 2.4, p = .03). According to study authors54 the higher odds of hospital admittance and acute hospital visits, observed in the subgroups of intervention participants may be explained by an increased awareness of the participants concerning the treatability of their health status, as a result of the nurse assessment.
In Cohen et al.,44 subgroup analyses focused on functional status (for the detailed description see previous section) age (> 75 versus ≤ 75 years), Charlson comorbidity index (low with score ≤ 2, and high with score > 2) and the year of enrollment. According to the study authors,44 none of these baseline characteristics had any modifying effect on the results of the intervention based on inpatient or outpatient geriatric evaluation and management. However, no statistical data corroborating this conclusion was provided.
In the study investigating geriatric intervention in individual sessions or group sessions, with participants determined as at risk or not at risk of frailty, with usual care as a control condition, Monteserin et al.51 performed logistic regression in order to identify multivariate predictors of likelihood of reversing from risk of frailty to healthy status (described by the authors as a “reversible risk of frailty”), evaluating data of patients at risk of frailty only, using information from the comprehensive geriatric assessment. The authors identified the following variables as multivariate predictors of reversible risk of frailty: younger age (OR = .89, 95% CI 0.82; 0.99), low consumption of medication at baseline (OR = .77, 95% CI 0.63; 0.93), not being at risk of depression at baseline (OR = 3.68, 95% CI 1.39; 9.70) and the intervention itself (OR = 3.08, 95% CI 1.21; 7.82).51
To obtain adjusted effects of two exercise approaches (Tai Chi and computerized balance training) on occurrence of falls and injurious falls, Wolf et al.55 examined potential baseline risk factors for falls, including variables such as age, gender, currently working for pay, volunteer status, cognitive status, scores in the depression test, scores in the instrumental ADL test, body mass index, trouble falling asleep, waking up during the night, feeling rested in the morning, cataract history, fallsin previous year, fear of falling and fall efficacy score. Fall occurrence in past year, fear of falling and trouble falling asleep were identified as significant risk factors for fall occurrence. Injurious falls were associated with falls in the last year (RR = 3.104, 95% CI 1.476; 6.530, p = .003) and fear of falling (RR = 1.466, 95% CI 1.039; 2.040, p = .029), but not with trouble falling asleep (RR = 0.915, 95% CI 0.598; 1.399, p = .680). However, there were no significant treatment differences for time to one of more falls before and after adjusting for covariates (see Table 7).55
Chan et al.42 observed that at the end of the three-month intervention the improvement rate in the exercise and nutrition consultation group was 45% and in the problem solving therapy group it was 44%. However, after adjusting the post-treatment results for multiple confounding variables, including baseline characteristics, such as age, gender, Mini-Mental State Examination scores, healthcare resource utilization, EuroQol Quality of Life Scale score, fat free mass, body mass index, one-leg stand and 25 (OH) Vitamin D levels, only improvement in the exercise and nutrition consultation group, as compared to the control group, remained significant.
In the study examining the effectiveness of continuum care provided to the older adults discharged from emergency departments, with usual care as a control condition, Eklund et al.29 tested whether the baseline differences in the Mini-Mental State Examination score or self-rated health had influenced the ADL and frailty outcomes. The authors stated that no modifying effects of the referred variables were found; however no statistical data corroborating this conclusion were provided.
Finally, Kim and Lee23 investigated the preventive effect of protein-energy supplementation among frail older people with low socioeconomic status. To identify the factors that determined the changes in nutritional and functional status during the study period (12 weeks), the authors performed Spearman's correlations, showing that change of physical functioning was significantly correlated with protein intake (rs = .23; p = .037) and mean adequacy ratio calculated from nutrient adequacy ratio for the intake of energy, protein, and 11 micronutrients (rs = .25; p = .023). The study authors also found a significant correlation between change in mid-arm circumference and change in Short Physical Performance Battery (rs = .31; p = .004). On the other hand, no significant correlations between change of physical functioning and energy intake, essential amino acid intake, body weight or mid-arm circumference were found. There was also no correlation between change in Short Physical Performance Battery and change in dietary intake data or body weight.23
Economic evidence of interventions preventing frailty
Two of the included studies32,44 presented economic data. The characteristics of these studies are summarized in Table 8.
Table 8.
Characteristics of included studies examining economic data
| Study | Design | Sample | Assessments | Perspective | Endpoints |
| Cohen, et al., 200244 | RCT with two-by-two factorial design | N = 1338 (2% female)Mean age 74.2 years (SD not provided)Range of age not provided | At discharge and at 12 months | Not clear | - Basic and instrumental ADL score - Physical Performance Test score - Quality of life - Survival at one year - Health care resource utilization - Costs of care |
| Fairhall, et al., 201532 | Two-arm, parallel group RCT | N = 241 (68% female)Mean age calculated separately for each group varied from 83.2 (± 5.91) to 83.4 (± 5.81) yearsRange of age 71–101 years | At 3 and 12 months | Health and community care funder | - Health-related quality of life - Prevalence of frailty - Total cost per participant - QALYs |
ADL, activities of daily living; QALYs, quality adjusted life years; RCT, randomized control trial.
The study developed by Fairhall et al.32 compared costs and cost-effectiveness of a 12-month multifactorial interdisciplinary intervention targeting identified frailty characteristics versus usual care from the community services and general practitioner. This study included 241 older adults with frailty and with a life expectancy exceeding 12 months. The participants were recruited after discharge from the Division of Rehabilitation and Aged Care Service (Sydney, Australia) between January 2008 and June 2011.32 In the study conducted by Cohen et al.,44 the cost of an intervention consisting of inpatient and outpatient geriatric evaluation and management was examined, and was compared with inpatient and outpatient usual care. This second study had a two-by-two factorial design and included 1388 stabilized patients with frailty from 11 Veterans Affairs Medical Centers (USA) that received either care in an inpatient geriatric unit or usual inpatient care, followed by either care at outpatient geriatric clinic or usual outpatient care. The patients were enrolled in the study between August 1995 and January 1999.44
Data related to economic and health related outcomes reported in both studies are presented in Table 9. In relation to the study developed by Fairhall et al.,32 the total cost of a 12-month multifactorial interdisciplinary intervention targeting identified frailty characteristics was AUD183,422, with an average cost per participant of AUD1528. Between-group differences in costs related with hospital admissions, general practitioner consultations, nursing or other health professional appointments, permanent and respite residential care, transport and home help were not significant. Regarding meal delivery, the mean cost was $255 more in the intervention group (95%CI −89; −421, p = .003), compared with the control group. At 12 months the reduction of frailty prevalence in the intervention group was significantly higher than in the control group (absolute difference: 14.7%, 95%CI 2.4%; 27.0%, p = .02). However, at three months only a marginally significant between-group difference in frailty prevalence was observed (11.3%, 95%CI −23.3%; 0.7%, p = .07). The change in the levels of quality of life measured at three and at 12 months and the controlled for baseline score was not significantly different between the intervention and the control groups (at three months: −0.04, 95%CI −0.10; 0.03, p = .24; at 12 months: 0.01; 95%CI −0.07; 0.10, p = .74).32
Table 9.
Costs, quality adjusted life years and outcome data reported in included studies
| Study | Endpoints | Findings | |||
| GEMU vs UCIP | GEMC vs UCOP | ||||
| Cohen, et al., 200244 | Basic ADL score - at discharge - at 12 monthsInstrumental ADL score - at discharge - at 12 monthsPhysical performance - at discharge - at 12 monthsQuality of life Physical functioning - at discharge - at 12 months Physical limitations - at discharge - at 12 months Emotional limitations - at discharge - at 12 months Bodily pain - at discharge - at 12 months Energy - at discharge - at 12 months Mental health - at discharge - at 12 months Social activity - at discharge - at 12 months General health - at discharge - at 12 months | Mean change in score0.230.27−0.30−0.203.124.50−1.56.74.534.013.022.015.324.90.84.5−0.34.52.718.3−0.02−5.5 | Mean change in score0.15†0.25−0.30−0.201.75†4.24†−5.4†4.54.729.812.020.39.2†20.0†−2.6†1.8−1.52.51.016.4−3.4−7.1 | Mean change in score0.200.27 (0.05)*−0.28−0.18 (0.09)2.344.67 (2.13)−3.76.8 (9.7)5.931.3 (24.1)13.422.1 (9.2)11.921.9 (10.2)0.85.4 (3.6)−0.66.3 (5.7)2.418.3 (16.0)−0.5−4.4 (−5.5) | Mean change in score0.200.25 (0.03)−0.30−0.21 (0.08)2.604.07 (1.30)†−3.24.5 (7.2)3.232.5 (29.2)†11.620.2 (8.0)12.822.9 (9.6)−2.5†1.0† (3.2)−1.20.8† (1.5)†1.216.4 (13.8)−2.9†−8.2† (−7.1) |
| Survival at one year - Main effect of GEMU - Deaths (%) - Probability of survival - Relative risk of death (95% CI) - Main effect of GEMC - Deaths (%) - Probability of survival - Relative risk of death (95% CI) | 22%78.4 ± 1.61.02 (0.81–1.28) | 21%78.8 ± 1.6 | 22%78.0 ± 1.61.07 (0.86–1.35) | 21%79.2 ± 1.5 | |
| Resource utilization - Number of days in the hospital - Number of days of initial hospitalization - Number of medical consultations - Number of surgical consultations - Number of days in long-term care | Mean (±SD)35.3 ± 1.423.2 ± 12.8 ± (not provided)2.1 ± (not provided)15.0 ± 1.8 | Mean (±SD)28.3 ± 1.4†15.0 ± 0.9†1.3 ± (not provided)†1.2 ± (not provided)†17.1 ± 1.8† | Mean (±SD)15.4 ± 1.8 | Mean (±SD)16.8 ± 1.8 | |
| Costs of care** (dollars) - initial hospitalization - care after discharge - total | 13,449 ± 62122,816 ± 1,08036,265 ± 1,298 | 10,758 ± 592†26,533 ± 1,20137,292 ± 1,369 | 12,254 ± 58423,689 ± 1,09135,943 ± 1,292 | 11,954 ± 66325,654 ± 1,19437,608 ± 1,374 | |
| Study | Endpoints | Findings multifactorial interdisciplinary intervention | Usual care | ||
| Fairhall, et al., 201532 | Prevalence of frailty - at baseline - at 3 months - at 12 months | (%)1006462 | (%)1007577† | ||
| Health-related quality of life - at baseline - at 3 months - at 12 months | Mean (±SD)0.67 ± 0.230.56 ± 0.310.49 ± 0.32 | Mean (±SD)0.66 ± 0.230.47 ± 0.340.47 ± 0.34 | |||
| Total cost (in Australian dollars) of - Hospital admissions - General practitioner consultation - Nursing or other health professional appointment - Permanent resident care (high level care) - Permanent resident care (low-level care) - Respite residential care (low-level care) - Transport - Home help - Meal delivery | 2,314,122128,892135,44577,45835,442709314,905172,01840,238 | 2,229,381126,960105,420141,39964,859920612,402132,5059,923† | |||
| Total cost per participant (Australian dollars) - All participants - Frail subgroup - Very frail subgroup | 25,030 ± 29,82723,006 ± 26,32328,742 ± 35,416 | 22,885 ± 32,35418,550 ± 29,54031,133 ± 36,081 | |||
| QALYs over 12 months | 0.52 ± 0.26 | 0.54 ± 0.27 | |||
| Costs per extra person who transitioned out of frailty - All participants - Frail subgroup - Very frail subgroup | 0.34 ± 0.480.39 ± 0.490.26 ± 0.45 | 0.21 ± 0.410.28 ± 0.460.07 ± 0.26 | |||
†Between-group significant difference.
*Values in brackets: values adjusted for the length of s64tay.
**Costs include all costs of inpatient, outpatient, and long-term care provided by Veterans Affairs medical centers, as well as care in private nursing homes. The costs of inpatient and outpatient care at non–Veterans Affairs facilities are not included.
ADL, activities of daily living; GEMC, geriatric evaluation and management clinic; GEMU, geriatric evaluation and management unit; QALYs, quality adjusted life years; RCT, randomized control trial; SD, standard deviation; SF-36, Short Form 36 Health Survey; UCIP, usual inpatient care; UCOP, usual outpatient care.
According to Fairhall et al.,32 in the sample of all participants, the ICER per additional patient experiencing transition from frailty was $15,955. For the “frail” subgroup, this cost was $41,428; for the “very frail” subgroup (patients met more than three CHS criteria for frailty) the intervention was both more effective and less costly than the control condition. A cost-effectiveness acceptability curve showed that the multifactorial interdisciplinary intervention targeting identified frailty characteristics would be cost-effective, with 80% certainty with decision makers’ willingness to pay $50,000 per extra person transitioning from frailty. In the very frail subgroup this value was reduced to $25,000. The improvement in QALYs was similar in both the intervention and control groups. The lack of significant difference was also shown for the subgroup analysis.
The mean costs of initial hospitalization in geriatric evaluation and management units and in usual inpatient care, calculated by Cohen et al.,44 were of $13,449 (± 621) and $10,758 (± 592), respectively (the authors did not provide the information about currency). The observed difference was significant (p < .001). Care after discharge in the inpatient intervention group was similar to the mean cost calculated for the inpatient control group (p = 0.19). The similarity between the inpatient intervention and the inpatient control groups was also observed for mean total cost of care (p = 0.29). In relation to mean costs of care provided in the outpatient intervention and the outpatient control groups, no significant differences were observed (the mean costs of initial hospitalization: p = 0.72; mean costs of care after discharge: p = 0.88; mean total cost of care: p = 0.69). The results obtained by Cohen et al.44 suggested that the intervention based on geriatric evaluation and management provided during the hospitalization period and/or after discharge was more beneficial for frail patients than usual care, at least for the outcomes of functional capacity and mental health, without an increase in overall costs.
Discussion
This review set out to examine the effectiveness of interventions for frailty in older adults, additionally aiming to determine if there is enough information to provide answers to questions of the impact of levels of frailty on effectiveness of interventions, and what factors modify the effectiveness of interventions. Economic evidence was also queried by including studies that examined issues such as cost effectiveness of interventions, alongside the success of those interventions.
While the literature contained a large number of interventions for frail older adults, it became immediately apparent that there were few studies specifically addressing frailty using validated scales or measurements of frailty as initial measures and primary outcome, with a general older population (e.g. not a population with a specific illness such as cancer). After excluding 2121 records and 346 full-text articles that did not meet the study criteria, 32 studies were assessed for quality. Of these, a further 11 were excluded after careful quality appraisal, with the reasons for exclusion at this stage being related to non-experimental designs and low methodological quality. These issues, along with the large number of studies excluded due to lack of validated before-after measures of frailty, suggest that the field is still at an early stage of development, with observational studies being predominant rather than well-constructed randomized controlled intervention trials. The use of well-validated measures of frailty was explored in full in an umbrella review of reviews on screening tools for frailty58 with the conclusion that while there were effective prognostic tools available, further research was needed to investigate whether psychometric properties of these frailty measures translate into effective tools when embedded in the community-based prevention programs, and specifically for use to measure intervention outcomes.
Nevertheless, this review was able to identify 21 randomized control trials of interventions for frailty that met our inclusion criteria. Within these records, post intervention measurements were repeated a second time in eight studies, with the post intervention period varying, up to a follow-up period of four years. Setting and intervention types were heterogeneous, resulting in an inability to perform meta-analysis. However, the inclusion of such variety in studies enabled an overview of the types of interventions that seemed to have more success in preventing, delaying or reversing frailty than others.
What types of interventions for frailty worked?
Exercise and nutrition interventions were amongst the most successful interventions to reduce frailty. Interventions delivered in group sessions were more successful than exercise interventions delivered one-to-one. In comparison, two systematic reviews analyzing the efficacy of exercise-based interventions on frailty36,37 concluded that these interventions seemed to be beneficial for different physical performance-related outcomes; however it was still unclear what type of exercise, its frequency and duration were most effective. None of these reviews separately discussed the findings related to interventions delivered in group and delivered individually. Thus, more studies on this topic are needed before further conclusion on the most favorable physical exercise program can be drawn.
Exercise plus nutrition was shown to be a good combination of interventions to reduce frailty. Combining exercise with nutrition may benefit patients by leading to a range of improvements in frailty that are not achieved when using either exercise or nutrition. For example, a study by Buigues et al.59 (published after November 2015, the search limit date set for this review) found that patients who received a prebiotic product showed improvements in certain aspects of frailty (exhaustion, muscle strength) but not others (weight loss, walking speed, physical activity). The frailty elements that did not change in this study may have changed if participants had also received a physical exercise intervention.
Group discussions and health educational sessions were shown to have little impact on outcomes apart from self-reported tiredness. Home visit by nurses or other professionals had mixed results. While these studies did not examine the impact of home care provision, or prevention of crises, they demonstrated positive effects on frailty or its components, particularly in one case where an alert button was provided in addition to the visits. Even a single preventive home visit was seen to have an effect, but did not make any difference three months later, suggesting that the frequency of visits may need examining more closely.
The only cognitive training intervention included covered issues related to flexibility, day-to-day coping and compensating or planning, such as working memory, problem solving abilities and attention. This showed a significant impact on frailty indices and frailty reduction lasting up to the follow-up at 12 months. Similarly, a novel intervention using play activities program (described in a study published after November 2015, the search limit date set for this review),60 was shown to have a wide range of impacts on psychological functioning, including frailty, life satisfaction and quality of life. However, another psychological, but non-cognitive, intervention of problem solving “therapy” did not have an impact on frailty. The distinction is important here, with the former addressing cognitive function and providing cognitive stimulation, but the latter focusing on therapeutic problem solving support as related to mood and self-efficacy. However, it is noticeable that cognitive training, while broadly seen as effective and supportive of cognitive reserve and coping and compensation in older age,61 is not a common intervention for frailty. Nevertheless, physical interventions, particularly exercise, are known to have an impact on cognition and brain health in older adults62 and further study is needed to elucidate on the relationship between cognitive change and frailty impact. For example, one study63 demonstrated clear relationships between change in cognition and change in outcome frailty measures, in both positive and negative directions. Finally, the studies demonstrated that combinations of interventions were particularly useful, with evidence for exercise and nutrition and cognitive training standing out as having cumulative impacts on frailty.
Who did frailty interventions work for?
Studies reported a variety of age ranges, gender distribution, and variously included people at risk of frailty, pre-frail and frail people. In terms of age, interventions showed impact across the age ranges. The physical exercise programs were shown to be effective in studies with participants ranging in age from 65 upwards. For example, one study with the “oldest-old” institutionalized participants with a mean age of 91.9 years41 showed significant effects on components of physical frailty such as gait speed and grip strength. Some researchers examined age as a moderator of the impacts in their studies, although few reported detailed comparisons of age groups. In the study43 investigating geriatric intervention in individual sessions or group sessions was found that younger age within their older frail group was a predictor of the likelihood to revert to being robust from a state of frailty, although considerably less so than the impact of the intervention itself. Other studies42,44,55 reported examining the effect of age as one of a set of modifiers in analyses on impact of their intervention, but reported that there were no impacts on frailty outcomes.
No study directly compared effectiveness by gender, although some examined gender as a potential moderator or risk factor. Given that this was often in the context of a range of effects (e.g. Chan et al.33) whether or not gender moderates effects is not yet clear. However, while studies varied in terms of the numbers of men and women recruited into the study, physical activity and physical activity plus nutritional supplementation interventions were effective for both men and women. This is important given the higher prevalence of physical frailty for older women, and related higher falls and fracture risks.6,24,64 However, nutrition supplementation on its own had a less clear impact, with studies that included either 100%49 or a significant majority23 of women having mixed results, but a study with a more even mix of men and women53 showing an overall significant effect on frailty measures. Given the higher evidenced frailty amongst older women, and specific issues such as hormone related muscle mass loss and osteoporosis that are gender related, the usefulness of frailty interventions by gender is a crucial issue for further research.
In addition, other types of interventions varied by their gender distribution of participants, but there was no clear pattern of types of interventions that had different implications for men and women. For example, one study44 with mainly male patients examining inpatient multidisciplinary screening and detailed care plan worked well at least at discharge. A similar study with 55% women29 did not show effects. On the other hand, other multi-disciplinary personalized care support interventions with a mix of male and female patients did work well32 as did those with mainly women patients.21 However, there is no clear reason to hypothesize possible gender related differences in impact for care interventions as there may be for exercise plus nutrition, given the differences in physical frailty development in older women and men.
We examined the influence of initial levels of frailty on the impact of the interventions where this was available. While exercise and nutritional interventions clearly worked across levels of frailty, based on available information on the included participant samples, this was less clear for other types of intervention. Interventions based on individually tailored management in community based older adults seemed to have an impact on frailty outcomes when the participants were indicated as frail,21,32 but not when they were mixed frail and pre-frail,50 although there may have been other less obvious differences between the studies such as context (Belgium and Australia versus Taiwan), adherence to the intervention protocol of the staff delivering it (implementation fidelity),65 or the actual content of the management in the community. For example, the management in the community that occurred in the successful study32 had very salient structured and well supported physical exercise components with individual physiotherapist instruction that was not described in other studies. In a study on continuum care for older adults who were discharged from the emergency department29 no difference in frailty outcomes was seen. However, the authors speculated that the standard of ordinary care for control participants was high and as such may have confounded the results.
Three studies22,29,44 examined whether baseline differences in the level of frailty had modifying effects on intervention outcome measures. There was no impact of initial level of frailty on ADL based outcomes of the continuum care program.29 In another study44 low or normal baseline functional status (again assessed using ADLs) had no influence on the effects of an inpatient geriatric care and management intervention. Bonnefoy et al.'s analysis of good and poor compliers to their exercise plus protein supplementation study also provided insight into the impact of initial levels of frailty.22 The people who turned out to be good compliers had better baseline performance on the Physical Activity Scale for the Elderly and walking speed and improved significantly more than poor compliers and the control group. The difficulty in interpreting this finding is that initial frailty levels and compliance are confounded. The relationship between extent of frailty and amount of improvement possible in an exercise plus supplementation intervention remains unanswered, with calls for studies that carefully examine the impact of level of frailty on prognosis made in previous studies58 being reiterated here in the context of interventions. Nevertheless, it is possible to conclude that impacts are evidenced across the range of not frail, pre-frail and frail older adults, genders and age.
Do frailty interventions have further impacts on secondary outcomes?
In addition to frailty, studies described findings relating to secondary outcomes such as functional and mobility outcomes, quality of life, self-rated health, depression or other mental health-related outcomes, cognition, functional capacity/mobility, ADLs, analytical parameters including blood analyses components, body composition parameters and nutrition-related outcomes, and adverse events. Functional capacity and mobility were common secondary outcomes in the physical exercise41,42,46 and nutrition interventions,23,49 with participants even into extreme old age showing improved indices such as ability to rise from a chair and balance, gait performance and lower body strength, with some improvements still being evident up to nine months later. Exercise interventions producing such changes included resistance training, balance and gait retraining, and strength training. Other related secondary outcomes of the exercise-based programs included better dual task walking performance and reduction of falls.41 Computer based balance training55 and home based exercise plus supplementation22 did not have these positive outcomes for mobility and safety related indices, but some nutrition supplementation interventions examined did so even when applied in the absence of an exercise intervention.
Impact on independence indices such as ADLs was shown for one of the exercise interventions with nutrition consultation42 and by continuum care,29 and postponement of decline in ADLs was shown for a multicomponent exercise program in institutionalized people over the age of 85.41 Impact on ADLs was not reliably shown for exercise and nutrition interventions, multi-professional senior meetings and for problem solving therapy. Other interventions that had no impact on frailty were nevertheless shown to have potential for ADLs, notably, the home-based exercise with dietary protein supplementation postponed deterioration in instrumental ADLs.22
Quality of life was assessed in several studies but was only shown to improve in programs where participants were given individually tailored evaluation and management or personalized care, or specific emotionally based psychological therapy (the problem solving therapy).21,44 Self-rated health was also examined by some studies and only the interventions with a multi-professional seniors meeting and a single preventive home visit showed positive impacts on self-rated health.47 Mental health was also positively affected by the geriatric evaluation and management of clinical conditions developed with inpatients and outpatients from the Veterans Affairs Medical Centers, which also impacted quality of life,44 and the problem solving therapy which was specifically aimed at emotional health.42 In addition, a group session and individual session with a geriatrician also seemed to impact risk of depression with depression being more common in the control group than in the interventions groups.51 Cognitive function was measured only rarely41,42,48,52 and the prevention of its decrease was reported only in the multicomponent exercise-based intervention provided to institutionalized older adults aged over 85 years.
Physiological secondary outcomes were observed for assessments such as overall skeletal or leg muscle mass, but significant changes were not evident.
Impact on adverse outcomes such as falls or hospitalizations was observed in several studies. Tai Chi was confirmed as useful for reducing falls risk,55 but resistance training and computerized balance training was not.55 Likelihood of hospitalization was not clearly reduced by any of the studies, but length of stay and number of consultations were observed to be higher in the inpatient intervention participants, although their overall care utilization was no greater than controls as they spent less time in long term care.44 The individual educational session by a geriatrician conducted with participants at risk of frailty, showed a positive influence on adverse outcomes in that participants from the control group had a higher risk of death or admission to a nursing home or home care program.51
What is the economic evidence regarding interventions for frailty?
Both studies reporting economic analyses32,44 focused on individually tailored management of frailty. The study targeting frailty characteristics with older adults in the community was effective for reducing frailty prevalence and cost savings,32 with average costs incurred in delivering the intervention being $1528 per participant. The obtained data suggested that the examined therapy, as compared to usual care, provided good value for money, especially for very frail persons. The study examining geriatric evaluation and management in inpatient and outpatient care units was shown to have a more favorable impact for frailty outcome than usual care, but only for the hospitalized patients.44 On the other hand, after receiving the individual tailored treatment, both inpatients and outpatients experienced significant improvement in quality of life. Total costs at one year were similar for the intervention (about $36,000) and usual care (about $37,000) groups, with the initial hospitalization of the inpatient subgroup being most costly in the intervention group than in usual care.
Limitations of the included studies
The studies included in this review presented several methodological weaknesses, with the most prominent being lack of participant blinding to treatment allocation, or unclear information about this issue (however, in most cases due to the nature of the applied interventions, the practical difficulties of the blinding process were recognized). Only eight studies provided clear information about the tools used for the assessment, indicating that their versions were culturally adapted or validated, while only nine studies clearly stated about equality of treatment between the intervention and the control groups, other than the intervention in question. Thus, we cannot rule out the possibility of bias arising from selection, performance and detection within the included studies.
In relation to studies reporting data on the economic component of this review, one such study was excluded due to insufficient methodological quality of the clinical/medical component, and only two were included for analysis. Both studies presented data on individually tailored management of the clinical condition. However, due to the different characteristics of the included samples (participants recruited from the community vs inpatient and/or outpatient care) it was not possible to proceed with a meta-analysis and obtain a pooled estimate of effects. In one of these studies44 various methodological weaknesses were observed. In addition, neither of the studies conducted sensitivity analyses to investigate uncertainty in estimates of costs or consequences, and both studies failed to present the issues of concern to users. This clearly reduces the validity of the extracted evidence, and sends a strong message that studies evaluating the economic aspects of interventions for frailty are needed.
Limitations of the review
The current review has some limitations. First, the search was undertaken in 2015 and as such it is over 12 months old. To overcome this limitation, the MEDLINE and CINAHL databases were searched for studies published in English from December 2015 to February 2017, using a phrase AB frail∗ AND AB (intervention OR treatment OR therapy OR program) AND AB old∗. The studies that met inclusion criteria of this review (n = 2) were introduced in the discussion.59,60 However, the review authors did not evaluate the methodological quality of these two studies and therefore their findings need to be treated with some caution. Second, the search was limited to articles published in the languages known by the group members and only papers published in English were included. The exclusion of papers in other languages could have limited access to studies with significant findings related to our aim that were developed in cultural and socio-economic contexts different from those considered in this review. Third, a large number of studies was excluded because of the lack of the operational definition of frailty used to select participants and/or because of the use of different criteria for frailty assessment before and after the intervention. This fact is highly important since it suggests that the possibility that the frailty concept is excessively used for the description of ageing processes, and not only for the description of a specific and assessed age-related state of decreased physiological reserves characterized by a weakened response to stressors and an increased risk of poor clinical outcomes. This fact also reflects the lack of consensus on frailty definition, which limits substantially the capacity to compare the obtained findings and to subsequently generalize them to the different clinical and economic contexts. For example, from 21 studies included in this review only nine used the same overall operational definition of frailty (based on CHS phenotypic indicators), and even those nine studies showed a significant variation on the operationalization of specific indicators used in the assessment and on the definition of cut-off points. Moreover, there were some outcomes (such as ADLs) that in some studies were considered as indicators of frailty,21,44 and in other studies22,29,41,42 as complementary outcomes. We decided to follow the structure proposed in the protocol34 and report these outcomes in separate sections (dedicated to primary versus secondary outcomes), which perhaps made the findings related to specific outcomes more difficult to compare.
Finally, due to the variation in outcomes assessed across included studies, and the reduced number of studies focusing on the same intervention and different characteristics of included samples, we were unable to conduct meta-analyses to more efficiently explore data from the included studies. Consequently, it was impossible to estimate the effect size difference between individuals receiving specific interventions and individuals in control conditions, which reduces the statistical power and generalizability of this review's findings.
Conclusion
This review has demonstrated mixed effectiveness of frailty interventions. We can conclude that physical exercise interventions are generally effective in reducing or reversing/postponing frailty but only where classes or group based interventions are used – evidence for home based or computerized training was not found. Geriatric management and continuum care was not found to be universally effective in terms of changing frailty status but differences between healthcare systems and interventions in the studies that did and did not find effects on frailty need consideration. For example, some authors65 noted that in instances where there were functional improvements, but not changes in frailty status, it might be that the intervention care and the background healthcare were not sufficiently different (particularly in developed countries with a high standard of care) to achieve a change in global physical frailty phenotype scores. Likewise for nutritional supplementation studies, those involving people with poor background nutrition will show greater effects than those in people who are generally well-nourished.
Home visits were widely supported, even at quite a low level such as a single visit, with added features such as provision of an alert button possibly increasing nurse involvement and patient confidence. Secondary impacts on ADLs or physical mobility were commonly supported, suggesting the further impact of successful interventions, but consistency in the range of secondary outcomes assessed was not found. Finally, the impact of frailty interventions on secondary issues such as quality of life, depression or cognition is much needed, and analyses that demonstrate the relationships between changes in frailty as a result of the intervention and these secondary outcomes that contribute to the wellbeing of older persons and costs to healthcare are also called for.
Recommendations for practice
This review has confirmed the view that frailty is malleable, with effects of interventions on frailty assessments demonstrated for men and women, for frail and pre-frail and for some very old participants indeed, including those in hospital or in long term institutional care. This accumulation of findings, albeit from a range of interventions, suggests that intervention for frailty is worthwhile across the range of frail patients, with strong evidence for physical exercise plus nutrition, and other combinations of evidence based intervention such as cognitive training. However, we can also make some suggestions as to what does not work. For example, exercise interventions without group support seemed less likely to work, and multi-disciplinary care worked well for frailty when it included specific interventions such as supported exercise, but not when only continuum care was coordinated. However, such care based interventions that did not have an impact on frailty itself still had other positive impacts, for example, on independent function. The economic evaluations of individually tailored management of frailty condition developed with older adults recruited from different settings (primary care, hospital care) was shown to be dominant as compared to usual care, with the intervention provided to older adults from the community being more effective and more probably cost saving, and the intervention conducted with inpatients and outpatients being more effective and equal cost. However, further research with more rigorous methodology is required to reinforce the current evidence and to increase transferability of findings.
Based on the findings of this systematic review, the following recommendations for practice can be made:
Physical exercise programs provided in groups to pre-frail and frail older adults that are institutionalized32 or that live in community42,46,49 are an effective intervention for reducing frailty level (Level of Evidence – 1a) or, at least, for positively changing some of the frailty indicators. Thus, based on current evidence, health professionals are strongly recommended to provide physical exercise program conducted in classes or in groups to prevent progression of pre-frailty and frailty in community dwelling and institutionalized older adults (GRADE A).
Physical exercise programs delivered in classes with home-based practice to non-frail, pre-frail and frail older adults from the community53,55 are an effective intervention for reducing frailty level (Level of Evidence – 1c), improving or postponing decline of grip strength, improving gait speed and reducing fear of falling (Level of Evidence – 1c). Thus, based on current evidence, health professionals may provide physical exercise programs conducted in classes with home-based practice to prevent progression of pre-frailty and frailty in community dwelling and non-institutionalized older adults (GRADE B).
Physical exercise programs provided individually to non-frail, pre-frail and frail community dwelling older adults43,55 have been shown to have no impact on mobility (Level of Evidence – 1c), functional status or biomedical and psychosocial variables (Level of Evidence – 1c). Thus, based on current evidence, the recommendation of physical exercise programs provided individually for preventing progression of pre-frailty and frailty in community dwelling older adults is not supported.
Nutritional supplementation provided to pre-frail and frail older adults from the community23,49,53 is an effective intervention for increasing physical activity (Level of Evidence – 1a), for reducing long-term exhaustion and for improving energy intake (Level of Evidence – 1c); however, it seems to have no impact on body weight. Thus, based on current evidence, health professionals are strongly recommended to provide nutritional supplementation to prevent progression of pre-frailty and frailty in community dwelling older adults (GRADE A).
Group sessions for persons not at risk of frailty and individual educational sessions by a geriatrician for persons at risk of frailty, provided to non-institutionalized older adults,51 are an effective intervention for reverting frailty condition (Level of Evidence – 1c), and the group sessions with one home visit conducted with older adults from the community40,47 is an effective therapy for postponing progression in tiredness in daily activities for up to one year (Level of Evidence – 1c/2RCTs). Thus, based on current evidence, health professionals may provide group sessions and individual educational sessions to prevent progression of pre-frailty and frailty in community dwelling and non-institutionalized older adults (GRADE B).
Home visit(s) from a nurse or other health professionals, provided to frail45,54 and non-frail40,47 older adults from the community, have been shown to have no impact on frailty core indicators (Level of Evidence – 1c/2RCTs), on disability in ADLs (Level of Evidence – 1c) or on physical and mental component of health status (Level of Evidence – 1c). However, a single preventive home visit by a trained professionals delivered to non-frail older adults from the community40 has been shown to be an effective therapy for postponing progression in tiredness in daily activities for up to one year (Level of Evidence – 1c), and the home visits from a nurse combined with use of alert button provided to frail older adults have been shown to be an effective therapy for reducing frailty prevalence (Level of Evidence – 1c). Thus, based on current evidence, health professionals may provide home visit(s) for preventing progression of pre-frailty and frailty in older adults (GRADE B).
Combined multidisciplinary treatment integrating physical activity and nutritional supplementation is an effective intervention for preventing decrease in maximal walking time in community dwelling older adults that are at risk of becoming frail31 (Level of Evidence – 1c), and for improving some frailty indicators in community dwelling women with frailty49 (Level of Evidence – 1c). Combined multidisciplinary treatment including nutritional supplementation, physical training and cognitive training is an effective intervention for reducing frailty level, especially for improving knee strength and energy level, in pre-frail and frail older adults from the community53 (Level of Evidence – 1c). Continued long-term intervention of music-based multitask training provided to community dwelling older adults at increased risk of falling48 is an effective intervention for preventing decline in gait speed and grip strength, and for reverting frailty status in pre-frail persons48 (Level of Evidence – 1c). Thus, based on current evidence, health professionals may provide combined multidisciplinary treatment for preventing progression of pre-frailty and frailty in community dwelling older adults (GRADE B).
Cognitive training provided to pre-frail and frail older adults from the community53 is an effective intervention for reducing frailty level and improving knee strength (Level of Evidence – 1c). Thus, based on current evidence, health professionals may provide cognitive training to prevent progression of pre-frailty and frailty in community dwelling older adults (GRADE B).
Problem solving therapy provided to frail older adults from the community42 has been shown to have no impact on improvement in frailty, gait speed, grip strength, weight, exhaustion or physical activity level (Level of Evidence – 1c). Thus, based on current evidence, the recommendation of problem solving therapy for preventing progression of pre-frailty and frailty in community dwelling older adults is not supported.
Hormone replacement with atamestane and/or dehydroepiandrosterone52 has been shown to have no impact on isometric grip strength, leg extension power or physical performance of frail independently living men (Level of Evidence – 1c). Thus, based on current evidence, the recommendation of the hormone replacement for preventing progression of pre-frailty and frailty in community dwelling older adults is not supported.
Individually tailored management of clinical condition is an effective intervention for improving physical performance in frail inpatients and outpaitents44 (Level of Evidence – 1c), and for reducing basic ADL dependency in older adults who are from the community and who have functional problems21 or who are frail and require hospital care44 (Level of Evidence – 1c), but it seems to have no impact on instrumental ADLs44 (Level of Evidence – 1c). On the other hand, individually tailored management of clinical condition has mixed effects for decreasing the core frailty indicators in pre-frail and frail older adults from the community29,32,50 and hospitals29 (Level of Evidence – 1c/3RCTs). In addition, individually tailored management of frailty condition, as compared to usual care, has a high probability to be cost saving when provided to community dwelling older adults32 (Level of Evidence - 6), does not increase the total costs of care when developed with inpatients44 (Level of Evidence - 6), and is equal cost when delivered to oupatients44 (Level of Evidence - 6). Thus, based on current evidence, we recommend adopting individually tailored management of frailty condition on a larger-scale basis (GRADE B).
Recommendations for research
Despite the positive findings, there are still many unresolved issues. The impact of an initial level of frailty or gender on the benefits of different interventions still needs clarification to inform personalized frailty intervention. The interaction between outcomes, such as impact of physical exercise or nutrition on mediators such as cognition, depression or self-efficacy in terms of the outcomes on frailty also needs further investigation. Significantly, in such extensive literature there were remarkably few intervention types that fulfilled the inclusion criterion of measuring frailty before and after an intervention, and few that employed careful RCT methods, preventing full systematic comparison or conclusions. Finally, there is a need for well-conducted economic evaluations of frailty interventions performed in different decision making contexts.
Acknowledgements
We acknowledge the support of the FOCUS project (Frailty management Optimisation through EIPAHA Commitments and Utilisation of Stakeholders input) which is a three-year project co-financed by the Consumers, Health, Agriculture and Food Executive Agency (CHAFEA), under the power delegated by the European Commission (Grant Agreement 664367 - FOCUS).

We acknowledge the contribution of other members of Focus project: Alessandro Nobilia, Ana González Segurab, Ana M. Martinez-Arroyoc, Donata Kurpasd, Enrique de la Cruz Martínezb, James Browne, Lex van Velsenf, Maria Bujnowskad, Rachel Shawe, Vicente Gilc, Vicente Llorensc who were co-responsible for elaboration of PICO questions and structuring of PICO components of this systematic review protocol.
a IRCCS Istituto Di RicercheFarmacologiche “Mario Negri”
b EVERIS Spain S.L.U
c ESAM Tecnología S.L.
d Wroclaw Medical University
e Aston Research Centre for Healthy Ageing, Aston University
f Roessingh Research and Development
The authors would like to acknowledge Daniela Cardoso for her contribution to the protocol development, and Ana Gois and Mariana Santos for their collaboration under supervision in the organization of the analyzed materials.
Appendix I: Search strategy
Search – November 25th, 2015
MEDLINE
CINAHL
Embase

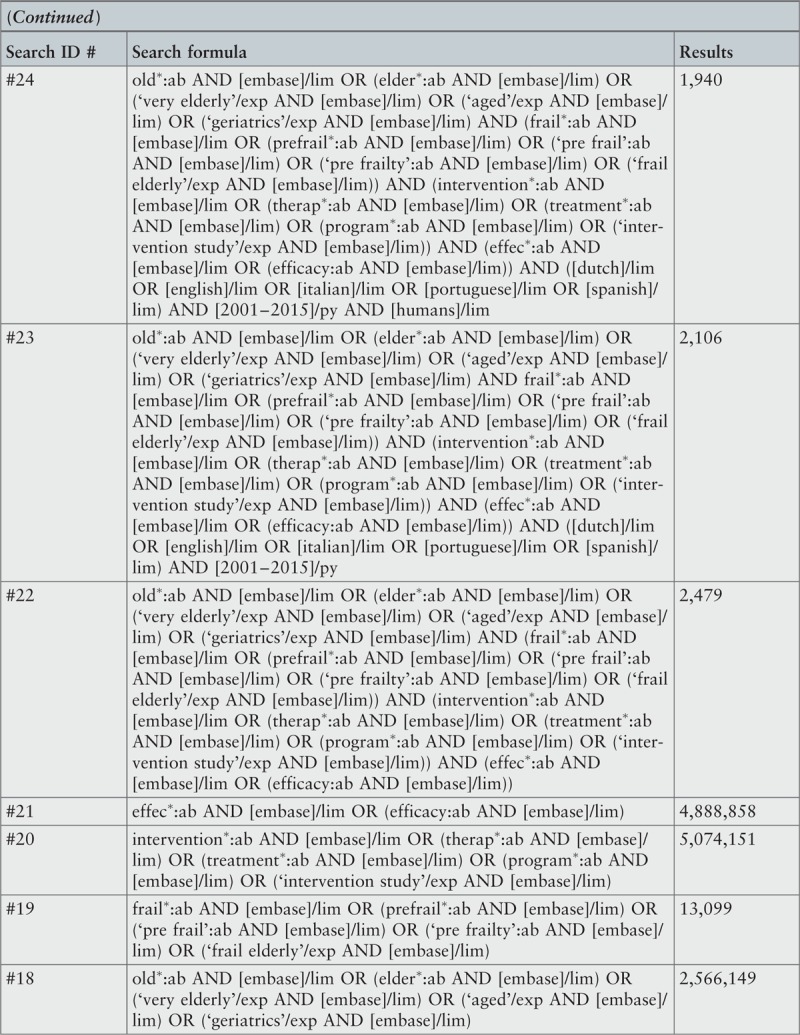
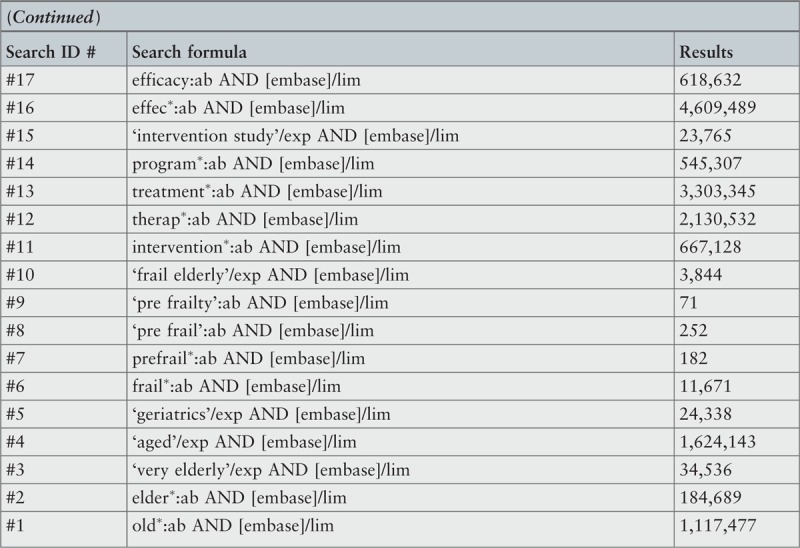
Cochrane Central Register of Controlled Trials

Scopus

SciELO

OpenGrey

Banco de teses da CAPES (www.capes.gov.br)

Dissertation Abstracts Online (e-Thos)

ProQuest –Theses and Dissertations

Appendix II: Excluded studies based on assessment of methodological quality
Pseudo-randomized control trials (one group)
Nomura T, Nagano K, Takato J, Ueki S, Matsuzaki Y, Yasumura S. The development of a Tai Chi exercise regimen for the prevention of conditions requiring long-term care in Japan. Arch Gerontol Geriatr. 2011;52(3):e198–203.
Reason for exclusion: Methodological appraisal value below minimum cut-off score. It was unclear whether the outcomes were measured in a reliable way. The study authors did not describe and did not include in the analysis the outcomes of people who withdrew. The critical appraisal items related with randomization, blinding, allocation and comparability of the groups were not considered as the study was conducted with one group only.
De Vries N, van Ravensberg CD, Hobbelen JS, van der Wees PJ, Olde Rikkert MG, Staal JB, et al. The Coach2Move Approach: Development and Acceptability of an Individually Tailored Physical Therapy Strategy to Increase Activity Levels in Older Adults With Mobility Problems. J Geriatr Phys Ther. 2015;38(4):169–82.
Reason for exclusion: Methodological appraisal value below minimum cut-off score. It was unclear whether the outcomes were measured in a reliable way and whether the appropriate statistical analysis were used. The critical appraisal items related with randomization, blinding, allocation and comparability of the groups were not considered as the study was conducted with one group only.
Before and after studies
Sugimoto H, Demura S, Nagasawa Y, Shimomura M. Changes in the physical functions of pre-frail elderly women after participation in a 1-year preventative exercise program. Geriatr Gerontol Int. 2014;14(4):975–82.
Reason for exclusion: Methodological appraisal value below minimum cut-off score. The composition of the study groups was intentionally different (healthy and pre-frail participants), thus all the critical appraisal items related with randomization, allocation and comparability of the groups were rated negatively. It was unclear whether the appropriate statistical analysis were used.
Yamada M, Arai H, Uemura K, Mori S, Nagai K, Tanaka B, et al. Effect of resistance training on physical performance and fear of falling in elderly with different levels of physical well-being. Age Ageing. 2011;40(5): 637–41.
Reason for exclusion: Methodological appraisal value below minimum cut-off score. The composition of the study groups was intentionally different (robust and frail participants), thus all the critical appraisal items related with randomization, allocation and comparability of the groups were rated negatively. The outcomes of people who withdrew were not described and included in the analysis. It was unclear whether the outcomes were measured in a reliable way.
Pseudo-randomized control trial (two groups)
Yamada M, Arai H, Sonoda T, Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc. 2012;13(6):507–11.
Reason for exclusion: In addition to lack of group randomization there was unclear information about allocation and blinding procedures, and insufficient data about tools used for the outcomes assessment. It was also unclear whether the groups were treated identically other than for the named interventions.
Randomized controlled trials
Binder E, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–8.
Reason for exclusion: Not all outcomes were measured in the same way for all groups. There was also unclear information about allocation and blinding procedures, and about the treatment of groups other than for the named interventions.
Chin A Paw MJM, de Jong N, Schouten EG, Hiddink GJ, Kok FJ. Physical exercise and/or enriched foods for functional improvement in frail, independently living elderly: a randomized controlled trial. Arch Phys Med Rehabil. 2001;82(6):811–7.
Reason for exclusion: Those assessing outcomes were not blind to treatment allocation. In addition, it was unclear if the assignment to treatment group was truly random, if participants were blinded to treatment allocation, if the allocation to treatment group was concealed from the allocator, if the outcomes of people who withdrew were described and included in the analysis, and if the groups were treated identically other than for the named interventions.
Kono A, Kanaya Y, Fujita T, Tsumura C, Kondo T, Kushiyama K, et al. Effects of a preventive home visit program in ambulatory frail older people: A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2012;67(3):302–9.
Reason for exclusion: The outcomes were not measured in a reliable way. There was unclear information about allocation and blinding procedures. In addition, it was unclear if the outcomes of people who withdrew were described and included in the analysis and if the control and treatment groups were comparable at entry.
Kwon J, Yoshida Y, Yoshida H, Kim H, Suzuki T, Lee Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: A randomized controlled trial. J Am Med Dir Assoc. 2015;16(3):263e1–8.
Reason for exclusion: The outcomes of people who withdrew were not described and included in the analysis. It was unclear if the assignment to treatment group was truly random, if participants were blinded to treatment allocation, if the allocation to treatment group was concealed from the allocator, if the groups were treated identically other than for the named interventions, and if the outcomes were measured in a reliable way.
Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of Tai Chi training within senior housing facilities. J Am Geriatr Soc. 2014;62(8):1484–9.
Reason for exclusion: The outcomes of people who withdrew were not described and included in the analysis, and the control and treatment groups were not comparable at entry. It was unclear if the assignment to treatment group was truly random, if participants were blinded to treatment allocation, if the allocation to treatment group was concealed from the allocator, and if the groups were treated identically other than for the named interventions. It was also unclear whether the statistical analysis used was appropriate (the authors used the parametric statistical tests in samples with n < 30, without reference to meeting the assumptions underlying these tests).
Rydwik E, Lammes E, Frändin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pliot treatment trial. Aging Clin Exp Res. 2008;20(2):159–170.
Reason for exclusion: The allocation to treatment groups was not concealed from the allocator, and the control and treatment groups were not comparable at entry. It was unclear if the assignment to treatment group was truly random, if participants and those assessing outcomes were blinded to treatment allocation, if the groups were treated identically other than for the named interventions, and if the outcomes were measured in a reliable way.
Appendix III: Characteristics of included studies
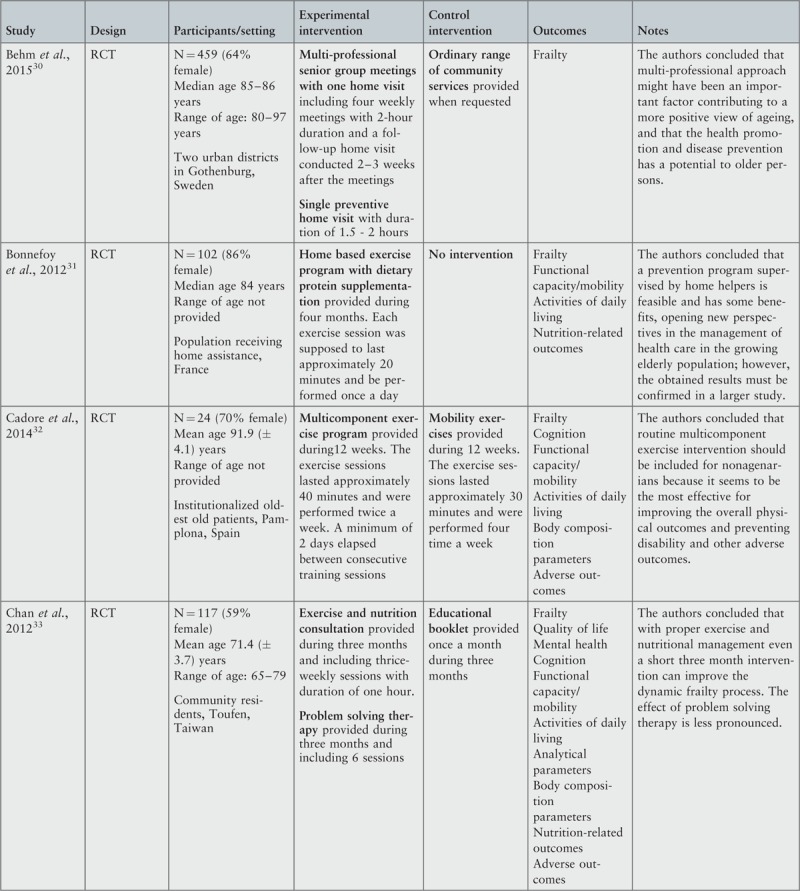
Footnotes
There are no conflicts of interest.
References
- 1.Rodriguez-Manas L, Feart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci 2013; 68 1:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59 3:255–263. [DOI] [PubMed] [Google Scholar]
- 3.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A: Biol Sci Med Sci 2007; 62 7:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62 7:722–727. [DOI] [PubMed] [Google Scholar]
- 5.Lang PO, Michel JP, Zekry D. Frailty syndrome: A transitional state in a dynamic process. Gerontology 2009; 55 5:539–549. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J GerontolBiol Med Sci 2001; 56 3:M146–M156. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Windham BG, Fried LP. Frailty in older persons. Genus 2005; 61 1:39–53. [Google Scholar]
- 8.Varadhan R, Seplaki CS, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev 2008; 129 11:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381 9868:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsitz LA. Dynamic models for the study of frailty. Mech Ageing Dev 2008; 129 11:675–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langlois F, Vu TTM, Kergoat MJ, Chassé K, Dupuis G, Bherer L. The multiple dimensions of frailty: physical capacity, cognition, and quality of life. International Psychogeriatrics 2012; 24 9:1429–1436. [DOI] [PubMed] [Google Scholar]
- 12.Lin F, Roiland R, Chen DG, Qiu C. Linking cognition and frailty in middle and old age: metabolic syndrome matters. Research article. Geriatric Psychiatry 2015; 30 1:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halil M, Kizilarslanoglu MC, Kuyumcu ME, Yesil Y, Jentoft AJ. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging 2015; 19 3:276–283. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment – A review of the evidence and causal mechanisms. Ageing Research Reviews 2013; 12 4:840–851. [DOI] [PubMed] [Google Scholar]
- 15.Ávila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: The Three-City Study. J Am Geriatr Soc 2009; 57 3:453–461. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg SA, Wershof Schwartz AW, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: A systematic literature review. Progress in Geriatrics 2011; 59 11:2129–2139. [DOI] [PubMed] [Google Scholar]
- 17.Collard RM, Comijs HC, Naarding P, Penninx BW, Milaneschi Y, Ferrucci L, et al. Frailty as a predictor of the incidence and course of depressed mood. J Am Med Direct Ass 2015; 16 6:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drubbel I, Numans ME, Kranenburg G, Bleijenberg N, de Wit NJ, Schuurmans MJ. Screening for frailty in primary care: a systematic review of the psychometric properties of the frailty index in community-dwelling older people. Bmc Geriatrics 2014; 14 1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35 5:526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobbens RJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MTh, Schols JMGA. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11 5:344–355. [DOI] [PubMed] [Google Scholar]
- 21.Vriendt P, Peersman W, Florus A, Verbeke M, Van de Velde D. Improving health related quality of life and independence in community dwelling frail older adults through a client-centred and activity-oriented program. A pragmatic randomized controlled trial. J Nutr Health Aging 2016; 20 1:35–40. [DOI] [PubMed] [Google Scholar]
- 22.Bonnefoy M, Boutitie F, Mercier C, Gueyffier F, Carre C, Guetemme G, et al. Efficacy of a home-based intervention programme on the physical activity level and functional ability of older people using domestic services: a randomised study. J Nutr Health Aging 2012; 16 4:370–377. [DOI] [PubMed] [Google Scholar]
- 23.Kim Ch-O, Lee K-R. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol A Biol Sci Med Sci 2013; 68 3:309–316. [DOI] [PubMed] [Google Scholar]
- 24.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc 2012; 60 8:1487–1492. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen PT, Lund H, Aadahl M, Sørensen EE. The experience of daily life of acutely admitted frail elderly patients one week after discharge from the hospital. Int J Qualitative Stud Health Well-being. 2015; 10:27370 Available from: http://dx.doi.org/10.3402/qhw.v10.27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar Ch, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BC Medicine 2013; 11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesari M, Vellas B, Hsu F-C, Newman AB, Doss H, King AC, et al. A physical activity intervention to treat the frailty syndrome in older persons—results from the LIFE-P study. J Gerontol A Biol Sci Med Sci 2015; 70 2:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, Gregori D, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med 2010; 11 10:739–747. [DOI] [PubMed] [Google Scholar]
- 29.Eklund K, Wilhelmson K, Gustafsson H, Landahl S, Dahlin-Ivanoff S. One-year outcome of frailty indicators and activities of daily living following the randomized controlled trial; “Continuum of care for frail older people”. BMC Geriatr 2013; 13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastos-Barbosa RG, Ferriolli E, Coelho EB, Moriguti JC, Nobre F, Lima NK. Association of frailty syndrome in the elderly with higher blood pressure and other cardiovascular risk factors. Am J Hypertens 2012; 25 11:1156–1161. [DOI] [PubMed] [Google Scholar]
- 31.Peklar J, O’Halloran AM, Maidment ID, Henman MC, Kenny RA, Kos M. Sedative load and frailty among community-dwelling population aged ≥65 years. J Am Med Dir Assoc 2015; 16 4:282–289. [DOI] [PubMed] [Google Scholar]
- 32.Fairhall N, Sherrington C, Kurrle S, Lord SR, Lockwood K, Howard K, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc 2015; 16 1:41–48. [DOI] [PubMed] [Google Scholar]
- 33.The Joanna Briggs Institute Joanna Briggs Institute Reviewers’ Manual 2014. Adelaide: The Joanna Briggs Institute; 2014. [Google Scholar]
- 34.Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, et al. Effectiveness of the interventions to prevent progression of pre-frailty and frailty in older adults: a systematic review protocol. JBI Database System Rev Implement Rep 2016; 14 1:4–19. [DOI] [PubMed] [Google Scholar]
- 35.Arantes P, Alencar M, Dias R, Dias J, Pereira L. Physical therapy treatment on frailty syndrome: systematic review. Rev Bras Fisioter 2009; 13 5:365–375. [Google Scholar]
- 36.Labra C, Guimaraes-Pinheiro Ch, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr 2015; 15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giné-Garriga M, Roqué-Fíguls M, Coll-Planas L, Sitjà-Rabert M, Salvà A. Physical Exercise Interventions for Improving Performance-Based Measures of Physical Function in Community-Dwelling, Frail Older Adults: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil 2014; 95 4:753–769. [DOI] [PubMed] [Google Scholar]
- 38.Chin A, Paw M, van Uffelen J, Riphagen I, Mechelen W. The Functional Effects of Physical Exercise Training in Frail Older People. A Systematic Review. Sports Med 2008; 38 9:781–793. [DOI] [PubMed] [Google Scholar]
- 39.The Joanna Briggs Institute Joanna Briggs Institute reviewers’ manual 2014. The systematic review of economic evaluation evidence. Adelaide: The Joanna Briggs Institute; 2014. [Google Scholar]
- 40.Behm L, Eklund K, Wilhelmson K, Zidén L, Gustafsson S, Falk K, et al. Health promotion can postpone frailty: Results from the RCT elderly persons in the Risk Zone. Public Health Nurs 2016; 33 4:303–315. [DOI] [PubMed] [Google Scholar]
- 41.Cadore E, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gómez M, et al. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr) 2014; 36 2:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan D, Tsou HH, Yang RS, Tsauo JY, Chen ChY, Hsiung Ch, et al. A pilot randomized controlled trial to improve geriatric frailty. BMC Geriatr 2012; 12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clegg A, Barber S, Young J, Iliffe S, Forster A. The Home-based Older People's Exercise (HOPE) trial: a pilot randomized controlled trial of a home-based exercise intervention for older people with frailty. Age Aging 2014; 43 5:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen H, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med 2002; 346 12:905–912. [DOI] [PubMed] [Google Scholar]
- 45.Favela J, Castro LA, Franco-Marina F, Sánchez-García S, Juárez-Cedillo T, Espinel Bermudez C, et al. Nurse home visits with or without alert buttons versus usual care in the frail elderly: a randomized controlled trial. Clin Interv Aging 2013; 8:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giné-Garriga M, Guerra M, Pagès E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act 2010; 18 4:401–424. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson S, Wilhelmson K, Eklund K, Gosman-Hedström G, Zidén L, Kronlöf GH, et al. Health-promoting interventions for persons aged 80 and older are successful in the short term--results from the randomized and three-armed Elderly Persons in the Risk Zone study. J Am Geriatr Soc 2012; 60 3:447–454. [DOI] [PubMed] [Google Scholar]
- 48.Hars M, Herrmann FR, Fielding RA, Reid KF, Rizzoli R, Trombetti A. Long-term exercise in older adults: 4-year outcomes of music-based multitask training. Calcif Tissue Int 2014; 95 5:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, et al. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: A randomized double blind, placebo-controlled, follow-up trial. PLoS ONE 2015; 10 2:e0116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li CM, Chen CY, Li CY, Wang WD, Wu SC. The effectiveness of a comprehensive geriatric assessment intervention program for frailty in community-dwelling older people: a randomized, controlled trial. Arch Gerontol Geriatr 2010; 50 Suppl 1:S39–42. [DOI] [PubMed] [Google Scholar]
- 51.Monteserin E, Brotons C, Moral I, Altimir S, San José A, Santaeugenia S, et al. Effectiveness of a geriatric intervention in primary care: a randomized clinical trial. Fam Pract 2010; 27 3:239–245. [DOI] [PubMed] [Google Scholar]
- 52.Muller M, van den Beld AW, van der Schouw YT, Grobbee DE, Lamberts SW. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. J Clin Endocrinol Metab 2006; 91 10:3988–3991. [DOI] [PubMed] [Google Scholar]
- 53.Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. Am J Med 2015; 128 11:1225–1236. [DOI] [PubMed] [Google Scholar]
- 54.Van Hout HP, Jansen AP, van Marwijk HW, Pronk M, Frijters DF, Nijpels G. Prevention of adverse health trajectories in a vulnerable elderly population through nurse home visits: A randomized controlled trial [ISRCTN05358495]. J Gerontol A Biol Sci Med Sci 2010; 65 7:734–742. [DOI] [PubMed] [Google Scholar]
- 55.Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T, et al. Selected as the best paper in the 1990 s: reducing frailty and falls in older persons: an investigation of tai chi and computerized balance training (reprinted from JAGS 1996). Am Geriatr Soc 2003; 51 12:1794–1803. [DOI] [PubMed] [Google Scholar]
- 56.Rockwood K, Mitnitski A, MacKnight C. Some mathematical models of frailty and their clinical implications. Rev Clin Gerontol 2001; 12 2:109–117. [Google Scholar]
- 57.Nelson E, Wasson J, Kirk J, Keller A, Clark D, Dietrich A, et al. Assessment of function in routine clinical practice: description of the COOP chart method and preliminary findings. J Chron Dis 1987; 40 Suppl1:55S–69S. [DOI] [PubMed] [Google Scholar]
- 58.Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, et al. Predicting risk and outcomes for frail older adults: an umbrella review of available frailty screening tools. JBI Database System Rev Implement Rep 2017; 15 4:1154–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland A, Navarro-Martínez R, Martínez-Martínez M, et al. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int J Mol Sci 2016; 17 6:E932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tse M, Ng S, Lee P, Lai C, Kwong E, Liu J, et al. Play activities program to relieve chronic pain and enhance functional mobility and psychological well-being for frail older adults: A pilot cluster randomized controlled trial. J Am Geriatr Soc 2016; 64 10:e86–e88. [DOI] [PubMed] [Google Scholar]
- 61.Bennet DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol 2014; 127 1:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006; 61 11:1166–1170. [DOI] [PubMed] [Google Scholar]
- 63.Holland CA, Carter M, Cooke R, Leask G, Powell R, Shaw R, et al. Collaborative Research between Aston Research Centre for Healthy Ageing (ARCHA) and the ExtraCare Charitable Trust, The final report, Aston University 2015, Aston Research Centre for Healthy Ageing; 2015. Available at http://www.aston.ac.uk/lhs/research/centres-facilities/archa/extracare-project/ [Accessed 13 November 2016]. [Google Scholar]
- 64.Romero-Ortuno R, Walsh C, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the survey of health, ageing and retirement in Europe (SHARE). BMC Geriatr 2010; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gwyther H., Bobrowicz-Campos E, Marcucci M, Apóstolo J, Cooke R, Santana S, et al. A realist review to understand the efficacy and outcomes of European frailty interventions in the country, healthcare organisational and patient contexts. Submitted. [Google Scholar]


