Abstract
Background
The clinical effect of radiographic contact of glioblastoma (GBM) with neurogenic zones (NZ)—the ventricular-subventricular (VSVZ) and subgranular (SGZ) zones—and the corpus callosum (CC) remains unclear and, in the case of the SGZ, unexplored. We investigated 1) if GBM contact with a NZ correlates with decreased survival; 2) if so, whether this effect is associated with a specific NZ; and 3) if radiographic contact or invasion by GBM of the CC, the largest identifiable white matter tract, is associated with decreased survival.
Methods
We retrospectively identified 207 adult patients who underwent cytoreductive surgery for GBM followed by chemotherapy and/or radiation. Age, preoperative Karnofsky performance status score (KPS), and extent of resection were recorded. Preoperative MRIs were blindly analyzed to calculate tumor volume and contact with VSVZ, SGZ, CC, and cortex. Overall (OS) and progression free (PFS) survivals were calculated and analyzed with multivariate Cox analyses.
Results
Among 207 patients, 111 had GBM contacting VSVZ (VSVZ+GBMs), 23 SGZ+GBMs, 52 CC+GBMs, and 164 cortex+GBMs. VSVZ+, SGZ+, and CC+ GBMs had significantly larger volume relative to non-contacting controls. In addition to age, KPS, gross total resection, chemotherapy, and radiation, multivariate Cox survival analyses revealed contact with VSVZ as an independent predictor of lower OS and the only predictor of lower PFS and early recurrence.
Conclusions
GBM contact with the VSVZ, but not SGZ, CC, or cortex, is associated with early recurrence and decreased survival. We hypothesize that the VSVZ niche has unique properties that contribute to GBM pathobiology in adults.
Keywords: Glioblastoma, Stem Cells, Ventricular-Subventricular Zone, Subgranular Zone, Survival, Subventricular Zone
INTRODUCTION
The ventricular-subventricular zone (VSVZ) [1, 2] and subgranular zone (SGZ) [3] are neurogenic zones that harbor neural stem cells in the adult human brain. The VSVZ, the larger of the two regions, is located in the lateral walls of the lateral ventricles, while the SGZ is found in hippocampal dentate gyrus [1–4].
Several studies have demonstrated that glioblastoma (GBM) tumor cells exhibit a tropism for the VSVZ by spreading along the white matter tracts, specifically the corpus callosum (CC) [5–7]. Patients with GBMs spreading along the CC have been shown to have decreased survival, [8–11] although the basis of this effect remains unclear. While some studies suggest a greater malignant potential of GBMs with VSVZ contact (VSVZ+ GBMs), the majority of these studies were conducted on small cohorts (<100 total patients) [12]. Further, the cumulative clinical data is limited by heterogeneous accounting of common, well-known survival confounders and other clinical variables such as extent of resection and tumor volume [12–18]. On the other hand, the clinical impact of GBM contact with SGZ is relatively unexplored.
A report by Lim et. al., proposed an interaction between the VSVZ and cortical contact, classifying GBMs into 4 groups—VSVZ+/cortex+, VSVZ+/cortex−, VSVZ−/cortex+, and VSVZ−/cortex−—based on the observation that VSVZ+/cortex+ GBMs were most likely to be multifocal and have distal recurrences compared to VSVZ−/cortex− GBMs [19]. While this classification is commonly used, [20–23] whether an interaction between VSVZ and cortex exists that is predictive of survival, tumor multifocality, and distal recurrences remains uninvestigated.
Therefore, we studied the influence on survival of radiographic contact of GBM with these two neurogenic zones as well as the CC with goals of answering four main questions: first, is GBM contact with a neurogenic zone sufficient to decrease patient survival; second, is any observed effect specific to a particular neurogenic zone? Third, is radiographic contact or invasion of the CC by GBM associated with decreased survival, and finally is there an interaction between VSVZ and cortex that is predictive of survival, tumor multifocality, and distal recurrence?
METHODS
Patient Population Selection
This study was conducted in accordance with the ethics and regulations of Vanderbilt University Medical Center and was approved by the Institutional Review Board. We retrospectively identified 207 adult (≥ 18 years of age) patients enrolled in our institutional tumor registry after informed consent who underwent a cytoreductive surgery for pathology-proven supratentorial GBM (WHO grade IV glioma) between 2001 and 2014. Patients were followed through January 2016. Those who underwent non-resective biopsies or who had incomplete medical records lacking preoperative clinical data and pre- and immediate postoperative imaging were excluded. All patients underwent maximal safe resection of their GBM by four specialized tumor neurosurgeons followed by treatment with chemotherapy (temozolomide) and radiation.
Clinical Data Collection
Patients’ age at the time of surgery, sex, preoperative Karnofsky performance status score (KPS; 0–100 in increments of 10), extent of tumor resection (EOR), time to death or last follow-up, and time to radiographic progression or last MRI without progression were collected. KPS was assigned by a physician at the time of evaluation. EOR was evaluated independently by both a neurosurgeon and neuroradiologist on the basis of MRI obtained within 24 hours of surgery. Gross total resection (GTR) was defined as having no residual contrast enhancement. Near total resection (NTR) was defined as enhancing tumor residual of less than 5% or when the senior radiologist cannot rule out minute residual tumor. Subtotal resection (STR) was defined as a resection of less than 95% of the enhancing tumor. Overall survival (OS) was calculated as the time to death from any cause. Progression-free survival (PFS) was calculated as the time to first definitive radiographic disease progression confirmed by neuroradiologist and neuro-oncologist without suspicion for pseudoprogression or as the time of death if no radiographic progression was noted.
Radiological Data Collection
Patients’ preoperative (< 3 weeks) T1-weighted gadolinium-enhanced MRIs were analyzed blindly, without knowledge of patients’ baseline characteristics or clinical outcomes mentioned above. OsiriX Lite software (version 7.0, Pixmeo, Geneva, Switzerland) was used to calculate tumor volume, assess tumor multifocality, and assess contact of the post-contrast enhancement of the GBM with the lateral walls of the lateral ventricle (VSVZ), medial edge of the hippocampus (SGZ), cortex, and corpus callosum (CC). These contact assessments are commonly performed in other studies [18, 24] and examples are depicted in Figure 1. The original study classifying GBMs into four classes based on VSVZ and cortical contact provided the rationale for evaluating cortical contact [19]. Both axial (typically ≤ 3 mm cuts) and reformatted coronal and sagittal sequences were assessed. Although no consensus exists to denote tumor multifocality, it was designated here by the presence of two or more noncontiguous contrast-enhancing lesions separated by at least 2 cm. Contact status of patients with multifocal tumors was denoted according to presence or absence of a lesion contacting the VSVZ, SGZ, CC, or cortex. Tumor volume was calculated by delineating the outer edge of tumor contrast enhancement and using the semiautomated volume rendering function in OsiriX Lite. Tumor volumes of all multifocal lesions in a patient were summated. Postoperative T1-weighted contrasted MRIs were assessed for first radiographic evidence of tumor recurrence agreed by both a neuroradiologist and neuro-oncologist. Further, any emergence of noncontiguous recurrences was noted by examining all postoperative MRIs. All imaging was interpreted by an independent board-certified, subspecialized neuroradiologist without knowledge of the study rationale or hypotheses.
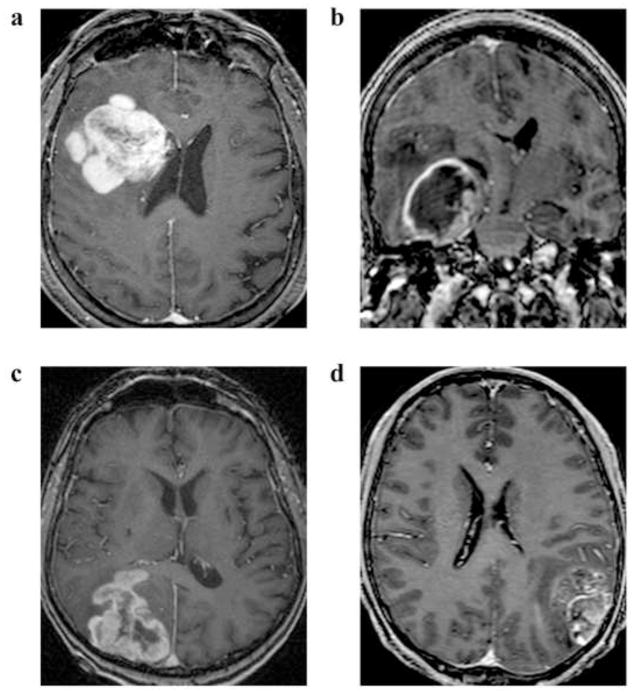
Fig. 1. Examples of T1 post-contrast MRIs of glioblastomas.
Glioblastomas with contrast enhancement contacting the (A) ventricular-subventricular zone, (B) subgranular zone, (C) corpus callosum, and (B, C, D) cortex.
Data Analysis
Data were analyzed and plotted using GraphPad Prism version 7.00 for Windows (GraphPad Software, San Diego, CA, USA). Distributions of age, KPS, tumor volume, and EOR were tested for normality with D’Agostino-Pearson omnibus K2 test and failed the normality test; hence, analyses to compare distributions of these variables were conducted with two-tailed Mann-Whitney U or Kruskal-Wallis tests. Frequencies of multifocal GBMs and distal recurrences were analyzed with two-tailed Fisher’s exact test or chi-squared (X2) test. Survival data were plotted using right-censored Kaplan-Meier curves and median survival times were calculated. If the time to death (OS) or radiographic progression (PFS) were unknown, they were censored at the time of last follow-up after diagnosis or at the time of last follow-up MRI, respectively. Both univariate and multivariate Cox survival analyses with all variables with a p-value < 0.1 on univariate analyses were conducted using R version 3.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance α was set at 0.05 for all statistical analyses. For simultaneous, multiple hypothesis testing in multivariate Cox analyses, a Benjamini–Hochberg procedure was applied to control false discovery rate (FDR) [25]. The Benjamini-Hochberg critical value for a FDR was set conservatively at 5%. Nomograms of the multivariate analyses were generated using R, utilizing the rms library (version 4.5).
RESULTS
A total of 207 adult patients (116 males, 91 females) who underwent a cytoreductive resection of their GBM were identified. In medians with interquartile range, overall age was 61.8 [51.2–70.4], KPS was 70 [60–80], and tumor volume was 32.5 cm3 [14.9–52.2]. Multifocal tumor presentation was noted in 19 patients (9.2%), and 45 patients (21.7%) had distal recurrences after resection. GTR was achieved in 21.3%, NTR in 33.3%, and STR in 45.4%. Median OS and PFS were 405 and 162 days, respectively.
Patient and Tumor Characteristics
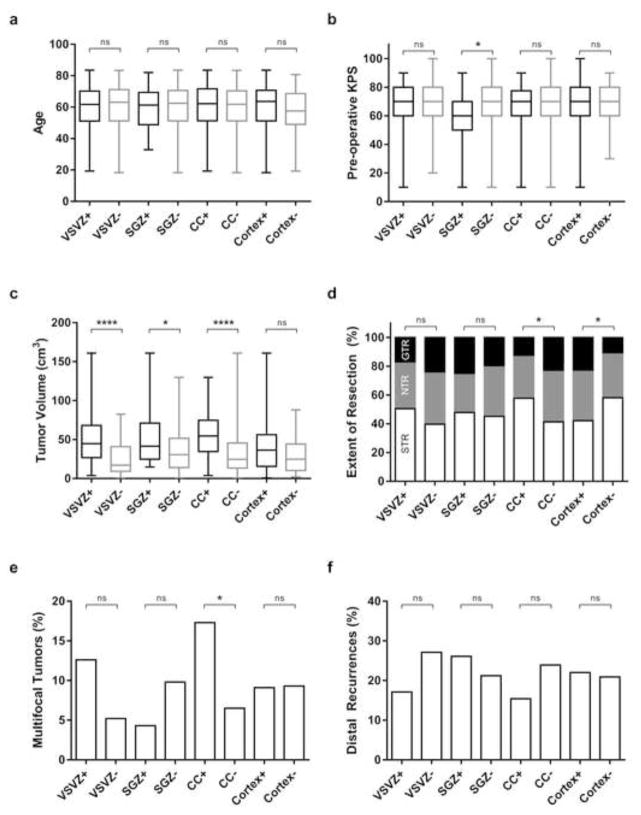
Among 207 patients, 111 had GBM contacting VSVZ+, 23 SGZ+, 52 CC+, and 164 cortex+ GBMs. Age, preoperative KPS, EOR, tumor volume distributive statistics together with number of patients with multifocal GBMs and distal recurrences are plotted in Figure 2 grouped based on radiographic contact with the VSVZ, SGZ, CC, and cortex. Age and pre-operative KPS did not differ in patients with VSVZ+, SGZ+, CC+, and cortex+ GBMs compared to their respective non-contacting controls, except in patients with SGZ+ GBMs who were noted to have lower KPS (p = 0.03; Figure 2a, b). While tumor volume did not significantly differ whether GBMs contacted cortex or not, the volumes of VSVZ+, SGZ+, and CC+ GBMs were significantly larger than their respective controls (p < 0.0001, 0.03, < 0.0001, respectively, Figure 2c). CC+ GBMs displayed a significantly increased frequency of multifocality (17.3% vs. 6.5% for CC− GBMs; p = 0.03) while VSVZ+ GBMs demonstrated a trend towards multifocality (12.6% vs. 5.2% for VSVZ− GBMs; p = 0.09). Patients with cortex+ and CC− GBMs overall received greater EORs compared to their respective controls; however, the number of GTRs did not significantly differ in patients with GBMs with or without VSVZ, SGZ, CC, or cortical contact (Figure 2d). No significant association was observed between tumor volume and EOR (p = 0.22; two-tailed Kruskal-Wallis test).
Fig. 2. Comparisons of age at diagnosis (a), pre-operative Karnofsky performance status score (KPS) (b), tumor volume (c), extent of resections (d), multifocal tumors (e), and distal recurrences (f) between glioblastoma patients with and without ventricular-subventricular zone (VSVZ), subgranular zone (SGZ), corpus callosal (CC), and cortical contact.
Box and whisker plots are used to represent medians, interquartile range, and maximum and minimum values (A–C). Percentages of subtotal (STR), near-total (NTR), and gross total resections (GTR) are depicted in D. P values of two-tailed Mann-Whitney U test (in a, b, c, d) and two-tailed Fisher’s exact test (in e, f) are noted as ns = non-significant; * 0.03; and **** ≤ 0.0001.
Overall and Progression Free Survival
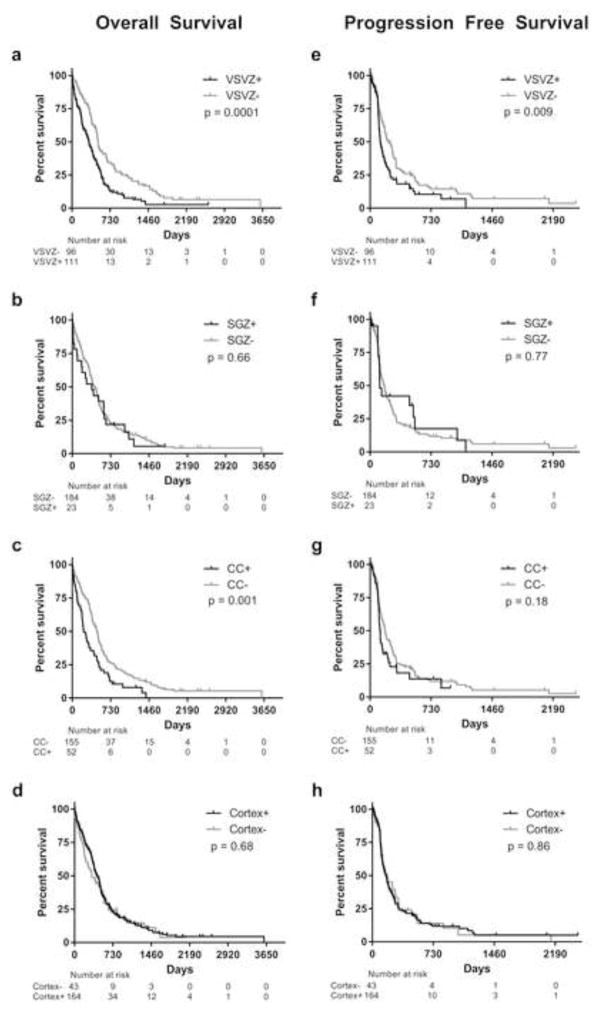
Kaplan-Meier OS and PFS curves are shown in Figure 3 and median survival days are listed in Supplemental Table S1. Results of univariate and multivariate Cox survival analyses including variables with p-values < 0.1 on univariate analyses are tabulated in Table 1. Increasing age, lower KPS, lack of radiation, chemotherapy, GTR, and VSVZ contact were independent predictors of lower OS. While CC contact was associated with lower OS in univariate analysis, it was not a significant predictor in multivariate analysis. Interestingly, of the 52 CC+ GBMs, 48 (92%) were also VSVZ+, while of the 155 CC− GBMs, only 63 (41%) were VSVZ+ (p < 0.0001; two-tailed Fisher’s test). Further, CC contact did not modify the effect of VSVZ+ GBMs on overall survival (Supplemental Figure S1). Whether CC contact modifies the effect of VSVZ− GBMs on survival could not be statistically computed due to very low power of such analysis, as there are only 4 patients with VSVZ−/CC+ GBMs. A nomogram of the variables found to be significant in multivariate Cox analysis was created to show the relative clinical effect of each variable and its linear contribution in predicting OS in our cohort (Supplemental Figure S2; for interpretation and use, refer to Gorlia, et. al., 2008 [26, 27]). With regards to recurrence, only VSVZ contact significantly predicted PFS. Overall, the results presented in Table 1 were similar with regards to the significance of the variables in correlating with OS and PFS when patients treated in the pre-temozolomide era (before March 2015, when the drug received FDA approval) were excluded (data not shown). No significant differences were observed in the frequency of postoperative distant recurrences in VSVZ+, SGZ+, CC+ and cortex+ GBMs compared to their non-contacting controls.
Fig. 3. Kaplan-Meier overall (A–D) and progression free (E–H) survival curves of glioblastoma patients with and without ventricular-subventricular zone (VSVZ), subgranular zone (SGZ), corpus callosal (CC), and cortical contact.
Censored values are indicated by tick marks.
Table 1.
Statistical results of univariate and multivariate cox survival analyses.
| Overall Survival (Hazard Ratio [95% CI]) | Progression Free Survival (Hazard Ratio [95% CI]) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | p-Value | Multivariate | p-Value | Univariate | p-Value | Multivariate | p-Value | |
| VSVZ contacta | 1.78 [1.32–2.38] | 0.0001 | 1.61 [1.14–2.26] | 0.006 | 1.55 [1.12–2.15] | 0.009 | 1.51 [1.09–2.10] | 0.01 |
| Radiationa | 0.23 [0.15–0.34] | <0.0001 | 0.36 [0.21–0.62] | 0.0002 | 0.53 [0.27–1.05] | 0.07 | 0.69 [0.33–1.46] | 0.34 |
| Chemotherapya | 0.30 [0.22–0.42] | <0.0001 | 0.49 [0.31–0.80] | 0.004 | 0.67 [0.43–1.05] | 0.08 | 0.76 [0.47–1.22] | 0.25 |
| EORb – GTR | 0.50 [0.34–0.74] | 0.0006 | 0.46 [0.31–0.69] | 0.0002 | 0.78 [0.52–1.17] | 0.23 | – | – |
| KPSc | 0.99 [0.98–1.00] | 0.002 | 0.99 [0.98–1.00] | 0.02 | 1.01 [1.00–1.02] | 0.28 | – | – |
| Agec | 1.02 [1.01–1.03] | <0.0001 | 1.01 [1.00–1.03] | 0.02 | 1.00 [0.99–1.01] | 0.61 | – | – |
| CC contacta | 1.75 [1.25–2.44] | 0.001 | 0.93 [0.63–1.39] | 0.74 | 1.31 [0.88–1.95] | 0.18 | – | – |
| EORb – NTR | 0.75 [0.54–1.03] | 0.08 | 0.79 [0.56–1.10] | 0.16 | 0.78 [0.54–1.12] | 0.18 | – | – |
| SGZ contacta | 1.11 [0.70–1.75] | 0.66 | – | – | 0.92 [0.54–1.57] | 0.77 | – | – |
| Cortex contacta | 0.93 [0.65–1.32] | 0.68 | – | – | 1.04 [0.70–1.53] | 0.86 | – | – |
| Volumec | 1.00 [1.00–1.00] | 0.98 | – | – | 1.00 [0.99–1.01] | 0.93 | – | – |
Hazard ratios are compared to their respective negative controls.
Gross total (GTR) and near total (NTR) resection were compared to subtotal resection(STR).
Designates variables treated continuously in the analysis.
– Variables not included in the multivariate cox analyses. EOR = extent of resection; KPS = Karnofsky performance status score. Bold p-values indicate meeting significance after controlled for false discovery rate (see methods for details).
VSVZ and Cortical Contact Interactions
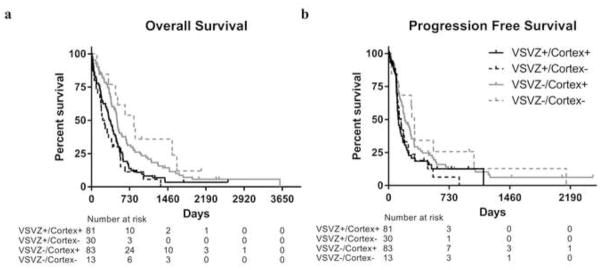
We categorized GBMs into four groups proposed previously based in VSVZ and cortical contact [19]. Similar to prior observation, [19] we also observed that VSVZ+/cortex+ GBMs were more likely to be multifocal (n=10/81) compared to VSVZ−/cortex− GBMs (n=0/13). However, when considering incidences of multifocality of VSVZ+/cortex− (n=4/30) and VSVZ−/cortex+ GBMs (n=5/83), no statistical interaction between VSVZ and cortical contact status with respect to multifocality was noted (p > 0.25, X2 test). Same was observed for distal recurrences [VSVZ+/cortex+ (n=14/81), VSVZ+/cortex− (n=5/30), VSVZ−/cortex+ (n=22/83), and VSVZ−/cortex− (n=4/13); p > 0.25, X2 test)]. Further, we observed no significant interaction between VSVZ and cortical contact status with respect to OS or PFS (Figure 4, Supplementary Table S2), consistent with other reports [21, 23].
Fig. 4. Kaplan-Meier overall (a) and progression free (b) survival curves of glioblastoma patients with and without VSVZ and/or cortical contact.
Censored values are indicated by tick marks.
DISCUSSION
We demonstrate in this study that radiographic contact of GBM with the neurogenic zone of the VSVZ is independently associated with earlier recurrence and decreased survival. Of the two neurogenic zones, these clinical effects were specific to the VSVZ but not the SGZ. Further, these outcomes were not associated with CC or cortical contact. We conclude that the VSVZ has unique properties that contribute to GBM pathobiology.
The finding that VSVZ contact specifically, rather than neurogenic zones generally, is a negative prognostic factor is surprising, as many microenvironmental factors are potentially common between these two regions. Both the VSVZ and SGZ neural stem cells are thought to be rich in specialized vascular contacts and to retain some neurogenic potential in the adult brain, suggesting a reservoir of proliferative signals [28, 29]. Although debated, [30] the density of proliferative cells and its age-related changes are similar between VSVZ and SGZ in humans [31]. However, anatomical distinctions between these two niches do include contact with the cerebrospinal fluid (CSF), which is extensive in the case of VSVZ and is a key feature of normal neural stem cells, and the presence of a “gap layer” filled with astrocytic processes in the VSVZ which emerges as neurogenesis declines postnatally [1, 2]. We hypothesize that either or both of these features might provide unique environments which support the growth of highly malignant cells through specific mechanistic stimuli (such as growth factors enriched in the CSF) or structural effects (e.g. providing an environment with fewer hindrances to invasion).
Several observations support these hypotheses. For example, widespread dissemination of tumor cells through CSF or along the length of VSVZ has been hypothesized [19, 32, 33]. Indeed, we noted contrast enhancement of the ipsilateral ventricular ependymal surface in a subset VSVZ+ GBMs (Supplemental Figure S3b–d). MRI-identified ependymal tumor dissemination is also observed and reported in the literature and has been associated with shorter survival [34]. While studies have demonstrated VSVZ+ GBMs are associated with a greater frequency of distal recurrences [19, 22, 35, 36], in our study, no difference was noted.
Interestingly, unlike SGZ, CC, and the cortex, we occasionally noted tumor contrast enhancement selectively and in a non-spherical growth extending towards and contacting the VSVZ (Supplemental Figure S3a, b). Others have also noted this “stalk”-like contact [37]. Such tropism was not observed with SGZ, CC, or cortex. Indeed, in multiple orthotopic xenograft studies, GBM tumor cells have demonstrated this tropism for the VSVZ, [5–7] lending support to the hypothesis that VSVZ may have specific, intrinsic, tumor-supporting stimuli.
We cannot infer from our data that the greater tumor volume is caused by VSVZ contact, as the converse hypothesis is equally suggestive; i.e., VSVZ contact may be the eventual result of tumor growth. In fact, tumors with contact with all the radiographic variables studied—VSVZ, SGZ, CC, and cortex—exhibited larger volumes compared to their respective controls. These locations represent medial and lateral edges of the brain, and with tumor growth, these boundaries are increasingly likely to be contacted by the tumor.
GBM contact with the CC did not influence survival. Few studies, however, have demonstrated that patients with CC+ GBMs have decreased survival [8–11]. Although the basis for this effect remains unclear, our analysis demonstrated a significant association of CC contact with VSVZ contact of GBMs (92% in this study). By nature of their anatomic proximity, VSVZ contact is likely coincident with CC contact in many cases, thus their analytic relationship with survival may demonstrate collinearity, confounding the survival outcome noted with CC contact. One recent study noted that 100% of the CC+ GBMs studied were also VSVZ+ GBMs, and that CC contact was not predictive of survival [18].
Cortical contact of GBM was not predictive of extent of resection, tumor multifocality, distal recurrences, or survival. Therefore, our results demonstrated that categorizing GBMs as VSVZ+ and VSVZ− resulted in statistically similar clinical associations as categorizing GBMs into VSVZ+/cortex+, VSVZ+/cortex−, VSVZ−/cortex+, and VSVZ−/cortex−, suggested originally by Lim, et. al., [19] and which continues to be used [20–23].
The results herein may be confined by the inherent limitations related to a retrospective study design encompassing a large time span. Hence, for a majority of the patients in this study (over 70%), the molecular status—MGMT promotor methylation and IDH mutation—of their GBMs is unknown. Therefore, this study cannot ascertain if the effects of VSVZ contact on survival are confounded by these molecular predictors. However, several studies have reported differences in MGMT promoter methylation [17, 35, 38] and IDH mutations [17, 23] in VSVZ+ and VSVZ− GBMs. Taking them cumulatively, these studies do not show a significant correlation between MGMT promoter methylation and VSVZ contact (MGMT promotor methylation noted in 66/363 VSVZ+ and in 40/254 VSVZ− GBMs cumulatively; Mantel-Haenszel odds ratio, random effects, 0.90 [0.42–1.94], p = 0.79) or IDH mutation and VSVZ contact (IDH mutation noted in 9/80 VSVZ+ and in 36/133 VSVZ− GBMs cumulatively; Mantel-Haenszel odds ratio, random effects, 0.63 [0.06–6.17], p = 0.69). Further, some of the radiologically-classified groups were small in number. Therefore, further larger studies are warranted to confirm the results herein.
Considering these limitations, this cohort represents one of the largest populations in which our unique hypothesis has been tested. Our results demonstrate that patients with GBMs contacting the VSVZ and SGZ neurogenic zones exhibit divergent clinical patterns of tumor recurrence and survival. Early recurrence and lower survival is specific to VSVZ but not SGZ. Our results indicate the necessity of dissecting the differential biological changes in GBMs contacting these two neurogenic zones and determining whether the worse outcome with VSVZ contact of GBMs is a manifestation of the VSVZ’s neurogenic (i.e., stem cell and growth-factor rich microenvironment) or non-neurogenic (i.e., its strategic dissemination-promoting location with vascular and CSF access, and unique migration-supporting extracellular matrix [39]) properties.
Supplementary Material
Acknowledgments
Funding: Vanderbilt Institute for Clinical and Translational Research grant support from the National Center for Advancing Translational Sciences [CTSA award No. UL1TR000445 to AMM]; Vanderbilt-Ingram Cancer Center Ambassadors, Vanderbilt-Ingram Cancer Center Discovery Grant, and National Institutes of Health [Cancer Center Support Grant P30 CA068485 and NINDS R01096238 to RAI]. We acknowledge Li Wang and Dr. Chang Yu of Vanderbilt Department of Biostatistics for statistical guidance.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 4.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nature reviews Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 5.Kroonen J, Nassen J, Boulanger YG, Provenzano F, Capraro V, Bours V, Martin D, Deprez M, Robe P, Rogister B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. International journal of cancer Journal international du cancer. 2011;129:574–585. doi: 10.1002/ijc.25709. [DOI] [PubMed] [Google Scholar]
- 6.Sadahiro H, Yoshikawa K, Ideguchi M, Kajiwara K, Ishii A, Ikeda E, Owada Y, Yasumoto Y, Suzuki M. Pathological features of highly invasive glioma stem cells in a mouse xenograft model. Brain tumor pathology. 2014;31:77–84. doi: 10.1007/s10014-013-0149-x. [DOI] [PubMed] [Google Scholar]
- 7.Goffart N, Kroonen J, Di Valentin E, Dedobbeleer M, Denne A, Martinive P, Rogister B. Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling. Neuro-oncology. 2015;17:81–94. doi: 10.1093/neuonc/nou144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mickevicius NJ, Carle AB, Bluemel T, Santarriaga S, Schloemer F, Shumate D, Connelly J, Schmainda KM, LaViolette PS. Location of brain tumor intersecting white matter tracts predicts patient prognosis. Journal of neuro-oncology. 2015;125:393–400. doi: 10.1007/s11060-015-1928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaichana KL, Jusue-Torres I, Lemos AM, Gokaslan A, Cabrera-Aldana EE, Ashary A, Olivi A, Quinones-Hinojosa A. The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? Journal of neuro-oncology. 2014;120:625–634. doi: 10.1007/s11060-014-1597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allahdini F, Amirjamshidi A, Reza-Zarei M, Abdollahi M. Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: does maximal resection of the tumor lengthen the median survival? World neurosurgery. 2010;73:128–134. doi: 10.1016/j.wneu.2009.06.001. discussion e116. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishna R, Barber J, Kennedy G, Rizvi A, Goodkin R, Winn RH, Ojemann GA, Berger MS, Spence AM, Rostomily RC. Imaging features of invasion and preoperative and postoperative tumor burden in previously untreated glioblastoma: Correlation with survival. Surgical neurology international. 2010:1. doi: 10.4103/2152-7806.68337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry AM, Hale AT, Chambless LB, Weaver KD, Thompson RC, Ihrie RA. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. Journal of neuro-oncology. 2016 doi: 10.1007/s11060-016-2278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. Journal of neurosurgery. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 14.Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD, Drappatz J, Kesari S. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. Journal of neuro-oncology. 2011;104:261–269. doi: 10.1007/s11060-010-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tejada-Solis S, Aldave-Orzaiz G, Pay-Valverde E, Marigil-Sanchez M, Idoate-Gastearena MA, Diez-Valle R. Prognostic value of ventricular wall fluorescence during 5-aminolevulinic-guided surgery for glioblastoma. Acta neurochirurgica. 2012;154:1997–2002. doi: 10.1007/s00701-012-1475-1. discussion 2002. [DOI] [PubMed] [Google Scholar]
- 16.Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, Li G, Harsh GRt, Thompson RC, Lim M, Quinones-Hinojosa A. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2013;20:1422–1426. doi: 10.1016/j.jocn.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Li X, Qiu B, Jiang T, Wu A. Can lateral ventricle contact predict the ontogeny and prognosis of glioblastoma? Journal of neuro-oncology. 2015;124:45–55. doi: 10.1007/s11060-015-1818-x. [DOI] [PubMed] [Google Scholar]
- 18.Liang TH, Kuo SH, Wang CW, Chen WY, Hsu CY, Lai SF, Tseng HM, You SL, Chen CM, Tseng WI. Adverse prognosis and distinct progression patterns after concurrent chemoradiotherapy for glioblastoma with synchronous subventricular zone and corpus callosum invasion. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015 doi: 10.1016/j.radonc.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncology. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappadakunnel M, Eskin A, Dong J, Nelson SF, Mischel PS, Liau LM, Ngheimphu P, Lai A, Cloughesy TF, Goldin J, Pope WB. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. Journal of neuro-oncology. 2010;96:359–367. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-oncology. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler U, Lutz K, Pichlmeier U, Stummer W, Franz K, Reulen HJ, Bink A Group ALAGS. Anatomic features of glioblastoma and their potential impact on survival. Acta neurochirurgica. 2015;157:179–186. doi: 10.1007/s00701-014-2271-x. [DOI] [PubMed] [Google Scholar]
- 23.Pina Batista KM, Vega IF, de Eulate-Beramendi SA, Morales J, Kurbanov A, Asnel D, Meilan A, Astudillo A. Prognostic significance of the markers IDH1 and YKL40 related to the subventricular zone. Folia neuropathologica/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2015;53:52–59. doi: 10.5114/fn.2015.49974. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015 doi: 10.1016/j.radonc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 26.Bredel M. Nomograms as clinicobiological predictors of survival in glioblastoma. The Lancet Oncology. 2008;9:5–6. doi: 10.1016/s1470-2045(07)70390-x. [DOI] [PubMed] [Google Scholar]
- 27.Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A, Lacombe D, Stupp R. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. The Lancet Oncology. 2008;9:29–38. doi: 10.1016/s1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 28.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nature reviews Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 30.Curtis MA, Low VF, Faull RL. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Developmental neurobiology. 2012;72:990–1005. doi: 10.1002/dneu.22028. [DOI] [PubMed] [Google Scholar]
- 31.Ernst A, Frisen J. Adult neurogenesis in humans-common and unique traits in mammals. PLoS biology. 2015;13:e1002045. doi: 10.1371/journal.pbio.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacoangeli M, Di Rienzo A, Colasanti R, Zizzi A, Gladi M, Alvaro L, Nocchi N, Di Somma LG, Scarpelli M, Scerrati M. Endoscopy-verified occult subependymal dissemination of glioblastoma and brain metastasis undetected by MRI: prognostic significance. OncoTargets and therapy. 2012;5:449–456. doi: 10.2147/OTT.S39429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi Y, Nakada M, Tanaka S, Uchiyama N, Hayashi Y, Kita D, Hamada J. Implication of 5-aminolevulinic acid fluorescence of the ventricular wall for postoperative communicating hydrocephalus associated with cerebrospinal fluid dissemination in patients with glioblastoma multiforme: a report of 7 cases. Journal of neurosurgery. 2010;112:1015–1019. doi: 10.3171/2009.8.JNS09516. [DOI] [PubMed] [Google Scholar]
- 34.Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, Chang SM. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. Journal of neurosurgery. 2005;102:622–628. doi: 10.3171/jns.2005.102.4.0622. [DOI] [PubMed] [Google Scholar]
- 35.Adeberg S, Konig L, Bostel T, Harrabi S, Welzel T, Debus J, Combs SE. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. International journal of radiation oncology, biology, physics. 2014;90:886–893. doi: 10.1016/j.ijrobp.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Sonoda Y, Saito R, Kanamori M, Kumabe T, Uenohara H, Tominaga T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurologia medico-chirurgica. 2014;54:302–309. doi: 10.2176/nmc.oa.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barami K, Sloan AE, Rojiani A, Schell MJ, Staller A, Brem S. Relationship of gliomas to the ventricular walls. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2009;16:195–201. doi: 10.1016/j.jocn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Fahrendorf D, Hesselmann V, Schwindt W, Wolfer J, Jeibmann A, Kooijman H, Kugel H, Heindel W, Bink A. Variations of ITSS-Morphology and their Relationship to Location and Tumor Volume in Patients with Glioblastoma. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2015 doi: 10.1111/jon.12228. [DOI] [PubMed] [Google Scholar]
- 39.Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia. 2015;63:1330–1349. doi: 10.1002/glia.22839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.