Abstract
Background
Exercise capacity as measured by peak oxygen uptake (Vo2) is similarly impaired in patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). However, characterization of how each component of Vo2 changes in response to incremental exercise in HFpEF versus HFrEF has not been previously defined. We hypothesized that abnormally low peripheral o2 extraction (arterio-mixed venous o2 content difference, [C(a-v)o2]) during exercise significantly contributes to impaired exercise capacity in HFpEF.
Methods and Results
We performed maximum incremental cardiopulmonary exercise testing with invasive hemodynamic monitoring on 104 patients with symptomatic NYHA II to IV heart failure (HFpEF, n=48, peak Vo2=13.9±0.5 mL kg−1 min−1, mean±SEM, and HFrEF, n=56, peak Vo2=12.1±0.5 mL kg−1 min−1) and 24 control subjects (peak Vo2 27.0±1.7 mL kg−1 min−1). Peak exercise C(a-v)o2 was lower in HFpEF compared with HFrEF (11.5±0.27 versus 13.5±0.34 mL/dL, respectively, P<0.0001), despite no differences in age, hemoglobin level, peak respiratory exchange ratio, Cao2, or cardiac filling pressures. Peak C(a-v)o2 and peak heart rate emerged as the leading predictors of peak Vo2 in HFpEF. Impaired peripheral o2 extraction was the predominant limiting factor to exercise capacity in 40% of patients with HFpEF and was closely related to elevated systemic blood pressure during exercise (r=0.49, P=0.0005).
Conclusions
In the first study to directly measure C(a-v)o2 throughout exercise in HFpEF, HFrEF, and normals, we found that peak C(a-v)o2 was a major determinant of exercise capacity in HFpEF. The important functional limitation imposed by impaired o2 extraction may reflect intrinsic abnormalities in skeletal muscle or peripheral microvascular function, and represents a potential target for therapeutic intervention.
Keywords: diastole, exercise, heart failure
Heart failure with preserved left ventricular ejection fraction (HFpEF) is an increasingly common condition with similar incidence and prognosis to heart failure with reduced left ventricular ejection fraction (HFrEF).1–4 A major source of morbidity in both HFpEF and HFrEF is impaired functional capacity, which is best quantified by the degree of impairment in peak VO2.5–7 Mechanistic studies of exercise intolerance in HFpEF have primarily focused on central cardiovascular abnormalities, including chronotropic incompetence6 and impaired stroke volume (SV) augmentation in the setting of decreased left ventricular (LV) compliance.5,8 More recently, impaired systolic reserve function and abnormal LV-central vascular coupling have also been implicated in causing impaired exercise capacity in HFpEF.9
In assessing the capacity to augment VO2 in HFpEF, it is important to consider relative increases in each of the 3 components of VO2 (ie, heart rate [HR], SV, and arterio-mixed venous oxygen content difference: (C[a-v]O2). In normal individuals, the degree to which peripheral oxygen extraction (ie, C(a-v)O2) increases in response to exercise (≈2.5×)10–12 is much greater than changes in SV (≈1.3×)11,13 and similar to increases in HR (≈2.5×). Several previous studies have found that patients with HFpEF are not able to increase HR and SV normally during exercise,5,6,8,14 which implies a greater reliance in the ability to increase C(a-v)O2 to augment VO2. However, the role of C(a-v)O2 in determining exercise capacity in HFpEF remains incompletely understood.15–17
In HFpEF, 2 studies15,16 that derived C(a-v)O2 indirectly have suggested that C(a-v)O2 is abnormally low in HFpEF, whereas a third study found that it was not impaired.17 To date, no studies have performed direct serial measurements of C(a-v)O2 throughout exercise in HFpEF and HFrEF to define O2 extraction patterns.
On the basis of the heterogeneous pathogenesis of HFpEF, and the recognized role of peripheral O2 extraction augmentation in increasing VO2 during exercise, we hypothesized that patients with HFpEF would be limited primarily by an inability to augment peripheral O2 extraction appropriately [ie, C(a-v)O2 <14 mL/dL or CvO2 >5 mL/dL].18 To address this hypothesis, we measured respiratory gas exchange parameters, arterial and mixed venous O2 saturations [C(a-v)O2], as well as HR and SV at 1-minute intervals throughout maximum incremental exercise in patients with symptomatic HFpEF and compared them with patients with HFrEF and normal controls. The primary objective of this study was to delineate the relative contributions of each component of VO2 to peak exercise capacity in patients with heart failure (HF).
Methods
Patient Population and Study Design
Consecutive patients who underwent cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring at Massachusetts General Hospital and chronic NYHA class II to IV symptoms were included in the study. We classified patients based on left ventricular ejection fraction and resting and exercise pulmonary capillary wedge pressure (PCWP) as (1) HFrEF: Chronic NYHA II to IV LV systolic dysfunction, left ventricular ejection fraction <0.45 on standard pharmacotherapy; (2) HFpEF: Chronic NYHA II to IV symptoms, left ventricular ejection fraction >0.50, and >15 mm Hg PCWP at rest. Exclusion criteria consisted of the following: (1) incomplete pulmonary arterial catheter pressure measurements; (2) documented intracardiac shunting; (3) severe valvular heart disease; (4) known active flow limiting CAD; (5) submaximal exercise as evidenced by peak respiratory exchange ratio (RER) <1.0; (6) the presence of a pulmonary mechanical limitation to exercise as defined by VE/(forced expiratory volume in 1 s [FEV1]×35)>0.7 at the anaerobic threshold.19,20 The control group was included to determine the extent to which hemodynamic measurements and O2 utilization during exercise in HFpEF subjects differed from normal controls. Controls consisted of subjects referred for CPET to evaluate dyspnea on exertion during the same period of time as the HFpEF group. Controls were required to have normal LV function, normal resting and exercise PCWP and normal exercise capacity as reflected by a peak VO2 >80% of that predicted on the basis of age, sex, and height.18
Cardiopulmonary Exercise Testing
All patients underwent placement of a pulmonary arterial catheter via the internal jugular vein and placement of a systemic arterial catheter via the radial artery. First-pass radionuclide ventriculography of both ventricles was performed immediately before cycle ergometry testing as previously described.21 Subjects then underwent maximum incremental upright cycle ergometry CPET (5–25 Watts/min continuous ramp after an initial 3-minute period of unloaded exercise, MedGraphics, St. Paul, MN) with simultaneous hemodynamic monitoring (Witt Biomedical Inc, Melbourne, FL) as previously described.21,22 None of the subjects developed angina, arrhythmia, hypotension, or significant electrocardiographic changes during exercise. Right atrial pressure, mean pulmonary arterial pressure, PCWP, and systemic arterial pressures were measured in the upright position, at end-expiration, while patients were seated on the cycle, at rest, and at 1-minute intervals during exercise. Fick cardiac outputs (CO)11,23 were also determined at 1-minute intervals throughout exercise by measuring oxygen uptake (VO2) and simultaneous radial arterial and mixed venous O2 content to calculate the C(a-v)O2. Peak VO2 was defined as the highest O2 uptake, averaged over 30 s, during the last minute of symptom-limited exercise, as previously described.22 Age-predicted maximal HR was defined as 220 minus age in years. Chronotropic response index was derived as the proportion of HR reserve used at peak exercise based on (HRpeak−HRrest)/([220−age]−HRrest)×100.24,25 Chronotropic response index <62% and <80% were considered abnormal in the presence and absence of β-blocker use, respectively.24,26–28
Arterio-Mixed Venous Oxygen Content
Arterial O2 content (CaO2)29 is the amount of O2 carried by blood to the periphery and was calculated as (hemoglobin×1.39×SaO2)+(0.003×PaO2). Similarly mixed venous O2 content (CvO2) represents the O2 content of blood returning from the peripheral tissues to the right heart which was calculated as (hemoglobin×1.39×SvO2)+(0.00 3×PvO2). Given a normal circulating hemoglobin level of ≈15 g/dL, an arterial saturation of 96% and mixed venous saturation of 72%, the normal resting CaO2 is 20 mL/dL and CvO2 is 15 mL/dL, which results in a normal resting C(a-v)O2 value of 5 mL/dL.
During exercise, peripheral tissues extract more O2 to maintain aerobic metabolism, which leads to a decrease in mixed venous saturation to ≈24% with a resultant reduction in CvO2 from 15 mL/dL at rest to 5 mL/dL at peak exercise in normal individuals.30 Thus peak exercise C(a-v)O2 in a normal person with a hemoglobin of 15 g/dL is 15 mL/dL (ie, approximately equal to the hemoglobin level).18,31 The amount of O2 extracted by tissues at peak exercise relative to O2 delivered (ie, extraction ratio, peak C(a-v)O2/CaO2) is normally 75%.
Statistical Methods
STATA 10 (Statacorp, College Station, TX) was used for statistical analysis. The Wilk–Shapiro test was used to assess the normality of distribution of the data. All continuous, normally distributed measurements are presented as the mean±SEM. Categorical data are reported as percentages. Group baseline characteristics were compared using either the Student t test, Mann–Whitney U test, or Fisher exact test, as appropriate. For clinical characteristics, comparisons between groups for continuous variables were performed using ANOVA with post hoc pairwise comparisons, unpaired 2-sample t tests or the Wilcoxon signed rank test, as appropriate. Pearson or Spearman correlation coefficients were calculated, based on whether the data were either normally or not normally distributed, respectively. Partial R2 values were obtained from a multiple linear regression model that included age, sex, HRmax, SVmax, and C(a-v)O2max. Subgroup analysis was performed comparing HF patients with higher and lower CvO2. A P<0.05 was considered significant. This study was approved by the Partners Healthcare Institutional Review Board, the authors had full access to the data and take responsibility for its integrity and for the article as written.
Results
Population Characteristics
Baseline characteristics for all HFpEF (n=48), HFrEF (n=56), and control subjects (n=24) are reported in Table 1. All patients surpassed their ventilatory anaerobic thresholds and demonstrated an average peak RER of 1.15 to 1.16 in all 3 groups, indicating maximum or near maximum exercise effort across the 3 groups.19,20 HFpEF subjects had more elevated body mass index and a female predominance (60%) compared with patients with HFrEF, consistent with the known distinct demographic characteristics of HFpEF and HFrEF populations.1,15,16,32
Table 1.
Demographics of Heart Failure and Control Subjects
| Characteristics | HFpEF (48) | HFrEF (56) | Controls (24) |
|---|---|---|---|
| Age, y | 63±12* | 59±12 | 55±18 |
| Male (number, %) | 20 (40)* | 45 (81)†‡ | 15 (62) |
| Race (White, %) | 46 (96) | 50 (88) | 23 (96) |
| BMI, kg/m2 | 33.7±7.6* | 27.8±6‡ | 27.6±3.0 |
| Comorbidities % | |||
| Hypertension | 29 (60)* | 34 (61)† | 9 (37) |
| Diabetes mellitus | 14 (25)* | 12 (21)† | 0 |
| Hyperlipidemia | 25 (52)* | 32 (57)† | 6 (25) |
| Atrial fibrillation | 12 (26)* | 11 (19)† | 0 |
| Heart failure pharmacotherapy % | |||
| Diuretics | 30 (63)* | 48 (86)†‡ | 1 (4) |
| ACE inhibitor or ARB | 14 (29) | 45 (80)†‡ | 7 (29) |
| β-adrenergic receptor blocker | 25 (52)* | 51 (91)†‡ | 4 (17) |
| Aldosterone antagonist | 4 (8) | 30 (54)†‡ | 0 |
| Digoxin | 6 (12)* | 28 (50)†‡ | 0 |
| LVEF % | 62±7* | 29±6†‡ | 67±6 |
| Resting Supine PCWP, mm Hg | 20±2.7* | 22±8.9† | 10±3.9 |
| Hemoglobin, gm/dL | 13.2±1.4 | 12.9±2.2 | 13.2±1.5 |
| Peak VO2, mL kg−1 min−1 | 13.9±3.5* | 12.1±3.7†‡ | 27.0±8.3 |
| Max watts achieved | 82±32* | 75±37† | 166±57 |
| Peak exercise RER | 1.15±0.07 | 1.16±0.14 | 1.15±0.05 |
| Peak exercise lactate, mM | 5.3±2.7* | 4.8±1.5† | 7.6±1.5 |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; PCWP, pulmonary capillary wedge pressure; and RER, respiratory exchange ratio.
P<0.05 between HFpEF and controls.
P<0.05 between HFrEF and controls.
P<0.05 between HFpEF and HFrEF.
Functional capacity as indicated by peak VO2 was reduced in HFpEF (13.9±0.5 mL kg−1 min−1) and in HFrEF (12.1±0.5 mL kg−1 min−1) compared with controls (27.0±1.7 mL kg−1 min−1, P<0.05 for both comparisons, Table 1). The measurement of VO2, HR, CaO2, and CvO2 during each minute of exercise and application of the Fick Principle [ie, VO2 = HR×SV×C(a-v)O2] permitted analysis of each component of VO2 during exercise in the 3 groups.
Arterial and Mixed Venous Oxygen Content at Rest and at Peak Exercise
All 3 groups had similar CaO2 values at rest and at peak exercise, reflecting mildly reduced hemoglobin levels and normal systemic arterial O2 saturations (Tables 1 and 2). Resting CvO2 was lowest in HFrEF (9.4±0.3 mL/dL), and similar in HFpEF and controls (11.6±0.3 and 12.1±0.36 mL/dL, P=0.70, Table 2). Compared to controls and patients with HFrEF, patients with HFpEF had the lowest average C(a-v)O2 and highest peak exercise CvO2, indicating relatively impaired maximum peripheral O2 extraction in HFpEF (Table 2; Figure 1). Maximum C(a-v)O2 was less than the predicted value [ie, maximum C(a-v)O2=hemoglobin level,18 and CvO2 >5 mL/dL, see Methods Section] in 75% of HFpEF versus 21% of HFrEF and 33% of controls (P<0.001). Peak C(a-v)O2 was not related to peak CO in any of the groups, indicating that at peak exercise these variables are dissociated, and not reciprocally related as they are at rest and during low-level exercise.
Table 2.
Hemodynamic, Gas Exchange, and Ventriculography Measurements of HF and Control Subjects
| Rest
|
Exercise
|
|||||
|---|---|---|---|---|---|---|
| Characteristics | HFpEF | HFrEF | Controls | HFpEF | HFrEF | Controls |
| VO2 mL/min | 307±11 | 281±9* | 298±9 | 1227±61† | 1021±50*‡ | 2049±122 |
| VO2 mL kg−1 min−1 | 3.4±0.1 | 3.4±0.1* | 3.7±0.12 | 13.9±0.5† | 12.1±0.5*‡ | 27.0±1.7 |
| CO, L/min | 5.1±0.2 | 3.7±0.1*‡ | 5.3±0.2 | 10.7±0.5† | 7.7±0.3*‡ | 15.2±0.7 |
| CI, L min−1 m−2 | 2.7±0.1 | 1.9±0.1*‡ | 2.7±0.1 | 5.2±0.2† | 3.8±0.1*‡ | 7.8±0.4 |
| SV, mL | 69±2.6 | 51±2*‡ | 74±2.8 | 88±3.6† | 68±2.8*‡ | 103±4.28 |
| SVI, mL/m2 | 36.6±1.3 | 25.6±1.0*‡ | 38.1±1.6 | 42.7±1.2† | 34.3±1.2*‡ | 52.4±2.0 |
| HR, beats/min | 75±2 | 75±0.5 | 73±1.8 | 121±3.6† | 113±3.2* | 148±3.47 |
| CaO2 mL/dL | 17.8±0.3 | 17.1±0.4 | 17.8±0.38 | 18.3±0.28 | 18.3±0.37 | 19.0±0.34 |
| CvO2 mL/dL | 11.6±0.3 | 9.4±0.3*‡ | 12.1±0.4 | 6.8±0.3† | 4.7±0.2*‡ | 5.7±0.2 |
| C(a-v)O2 mL/dL | 6.2±0.2 | 7.8±0.2*‡ | 5.7±0.2 | 11.5±0.3† | 13.5±0.3‡ | 13.3±0.3 |
| SBP, mm Hg | 150±4 | 123±3.2*‡ | 152±3.3 | 184±5.3 | 148±4.5*‡ | 190±4.9 |
| DBP, mm Hg | 74±2 | 67±1.5*‡ | 78±1.2 | 84±2.9 | 76±2.5*‡ | 86±2.2 |
| MAP, mm Hg | 99±2 | 86±2*‡ | 102±2 | 117±3.3 | 100±2.9*‡ | 120±2.2 |
| RVEF (%) | 50±1 | 38±1*‡ | 52±1.6 | 49±1.1† | 38±1.3*‡ | 55±1.4 |
| LVEF (%) | 62±1† | 30±1*‡ | 67±1.2 | 64±1.1† | 33±1*‡ | 70±1.2 |
| LVEDV, mL | 133±5 | 264±12*‡ | 117±6.5 | 152±4.5 | 275±11.4*‡ | 138±6.1 |
| LVEDVI, mL/m2 | 65±2.4 | 133±5.4*‡ | 57±3.7 | 74±2.0 | 140±5.6*‡ | 67±4.5 |
| RAP, mm Hg | 10±0.4† | 9±0.7* | 4±0.6 | 13±0.3† | 16±0.9*‡ | 8±0.6 |
| PAP, mm Hg | 30±0.9† | 35±1.7*‡ | 17±1.2 | 43±1.4† | 49±1.3*‡ | 31±0.6 |
| PCWP, mm Hg | 20±0.4† | 22±1.2* | 10±0.8 | 29±1.4† | 30±1.2* | 16±0.6 |
| Elastance (Ea), mm Hg/mL | 2.2±0.1† | 2.4±0.1* | 1.9±0.1 | 2.0±0.1† | 2.1±0.1* | 1.7±0.1 |
CI indicates cardiac index; CO, cardiac output; DBP, diastolic blood pressure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; LVEDV, left ventricular end diastolic volume; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; PAP, pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RVEF, right ventricular ejection fraction; SBP, systolic blood pressure; SV, stroke volume; and SVI, stroke volume index.
P<0.05 between HFrEF and controls.
P<0.05 between HFpEF and controls.
P<0.05 between HFpEF and HFrEF.
Figure 1.
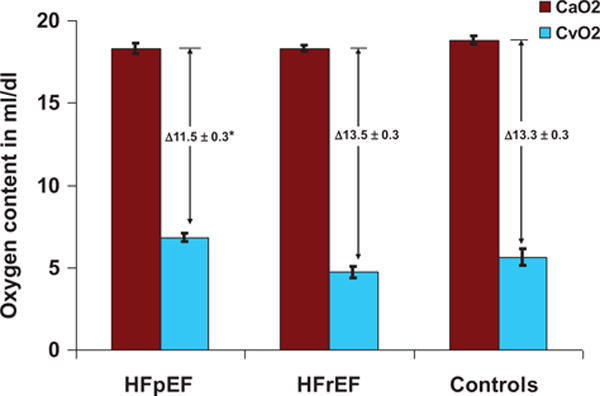
Arterial oxygen content (CaO2) and mixed venous oxygen content (CvO2) at peak exercise in patients with heart failure with preserved ejection fraction (HFpEF), HFrEF, and controls. *P<0.05 for comparison of HFpEF with HFrEF and controls.
CO/VO2 slope, also termed exercise factor, was 5.6±0.2 in controls consistent with the values reported by previous investigators.12,33,34 CO/VO2 slope was 6.1±0.2 in HFpEF (P=0.068 compared with controls, P<0.0001 compared with HFrEF) and 5.0±0.17 in HFrEF (P=0.02 compared with controls). Higher CO/VO2 slope in HFpEF compared with HFrEF is indicative of a reduced relative contribution of C(a-v)O2 to VO2 in HFpEF throughout exercise. Among patients in whom peak C(a-v)O2 was below predicted and constituted the primary cause of reduced peak VO2, the CO/VO2 slope was 8 (Figure I in the Data Supplement), indicating a disproportionate reliance on CO increment throughout exercise to compensate for abnormal C(a-v)O2.
Chronotropic Response During Exercise
HR at rest was similar in all 3 groups (Table 2). Failure to reach 85% of predicted HR was similarly common in HFpEF (67%) and HFrEF (75%, P=0.35). After accounting for β-blocker use, 73% of patients with HFpEF and 75% in patients with HFrEF met diagnostic criteria for chronotropic incompetence, consistent with findings from previous studies of exercise response patterns in HF.6,15,35,36
SV and Filling Pressures During Exercise
Resting SV in HFpEF was higher than resting SV in HFrEF and similar to that in controls (Table 2). At peak exercise, patients with HFpEF achieved higher SV than HFrEF subjects (88±3.6 mL versus 68±2.8 mL; P<0.001) but lower than controls (103±4.3 mL; P=0.03 compared with HFpEF; Table 2). The observed differences in SVs in HFpEF and HFrEF occurred in the setting of similar resting and exercise PCWP.
Integrated Responses: CO Versus Extraction Reserve Capacity During Exercise
We examined reserve capacity of each component of VO2 independently of resting values by assessing change in HR, SV, and C(a-v)O2 from rest to peak exercise in the 3 groups (Figure 2). In normal middle-aged controls in our study, VO2 increased 592±42% from rest to peak exercise, consistent with previous studies.13,31 This increase was because of a 109±8% increase in HR, a 39±4% increase in SV, and a 138±9% increase in C(a-v)O2 during exercise. In contrast, patients with HFpEF had a 311±20% increase in resting VO2 during exercise because of a 63±5% increase in HR, a 32±5% increase in SV, and a 91±6% increase in C(a-v)O2. Patients with HFrEF had a 264±14% increase in VO2 attributable to a 53±4% increase in HR, a 40±5% increase in SV, and a 77±5% increase in C(a-v)O2 (Figure 2). Notably, in all groups the magnitude of increase in C(a-v)O2 in response to exercise was greater than the magnitude of increase in HR or SV; thereby highlighting the important contribution of increase in C(a-v)O2 to augmenting VO2 during exercise.
Figure 2.
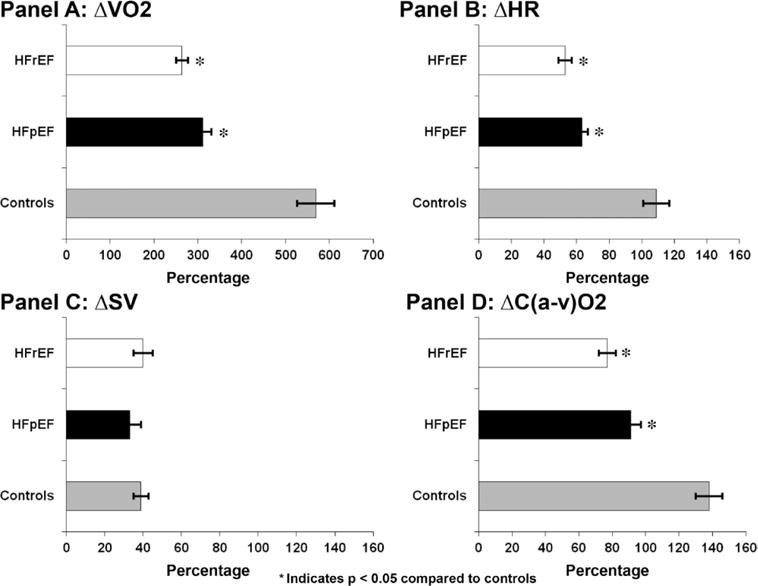
Percentage increase in VO2 and each of its components, heart rate (HR), stroke volume (SV) and arterio-mixed venous saturation difference (C(a-v)O2) from rest to peak exercise, *P<0.05.
Assessment of convective oxygen delivery (ie, CO×CaO2) and diffusive oxygen transport (represented by fall in CvO2) is an alternative, mechanistic way to analyze components of O2 utilization.37 Multipoint plots of CvO2 versus VO2 in the 3 groups indicate that diffusive O2 transport is most impaired in HFpEF, whereas convective O2 delivery is lowest in HFrEF (Figure 3). The extent to which VO2 would increase upon normalization of convective O2 delivery and diffusive O2 utilization in HFpEF is illustrated in Figure 3 and highlights the greater relative abnormality in diffusive O2 transport than in convective O2 delivery in HFpEF.
Figure 3.
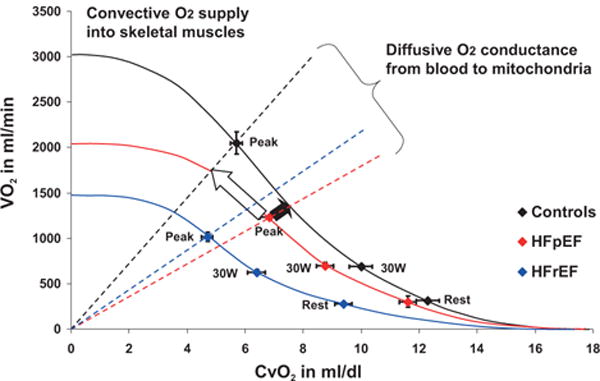
Illustration of the convective and diffusive components that interact to determine exercise capacity (VO2) in heart failure and controls. Mean values for CvO2 and VO2 at rest, 30 W, and peak exercise are used to construct Fick principal lines, which indicate convective O2 delivery and are curvilinear because they directly reflect the hemoglobin dissociation curve. The vertical lines extending from the origin to the VO2-CvO2 plot at peak exercise indicate maximum diffusive oxygen delivery as determined by the Fick law, with a steeper relationship indicating better O2 diffusion. Black arrow indicates the increment in peak VO2 in heart failure with preserved ejection fraction (HFpEF) if convective O2 delivery was corrected to that of normal controls. White arrow indicates the increment in peak VO2 if O2 diffusion was normalized in HFpEF.
Predictors of Peak VO2
Partial R2 values describing age and sex-adjusted relationships between peak VO2 and individual components of peak VO2 are displayed in Table 3. In HFpEF, peak VO2 related to maximum C(a-v)O2 (partial R2=0.28; P=0.0002) and peak HR (partial R2=0.35; P<0.0001) and there was a trend toward association with maximum SV (partial R2=0.07, P=0.077). In normal controls, by way of contrast, peak C(a-v)O2 tended to be more constant (mean 13.3±0.3 mL/dL) and predictably related to hemoglobin levels (mean 13.2g/dL),18 with a lower, partial R2 value (0.19, P=0.056) relative to peak VO2.
Table 3.
Heart Rate, Stroke Volume, and C(a-V)O2 Association With Peak VO2
| Characteristics | HFpEF
|
HFrEF
|
Controls
|
|||
|---|---|---|---|---|---|---|
| Partial R2 | P Value | Partial R 2 | P Value | Partial R 2 | P Value | |
| HR max | 0.350 | <0.0001 | 0.307 | <0.0001 | 0.342 | 0.006 |
| SV max | 0.072 | 0.077 | 0.379 | <0.0001 | 0.436 | 0.001 |
| C(a-v)O2 max | 0.281 | 0.0002 | 0.253 | 0.0001 | 0.187 | 0.056 |
HR, SV, and C(a-v)O2 are adjusted for age and sex both and VO2 is indexed to body weight. HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; and SV, stroke volume.
Blood Pressure and Diffusive Oxygen Transport in HFpEF
To further investigate impaired diffusive O2 transport in HFpEF in isolation, we stratified patients with HFpEF into 2 groups based on median peak exercise CvO2 of 6.8 mL/dL. The higher CvO2 subgroup did not differ from the lower CvO2 subgroup in age, sex, left ventricular ejection fraction, CO, or cardiac filling pressures but hemoglobin was slightly higher in the higher CvO2 group (Table I in the Data Supplement). The subset of patients with HFpEF with higher CvO2 had similar lactate and peak RER to the lower CvO2 group, which argues against reduced effort during exercise as an explanation for the attenuated fall in CvO2 during exercise in the high CvO2 group. The most striking difference between HFpEF CvO2 subgroups was that elevated CvO2 was associated with a disproportionate hypertensive response during exercise with elevation of diastolic blood pressure (DBP) (93±4 mm Hg versus 76±3; P=0.001), systolic blood pressure (196±7 versus 171±7 mm Hg; P=0.01), and mean arterial pressure (127±4 versus 107±4 mm Hg; P=0.001) at peak exercise (Figure 4; Table I in the Data Supplement). Among patients with peak exercise DBP in excess of 100 mm Hg, CvO2 was 8.5±0.3 mL/dL, compared with CvO2 of 6.5±0.4 mL/dL (P=0.005) in patients with exercise DBP≤100 mm Hg. When analyzed as a continuous variable, CvO2 was directly correlated with peak exercise DBP (Pearson r=0.49; P=0.0005) and extraction ratio was inversely related to exercise DBP (Pearson r=−0.41; P=0.004).
Figure 4.
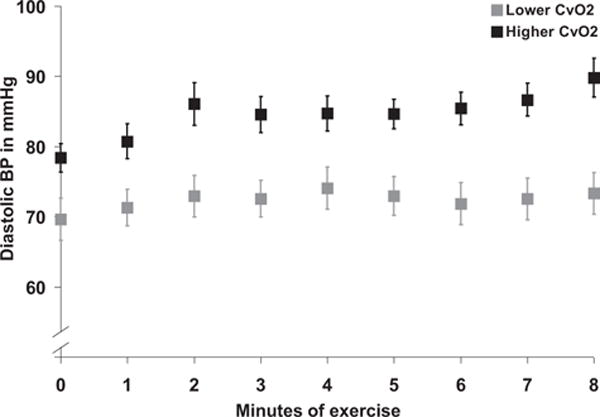
Diastolic blood pressure (BP) at rest and during incremental exercise in 2 subgroups of heart failure with preserved ejection fraction stratified by median mixed venous oxygen con-tent at peak exercise.
Discussion
In comprehensively characterized cohorts with HFpEF, HFrEF, and controls, we found that relative augmentation in peripheral oxygen extraction [C(a-v)O2] exceeded that of HR or SV during maximum incremental exercise in all 3 groups. Impaired peripheral O2 extraction was present in 75% of HFpEF subjects in our study and was attributable to impaired diffusive O2 transport and utilization (Figures 1 and 3). In contrast to the close association that we observed between peak VO2 and C(a-v)O2 in HFpEF, we found relatively modest or absent associations between peak VO2 and LV filling pressures or LV SV in HFpEF. Taken together, our findings highlight the potentially important role of targeting peripheral O2 extraction to augment impaired exercise capacity in HFpEF, particularly in light of failure of other interventions directed at central cardiac function to improve exercise capacity in HFpEF.32,38–40
The validity of our findings defining relative components of VO2 augmentation in HFpEF, HFrEF, and normals is supported by (1) rigorous entry criteria with confirmation of diagnoses with invasive hemodynamic assessment and ventriculography on the day of enrollment; (2) direct repeated measurements of CaO2, CvO2, and CO at 1-minute intervals throughout exercise; (3) use of physiologically relevant upright exercise with maximum effort confirmed by mean RERs ≥1.15 in each group; and (4) consistency of our findings with other studies with regard to demographic variables of HF subgroups and absolute levels of peak C(a-v)O2 during exercise in normals.
Exercise Capacity in HF
Limitation in exercise capacity is a cardinal manifestation of HF that is closely related to poor quality of life and mortality.41,42 The degree of reduction in exercise capacity in HFpEF in our study was similar to that reported in previous studies,3,5,15,16,32 and was intermediate between 2 recent interventional trials in HFpEF with rigorous entry criteria.43,44 In HFrEF, exercise capacity was also similar to that reported in previous studies,45,46 confirming that the peak VO2 values measured in our study were representative of the broader HF populations.
Association of Central Cardiac Function With Exercise Capacity in HF
Our finding that 73% of patients with HFpEF and 75% of patients with HFrEF had chronotropic incompetence, after accounting for β-blocker use, and that peak HR was strongly associated with peak VO2 in HFpEF, confirms previous studies demonstrating an important influence of chronotropic response on exercise capacity in HF.6,35,36,47,48 By confining our study to individuals who exceeded their ventilatory anaerobic threshold and an RER of 1.0, we can be confident that impaired chronotropic responses did not reflect lack of maximum effort or premature cessation of exercise because of pulmonary or orthopedic limitations.
SV in patients with HFpEF compared with controls was similar at rest but lower at peak exercise. Previous elegant studies have elucidated mechanisms by which SV is impaired in HFpEF at rest and during exercise, including abnormal ventriculo-vascular coupling,6 impaired relaxation,8,46 and impaired augmentation in systolic function.5,49 However, not all studies to date have found impaired SV responses to exercise in HFpEF50,51 and we found that within patients with HFpEF, peak SV was not significantly related to peak VO2. Furthermore, the percent-age increases in SV from rest to peak exercise within the groups were modest and similar between patients with HF and controls (39±4% in controls, 32±5% in HFpEF, and 40±5% in HFrEF; Figure 2).13,31 The modest increments in SV in response to exercise (32% to 40%) across the 3 groups indicate that the range of SV reserve capacity is more narrow than that for HR (53% to 109%) or C(a-v)O2 (77% to 138%; Figure 2). Hence, targeting impaired SV augmentation in response to exercise may be of limited benefit in a broad population of patients with HFpEF.
Peripheral Oxygen Extraction in HFpEF
Reduced C(a-v)O2 was the leading cause of impaired exercise capacity (ie, the degree of impairment in C(a-v)O2 was greater than that in CO as a % of predicted) in 40% of patients with HFpEF in our study and in only 2% of patients with HFrEF. Furthermore, in patients with HFpEF, we found that normalization of impaired O2 diffusion would result in a greater increment in peak VO2 than normalization of convective O2 delivery (Figure 3).
After convective delivery of O2 to skeletal muscle, diffusive O2 transport and utilization is dependent on the pathway consisting of skeletal muscle tissue microcirculatory O2 exchange vessels (ie, arterioles, venules, capillaries) and muscle units. O2 is transported passively by diffusion in this physically short pathway.37,52 In light of the large-scale blood flow redistribution to skeletal muscles during exercise, our finding that impaired diffusive O2 transport in HFpEF was closely related to an exaggerated systemic blood pressure increment during exercise (Figure 4), suggesting a potential role of impaired skeletal muscle vasodilatory capacity in small resistance vessels in mediating reduced peak C(a-v)O2 in HFpEF. Vasoconstrictor sympathetic tone and intrinsic microvascular control mechanisms have been shown to modulate the balance between O2 delivery an O2 demand within organs10, which suggests that skeletal muscle sympatholysis during exercise may be dysregulated in patients with HFpEF with impaired O2 extraction. In further support of sympathetic dysregulation and poorly co-ordinated vasoconstriction, elevated norepinephrine levels have been reported in patients with HFpEF at rest. Alternatively, diffusing capacity of the microvascular network may be limited by heterogeneity in microcirculatory blood flow recognized to occur in proinflammatory states. Finally, morphological and histochemical changes in skeletal muscle have also been described in HFrEF,53,54 including marked abnormalities in skeletal muscle mass, composition, capillary density, fiber type, oxidative metabolism, mitochondrial mass, and mitochondrial function as reviewed by Clark et al.55 These pathological peripheral abnormalities are distinct from the influence of deconditioning alone.56,57 Detailed investigations of skeletal muscle in HFpEF are limited, although intriguing in that Bhella et al15 first reported reduced oxidative metabolism by MRI in 2 patients with HFpEF and more recently abnormal skeletal muscle mass, adiposity, fiber type, and capillary density have been observed in HFpEF.58,59
Previous HFpEF studies in which C(a-v)O2 was estimated via noninvasive CO measurement have led to widely variable estimates of C(a-v)O2 levels in normals and in HFpEF.15,16 Peak exercise C(a-v)O2 values should be equal to hemoglobin levels in normal individuals.18,31 In 1 previous study that directly measured C(a-v)O2 in a subset of patients studied, C(a-v)O2 levels in controls and HFpEF were similarly low (10.1±0.3 versus 9.9±0.3 mL/dL; P=0.7).17 However, the study by Abudiab et al relied on exercise in a semisupine position and control subjects only exercised to 80 Watts, which may not have elicited maximum C(a-v)O2 as we observed C(a-v)O2 to increase in a linear fashion throughout maximum incremental exercise in our study (data not shown). In other HFpEF studies with a control group, the peak C(a-v)O2 values in controls7,15,17 were also 30% lower than their hemoglobin levels, which is much lower than to the ≈6% reduction in C(a-v)O2 expected with deconditioning alone.18 In previous small studies in HFpEF that deployed maximum upright exercise, C(a-v)O2 is consistently depressed.5,50,51 Our findings of an inverse initial relationship between C(a-v)O2 and CO that is no longer present at peak exercise points to the importance of performing maximum effort exercise to ascertain peak O2 extraction capacity in study populations.
Clinical Implications
Within the constraints of currently applied definitions of HFpEF,2,60 a single dominant pathophysiological mechanism governing exercise intolerance in HFpEF is unlikely to exist. The heterogeneity of the HFpEF population poses a major challenge to development of therapies to treat the entire HFpEF population.32,38–40 A potential pathway forward is to carefully identify subjects in whom the majority of reduction in peak VO2 is attributable to an abnormality in 1 component of peak VO2. In this study, CPET with invasive hemodynamic measurements permitted us to probe the reserve capacity of each component of VO2 to subphenotype patients on the basis of the dominant mechanism limiting exercise capacity. This approach may refine patient selection for targeted HFpEF therapeutics, for example, HFpEF could be subclassified into those with primarily impaired peripheral O2 extraction, chronotropic incompetence, or impaired SV among patients able to complete maximum incremental exercise without orthopedic or pulmonary mechanical limitation.
This study highlights the significant role of impaired C(a-v)O2 augmentation in contributing to exercise intolerance in ≈40% of an HFpEF population similar to those recently studied in HFpEF trials. Further studies are needed to determine the relative effect of targeting different aspects of the O2 diffusion unit. A recent study by Haykowsky et al61 found that improved peripheral function [estimated C(a-v)O2] primarily accounted for observed improvements in peak VO2 after exercise training in an HFpEF cohort. In light of the plasticity of skeletal muscle, targeting oxygen diffusion abnormalities in HF is particularly attractive. Positive studies with iron repletion in HFrEF, which promotes aerobic enzymatic activity and O2 storage in myoglobin offer promise for the possibility of extending this intervention to HFpEF.62 With regard to improving diffusional O2 transport to muscle in HF, decreasing O2 affinity (right shifting the O2 dissociation curve) has been shown to improve exercise capacity in mice with HF.30 Alternatively, patients with HFpEF with an exaggerated blood pressure response to exercise and impaired O2 diffusion may be particularly amenable to treatment with vasodilator interventions (ie, with nitrates, such as the NHLBI Heart Failure Network NEAT Trial, NCT02053493) to target skeletal muscle resistance vessels.
In contrast, patients in whom the dominant component of VO2 impairment is chronotropic incompetence,48 pacing or reduction in heart rate–lowering medications may promote improved exercise capacity, as will be tested in the RAPID Trial (NCT02145351). Finally, while SV emerged as the least dynamic of the 3 Fick variables, if SV remains fixed because of a noncompliant ventricle, then attempting to promote improvement in myocardial relaxation properties during exercise may be warranted.
Limitations
Our study has several limitations. Results were derived from small patient cohort referred to a tertiary care center, which may not be representative of the general patients with HFpEF found in the community, and we tested multiple hypotheses regarding associations between C(a-v)O2 and physiological parameters, increasing the chance of type 1 error. Our control population was limited in size (n=24) because of the infrequency with which subjects without significant cardiopulmonary disease undergo CPET with invasive hemodynamic monitoring. Using clinically referred patients who were physiologically normal as controls may underestimate differences between patients with HF and controls. The sampling of systemic venous blood does not permit localization of the peripheral abnormality in oxygen utilization in HFpEF. However, the majority of blood is directed to skeletal muscle during exercise and splanchnic and renal vasoconstriction have been shown to occur normally in HFrEF.10 Although none of the patients with HFpEF in our study had a known diagnosis of a mitochondrial disease or muscular dystrophy, it is possible that some of these patients may have had underlying conditions other than HFpEF that impaired skeletal muscle oxygen extraction. Finally, direct assessments of skeletal muscle and its perfusion were not available in our study to investigate potential histopathologic correlates of impaired O2 diffusion. This will be an important topic of future investigations aimed at further characterizing impaired O2 diffusion in HFpEF.
Conclusions
Patients with HFpEF demonstrated abnormally low peripheral oxygen extraction [C(a-v)O2] during exercise compared with HFrEF subjects and normal controls. This finding highlights the importance of looking beyond impairments in LV function and CO in evaluating functional limitations in patients with HFpEF. Our findings further indicate that improving abnormal O2 extraction may be an important therapeutic target in the notoriously difficult-to-treat patients with HFpEF.
Supplementary Material
CLINICAL PERSPECTIVE.
Within the constraints of currently applied definitions of heart failure with preserved left ventricular ejection fraction (HFpEF), a single dominant pathophysiological mechanism governing exercise intolerance is unlikely to exist. A potential pathway forward is to carefully identify subjects in whom the majority of reduction in peak VO2 is attributable to an abnormality in 1 component of peak VO2. In this study, cardiopulmonary exercise testing with invasive hemodynamic measurements permitted us to probe the reserve capacity of each component of VO2 to subphenotype patients on the basis of the dominant mechanism limiting exercise capacity. In comprehensively characterized cohorts with HFpEF, heart failure with reduced left ventricular ejection fraction (HFrEF), and controls, we found that relative augmentation in peripheral oxygen extraction [arterial-mixed venous oxygen content difference, C(a-v)O2] exceeded that of heart rate or stroke volume during maximum incremental exercise in all 3 groups. Impaired peripheral O2 extraction was present in 75% of HFpEF subjects in our study and was attributable to impaired diffusive O2 transport and utilization. There were only modest associations between peak VO2 and left ventricular filling pressures or left ventricular stroke volume in HFpEF. Taken together, our findings highlight the potentially important role of targeting C(a-v)O2 to augment impaired exercise capacity in HFpEF. This approach may refine patient selection for targeted HFpEF therapeutics; for example, HFpEF could be subclassified into those with primarily impaired peripheral O2 extraction, chronotropic incompetence, or impaired stroke volume among patients able to complete maximum incremental exercise.
Acknowledgments
Sources of Funding
This work was supported by K23 HL091106, R01 HL119154, the Hassenfeld Clinical Scholars Program (Dr Lewis and A.S. Eisman), and the Massachusetts General Hospital Cardiac Performance Program (Drs Baggish, Weiner, and Lewis)
Footnotes
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.114.001825/-/DC1.
Disclosures
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschöpe C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 11.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated non-invasively from oxygen uptake during exercise. J Appl Physiol (1985) 1997;82:908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 12.Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- 13.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss HP, Tschöpe C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp B. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 19.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 20.Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity; a report to the thoracic society. Thorax. 1957;12:290–293. doi: 10.1136/thx.12.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 23.Agostoni PG, Wasserman K, Perego GB, Guazzi M, Cattadori G, Palermo P, Lauri G, Marenzi G. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond) 2000;98:545–551. [PubMed] [Google Scholar]
- 24.Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiol Clin. 1992;10:705–717. [PubMed] [Google Scholar]
- 25.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 26.Elhendy A, Mahoney DW, Khandheria BK, Burger K, Pellikka PA. Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol. 2003;42:823–830. doi: 10.1016/s0735-1097(03)00832-5. [DOI] [PubMed] [Google Scholar]
- 27.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 28.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 29.Finch CA, Lenfant C. Oxygen transport in man. N Engl J Med. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Takeda T, Omiya S, Hikoso S, Yamaguchi O, Nakano Y, Higuchi Y, Nakai A, Abe Y, Aki-Jin Y, Taniike M, Mizote I, Matsumura Y, Shimizu T, Nishida K, Imai K, Hori M, Shirasawa T, Otsu K. Reduction in hemoglobin-oxygen affinity results in the improvement of exercise capacity in mice with chronic heart failure. J Am Coll Cardiol. 2008;52:779–786. doi: 10.1016/j.jacc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Beck KC, Randolph LN, Bailey KR, Wood CM, Snyder EM, Johnson BD. Relationship between cardiac output and oxygen consumption during upright cycle exercise in healthy humans. J Appl Physiol (1985) 2006;101:1474–1480. doi: 10.1152/japplphysiol.00224.2006. [DOI] [PubMed] [Google Scholar]
- 32.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, RELAX Trial Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taivassalo T, Abbott A, Wyrick P, Haller RG. Venous oxygen levels during aerobic forearm exercise: an index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol. 2002;51:38–44. doi: 10.1002/ana.10027. [DOI] [PubMed] [Google Scholar]
- 34.Epstein SE, Beiser GD, Stampfer M, Robinson BF, Braunwald E. Characterization of the circulatory response to maximal upright exercise in normal subjects and patients with heart disease. Circulation. 1967;35:1049–1062. doi: 10.1161/01.cir.35.6.1049. [DOI] [PubMed] [Google Scholar]
- 35.Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 36.Al-Najjar Y, Witte KK, Clark AL. Chronotropic incompetence and survival in chronic heart failure. Int J Cardiol. 2012;157:48–52. doi: 10.1016/j.ijcard.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- 38.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo-DHF Investigators Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 40.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 41.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 42.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55:1037–1042. doi: 10.1016/0002-9149(85)90742-8. [DOI] [PubMed] [Google Scholar]
- 43.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 45.Mancini D, Katz S, Donchez L, Aaronson K. Coupling of hemodynamic measurements with oxygen consumption during exercise does not improve risk stratification in patients with heart failure. Circulation. 1996;94:2492–2496. doi: 10.1161/01.cir.94.10.2492. [DOI] [PubMed] [Google Scholar]
- 46.Farr MJ, Lang CC, Lamanca JJ, Zile MR, Francis G, Tavazzi L, Gaasch WH, St John Sutton M, Itoh H, Mancini D, MCC-135 GO1 Investigators Cardiopulmonary exercise variables in diastolic versus systolic heart failure. Am J Cardiol. 2008;102:203–206. doi: 10.1016/j.amjcard.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 47.Dobre D, Zannad F, Keteyian SJ, Stevens SR, Rossignol P, Kitzman DW, Landzberg J, Howlett J, Kraus WE, Ellis SJ. Association between resting heart rate, chronotropic index, and long-term outcomes in patients with heart failure receiving β-blocker therapy: data from the HF-ACTION trial. Eur Heart J. 2013;34:2271–2280. doi: 10.1093/eurheartj/ehs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ennezat PV, Lefetz Y, Maréchaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Véhier C, Jude B, Nevière R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 54.Drexler H, Faude F, Höing S, Just H. Blood flow distribution within skeletal muscle during exercise in the presence of chronic heart failure: effect of milrinone. Circulation. 1987;76:1344–1352. doi: 10.1161/01.cir.76.6.1344. [DOI] [PubMed] [Google Scholar]
- 55.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 56.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 57.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 58.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–1216. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 61.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P, FAIR-HF Trial Investigators Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


