Ibrutinib, a Bruton tyrosine kinase inhibitor, is a new oral agent approved for the treatment of chronic lymphocytic leukemia (CLL).1 Ibrutinib is well-tolerated, with the majority of its adverse events within grade 1 or 2 severity.2 The most common nonhematologic side effects are diarrhea, arthralgia, rash, and brittle nails.2, 3 Three subtypes of ibrutinib-related dermatitis have been reported in the literature: 1) asymptomatic nonpalpable petechial rash, 2) leukocytoclastic vasculitis–like pruritic violaceous palpable purpura, and 3) painless nonpruritic edematous papules with centripetal spread.4, 5 We report a previously undescribed case of a pityriasis rosea (PR)–like dermatitis associated with the use of ibrutinib.
Case report
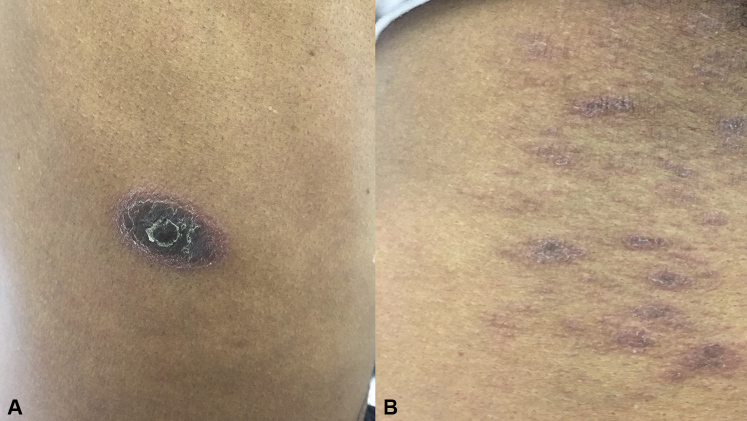
A 62-year-old man with a history of relapsed CLL who had been taking ibrutinib 420 mg daily for 8 months presented with a 1-month history of a pruritic plaque on his right flank. Subsequently, he developed more lesions over his right lower abdomen and the upper aspect of his left chest. The patient denied any oral or genital lesions. He also denied any viral infection before onset of the rash. The patient had initially been treated with chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab and was then treated with vincristine and rituximab at relapse. Chemotherapy was discontinued 2 years before the onset of the rash. Comorbidities included hypertension, hyperlipidemia, and hypogammaglobulinemia. Other concurrent medications included infusions of intravenous immunoglobulin every 4 weeks without rash during or after the infusion. He was also taking long-term amlodipine, hydrochlorothiazide, and lovastatin, preceding his ibrutinib by many years. The physical examination revealed a large, isolated, 3-cm violaceous plaque with a trailing central scale over the right flank (Fig 1, A) and multiple grouped violaceous scaly papules each measuring 0.5 to 1 cm under the right axilla (Fig 1, B) and over the upper aspect of the left chest.
Fig 1.
Clinical images of the rash over the right flank and right axilla. A, A 3-cm violaceous plaque with an inward-facing central scale on the right flank. B, Multiple grouped violaceous scaly papules each measuring 0.5 to 1 cm under the right axilla.
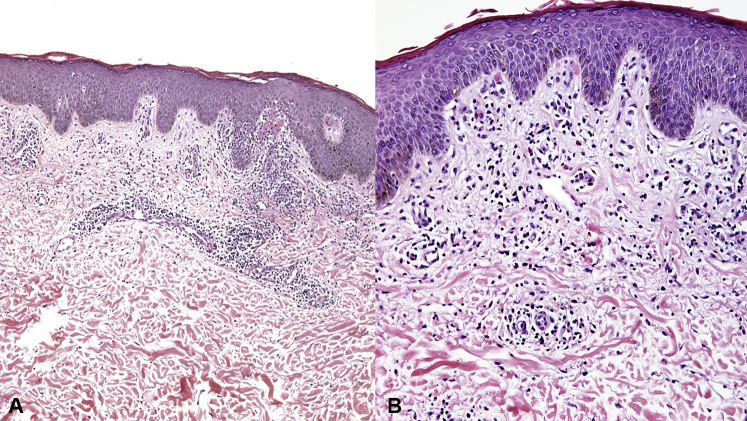
A punch biopsy specimen was obtained and histopathologic examination revealed chronic spongiotic dermatitis with eosinophils, consistent with a drug eruption (Fig 2). The patient was continued on ibrutinib and treated with triamcinolone acetonide 0.1% cream twice daily for 1 month. During his 1-month follow-up, the patient had persistence of his rash. He endorsed less pruritus and no new lesions. The patient had resolution of the rash during his 3-month follow-up, and no interruption of ibrutinib was required.
Fig 2.
Histopathologic images of punch biopsy specimens obtained from the right flank. A, Superficial perivascular infiltrate with spongiosis. B, Perivascular cell infiltrates consisting of lymphocytes and eosinophils, suggestive of drug eruption. (Hematoxylin–eosin stain; original magnification: A, ×4; B, ×10.)
Discussion
Ibrutinib is a new drug that has been approved by the US Food and Drug Administration for several hematologic malignancies, including CLL,1 mantle cell lymphoma,6 and Waldenström macroglobulinemia.7 It is also being studied for chronic graft versus host disease.8 Cutaneous adverse events for ibrutinib are reported in about 2% to 27% of treated patients.4 We present a previously undescribed rash with ibrutinib, consisting of a PR-like pattern. The rash in our patient appeared 240 days after treatment with ibrutinib. Ibrutinib-induced skin rash is known to have a variable onset that sometimes can be delayed.4 Iberri et al4 reported a range of 44 to 384 days for the onset of the nonpalpable petechial rash and 6 to 404 days for the palpable purpuric rash.4
One suggested mechanism of ibrutinib-induced drug eruption is through epidermal growth factor receptor (EGFR) inhibition.9 Although ibrutinib is highly selective for Bruton tyrosine kinase, it exerts off-target effects on other kinases, such EGFR.10 EGFR inhibitors have well-established cutaneous side effects.11 Another suggested mechanism of ibrutinib-induced drug eruption is through its ability to inhibit c-Kit and platelet-derived growth factor receptor (PDGFR). The tyrosine kinase inhibitor imatinib, used in the treatment of chronic myeloid leukemia, has been reported to induce a PR-like eruption.12 Imatinib-induced PR-like eruption has been attributed to its ability to inhibit c-Kit and PDGFR in keratinocytes.12 Akin to imatinib, ibrutinib has been shown to inhibit c-Kit and PDGFR.10
Ibrutinib rashes are usually grade 1 or 2 in severity and can be treated symptomatically with topical steroids and antihistamines.1, 4 As in our patient, the majority of ibrutinib-associated rashes resolve with symptomatic treatment without discontinuation of the ibrutinib.4 Patients with severe drug rash may require systemic steroids, temporary ibrutinib interruption, or a dose adjustment.4 Dermatologists should be aware of the different manifestation of ibrutinib-associated eruptions and their management because of the expanding use of this drug.
Footnotes
Funding sources: None.
Dr Murina is a speaker for AbbVie, Celgene, and Novartis. All other authors have no conflicts of interest.
References
- 1.Saba N., Ghias M., Manepalli R. Auranofin induces a reversible in-vivo stress response that correlates with a transient clinical effect in patients with chronic lymphocytic leukemia. Blood. 2013;122:3819. [Google Scholar]
- 2.Farooqui M.Z., Valdez J., Martyr S. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16:169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitar C., Farooqui M.Z., Valdez J. Hair and nail changes during long-term therapy with ibrutinib for chronic lymphocytic leukemia. JAMA Dermatol. 2016;152:698–701. doi: 10.1001/jamadermatol.2016.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iberri D.J., Kwong B.Y., Stevens L.A. Ibrutinib-associated rash: a single-centre experience of clinicopathological features and management. Br J Haematol. 2016 doi: 10.1111/bjh.14302. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Mannis G., Wu D., Dea T., Mauro T., Hsu G. Ibrutinib rash in a patient with 17p del chronic lymphocytic leukemia. Am J Hematol. 2015;90:179. doi: 10.1002/ajh.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M.L., Rule S., Martin P. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treon S.P., Tripsas C.K., Meid K. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 8.Schutt S.D., Fu J., Nguyen H. Inhibition of BTK and ITK with ibrutinib is effective in the prevention of chronic graft-versus-host disease in mice. PLoS One. 2015;10:e0137641. doi: 10.1371/journal.pone.0137641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen A.B., Stausbol-Gron B., Riber-Hansen R., d'Amore F. Ibrutinib-associated skin toxicity: a case of maculopapular rash in a 79-Year old Caucasian male patient with relapsed Waldenström's macroglobulinemia and review of the literature. Dermatol Rep. 2017;9:6976. doi: 10.4081/dr.2017.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Cruz O.J., Uckun F.M. Novel Bruton's tyrosine kinase inhibitors currently in development. Onco Targets Ther. 2013;6:161–176. doi: 10.2147/OTT.S33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C., Soria J.C., Spatz A. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho A.Y., Kim D.H., Im M., Lee Y., Seo Y.J., Lee J.H. Pityriasis rosea–like drug eruption induced by imatinib mesylate (Gleevec) Ann Dermatol. 2011;23(suppl 3):S360–S363. doi: 10.5021/ad.2011.23.S3.S360. [DOI] [PMC free article] [PubMed] [Google Scholar]