Introduction
Eosinophilic annular erythema (EAE) is a relatively new entity, clinically presenting as a figurate erythema. The cause of EAE remains obscure, but it has been associated with several chronic conditions and malignancies. We describe an unusual case of EAE developing before the onset of autoimmune hepatitis.
Report of a case
A 22-year-old man with history of acne was referred to our dermatology department for possible patch testing and evaluation of his widespread rash. Three months before referral, the patient had an eruption of well-demarcated plaques on his scalp, face, neck, and extremities, associated with pruritus. The patient's eruption was refractory to topical corticosteroids (desoximetasone, clobetasol, and triamcinolone). The patient denied similar eruption in family members or close contacts. Additional medical history, including new medications, changes in personal products, and new sexual contacts, was noncontributory, and detailed review of systems was found to be negative.
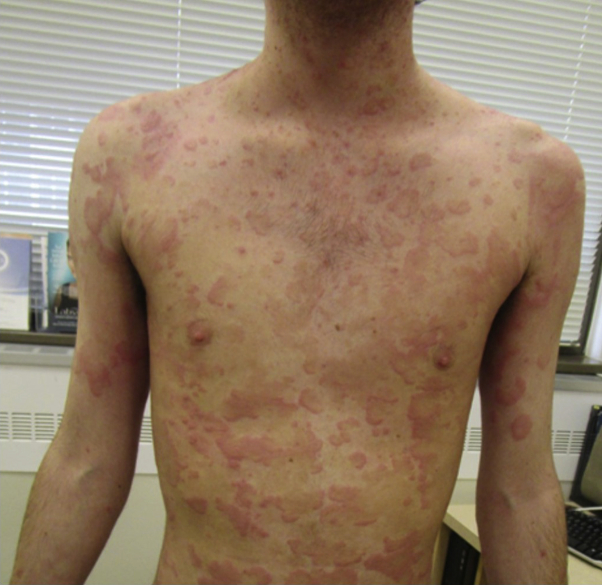
Physical examination found annular erythematous plaques with central clearing on the trunk and extremities, including the palms of both hands (Fig 1). The face, scalp, and inguinal area were spared. Results of laboratory tests, which included complete blood count, complete metabolic panel, rapid plasma reagin, hepatitis panel, HIV antibody, herpes simplex virus antibody, and Lyme titers, were normal, with the exception of eosinophilia (13%).
Fig 1.
Eosinophilic annular erythema (EAE). Multiple erythematous annular plaques with central clearing involving the neck, trunk, and extremities.
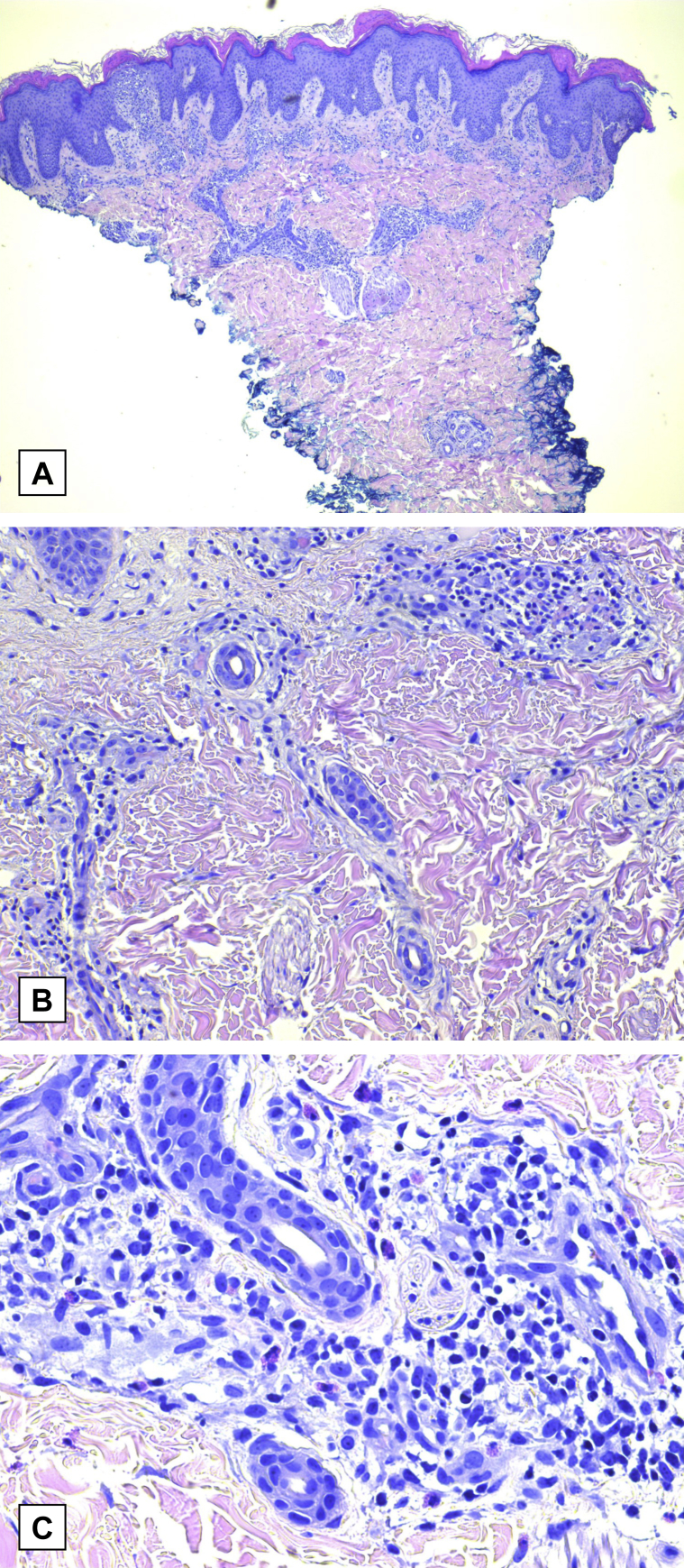
Biopsies of the back and abdomen performed by his outside dermatologist found subacute spongiotic dermatitis with parakeratosis, preservation of the dermoepidermal junction, and superficial to midperivascular lymphohistiocytic cell infiltrate (Fig 2, A-C). At the level of the dermis, interstitial mucin deposition and scattered eosinophils were visible, with notable absence of flame figures. The diagnosis of eosinophilic annular erythema was favored given his clinical presentation and pathology results.
Fig 2.
A, Histopathology shows marked alterations in the epidermis (spongiosis and parakeratosis) and superficial and deep inflammatory infiltrate within the dermis. B and C, Perivascular and interstitial infiltrate composed of eosinophils and lymphocytes with mucin noted between dermal collagen bundles. (A to C, Hematoxylin-eosin stain; original magnifications: A, ×4; B, ×20; and C, ×40.)
The patient had considerable improvement on oral prednisone, but his condition flared when this medication was tapered. A trial of hydroxychloroquine, 200 mg twice daily, was discontinued after 4 weeks of therapy because of lack of effective response. Methotrexate, 10 mg weekly, was found to be efficacious. Unfortunately, leukocytosis and worsening eosinophilia developed (increasing from 8.1 × 103/μL to 23.2 × 103/μL and 42% to 65%, respectively), leading to discontinuation of methotrexate after 2 weeks of therapy. While on methotrexate, the patient had blisters on his feet, bilateral ankle swelling, and stiff knees. He was referred to the oncology department, given reports of malignancy in the literature associated with eosinophilic annular erythema and his leukocytosis. Two weeks after cessation of methotrexate, the patient's leukocytosis resolved and his eosinophilia declined to 20%.
Over the next 10 weeks, the patient was treated with fluocinonide cream and mycophenolate mofetil, 500 mg twice daily decreased to once daily (because of increased aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase to 107 IU/L, 91 IU/L, and 199 IU/L, respectively). Despite moderate improvement in his skin, the patient was advised to discontinue mycophenolate mofetil and was referred to the gastroenterology department for increasing elevation of liver enzymes (aspartate aminotransferase, 212 IU/L; alanine aminotransferase, 145 IU/L; and alkaline phosphatase, 207 IU/L). Drug-induced hepatitis was excluded as a causative factor because of continued elevation of liver enzymes for 2 months after discontinuation of mycophenolate mofetil. Investigation found positive smooth muscle antibody, elevation of serum IgG (2037 mg/dL), and a liver biopsy showing chronic nonspecific hepatitis consistent with a diagnosis of autoimmune hepatitis. The patient was started on prednisone, 60 mg once daily, and is now having resolution of his rash with mild postinflammatory hyperpigmentation.
Discussion
EAE is a chronic and recurrent skin disease. Clinically, it presents as urticarial, annular papules and plaques on the trunk and extremities.1, 2 The lesions are typically asymptomatic or mildly pruritic.3 Over time, these lesions can adopt an annular or polycyclic pattern with central clearing.4 During resolution, transient pigment changes may be observed, but no atrophy or scarring occurs.4 Similar to most figurate erythemas, EAE is of unknown etiology. However, EAE is hypothesized to be the result of a hypersensitivity reaction to an unknown antigen.5 EAE can be differentiated from other figurate erythemas by clinical history (excluding tick exposure), absence of scale, presence of eosinophils in the dermis, and negative Lyme titers, as seen in our patient.2, 6
It is debated whether EAE is a subset of Wells syndrome or if it is a distinct entity in itself. Wells syndrome, also known as eosinophilic cellulitis, is an inflammatory dermatitis that has a wide range of clinical presentations. However, Wells syndrome typically presents as acute erythematous plaques usually resolving with hyperpigmentation and residual atrophy, as opposed to EAE, which resolves with no sequlae.2 Wells syndrome can occur in patients of any age, and lesions rarely affect the face.7 The histopathologic findings of Wells syndrome include dermal edema, eosinophils, and “flame figures” formed by eosinophilic granules coating collagen.8 In contrast, EAE is characterized by superficial and deep perivascular lymphocytic infiltrate with numerous eosinophils.2 Although both conditions have tissue eosinophilia, EAE is also distinguished from Wells syndrome by the absence of flame figures and diffuse inflammatory infiltrate on histopathology as seen in our case.2, 5 However, multiple cases of EAE have been reported with the histopathologic findings of flame figures.6, 9, 10 This variation in findings is thought to be because of the timing of when the biopsy specimens are taken, with earlier EAE lesions being devoid of flame figures and well-developed EAE lesions containing them.5, 6
Although EAE can be self-limited, it often runs a chronic relapsing and remitting course with variable response to treatment.3 Both antimalarials and systemic steroids are reported to clear the lesions.4, 5, 6 However, early relapse after discontinuation of these treatments is common.4, 6, 9 Additional treatments reported to have some success include dapsone, indomethacin, nicotinamide, and ultraviolet B phototherapy.3, 4, 9, 11 Of note, El-Khalawany et al6 observed that treatment of associated diseases (including chronic gastritis, diabetes mellitus, chronic hepatitis C virus infection, and chronic kidney disease) increased the time of remission and decreased the rate of relapse in their EAE patients treated with prednisone alone or in conjunction with cyclosporine or hydroxychloroquine.
Multiple chronic conditions are reported to have a possible association with EAE, including autoimmune thyroid disease, borreliosis, chronic gastritis caused by Helicobacter pylori infection, diabetes mellitus, hepatitis C infection, chronic kidney disease, thymoma, and autoimmune pancreatitis.1, 6, 11 Coexisting malignancy and EAE have also been reported, specifically, clear cell renal carcinoma and metastatic prostate cancer.9, 10 To our knowledge, there are no reports of EAE in association with autoimmune hepatitis. Additionally, this case shows the unique development of hepatic disease 7 months after onset of EAE. Furthermore, the correlation between adequate treatment of the patient's autoimmune hepatitis and resolution of his rash suggests the importance for awareness of EAE as a presenting sign for evolving systemic disease in the absence of a malignancy or chronic condition at initial presentation.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Olabola Awosika's fellowship is funded by Janssen Biotech, Inc. Dr Alison Ehrlich discloses that she is a speaker for Abbvie, Lilly, and Celgene and a principal investigator for Abbvie, Dermira, UCB, Merck, DUSA, Regeneron, Lilly, Leo Pharma, and Janssen. The other authors report no conflicts of interest in this work.
References
- 1.Iga N., Otsuka A., Kaku Y., Miyachi Y., Kabashima K. Eosinophilic annular erythema limited on the palms and the soles and possibly associated with thymoma. J Eur Acad Dermatol Venereol. 2016;30(7):1213–1214. doi: 10.1111/jdv.13139. [DOI] [PubMed] [Google Scholar]
- 2.Sempau L., Larralde M., Luna P.C., Casas J., Staiger H. Eosinophilic annular erythema. Dermatol Online J. 2012;18(3):8. [PubMed] [Google Scholar]
- 3.Manriquez J., Berroeta-Mauriziano D., Andino-Navarrete R., Vera-Kellet C. Eosinophilic annular erythema: complete clinical response with dapsone. Int J Dermatol. 2015;54(4):e96–e98. doi: 10.1111/ijd.12736. [DOI] [PubMed] [Google Scholar]
- 4.Thomas L., Fatah S., Nagarajan S., Natarajan S. Eosinophilic annular erythema: successful response to ultraviolet B therapy. Clin Exp Dermatol. 2015;40(8):883–886. doi: 10.1111/ced.12668. [DOI] [PubMed] [Google Scholar]
- 5.Karataş Toğral A., Seçkin D. Eosinophilic annular erythema: A late but complete response to hydroxychloroquine. Australas J Dermatol. 2017;58:228–230. doi: 10.1111/ajd.12445. [DOI] [PubMed] [Google Scholar]
- 6.El-Khalawany M., Al-Mutairi N., Sultan M., Shaaban D. Eosinophilic annular erythema is a peculiar subtype in the spectrum of Wells syndrome: a multicentre long-term follow-up study. J Eur Acad Dermatol Venereol. 2013;27(8):973–979. doi: 10.1111/j.1468-3083.2012.04616.x. [DOI] [PubMed] [Google Scholar]
- 7.Sommer S., Wilkinson S.M., Merchant W.J. Eosinophilic cellulitis following the lines of Blaschko. Clin Exp Dermatol. 1999;24(6):449–451. doi: 10.1046/j.1365-2230.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 8.Sinno H., Lacroix J.P., Lee J. Diagnosis and management of eosinophilic cellulitis (Wells' syndrome): A case series and literature review. Can J Plast Surg. 2012;20(2):91–97. doi: 10.1177/229255031202000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rongioletti F., Fausti V., Kempf W., Rebora A., Parodi A. Eosinophilic annular erythema: an expression of the clinical and pathological polymorphism of Wells syndrome. J Am Acad Dermatol. 2011;65(4):e135–e137. doi: 10.1016/j.jaad.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 10.González-López M.A., López-Escobar M., Fernández-Llaca H., González-Vela M.C., López-Brea M. Eosinophilic annular erythema in a patient with metastatic prostate adenocarcinoma. Int J Dermatol. 2015;54(3):e80–e82. doi: 10.1111/ijd.12640. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa K., Fukumoto T., Yoshida M., Matsumoto Y., Shobatake C., Asada H. Eosinophilic annular erythema in a patient with autoimmune pancreatitis: Nicotinamide therapy may be beneficial for achieving remission. J Dermatol. 2016;43:1380–1381. doi: 10.1111/1346-8138.13411. [DOI] [PubMed] [Google Scholar]