Introduction
Mucous membrane pemphigoid (MMP) is a rare, autoantibody-mediated disease characterized by mucocutaneous blistering including oral, ocular, laryngeal, and skin involvement. Treating MMP is challenging, with few effective therapies available. We report successful treatment of a patient with treatment-refractory MMP with the proteasome inhibitor, bortezomib.
Case report
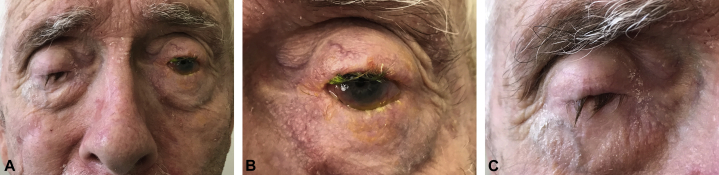
A 68-year-old man presented with a 7-year history of conjunctival inflammation with progressive development of skin and oral erosions and epistaxis (Fig 1). Skin biopsies found papillary dermal edema, an eosinophilic dermal infiltrate, and eosinophilic spongiosis, suggestive of bullous pemphigoid (BP). Direct immunofluorescence testing of a skin biopsy from the neck found linear IgA and IgG deposition along the dermoepidermal junction. Direct immunofluorescence of a gingival biopsy, however, was negative. BP180/230 antibodies were not detected; antilaminin serology testing was not available. Extensive mucosal involvement suggested a unifying diagnosis of MMP. Despite 2 years of treatment with doxycycline (100 mg twice a day), methotrexate (27.5 mg/wk intramuscularly), rituximab (4 doses of 750 mg over 2 months), and cyclophosphamide (750 mg × 1 dose followed by 5 months of weekly infusions of 1 g), the patient had limited improvement in skin and ocular inflammation. Progressive ocular scarring prompted hospital admission for methylprednisolone (1 g intravenously daily for 3 days) and intravenous immunoglobulin (2 g/kg total dose infused over 4 days), resulting in reduced photophobia, stabilization of visual acuity, and healing of oral and nasal ulcers. Because his ocular and skin disease recurred and remained poorly controlled despite further treatment with oral prednisone (60 mg/d), rituximab (1 g given twice in 1 month repeated 6 months later) and topical corticosteroids, the patient was admitted for empiric plasmapheresis despite having undetectable serum autoantibodies. During that admission, progressively worsening dyspnea with impaired resting oxygen saturation levels (80%) prompted a pulmonary workup. Pulmonary function testing, chest computed tomography, and bronchoscopy suggested evidence of pemphigoid-related bronchiolitis. The patient also had steroid-induced myopathy, necessitating rapid taper and discontinuation of oral corticosteroids. Intraocular corticosteroid injections were initiated, and rituximab treatment (900 mg/wk for 8 weeks then given monthly for 6 months) was restarted.
Fig 1.
A, Severe eye involvement. B, Left eye, close-up. C, Right eye with maximal opening of the eyelids, close up.
Photo credit: Timothy H. Schmidt, MD, PhD.
Given the patient's refractory disease and his continued wishes to pursue aggressive therapy, a multidisciplinary team initiated bortezomib treatment with rapid improvement of the patient's skin lesions, ocular inflammation, and pulmonary symptoms. The patient received 4 cycles of bortezomib infusions (1.3 mg/m2 on days 1, 4, 8, and 11) over 10 months with moderate adverse effects including fever, malaise, myalgias, arthralgias, and rash. No new pemphigoid-related lesions were detected after the fourth cycle of treatment. Over the following months, although measurable visual acuity was unchanged, the patient reported marked improvement in vision; reduction in conjunctival inflammation was noted. One year after starting bortezomib, the patient's forced expiratory volume in the first second had increased from 1.92 L to 2.41 L. Fourteen months after the final infusion, the patient had erythematous gingiva and conjunctiva but was otherwise in clinical remission.
Discussion
Ocular, nasal, and oral involvement in MMP is seen in 65%, 20% to 40%, and 85% of patients, respectively.1 Few case reports describe MMP-related subepithelial bullae with underlying chronic bronchial inflammation as observed in our patient.2
MMP is caused by loss of immunologic tolerance to structural proteins including BPAg2, laminin 332, laminin 311, and integrin β4.3 Although many of the same proteins are autoimmune targets in BP, MMP preferentially affects the mucosa and causes substantial scarring. In MMP, IgG or IgA autoantibody binding may occur in the lower lamina lucida and lamina densa, a deeper location of inflammation than what is seen in BP. Complications may arise from active erosive disease and subsequent scarring. Although the term MMP is preferred by consensus expert groups, there likely exist distinct variants of MMP that are treatment recalcitrant, result in profound scarring, and may stem primarily from differential autoimmune targeting of antigens in skin, eye, mouth, and airways.4
Available treatments for MMP closely parallel those of BP and aim to reduce antibody production. Patients with extensive or vision-threatening disease require aggressive systemic therapies.5 Although prednisone, doxycycline, methotrexate, and cyclophosphamide have some utility in MMP, few drugs have shown efficacy specifically for MMP.5 Methotrexate and the combination of rituximab and intravenous immunoglobulin have been used successfully in cases of severe MMP with ocular involvement.5, 6, 7 Long-term follow-up data for rituximab in MMP has not been reported. There is limited evidence for plasmapheresis in MMP management.
In our case, more established treatments for MMP failed to control the patient's disease, so we turned to bortezomib, a proteasome inhibitor. US Food and Drug Administration approved for the treatment of multiple myeloma and mantle cell lymphoma, proteasome inhibitors are primarily used for their anticancer activities. Bortezomib inhibits the 26S proteasome, a key enzyme in degrading intracellular proteins.8 The accumulation of unfolded proteins triggers downstream apoptotic pathways. Side effects of bortezomib include fatigue, weakness, rash, fever, myalgia, gastrointestinal disturbances, and peripheral neuropathy.
Bortezomib is currently being studied as a potential therapy for antibody-mediated autoimmune diseases, including systemic lupus erythematosus and autoimmune thrombocytopenia.9, 10 Bortezomib induces immunosuppression through several different mechanisms. First, it inhibits proliferation of immune cells by reducing class II major histocompatibility complex expression and suppressing the proinflammatory nuclear transcription factor kappa B pathway.8, 9 Bortezomib also reduces production of pathogenic antibodies involved in autoimmune disease through 2 mechanisms: first, through the induction of cellular stress and apoptosis of antibody-producing cells, and second, by reducing the efficiency of proteolytic peptide processing and presentation in B and T cells.10 Studies show that proteasome inhibitors, particularly bortezomib, preferentially target plasma cells because of their high activity of protein synthesis. Our patient was refractory to rituximab, suggesting that his disease may be driven by long-lived plasma cells rather than B cells and short-lived plasmablasts. Thus, we hypothesized that using bortezomib to target plasma cells would help our patient. Bortezomib may be a potential therapeutic option for cases of refractory MMP and other autoantibody-mediated diseases that do not respond to rituximab. Larger studies and randomized controlled trials are needed to better understand the efficacy and safety of bortezomib for autoantibody-mediated diseases like MMP.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Xu H.H., Werth V.P., Parisi E. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57(40):611–630. doi: 10.1016/j.cden.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Carvalho C.R., Amato M.B., Da Silva L.M. Obstructive respiratory failure in cicatricial pemphigoid. Thorax. 1989;44(7):601–602. doi: 10.1136/thx.44.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kourosh A.S., Yancey K.B. Pathogenesis of mucous membrane pemphigoid. Dermatol Clin. 2011;29(3):479–484. doi: 10.1016/j.det.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Murrell D.F., Marinovic B., Caux F. Definitions and outcome measures of mucous membrane pemphigoid: recommendations of an international panel of experts. J Am Acad Dermatol. 2015;72:168–174. doi: 10.1016/j.jaad.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Neff A.G., Turner M., Mutasim D.F. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag. 2008;4(3):617–626. doi: 10.2147/tcrm.s1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCluskey P., Chang J.H., Singh R. Methotrexate therapy for ocular cicatricial pemphigoid. Ophthalmology. 2004;111:796–801. doi: 10.1016/j.ophtha.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Maley A., Warren M., Haberman I. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid. J Am Acad Dermatol. 2016;74(5):835–840. doi: 10.1016/j.jaad.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Milano A., Perri F., Capnigro F. The ubiquitin-proteosome system as a molecular target in solid tumors: an update on bortezomib. Onco Targets Ther. 2009;2:171–178. doi: 10.2147/ott.s4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neubert K., Meister S., Moser K. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 10.Ratnasingam S., Walker P., Tran H. Bortezomib-based antibody depletion for refractory autoimmune hematological diseases. Blood Advances. 2016;1(1):31–35. doi: 10.1182/bloodadvances.2016001412. [DOI] [PMC free article] [PubMed] [Google Scholar]