Case report
We present a case of late-onset cobalamin C deficiency presenting with a unique shellac-like erosive desquamation and mental status changes that was dramatically responsive to treatment. We discuss cobalamin C deficiency and compare the cutaneous findings with other similar nutritional deficiency states. The brown shellac-like desquamation should prompt dermatologists to consider this diagnosis in the setting of a suspected nutritional deficiency.
A 21-year-old wheelchair-bound man with a history of autoimmune cerebritis, seizure disorder, and recurrent deep-vein thrombosis presented with intractable diarrhea and an acute and progressive reduction in mentation. The patient was previously able to use minimal verbal communication, but upon admission, he was somnolent. Three years prior, he was much more independent, working at a fast-food chain. Patient had been stable taking home medications, which included lacosamide, levetiracetam, topiramate, phenobarbital, diazepam, clobazam, warfarin, escitalopram, and mirtazapine. On previous admission for nonconvulsive status epilepticus, the patient was found to have hyperammonemia. At that time, urine organic acids revealed an elevated lactic acid 128 mg/dL (reference range 0-50 mg/dL) and methylmalonic acid (MMA) 2463 mg/dL (reference range 0-5 mg/dL). These findings suggested a methylmalonic acidemia, although no further testing was performed. On this admission, causes of altered mentation were extensively investigated by internal medicine and neurology. There was no evidence of status epilepticus or flare of the patient's autoimmune cerebritis on electroencephalogram, computed tomography, and magnetic resonance imaging. However, unusual widespread skin desquamation prompted dermatology consultation.
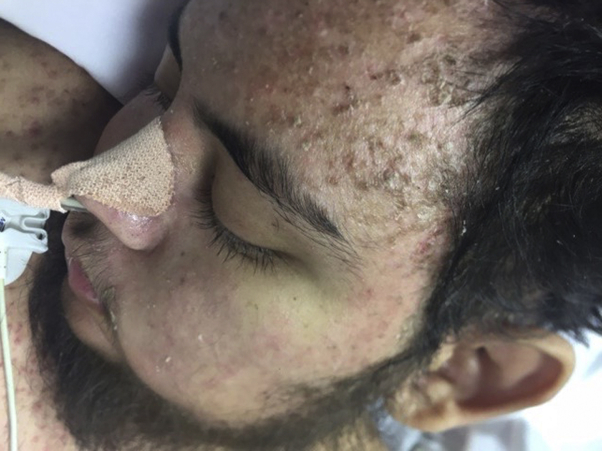
On physical exam, the patient was unarousable by verbal or tactile stimuli, though withdrew from pain. He had thick, yellow seborrheic dermatitis-like scaling on his scalp, hyperpigmented scales on the face (Fig 1), and mild cheilitis.
Fig 1.
Seborrheic-like dermatitis on face of patient with cobalamin C deficiency.
The buttocks, posterior thighs, and inguinal areas had distinctive, intertriginous, hyperpigmented patches with thick, brown, superficially erosive, shellac-like desquamation (Figs 2 and 3).
Fig 2.

Shellac-like desquamation (close-up) of buttocks of patient with cobalamin C deficiency.
Fig 3.
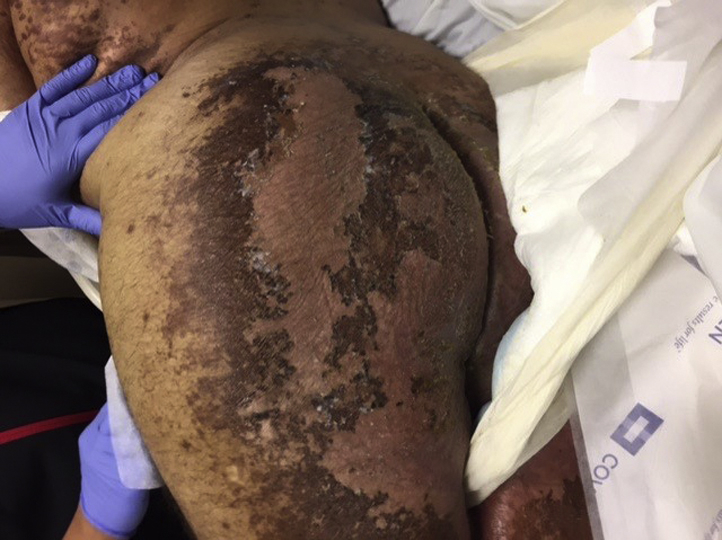
Superficial, erosive desquamating dermatitis of flank and buttock of patient with cobalamin C deficiency.
Pertinent lab findings on admission included 2+ level of ketones on urinalysis, serum MMA of 53.93 μmol/L (reference range 0-0.4 μmol/L), homocysteine of 81.7 mcmol/L (reference range 3.7-13.9 mcmol/L), and ammonia of 115 mcmol/L (reference range 11-32 mcmol/L). Vitamin B12 was 478 pg/mL (reference range 247-911 pg/mL), zinc was 49 μg/dL (reference range 60-120 μg/dL), and biotin was 763.3 pg/mL (reference range 221.0-3004.0 pg/mL).
The lab findings were consistent with methylmalonic acidemia from cobalamin C deficiency, and the patient was started on treatment for this correctable metabolic encephalopathy. He was treated with hydroxocobalamin intramuscularly 2 mg/day, betaine 3 g twice daily (to promote the conversion of homocysteine to methionine through the betaine-homocysteine methyltransferase), L-carnitine 800 mg every 4 hours, vitamin B6 12.5 mg/day, and folic acid 5 mg/day (to promote the remethylation pathway). These agents were used to bypass the defective enzyme and avoid buildup of toxic metabolites.
For his hyperammonemia, he was given lactulose 15 mL twice a day, as well as metronidazole 500 mg 3 times a day to reduce gut flora that might produce propionic acid, which cannot be metabolized.1 His homocysteine levels diminished with treatment from 81.7 mcmol/L to 54.7 mcmol/L to 44.1 mcmol/L, and his cutaneous findings improved considerably (Fig 4).
Fig 4.
Healing of dermatitis on buttocks of patient upon treatment of cobalamin C deficiency.
Within days of therapy, the patient was remarkably more alert, responding to commands with few words. Clinical improvement paralleled the improvement in homocysteine levels. On subsequent hospital admission, he showed continued progress, communicating with more complex language.
Discussion
Increased MMA and homocysteine levels and a normal vitamin B12 level is the classic pattern of cobalamin C disease.2 The disorder has been traced to the MMACHC gene located on chromosome 1P. The patient underwent genetic testing, and MMACHC gene sequencing confirmed this defect showing 2 pathogenic variants: nucleic acid change c.352delC, resulting in the heterozygous amino acid alteration p.G1n118fs, and nucleic acid change c.482G>A, resulting in the heterozygous amino acid alteration p.Arg161Gln. Together these 2 variants resulted in methylmalonic aciduria and homocystinuria, cobalamin C type.
MMA consists of a group of rare autosomal recessive disorders that affect the ability to metabolize branched chain amino acids. Combined methylmalonic aciduria with homocystinuria is characteristic of cobalamin C deficiency, the most common inborn error of cobalamin metabolism. The early-onset type is more severe, often presenting in infancy; the late-onset is more rare but subtle and progressive in onset. Like our patient, the late-onset type often presents with behavioral and psychiatric manifestations, frequently with progressive encephalopathy, delirium, dementia, and gait disturbances.2 Thus, the diagnosis can be difficult and often delayed or missed. Patients might develop skin involvement concurrent with the development of the systemic symptoms. The skin involvement in cobalamin C disease has been reported to present in 1 of 2 patterns of erosive desquamative dermatitis: resembling staphylococcal scalded skin syndrome or a diffuse erythema with superficial erosions, desquamation, and cheilitis resembling acrodermatitis enteropathica.3 Our patient did not fit either of these classic patterns and instead showed a distinctive, brown shellac-like desquamation.
Nutritional deficiencies can also have characteristic skin findings with intertriginous involvement (Table I).4 Zinc deficiency manifests as acrodermatitis enteropathica with symmetric, intertriginous dermatitis, specifically around the perineum, along with treatment-resistant seborrheic dermatitis, and diarrhea. Niacin (vitamin B3) deficiency or pellagra presents with photo-distributed dermatitis that can have a parchment-like appearance, neuropathy, diarrhea, and dementia. Biotin (vitamin B7) deficiency can also manifest with severe seborrheic dermatitis and changes in periorificial, perianal, and intertriginous locations.4 A seborrheic dermatitis might be seen in hyperammonemia, which can also be associated with seizures, ataxia, depression, lassitude, nausea, and vomiting. Similarly, deficiencies in riboflavin (vitamin B2), pyridoxine (vitamin B6), biotin, and essential fatty acids all manifest with intertriginous dermatitis, and such findings should prompt a consideration of nutritional disease.4 We checked biotin levels in our patient as long-term use of antiepileptic medication has been associated with biotin deficiency.5
Table I.
Metabolic and nutritional deficiencies with intertriginous skin involvement
| Deficiency | Dermatological findings | Systemic findings | Diagnostic work-up | Treatment recommendations |
|---|---|---|---|---|
| Zinc | Symmetric dermatitis of acral, perioral, and intertriginous areas, including perineum; recalcitrant seborrheic dermatitis; alopecia; paronychia | Anorexia, dysgeusia, impaired wound healing, immune deficiency, photophobia, gastrointestinal upset | Plasma zinc (drawn in morning given diurnal variation in levels); low alkaline phosphatase (a zinc-dependent enzyme) might be helpful finding | Genetic form: 3 mg/kg po/IV zinc lifelong; acquired form: 0.5-1.0 mg/kg po zinc |
| Niacin (vitamin B3) | Photo-distributed dermatitis with perianal and personal inflammation and erosions; cheilitis; atrophic glossitis | Peripheral neuropathy, anorexia, irritability, abdominal pain, dementia (late) | Urinary excretion of niacin metabolites; serum niacin levels unreliable | Mild cases: 50 mg po nicotinic acid tid; advanced cases: 50-100 mg IM nicotinic acid 3-4 days then same dose orally |
| Biotin (vitamin B7) | Seborrheic dermatitis-like rash of scalp, perioral, flexural perianal areas; alopecia | Depression, anorexia, paresthesias, hypotonia, seizures, ataxia | Urinary biotin metabolites; serum biotin levels unreliable | Children: 5-20 mg/day po/IM biotin; adults: 10-40 mg/day po/IM biotin |
| Cobalamin C | SSSS-like erosive desquamative dermatitis or diffuse erythema with superficial erosions and cheilitis or brown shellac-like desquamation favoring intertriginous skin | Early onset: developmental delay, hypotonia, seizures, visual defects; Late-onset: behavioral disturbances, progressive encephalopathy, dementia, abnormal gait | Newborn screening; serum methylmalonic acid; serum homocysteine; serum ammonia; biochemical and molecular testing | Hydroxocobalamin 2 mg IM QD, betaine 3g bid, L-carnitine 800 mg Q4hr, vitamin B6 12.5mg po QD, folic acid 5 mg po QD |
bid, Twice a day; IM, intramuscularly; IV, intravenously; po, oral; Q, every; QD, every day; SSSS, staphylococcal scalded skin syndrome; tid, 3 times a day.
Although cobalamin C deficiency is the most common inherited disorder of vitamin B12 metabolism and the most common form of MMA, the late-onset form displays a broad phenotypic spectrum of varied neuropsychiatric manifestations. Screening occurs with plasma amino acids, total plasma homocysteine level, and urine organic acids, with the diagnosis confirmed by findings of hyperhomocysteinemia, low plasma methionine, and elevated MMA.6 Final confirmation occurs by genotyping of the MMACHC gene.6 Recent developments in newborn screening allow for early detection of inborn errors of metabolism and have been mandated in Texas since 2015.7 Our patient was not screened because the mandate was implemented years after his birth.
Early detection of MMA cobalamin C is essential because patients respond well to hydroxocobalamin therapy, often with early neurologic improvement and resolution of systemic symptoms. Changes seen on magnetic resonance imaging have also been shown to be reversed with treatment.2
By increasing awareness that skin eruptions resembling acrodermatitis enteropathica can be an initial systemic sign of an inborn error of metabolism, physicians can diagnose and potentially limit resultant neuropsychiatric damage. This particularly distinctive, brown shellac-like erosive desquamation should raise suspicion for aminoacidopathies.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Fraser J.L., Venditti C.P. Methylmalonic and propionic acidemias: clinical management update. Curr Opin Pediatr. 2016;6:682–693. doi: 10.1097/MOP.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurkas E., Kartal A., Aydin K. Reversible clinical and magnetic resonance imaging findings in late-onset cobalamin C defect. Genet Couns. 2015;26:425–430. [PubMed] [Google Scholar]
- 3.Howard R., Frieden I.J., Crawford D. Methylmalonic acidemia, cobalamin C type, presenting with cutaneous manifestations. Arch Dermatol. 1997;133:1563–1566. [PubMed] [Google Scholar]
- 4.Lakdawala N., Grant-Kels J. Acrodermatitis enteropathica and other nutritional diseases of the folds (intertriginous areas) Clin Dermatol. 2015;33:414–419. doi: 10.1016/j.clindermatol.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Schulpis K.H., Karikas G.A., Tjamouranis J., Regoutas S., Tsakiris S. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42(10):1359–1362. doi: 10.1046/j.1528-1157.2001.47000.x. [DOI] [PubMed] [Google Scholar]
- 6.Rahmandar M.H., Bawcom A., Romano M.E., Hamid R. Cobalamin C deficiency in an adolescent with altered mental status and anorexia. Pediatrics. 2014;134:e1709–e1714. doi: 10.1542/peds.2013-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.All Texas Newborns Are Screened for These Disorders.https://www.dshs.texas.gov/newborn/screened_disorders.shtm. N.p., 22 Feb. 2017. Web.