Introduction
Locally advanced or metastatic basal cell carcinoma (BCC) has traditionally been difficult to treat. Options for unresectable tumors have been limited to cisplatin-based chemotherapy or palliative radiation therapy. With the advent of molecularly targeted drugs to the hedgehog (Hh) pathway, another option is now available. In some cases, these new drugs have already provided patients with significant increases in overall survival rates.1, 2
Vismodegib was the first drug within this group to be commercially available in January of 2012. It inhibits a transmembrane protein known as smoothened (SMO),3 which in turn down regulates the expression of Gli genes that are responsible for promoting tumorigenesis.4 Sonidegib, which targets the same SMO protein, was the second to be approved by the US Food and Drug Administration (FDA) for patients in 2015. In addition to these newly approved drugs, itraconazole, an older antifungal medication, has recently been identified as an inhibitor of the same pathway, albeit through a different mechanism, to reduce BCC tumor load in humans.5
Here we present a case of a patient with locally advanced BCC of the ethmoid sinus and brain who subsequently had resistance to vismodegib but then responded to a second round of combination treatment using sonidegib and itraconazole.
Case report
An 87-year-old white man presented to our clinic with an inoperable advanced BCC involving the sinuses and brain. Five years earlier, he was placed on vismodegib through a clinical trial for the same tumor, which at that time only involved the nasal cavity and sinuses. He responded dramatically to treatment with approximately 70% reduction in tumor size within the first 3 months. Treatment was continued for more than a year, but effects diminished over time, and because of the negative side effects of therapy, vismodegib was discontinued. The patient was then treated with electron beam radiation therapy with a total dose of 70 Gy.
Two years later, the BCC recurred, occupying the left nasal cavity, ethmoid sinus, and frontal sinus and extending into the left orbit. The patient was placed back on vismodegib for 6 months; however, the tumor continued to progress. Radiation therapy was not an option because of cumulative dose limitations to critical structures. Because the patient did not respond to an inhibitor of the Hh pathway, he was placed on a new medication with a different mechanism of action, pembrolizumab. After 3 cycles, a positron emission tomography scan showed further progression of the tumor with a new lesion identified in the right frontal lobe of the brain that was approximately 2 cm in size. Given tumor invasion into the brain, and inability to treat with further radiation therapy, hospice was discussed and offered.
The patient was interested in pursuing further medical options. Thus, he was started on 2 different Hh pathway inhibitors at the same time, sonidegib and itraconazole. Sonidegib was given as a daily dose of 200 mg. Because there was a concern for drug-drug interaction, itraconazole was pulse dosed at 100 mg/d for 2 weeks followed by a rest period for 2 weeks. This regimen for itraconazole was repeated every month. The patient was monitored with blood work and experienced no major adverse side effects.
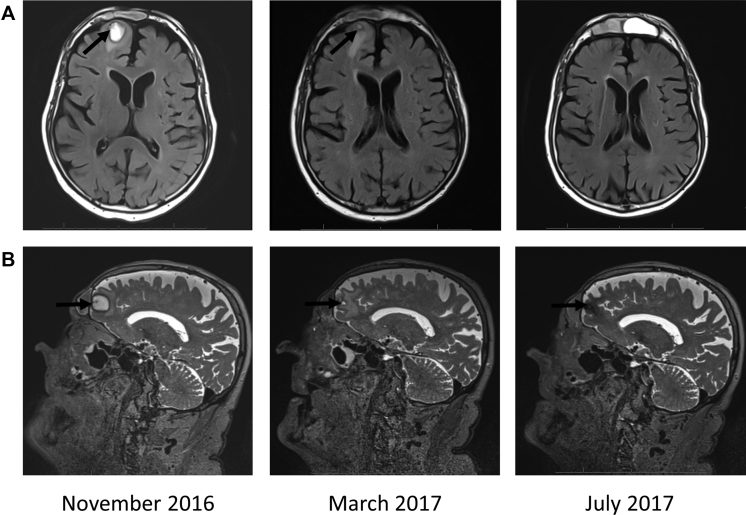
After 3 months, there was a significant improvement shown by magnetic resonance imaging (Fig 1). The frontal lobe tumor regressed while the tumor in the sinus stabilized. After approximately 8 months of treatment, the intracranial lesion, which initially measured 1.8 × 1.3 × 1.9 cm and was T2 hyperintense, was now no longer visible on the T2-weighted sequences, and the intranasal and sinus lesions were largely stable to slightly improved.
Fig 1.
Magnetic resonance imaging of advanced BCC of the frontal lobe during treatment with sonidegib and itraconazole. T2-weighted axial (A) or sagittal (B) images of the brain, face, and orbits show regression of the intracranial lesion over time. Arrow indicates the lesion within the frontal lobe.
Discussion
This report provides evidence that patients with advanced or metastatic BCC who initially do not respond to an Hh pathway inhibitor may benefit from other drugs within the same class. Often in therapy, the decision is made to try a different drug class after one fails. Our patient was given pembrolizumab after not responding to an Hh inhibitor. Pembrolizumab is a humanized monoclonal antibody that attaches and binds the programmed cell death protein 1 receptor on lymphocytes and blocks immune suppression by the tumor. It has been dramatically effective in some metastatic melanomas,6 but in this case was ineffective in halting the growth of this patient's advanced BCC. We returned to targeting the Hh pathway because it initially showed response but this time used drugs with alternate targets. Although vismodegib, sonidegib, and itraconazole all act to inhibit the action of the SMO protein, their mechanisms of action are slightly different. Vismodegib and sonidegib are distinct molecules and thought to act at different binding sites on the SMO protein. In addition, different amino acid mutations on the SMO protein are required to confer resistance to either vismodegib7 or sonidegib,8 also suggesting that they act at different binding sites. Itraconazole is believed to inhibit the Hh by preventing the transport of the SMO protein to the cilia, a mechanism known as cilial transportation inhibition.9 Future patients with advanced BCC who become refractory to one Hh therapy should be considered candidates for other drugs within this same pathway.
Combination therapy is not a new concept in medicine. This approach has been used successfully in the treatment of infectious disease, as in HIV, and traditional chemotherapy in oncology. By targeting multiple sites within the same pathway, the hope is that the desired effect is synergistic, and there is less chance of resistance. This is particularly important in cancer therapy, in which genetic and protein mutations can result in escape from drug effectiveness. Advanced BCC is no different than other cancers. Reports have already identified multiple mutations in amino acids within the SMO protein that are responsible for resistance to both vismodegib and sonidegib.7, 8 Given this observation, multiple drug therapy may be a more ideal approach for the Hh pathway. A longer-term follow-up of this patient as well as future studies may help determine if using more than one drug at a time will provide a longer-lasting response.
This patient is advanced in age with borderline kidney function, and there was some initial concern about placing him on both sonidegib and itraconazole. Itraconazole is a strong inhibitor of the cytochrome P-450 CYP3A pathway in the liver. Sonidegib is metabolized through the same system.10 We, therefore, pulsed itraconazole with a low dose of 100 mg/d, 2 weeks on and 2 weeks off. The patient tolerated this regimen well with minimal side effects. Further experimentation will determine optimal dosing combinations in the future.
Our patient had a positive response using both itraconazole and sonidegib for advanced BCC after failure of vismodegib. This observation supports that combination therapy to inhibit the Hh pathway can be well tolerated, the use of itraconazole and/or sonidegib may be an option in the treatment of BCC extension within the brain, and alternative Hh inhibitors may produce a positive response even when another has failed.
Acknowledgments
The authors thank Drs Clark C. Otley, Tri H. Nguyen, and Michael R. Migden for review, discussion, and insights with this case.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Sekulic A., Migden M.R., Oro A.E. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Migden MR, Guminski AD, Gutzmer R, et al. Randomized, double-blind study of sonidegib (LDE225) in patients (pts) with locally advanced (La) or metastatic (m) basal-cell carcinoma (BCC). Presented at the 50th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, Abstract #9009a. 2014.
- 3.Von Hoff D.D., LoRusso P.M., Rudin C.M. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 4.Tang J.Y., Mackay-Wiggan J.M., Aszterbaum M. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D.J., Kim J., Montoya K.S.J. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32(8):745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 6.Robert C., Schachter J., Long G.V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 7.Pricl S., Cortelazzi B., Col V.D. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2014;9(2):389–397. doi: 10.1016/j.molonc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amakye D., Jagani Z., Dorsch D. Unraveling the therapeutic potential of the hedgehog pathway in cancer. Nat Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 9.Kim J., Aftab B.T., Tang J.Y. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odomzo [package insert] 2015. Novartis Pharmaceuticals Corporation. East Hanover, New Jersey. [Google Scholar]