Introduction
Paraneoplastic pemphigus (PNP) is an autoimmune blistering syndrome with 5 well-described clinicopathologic phenotypes. Nguyen et al categorized these subtypes as pemphigus-like, pemphigoid-like, erythema multiforme-like, graft-vs-host-disease–like, and lichen planus–like.1 However, there is increasing recognition of PNP simulating Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Herein, we propose SJS/TEN-like PNP as a distinct subtype of PNP. We present 2 new cases of SJS/TEN-like PNP and review the previously reported cases of this subtype. Clinicopathologic factors that merit consideration for PNP in this context include a history of associated underlying neoplasia, the absence of a new drug, and histopathology indicative of chronicity or acantholysis. In patients with these features and clinical morphology typical of SJS/TEN, serologic evaluation for IgG autoantibodies against envoplakin (EP), desmoglein (DSG) 1, and DSG3 should be considered in order to exclude SJS/TEN-like PNP.
Patient 1
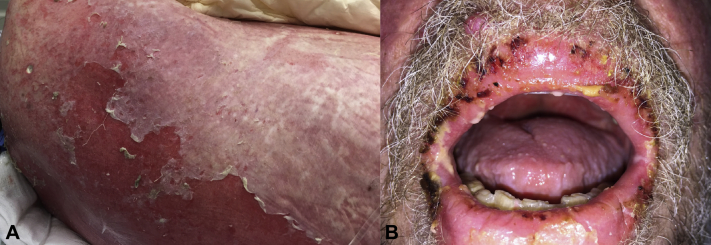
A 66-year-old man with chronic lymphocytic leukemia (CLL) had an eruption on his trunk. Aside from ibrutinib, no other medications were associated with the onset of this eruption. An initial biopsy demonstrated lichenoid interface dermatitis with keratinocyte apoptosis. Topical corticosteroids and discontinuation of ibrutinib were unsuccessful at treating the patient's condition. The eruption progressed and eventuated in the sloughing of sheets of skin covering >90% of the body surface area (Fig 1, A). A painful stomatitis and hemorrhagic erosions involving the lips were also present (Fig 1, B). After a presumptive diagnosis of TEN, the patient was admitted for in-patient management.
Fig 1.
A, Erythroderma with widespread and confluent epidermal detachment resembles toxic epidermal necrolysis. B, Severe mucositis including erosive cheilitis with hemorrhagic crust was also present.
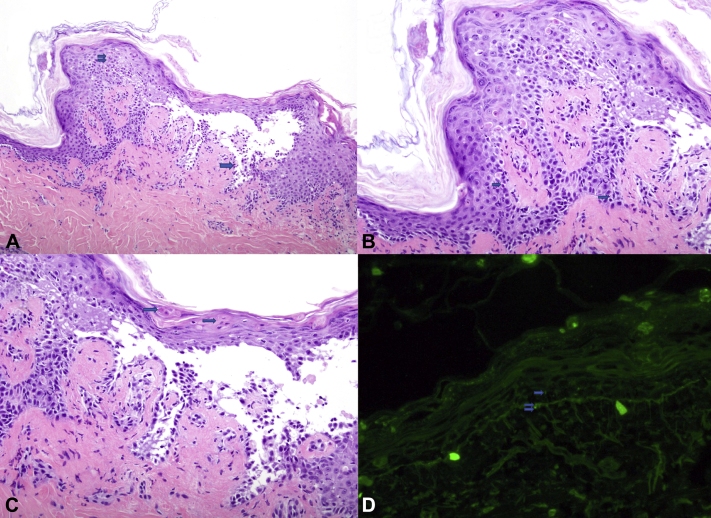
A repeat biopsy demonstrated an intraepidermal vesicle secondary to suprabasilar acantholysis associated with vacuolar interface dermatitis with keratinocyte necrosis. Parakeratosis and dysmaturation, indicative of chronicity, were also evident (Fig 2, A-C). Direct immunofluorescence highlighted cell surface and junctional reactivity with IgG (Fig 2, D). Serum studies were subsequently performed: IIF (indirect immunofluorescence) on rat bladder was nonreactive, but immunoblotting and enzyme-linked immunosorbent assay (ELISA) demonstrated positive index values for IgG against EP and periplakin (PP) and for IgG against DSG1, DSG3, and EP, respectively. These immunopathologic and serologic findings confirmed the diagnosis of PNP. Treatment was initiated with intravenous immunoglobulin 2 g/kg divided over 4 days and high-dose systemic corticosteroids; ibrutinib was restarted for the CLL underlying PNP. Despite improvement in cutaneous manifestations, the patient's hemodynamic status worsened, and he died ∼2 months after the onset of PNP.
Fig 2.
A, Suprabasilar acantholysis with tombstoning of the basilar keratinocytes (single arrow) and keratinocyte necrosis (double arrow) are present together. B, Vacuolar interface dermatitis (single arrow) underlies keratinocyte apoptosis. C, Overlying the intraepidermal blister, there is parakeratosis and dymaturation, with dyskeratotic cells in the stratum corneum (single arrow), indicative of chronicity. D, Weak focal cell surface (single arrow) and junctional (double arrow) reactivity. (A-C, Hematoxylin-eosin stain; original magnification: A, ×100; B and C, ×200.) (D, Anti-IgG direct immunofluorescence; original magnification: ×100.)
Patient 2
A 62-year-old woman with a history of untreated CLL presented for a progressive and painful eruption of 3 months' duration, which began on a lower extremity and then spread to the trunk and upper extremities. No triggering medication could be elicited. Management with topical and systemic corticosteroids (prednisone 50 mg/day) was unsuccessful at treating the condition.
Subsequent progression resulted in erythroderma, along with vaginal, ocular, and oral mucosal erosions. Punch biopsy revealed acantholysis and suprabasilar clefting. IIF on rat bladder demonstrated cell surface reactivity, and an immunoblot for IgG against PP and EP and ELISA for IgG against EP demonstrated positive indices, confirming the diagnosis of PNP. Treatment was initiated with a cycle of intravenous immunoglobulin in tandem with a chemotherapy regimen for CLL: rituximab, cyclophosphamide, and prednisone. Three months after her hospital discharge, the patient continues to do well, with resolution of her skin rash and control of her CLL.
Discussion
PNP is typified by painful mucositis, a polymorphic skin eruption, interface dermatitis with or without acantholysis, immunopathology involving plakin family proteins, and an underlying neoplasm, which is most frequently malignant. The mucositis usually affects the oral cavity but might affect the genital or ocular mucosae.2 Plakins, which serve as antigenic targets in PNP, include EP, PP, desmoplakin 1 and 2, and bullous pemphigoid antigen. Nonplakin antigens include DSG1 and DSG3. Bladder, a transitional epithelium, expresses plakin family proteins but not DSG1 or DSG3, which are specific to stratified squamous epithelium. Therefore, cell surface reactivity detected by IIF on rat bladder can confirm the presence of pathogenic circulating autoantibodies directed against plakins. More recently, α-2-macroglobulin-like protein 1 has been identified as an antigenic target in PNP.3 The most commonly associated underlying neoplasms are non-Hodgkin lymphoma, CLL, Castleman disease, thymoma, sarcomas, and Waldenstrom macroglobulinemia. Although bronchiolitis obliterans is a frequent cause of death in PNP,4 neither of our patients developed this pulmonary disease.
Immunoblotting, IIF, immunoprecipitation, and ELISA are serologic studies that can be used to identify the defining autoantibodies in PNP.5 Immunoblotting for EP or PP is almost 100% sensitive and is considered the current standard for diagnosis, but false positive results lower the specificity to 82%-91%.6, 7, 8 IIF on rat bladder is commonly used to confirm PNP, given a recorded specificity near 100%; however, sera from patients with SJS/TEN has been shown to react with rat bladder, making this study less useful in distinguishing these 2 conditions.8, 9 The histology of PNP is nonspecific and is lichenoid as often as it is acantholytic; both reaction patterns are present in 60% of cases, as they were in patient 1. Keratinocyte necrosis is associated with a poorer prognosis. Direct immunofluorescence has a 97% specificity for PNP when both cell surface and junctional IgG (or C3) are identified. However, the sensitivity of this finding is <50%.7, 8, 10 ELISA for EP, helpful in both patients described, demonstrates variable sensitivity (30%-100%), but high specificity (90%-100%).6, 8, 11
Five previously described cases of SJS/TEN-like PNP are described alongside the 2 patients in this series in Table I.12, 13, 14, 15, 16 In all 7 cases, an underlying neoplasm was identified, but only 3 were identified before the diagnosis of PNP. In cases with reported histopathology (6 of 7), both acantholysis and keratinocyte necrosis were identified. When performed, serology demonstrated IgG autoantibodies against EP and PP in 5 out of 7 cases.
Table I.
| Source | Age, y | Sex | Tumor | Clinical presentation | Histopathology | Serology∗ | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Yamada et al12 | 57 | M | B-cell lymphoma† | Fever; oral, genital, ocular erosions; desquamative erythroderma | Subepidermal blister; keratinocyte necrosis | DSG3+, DSG1−, rat bladder IIF+, EP+, PP+ | Corticosteroids, plasmapheresis, rituximab, IVIg | Died of MSOF |
| Hayanga et al13 | 45 | F | Follicular lymphoma† | Oral, genital, ocular erosions; desquamative erythroderma | Suprabasilar blister with acantholysis; keratinocyte necrosis | DSG3+, DSG1+ | Corticosteroids, rituximab | Discharged after 8 days, further follow-up unavailable |
| Mar et al14 | 11 | F | Inflammatory fibrosarcoma† | Dyspnea; oral, genital, and ocular erosions | Suprabasilar blister with acantholysis | DSG1+, PP+, EP+ | Corticosteroids, methotrexate, oral gold, tumor resection | Died of respiratory failure |
| Lyon et al15 | 56 | M | CLL‡ | Fever; oral erosions, erosions of 60% BSA | NR | Rat bladder IIF+ | Corticosteroids, cyclosporine | Alive and well at 2- year follow-up |
| Chorzelski et al16 | 31 | M | Castleman tumor† | Oral, genital, ocular erosions; periungual erosions and bullae | Acantholysis keratinocyte necrosis | DSG3+, DSG1+, PP+, EP+, rat bladder IIF+ | Corticosteroids, tumor resection, plasmapheresis, cyclosporine | Died of respiratory failure |
| McLarney et al, 2017 | 66 | M | CLL‡ | Oral and genital erosions; involvement of >90% BSA | Subrabasilar blister with acantholysis; keratinocyte necrosis | DSG3+, DSG1+, PP+, EP+, rat bladder IIF− | IVIg, corticosteroids, ibrutinib | Died of MSOF |
| McLarney et al, 2017 | 62 | F | CLL‡ | Oral, genital, ocular erosions; erythroderma | Suprabasilar blister with acantholysis | PP+, EP +, rat bladder IIF+§ | Corticosteroids, IVIg, rituximab, cyclophosphamide | Alive and well at 3-month follow-up |
BSA, Body surface area; CLL, chronic lymphocytic leukemia; DSG1, desmoglein 1; DSG3, desmoglein-3; ELISA, enzyme-linked immunosorbent assay; EP, envoplakin; IIF, indirect immunofluorescence; IVIg, intravenous immunoglobulin; MSOF, multisystem organ failure; NR, not reported; PNP, paraneoplastic pemphigus; PP, periplakin; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
DSG1 and DSG3 autoantibodies were assessed by ELISA, and EP and PP autoantibodies were assessed by ELISA, immunosorbent assay, or both.
Undiagnosed before onset of PNP.
Known diagnosis before onset of PNP.
Direct immunofluorescence was not performed with samples from this patient.
SJS/TEN-like PNP is challenging to distinguish from true SJS/TEN for several reasons. Although severe mucositis is common to both entities, PNP is very rare relative to SJS/TEN, which might prompt a presumptive clinical diagnosis of the more common disease. Keratinocyte necrosis and interface dermatitis are overlapping features of PNP and SJS/TEN on light microscopy; acantholysis indicative of pemphigus might not develop until PNP is advanced. An underlying neoplasm might not be recognized before the onset of mucocutaneous manifestations. Furthermore, in patients with a known history of underlying malignancy, their medication could be a confounding factor. Finally, use of disease-defining serologic studies might be limited by cost and access, and their interpretation and applications requires knowledge of pertinent sensitivities and specificities, as described above. Table II2, 5, 9, 13, 17 compares the clinical, histologic, and serologic features of SJS/TEN and SJS/TEN-like PNP: notably, IIF on rat bladder might be reactive and autoantibodies against α-2-macroglobulin-like protein 1 and PP might be identified in the sera of patients with SJS/TEN.9 In this context, the most helpful distinguishing features are chronicity by history and/or histopathology (ie, parakeratosis), acantholysis, and identification of IgG autoantibodies against EP, DSGs, or both.
Table II.
| Category | SJS/TEN | SJS/TEN-like PNP |
|---|---|---|
| Epidemiology | Common | Rare |
| History of known neoplasm | Variable | Variable |
| Common drug trigger∗ | Present | Variable |
| Mucosal involvement | Always present | Always present |
| Histopathology | Interface dermatitis with epidermal necrosis, normal stratum corneum | Interface dermatitis with epidermal necrosis,† suprabasilar acantholysis,† parakeratosis, and dysmaturation (well-established) |
| Serology‡ | PP+, rat bladder IIF+, A2ML1+, EP−, DSG1−, and DSG3− | PP+, rat bladder IIF+, A2ML1+, EP+, DSG1+, and DSG3+ |
| Involvement of other organ systems | Renal, gastrointestinal, respiratory | Pulmonary (bronchiolitis obliterans) |
| Effect of drug discontinuation | Beneficial | Not beneficial |
| Management | Discontinue offending drug, cyclosporine, corticosteroids | Treat underlying neoplasm, IVIg, rituximab |
| Prognosis and course | SJS: 1%-5% mortality TEN: 25%-30% mortality Acute and self-limited |
62%-90% mortality, chronic and relentlessly progressive |
A2ML1, α-2-Macroglobulin-like-protein 1; DSG1, desmoglein 1; DSG3, desmoglein-3; ELISA, enzyme-linked immunosorbent assay; EP, envoplakin; IIF, indirect immunofluorescence; IVIg, intravenous immunoglobulin; PNP, paraneoplastic pemphigus; PP, periplakin; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
Commonly implicated drugs include allopurinol, nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, lamotrigine, phenobarbital, phenytoin, carbamazepine, nevirapine, minocycline, sulfasalazine, and fluoroquinolones.
Either pattern, or both, might be present in representative biopsies.
DSG1, DSG3, and A2ML1 autoantibodies are assessed by ELISA, and EP and PP autoantibodies are assessed by ELISA, immunosorbent assay, or both.
In conclusion, SJS/TEN-like PNP represents a diagnostically challenging subtype. Recognition is important, given the guarded prognosis and association with internal malignant neoplasia, frequently unidentified before the onset of mucocutaneous manifestations.
Footnotes
Funding sources: Supported by the open access publishing fund at the University of Florida (http://cms.uflib.ufl.edu/ScholComm/UFOAPF).
Conflicts of interest: None declared.
References
- 1.Nguyen V.T., Ndoye A., Bassler K.D. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001;137:193–206. [PubMed] [Google Scholar]
- 2.Sehgal V.N., Srivastava G. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Int J Dermatol. 2009;48:162–169. doi: 10.1111/j.1365-4632.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 3.Kershenovich R., Hodak E., Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13:477–481. doi: 10.1016/j.autrev.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Anhalt G.J. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc. 2004;9:29–33. doi: 10.1111/j.1087-0024.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- 5.Poot A.M., Diercks G.F., Kramer D. Laboratory diagnosis of paraneoplastic pemphigus. Br J Dermatol. 2013;169:1016–1024. doi: 10.1111/bjd.12479. [DOI] [PubMed] [Google Scholar]
- 6.Powell J.G., Grover R.K., Plunkett R.W., Seiffert-Sinha K., Sinha A.A. Evaluation of a newly available ELISA for envoplakin autoantibodies for the diagnosis of paraneoplastic pemphigus. J Drugs Dermatol. 2015;14:1103–1106. [PubMed] [Google Scholar]
- 7.Choi Y., Nam K.H., Lee J.B. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012;39:973–981. doi: 10.1111/j.1346-8138.2012.01655.x. [DOI] [PubMed] [Google Scholar]
- 8.Joly P., Richard C., Gilbert D. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619–626. doi: 10.1067/mjd.2000.107488. [DOI] [PubMed] [Google Scholar]
- 9.Park G.T., Quan G., Lee J.B. Sera from patients with toxic epidermal necrolysis contain autoantibodies to periplakin. Br J Dermatol. 2006;155:337–343. doi: 10.1111/j.1365-2133.2006.07323.x. [DOI] [PubMed] [Google Scholar]
- 10.Poot A.M., Siland J., Jonkman M.F., Pas H.H., Diercks G.F. Direct and indirect immunofluorescence staining patterns in the diagnosis of paraneoplastic pemphigus. Br J Dermatol. 2016;174:912–915. doi: 10.1111/bjd.14282. [DOI] [PubMed] [Google Scholar]
- 11.Probst C., Schlumberger W., Stocker W. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin Chim Acta. 2009;410:13–18. doi: 10.1016/j.cca.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Yamada T., Nakamura S., Demitsu T. Paraneoplastic pemphigus mimicking toxic epidermal necrolysis associated with B-cell lymphoma. J Dermatol. 2013;40:286–288. doi: 10.1111/1346-8138.12083. [DOI] [PubMed] [Google Scholar]
- 13.Hayanga A.J., Lee T.M., Pannucci C.J. Paraneoplastic pemphigus in a burn intensive care unit: case report and review of the literature. J Burn Care Res. 2010;31:826–829. doi: 10.1097/BCR.0b013e3181eed4b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar W.A., Glaesser R., Struble K., Stephens-Groff S., Bangert J., Hansen R.C. Paraneoplastic pemphigus with bronchiolitis obliterans in a child. Pediatr Dermatol. 2003;20:238–242. doi: 10.1046/j.1525-1470.2003.20311.x. [DOI] [PubMed] [Google Scholar]
- 15.Lyon C.C., Carmichael A.J. Toxic epidermal necrolysis and paraneoplastic pemphigus. Lancet. 1998;352(9122):149. doi: 10.1016/S0140-6736(98)85059-9. [DOI] [PubMed] [Google Scholar]
- 16.Chorzelski T., Hashimoto T., Maciejewska B., Amagai M., Anhalt G.J., Jablonska S. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393–400. doi: 10.1016/s0190-9622(99)70111-8. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman S., Sekula P., Venhoff M. Systemic Immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis. JAMA Dermatol. 2017;153(6):514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]