Introduction
Chronic arsenic exposure is rare in developed nations. The few cases of arsenical keratosis in the United States have primarily been attributed to occupational and medicinal exposures.1 Here, we describe a case of an elderly man in whom arsenical keratoses and cutaneous cancers developed decades after being treated with oral Fowler solution, for the treatment of acne during adolescence.
Case
A man in his 80s had a 45-year history of numerous erythematous scaly papules and plaques on his trunk and extremities (Fig 1, Fig 2, Fig 3). Previously, the patient had undergone left hemiscrotectomy that showed T2 multifocal, invasive, moderately differentiated squamous cell carcinoma (SCC). He was born and raised in the United States and reported a history of acne vulgaris in his young adulthood that was treated with oral Fowler solution for many years.
Fig 1.
Numerous scattered erythematous scaly papules and plaques, as well as hypopigmented patches, on the torso of patient with arsenical keratosis.
Fig 2.
Numerous scattered erythematous scaly papules and plaques on back of patient with arsenical keratosis.
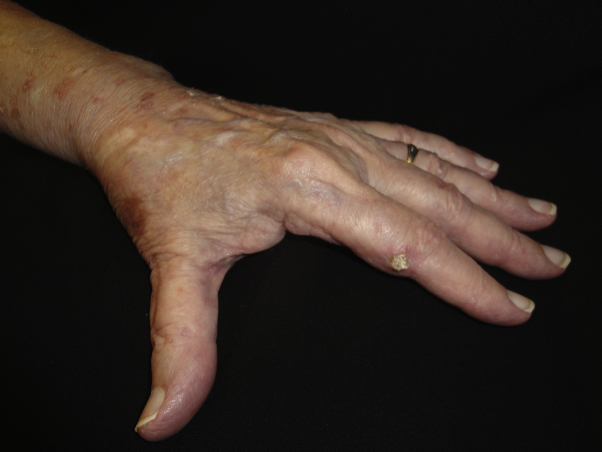
Fig 3.
Thick hyperkeratotic papule on volar aspect of second digit of patient with arsenical keratosis.
Physical examination revealed an otherwise healthy elderly white man with numerous thick, yellow scaly papules and plaques on an erythematous base on the face, neck, chest, back, arms, hands, thighs, buttocks, legs, and feet. He also had well-circumscribed hypopigmented macules and patches scattered over his torso and lower arms.
A shave biopsy specimen that was obtained from a lesion on his back revealed thick, compact parakeratotic hyperkeratosis and acanthosis with lack of nuclear maturation; hyperchromatic and pleomorphic nuclei; and occasional mitoses. There was also involvement of the papillary and reticular dermis with an inflammatory infiltrate in the dermis. These findings were consistent with the diagnosis of invasive SCC (Fig 4).
Fig 4.
Hematoxylin-eosin stain consistent with invasive squamous cell carcinoma.
After a lengthy discussion of treatment and prevention strategies, the patient opted to undergo surgical excision of the skin cancers and a trial of nicotinamide for chemoprevention instead of oral retinoids due to its affordability and better side effect profile.2
Discussion
The 3 main sources of chronic arsenic exposure are environmental, medicinal, and occupational hazard.1 Of these, environmental arsenic contamination through groundwater is by far the most common cause of chronic arsenic poisoning worldwide.1 Chronic arsenic exposure is very rare in developed nations and the cases of arsenical keratosis in the United States have primarily been attributed to occupational and medicinal exposures.1 During the early 20th century, the most common arsenic-containing preparation in Western medicine was called Fowler solution, which contained inorganic trivalent arsenic (1% potassium arsenite).3 This medication was used topically and orally to treat various dermatologic disorders and inhaled to treat asthma.4 Today, medicinal arsenic is only approved by the Food and Drug Administration for the treatment of relapsed or refractory acute promyelocytic leukemia.
Unlike acute arsenic poisoning, which might become symptomatic and even fatal within hours to days, chronic arsenic exposure has an insidious course and might take 30-50 years before becoming clinically apparent.1 Despite the insidious nature, the chronic form can be equally as devastating as the acute form. Indeed, chronic exposures to inorganic arsenic, especially in trivalent form, has been associated with malignancies of the skin, lung, liver, urinary tract, and other organs.5 Because the earliest and most common manifestations are often dermatologic, dermatologists play a pivotal role in making an early diagnosis. A frequent dermatologic sign of chronic arsenic exposure is cutaneous melanosis of the trunk, which is characterized by hyperpigmented patches often affecting the nipples and skin folds. Guttate hypopigmentation or even depigmentation might overlie these hyperpigmented patches resulting in leucomelanosis and is classically described as raindrops on a dusty road.4, 5 Arsenical keratoses might also develop and are characterized as multiple, small (2-10 mm), yellowish, papules, which can appear on sites prone to friction or trauma. While the palms and soles are most commonly affected, arsenical keratoses can appear anywhere on the body including the trunk, scrotum, and other nonexposed sites. Over time, these lesions can enlarge and coalesce into verrucous or corn-like plaques and might undergo malignant transformation, usually in the form of SCC.4 Of note, arsenic exposure has also been associated with an increased risk for basal cell carcinoma. Cutaneous malignancies in these patients are frequently found in multiples and can involve both exposed and nonexposed areas of the body, which differs from patients who do not have a history of arsenic exposure as they typically present with singular cutaneous malignancies on exposed parts of the body.4, 5
Although there are no randomized controlled trials that specifically investigate chemoprevention and therapeutic options for cutaneous malignancies in patients who have a history of chronic arsenic exposure, the standard of care includes oral retinoids for chemoprevention and surgical treatment of cutaneous malignancies. In 2015, a phase 3 randomized clinical trial successfully showed that oral nicotinamide (an amide form of vitamin B3) was safe and effective in reducing the rates of new nonmelanoma skin cancers and actinic keratosis in high-risk patients.2 Further studies are needed to determine the efficacy of this treatment in patients with chronic arsenic exposure.
Acknowledgments
We would like to thank the patient for allowing us to publish this information.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Guha Mazumder D.N. Chronic arsenic toxicity & human health. Indian J Med Res. 2008;128(4):436–447. [PubMed] [Google Scholar]
- 2.Chen A.C., Martin A.J., Choy B. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 3.Bates M.N., Smith A.H., Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992;135(5):462–476. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz R.A. Arsenic and the skin. Int J Dermatol. 1997;36(4):241–250. doi: 10.1046/j.1365-4362.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Pratt M., Wadden P., Gulliver W. Arsenic keratosis in a patient from Newfoundland and Labrador, Canada case report and review. J Cutan Med Surg. 2016;20(1):67–71. doi: 10.1177/1203475415599342. [DOI] [PubMed] [Google Scholar]