Abstract
Objective
To evaluate the ability of the eluate from fibrin-rich plasma (FRP) membrane to induce proliferation and differentiation of isolated human adipose-derived stem cells (ASCs) into chondrocytes.
Method
FRP membranes were obtained by centrifugation of peripheral blood from two healthy donors, cut, and maintained in culture plate wells for 48 h to prepare the fibrin eluate. The SCATh were isolated from adipose tissue by collagenase digestion solution, and expanded in vitro. Cells were expanded and treated with DMEM-F12 culture, a commercial media for chondrogenic differentiation, and eluate from FRP membrane for three days, and labeled with BrdU for quantitative assessment of cell proliferation using the High-Content Operetta® imaging system. For the chondrogenic differentiation assay, the SCATh were grown in micromass for 21 days and stained with toluidine blue and aggrecan for qualitative evaluation by light microscopy. The statistical analysis was performed using ANOVA and Tukey's test.
Results
There was a greater proliferation of cells treated with the eluate from FRP membrane compared to the other two treatments, where the ANOVA test showed significance (p < 0.001). The differentiation into chondrocytes was visualized by the presence of mucopolysaccharide in the matrix of the cells marked in blue toluidine and aggrecan.
Conclusions
Treatment with eluate from FRP membrane stimulated cell proliferation and induced differentiation of the stem cells into chondrocytes, suggesting a potential application of FRP membranes in hyaline cartilage regeneration therapies.
Keywords: Platelet-rich plasma, Membranes, Cartilage, Regeneration
Resumo
Objetivo
Avaliar a capacidade do eluato proveniente da membrana de plasma rico em fibrina (PRF) de induzir proliferação e diferenciação das células-tronco humanas isoladas de tecido adiposo (CTDAh) em condrócitos.
Método
As membranas de PRF foram obtidas por centrifugação de sangue periférico de dois indivíduos saudáveis, cortadas, colocadas em poços de placa de cultivo por 48 h para obtenção do eluato de fibrina. As CTDAh foram isoladas do tecido adiposo por digestão com solução de colagenase e expandidas in vitro. As células foram expandidas e tratadas com meio de cultivo DMEM-F12, meio comercial para diferenciação condrocítica, e eluato de fibrina durante três dias e marcadas com BrdU para avaliação quantitativa da proliferação celular com o uso do sistema de imagens High-Content Operetta®. Para o ensaio de diferenciação condrogênica, as CTDAh foram cultivadas em micromassa por 21 dias e coradas com azul de toluidina e agrecana para avaliação qualitativa em microscópio óptico. As avaliações estatísticas foram feitas por meio dos testes Anova e Tukey.
Resultados
Houve uma maior proliferação das células tratadas com o eluato de fibrina comparativamente com os outros dois tratamentos, nos quais o teste Anova apontou significância (p < 0,001). A diferenciação em condrócitos foi visualizada pela presença de mucopolissacarídeos na matriz das células tratadas com meio de diferenciação ou eluato e marcação positiva para agrecana.
Conclusões
O tratamento com o eluato da membrana de fibrina estimulou a proliferação celular e induziu a diferenciação das células-tronco em condrócitos, o que sugere uma potencial aplicação da membrana de PRF nas terapias de regeneração de cartilagem hialina.
Palavras-chave: Plasma rico em plaquetas, Membranas, Cartilagem, Regeneração
Introduction
Among the degenerative diseases that affect the elderly, degradation of articular cartilage tissue, a process known as osteoarthritis or osteoarthrosis (OA) is one of the most common.1
The functionality of the cartilage depends on the integrity of its extracellular matrix (ECM) and on the arrangement of its molecular components. The susceptibility of articular cartilage to progress to OA is due to its limited autoregeneration capacity, caused by the low mitotic activity of chondrocytes and their avascular nature.2, 3
In large weight-bearing joints that are subjected to friction, cartilage defects do not regenerate spontaneously and require therapeutic intervention. Conventional treatment for the repair of cartilage defects, such as non-surgical approaches (e.g., glucosamine, steroids, and hyaluronic acid injections) or surgical treatment (e.g., debridement) only relieve pain and do not restore the joint surface.4 Therefore, traditional techniques are palliative. Washing and chondroplasty promote symptomatic relief of pain without hyaline tissue formation. These techniques remove the superficial layer of the cartilage, including the collagen fibers, which are responsible for the mechanical resistance and create a cartilage tissue with inferior functionality. The subchondral debridement or microfracture technique has been considered as a stimulant for the production of hyaline-like tissue, whose properties and durability are comparable to that of normal cartilage. However, in many cases a formation of fibrocartilaginous tissue that degenerates over time has been observed. Autologous osteochondral transplantation and mosaicplasty (cartilage autotransplantation) may restore cartilage tissue, but its applications are restricted to small defects; there are also some concerns regarding morbidity of the donor site.5 The treatment of OA and local articular cartilage defects remains challenging. There are currently no surgical or non-surgical treatments that repair or restore the damaged surface.
Accordingly, current treatment methods do not provide a satisfactory long-term outcome; this fact has stimulated studies into innovative approaches in tissue engineering with the use of biomaterials as frameworks for new tissue formation.6, 7 Some of the biomaterials are natural and autologous, such as a membrane of fibrin-rich plasma (FRP). The fibrin membrane can be readily isolated from plasma from the patient's peripheral blood through centrifugation, when a dense FRP membrane is formed and can be readily used after exudation. The FRP exudate contains significant amounts of growth factors in addition to the glycoprotein matrix, particularly fibronectin and vitronectin, two key proteins that allow extracellular and cell-matrix contact.8
Fibrin membrane, therefore, is an autologous natural biomaterial that is rich in glycoproteins and growth factors, easy to prepare, and inexpensive. Due to its autologous nature, there is no risk of infection or onset of autoimmune processes. Therefore, this study is aimed at evaluating the ability of FRP membrane to induce proliferation and differentiation of human stem cells, derived from adipose tissue, into chondrocytes.
Materials
This study was approved by the Research Ethics Committee of PUC-PR, under opinion No. 1,348,208.
Peripheral blood was collected from two healthy volunteers to obtain the fibrin membrane. The participants were chosen according to the inclusion criteria: age between 18 and 55 years; minimum of 54 kg body mass; no chronic or autoimmune diseases; non-smokers; and no use of aspirin or other drugs that may interfere with the coagulation process in the last two weeks. The exclusion criteria were as follows: pregnancy; presenting with arterial and/or peripheral venous insufficiency; or continuous use of anticoagulant drugs.
Human stem cells derived from adipose tissue (ASC) were obtained by liposuction surgery from a single donor, from which they were isolated and expanded. The donor accepted to donate and signed an Informed Consent Form.
Methods
Preparation of fibrin membranes and eluate
A total of 80 mL of blood from the antecubital (superficial) vein of each participant was taken with the aid of a Scalp 21G device (BD Vacutainer®) for filling eight 10-mL plastic tubes, properly sterilized and identified with codes, for vacuum blood collection (BD Vacutainer®). The tubes contained no anticoagulants, but did contain a clot activator. The collected blood was immediately centrifuged (Spinlab digital centrifuge) at 770 × g for 12 min at a controlled room temperature of 20 °C. After centrifugation, the FRP clot was removed with sterile tweezers and, with a sterile scissors, the small part of red cells adhered to its end was removed. The clots were then pressed with a sterilized stainless steel plate (Box PRF BmdCon®) for serum withdrawal (exudate extraction). The FRP membrane was then obtained.9 The membranes were used immediately after their production to obtain the eluate, in order to prevent any protein degeneration, which could occur in a maximum of 3 h.
Eight FRP membranes from the two participants, once extracted and exuded, were cut with a sterile biopsy punch (No. 6 mm Miltex®) into three 6 mm circles and placed in 6-well culture plates (GreinerBio-One). Then, 5 mL of DMEM-F12 culture medium without SBF was added to each well. The supernatants were removed from the plates after 48 h, transferred into 50-mL tubes, and stored at temperatures between 2 and 8 °C.10 In this way, the eluate from each individual was obtained.
Isolation and expansion of stem cells derived from human adipose tissue (ACS)
The vial with 50 mL of the adipose tissue (AT) was processed in a laminar flow, using the enzymatic digestion method in accordance with Rebelatto et al.11 In this process, the AT was washed three times with 150 mL of phosphate saline solution (PBS – Gibco™ Life Technologies, Grand Island, USA) and dissociated with type I collagenase (1 mg for each mL of fat; Gibco™ Invitrogen, NY, USA) for 30 min at 37 °C, with constant stirring. After digestion, the lower liquid part was removed and filtered with cell strainer (100-μm mesh, BD Falcon, BD Biosciences Discovery Labware, Bedford, USA). The cell suspension was centrifuged at 800 × g for 10 min, and the contaminating erythrocytes were removed after lysis with a pH 7.3 buffer.12 Cells were washed and re-filtered with cell strainer (40-μm mesh, BD Falcon™, BD Biosciences Discovery Labware, Bedford, USA). The resulting cells were cultured at 1 × 105 cells/cm2 density in T75 culture flasks (TPP, Trasadingen, Switzerland) in DMEM-F12 medium (Gibco™ Invitrogen, NY, USA), supplemented with 10% fetal bovine serum (Gibco™ Invitrogen, NY, USA), 1% penicillin (100 units/mL), and streptomycin (100 μg/mL; Gibco™ Invitrogen, NY, USA).
The ASCs were stored in an incubator with 5% CO2 tension, 37 °C, and 95% humidity. After 72 h of culturing, the non-adherent cells were removed and discarded. The medium was exchanged two times a week, and the cells were stored until reaching confluence between 80% and 90%. Subsequently, the cells were dissociated (detached from the bottom of the flask) with 0.25% trypsin/EDTA (Invitrogen™, NY, USA) and re-plated into other culture flasks, which characterized the first pass (P1). After the third dissociation (P3), the cells were suspended in DMEM-F12 medium with 15% SFB and counted in Neubauer's chamber.
ACS cultivation with FRP eluate
A total of 3000 cells per well were distributed in 96-well culture plates (GreinerBio-One) with DMEM-F12 medium and 15% FBS, which were stored in an incubator with 5% CO2 tension for 12 h for cell adhesion.
After 12 h of culture, the medium was removed with serum and serum-free medium (SBF-free) was added for cell starvation, in order to avoid interference of serum growth factors in cell proliferation. After 24 h, 150 μL of the eluate from the membranes obtained from the two donors were added into each well (test wells), and 150 μL of SBF medium into the control wells. Cells were cultured for three days. The membrane eluate and culture medium were changed daily.13 After three days of cultivation, bromodeoxyuridine (BrdU) staining was performed to assess cell proliferation.
For the cell proliferation test, the cells were plated in technical sextuplicates. The results with total number of nuclei and percentage of BrdU-stained cells were plotted as the mean of the technical replicates.
Cell staining with BrdU and evaluation of cell proliferation
After three days of cultivation, BrdU staining was performed for 24 h to evaluate cell proliferation. To this end, 50 μL per well of BrdU (Eugene, Life Technologies, Oregon, USA) at 100 μM concentration were added. After 24 h, cells were washed with PBS and fixated with 4% paraformaldehyde (Sigma–Aldrich, St. Louis, USA) for 30 min, at room temperature. Once fixated, the cells were washed with PBS and, subsequently, with distilled water, and agitated for 5 min. The cells were then washed twice with HCl 2 N at 50 °C and agitated for 10 min and then washed and agitated with borate buffer at 50 °C for another 10 min.
Cells were permeabilized with 0.3% TBS-Triton (Sigma–Aldrich) and agitated for 10 min. Subsequently, nonspecific sites were blocked with TBS 10 mM + 5% goat serum + 1% bovine albumin + 0.1% Triton and agitated for 1 h. The cells were then incubated with BrdU antibody conjugated with AlexaFluor 488 (1:200 dilution; Life Technologies, OR, USA) for 1 h, at room temperature. After the staining process, the cells were washed again with PBS.
For visualization of the nucleus, DAPI (1 μg/μL) (Eugene, Life Technologies, OR, USA) was used for 5 min at room temperature. The cells were then washed with PBS and distilled water.
For the cell proliferation assay, the 96-well plate was scanned with the Hight-Content Operetta imaging system (kindly provided by Perkin Elmer®) with 10× magnification; nine fields of view/well were evaluated. The images were analyzed using the Harmony 3.5.2 software (Perkin Elmer®).
Specific markers were used, and the Hight-Content Operetta® imaging system evidenced the nuclei found in each well of the cell culture dish. How markers were used: DAPI was used as a control for cell nuclei staining; BrdU + anti-BrdU, where BrdU is incorporated into the thymine of the DNA of the proliferating cells and therefore indicates the cells in cell division; and only anti-BrdU was used as negative control, a secondary antibody with fluorophore (green) when bound to BrdU.
The data were analyzed with the software Graphpad Prism version 7, with the ANOVA test, followed by Tukey's test. Differences were considered to be statistically significant when p ≤ 0.05. Data were expressed as the total number of stained nuclei and the percentage of proliferating cells during the BrdU pulse.
Assessment of the differentiation of stem cells in chondrocytes
For the chondrogenic differentiation, the ASCs were isolated and expanded as previously described. These cells were cultured in micromass. Approximately 2 × 105 cells diluted in 0.5 mL culture medium were centrifuged at 400 × g for 10 min in a 15 mL polypropylene tube to form the cell pellet. The cells were cultured for 21 days under three conditions: commercial chondrogenic differentiation medium (Differential Basal Medium Chondrogenic; Lonza, Walkerville, USA), DMEM-F12 medium + 15% SFB (control), and fibrin eluate obtained from two individuals. For each condition, 0.5 mL of solution was added to the cell pellet, homogenized, and centrifuged again at 400 × g for 10 min. The tubes with the cells were incubated at 37 °C, with 5% CO2.
Without moving the cell pellet, the medium was changed every three days. At day 21, the cell aggregate was fixated in 10% formaldehyde for 1 h at room temperature, dehydrated in serial dilutions of ethyl alcohol, made transparent in xylol, and infiltrated with liquid paraffin. The paraffin blocks were sectioned with a microtome (4 μm) and the slides were stained using the toluidine blue staining technique (Sigma–Aldrich, St. Louis, USA) in order to expose the matrix of mucopolysaccharides; immunocytochemical staining with aggrecan antibody (Invitrogen™, NY, USA; 1:100) was used to assess the presence of extracellular matrix synthesized by chondrocytes.
Results
After the FRP membranes were extracted from the tubes, it was possible to visualize the three membrane regions identified by Dohan et al.14 as: red thrombus, characterized by an aggregation of red blood cells and platelets to the fibrin matrix; fibrin clot, an acellular area formed by fibrin matrix; and mixed area (buffy coat), an intermediate between red and white.
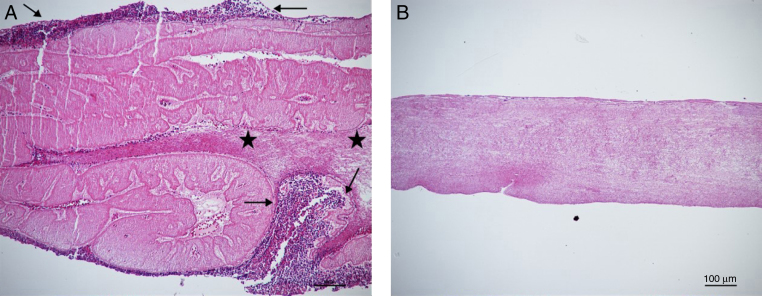
Microscopic analysis of the FRP membrane (Fig. 1) confirmed the formation of the regions observed macroscopically. Histological sections demonstrated that the lower part of the fibrin matrix (FRP) was occupied by whitish streaks (“mixed area”) and aggregates of cellular fragments (red thrombus). However, the upper part of the clot, formed by the fibrin network, did not have platelets or other cellular bodies.
Fig. 1.
Microscopic analysis of the fibrin membrane. (A) Platelet accumulation in the lower region (arrows) in transition with the mixed region, which shows whitish streaks (stars); (B) Superior area of the clot with the extensive fibrin network. Staining: Hematoxylin-Eosin. Magnified 10×.
Initially, the cell proliferation assays were made with the ASCs directly on the fibrin membrane; however, autofluorescence was observed after staining with antibodies, which prevented the counting of the cells by the Operetta® inverted infrared microscope. As an option, the technique of Gassling et al.13 was adapted, using the eluate from the fibrin membrane instead of the membrane itself.
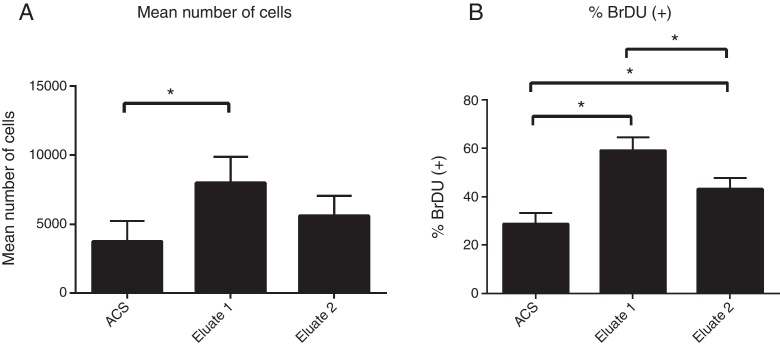
Table 1 shows the mean count of stained nuclei in each well per treatment at the time of cultivation. In the present study, it was observed that the fibrin eluate treatment was significant for the number of cells marked in relation to the control group, with p = 0.002 for the ANOVA test (n = 6). Tukey's post test indicated a significant difference between the ACS + Eluate 1 group and the other groups, as shown in Fig. 2A.
Table 1.
Total number of nuclei stained immediately after cultivation.
| Well 1 | Well 2 | Well 3 | Well 4 | Well 5 | Well 6 | |
|---|---|---|---|---|---|---|
| ACS | 1266 | 3433 | 4299 | 5372 | 5008 | 2986 |
| ACS + Eluate 1 | 5459 | 9356 | 9848 | ND | 8620 | 6699 |
| ACS + Eluate 2 | 5268 | 2967 | 6367 | 5745 | 6111 | 7171 |
Fig. 2.
Statistical results showing: (A) Total cells stained by condition of culture; (B) Percentage of 24 h proliferation cells (BrDU-stained).
Fibrin eluate treatments also presented a higher percentage of labeled nuclei in cell proliferation during the BrdU pulse (24 h) when compared with the control group (Table 2). The ANOVA test showed significance, with value of p < 0.0001 (n = 6). Tukey's post test revealed that the groups (ACS + Eluate 1 and ACS + Eluate 2) were statistically different when compared with the control and with each other, as shown in Fig. 2B. A greater cell division, and therefore, cell proliferation, was observed when ACS was in contact with the fibrin eluate.
Table 2.
Percentage of BrdU-stained cells in cell proliferation (24 h).
| Well 1 | Well 2 | Well 3 | Well 4 | Well 5 | Well 6 | |
|---|---|---|---|---|---|---|
| ACS | 37 | 28 | 26 | 27 | 26 | 32 |
| ACS + Eluate 1 | 62 | 51 | 55 | ND | 64 | 63 |
| ACS + Eluate 2 | 47 | 40 | 42 | 49 | 38 | 51 |
Microscopic analysis of the histological slides of the chondrogenic differentiation assay demonstrated that the three assay conditions (control, Gibco™ commercial differentiation medium, and fibrin eluate) were able to induce the differentiation of stem cells into chondrocytes.
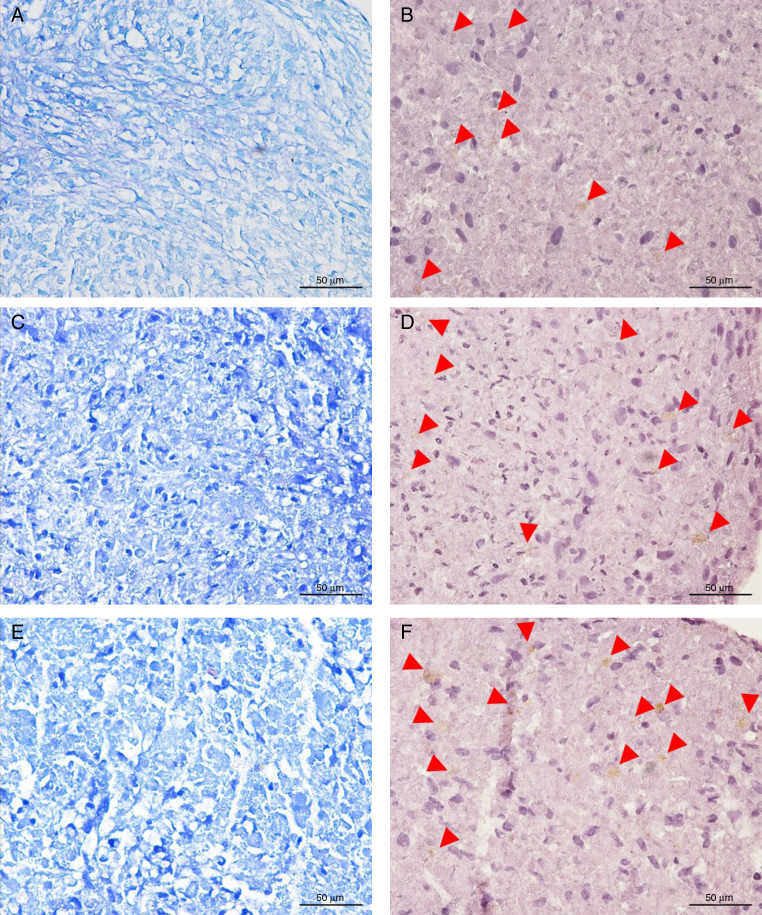
It is possible to observe in the microscopic images of Fig. 3, in toluidine blue, that cells grown in commercial differentiation medium and in fibrin eluate presented a more organized extracellular matrix (C and E), with few spaces between the cells, when compared with cells cultured only with SFB (control) medium. The control cells (A) presented a matrix with enough space between the cells and with less intense staining than the other assays (differentiation medium and fibrin eluate), which indicates less mucopolysaccharides in the extracellular matrix. The staining with toluidine blue marks acidic groups of cellular components, such as the region of the cell nucleus, and has affinity for mucopolysaccharides found in the extracellular cartilaginous matrix, such as proteoglycans and glycosaminoglycans.11
Fig. 3.
Microscopic evaluation of a chondrogenic differentiation assay: (A, C, and E) Extracellular matrix evidenced by toluidine blue staining, which shows higher intensity in the differentiation and eluate; (B, D, and F) Immunocytochemical staining for aggrecan, extracellular matrix protein, expressed by cells in chondrocyte differentiation (arrows). Magnified 40×.
Aggrecan immunocytochemical staining suggested that all assays had glycoprotein expression in the extracellular matrix. The staining was visualized with low intensity (arrows in B, D, and F), as this was a 21-day experimental cell culture. Comparing the images, it is possible to identify points that vary in tone (from light to medium) in the cytoplasm of several cells. Cells in the medium containing eluate presented aggrecan expression, and the onset of chondrocytic differentiation was confirmed by toluidine blue staining.
Discussion
After the compression exudate was removed, 3-cm2 membranes were obtained. The FRP membrane was shown to have sufficient texture and elasticity to be used as a biomaterial, as suggested by Khorshidi et al.15
In addition to these physical characteristics, Dohan et al.16 demonstrated that, after centrifugation, platelets are activated on the FRP membrane and growth factors (TGF-β, PDGF, IGF) and cytokines are gradually released. During this process, an intercellular reaction occurs between the fibrin, the cytokines, and the extracellular matrix of the involved site. Thus, FRP progressively acts on the modulation of an inflammatory tissue response, both by attracting new cells to regenerate adjacent tissue and by contributing to the defense against bacteria and other pathogens.17
In a third study, Dohan et al.,18 investigated which cytokines were present in the FRP membrane. Pro-inflammatory (IL-1, IL-6, and TNF-α) and anti-inflammatory cytokines (IL-4) were found, as well as the growth factor responsible for local angiogenesis (VEGF), resulting in an efficient response against tissue inflammation.
Gassling et al.10 compared the growth of human periosteal cells in FRP membranes with their growth in commercial collagen membranes. Their study assessed cell viability, lactic dehydrogenase (LDH) dosage biocompatibility, and cell proliferation by BrdU. The results showed that both membranes presented viable cells; apoptosis was not observed. BrdU staining indicated greater cell proliferation in FRP membranes than in collagen membranes, whereas MTT tests confirmed higher metabolic activity also for fibrin membranes.
Similar to the previous study, Gassling et al.13 proposed the use of fibrin membrane eluate to culture osteoblasts. Their study compared the compatibility and cell proliferation between fibrin and commercial collagen membranes. Their results showed a higher cellular proliferation by BrdU for the FRP membrane, as well as a greater cell differentiation in alkaline phosphatase test.
In the present study, a significant difference in cell proliferation was observed between the control group and the eluate groups; the eluate 1 group presented the highest number of nuclei in proliferation stained by BrdU. The difference in results suggests that the fibrin clot may vary (according to the donor) in platelets, cytokines, and growth factors counts, which are paramount for the cellular mechanism of cell proliferation, as membranes of two donors were produced.
The formation of the fibrin clot involves a slow polymerization of fibrin in the tube, resulting from coagulation by autologous thrombin concentrations, which influence the three-dimensional structure of the fibrin mesh obtained. The formed fibrin can assume two distinct architectures: condensed tetramolecular, with bilateral junctions, and connected trimolecular, with equilateral junctions. FRP with bilateral junctions is formed in high concentrations of thrombin, resulting in a very rigid fibrin network that does not favor the passage of growth factors to cellular medium. In turn, in a low concentration of thrombin, the fibrin network formed with equilateral junctions is thin and flexible, facilitating the infiltration of growth factors and chemotaxis of other cells to the site.16
The results of cell proliferation in contact with FRP were also corroborated by the study of Ehrenfest et al.,19 which analyzed its effect in primary culture of different human cell types (gingival fibroblasts, dermal pre-keratinocytes, pre-adipocytes, and maxillofacial osteoblasts) and demonstrated the ability of FRP to induce the proliferation of all cell types, especially osteoblasts, suggesting that growth factors promote angiogenesis, proliferation, and cell differentiation.
Xu et al. used ACS derived from mammary adipose tissue and FRP.20 These authors treated stem cells with the ginsenoside Rg1 (a sterol compound extracted from ginseng) and with FRP. Both were able to promote cell proliferation and differentiation; the expression of collagen and vascular endothelial growth factor (VEGF) was observed.
During chondrogenic differentiation, chondrocytes synthesize the extracellular matrix, while other growth factors act synergistically for this event. Froelichet et al.21 cultured ACS with a biomaterial composed of gel fibrin and polyurethane and added TGF-β and BMP-2 growth factors for 35 days. Their assay proved the ability of ACS to differentiate into chondrocytes and that the composite (fibrin + polyurethane) serves as a framework for cell growth; these authors also detected the deposition of aggrecan and glycosaminoglycan in the extracellular matrix.
Chondrocyte differentiation was also observed with the use of platelet-rich plasma (PRP). In an in vitro study, Mishra et al.22 observed that PRP was able to proliferate and differentiate stem cells into chondrocytes, using cell cycle markers (Sox-9) and structural component of the articular cartilage (aggrecan).
The influence of collagen on pre-differentiated chondrocyte micromasses was studied by Hui et al.23 From histological and immunocytochemical assays, these authors were able to observe positive staining in extracellular matrix components of the cellular micromass, such as GAG, type II collagen, and aggrecan, with high levels of expression. This finding corroborates the histological and immunocytochemical results of the present study, in which the extracellular matrix was intensely stained in toluidine blue and aggrecan, suggesting that fibrin eluate induced the stem cells to differentiate into chondrocytes.
In a cell proliferation study, Wu et al.24 demonstrated that the FRP membrane is capable of differentiating cells into osteoblasts, as well as regulating the expression of the proteins that synthesize the extracellular matrix, resulting in injured tissue regeneration.
Conclusion
The fibrin membrane extraction method (from peripheral blood) used in the present study was shown to be effective; the membranes were produced with structural characteristics similar to those described in the literature. The experimental protocol chosen, in which fibrin membrane eluate was used instead of the membrane itself, was necessary for cell visualization and counting, which is hindered by the autofluorescence of the membrane.
The growth of ACS with the addition of the fibrin membrane eluate showed a significant difference in cell proliferation in relation to the control group (p = 0.002 for the ANOVA test, with n = 6). This result demonstrates the ability of the fibrin membrane to promote tissue regeneration. Tukey's post test revealed a significant difference between the ACS + Eluate 1 group and the other groups (control and ACS + Eluate 2), as well as the fact that the physiological conditions of the participants interfered in FRP properties.
The beginning of the chondrocytic differentiation in the wells with the fibrin eluate was confirmed by the expression of glycoproteins present in the extracellular cartilaginous matrix, visualized with the toluidine blue and aggrecan staining. Therefore, the fibrin membrane is capable of inducing the differentiation of stem cells into chondrocytes and favors the regeneration of cartilaginous tissue, especially when an injection of these cells is used in a damaged joint.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Fuller R. Osteoartrose. In: Greve J.M.D.A., editor. Tratado de medicina de reabilitação. Roca; São Paulo: 2007. pp. 889–904. [Google Scholar]
- 2.Myers K.R., Sgaglione N.A., Grande D.A. Trends in biological joint resurfacing. Bone Joint Res. 2013;2(9):193–199. doi: 10.1302/2046-3758.29.2000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betsch M., Schneppendahl J., Thuns S., Herten M., Sager M., Jungbluth P. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLOS ONE. 2013;8(8):e71602. doi: 10.1371/journal.pone.0071602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei M., He F., Li J., Tidwell J.E., Jones A.C., McDonough E.B. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19(9–10):1144–1154. doi: 10.1089/ten.TEA.2012.0351. [DOI] [PubMed] [Google Scholar]
- 5.Gobbi A., Karnatzikos G., Scotti C., Mahajan V., Mazzucco L., Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286–299. doi: 10.1177/1947603510392023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge Z., Li C., Heng B.C., Cao G., Yang Z. Functional biomaterials for cartilage regeneration. J Biomed Mater Res A. 2012;100(9):2526–2536. doi: 10.1002/jbm.a.34147. [DOI] [PubMed] [Google Scholar]
- 7.Fedorovich N.E., Alblas J., Hennink W.E., Oner F.C., Dhert W.J. Organ printing: the future of bone regeneration? Trends Biotechnol. 2011;29(12):601–606. doi: 10.1016/j.tibtech.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Del Corso M., Toffler M., Ehrenfest D.M. Use of autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites. J Implant Adv Clin Dent. 2010;1(9):27–35. [Google Scholar]
- 9.Dohan Ehrenfest D.M., Del Corso M., Diss A., Mouhyi J., Charrier J.B. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81(4):546–555. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 10.Gassling V., Douglas T., Warnke P.H., Açil Y., Wiltfang J., Becker S.T. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21(5):543–549. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 11.Rebelatto C.K., Aguiar A.M., Moretão M.P., Senegaglia A.C., Hansen P., Barchiki F. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233(7):901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 12.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gassling V., Hedderich J., Açil Y., Purcz N., Wiltfang J., Douglas T. Comparison of platelet rich fibrin and collagen as osteoblast-seeded scaffolds for bone tissue engineering applications. Clin Oral Implants Res. 2013;24(3):320–328. doi: 10.1111/j.1600-0501.2011.02333.x. [DOI] [PubMed] [Google Scholar]
- 14.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Khorshidi H., Raoofi S., Bagheri R., Banihashemi H. Comparison of the mechanical properties of early leukocyte- and platelet-rich fibrin versus PRGF/endoret membranes. Int J Dent. 2016;2016:1849207. doi: 10.1155/2016/1849207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Badade P.S., Mahale S.A., Panjwani A.A., Vaidya P.D., Warang A.D. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J Dent Res. 2016;27(3):300–304. doi: 10.4103/0970-9290.186231. [DOI] [PubMed] [Google Scholar]
- 18.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: Leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51–e55. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Dohan Ehrenfest D.M., Diss A., Odin G., Doglioli P., Hippolyte M.P., Charrier J.B. In vitro effects of Choukroun1s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):341–352. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Xu F.T., Liang Z.J., Li H.M., Peng Q.L., Huang M.H., Li D.Q. Ginsenoside Rg1 and platelet-rich fibrin enhance human breast adipose-derived stem cell function for soft tissue regeneration. Oncotarget. 2016 doi: 10.18632/oncotarget.9360. PubMed PMID: 27191987 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Froelich K., Setiawan L.E., Technau A., Tirado M.R., Hackenberg S., Hagen R. Influence of different growth factors on chondrogenic differentiation of adipose-derived stem cells in polyurethane-fibrin composites. Int J Artif Organs. 2012;35(12):1047–1060. doi: 10.5301/ijao.5000132. [DOI] [PubMed] [Google Scholar]
- 22.Mishra A., Tummala P., King A., Lee B., Kraus M., Tse V. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. [Google Scholar]
- 23.Hui T.Y., Cheung K.M., Cheung W.L., Chan D., Chan B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29(22):3201–3212. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Wu C.L., Lee S.S., Tsai C.H., Lu K.H., Zhao J.H., Chang Y.C. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012;57(2):207–212. doi: 10.1111/j.1834-7819.2012.01686.x. [DOI] [PubMed] [Google Scholar]