Abstract
Epithelial surfaces line the body and provide a critical interface between the body and the external environment which is essential to maintaining the symbiotic relationship between the host and the microbiome. Tissue-resident epithelial γδ T cells represent a major T cell population in epithelia and are ideally positioned to perform barrier surveillance and aid in tissue homeostasis and repair. In this review we focus on the intraepithelial γδ compartment in the two largest epithelial tissues in the body, namely the epidermis and intestine, and provide a comprehensive overview of the crucial contributions of intraepithelial γδ cells at these sites to tissue integrity and repair, host homeostasis and host protection in the context of the symbiotic relationship with the microbiome and during pathogen clearance. Finally, we address epithelia-specific butyrophilin-like molecules and touch upon their emerging role in selectively shaping and regulating epidermal and intestinal γδ T cell repertoires.
γδ T cells are among the very first T cells to develop in the thymus. In both humans and mice, γδ T cells comprise a minor part (1–5%) of the circulating T cell compartment found in blood and secondary lymphoid organs. However, specific subsets of γδ T cells are present in much higher numbers (10–100%) in epithelial tissues such as the epidermis of the skin, the gastrointestinal tract and the reproductive track where they express tissue-specific T cell receptors that in many cases show little to no diversity1.
Epithelial γδ T cell subsets are part of a larger group of epithelial residing lymphocytes termed intra-epithelial lymphocytes (IEL)2. Epithelial tissues are comprised of a tight network of constantly renewing cells that line the body and effectively create a “wall” to the outside environment. In direct contact with the outside environment, the epithelia prevents water and nutrient loss while at the same time providing essential protection from physical damage and pathogen entry3, 4. Exposure to the outside environment also infers that the epithelium is in constant contact with the enormous amount of microbes that line our epithelial surfaces, collectively termed the microbiome. Despite profound host reliance on microbial commensals that carry out essential host beneficial functions, these potentially pathogenic microbes also pose a constant threat of invasion and therefore impose the need for tight regulation of tissue integrity and the epithelial immune response, which is mediated by the uniquely positioned IEL compartment5, 6.
Although our understanding of γδ T cell development, maturation, activation and effector function has increased within recent years, many aspects remain unknown. A major confounder to this fact has been the lack of identified epithelial γδ T cell activating antigens. Recent hints as to how molecules possibly activate and select for specific γδ T cell subsets has come from the identification of butyrophilin-like (btnl) molecules. Combined with the apparent γδ T cell regulatory capacity, the specific expression pattern of individual btnl molecules in distinct epithelial tissues such as skin and intestine has revealed a role for these molecules in shaping local γδ IEL compartments by selectively promoting maturation and expansion of tissue specific γδ T cells7–9
In this review we focus on the γδ IEL compartment in the two largest epithelial tissues in the body, namely the epidermis and intestine, with particular emphasis on the murine system, and discuss just how crucial the contributions of γδ IEL at these sites are to tissue integrity, host homeostasis and host protection in the context of the symbiotic relationship with the microbiome and during pathogen clearance. Furthermore, this review touches upon the emerging role of Butyrophilin-like (btnl) molecules in γδ T cell activation, and how the tissue specific expression of these molecules possibly contribute to shaping organ-specific γδ T cell compartments.
Epithelial tissues – Skin and intestine
Epithelial tissues of tightly linked cells collectively create a barrier to the environment both outside (e.g. skin) and inside (e.g. intestine, lungs, uterus) the body. These tissues differ from one to another in cellular composition, shape and thickness which allows for specialized needs at different anatomical sites. On the one hand, the epidermis of the skin is composed of a multi-cell layer that forms a tight but not impermeable seal that is ideal to provide protection against physical damage and water loss. In contrast, the intestinal epithelium consists of a single-cell layer which forms a leaky barrier that is essential to the exchange of nutrients and fluids. A common trait however, is the positioning of the tissue on the basement membrane and the presence of T cells throughout the tissue10,11
The skin provides a first line of defense against physical and chemical compounds as well as protecting against the many potentially pathogenic microbes that inhabit the skin. Separated by the basement membrane, skin is divided into two major compartments, the epidermis and the dermis. The epidermis is composed of four different layers of differentiating keratinocytes which account for ~95% of all cells in the epidermal compartment with constant shedding of dead cells from the outer most layer and replacement from layers below. Among the remaining 5% of epidermal residing immune cells are Langerhans cells (LC) and T cells11, 12. In naïve wild type (WT) mice the epidermal T cell compartment is dominated by a highly specialized γδ T cell subset termed dendritic epidermal T cells (DETC)13(FIG.1a). The immune cell composition of the epidermis is subject to species specific differences and thus no direct equivalent of DETC is present in human skin. However, human epidermis is home to both γδ and αβ T cells and human epidermal resident T cells show effector functions very similar to that of DETC14. The underlying dermal connective tissue is enriched for collagen that provides the structural framework for lymphatic and vascular vessels, through which migrating cells traffic to and from skin and as a consequence the dermal compartment of skin shows greater cell diversity than epidermis. Immune cells residing in the dermis under steady-state conditions include dermal subsets of dendritic cells, mast cells, innate lymphoid cells (ILC), γδ T cells, αβ T cells, B cells, macrophages and NK cells15(FIG.1a).
Figure 1. Skin and intestinal epithelial composition with immune cell distribution.
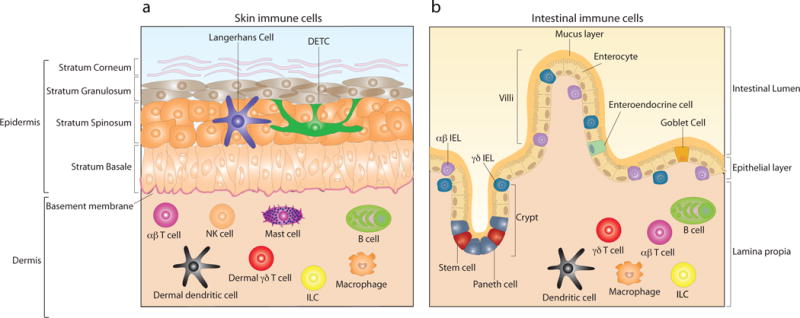
a) Skin is composed of two major compartments, the epidermis and the dermis, which are separated by the basement membrane. The outer most layer is the epidermis, which is further subdivided into four layers; stratum corneum, strata granulosum, strata spinosum and stratum basale. The cellular composition of epidermis is dominated by keratinocytes, accounting for ~95% of all cells in the epidermal compartment. Among additional cells residing in murine epidermis are Langerhans cells and dendritic epidermal γδ T cells (DETC). Due to the presence of blood and lymphatic vessels, through which circulating lymphocytes traffic to and from skin, the cellular composition of the dermal compartment is more diverse than the epidermis. Dermal residing immune cells include dermal dendritic cells, γδ T cells, αβ T cells, innate lymphoid cells (ILC), mast cells, macrophages, B cells and NK cells. b) The epithelium of the gastrointestinal tract is composed of a single cell-layer which separates the intestinal lumen from the underlying lamina propia. The intestinal epithelial layer forms both crypts and villi. Pluripotent stem cells are located at the base of crypts and give rise to 4 distinct epithelial cell subsets; enterocytes, which represent the majority of epithelial cells, Goblet cells, enteroendocrine cells and Paneth cells. Both γδ and αβ intraepithelial lymphocytes (IEL) are interspersed throughout the epithelium and are positioned both within and directly below the epithelium. Beneath the intestinal epithelium, the lamina propia is home to a plethora of immune cells which include dendritic cells, γδ T cells, αβ T cells, ILC, macrophages and B cells.
The single-cell layer of the gastrointestinal (GI) tract is not only the largest epithelial barrier but also the most vigorously dividing tissue of adult mammals with a complete cellular renewal every 4–5 days10. The architecture of the GI tract consists of crypts and villi. While crypts are located at the base of invaginations of the epithelium into the underlying connective tissue, villi form finger-like protrusions into the gut lumen that create the vast surface area of the GI tract (FIG.1b). The majority of cells in the GI tract are enterocytes which facilitate absorption of nutrients and water from the lumen. Anatomically the GI tract is divided into two major compartments, the small intestine (SI) and the colon, also known as the large intestine4, 10. Like the skin, intestinal epithelium and the underlying tissue is home to a plethora of immune cells e.g. B cells, ILC, macrophages, dendritic cells, γδ- and αβ T cells16. In contrast to mouse skin where DETC are the only T cells in the epidermis, the IEL compartment of mouse and human intestine is more comparable and both are composed of γδ and αβ T cells which are interspersed between epithelial cells and also just below the basement membrane17 (FIG.1b).
γδ T cells in the epithelial layer of skin and intestine
Mouse γδ T cell development initiates at the time of seeding of the fetal thymus at embryonic day 13 (E13) with distinct γδ T cell subsets developing in overlapping sequence and, while the earlier emerging subsets are restricted to development in the embryonic thymus, the latter are replenished from the thymus throughout life18, 19. The early emerging populations are exported to the periphery where they migrate into and populate epithelial tissue while the majority of latter developing populations circulate the blood and secondary lymphoid organs20, 21. The murine γδ T cell subsets are distinguished by their use of different Vγ usage whereas human γδ T cell subsets are often distinguished by Vδ usage. In regard to murine γδ T cells, it is important to note the existence of two different γδ nomenclatures22, 23. While we employ the Garman nomenclature22 in this review, a comparison of the two classifications is provided in Table1.
Table 1.
Comparison of the two most commonly used T cell receptor γ nomenclatures
Development and homeostasis
Expressing a canonical Vγ3Vδ1 TCR, DETC precursors are the very first T cells to develop in the mouse thymus. Vγ3+ thymocytes are solely generated during the early fetal stages of thymic development from E13–E18 and migrate to the epidermis where a defined homeostatic density is maintained throughout life24, 25. In mice, intestinal homing γδ IEL populate the gut around birth and, while capable of paring with several Vδ-genes, intestinal γδ IEL almost exclusively express a Vγ5 TCR24, 26. The developmental route of gut γδ IEL has been the subject of controversy for many years and a number of studies showing both thymic dependence and independence have been published (FIG.2).1, 27, 28. IL-7 receptor (IL-7R) signaling is necessary for V-J recombination and transcription of the TCRγ gene during thymic development and both IL-7 deficient and IL-7 receptor deficient animals are devoid of γδ T cells29–31. Vγ3+ thymocyte IL-7R signaling is mediated via the JAK/STAT pathway, evident by the complete lack of fetal thymic precursors and adult DETC in JAK3 deficient and STAT5 deficient mice32, 33. Interestingly, while DETC precursor development is independent of IL-15, this cytokine regulates the Vγ5 gene repertoire through STAT5 dependent Vγ5 specific chromatin modifications, enhancing subsequent gene rearrangement accessibility34 and STAT5 deficient mice are devoid of intestinal γδ IEL32. When seeded in the epithelium, both DETC and Vγ5+ IEL depend on a specific cytokine milieu in order to proliferate and maintain homeostatic numbers. Primarily mediated via IL-15 produced by neighboring epithelial cells, the necessity for IL-15 is apparent by the loss of tissue residing DETC and Vγ5+ IEL in IL-15 deficient and IL-15 receptor deficient mice35–39. Epithelial persistence is additionally dependent on the ligand activated transcription factor aryl hydrocarbon receptor (AhR), and while no developmental or trafficking defects are observed, AhR deficient mice show complete loss of DETC and Vγ5+ IEL in adult mice40, 41. Conditional knockout mice further clarified that epithelial maintenance of both DETC and Vγ5+ IEL is critically dependent on cell-intrinsic AhR activity41. Found in the cytosol, AhR is a highly conserved basic helix-loop-helix transcription factor. Upon ligand binding in the cytosol AhR translocates to the nucleus where it forms a dimer with the AhR receptor nuclear translocater and activates transcription of a battery of genes by binding to upstream AhR-enhancer elements16, 42, 43. The underlying cause for diminished DETC and Vγ5 IEL numbers in AhR deficient mice appear to differ between the γδ T cell subsets and while the DETC reduction is caused by a failure to proliferate when seeded in the epidermis40, Vγ5 IEL show no proliferative impairment indicating that the causative effect on Vγ5 IEL is likely a reduced survival potential41
Figure 2. Thymic development and epithelial migration.

In the fetal thymus, Vγ3Vδ1+ dendritic epidermal T cell (DETC) progenitors mature by receiving adequate stimuli through the T cell receptor and by conditioning by Skint1. Thymic DETC precursor maturation includes up-regulation of sphingosine-1 phosphate receptor 1 (S1PR1) which facilitates thymic egress. The chemokine receptor, CCR10, is also up-regulated and aids in directing the migration of DETC precursors to the epidermis through the recognition of the CCR10 ligand, CCL27, expressed by keratinocytes. Once positioned in the epidermis, DETC down-regulate CCR10 corresponding with up-regulation of the chemokine receptor CCR4. Development of intestinal γδ intraepithelial lymphocytes (IEL) in can occur both within the adult thymus and extra-thymic, and gives rise to γδ IEL expressing the chemokine receptor CCR9. Unlike DETC precursors, thymic egress of γδ IEL is independent of S1PR1. γδ IEL migration to intestinal epithelium is directed by CCR9 and the recognition of the ligand CCL25 expressed by intestinal epithelial cells which allows entry and seeding within the epithelium. An early proliferative boost of γδ IEL is then facilitated by the timely expression of butyrophilin-like molecule 1 (Btnl1) on enterocytes whereby homeostatic γδ IEL numbers are obtained.
Epithelial migration and seeding
Originally, expression of the DETC specific Vγ3Vδ1 TCR was believed essential to epidermal localization. However, later studies contradicted this belief by showing that both Vγ3 deficient and Vδ1 deficient mice have normal epidermal numbers of γδ T cells that express TCRs other than the canonical Vγ3Vδ1 DETC TCR44, 45. Although the DETC TCR ligand to this day is unknown, TCR signaling appears necessary as fetal thymic DETC precursors in TCR signaling defect mice show impaired skin homing properties46. In line with this, receiving adequate signaling in the fetal thymus correlates with increased transcription of sphingosine-1 phosphate receptor 1 (S1PR1)47–49, a receptor highly involved in thymic egress50 (FIG.2). To populate the skin, DETC are faced with the task of entering the dermal microvascular endothelium and from there extravasating and migrating into the epidermis. A crucial part in this process is the expression of E- and P-selectin ligands which bind to corresponding selectins expressed on the endothelium51 as evidenced by the greatly reduced number of DETC in skin of mice that express defective E and P selectin ligands47.
While adhesion molecules require close proximity of cells to act, leukocyte migration over large distances is largely mediated by chemokines. DETC precursors up-regulate the chemokine receptor CCR10 before exiting the thymus48, 49 and the ligand CCL27 is highly expressed by keratinocytes52. The importance of CCR10/CCL27 in directing DETC precursors into the epidermis is apparent in young mice lacking CCR10 which have severely reduced epidermal DETC numbers corresponding with dermal retention53. However, the few Vγ3+Vδ1+ T cells that are capable of migrating to the epidermis in the absence of CCR10 do expand locally and achieve normal homeostatic numbers in adult mice47, 53. Although only a minor proportion of Vγ3+ thymocytes express CCR4 in the fetal thymus, the non-redundant role of CCR4 expression on DETC precursor migration to the skin is obvious by the drastic reduction of DETC in both newborn and adult skin of CCR4 deficient mice47. Interestingly, adult DETC down-regulate CCR10 while nearly all are CCR4 positive, possibly suggesting a dynamic chemokine receptor expression on adult DETC distinct from their thymic precursors with potential importance for DETC maintenance in the skin53 (FIG.2).
Unlike for DETC, Vγ5+ IEL thymic egress is independent of S1PR154. The composition of IEL in the large and small intestine differ and while γδ IEL are found in both compartments, the small intestine is particularly enriched for γδ IEL55. Vγ5+ IEL migration to the small intestine is in part directed by CCR9 expressed on the trafficking gut γδ IEL and secretion of the specific ligand CCL25 by the small intestine epithelial cells56–59. However, overall little is known about what drives Vγ5+ IEL intestinal homing and the fact that mice lacking either CCR9 or CCL25 only show partial reduction in gut γδ IEL suggests functional redundancy with other chemo-attractant receptors and ligands58, 59. The observation that the small intestinal homing receptor CCR9 is preferentially expressed by thymocytes that are phenotypically antigen naïve, and that CCR9 expression negatively correlates with IEL TCR-ligand interaction in the thymus, further suggests that some Vγ5+ intestinal IEL develop in the absence of positive selection and that this might favor trafficking to the small intestine60 (FIG.2). In the adult mouse, small intestinal homing of newly developed Vγ5+ γδ T cells is also dependent on integrin α4β7 which mediates recirculation through the gut-associated lymphoid tissue where specific priming of Vγ5+ IEL reinforces small intestinal tropism by further inducing CCR9 and α4β7 expression61.
Tissue homeostasis and repair
Present at the very frontier and in close contact with neighboring epithelial cells, tissue-resident γδ T cells are ideally positioned to partake in the upkeep of tissue homeostasis and epithelial repair and DETC and intestinal γδ IEL share several common features that aid in maintenance of their respective epithelial barriers62–64.
Epidermal γδ T cells
In steady-state skin, DETC dendrites extend both basally and apically towards the stratum corneum and, while apical dendrites are immobile and anchor DETC to the tight junctions formed by keratinocytes, basal protrusions are highly mobile65. DETC are sessile under homeostatic conditions65,66, however their highly dendritic morphology allows for simultaneous contact with several neighboring cells e.g. keratinocytes, Langerhans cells and melanocytes, thereby increasing DETC sensitivity to tissue stress and pathology. In the absence of DETC, the epidermal compartment of mice deficient for all γδ T cell subsets (TCRδ−/−), is populated by αβ T cells with diverse TCR expression. However, gradual decline and eventual loss of these epidermal replacement T cells suggest that an antigen-responsive γδ TCR might not be necessary for T cell trafficking to the epidermis, but is necessary to maintain homeostatic numbers in the epidermis throughout life67. The necessity of a fully functional epidermal T cell compartment is evident in TCRδ−/− mice where the lack of DETC results in increased keratinocyte apoptosis due to insulin-like growth factor 1 (IGF-1) deficiency. Produced by DETC, IGF-1 also acts to reduce intrinsic apoptosis thereby providing an autocrine feedback loop aiding the upkeep of homeostatic DETC numbers in the skin64 (FIG.3a).
Figure 3. Epithelial maintenance and repair.

a) In healthy tissue, dendritic epidermal T cells (DETC) are highly dendritic with dendrites extending both basally and apically towards the stratum corneum. Maintenance of DETC homeostatic numbers is dependent upon IL-15, produced by keratinocytes, insulin-like growth factor 1 (IGF1) produced by the DETC themselves and ligand activation and cell intrinsic signaling through the transcription factor aryl hydrocarbon receptor (AhR). Additionally, IGF1 acts on keratinocytes to increase proliferation and reduce apoptosis. The sensing of stressed or damaged keratinocytes leads to DETC activation which, in vivo, is visualized by the dramatic morphological change in DETC from dendritic to round. Upon activation, DETC at the wound edge produce IGF1 and keratinocyte growth factor 1 and 2 (KGF1/2), which all facilitate keratinocyte proliferation and timely wound closure. A subset of activated DETC further produces IL-17A. Acting on keratinocytes, IL-17A induces production of the antimicrobial peptides (AMP) Regenerating islet-derived protein 3 gamma (RegIII3γ) and β-defensin thereby providing antimicrobial protection to the damaged tissue. In addition, RegIIIγ acts directly on keratinocytes to induce proliferation and mediate re-epitheliazation of wounded skin. b) Intestinal γδ intraepithelial lymphocyte (IEL) survival and retention in healthy tissue depends on cell-intrinsic AhR activation and IL-15 production by neighboring epithelial cells. In return, γδ IEL secrete KGF1 which induces intestinal epithelial cell (IEC) proliferation and increases barrier integrity, while further facilitating epithelial repair following tissue damage. Through production of tumor growth factor β-1 (TGFβ-1) γδ IEL also act to dampen the pro-inflammatory (IFNγ) and cytolytic (Granzyme B) potential αβ IEL thereby reducing additional damage and tissue destruction caused by an excessive inflammatory immune response.
Damaged or stressed keratinocytes express DETC TCR specific ligand capable of DETC activation in a non MHC-restricted manner68. The ability of DETC to respond to such damage and stress has proven crucial during wound healing where TCRδ−/− mice show a significant 2–3 day delay in wound closure caused by reduced keratinocyte proliferation and re-epithelization. Upon wounding, DETC residing in close proximity to the wound actively contribute to healing by producing numerous cytokines, chemokines and growth factors, including keratinocyte growth factors 1 and 2 (KGF1/KGF2)68–71 (FIG.3a). Similar to αβ T cells, complete activation of DETC requires coordinated interaction of molecules in addition to the TCR. However, DETC do not express the accessory molecules CD4, CD8 and CD28, which are integral to effective αβ T cell activation72. The absence of these molecules suggests that other surface expressed molecules contribute to DETC activation. Indeed, to date, DETC expression of three accessory molecules; junctional adhesion molecule-like protein (JAML)73, 74, the semaphorin CD10075 and the C-type lectin-like NKG2D receptor76 have been identified to show great importance to DETC activation and wound healing. Common for all three co-stimulatory receptors is the acute up-regulation of their respective ligands on keratinocytes at the wound margin. Thus, binding of JAML to its ligand Coxsackie and adenovirus receptor (CAR) leads to DETC proliferation and production of pro-inflammatory cytokines and KGF-1, while blocking JAML/CAR interaction leads to diminished DETC activation and delayed wound closure kinetics similar to that observed for TCRδ−/− mice74. Similarly, blocking the interaction between NKG2D and its ligand H60c significantly impairs in vivo KGF secretion by DETC and wound repair is again delayed akin to that of TCRδ−/− mice76. In situ, an early hallmark of DETC activation is the rapid rounding of these characteristically dendritic T cells63. Signaling through CD100 on DETC induces rounding via ERK kinase and cofilin and a defect in rounding is evident in the absence of CD100-mediated signals. This drastic morphological change is induced by up-regulation of the CD100 ligand PlexinB2 on keratinocytes, and possibly facilitates intraepidermal migration of activated DETC to sites of injury and provides a mechanistic explanation for the defective wound healing observed in CD100 deficient (CD100−/−) mice75.
In addition to mediating wound closure, DETC expression of NKG2D also facilitates cutaneous tumor clearance77, 78. In this regard, it is tempting to speculate on the possible involvement of JAML and CD100 in DETC mediated cutaneous tumor clearance and further investigations should be directed to clarify the possible role of these receptors and their ligands during tumor surveillance. That γδ T cells in general constitute a crucial part of the immune response against tumors is evident from a recent seminal study reporting that intra-tumoral γδ T cells are the most favorable prognostic immune population across 39 cancer types in humans79. As such, the role of γδ T cells in cancer immunity is the focus of ongoing studies by numerous groups80.
As previously mentioned, the composition of T cell subsets in the skin differs between mouse and human. In human skin, although αβ T cells dominate in both dermis and epidermis, γδ T cells are present in both compartments81. Human skin residing γδ T cells are unique in that they express a Vδ1 TCR with an oligoclonal repertoire distinct from that of circulating γδ T cells82. While the wound healing contributions of DETC in the mouse are well established, the functional capabilities and role of human epidermal γδ and αβ T cells are just beginning to be elucidated. Similar to DETC, human epidermal T cells produce IGF-1 and promote wound healing upon activation. Strikingly, both γδ and αβ T cells isolated from acute, but not from chronic wounds, actively produce IGF-1. In fact, isolated T cells from chronic wounds were found to be completely unresponsive to stimulation14. Thus, much like DETC, both epidermal γδ and αβ T cells facilitate wound closure in human skin and the unresponsive state of epidermal T cells in chronic wounds further solidifies the importance of skin-resident T cells to wound healing and possibly provide therapeutic targets for improving wound healing.
Intestinal γδ IEL
Since the intestinal epithelium is composed of a single cell-layer, γδ IEL are only able to directly interact with two epithelial cells at any given time. However, unlike DETC, intestinal γδ IEL are highly motile and migrate through the intestinal epithelium via occludin mediated cell/cell contact with epithelial cells, facilitating extensive surveillance of the intestinal epithelium83. The importance of intestinal γδ T cells to tissue homeostasis is evident by the reduced proliferation of epithelial cells in both the small intestine and colon of TCRδ−/− mice62, 84. Intestinal γδ T cells are highly capable of producing KGF-1 upon activation69. Combined with the fact that γδ IEL-derived KGF-1 expression is highest in the crypt and gradually decreases towards the tip of the villi85, the decreased proliferation and migration of epithelial crypt cells in naïve TCRδ−/− mice84 might be explained by the lack of γδ-derived KGF-1 (FIG.3b). The essential contributions of γδ IEL to intestinal homeostasis is further highlighted by the increased gut permeability attributed to a decrease in intestinal tight junctional complexes observed in TCRδ−/− mice86. This perturbation correlates with increased susceptibility to the development of spontaneous colitis in aged mice87.
A widely used and well characterized model to study intestinal epithelial repair is the Dextran Sulfate Sodium (DSS) mouse model of colitis. Treatment with DSS in the drinking water results in intestinal damage to the colon and the subsequent removal of DSS allows for studying epithelial repair. DSS-treated TCRδ−/− or KGF-1-deficient (KGF1−/−) mice experience exacerbated tissue damage and the repair of damaged epithelium is dramatically impaired62. Production of KGF-1 by γδ IEL is in part mediated through CD100 and DSS induced colitis in CD100−/− mice closely resembles that of γδ-deficient mice showing increased colon ulceration and mucosal infiltration by inflammatory cells62, 88. Gut γδ IEL also express JAML, and CAR is constitutively expressed by intestinal epithelial cells74, 89. Given that JAML ligation leads to KGF-1 production by DETC, it is likely that JAML also aids in gut γδ IEL-mediated tissue repair74. Interestingly, intestinal γδ IEL but not αβ IEL are capable of producing KGF-1 upon activation69. Thus, coupled with the observation that DSS-treated αβ T cell-deficient mice show a phenotypically similar damage and repair profile to that of WT mice, the necessity of γδ IEL derived KGF-1 to intestinal epithelial repair is clearly demonstrated and illustrates a unique role for γδ T cells in epithelial tissue repair62, 63, 69 (FIG.3b). Furthermore, as mentioned earlier adult AhR deficient mice have severely reduced numbers of Vγ5+ IEL in the intestine. When treated with DSS, AhR deficient mice experience accelerated weight loss, extreme shortening of the colon and severe hemorrhage which lead to them reach the humane endpoint. However, the transfer of WT gut IEL result in a full recovery of all animals, whereby further emphasizing the importance of Vγ5 IEL function in intestinal barrier upkeep and survival41.
In several mouse models of intestinal inflammation and colitis, the lack of γδ T cells correlates with increased levels of IFNγ in the intestinal epithelium90 and the transfer of γδ IEL to TCRδ−/− mice results in reduced colitis correlating with decreased IFNγ and increased TGFβ-1 production by intestinal IEL87. These observations also translate to humans where a specific subset of gut γδ T cells, that express the regulatory receptor NKG2A, are capable of dampening the pro-inflammatory (IFNγ) and cytotoxic (Granzyme B) potential of αβ intestinal IEL, in patients with celiac disease, through the production of TGFβ-191. Thus, in addition to promoting tissue repair, intestinal γδ IEL also act to dampen the potentially pro-inflammatory and cytotoxic effector functions of αβ IEL (FIG.3b). In summary, the intimate contact between epithelial γδ T cells and neighboring cells allow for effective communication and aid in maintaining tissue homeostasis and the timely return to a steady-state condition following injury or stress.
Butyrophilin-like molecules shape epidermal and gut γδ IEL compartments
The observation that mice on the FVB genetic background from Taconic (FVB.Tac) are uniquely depleted of DETC, and that this defect is due to a failure of DETC progenitors to mature92, lead to identification of the selection and upkeep of intraepithelial T cells 1 (Skint1) gene7. Skint1 is exclusively transcribed by keratinocytes and thymic epithelial cells where it uniquely functions to promote IFNγ-producing potential and TCR-hyporesponsiveness of DETC progenitors92–94. Under homeostatic conditions mature DETC exist in a semi-activated state. By use of intravital-microscopy, one study identified structures of phosphorylated Tyrosine-rich aggregates located on projections (PALPs) in DETC dendrites65. Due to PALP co-localization with clustered TCR expression and the constitutive Lck-dependent phosphorylation of the DETC TCR CD3 complex, it was suggested that DETC receive constitutive TCR signaling via ligand recognition on surrounding epithelial cells, causing the semi-activated state of DETC in healthy skin65. However, staining with soluble DETC TCR tetramers shows no ligand expression in healthy skin while a rapid and transient ligand expression by keratinocytes is observed within 1–3 hours following wounding95. These seemingly contrasting findings might be explained by the possibility that DETC TCR ligand is not readily detectable by soluble DETC TCR tetramers in healthy skin due to low level expression and complete engagement with DETC TCR in PALPs. In addition, none of the above mentioned studies have identified a DETC TCR ligand and the existence of several DETC TCR ligands remain a possibility. If so, different ligands might be differentially expressed and recognized by DETC during homeostasis and wound healing. Due to the crucial role in Vγ3Vδ1+ thymocyte development and the exclusive expression in the thymus and epidermis Skint1 was suggested to bind the DETC TCR and mediate constitutive TCR signaling65. However, DETC TCR ligand can be detected on the cell surface of keratinocytes at the wound edge in FVB.Tac mice95. As Skint1 has neither been detected on the cell surface of keratinocytes nor been found to directly bind the DETC TCR92, 93, 96, these observations indicate that Skint1 might not be a ligand, or at least not the only ligand, of the DETC TCR. Interestingly, mice selectively deficient for epidermal Skint1 only show a minor delay in wound healing. In contrast, a more pronounced delay is evident in Skint3 and Skint9 epidermal depleted animals. This delay correlates with abnormal DETC activation/maintenance upon wounding which is not observed in Skint1 depleted mice97. These findings indicate that although Skint1 plays a role in DETC progenitor maturation in the thymus98 other Skints are capable of modulating DETC activation in the skin. Thus, given the apparent differential expression pattern and in situ DETC stimulatory capacity of Skint molecules, it will be interesting for future studies to investigate specific DETC/Skint interactions and to elucidate how such interactions induce and modulate DETC activation.
Skint genes are not present in humans7, 8 but Skint1 does show strong homology with a subfamily of butyruphilin-like (btnl) molecules which are conserved in humans9, 96, 99. In mice, several btnl transcripts are largely restricted to gut epithelium with highest expression in the post-mitotic enterocytes of the small intestinal villi8, 96, and the selective expression of Btnl1 by small intestinal villi, at an early time point of life, was recently found to critically and selectively promote Vγ5+ IEL maturation and expansion. Btnl induced activation further corresponded with TCR down-regulation and up-regulation of the T cell activation marker CD25 and an increased production of IFNγ, GM-CSF and CCL4. The translational value of Btln genes is evident from the finding that a subset of human colonic γδ T cells expressing a Vγ4+Vδ2− TCR is specifically activated by BTNL3 and BTLN8 in vitro8.
Similar to Skint1, even though murine and human btnl/BTNL induced intestinal γδ IEL activation resembles that of TCR-mediated activation8, investigations have failed to report direct Btnl/γδ IEL-TCR interactions. One hint as to how Btln functions could be performed comes from the human protein butyrophilin 3A1 (BTN3A1). The cytosolic tail of BTN3A1 contains a B30.2 domain, the importance of which is best characterized in the tripartite motif molecules (TRIM) where the different B30.2 domains appear to have evolved to bind domain-specific ligands with high affinity in a manner similar to pattern recognition receptors9, which is capable of activating circulating human γδ T cells by binding organic pyrophosphate molecules commonly known as phosphoantigens100–102. Interestingly, intestinal γδ IEL activating Btnl/BTNL molecules all contain cytoplasmic B30.2 domains, indicating that phosphoantigens might play a broader role in γδ T cell regulation. Thus, although the majority of human gut γδ IEL are Vδ1+, which do not react to BTN3A1, it is possible that the BTNL3/8 induced activation occurs by corresponding mechanisms102. However, similar capabilities of individual B30.2 domains await confirmation before any such conclusion can be drawn. Given that BTNL/Btnl/Skint molecules are part of the B7-family, which is defined by molecules with either positive or negative T cell activation potential103, it is likely that individual BTNL/Btnl/Skint molecules can confer either positive or negative T cell stimuli through co-stimulatory action. Indeed, both T cell inhibitory or activational properties of individual Btnl/BTNL molecules have been confirmed in both mice and humans in vitro104–107. As noted by the Hayday lab8, if the regulatory nature of Skints and Btln on T cells is through accessory signals, these molecules could be the first co-stimulatory receptors to show TCR-specificity and play a role in selectively shaping individual IEL compartments.
Microbial tolerance and clearance
The microbiome consists of complex bacterial, archaeal, fungal, viral and protozoan communities and has co-evolved with the host mammalian genome through millions of years to colonize the host interface to the outside environment108. While the microbiome benefits from this symbiotic relationship by inhabiting a nutrient rich environment it also provides key host beneficial functions109. However, these potentially pathogenic microbes also pose a constant threat of invasion and therefore impose the need for tight regulation of tissue integrity and the ability to combat opportunistic penetration of microbes across the epithelial tissue during homeostasis and following barrier disruption.
γδ T cells and gut microbes
In the intestinal tract, the symbiotic relationship between the host and gut commensals is in part maintained by minimizing bacterial-epithelial cell contact. Facilitated by the production of mucus, IgA and antimicrobial peptides (AMP), this spatial separation is vital in limiting adaptive immune responses to the microbiota109–112. Interestingly, while the microbiome has an immense effect on the composition and number of αβ IEL, gut γδ IEL development and numbers in germ-free mice do not differ from that of WT mice, indicating that the microbiome has little to no effect on gut γδ IEL homeostatic numbers110. Small intestinal γδ IEL are however conditioned by the microbiome and introducing microbiota harvested from conventionally raised mice into germ-free mice elicits a strong induction of several AMP, including that of Regenerating islet-derived protein 3 gamma (RegIIIγ)113 (FIG.4a). RegIIIγ, and the human equivalent RegIIIα, are C-type lectins that recognize Gram-positive bacterial targets and form a hexameric membrane-penetrating pore to directly kill bacteria114–116. RegIIIγ production by small intestinal γδ IEL is mediated by intestinal epithelial cells, particularly in response to bacteria capable of penetrating the mucus layer and invading intestinal epithelial cells and thus TCRδ−/− mice show a clear increase in the number of intestinal intracellular bacteria upon co-housing of previously separated mice113. RegIIIγ is also up-regulated by colon-resident γδ IEL following DSS-induced colonic damage and this correlates with a microbiota-dependent transcriptional change of genes primarily involved with immune regulation and recruitment of inflammatory cells. In addition, increased bacterial burden is observed in TCRδ−/− mice during the onset of severe DSS-induced intestinal damage117 (FIG.4a). Combined, these studies suggest that intestinal γδ IEL are early responders essential to limiting mucosal penetration by intestinal bacteria during both tissue homeostasis and following epithelial damage.
Figure 4. Proposed model of DETC and intestinal γδ IEL participation in microbial tolerance and clearance.

a) Dendritic epidermal T cells (DETC) are capable of responding to bacterial insult and directly respond to Gram-negative bacteria via recognition of the lipopolysaccharide (LPS) component of the bacterial cell membrane, which leads to production of effector cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-2. DETC also respond to Staphylococcus aureus infection and aid in the recruitment of neutrophils by producing IL-17A. The abundant presence of the skin commensal, Staphylococcus epidermis (S. epidermis) tunes IL-1 signaling in the skin by inducing keratinocyte production of IL-1α and IL-1 Receptor antagonist (IL-1RA), which in turn induce both DETC and dermal γδ T cells to produce IL-17A. In doing so, S. epidermis mediates effector T cell responses in the skin and promotes protection against Leishmania major infection. S. epidermis also aids proper wound healing by providing an optimal inflammatory environment through the production of small molecules that act on keratinocytes via Toll-like receptor 2 (TLR2) to dampen the TLR3-induced production of the pro-inflammatory cytokines TNFα and IL-6. Germ-free mice have faster wound healing and no scarring when compared to conventionally housed mice, indicating that the skin microbiota has a negative effect on wound healing and scaring. To date, the role of the host microbiome in DETC function is unknown. b) The microbiome conditions γδ IEL effector functions through an intestinal epithelial cell-intrinsic myeloid differentiation primary response gene 88 (MyD88) pathway. Microbiome conditioning of γδ IEL leads to production of several AMP, including Regenerating islet-derived protein 3 gamma (RegIIIγ) and collectively facilitate early protection against invasion by intestinal-resident bacteria. Activated γδ IEL further induce an anti-viral response by producing Type I/III IFNs which in turn induce up-regulation of anti-viral IFN-responsive genes in intestinal epithelial cells. Upon intestinal epithelial damage, γδ IEL again aid in host protection by mounting an anti-bacterial response which includes RegIIIγ production. This response is also shaped by the microbiome which acts directly on γδ IEL, through a MyD88-dependent mechanism, to limit early penetration by opportunistic bacteria following tissue injury. γδ IEL further produce pro-inflammatory cytokines (IL-1β) and chemokines that recruit additional effector cells to the site of damage.
Small intestinal γδ IEL transcriptional profiles show high expression of several cytolytic genes such as Granzyme A and B, indicating a cytotoxic potential towards pathogens and infected cells118, 119. Even so, evidence of direct lysis by γδ IEL in vivo is sparse, but using a CD3-redirected lysis assay, pioneering experiments found that small intestinal γδ IEL can constitutively kill target cells120. Furthermore, the protective role of γδ IEL is evident in infectious mouse models and, despite the fact that γδ and αβ small intestinal IEL have similar cytolytic gene expression profiles119, only γδ- but not αβ-deficient mice are more susceptible to infection86, 113, 118.
The limited knowledge on effector functions of intestinal γδ IEL has in large part been due to the inability to sustain viable cells in vitro, especially upon receiving TCR stimuli. Within recent years a protocol was developed which enables the continued culture of γδ IEL and in vitro activation assays96. This development has allowed for studying γδ IEL activational consequences and has demonstrated a role of γδ IEL in the anti-viral response. Indeed, 22 out of the 50 most up-regulated genes in intestinal epithelial cells cultured with conditioned IEL medium were related to anti-viral functions and inferred viral resistance by intestinal epithelial cells to infection both in vitro and in vivo121 (FIG.4a).
Given the intra-epithelial and basolateral location, γδ IEL are ideally positioned to compartmentalize and limit microbial pathogenic exposure to the systemic immune compartment whereby providing host-protective function. How this is done is currently unknown and whether intestinal γδ IEL host protection is mediated directly by RegIIIγ and other AMP or by cytolytic functions has yet to be directly demonstrated.
γδ T cells and skin microbes
Although skin provides a nutrient poor and much harsher host environment than the intestinal tract it is still populated by a rich diversity of commensals with great importance to cutaneous health15. A proper balance of the microbial composition is important to host homeostasis and thus the state of dysbiosis of the skin microbiome has been associated with several skin disorders including atopic dermatitis (AD)122–124, psoriasis125 and rosacea126.
Staphylococcus epidermis (S. epidermis) represents the most commonly isolated bacterial species from human healthy skin and this bacterium is highly capable of modulating host immune response in a host-beneficial manner127–129. S. epidermis does so in part by producing small TLR2-activating molecules that not only increase keratinocyte production of specific AMP, leading to increased host-resistance to infection by select bacterial and viral pathogens128, but also modulates keratinocyte-mediated inflammation by inhibiting pro-inflammatory cytokine release in response to necrotic and damaged tissue127 (FIG.4b). Furthermore, S.epidermis controls IL-1 signaling to promote pro-inflammatory effector T cell responses in the skin and germ-free mice show increased bacterial burden when infected with Leishmania major (L. major), due to decreased production of IL-17A and IFNγ by skin resident γδ and αβ T cells129. Not dissecting the epidermis or dermis individually, the study did not analyze possible specific interactions between the microbiome and DETC but did show an overall decrease in the number of IL-17A producing γδ T cells in the skin of germ-free mice129. Both RegIIIα in humans and RegIIIγ in mice are rapidly up-regulated by keratinocytes in wounded skin and create an autocrine feedback loop in which terminal differentiation is inhibited and proliferation is increased. This mechanism is governed by IL-17A receptor signaling in keratinocytes and thus mediated by IL-17A producing cells in the injured tissue130 (FIG.4b). Although the ability of DETC to produce IL-17A has been controversial, a subset of DETC is capable of producing large amounts of IL-17A following wounding and during the contact hypersensitivity response, particularly when TCR ligation occurs alongside IL-1β stimulation131, 132. Upon wounding, DETC-derived IL-17A induces keratinocyte production of several AMP, including RegIIIγ, thereby actively promoting the antimicrobial response and subsequent keratinocyte proliferation upon tissue damage131 (FIG.3a). Although not specifically investigated, the ability of S. epidermis to control IL-1-signaling, and consequently IL-17A production by skin residing γδ T cells129, provides a likely mechanisms by which host and skin commensal cross-talk influences DETC function in a host-beneficial manner to facilitate wound healing. Given the fact that dermal γδ T cells are also capable of producing IL-17A133, 134 and because S. epidermis is also found in the dermis of healthy skin135, the decreased number of IL-17A+ skin residing γδ T cells in germ-free mice129 is likely to be caused by decreased IL-17A production by both dermal γδ T cells and DETC (FIG.4b).
DETC also demonstrate anti-bacterial activity through recruitment of neutrophils to the skin upon Staphylococcus aureus infection. In addition DETC can directly respond to Gram-negative bacteria through LPS stimuli which can act alone, but with a better response when TCR stimuli is also provided, to promote cytokine production136, 137 (FIG.4b). Lytic capability of DETC was proven in early studies which found that DETC cultured with Concavalin A and IL-2 exhibited great cytotoxic activity in vitro138, 139. Latter studies further elucidated on the importance of DETC cytolytic functions in regulation and killing of cutaneous malignancies through NKG2D/ligand interactions77, 78, 140. Interestingly, DETC activation and cytotoxic activity is not strictly linked to TCR ligand engagement, and TCR-independent activation can occur by NKG2D ligation alone141.
Although surprisingly little is known about how skin microbiota influence wound healing, one might speculate that within the community of commensals the presence of some microbes promotes wound healing while others delay it. Because prolonged and dys-regulated expression of pro-inflammatory cytokines leads to increased neutrophils influx and subsequent tissue damage, it is possible that S. epidermis derived small TLR2-activating molecules provide further host-beneficial function by dampening keratinocyte-mediated inflammation and, although wound closure kinetics and scaring has not been assessed, it might aid in reducing excessive inflammation and scaring in damaged tissue127. This notion is supported by the recent observation that mice administered antibiotics orally, which results in decreased bacterial density and altered microbial composition in scars by a shift in the dominating phyla from Staphylococcus to Lactobacillaceace, have decreased levels of IL-17A and RegIIIγ and experience a delay in wound healing142. However, germ-free mice were recently found to have faster wound healing and less scarring when compared to conventionally housed mice143, suggesting that although individual microbes can be host-beneficial the skin microbiota as a whole may have a negative effect on wound healing and scaring (FIG.4b). These observations raise the possibility that select microbes or microbial products can be used in wound healing treatment, at least to reduce unwanted excessive inflammation. This might prove tricky and a key feature of such treatment will be to reduce potential detrimental aspects of inflammation while at the same time ensuring no additional risk to wound infection127. Furthermore, dissecting epidermal and dermal tissue separately will provide a more detailed understanding of how microbes interact with individual γδ T cell subsets in the skin with possible implications to wound healing and anti-microbial responses.
Conclusion
Tissue-resident epithelial γδ T cells are ideally positioned for surveillance of epithelial tissues. Not only do these cells provide essential epithelial growth factors, which are crucial to maintaining tissue homeostasis and the timely return to steady-state condition following damage, but also rapidly act to compartmentalize and limit microbial pathogenic exposure to the systemic immune compartment. Epithelial γδ T cells are activated in response to tissue damage through the TCR and by the expression of specific accessory molecules for which ligand expression is up-regulated on injured epithelial cells. Although TCR ligands for epithelial γδ T cell subsets remain elusive, new co-stimulatory receptors-ligand pairs have been identified that modulate effector functions. Individual Btnl molecules are both capable of activating and selectively shaping the specific repertoire of both epidermal and intestinal epithelial tissue-resident γδ T cells in mice. However, given that no direct TCR-binding has been observed and no ligands for Btnl molecules have been identified, how such actions are performed is currently unknown.
Although the symbiotic relationship between the host and the microbiome has evolved over millions of years, only now is it becoming clear just how important host-microbiome interactions are to host homeostasis and pathogen protection. The observation that select microbes provide host beneficial function by limiting excessive inflammatory responses to tissue injury provides a possible future therapeutic avenue to improve wound healing.
Within the past few years we have started to gain new information about how specific host and microbial derived molecules shape the unique effector functions of epithelial γδ T cell subsets. Continued research into the specific mechanisms by which murine tissue resident epithelial γδ T cell subsets orchestrate barrier maintenance and repair will allow for a better understanding of the function of human γδ IEL in both health and disease. Thus, research in progress is focused on maximizing the potential of epithelial γδ T cells to improve regeneration of barrier tissue, detection of epithelial malignancies and pathogen protection. Therefore, although much remains to be learned, the future looks bright for the therapeutic potential of epithelial γδ T cells.
Key points.
Although sparse among circulating T cells, specific subsets of γδ T cells are present in much higher numbers and constitute between 10–100% of T cells in epithelial tissues such as the epidermis of the skin and the gastrointestinal tract and show unique effector function.
Epithelial-resident γδ T cells play vital roles in tissue homeostasis and re-epitheliazation following tissue damage and are thus critical to the upkeep of epithelial barrier function and host survival.
New co-stimulating receptor-ligand pairs have been identified that drive activation and effector function of epithelial-resident γδ T cells and the timely return to steady-state conditions following tissue injury.
Butyrophilin-like (Btnl) molecules are part of the B7-family of accessory molecules and immune-modulatory functions for individual Btnl molecules exist in both humans and mice. Although the precise mechanism remains unknown, the specific expression of individual Btln family members in epithelial tissue selectively shape and expand epithelial-specific γδ T cell repertoires.
Epithelial-resident γδ T cell subsets are uniquely positioned to mediate host microbial tolerance, while at the same time retaining the ability to mount a rapid response against invading pathogens and thus provide early protection against pathogen entry.
Acknowledgments
The authors wish to thank the following funding sources: NIH grants AI036964, AI1064811, and AI129401, The Danish Council for Independent Research 4183-00308B and Lundbeckfonden R182-2014-3467.
Biographies
Morten M. Nielsen completed his Master’s degree and doctoral studies at The University of Copenhagen, Copenhagen, Denmark. He then moved to The Scripps Research Institute, La Jolla, California, USA to take a postdoctoral position in the laboratory of Dr. Wendy L. Havran. His research focuses on identifying cellular interactions in human skin that drive skin-resident T cell activation and wound healing.
Deborah A. Witherden completed her doctoral studies at The University of Melbourne, Melbourne, Australia and postdoctoral studies at IGBMC, Strasbourg, France. From there she moved to The Scripps Research Institute, La Jolla, California, USA and joined the laboratory of Dr. Wendy L. Havran where she is currently a Senior Staff Scientist. Her research focuses on the molecular interactions driving the activation and effector functions of epithelial–resident γδ T cells.
Wendy L. Havran is Professor of Immunology and Microbiology and Associate Dean of Graduate and Postdoctoral Studies at the Scripps Research Institute. She completed her Ph.D.in Immunology at the University of Chicago followed by postdoctoral research at the University of California Berkeley. Her lab focuses on the regulation of immune responses in epithelial barrier tissues.
Reference List
- 1.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 2.Jameson JM, Sharp LL, Witherden DA, Havran WL. Regulation of skin cell homeostasis by gamma delta T cells. Front Biosci. 2004;9:2640–2651. doi: 10.2741/1423. [DOI] [PubMed] [Google Scholar]
- 3.Roberts N, Horsley V. Developing stratified epithelia: lessons from the epidermis and thymus. Wiley Interdiscip Rev Dev Biol. 2014;3:389–402. doi: 10.1002/wdev.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 5.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marco BR, et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell. 2016;167:203–218. doi: 10.1016/j.cell.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes DA, Reith W, Trowsdale J. Regulation of Immunity by Butyrophilins. Annu Rev Immunol. 2016;34:151–172. doi: 10.1146/annurev-immunol-041015-055435. [DOI] [PubMed] [Google Scholar]
- 10.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E. Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Curr Top Dev Biol. 2016;116:357–374. doi: 10.1016/bs.ctdb.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301. doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 13.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toulon A, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol. 2016;16:353–366. doi: 10.1038/nri.2016.48. [DOI] [PubMed] [Google Scholar]
- 16.Veldhoen M, Brucklacher-Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol. 2012;12:696–708. doi: 10.1038/nri3299. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, et al. Disturbance of intraepithelial lymphocytes in a murine model of acute intestinal ischemia/reperfusion. J Mol Histol. 2014;45:217–227. doi: 10.1007/s10735-013-9544-1. [DOI] [PubMed] [Google Scholar]
- 18.Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G627–G630. doi: 10.1152/ajpgi.00224.2005. [DOI] [PubMed] [Google Scholar]
- 19.Wiest DL. Development of gammadelta T Cells, the Special-Force Soldiers of the Immune System. Methods Mol Biol. 2016;1323:23–32. doi: 10.1007/978-1-4939-2809-5_2. [DOI] [PubMed] [Google Scholar]
- 20.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 21.Itohara S, et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 22.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 23.Hayday AC, et al. Unusual organization and diversity of T-cell receptor alpha-chain genes. Nature. 1985;316:828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- 24.Asarnow DM, et al. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 25.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 26.Takagaki Y, DeCloux A, Bonneville M, Tonegawa S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989;339:712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- 27.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20:185–191. doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H, et al. Curriculum vitae of intestinal intraepithelial T cells: their developmental and behavioral characteristics. Immunol Rev. 2007;215:154–165. doi: 10.1111/j.1600-065X.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 29.Maki K, et al. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maki K, Sunaga S, Ikuta K. The V-J recombination of T cell receptor-gamma genes is blocked in interleukin-7 receptor-deficient mice. J Exp Med. 1996;184:2423–2427. doi: 10.1084/jem.184.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shitara S, et al. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta+ intraepithelial lymphocytes. J Immunol. 2013;190:6173–6179. doi: 10.4049/jimmunol.1202573. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, et al. STAT5 is required for thymopoiesis in a development stage-specific manner. J Immunol. 2004;173:2307–2314. doi: 10.4049/jimmunol.173.4.2307. [DOI] [PubMed] [Google Scholar]
- 33.Ye SK, et al. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/s1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Nguyen H, Kang J. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of gamma delta intestinal intraepithelial lymphocytes. Nat Immunol. 2005;6:1263–1271. doi: 10.1038/ni1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De CA, et al. Developmental and functional defects of thymic and epidermal V gamma 3 cells in IL-15-deficient and IFN regulatory factor-1-deficient mice. J Immunol. 2002;168:6486–6493. doi: 10.4049/jimmunol.168.12.6486. [DOI] [PubMed] [Google Scholar]
- 36.Kawai K, et al. Requirement of the IL-2 receptor beta chain for the development of Vgamma3 dendritic epidermal T cells. J Invest Dermatol. 1998;110:961–965. doi: 10.1046/j.1523-1747.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 39.Schluns KS, et al. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadow S, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J Immunol. 2011;187:3104–3110. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Hooper LV. You AhR what you eat: linking diet and immunity. Cell. 2011;147:489–491. doi: 10.1016/j.cell.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Stange J, Veldhoen M. The aryl hydrocarbon receptor in innate T cell immunity. Semin Immunopathol. 2013;35:645–655. doi: 10.1007/s00281-013-0389-1. [DOI] [PubMed] [Google Scholar]
- 44.Girardi M, Lewis JM, Filler RB, Hayday AC, Tigelaar RE. Environmentally responsive and reversible regulation of epidermal barrier function by gammadelta T cells. J Invest Dermatol. 2006;126:808–814. doi: 10.1038/sj.jid.5700120. [DOI] [PubMed] [Google Scholar]
- 45.Itohara S, et al. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 46.Xia M, et al. Differential roles of IL-2-inducible T cell kinase-mediated TCR signals in tissue-specific localization and maintenance of skin intraepithelial T cells. J Immunol. 2010;184:6807–6814. doi: 10.4049/jimmunol.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Campbell JJ, Kupper TS. Embryonic trafficking of gammadelta T cells to skin is dependent on E/P selectin ligands and CCR4. Proc Natl Acad Sci U S A. 2010;107:7443–7448. doi: 10.1073/pnas.0912943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Y, et al. Cutting edge: Intrinsic programming of thymic gammadeltaT cells for specific peripheral tissue localization. J Immunol. 2010;185:7156–7160. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 51.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 52.Morales J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunisawa J, et al. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–2348. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogata M, Itoh T. Gamma/delta intraepithelial lymphocytes in the mouse small intestine. Anat Sci Int. 2016;91:301–312. doi: 10.1007/s12565-016-0341-2. [DOI] [PubMed] [Google Scholar]
- 56.Staton TL, et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–488. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- 57.Wurbel MA, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Wurbel MA, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 59.Wurbel MA, Malissen M, Guy-Grand D, Malissen B, Campbell JJ. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178:7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen KD, Shin S, Chien YH. Cutting edge: Gammadelta intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J Immunol. 2009;182:7348–7351. doi: 10.4049/jimmunol.0900465. [DOI] [PubMed] [Google Scholar]
- 61.Guy-Grand D, et al. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J Exp Med. 2013;210:1839–1854. doi: 10.1084/jem.20122588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jameson J, et al. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 64.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 65.Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal gammadelta TCRs. Nat Immunol. 2012;13:272–282. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive gamma delta TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 68.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 69.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 70.Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–992. [PubMed] [Google Scholar]
- 71.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 72.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 73.Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witherden DA, et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal gammadelta T cell function. Immunity. 2012;37:314–325. doi: 10.1016/j.immuni.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida S, et al. Involvement of an NKG2D ligand H60c in epidermal dendritic T cell-mediated wound repair. J Immunol. 2012;188:3972–3979. doi: 10.4049/jimmunol.1102886. [DOI] [PubMed] [Google Scholar]
- 77.Girardi M, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 78.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 79.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 81.Elbe A, Foster CA, Stingl G. T-cell receptor alpha beta and gamma delta T cells in rat and human skin–are they equivalent? Semin Immunol. 1996;8:341–349. doi: 10.1006/smim.1996.0045. [DOI] [PubMed] [Google Scholar]
- 82.Holtmeier W, et al. The TCR-delta repertoire in normal human skin is restricted and distinct from the TCR-delta repertoire in the peripheral blood. J Invest Dermatol. 2001;116:275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 83.Edelblum KL, et al. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109:7097–7102. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Komano H, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 86.Dalton JE, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–829. doi: 10.1053/j.gastro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Inagaki-Ohara K, et al. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 88.Meehan TF, et al. Protection against colitis by CD100-dependent modulation of intraepithelial gammadelta T lymphocyte function. Mucosal Immunol. 2014;7:134–142. doi: 10.1038/mi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pazirandeh A, et al. Multiple phenotypes in adult mice following inactivation of the Coxsackievirus and Adenovirus Receptor (Car) gene. PLoS One. 2011;6:e20203. doi: 10.1371/journal.pone.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhl AA, et al. Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J Leukoc Biol. 2007;81:168–175. doi: 10.1189/jlb.1105696. [DOI] [PubMed] [Google Scholar]
- 91.Bhagat G, et al. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis JM, et al. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 93.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Wencker M, et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. 2014;15:80–87. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Komori HK, et al. Cutting edge: dendritic epidermal gammadelta T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunol. 2012;188:2972–2976. doi: 10.4049/jimmunol.1100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bas A, et al. Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes. Proc Natl Acad Sci U S A. 2011;108:4376–4381. doi: 10.1073/pnas.1010647108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keyes BE, et al. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell. 2016;167:1323–1338. doi: 10.1016/j.cell.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barbee SD, et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adams EJ, Gu S, Luoma AM. Human gamma delta T cells: Evolution and ligand recognition. Cell Immunol. 2015;296:31–40. doi: 10.1016/j.cellimm.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rhodes DA, et al. Activation of human gammadelta T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol. 2015;194:2390–2398. doi: 10.4049/jimmunol.1401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sandstrom A, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vavassori S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 103.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arnett HA, et al. BTNL2, a butyrophilin/B7-like molecule, is a negative costimulatory molecule modulated in intestinal inflammation. J Immunol. 2007;178:1523–1533. doi: 10.4049/jimmunol.178.3.1523. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen T, Liu XK, Zhang Y, Dong C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol. 2006;176:7354–7360. doi: 10.4049/jimmunol.176.12.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamazaki T, et al. A butyrophilin family member critically inhibits T cell activation. J Immunol. 2010;185:5907–5914. doi: 10.4049/jimmunol.1000835. [DOI] [PubMed] [Google Scholar]
- 107.Chapoval AI, et al. BTNL8, a butyrophilin-like molecule that costimulates the primary immune response. Mol Immunol. 2013;56:819–828. doi: 10.1016/j.molimm.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 109.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 110.Bandeira A, et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moor K, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017 doi: 10.1038/nature22058. [DOI] [PubMed] [Google Scholar]
- 112.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 113.Ismail AS, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukherjee S, et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505:103–107. doi: 10.1038/nature12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fahrer AM, et al. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci U S A. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRgammadelta+ and TCRalphabeta+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 120.Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989;243:1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- 121.Swamy M, et al. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat Commun. 2015;6:7090. doi: 10.1038/ncomms8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kong HH, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–530. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 124.Nakatsuji T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3:e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69:1025–1032. doi: 10.1016/j.jaad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Lai Y, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–1382. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lai Y, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lai Y, et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37:74–84. doi: 10.1016/j.immuni.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacLeod AS, et al. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J Clin Invest. 2013;123:4364–4374. doi: 10.1172/JCI70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nielsen MM, et al. IL-1beta-dependent activation of dendritic epidermal T cells in contact hypersensitivity. J Immunol. 2014;192:2975–2983. doi: 10.4049/jimmunol.1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai Y, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Brien RL, Born WK. Dermal gammadelta T cells—What have we learned? Cell Immunol. 2015;296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakatsuji T, et al. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013;4:1431. doi: 10.1038/ncomms2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leclercq G, Plum J. Stimulation of TCR V gamma 3 cells by gram-negative bacteria. J Immunol. 1995;154:5313–5319. [PubMed] [Google Scholar]
- 138.Nixon-Fulton JL, Hackett J, Jr, Bergstresser PR, Kumar V, Tigelaar RE. Phenotypic heterogeneity and cytotoxic activity of Con A and IL-2-stimulated cultures of mouse Thy-1+ epidermal cells. J Invest Dermatol. 1988;91:62–68. doi: 10.1111/1523-1747.ep12463290. [DOI] [PubMed] [Google Scholar]
- 139.Takashima A, Nixon-Fulton JL, Bergstresser PR, Tigelaar RE. Thy-1+ dendritic epidermal cells in mice: precursor frequency analysis and cloning of concanavalin A-reactive cells. J Invest Dermatol. 1988;90:671–678. doi: 10.1111/1523-1747.ep12560835. [DOI] [PubMed] [Google Scholar]
- 140.Gao Y, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ibusuki A, et al. NKG2D triggers cytotoxicity in murine epidermal gammadelta T cells via PI3K-dependent, Syk/ZAP70-independent signaling pathway. J Invest Dermatol. 2014;134:396–404. doi: 10.1038/jid.2013.353. [DOI] [PubMed] [Google Scholar]
- 142.Zhang M, et al. Oral antibiotic treatment induces skin microbiota dysbiosis and influences wound healing. Microb Ecol. 2015;69:415–421. doi: 10.1007/s00248-014-0504-4. [DOI] [PubMed] [Google Scholar]
- 143.Canesso MC, et al. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol. 2014;193:5171–5180. doi: 10.4049/jimmunol.1400625. [DOI] [PubMed] [Google Scholar]


