Abstract
Triple-negative breast cancer (TNBC) is an aggressive disease lacking targeted therapy. In this study, we developed a CAR T cell-based immunotherapeutic strategy to target TEM8, a marker initially defined on endothelial cells in colon tumors that was discovered recently to be upregulated in TNBC. CAR T cells were developed that upon specific recognition of TEM8 secreted immunostimulatory cytokines and killed tumor endothelial cells as well as TEM8-positive TNBC cells. Notably, the TEM8 CAR T cells targeted breast cancer stem-like cells, offsetting the formation of mammospheres relative to non-transduced T cells. Adoptive transfer of TEM8 CAR T cells induced regression of established, localized patient-derived xenograft tumors (PDX) as well as lung metastatic TNBC cell line-derived xenograft tumors, by both killing TEM8+ TNBC tumor cells and targeting the tumor endothelium to block tumor neovascularization. Our findings offer a preclinical proof of concept for immunotherapeutic targeting of TEM8 as a strategy to treat TNBC.
Introduction
Triple-negative breast cancers (TNBC) are estrogen and progesterone receptor-negative and lack amplification of the human epidermal growth factor receptor 2 (HER2) gene. Accounting for 15–20% of cases, TNBC is associated with a particularly aggressive phenotype and a higher incidence of recurrence (1,2). To date there are no approved targeted therapies for TNBC, new therapies are thus desperately needed.
In 2000, St. Croix identified tumor endothelial marker 8 (TEM8) as a marker of tumor-associated versus normal endothelium in colorectal cancer (3). Also known as anthrax toxin receptor 1 (ANTXR1), TEM8 is an integrin-like cell surface protein and has been shown to play a role in endothelial cell migration and invasion (3–5). TEM8 is conserved in mice and humans; sharing an overall 98% amino acid sequence homology (6). Blocking and knocking out TEM8 resulted in a decline in tumor growth in several preclinical cancer models (5–10). Importantly, deletion of the TEM8 gene in mice did not result in defects in angiogenesis associated with wound healing or normal development (9,10).
Mounting evidence implicates a role for TEM8 in TNBC pathogenesis. TEM8 is elevated in invasive breast cancer tissue relative to normal tissue and correlates with a higher incidence of recurrence in basal breast cancer (11,12). Overexpressing TEM8 in a preclinical breast cancer model increased tumor growth and metastasis (13). In 2013, Chen et al. identified TEM8 as a marker of breast cancer stem-like cells (BCSC) (14). Taken together, these data support TEM8 as a potential target not only of TNBC tumor-associated vessels, but also TNBC tumor cells and potentially BCSCs.
In this study, we sought to determine whether T cells rendered specific to TEM8 using chimeric antigen receptors (CARs) could serve as a novel targeted therapy for TNBC. Specifically, we explored the effector functions of TEM8 CAR redirected T cells against TNBC cells as well as against the tumor associated vasculature.
Materials and Methods
Tissue microarray
Frozen tissue microarrays (BRF404b) containing primary breast cancer cases and normal adjacent breast tissue controls were obtained from US Biomax, Inc. in Rockville, MD.
Immunofluorescence (IF)
Frozen tissue microarrays were stained as previously described (9). L2 in antibody form (mouse-human reverse chimeric antibody with human variable domains and mouse IgG2a constant domains) was not suitable for IF with high background observed in the negative control (secondary only), specifically when staining mouse tissues.
Western blot
Total cell lysates were prepared using Pierce IP Lysis Buffer supplemented with protease and phosphatase inhibitors (Thermo Scientific) and quantified with the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). The protein samples were separated via SDS-PAGE and then transferred to polyvinylidene difluoride membranes. Blots were incubated in Odyssey Blocking Buffer (LI-COR, Lincoln, NE) followed by overnight incubation with mouse anti-SB5 antibody diluted in blocking buffer. Blots were then exposed to the IRDye Secondary Antibodies (LI-COR) for 60 min at room temperature and washed again. Blots were detected using (LI-COR, Lincoln, NE) Odyssey Infrared Imaging System and analyzed using the ImageJ software.
Retroviral constructs
The monoclonal antibody L2 has previously been described (5,7,9). The L2 scFv was generated using the sequence for a single light chain of L2 antibody connected by a flexible glycine serine linker followed by a single heavy chain of the L2 antibody. L2 based constructs were assembled, synthesized, and sequence-verified as previously described (15–17). A retroviral vector encoding the fusion protein eGFP-Firefly Luciferase (eGFP.FFluc) previously described (18) was used to generate firefly luciferase expressing T cells, MDA-MB-468 and LMD231 cells (in vivo experiments).
Retrovirus production and T cell transduction
To produce retroviral supernatant, human embryonic kidney (HEK) 293T cells were co-transfected with either the L2 2G or L2 3G encoding SFG retroviral plasmids, Peg-Pam-e plasmid encoding MoMLV gag-pol and the plasmid containing the sequence for the RD114 envelope (19) T cells were transduced with retroviral vectors containing their respective CARs as described (20). In order to generate GFP-ffluc CAR T cells equal parts of eGFP-firefly reporter gene and CAR containing retroviral supernatants were used to co-transduce primary human T cells. T cells were then normalized for GFP expression and CAR density. Evidence of light output was confirmed prior to use using a luminometer following the addition of D-luciferin substrate.
Blood donors, cell lines and culture
Blood samples were obtained from healthy donors on a protocol approved by the institutional review board of Baylor College of Medicine. Written informed consent was obtained from all donors. All parental cell lines were used less than six months after receipt or resuscitation. Breast cancer cell lines ((Hs578T, MDA-MB-231, MDA-MB-436, MDA-MB-468 and SK-BR-3) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The lung metastasis-derived LMD231 cell line was a gracious gift from Dr. Harikrishna Nakshatri (Indiana University)(21). Breast cancer lines were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) with 10% fetal calf serum (Hyclone, Logan, UT) and 2mmol/l GlutaMAX (Invitrogen, Carlsbad, CA). Endothelial cell lines: HMMEC (ScienCELL; Carlsbad, CA) and HC6020 (CELL biologics; Chicago, IL) were cultured in Endothelial Cell Medium EGM Complete Medium (CC-3024; Lonza, USA), 10% FBS, Endothelial Cell Growth Supplement (ECGS), 90 Mg/mI, Na heparin, 30 Mg/ml Endothelial Cell Growth Supplement, 10 ng/ml epidermal growth factor (EGF), Vascular Endothelial Growth Factor (VEGF) (0.5ng/ml), 0.5% Bovine Serum Albumin (BSA) and Ascorbic Acid (1ug/ml). Raji and T cells were cultured in RPMI- 1640, 10% FCS and 2mmol/l GlutaMAX (Invitrogen).
Flow Cytometry
Samples were run on either the Gallios Flow Cytometer: 3 lasers, 10-color configuration (Beckman Coulter, Brea, CA) or the BD Accuri C6 Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ). Data analysis was done on ≥10,000 events using the Kaluza (Beckman Coulter, Brea, CA) and FlowJo (Tree Star, Ashland, OR) data analysis software, respectively. Cells were washed once with PBS containing 1% FBS (FACS buffer) prior to addition of antibodies. After 30 min - 1hr of incubation at 4°C in the dark the cells were washed for analysis.
Monolayer cytotoxicity assays
Cytotoxicity assays were performed as previously described (22). Non-transduced T cells were used to normalize the percentage of CAR positive cells. The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: (test release − spontaneous release)/ (maximal release − spontaneous release) × 100.
Cocultures/Enzyme-linked immunosorbent assay
Effector T cells (CAR expressing T cells or non-transduced T cells) from healthy donors were co-cultured with TEM8-positive and TEM8-negative cell lines at a 1:1 effector to target ratio in a 96 well plate. After 24 to 48 hours incubation, culture supernatants were harvested and ELISA determined the presence of IFN-γ and IL-2 as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Mouse models
All animal experiments were conducted on a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). Animals were regularly examined for any signs of stress and euthanized according to pre-set criteria. Subcutaneous model: Six to ten week female athymic nude mice were purchased from taconic (NCRNU-F Homozygous CrTac: NCr-Foxn1nu; Taconic, Hudson, NY) for animal studies. Mice received a subcutaneous injection of 2 × 105 MDA-MB-468 eGFP.FFluc labeled cells in 100 µL phenol-red free matrigel on day 0 (CORNING, Corning, NY). Nine days after tumor cell injection, animals received a single dose of 25 × 106 T cells for antitumor studies. Animals were imaged using the IVIS® Lumina II as previously described (17). For bioluminescence experiments, intensity signals were log-transformed and summarized using mean at baseline and multiple subsequent time points for each mouse group.
Human breast cancer patient-derived xenograft (PDX) models
Fresh PDX tumor fragments were transplanted in the epithelium-free, right inguinal fat pad of four-week-old SCID/Bg mice, and allowed to engraft as previously described (23). The slower growing TNBC PDX BCM-2665 was allowed to engraft for 21 days (~140mm3) prior to treatment. The more aggressive claudin-low TNBC PDX WHIM12 was allowed to engraft for 13 days (~165mm3). To account for the difference in two models growth rate BCM-2665 mice received 3 doses of 5×106 L2 3G CAR T cells post-transplant via intratumoral injection, while WHIM12 mice received 5 doses. Irrelevant CAR (CD19) T cells, non-transduced T cells and untreated tumors served as controls. Body weight and tumor volume measurements were taken twice a week for the duration of the studies. Tumors were harvested when they reached the maximum allowable size as per IACUC protocols.
TNBC lung metastasis model
5 ×105 LMD231.eGFPffluc cells were systemically administered to 10-week-old athymic nude mice. Starting on day 8, mice received 3 consecutive doses of 3 million L2 3G CAR (90% transduction) or non-transduced T cells. Evidence of disease was measured by bioluminescence imaging.
Statistical analysis
For cytokine release and cytotoxicity student’s t test was used to compare two groups at a time. For bioluminescence data signal intensity changes from baseline at each time point were calculated and compared using paired t-tests or Wilcoxon signed-ranks test. Log-rank test was used to compare the survival distribution between treatment groups.
Results
TEM8 is overexpressed in TNBC
To validate TEM8 as a target we stained 6 primary TNBC samples for TEM8 and compared the staining pattern to normal adjacent breast tissue. TEM8 (green) was detected in all TNBC cases, while little to no staining was observed in control tissue (Fig. 1A). To determine the location of TEM8 expression we stained primary TNBC vessels with anti CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) and plasmalemma vesicle-associated protein (PV1/MECA-32) antibodies as previously described (9,24). We observed two patterns of TEM8 expression in primary TNBC, a perivascular stromal signature (TNBC-1, 5 and 6) as well as diffuse staining throughout the tumor tissue (TNBC-2, 3 and 4) (Fig. 1A). Normal adjacent breast tissues did not express TEM8 (n=3). Based on these results we decided to test TNBC cell lines for TEM8 expression (5,25). As determined by western blot TNBC cell lines Hs578T, MDA-MB-231, MDA-MB-436 and MDA-MB-468 express TEM8 (564aa, 62kDa band) (Fig.1B). Normalized to the housekeeping gene GAPDH, Hs578T had the highest level of expression, followed by MDA-MB-231, MDA-MB-436 and MDA-MB-468 (Fig. 1C). The HER2-amplified luminal breast cancer cell line SKBr3 did not express appreciable levels of TEM8 (Fig. 1B & C).
Fig. 1. TEM8 is overexpressed in breast cancer but not normal breast tissues.
(A) Co-immunofluorescence of TEM8 (green) and CD31 and PV1 (red) (N=6 donors). Hoechst 33528 nuclear stain (blue). Figures are representative. 40× magnification. (B) Western blot of TNBC lines Hs578T, MDA-MB-231, MDA-MB-436, MDA-MB-468 and HER2-amplified breast cancer cell line, SKBr3. GAPDH served as a loading control. Data shown are representative of three blots using the same cell lines. (C) Quantification of Fig. 1B. (D) Flow cytometry of TEM8 surface expression by TNBC cell lines (Hs578T, MDA-MB-231, MDA-MB-436 and MDA-MB-468) and (E) endothelial cell lines: Human Umbilical Vein Endothelial Cells (HUVEC), Human Mammary Microvascular Endothelial Cells (HMMEC), Human Mammary Tumor-associated Endothelial Cells (HC 6020) and murine tumor endothelial cells (2H11 +ve control & bEND.3). Secondary antibody only control (black), red = test (L2 antibody). B-cell lymphoma Raji cells served as a negative control. Data shown are based on ≥ 10, 000 events gated on live cells on the basis of FSC and SSC properties.
We used flow cytometry to determine the surface expression of TEM8, by TNBC and tumor endothelial cell lines (Fig. 1D & 1E). Of the TNBC cell lines MDA-MB-468 expressed the highest level of TEM8 on the surface, followed by MDA-MB-231, MDA-MB-436 and Hs578T. We compared tumor-derived endothelial cells to normal endothelial cells. Breast tumor-associated endothelial cells (HC 6020) and murine tumor-associated endothelial cells (2H11) expressed TEM8 whereas normal human microvascular mammary endothelial cells (HMMEC) and human umbilical vein endothelial cells (HUVEC) did not (26). The murine brain tumor endothelial cell line bEND.3 expressed TEM8 albeit to a much lesser extent than the other tumor endothelial lines (Figure 1E). Assessment of TEM8 expression in a panel of normal tissues was negative for TEM8 (Supplementary Fig. S1A).
L2 2G and L2 3G CAR T cells effectively kill TNBC and TEC cell lines in vitro
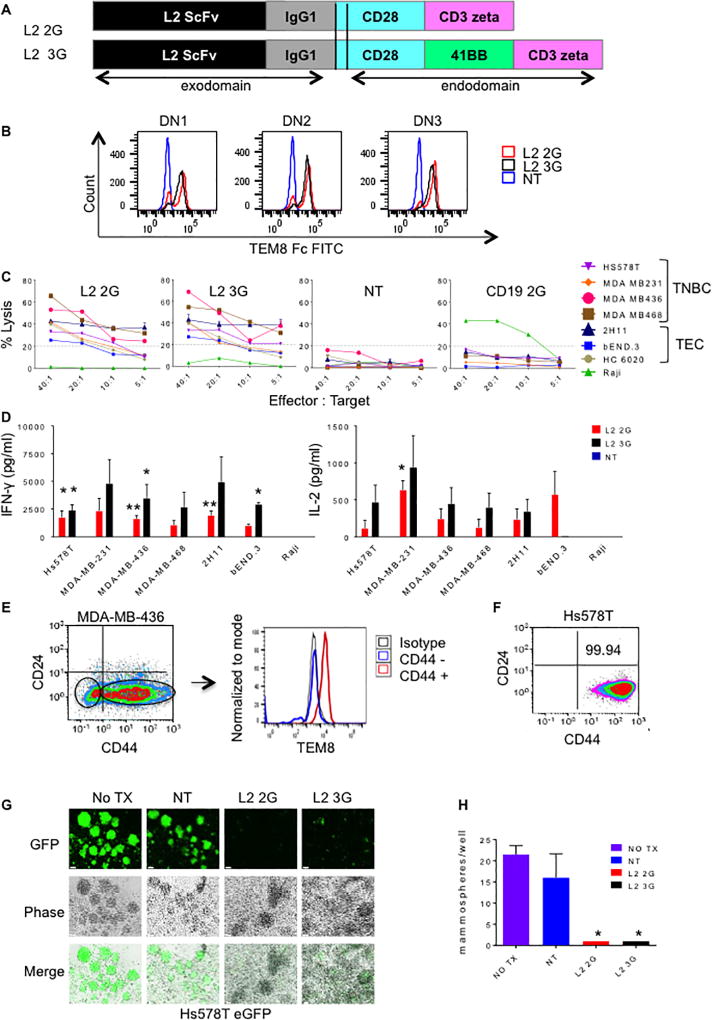
CARs consist of the single chain variable fragment (scFv) of a monoclonal antibody acting as a target-recognition extracellular domain fused with signaling domains traditionally derived from the T cell receptor complex (27). T cells can be genetically modified to express CAR molecules on their surface, so that upon binding of the target antigen killing is induced (19,27). Use of a CD28 CD3-ζ signaling domain has been shown to mediate both the killing and proliferative capacity of CAR T cells (28), however addition of a TNFα receptor-like costimulatory moiety, specifically 41BB, has demonstrated increased in vivo persistence (29). We created 2nd generation (CD28.CD3-ζ) and 3rd generation (CD28.41BB.CD3-ζ) TEM8 specific CAR molecules derived from the scFv of the TEM8 antibody L2, which we hereafter refer to as L2 2G and L2 3G, respectively (Fig. 2A) (9). Primary human T cells from three healthy donors were transduced with either L2 2G or L2 3G CAR transgenes with similar transduction rates (Fig. 2B).
Fig. 2. L2 CAR T cells target TNBC.
(A) L2 second (2G) and third generation (3G) TEM8 specific CAR construct design. (B) FACS analysis to show specific binding of L2 2G (red) and 3G (black) CAR T cells to TEM8. NT cells served as a negative control (blue). (C) Standard 4 hr. 51Cr release assay for TNBC cell lines: Hs578T, MDA-MB-231, MDA-MB-436 and MDA-MB-468 and tumor endothelial cell lines 2H11 and bEND.3. NT and CD19 2G CAR T cells served as T cell controls. Raji B cell lymphoma cells are negative for TEM8 and positive for CD19. Mean values of three experiments done in triplicate are shown. (D) Cytokines IFNγ and IL-2 released as measured by ELISA. (E) MDA-MB-436 cells were tristained for CD44, CD24 and TEM8. 1st panel shows CD44/CD24 staining with gating for CD44+ and CD44− cells. 2nd panel shows histogram plot of TEM8 expression on CD44+ and CD44− negative fractions with isotype control.(F) Hs578T cells were stained for CD44 and CD24. (G) Microscopic appearance of enhanced GFP (eGFP) expressing Hs578T cells grown in non-adherent non-differentiating conditions that were treated with either TEM8 CAR T cells or NT T cells at a 5:1 effector: target ratio. Notice the reduction in mammosphere formation in the TEM8 CAR T cell treatment groups. (H) Quantification of mammosphere formation of each treatment group. Mean + SD. P values ≤ 0.05, 0.005, 0.0001 = *, **, ***, respectively. Unless otherwise indicated, statistical analysis was based on comparison to NT T cell controls.
In cytotoxicity assays, L2 2G and 3G CAR T cells effectively killed TNBC lines Hs578T, MDA-MB-231, MDA-MB-436 and MDA-MB-468, human breast tumor endothelial line HC 6020 and murine tumor endothelial cell lines 2H11 and bEND.3 (Fig. 2C). TEM8-negative Raji cells were not killed.
Next we assessed the CAR T cells’ cytokine release against TNBC and tumor endothelial cell lines (Fig. 2D). L2 3G CAR T cells released significantly higher amounts of IFNγ and IL-2 in response to all four TNBC cell lines and 2H11 when compare to L2 2G CAR T cells. In coculture with bEND.3 IFNγ only but not IL-2 was higher with L2 3G, and only L2 2G released IL2. The modest expression of TEM8 by bEND.3 cells coupled with the lower threshold of activation required for L2 2G cells, could explain why L2 2G, but not L2 3G cells were activated by bEND.3. NT T cells did not release detectable amounts of cytokines.
BCSC are associated with an aggressive phenotype, therapy resistance and poor prognosis in TNBC (30,31). As such we tested TNBC cell lines for expression of BCSC markers CD44 and CD24 (Supplementary Fig. S2A). In particular, independent of CD24 expression, CD44 has been identified as a stem cell marker associated with a poor prognosis in TNBC (32). TNBC cell line MDA-MB-436 cells contained sufficient populations of CD44+ and CD44− cells to analyze TEM8 surface expression. Relative to the CD44− fraction CD44+ cells had significantly higher levels of TEM8 as determined by flow cytometry (Fig. 2E). To test whether L2 CAR T cells could potentially target BCSC and inhibit mammosphere formation, a hallmark of BCSCs, Hs578T cells expressing a GFP reporter gene were grown in non-adherent non-differentiating conditions and cultured with either L2 CAR T cells or non-transduced control cells. Hs578T cells are enriched for stem-like cells and were >99 % CD44+ (Fig. 2F). L2 CAR T cells significantly reduced the number of mammospheres compared to NT T cells (Fig. 2G & 2H p < 0.05), and when compared to irrelevant CAR T cells (CD19) we saw the same results (Supplementary Fig. S2B).
L2 3G CAR T cells have superior efficacy in vivo
To compare L2 2G and L2 3G CAR T cells functionally both in vitro and in vivo, we normalized for GFP fluorescence and CAR expression (Supplementary Figs. S3A & S3B). In long term killing assays at a 1:1 and 1:5 effector: target ratios L2 2G CAR T cells killed TNBC cell lines MDA-MB-468 and HS578T more quickly than L2 3G CAR T cells (Fig. 3A). When testing proliferation against TEM8+ cell lines, L2 2G and L2 3G CAR T cells proliferated similarly as determined by flow cytometry using CellTrace Violet staining. We did detect a discernable difference however when using both low (0.2ug/ml) and high (1.0ug/ml) concentrations of recombinant TEM8 protein as a source of stimulation (Fig. 3B). L2 2G CAR T cells were able to proliferate more against lower levels of antigen. Non-transduced T cells served as a control, only proliferating in the presence of IL-2. To distinguish how these differences affect the T cell subset phenotype, specifically naïve, central memory and effector memory T cell subsets, we assayed L2 2G and L2 3G CAR T cells for expression of CCR7 and CD45RA (Supplementary Fig. S3C). Adding a 41BB-signaling moiety (L2 3G CAR) resulted in higher preservation of the central memory phenotype (CCR7+/CD45RA−) upon stimulation with Hs578T and MDA-MB-468 cells (Fig. 3C). This difference was nearing significance with a p value of 0.06 (n=3 donors).
Fig. 3. L2 3G CAR T cells have enhanced in vivo activity.
(A) Long-term cytotoxicity of L2 CAR T cells against TNBC cell lines Hs578T and MDA-MB-468 at a 1:1 and 1:5 effector target ratio. (B) Proliferation of T cells in the presence of recombinant TEM8 or TEM8+ TNBC lines. IL-2 serves as a positive control. (C) Difference in % central memory cells pre and post antigen exposure (ΔCM; CCR7+/CD45RA−). (D) 1×106 MDA-MB-468 cells were implanted into mice. On day 7, 5 ×106 L2 2G, L2 3G or NT eGFP.FFLuc T cells were injected intratumor and monitored for persistence via bioluminescence imaging over the course of a month. Mean + SEM. (E) In vivo bioluminescence imaging of xenograft tumors. Shown is the mean radiance (photons/second/cm2/selected region), with n=9–10 per group. (F) Bioluminescence data plotted as disease-free survival probability with evidence of disease indicated by a bioluminescence signal higher than the mean on the day of treatment (p value, Log-rank/Mantel-Cox Test). (G) Quantification of vessels in explanted tumors as determined by CD31 and MECA-32 staining (n=5). Unless indicated, Mean + SD is shown. P values ≤ 0.05, 0.005, 0.0001 = *, **, ***, respectively. Unless otherwise indicated, statistical analysis was based on comparison to NT T cell controls.
Lastly we compared L2 2G and L2 3G CAR T cells in vivo. To test the ability of these cells to persist upon chronic antigen exposure, L2 2G or L2 3G CAR T cells were injected into MDA-MB-468 tumor bearing mice. Labeled with firefly luciferase, T cells were monitored over time. L2 3G CAR T cells, relative to L2 2G CAR T cells persisted longer in vivo (n=5). Non-transduced T cells served as a control (Fig. 3D).
To investigate the impact of TEM8 CAR T cells on the growth of established and vascularized TNBC xenografts, eGFP.FFLuc-expressing TEM8+ MDA-MB-468 cells were injected subcutaneously into the flanks of 6–8 week old female athymic nude mice. On day 9, mice received a single dose of L2 2G or 3G CAR T cells, NT T cells or T cells expressing an irrelevant CAR (n=10 per group) intratumorally. Both L2 2G and L2 3G induced regression of MDA-MB-468 xenografts compared to NT T cells and mice that received no treatment (p=0.02, 36 days post treatment; p=0.01, 50 days post treatment, Fig. 3E). L2 3G cells were significantly better at controlling the tumor at day 59 (p=0.04). Furthermore, L2 3G but not L2 2G significantly improved survival relative to NT T cells (p=0.003; Log-rank/Mantel-Cox Test) (Fig. 3F). Tumors were explanted to analyze the effect of L2 CAR T cells on vessel density. We examined the effects of the CAR T cells on the vascular component of the tumor. H&E staining of tumor explants revealed dense tumor tissue in control groups, with nests of tumor cells and collapse of the tumor infrastructure in L2 treated tumors (Supplementary Fig. S4A). Immunofluorescence staining for CD31/MECA-32 revealed that L2 2G and L2 3G CAR T cell-treated groups had significantly decreased vascularization, (p = 0.0001 and 0.001, respectively), whereas tumors that received no treatment, NT T cells or T cells expressing an irrelevant CAR (HER2 2G) had an intact vascular supply (Fig. 3G & Supplementary Fig. S4B). TEM8 staining (green) revealed incomplete tumor targeting (Supplementary Fig. S4B). Further, while tumors recurred in mice treated with L2 CAR T cells as determined by bioluminescence imaging of the tumor cells, the supporting tumor microenvironment was affected as indicated by a smaller tumor volume as determined by external caliper measurements (Supplementary Fig. S4C).
TEM8 CAR T cells induce regression of established orthotopic TNBC PDXs and improve the survival of treated animals
PDX models have been shown to mimic much of the microenvironment of the tumor of origin (23). To test the ability of TEM8 CAR T cells to target patient-derived tumors, we evaluated the effect of treatment on two TNBC PDX models: BCM-2665 (generated by Dr. Michael Lewis at Baylor College of Medicine from a patient with locally advanced triple-negative breast cancer) and WHIM12 (generated by Dr. Matthew Ellis then at Washington University from an especially aggressive claudin-low breast cancer; Horizon; Cambridge, MA). Similar to the two patterns we observed in primary TNBC samples the BCM-2665 PDX induced host TEM8 expression and displayed a predominately perivascular expression, while TEM8 expression by WHIM12 PDX was expressed diffusely throughout the tissue, expressed by both the tumor cells and perivascularly (Fig. 4A).
Fig. 4. L2 CAR T cells induce regression and prolong survival in mice bearing TNBC patient-derived xenografts.
(A) Immunofluorescence staining of PDXs BCM-2665 (top panel) and WHIM12 (lower panel) for TEM8 (green) and vessels (red). (B) Bioluminescence imaging of BCM-2665 tumor bearing mice post 1st T cell dose (n=4 NT, n= 7 CAR). (C) Kaplan-Meier survival curve of mice bearing BCM-2665 tumors. (D) Bioluminescence imaging of WHIM12 tumor bearing mice post 1st T cell dose (n=5 NT, n= 10 CAR). (E) Kaplan-Meier survival curve of mice bearing WHIM12 tumors. Survival curves are shown as days post 1st T cell dose. Non-transduced T cell and sham-injected tumors serve as controls. (F) Immunofluorescence imaging of vessels (red) post T cell treatment of the BCM-2665 PDX model. Body weight and tumor volume measurements were taken twice a week for the duration of the study. Mean radiance for each of the treatment groups is displayed. Error bars represent Mean + SD. P values ≤ 0.05, 0.005, 0.0001 = *, **, ***, respectively. All microscope images were taken at 20× magnification, scale bar = 50 µm.
As L2 3G CAR T cells demonstrated superior efficacy over L2 2G CAR T cells in vivo, we proceeded with L2 3G CAR T cells for testing in orthotopic patient-derived xenograft models. Non-transduced T cells and sham injected (PBS-treated) served as a control. Body weight and tumor volume measurements were taken twice a week for the duration of the study. L2 3G CAR T cells induced regression in 6/7 BCM-2665 PDX tumor-bearing mice and significantly extended the median survival from 21 days to 60 days. 1/7 mice displayed no evidence of disease on day 60 (Figs. 4B & 4C). In the WHIM12 PDX model, L2 CAR T cells induced tumor stabilization relative to control groups and significantly extended median survival from 11 to 26.5 days, with 2/10 mice still alive at day 60 (Figs. 4D & 4E). Taking advantage of the stromal TEM8 signature of the BCM-2665 PDX, we tested whether TEM8 CAR T cells could specifically target the tumor microenvironment and decrease vascularization. As indicated by CD31 and MECA-32 staining (red), TEM8 CAR T cell treated tumors displayed evidence of vascular targeting with necrotic pockets devoid of vessels (Fig. 4F).
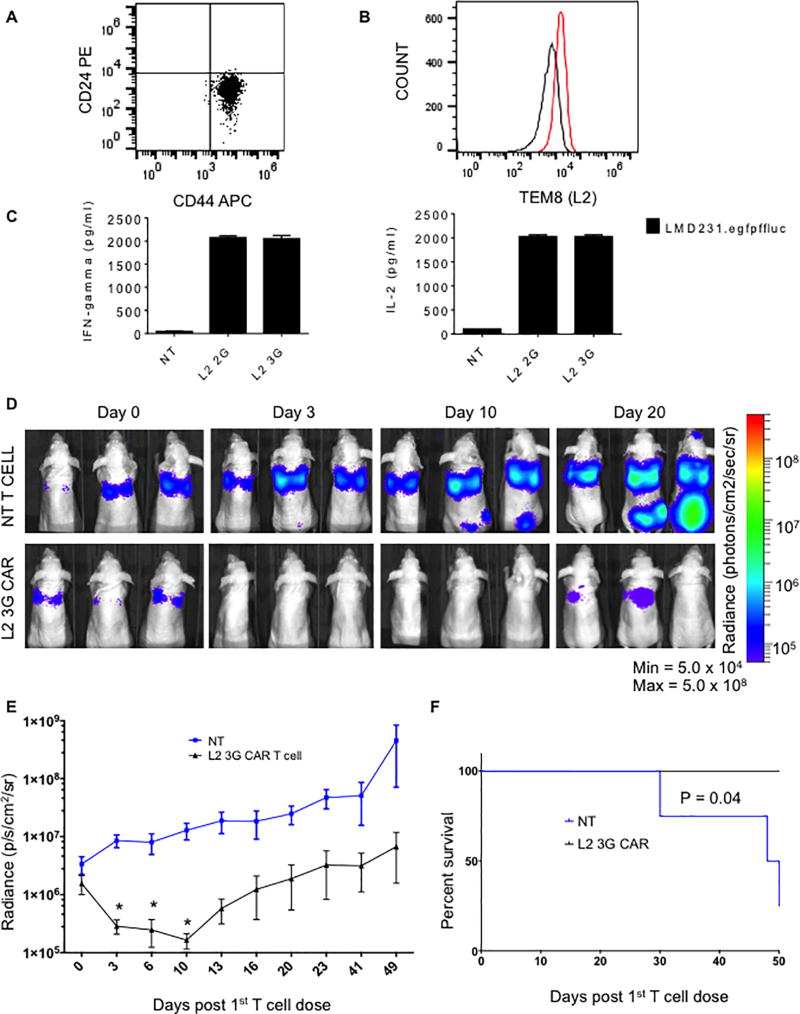
TEM8 CAR T cells induce regression in a lung metastasis model of TNBC
The TNBC lung metastasis-derived cell line LMD231 was used to test our CAR T cells in a systemic model. Similar to the parental line MDA-MB-231, LMD231 cells were predominately CD44+/CD24− and TEM8 positive (Fig. 5A& 5B). To determine whether this level of expression was detectable by our CAR T cells, LMD231 cells were incubated at a 1:1 ratio with L2 CAR T cells overnight. L2 CAR T cells recognized and released high levels (>2000 pg/ml) of IFNγ and IL-2 as determined by ELISA (Fig. 5C). LMD231.eGFPfffluc cells were injected systemically via tail vein into athymic nude mice. Tumors were allowed to engraft and vascularize for eight days, after which they received 3 consecutive doses of 3×106 L2 3G CAR T cells or NT T cells. Tumor burden was measured using bioluminescence imaging. L2 3G CAR T cells induced significant tumor regression in mice such that no mice developed detectable metastasis to the bones and brain of mice as observed in the control group (Fig. 5D & 5E). Lastly, this provided a significant survival advantage (n=4, p< 0.05) (Fig. 5F).
Fig. 5. L2 CAR T cells induce regression and cordon metastasis to the bone and brain in a systemic model of TNBC.
(A) Flow cytometry staining of the LMD231 cell line for CD44 and CD24. (B) Staining for TEM8 on the LMD231 cell line, isotype (black) and test (red). (C) ELISA of T cells cultured with LMD231 cells for 24hrs. (D) Representative images of mice treated with NT or L2 3G CAR T cells over time. (E) Graph of mean radiance signal (lungs only) from mice bearing LMD231.eGFPffluc xenografts treated with NT T cells (blue) or L2 3G CAR T cells (black) over time. (F) Kaplan-Meier survival curve. Error bars represent Mean + SEM. P values ≤ 0.05, 0.005, 0.0001 = *, **, ***, respectively.
Since murine and human TEM8 have a 98% amino acid sequence homology and L2 CAR T cells lyse murine tumor endothelial lines 2H11 and to a lesser extent bEND.3 cells in vitro, it is important that the mice be monitored for any signs of distress. We, nor the veterinary staff, detected any clinical signs of distress from the use of systemic administration of CAR T cells to these mice. Further to test for potential “on-target/off tumor toxicity” a dose of 10 × 106 L2 3G CAR T cells was administered to both female athymic nude mice and male SCID mice and tested for evidence of vascular injury using cytokine multiplex analysis and H&E staining of harvested major organs. Relative to L2 2G CAR T cells, L2 3G CAR T cells required higher levels of antigen to proliferate (Fig. 3B). However, we chose L2 3G CAR cells to test in toxicity studies for two reasons: these cells release on average higher levels of IFNγ, a key player in cytokine release syndrome (33), but also for their increased antitumor activity in vivo making them the most viable for clinical translation. Analysis of vascular injury markers and of harvested organs showed no evidence of vascular injury when compared to control mice (Supplementary Figs. S5A, S5B & S6).
Discussion
Here we report on the first TEM8 specific CAR molecule that is able to co-target both TNBC breast cancer stem cells and tumor-associated vasculature. TEM8 CAR T cells killed TNBC tumor cells, inhibited progenitor-mediated mammosphere formation, obliterated tumor neovasculature and induced regression of established PDXs in local and metastatic murine models of TNBC.
There is an increasing realization that solid tumors create or exploit complex multicellular niches for their growth and that understanding and targeting such niches is critical for improving therapeutic success. Expressed in targetable levels on TNBC tumor cells, BCSCs, as well as the tumor microenvironment, TEM8 represents a unique antigen for targeted cancer immunotherapy. Indeed, despite their single specificity, TEM8 CAR T cells function as a broad-spectrum multi-specific biologic that simultaneously targets various elements of the TNBC tumor and its microenvironment.
Importantly, TEM8 is upregulated in the stem cell compartment of TNBC (14). This population of cells has been linked not only to tumor initiation and progression, but also to metastasis and resistance to chemotherapy and radiotherapy, which explains at least in part the limited-efficacy of standard of care measures for TNBC (34–36). After conventional therapy, breast cancers have been reported to selectively display mesenchymal and tumor-initiating cell features; a population we show is preferentially TEM8 positive (37). Therefore, we anticipate that TEM8-expressing TNBC cells represent a viable target for immunotherapeutic intervention that will abrogate the compartment responsible for failure of conventional therapies.
L2 CAR T cells readily released immuno-stimulatory cytokines IFNγ and IL-2 upon encountering TEM8 and killed TEM8-positive TNBC and tumor endothelial cell lines, showing cross reactivity against murine and human TEM8. Favorably, the L2 scFv antibody fragment was derived from a fully human Fab antibody; a clinically appealing characteristic avoiding the induction of human anti-mouse antibodies and clearance by the immune system in future clinical application. Collectively, these features provided a sound justification to focus further evaluation of TEM8-targeted T cells in our preclinical studies on cells expressing the L2 scFv-based CAR.
While demonstrating comparable cytolytic capabilities as L2 2G T cells in vitro, L2 3G CAR T cells were more efficacious than the former against established TNBC xenografts in vivo. Notably, L2 3G CAR T cells displayed increased cytokine release and preservation of a more favorable central memory phenotype when compared to L2 2G T cells in in vitro experiments, which could explain their enhanced in vivo activity. L2 3G demonstrated a significantly higher degree of in vivo expansion and persistence than L2 2G cells that correlates with increased percentage of central memory cells.
Treatment with a single dose of L2 CAR T cells did not completely eradicate the established TNBC xenografts. In both, L2 2G CAR T cell- and L2 3G CAR T cell-treated groups, tumors eventually progressed. Nevertheless, nearly two months post-treatment, mice treated with L2 3G CAR T cells still had significantly smaller tumors than mice treated with NT T cells and staining for CD31 revealed that L2-treated tumors had significantly reduced vascularization.
Patient-derived tumor xenografts (PDX) models are an invaluable resource for assessing the potential efficacy of a therapy in a preclinical setting. Our L2 CAR T cells effectively induced regression and stasis in TNBC PDX models BCM-2665 and claudin-low WHIM12, respectively. Increased expression of ANTXR1, the TEM8 gene, has been correlated with a poorer survival outcome in TNBC and induction of TEM8 expression in murine 4T1 breast cancer cells increased growth and metastasis (11,13,14). Our CAR strategy targets TEM8 on the surface, however, TEM8 can also be expressed in other isoforms internally. The WHIM12 PDX in particular expresses high levels of intracellular TEM8, which could explain at least in part the reduced efficacy of CAR T cells against the WHIM12 PDX versus BCM-2665.
In conclusion, our study presents TEM8 as a promising target antigen for CAR-based cancer immunotherapy of TNBC. We showed that TEM8-specific (L2) CAR T cells are capable of killing TNBC tumor parenchyma, tumor endothelial cells as well as BCSCs, inducing regression of established TNBC xenografts. Thus, we propose TEM8 CAR T cells as an attractive targeted therapy for TNBC.
Supplementary Material
Acknowledgments
We would like to thank Drs. Malcolm Brenner, Motthafar Rimawi and Kent Osborne for their insight and professional advice and Mrs. Catherine Gillespie and Dr. Sujith Joseph for editing this manuscript. We would also like to thank summer students Daphine Mugayo, Yasmina Fayed, Jibwa Jakana-Lule and Jordan Clinton for their technical assistance.
Financial Support: This research was supported in part by the Stand Up To Cancer St. Baldrick's Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. Authors directly supported by this grant include N Ahmed and M Hegde. This research was also funded by the following T32 training grants: award numbers T32GM088129 and 5T32HL092332 from the National Institute of General Medical Sciences and National Heart, Lung and Blood Institute, respectively. Authors directly supported by both T32 grants include T Byrd and K Fousek. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, National Heart, Lung and Blood Institute or National Institutes of Health. The Cytometry and Cell Sorting Core at Baylor College of Medicine also supported this project with funding from the NIH (AI036211 and CA125123).
Conflict of Interest: NMA and TTB are listed inventors on a patent pertaining to vascular-targeted T-cell therapy, publication number: WO 2014164544 A.
References and Notes
- 1.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma S, Barry M, Gallagher DJ, Kell M, Sacchini V. An overview of triple negative breast cancer for surgical oncologists. Surg Oncol. 2015 doi: 10.1016/j.suronc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 4.Fu S, Tong X, Cai C, Zhao Y, Wu Y, Li Y, et al. The structure of tumor endothelial marker 8 (TEM8) extracellular domain and implications for its receptor function for recognizing anthrax toxin. PLoS One. 2010;5(6):e11203. doi: 10.1371/journal.pone.0011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, et al. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer research. 2004;64(3):817–20. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 6.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer research. 2001;61(18):6649–55. [PubMed] [Google Scholar]
- 7.Fernando S, Fletcher BS. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer research. 2009;69(12):5126–32. doi: 10.1158/0008-5472.CAN-09-0725. [DOI] [PubMed] [Google Scholar]
- 8.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14(11):1236–46. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary A, Hilton MB, Seaman S, Haines DC, Stevenson S, Lemotte PK, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer cell. 2012;21(2):212–26. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, et al. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer research. 2009;69(15):6021–6. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies G, Rmali KA, Watkins G, Mansel RE, Mason MD, Jiang WG. Elevated levels of tumour endothelial marker-8 in human breast cancer and its clinical significance. Int J Oncol. 2006;29(5):1311–7. [PubMed] [Google Scholar]
- 12.Gutwein LG, Al-Quran SZ, Fernando S, Fletcher BS, Copeland EM, Grobmyer SR. Tumor endothelial marker 8 expression in triple-negative breast cancer. Anticancer Res. 2011;31(10):3417–22. [PubMed] [Google Scholar]
- 13.Opoku-Darko M, Yuen C, Gratton K, Sampson E, Bathe OF. Tumor endothelial marker 8 overexpression in breast cancer cells enhances tumor growth and metastasis. Cancer Invest. 2011;29(10):676–82. doi: 10.3109/07357907.2011.626474. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Bhat-Nakshatri P, Goswami C, Badve S, Nakshatri H. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer research. 2013;73(18):5821–33. doi: 10.1158/0008-5472.CAN-13-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33(15):1688–96. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer research. 2007;67(12):5957–64. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(2):474–85. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101(5):1905–12. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Lewis MT. Establishment of Patient-Derived Xenograft (PDX) Models of Human Breast Cancer. Curr Protoc Mouse Biol. 2013;3(1):21–9. doi: 10.1002/9780470942390.mo120140. [DOI] [PubMed] [Google Scholar]
- 24.Newman PJ, Berndt MC, Gorski J, White GC, 2nd, Lyman S, Paddock C, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247(4947):1219–22. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 25.Yang MY, Chaudhary A, Seaman S, Dunty J, Stevens J, Elzarrad MK, et al. The cell surface structure of tumor endothelial marker 8 (TEM8) is regulated by the actin cytoskeleton. Biochimica et biophysica acta. 2011;1813(1):39–49. doi: 10.1016/j.bbamcr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter-Yohrling J, Morgenbesser S, Rouleau C, Bagley R, Callahan M, Weber W, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(6):2179–89. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 27.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20(10):1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 29.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma F, Li H, Wang H, Shi X, Fan Y, Ding X, et al. Enriched CD44(+)/CD24(−) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC) Cancer Lett. 2014;353(2):153–9. doi: 10.1016/j.canlet.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, et al. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol. 2012;43(3):364–73. doi: 10.1016/j.humpath.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, et al. Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer. Biomed Res Int. 2015;2015:158682. doi: 10.1155/2015/158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–79. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(6):1845–7. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28(25):4006–12. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34(6):732–40. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.