Abstract
UVC radiation is known to be highly germicidal. However, exposure to 254-nm-UVC light causes DNA lesions such as cyclobutane pyrimidine dimers (CPD) in human cells, and can induce skin cancer after long-term repeated exposures. It has been reported that short wavelength UVC is absorbed by proteins in the membrane and cytosol, and fails to reach the nucleus of human cells. Hence, irradiation with 222-nm UVC might be an optimum combination of effective disinfection and biological safety to human cells. In this study, the biological effectiveness of 222-nm UVC was investigated using a mouse model of a skin wound infected with methicillin-resistant Staphylococcus aureus (MRSA). Irradiation with 222-nm UVC significantly reduced bacterial numbers on the skin surface compared with non-irradiated skin. Bacterial counts in wounds evaluated on days 3, 5, 8 and 12 after irradiation demonstrated that the bactericidal effect of 222-nm UVC was equal to or more effective than 254-nm UVC. Histological analysis revealed that migration of keratinocytes which is essential for the wound healing process was impaired in wounds irradiated with 254-nm UVC, but was unaffected in 222-nm UVC irradiated wounds. No CPD-expressing cells were detected in either epidermis or dermis of wounds irradiated with 222-nm UVC, whereas CPD-expressing cells were found in both epidermis and dermis irradiation with 254-nm UVC. These results suggest that 222-nm UVC light may be a safe and effective way to reduce the rate of surgical site and other wound infections.
Keywords: 222-nm UVC, methicillin-resistant Staphylococcus aureus, wound infection, CPD, dermis
1. Introduction
Surgical site infections (SSI) are an important cause of morbidity in surgical patients and are responsible for an increased economic burden to the healthcare systems [1]. Most SSI result from commensal bacteria colonizing the patient’s own body. Staphylococcus aureus is frequently detected as a commensal skin microorganism and is the most common microbial cause of SSI, accounting for 15–20% of SSI occurring in hospitals [2, 3]. To date, methicillin-resistant S. aureus (MRSA) is a major problem worldwide. SSI caused by MRSA has been shown to significantly increase the length of post-operative hospital stay, costs, and mortality [4, 5]. As most clinical isolates of MRSA are resistant to multiple antibiotics and other antimicrobials, options for effective antimicrobial therapy are often limited [6]. Hence novel strategies for prevention and treatment of SSI and for wound care are required.
UVC light is electromagnetic irradiation at wavelengths of 200 to 280 nm. It has been known for the last 100 years that UV light, particularly UVC in the range of 240–280 nm is highly germicidal [7]. UVC light at the 254-nm wavelength is easily produced from a low-pressure mercury vapor lamp, and is commonly used to inactivate and kill many microbial species [8, 9]. The germicidal mechanism of UVC light relies on its DNA-damaging effect caused by a variety of mutagenic and cytotoxic DNA lesions such as cyclobutane pyrimidine dimers (CPD) (10). CPD is known to interrupt the transcription, translation, and replication of DNA, leading to cell death [9–10]. It was reported that irradiation of surgical wounds with germicidal UVC during orthopedic surgical procedures reduces the rate of SSI [11]. However, it has also been shown that exposure of human cells to 254-nm UVC causes the formation of mutagenic and cytotoxic DNA lesions, which (if repeated for a sufficiently long time) can lead to the initiation and progression of skin cancer [12].
It has been reported that penetration ability of short-UVC light (around 200–230 nm) through biological materials is very limited because short-UVC light is strongly absorbed by proteins, particularly by the peptide bonds, and other biomolecules [13, 14]. Short-UVC light is reduced by half in only about 0.3 μm of tissue [15, 16]. Buonanno et al. reported that 207-nm UVC light which is produced by krypton-bromine excimer lamp, can inactivate bacteria efficiently, moreover it also showed less cytotoxic and mutagenic damage to human keratinocytes [17, 18]. Although the effect of 207-nm UVC light on mammalian cells in subcutaneous tissues has not yet elucidated, these reports showed that irradiation to surgical sites with 207-nm UVC light during operative procedures could be an effective strategy to prevent SSI.
Recently, a krypton-chlorine (Kr-Cl) excimer lamp was developed to emit 222-nm UVC light [19]. The germicidal and cytotoxic property of 222-nm UVC light was investigated to clarify whether this light is harmful to bacterial cells but not to human cells. To date, the use of short-UVC irradiation for treatment of wound infections remains at an early stage. Most studies have been confined to in vitro and ex vivo models [17–20], and the direct effects of short-UVC light on infected wounds in vivo have not been investigated. In this study, we evaluated the bactericidal and DNA-damaging effects of 222-nm UVC light in MRSA-infected wounds in a mouse model. We demonstrated that 222-nm UVC light at 75 and 150 mJ/cm2 showed the efficient bactericidal activity on normal mouse skin. Although the bactericidal activity of 222-nm UVC at 75 and 150 mJ/cm2 measured immediately after irradiation in infected wounds was comparable and slightly less than that of 254-nm UVC, respectively, the bacterial numbers in the wounds on days 5, 8 and 12 in 222-nm UVC at 150 mJ/cm2 wounds were comparable or lower than those in 254-nm UVC wounds. Importantly, DNA damage to keratinocytes and other cells in the infected wound caused by 222-nm UV light was less severe than that caused by 254-nm UVC.
2. Materials and methods
2.1. Mice
BALB/c mice were purchased from Clea Japan, Tokyo, Japan. Mice were maintained under specific pathogen-free conditions at the Institute for Animal Experimentation, Hirosaki University Graduate School of Medicine. All animal experiments were carried out in accordance with the Institutional Animal Care and Use Committee [ethics committee of Hirosaki University].
2.2. UVC light source
Two types of lamps were used for UVC light irradiation; one was a krypton-chloride (Kr-Cl) excimer lamp which emits in the range from 200- to 230-nm whose maximum output wavelength is 222-nm, and another was a conventional low pressure mercury lamp (SUV-4) that emits a line spectrum at 254-nm. The 222-nm emitting device is called “SafeZoneUVC” (Ushio Inc. Tokyo, Japan), which is in the registration process for a trademark, and is composed of the lamp, air-cooling fan, mirrors and a custom band-pass filter. The filter was used to remove essentially all but the dominant 222-nm emission wavelength. The irradiance of 222-nm light was measured using an S-172/UIT250 accumulated UV meter (Ushio Inc.) and was found to be 5 mW/cm2 at 10 mm distance from the emission window. The irradiance for 254-nm light was evaluated by an S-254/UIT250 (Ushio Inc.) to be 3 mW/cm2 at 20 mm distance from the window.
Figure 1 shows the measured spectra emitted from the Kr-Cl excimer lamp equipped with the band-pass filter. It is emphasized that the spectrum shown here does not have any light of longer wavelengths which are more penetrating into living tissues and therefore potentially more harmful to human cells.
Fig. 1.
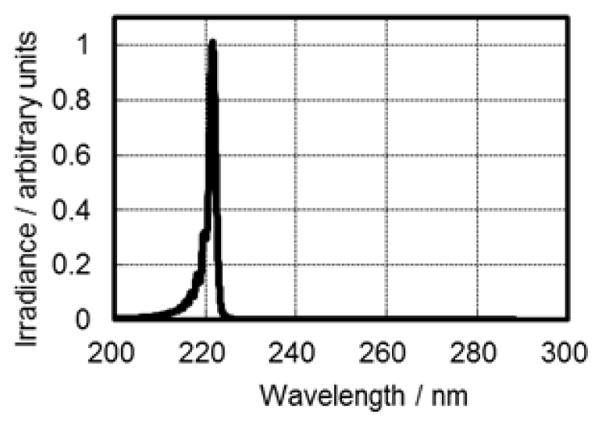
Measured spectra emitted from the Kr-Cl excimer lamp equipped with a band- pass filter.
2.3. Bacterial strain and culture condition
S. aureus strain 834, a clinical isolate from a sepsis patient that is classified as MRSA strain [21], was used for contamination of normal skin and for wound infections in mice. The bacterial cells were grown at 37°C in tryptic soy broth (BD Diagnosis Systems, Sparks, MD) for 15 h, harvested by centrifugation and washed with phosphate-buffered saline (PBS). The bacterial cells were then diluted with PBS to an appropriate concentration by spectrophotometric measurement at 550 nm.
2.4. Mouse model of full-thickness skin wound
Mice were anesthetized by intraperitoneal injection of a cocktail containing medetomidine chloride (Meiji Seika Pharm Co., Tokyo, Japan), Midazolam (SANDOZ, Tokyo, Japan) and Butorphanol tartrate (Meiji Seika) at a dose of 0.3 mg/kg, 4 mg/kg and 5 mg/kg, respectively. After shaving the dorsal surface using an electric fur clipper, an 8 mm-diameter circular full-thickness wound was made using a skin biopsy punch (Kai Industries, Gifu, Japan).
2.5. S. aureus inoculation and bacterial counts
Twenty μL of a bacterial suspension containing 5 × 105 colony-forming units (CFU) of S. aureus was deposited onto the shaved normal skin using a micropipette and smeared using the micropipette tip. One hour later, the skin was irradiated or sham-irradiated with 222-nm UVC at doses of 75, 150 and 450 mJ/cm2. Immediately after irradiation, the skin area of approximately 1.2 by 1.2 cm was excised. To evaluate the bactericidal effect of 222-nm UVC irradiation on dorsal skin wounds, twenty μL of a bacterial suspension containing 5 × 105 CFU of S. aureus was smeared onto the skin wound using a micropipette tip. The skin wounds were air-dried for 1 h, and irradiated with various fluences of 222-nm UVC light. Non-irradiated wounds and 254-nm UVC light were used as negative control and positive control, respectively. At immediately and on days 1, 3, 5, 8 and 12 after UVC irradiation, the skin wound area 1.2 by 1.2 cm was excised immediately after sacrifice of the animals. The excised skin samples were homogenized vigorously in PBS at 10× vol/wt. Bacterial numbers in the skin and wounds were enumerated by plating 10-fold serial dilutions of the homogenates on tryptic soy agar (BD Diagnosis System), counting colonies 24 h after incubation at 37°C and expressing as CFU.
2.6. Histological and immunohistochemical analysis
It is known that UVC radiation induces CPD and (6–4) photoproducts (6-4PPs) in DNA, which eventually can lead to skin carcinogenesis. Therefore, the effect of 222-nm UVC on DNA damage was observed after irradiation mouse dorsal skin with 222-nm UVC at 150 mJ/cm2. Skin samples with non-irradiation and 254-nm UVC irradiation at 150 mJ/cm2 were used as controls. Skin tissue was taken from non-irradiated and irradiated wounds on days 5 and 8 after infection and fixed with 10% phosphate-buffered formalin overnight at 4°C. Paraffin-embedded blocks of the skin tissue and 4 μm-thick paraffin sections were prepared. After deparaffinization and rehydration of the tissue sections, hematoxylin and eosin staining was performed. To analyze neutrophils in the skin tissue, the deparaffinized and rehydrated sections were incubated in PBS containing 5% bovine serum albumin (Sigma, St. Louis, MO) and 2% rat serum for 1 h at room temperature. The sections were then incubated with rat anti-mouse neutrophil monoclonal antibody Ly-6G (1:50 dilution; Hycult Biotechnology, Uden, Netherlands) for overnight at 4°C. After rinsing three times in PBS for 5 min each, samples were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rat secondary antibody (AbD Serotec, Oxford, UK) at room temperature for 1 h. Samples were then rinsed 3 times with PBS for 5 min each at room temperature. The color reaction was developed by the addition of diaminobenzidine and then counterstaining was performed with hematoxylin. To observe S. aureus in the skin tissues, the deparaffinized and rehydrated sections were incubated with rabbit anti-S. aureus antibody (Virostat, Portland, ME) for overnight at 4°C. After rinsing in PBS, the sections were incubated for 1 h at room temperature with HRP-conjugated goat anti-rabbit secondary antibody (AbD Serotec), and the color reaction was developed as described above. To detect CPD formation, skin sections were prepared as described above and antigen retrieval was performed by incubating the sections in proteinase K solution (1:1000; Qiagen, Hilden, Germany). The sections were then incubated with HRP-conjugated anti-CPD monoclonal antibody (Kaniya Biomedical Co., Seattle, WA) for overnight at 4°C. The color reaction was developed as described above. The CPD-positive cells were quantified by counting the cells in 10 random high-power (× 400) fields of each section.
2.7. Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analyses were performed by student’s t-test. P < 0.05 was considered significant.
3. Results
3.1. Bactericidal activity of 222-nm UVC irradiation against S. aureus on the surface of normal mouse skin
Bacterial numbers on the non-irradiated skin samples were approximately 4.5 log CFU, whereas the mean CFU after 75 mJ/cm2 of 222-nm UVC was less than 2.8 log CFU. To evaluate whether higher doses of UVC irradiation show even more effective bactericidal activity, S. aureus-inoculated skin samples were irradiated with 222-nm UVC at 150 and 450 mJ/cm2. The bactericidal effect of higher doses of 222-nm UVC irradiation was comparable to that of 75 mJ/cm2 (Fig. 2).
Fig. 2.
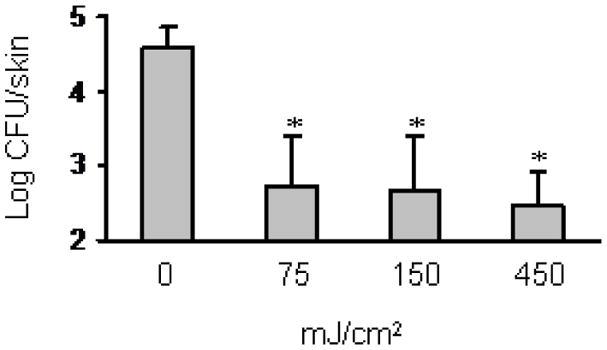
Bactericidal effect of 222-nm UVC irradiation on S. aureus inoculated onto mouse dorsal skin. S. aureus suspension was inoculated onto dorsal skin of mice, and air-dried for 1 h. The skin was irradiated with 222-nm UVC light at 75, 150 and 450 mJ/cm2. Bacterial counts in the skin were enumerated as described in the materials and methods. Data are expressed as the means ± standard deviation of a group of 5 mice. * P < 0.05.
3.2. Bactericidal effect of 222-nm UVC irradiation in dorsal skin wounds infected with S. aureus
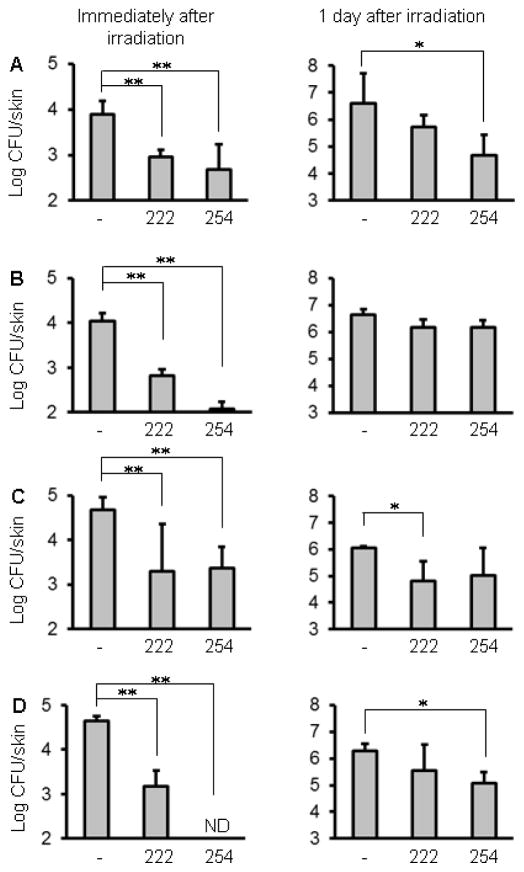
The bacterial counts of the skin wounds irradiated with 222-nm UVC at 75, 150, 750 and 1500 mJ/cm2 were approximately 1.0~1.5 log CFU less than those in the non-irradiated wounds immediately after irradiation (Fig. 3A–D). Irradiation with 254-nm UVC at 150 and 1500 mJ/cm2 reduced bacterial numbers more effectively than irradiation with 222-nm UVC. One day after inoculation, bacterial numbers increased compared to that found on day of inoculation, suggesting that S. aureus grows in both non-irradiated and irradiated wounds. However, the bacterial counts of the wounds irradiated with 222-nm UVC at 750 mJ/cm2 and 254-nm UVC at 75 and 1500 mJ/cm2 were less than those in the non-irradiated wounds (Fig. 3B–D). To evaluate the effect of 222- or 254-nm UVC on bacterial numbers along with wound healing, bacterial counts in the skin wounds were enumerated on days 3, 5, 8 and 12 after irradiation. As shown in Fig. 4, the bacterial counts increased on days 3 and 5. The mean bacterial counts in non-irradiated wounds reached up to 7 log CFU. The bacterial counts in the wounds irradiated with 75 mJ/cm2 were comparable to those in non-irradiated wounds, whereas the bacteria in the wounds irradiated with 222-nm or 254-nm UVC at 150 mJ/cm2 were significantly reduced, compared with the non-irradiated wounds. On day 8, bacterial numbers in the wounds irradiated with 254-nm UVC were significantly higher than those in the non-irradiated and 222-nm UVC irradiated wounds. On day 12, bacterial counts in the wounds irradiated with 222-nm or 254-nm UVC were significantly reduced compared with the non-irradiated wounds.
Fig. 3.
Bactericidal effect of 222-nm UVC irradiation on S. aureus inoculated onto skin wounds. Full-thickness wounds were made on mouse dorsal skin, and S. aureus suspension was spotted onto the wounds. The wounds were irradiated with 222-nm and 254-nm UVC light at 75 (A), 150 (B), 750 (C) and 1500 mJ/cm2 (D) at 1 h after inoculation. Bacterial counts were enumerated immediately and 1 day after irradiation. Data are expressed as the means ± standard deviation of a group of 3 to 4 mice. ** P < 0.01, * P < 0.05, ND; not detected.
Fig. 4.
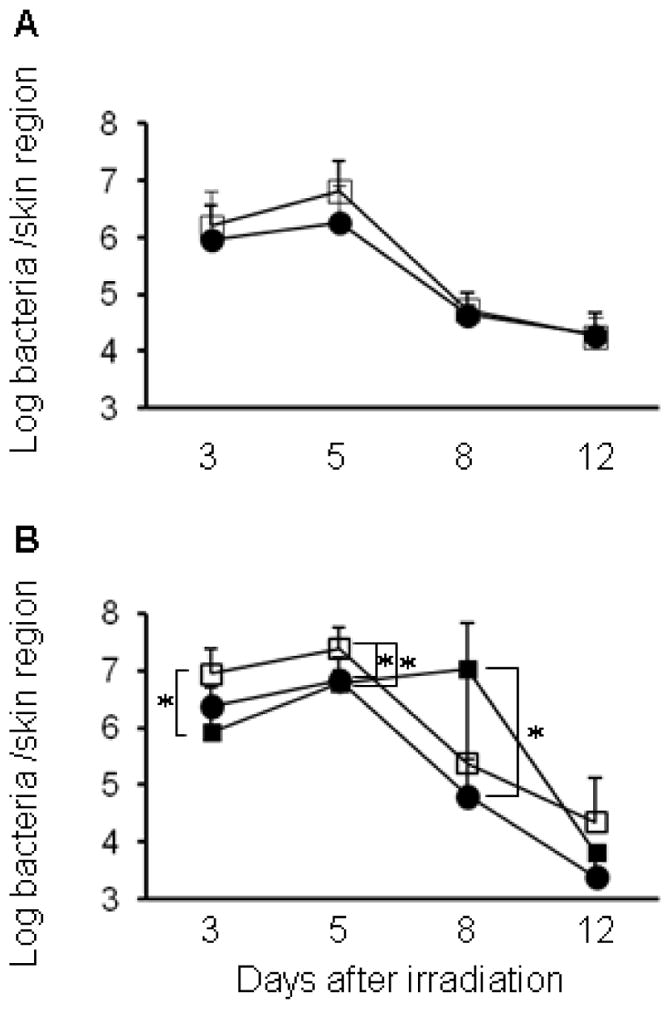
Time courses for bacterial count in S. aureus-infected wounds after 222-nm and 254-nm UVC irradiation. S. aureus suspension was inoculated onto the skin wounds of mice, and the wounds were irradiated with 75 mJ/cm2 of 222-nm (A) or 150 mJ/cm2 of 222-nm and 254-nm UVC light (B) at 1 h after inoculation. Skin samples were taken on days 3, 5, 8 and 12 after UVC irradiation, and bacterial counts in the wounds were enumerated. Open square: non-irradiated wound, closed square: 254-nm UVC irradiated wound and closed circle: 222-nm UVC irradiated wound. Data are expressed as the means ± standard deviation of a group of 3 to 4 mice. * P < 0.05.
3.3. Healing effect of 222-nm UVC irradiation on S. aureus-infected skin wounds
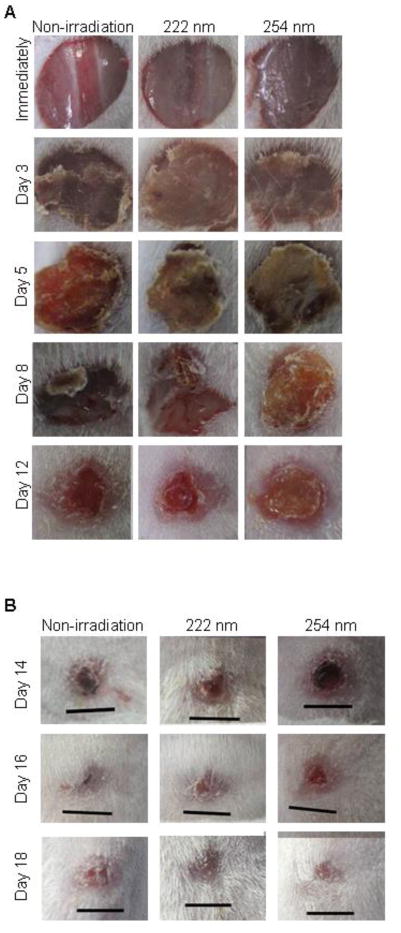
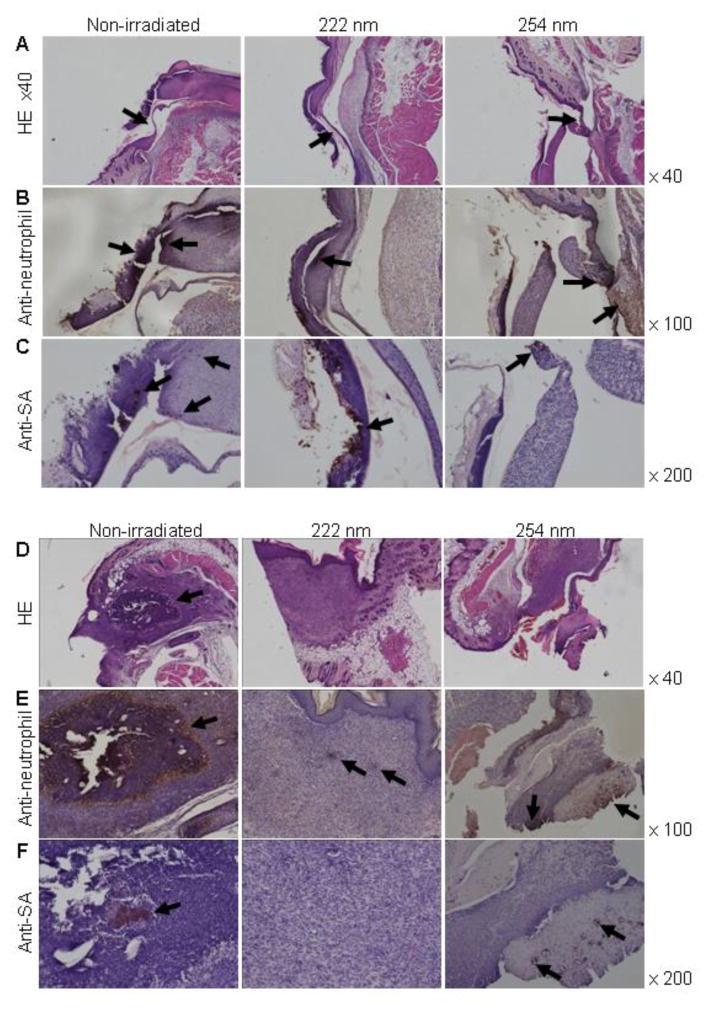
To evaluate the healing effect of 222-nm UVC irradiation on skin wounds infected with S. aureus, the wounds were observed until 18 days after irradiation. Fig. 5 shows the gross appearances of the skin wounds. Immediately after irradiation, the gross appearance of the non-irradiated wounds and wounds irradiated with 222-nm or 254-nm UVC were not obviously different. Scabs which were tightly adherent to the wound surface, were observed on the wounds from day 3 after irradiation. On day 8 after irradiation, the size of the scabs on 222-nm UVC irradiated and non-irradiated wounds reduced. The scabs of 222-nm UVC irradiated wounds and non-irradiated wounds loosely adhered to the wound tissue, whereas the scab on 254-nm UVC irradiated wound tightly adhered to the tissue. The small scabs adhered in 222-nm and 254-nm UVC irradiated and non-irradiated wound on days 16 after irradiation and disappeared in wounds of each group on day 18 after irradiation (Fig. 5B). To further evaluate the healing effect of 222-nm UVC irradiation on infected wounds, histological analysis was performed. Migration of epidermal cells toward the center of the non-irradiated and 222-nm UVC irradiated wounds was observed on day 5 after irradiation, whereas epidermal cells remained near to the wound margin and the epidermis did not spread over the 254-nm UVC irradiated wound tissue (Fig. 6A). The results suggest that migration of epidermal cells is impaired by 254-nm UVC irradiation but not by 222-nm UVC. Neutrophil infiltration was observed in the scab of 222-nm UVC irradiated wounds, whereas massive neutrophil infiltration was observed not only in the scab but also in the subcutaneous tissues of non-irradiated and 254-nm UVC irradiated wounds (Fig. 6B). Immunostaining of S. aureus showed that bacterial cells were detected in both the scab and subcutaneous tissue of the non-irradiated wound whereas bacteria were detected only in the scab tissue of wounds irradiated with 222- and 254-UVC on day 5 (Fig. 6C). Fig. 6D-F shows histology of the wounds on day 8 after irradiation. Abscess formation (Fig. 6D), neutrophil infiltration (Fig. 6E), and S. aureus cells (Fig. 6F), were seen in the non-irradiated subcutaneous tissues. Scab adherent to the wound tissue, neutrophil infiltration and S. aureus cells in the scab but not the subcutaneous tissue were seen in the 254-nm UVC irradiated wound, whereas re-epithelialization, granulation tissue formation and only a few neutrophils were observed in the wound irradiated with 222-nm UVC irradiation.
Fig. 5.
Gross appearances of S. aureus-infected skin wounds after UVC irradiation. S. aureus suspension was inoculated onto the skin wounds of mice, and the wounds were irradiated with 150 mJ/cm2 of 222-nm and 254-nm UVC light at 1 h after inoculation. Wounds were observed immediately and on days 3, 5, 8 and 12 after UVC irradiation (A). The skin wounds were entirely healed until day 18 after UVC irradiation (B). Scales bars represent 5 mm.
Fig. 6.
Histology of S. aureus-infected wounds after UVC irradiation. Mouse skin wounds were infected with S. aureus and then irradiated with 150 mJ/cm2 of 222-nm and 254-nm UVC light at 1 h after inoculation. Wound tissue samples were taken on day 5 (A–C) and day 8 (D–F) after irradiation. Tissue sections were stained with hematoxylin and eosin (A, D). Arrows indicate migration of epidermal cells. Immunohistochemistry was performed to detect neutrophils (B, E). Arrows indicate neutrophil infiltration. S. aureus cells were detected by immunohistochemistry (C, F). Arrows indicate S. aureus aggregates.
3.4. Effect of 222-nm UVC exposure on DNA damage
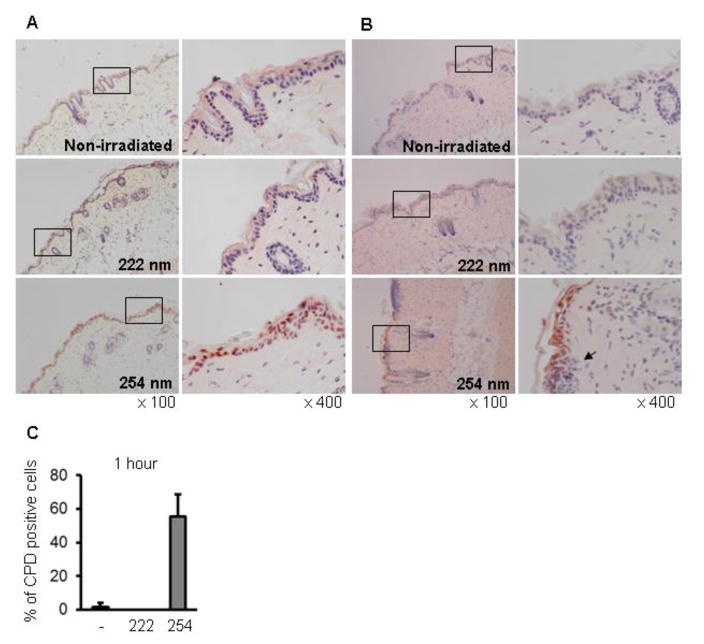
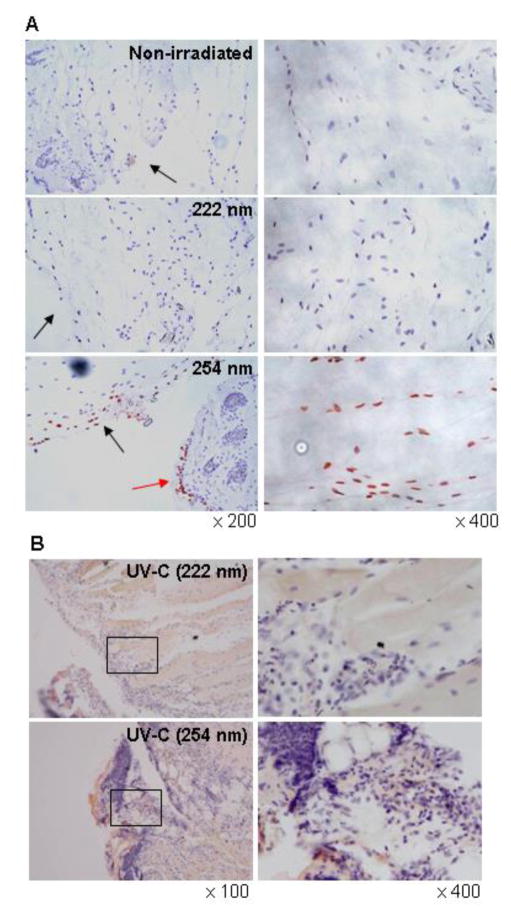
It is known that UVC radiation induces CPD and (6–4) photoproducts (6-4PPs) in DNA, which lead to skin carcinogenesis. Thus, we evaluated the effect of 222-nm UVC on DNA damage. Immediately after irradiation, CPD-expressing cells were detected in the epidermis irradiated with 254-nm but not 222-nm UVC, and the percentage of CPD-expressing cells in keratinocytes was approximately 60% (Fig. 7A and C). Inflammatory cell infiltration was observed under the basement membrane of skin irradiated with 254-nm UVC on day 1 after irradiation (Fig. 7B). We further evaluated the effect of 222-nm UVC irradiation on DNA damage in S. aureus-infected wound tissue. CPD-expressing cells were detected in the subcutaneous tissue irradiated with 254-nm but not 222-nm UVC at immediately after irradiation (Fig. 8A). One day after irradiation, CPD-expressing cells were not detected but massive inflammatory cell infiltration was detected in the 254-UVC irradiated subcutaneous tissue (Fig. 8B).
Fig. 7.
Effect of 222-nm UVC exposure on DNA damage in mouse dorsal skin. Mice dorsal skins were irradiated with 222-nm or 254-nm UVC at 150 mJ/cm2 and excised immediately (A) and on day 1 after irradiation (B). CPD-expressing cells were detected by immunohistochemical staining (red-brown). An arrow indicates inflammatory cell infiltration. (C) CPD-positive cells were quantified by counting the cells in 10 random high-power (× 400) fields of each section.
Fig. 8.
Effect of 222-nm UVC exposure on DNA damage in S. aureus infected wound tissue. Mouse skin wounds were made and infected with S. aureus. The wounds were irradiated with 150 mJ/cm2 of 222-nm and 254-nm UVC light at 1 h after infection. Wound tissue samples were taken immediately (A) and on day 1 after irradiation (B). CPD-retaining cells were detected by immunohistochemical staining (red-brown). Black arrows indicate wound surface. Red arrow indicates epidermis.
4. Discussion
SSI is the most common type of nosocomial infection occurring in hospitals, caused by contamination of surgical wounds with natural commensal bacteria and by hospital pathogens, of which S. aureus is one of the most frequently detected [22–25]. Since their discovery in the 1950s, SSI and wound infections have been more or less successfully treated with antibiotics. However, widespread use and overuse of antibiotics has led to the emergence of many drug-resistant bacteria. Over the past decade, multidrug-resistant bacteria such as MRSA have attracted increased attention in clinical settings leaving physicians with few therapeutic options. It has been reported that multidrug-resistant bacteria have the same level of sensitivity to UVC irradiation as their wild-type counterparts. Hence, UVC might become an alternative approach to current therapeutic approaches for SSI [20].
Taking into account the DNA-damaging effect of 254-nm UVC light on normal human cells, 200–222 nm UVC light becomes more attractive for prevention and therapy of wound infections as its bactericidal efficacy is comparable, but its adverse effects on human cells is significantly less. The results of Buonanno et al. showed that 207- and 222-nm UVC light could inactivate bacteria efficiently but do not appear to be significantly cytotoxic or mutagenic to human keratinocytes or to normal mouse skin [17–19]. Although the germicidal efficacy and skin safety of 207- and 222-nm UVC light have been confirmed, most of the previous experiments were only performed in vitro and using undamaged hairless mouse skin [17–20]. In this study, we evaluated the bactericidal and side effects of 222-nm UVC light on normal mouse skin and directly on infected wounds.
In our in vivo study, about 2 log CFU of S. aureus inoculated onto dorsal skin of mice was killed by the irradiation with 75 mJ/cm2 of 222-nm UVC (Fig. 2). However approximately 2.5 log CFU of S. aureus still survived on the dorsal skin after 222-nm UVC irradiation, and the bactericidal effect of the larger dose of 222-nm UVC (150 mJ/cm2 and 450 mJ/cm2) was not significantly different to that of the irradiation with 75 mJ/cm2. Because the skin surface is not completely smooth and is covered by intersecting grooves called “sulci cutis”, it might be possible that S. aureus cells were distributed in such a way within these sulci cutis grooves, or in hair follicles and were not irradiated with a sufficient dose of UVC to elicit the full bactericidal effect. In other words, some bacteria could have been shielded by the skin surface irregularities.
Several studies on the antimicrobial effect of 254-nm UVC light in wound infections were previously reported. By using a mouse model of burn infection, Dai et al. reported that irradiation with 254-nm UVC significantly reduced the fungal burden in burns infected with Candida albicans [26]. Moreover, 254-nm UVC light irradiation reduced the bacterial burden in skin wounds infected with Pseudomonas aeruginosa, S. aureus and Acinetobacter baumannii [27, 28]. In addition to the germicidal effect, irradiation of 254-nm UVC induced DNA lesions in the wounds [27, 28] but these were repaired in the following days. In addition to the bactericidal activity on the normal mouse skin, the effect of 222-nm UVC on wound infections was further investigated in our study. Immediately after irradiation, the bacterial numbers in the wounds irradiated with 222-nm UVC at 75, 150, 750 and 1500 mJ/cm2 were 0.9, 1.2, 1.4 and 1.6 log CFU lower than that in non-irradiated wounds, respectively. These results indicated that the bactericidal effect of 222-nm UVC light was less effective to S. aureus inoculated on skin wound than when the same bacteria were inoculated on normal mouse skin. It is known that wound exudate, a protein-rich fluid produced in response to tissue damage, is present in wound. It contains various types of cells and many kinds of proteins including inflammatory mediators as well as proteolytic enzymes [29]. Thus it might be possible that proteins in the wound exudate absorb UVC light thus protecting the bacteria, and the remaining bacterial cells which were not eradicated, proliferate and grow in the wound after UV irradiation. Fig. 3B and D demonstrated that 254-nm UVC at 150 and 1500 mJ/cm2 elicited a more effective bactericidal effect than irradiation with 222-nm UVC light when measured immediately after UV irradiation. These results suggest that 254-nm UVC light tends to show a more powerful antibacterial effect than that of 222-nm UVC light. On day 1 after irradiation, although the bacterial counts in wounds had increased in comparison to those found immediately after irradiation, the bacterial burden in the skin wounds irradiated with 222-nm UVC at 750 mJ/cm2 and 254-nm UVC at 75 and 1500 mJ/cm2 was 1 log CFU less than that in the non-irradiated wounds (Fig 3A–D).
The kinetics of bacterial burden in the wounds irradiated with 222-nm UVC at 75 mJ/cm2 and 222- and 254-nm UVC lights at 150 mJ/cm2 are shown in Fig. 4. The bacterial counts in the wound irradiated with 222-nm UVC at 75 mJ/cm2 were comparable to those in the non-irradiated wound, whereas the bacteria in the wounds irradiated with 222-nm and 254-nm UVC at 150 mJ/cm2 were reduced, compared with the non-irradiated wounds. Unexpectedly, the bacterial count in the wound irradiated with 254-nm UVC was higher than that in non-irradiated wounds on day 8. It is known that the scab that forms on the wound surface contains higher numbers of microorganisms than the underlying tissue [30]. One function of the scab appears to be to “wall off” and contain any bacteria until the wound is completely healed. It has also been suggested that prevention of such scab formation is able to reduce the risk of infection and induce faster wound healing [29]. As shown in Fig. 5, a scab was formed on both the irradiated and non-irradiated wounds on day 3 after infection. A decrease in the size of the scab was obvious in the non-irradiated and 222-nm UVC irradiated wounds, but not in the 254-nm UVC irradiated wounds at day 8. Thus, the increase of bacterial burden in the 254-nm UVC irradiated wounds on day 8 might be due to more bacterial growth in the scab since aggregates with S. aureus was also detected in the scab of 254-nm UVC irradiated wound (Fig. 6F). On the other hand, aggregates of S. aureus were found in the granulation tissues of non-irradiated wounds at day 8 after irradiation (Fig 6F). These bacterial aggregates are consistent with a larger neutrophil infiltration, which was detected in the granulation tissue of non-irradiated wound (Fig. 6E). These results suggest that decrease of bacterial burden in the non-irradiated wounds on day 8 might be due to reduction of the scab size. Moreover, bacterial burden in the 222-nm UVC irradiated wound on day 8 was lower than the wounds other treatments. This might be due to reduction of the scab size and also ability of 222-nm UVC to reduce the number of S. aureus cells which correlates with less inflammatory response seen in the 222-nm UVC wound in Fig. 6E.
In the wound healing process, epithelial cells from the wound edge begin to migrate over the surface of wound “burrowing a path” beneath the scab and the underlying granulation tissue [31]. It has been previously reported that UV exposure might be beneficial for wound healing by inducing hyperplasia, granulation tissue formation, sloughing of necrotic tissue and enhancing re-epithelialization [7, 20]. However, UVB irradiation of the skin induces a sterile inflammation resulting in a dose-dependent erythema [32], and irradiation with UV light induces the direct or indirect formation of several types of DNA lesions in human cells as well as in microorganisms. The form of DNA damage involves CPD and 6-4PPs, and CPD is most strongly involved in human skin cancer [12]. Our results demonstrated that irradiating with 254-nm UVC but not with 222-nm UVC impaired the burrowing or migration of keratinocytes across the wound (Fig. 6A), and the hard scab remained attached to the wound irradiated with 254-nm UVC on day 8 after irradiation (Fig. 5 and 6D). However, the timing of scab disappearance in the wounds irradiated with 222-nm UVC were comparable to 254-nm UVC irradiated wounds and non-irradiated wounds (Fig. 5B). It was suggested that irradiation with 222-nm but not 254-nm UVC could promote healing of infected wound in the early stage after injury, but could not have an effect on duration of entire wound healing. CPD-retaining cells accounted for approximately 60% of keratinocytes in the epidermis irradiated with 254-nm UVC at immediately after irradiation (Fig. 7A and C). CPD-expressing cells were still detected, and inflammatory cell infiltration was seen beneath the epidermis on day 1 after 254-nm UVC but not after 222-nm UVC irradiation (Fig. 7B). Fibroblasts are the main type of mesenchymal cells in the dermis. Fibroblasts produce fibrillar extracellular matrix (collagen) which plays a crucial role in repair of skin and wound healing [33]. Because UV-induced CPD is the most predominant and persistent type of DNA lesion, decrease in the repair rates of the lesions in dermal fibroblasts may cause severe structural distortions in the DNA molecule affecting important cellular processes such as DNA replication, transcription and ultimately leading to mutagenesis and tumorigenesis [34, 35]. As shown in Fig. 8, CPD-expressing cells were detected in the dermal tissue as well as the epidermal tissue of the wound immediately after irradiation with 254-nm but not 222-nm UVC. Although CPD- expressing cells were not detected in the dermal tissue of the wound at day 1 after irradiation, massive inflammatory cell infiltration was seen in the 254-nm but not 222-nm UVC irradiated wounds. These results suggest that irradiation with 254-nm but not 222-nm UVC induces prominent DNA damage and inflammation in wounded dermis as well as epidermis.
In this study, we have demonstrated that 222-nm UVC light emitted by an Xe-Cl lamp showed effective bactericidal activity in S. aureus-infected wounds with less mutagenic and cytotoxic effect compared to more conventional 254-nm UVC. Although further investigations will be required for any application of 222-nm UVC light in clinical settings, the Xe-Cl lamp has a high potential. This lamp is promising to reduce SSI and wound infections especially by multi-drug resistance bacteria.
Highlights.
Irradiation with 222-nm UVC showed a bactericidal effect against MRSA in vivo
Irradiation with 222-nm UVC reduced bacterial counts in MRSA-infected wounds
222-nm UVC light did not induce CPD in either epidermal or dermal cells, while 254-nm UVC did
Acknowledgments
Michael R Hamblin was funded by US NIH grants R01AI050875 and R21AI121700. Tianhong Dai was funded by US NIH grants R01AI123312 and R21AI109172, US DoD grants W81XWH-14-2-0008 and FA9550-16-1-0479.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. 2008;70(Suppl 2):3–10. doi: 10.1016/S0195-6701(08)60017-1. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Kaye KS. Staphylococcal surgical site infections. Infect Dis Clin North Am. 2009;23:53–72. doi: 10.1016/j.idc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Kaye KS, Anderson DJ, Choi Y, Link K, Thacker P, Sexton DJ. The deadly toll of invasive methicillin-resistant Staphylococcus aureus infection in community hospitals. Clin Infect Dis. 2008;46:1568–1577. doi: 10.1086/587673. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Sexton DJ, Kanafani ZA, Auten G, Kaye KS. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:1047–1053. doi: 10.1086/520731. [DOI] [PubMed] [Google Scholar]
- 5.Altemeier WA, Culbertson WR, Hummel RP. Surgical considerations of endogenous infections-sources, types, and methods of control. Surg Clin North Am. 1968;48:227–240. doi: 10.1016/s0039-6109(16)38448-1. [DOI] [PubMed] [Google Scholar]
- 6.Menichetti F. Current and emerging serious Gram-positive infections. Clin Microbiol Infect. 2005;11(Suppl 3):22–28. doi: 10.1111/j.1469-0691.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Avci P, Dai T, Huang YY, Hamblin MR. Ultraviolet radiation in wound care: sterilization and stimulation. Adv Wound Care (New Rochelle) 2013;2:422–437. doi: 10.1089/wound.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JC, Ossoff SF, Lobe DC, Dorfman MH, Dumais CM, Qualls RG, Johnson JD. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49:1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurzadyan GG, Görner H, Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141:244–251. [PubMed] [Google Scholar]
- 10.Pfeifer GP, You YH, Besaratinia A. Mutations induced by ultraviolet light. Mutat Res. 2005;571:19–31. doi: 10.1016/j.mrfmmm.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Ritter MA, Olberding EM, Malinzak RA. Ultraviolet lighting during orthopaedic surgery and the rate of infection. J Bone Joint Surg Am. 2007;89:1935–1940. doi: 10.2106/JBJS.F.01037. [DOI] [PubMed] [Google Scholar]
- 12.Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldfarb AR, Saidel LJ. Ultraviolet absorption spectra of proteins. Science. 1951;114:156–157. doi: 10.1126/science.114.2954.156. [DOI] [PubMed] [Google Scholar]
- 14.Setlow RB, Preiss JW. Spectra of some amino acids, peptides, nucleic acids, and protein in the vacuum ultraviolet. J Chem Phys. 1956;25:138–141. [Google Scholar]
- 15.Coohill TP. Virus-cell interactions as probes for vacuum-ultraviolet radiation damage and repair. Photochem Photobiol. 1986;44:359–363. doi: 10.1111/j.1751-1097.1986.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 16.Green H, Boll J, Parrish JA, Kochevar IE, Oseroff AR. Cytotoxicity, mutagenicity of low intensity, 248 and 193 nm excimer laser radiation in mammalian cells. Cancer Res. 1987;47:410–413. [PubMed] [Google Scholar]
- 17.Buonanno M, Randers-Pehrson G, Bigelow AW, Trivedi S, Lowy FD, Spotnitz HM, Hammer SM, Brenner DJ. 207-nm UV light-a promising tool for safe low-cost reduction of surgical site infections. I: in vitro studies. PLoS One. 2013;8:e76968. doi: 10.1371/journal.pone.0076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buonanno M, Stanislauskas M, Ponnaiya B, Bigelow AW, Randers-Pehrson G, Xu Y, Shuryak I, Smilenov L, Owens DM, Brenner DJ. 207-nm UV light-a promising pool for safe low-cost reduction of surgical site infections. II: in-vivo safety studies. PLoS One. 2016;11:e0138418. doi: 10.1371/journal.pone.0138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliasson B, Kogelschatz U. UV excimer radiation from dielectric-barrier discharges. Appl Phys B. 1988;46:299–303. [Google Scholar]
- 20.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10:185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun. 1995;63:1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionigi R, Rovera F, Dionigi G, Imperatori A, Ferrari A, Dionigi P, Dominioni L. Risk factors in surgery. J Chemother. 2001;13:6–11. doi: 10.1179/joc.2001.13.Supplement-2.6. [DOI] [PubMed] [Google Scholar]
- 23.Davis N, Curry A, Gambhir AK, Panigrahi H, Walker CR, Wilkins EG, Worsley MA, Kay PR. Intraoperative bacterial contamination in operations for joint replacement. J Bone Joint Surg Br. 1999;81:886–889. doi: 10.1302/0301-620x.81b5.9545. [DOI] [PubMed] [Google Scholar]
- 24.Anguzu JR, Olila D. Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr Health Sci. 2007;7:148–154. doi: 10.5555/afhs.2007.7.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lilani SP, Jangale N, Chowdhary A, Daver GB. Surgical site infection in clean and clean-contaminated cases. Indian J Med Microbiol. 2005;23:249–252. [PubMed] [Google Scholar]
- 26.Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d’Enfert C, Hamblin MR. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol. 2011;87:342–349. doi: 10.1111/j.1751-1097.2011.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai T, Garcia B, Murray CK, Vrahas MS, Hamblin MR. UVC light prophylaxis for cutaneous wound infections in mice. Antimicrob Agents Chemother. 2012;56:3841–3848. doi: 10.1128/AAC.00161-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai T, Murray CK, Vrahas MS, Baer DG, Tegos GP, Hamblin MR. Ultraviolet C light for Acinetobacter baumannii wound infections in mice: potential use for battlefield wound decontamination? J Trauma Acute Care Surg. 2012:661–667. doi: 10.1097/TA.0b013e31825c149c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadwick P, McCardle J. Exudate management using a gelling fibre dressing. The Diabetic Foot Journal. 2015;18:43–48. [Google Scholar]
- 30.van der Pol E, Mudde YD, Coumans FA, van Leeuwen TG, Sturk A, Nieuwland R. Wound scabs protect regenerating tissue against harmful ultraviolet radiation. Med Hypotheses. 2016;96:39–41. doi: 10.1016/j.mehy.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013;28:397–409. doi: 10.1016/j.jmbbm.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, Rust W, Hildebrandt T, Geisslinger G, Orengo C, Bennett DL, McMahon SB. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-B-induced inflammation. PLoS One. 2014;9:e93338. doi: 10.1371/journal.pone.0093338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichihashi M, Ando H, Yoshida M, Niki Y, Matsui M. Photoaging of the skin. Anti-Aging Medicine. 2009;6:46–59. [Google Scholar]
- 35.Ramasamy K, Shanmugam M, Balupillai A, Govindhasamy K, Gunaseelan S, Ganesan M, Mary RB, Prasad NR. Ultraviolet radiation-induced carcinogenesis: Mechanisms and experimental models. J Radiat Cancer Res. 2017;8:4–19. [Google Scholar]