Abstract
PD2 (Pancreatic Differentiation 2)/PAF1 (RNA Polymerase II-Associated Factor 1) is the core subunit of the human PAF1 complex (PAF1C) that regulates the promoter-proximal pausing of RNA polymerase II as well as transcription elongation and mRNA processing, and coordinates events in mRNA stability and quality control. As an integral part of its transcription regulatory function, PD2/PAF1 plays a role in post-translational histone covalent modifications as well as regulates expression of critical genes of the cell cycle machinery. PD2/PAF1 alone, and as a part of PAF1C, provides distinct roles in the maintenance of self-renewal of embryonic stem cells and cancer stem cells, and in lineage differentiation. Thus, PD2/PAF1 malfunction or its altered abundance is likely to affect normal cellular functions leading to disease states. Indeed, PD2/PAF1 is found to be up-regulated in poorly differentiated pancreatic cancer cells and has the capacity for neoplastic transformation when ectopically expressed in mouse fibroblast cells. Likewise, PD2/PAF1 is up-regulated in pancreatic and ovarian cancer stem cells. Here, we concisely describe multifaceted roles of PD2/PAF1 associated with oncogenic transformation, and implicate PD2/PAF1 as an attractive target for therapeutic development to combat malignancy.
Introduction
The human PAF1(RNA polymerase II-Associated Factor 1) complex (PAF1C), an assembly of five proteins (PAF1, CDC73, CTR9, LEO1, and SKI8 (1)), is highly conserved across different species (1–3). Similar to its yeast counterpart, PAF1C has a specific role in transcriptional elongation, mRNA maturation and processing, histone covalent modifications, and telomere silencing via its interaction with a variety of factors (2,4,5). The majority of interactions of PAF1C with its binding partners are primarily mediated through its PAF1 (also known as pancreatic differentiation 2 or PD2) subunit. Therefore, PD2/PAF1 has evolved as an integral part of the RNA polymerase II (Pol II)-associated molecular network and has been shown to be a key player in important cellular processes including oncogenic transformation, cell cycle, chromatin organization, stem cell self-renewal, and pluripotency (1,6–10). Of interest, ectopic expression of PD2/PAF1 in NIH3T3 cells leads to increased cellular proliferation and to aggravated tumorigenicity (9), which has underlined its role as a critical tumor promoter when up-regulated. Indeed, PD2/PAF1 is up-regulated in poorly differentiated pancreatic cancer (PC) cells (9), and an enhanced abundance of PD2/PAF1 induces pancreatic tumorigenesis and enhanced metastasis (11). PD2/PAF1 has also been implicated in development of other malignancies via its interaction and/or regulation of other proteins. For instance, PD2/PAF1 is predicted to be a prognostic marker for early stage non-small cell lung cancer (NSCLC) as it is aberrantly elevated in early-stage NSCLC tumor samples and promotes NSCLC tumorigenesis via c-MYC transcriptional activation (12). Similarly, PAF1C interacts with CxxC-RD2 region of mixed lineage leukemia (MLL), a region that is always retained in MLL-rearranged oncoproteins and this interaction is indispensable for MLL-rearranged oncoproteins-mediated leukemogenesis (13). In addition, PD2/PAF1 is found to be upregulated in pancreatic and ovarian cancer stem cells (CSCs) (7,14). Here, we discuss the varied functions of PD2/PAF1 with its involvement in oncogenesis, thus placing PD2/PAF1 at the crossroads of the tumor network.
Historical perspective on PD2/PAF1 with function in transcription
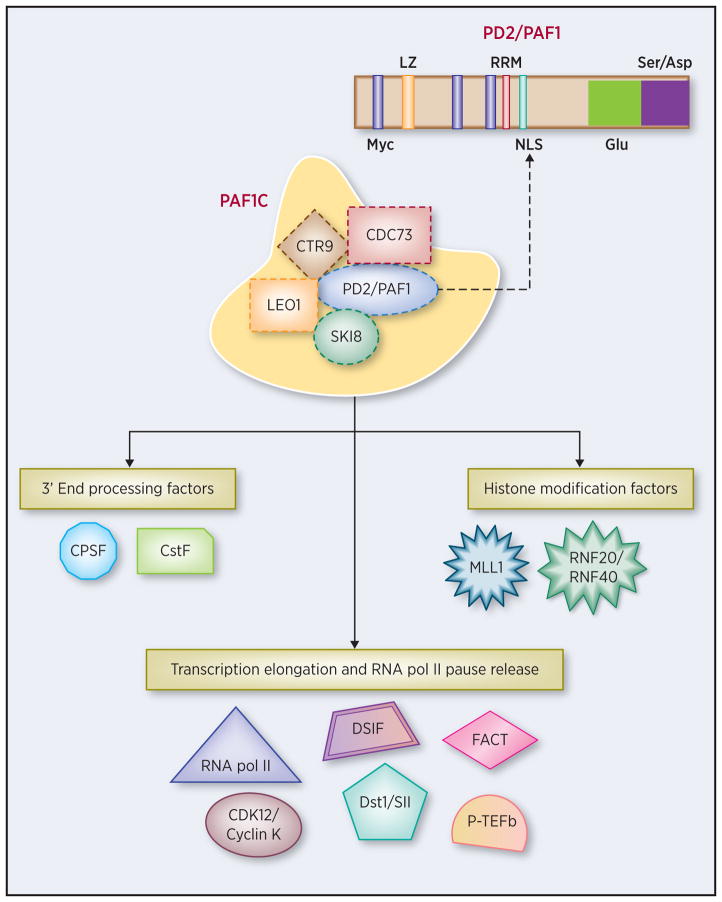
PD2/PAF1 was originally identified as one of the genes having a 30-fold overexpression in Panc1, a poorly differentiated PC cell line, compared to HPAF/CD11, a well-differentiated PC cell line (9). Mapping of the genomic location for PD2/PAF1 revealed that it is present in the 19q13.2 amplicon locus, which harbors the potent oncogene AKT2 (9), suggesting a concerted function between PD2/PAF1 and AKT2 in stabilization of the locus. Domain architecture of PD2/PAF1 (Figure 1) indicates possible interactions with RNA, concurrent with its function as a key molecular member of the cellular transcription network and mRNA stability (1).
Figure 1. PAF1 complex (PAF1C) and its interacting partners.
Human PAF1C consists of 5 components: PAF1 or PD2 (Pancreatic differentiation 2), CDC73, SKI8, LEO1, and CTR9. The domain organization of PD2/PAF1 consists of three Myc type helix-loop-helix, a leucine zipper (LZ), an RNA recognition motif (RRM), Ser/Asp-rich region, a Glu-rich domain and a nuclear localization signal (NLS). PAF1C interacts with a variety of factors involved in histone covalent modifications, transcription and mRNA 3′ end processing. These include histone chaperone FACT (Facilitates chromatin transcription), RNF20/RNF40 (that catalyzes histone H2B monoubiquitination), methyltransferases such as mixed lineage leukemia 1 (MLL1) that methylates histone H3 at K4, RNA Pol II, CDK12/Cyclin K complex, positive transcription elongation factor b (P-TEFb), elongation factors such as TFIIS (Dst1/SII) and DRB sensitivity-inducing factor (DSIF), human cleavage and poly (A) factors that include cleavage and polyadenylation specificity factor (CPSF) and cleavage stimulation factor (CstF).
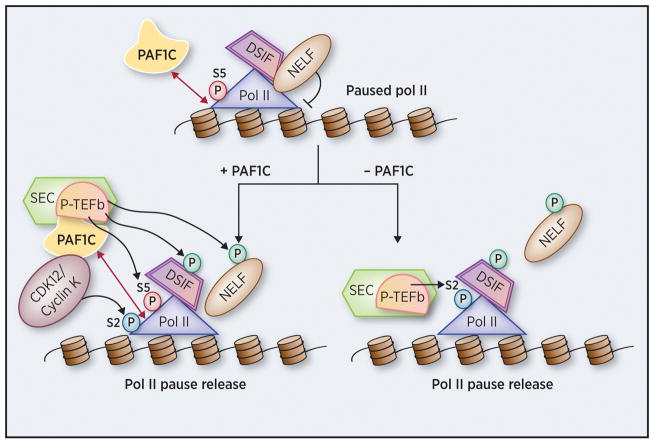
As a member of PAF1C, PD2/PAF1 is involved in transcription elongation via direct interactions with Pol II, Facilitates chromatin transcription (FACT), Positive Transcription Elongation Factor (P-TEFb) and other factors (15–17) (Figure 1). The roles of PD2/PAF1 in transcription have been reviewed elsewhere (15,18). We discuss here the less explored function of PAF1C as the regulator of promoter-proximal pause release of Pol II, a phenomenon essential for effective transcription elongation in metazoans. Pausing of Pol II at 20–60 nucleotides downstream of the transcription start site, known as Pol II promoter-proximal pausing, is mediated primarily via binding of DRB sensitivity-inducing factor (DSIF) and negative elongation factor (NELF) to Pol II. Pause release is thought to require phosphorylation of DSIF, NELF (and its subsequent dissociation), and C–terminal domain (CTD) serine 2 (S 2) of the largest subunit of Pol II. (Figure 2). Chen et al. have recently shown that loss of PD2/PAF1 in colorectal carcinoma HCT116 cells results in increased Pol II occupancy within 9,333 gene bodies (19). They further demonstrated that the Super Elongation Complex (SEC) is rapidly recruited to Pol II upon PD2/PAF1 loss (19). However, Yu et al. propose that PD2/PAF1 may function as a positive or negative regulator of Pol II promoter proximal pausing based on the physiological states and genetic backgrounds of cells (17). For instance, PD2/PAF1 knockdown in human acute lymphoblastic leukemia CCRF-CEM cells caused a decrease in Pol II pausing in a majority of genes, implying that PD2/PAF1 is a negative regulator of Pol II pausing release (17). In contrast to the results obtained in CCRF-CEM cells, knockdown of PD2/PAF1 in acute myeloid leukemia THP1 cells led to increased promoter-proximal pausing of Pol II (17). They also demonstrated that PAF1C is responsible for recruitment of CDK12-Cyclin K, which has been suggested as the predominant Pol II CTD Ser2 kinase. The role of PD2/Paf1 as the positive regulator of promoter-proximal paused Pol II release is further supported in yeast (16), wherein the N-terminal domain of an mRNA capping enzyme Cet1 recruits FACT, which in turn targets PAF1C to active genes for release of promoter proximally paused Pol II. In another study, MYC-PAF1C interaction was reported to inhibit transition to the elongating form of Pol II as proteasomal degradation of MYC promotes transcription elongation (20). Thus, there is ample literature suggesting that PD2/PAF1 plays specific roles in the regulation of Pol II promoter-proximal pause release. Such functions of PD2/PAF1 underline its importance in gene regulation in the pluripotency network. In the years following its discovery, PD2/PAF1 gained attention as a key oncogenic protein, overexpressed in various cancers such as pancreatic cancer, ovarian cancer, non-small cell lung cancer, and endocrine tumors (1,7,9,12,21). Our study demonstrating transformation of fibroblast cells on targeted overexpression of PD2/PAF1 further emphasized its neoplastic action and encouraged elucidation of its specific roles in tumor pathogenesis (Figure 3). Such outcomes could be mediated via multiple pathways (e.g., cell cycle, histone covalent modifications, chromatin remodeling, MAPK or mitogen activated protein kinase, estrogen signaling and others), as described below.
Figure 2. PD2/PAF1 as the regulator of RNA Pol II promoter-proximal pausing.
Although PD2/PAF1 is found to regulate promoter-proximal Pol II pause release, two opposing models have been proposed. In one model, positive transcription elongation factor b (P-TEFb) as a part of the super elongation complex (SEC) is responsible for serine 5 (S5) phosphorylation of the CTD (C-terminal domain) of the largest subunit of Pol II, DSIF and negative elongation factor (NELF), leading to subsequent dissociation of NELF. Further, P-TEFb recruits PAF1C to promoter proximal regions, which in turn recruits the CDK12/Cyclin K complex for serine 2 (S2) phosphorylation of the Pol II CTD, resulting in productive Pol II elongation (left panel) (17). In another model, the loss of PAF1C is essential for recruitment of SEC, which mediates Pol II CTD S2 phosphorylation (right panel) (19). Phosphorylation of DSIF and NELF (and subsequent dissociation) is also required for effective Pol II pause release along with S2 phosphorylation of the Pol II CTD.
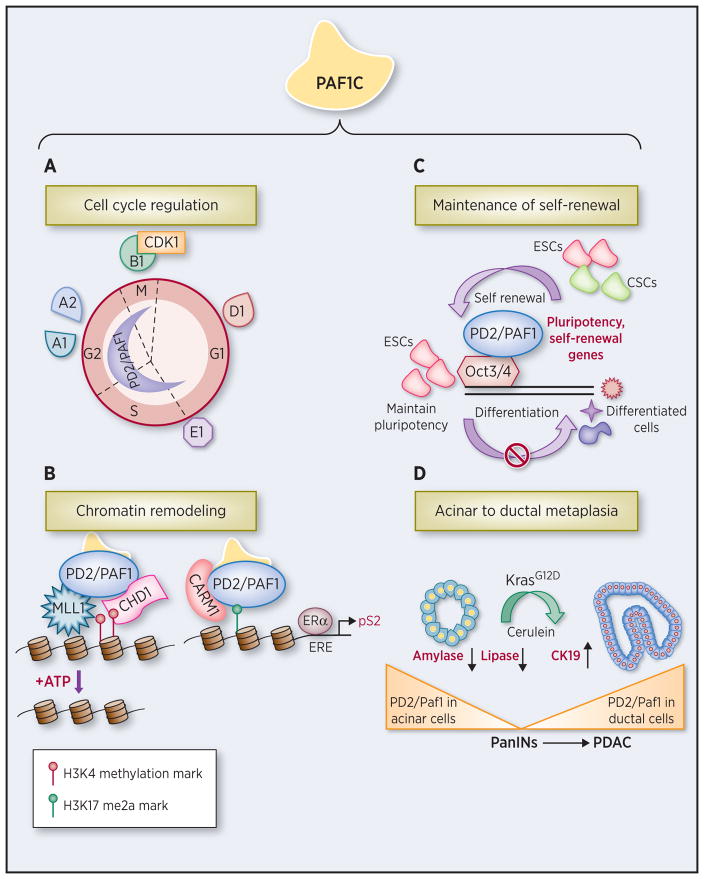
Figure 3. PD2/PAF1 as a master regulator of key cellular processes.
PD2/PAF1 and PAF1C have several distinct functions in key cellular processes. A. PD2/PAF1 controls cell cycle progression by specifically regulating a subclass of genes (cyclins A1, A2, D1, E1, B1, and cyclin dependent kinase, CDK1) implicated in cell cycle progression during G1/S, S/G2, and G2/M. B. PD2/PAF1 performs histone covalent modifications and chromatin remodeling via interaction with the histone methyltransferase MLL1 and regulates the expression as well as nuclear import of CHD1 (an ATPase dependent chromatin remodeling protein that specifically binds to methylated-K4 of histone H3). PAF1C is a ‘reader’ of the histone H3R17me2a mark (di-methylated arginine of histone H3) and is recruited to this specific histone modification mark by CARM1 (coactivator-associated arginine methyltransferase 1) to regulate downstream transcription of ERα (estrogen receptor α) target genes such as pS2. ERE, estrogen responsive element. C. PD2/PAF1 interacts with Oct3/4 in mouse embryonic stem cells (ESCs) and ovarian cancer stem cells (CSCs), and thereby promotes self-renewal possibly by regulating self-renewal genes. D. PD2/Paf1 is expressed in acinar cells of normal pancreas. However, in the case of progressive inflammation driven by cerulein treatment in the background of Kras mutation, PD2/Paf1 is expressed in amylase and CK19 double-positive metaplastic ducts that represent intermediate structures in the process of acinar to ductal metaplasia (ADM). PD2/Paf1 expression is lost and regained during acinar trans-differentiation in PC initiation and it mediates regulation of lineage-specific markers. PanIN, pancreatic intraepithelial neoplasia; and PDAC, pancreatic ductal adenocarcinoma.
PD2/PAF1 in regulation of cell cycle
As mentioned above, PD2/PAF1 is located in chromosome 19 concurrently with the Akt2 gene, which is an isoform of the serine-threonine protein kinase B family with a well-established role in regulating cell cycle (1,22). Thus, PD2/PAF1 is likely to be involved in regulation of the cell cycle. Indeed, PD2/PAF1 has been found to control cell cycle genes coherent with its temporally as well as spatially oscillatory expression patterns during cell cycle progression, similar to the expression profile for cyclins. PD2/PAF1 specifically regulates a subclass of genes (cyclins A1, A2, D1, E1, B1, and cyclin dependent kinase CDK1) directly implicated in cell cycle progression during G1/S, S/G2, and G2/M (Figure 3A) (8). Expression of PD2/PAF1 also delays DNA replication but favors G2/M transition, in part through microtubule assembly and mitotic spindle formation (8). One of the hallmarks of tumorigenesis is uncontrolled cell proliferation due to loss of cell cycle regulation. Thus, upregulated PD2/PAF1 in pancreatic and other cancers might be involved in promoting cell propagation during oncogenesis owing to its cell cycle regulatory function.
PD2/PAF1 in regulation of histone covalent modifications and chromatin remodeling
PAF1C facilitates the process of transcription elongation through recruitment of several factors that perform post-translational histone covalent modifications (4,6,23–25). PD2/PAF1 as a part of the PAF1C has been found to regulate ubiquitylation of histone H2B through interaction with RNF20/RNF40, which facilitates further downstream histone covalent modifications including H3K4 and H3K79 methylation (24–26). The PD2/PAF1 subunit of both yeast and human PAF1C has been shown to be involved in histone H3K4 and H3K79 methylation (6,18,24,25,27). Furthermore, PD2/PAF1 regulates the dimethylation and trimethylation of H3K4 through interaction with histone methyltransferase (6). Our study further demonstrates that interaction between PD2/PAF1 and CHD1 regulates nuclear import of CHD1, an ATPase-dependent chromatin remodeling protein, and their interaction is responsible for nucleosomal structure rearrangement, which can lead to subsequent changes in gene expression (Figure 3B) (6,28). Another independent study has shown that PAF1C associates with the MLL complex, via contact with the PD2/PAF1 subunit at the HOX gene locus to control leukemogenesis (13).
PD2/PAF1 as an effector of MAPK and estrogen signaling pathways
PAF1C is also found to act as a critical mediator in key signaling pathways related to oncogenesis. For example, a recent study by Kim et al. shows that yeast Mpk1, a MAPK activated in response to cellular stress, regulates transcription elongation in conjunction with PAF1C via its interaction with PD2/Paf1 subunit through a conserved D motif (29). Of interest, complementation studies performed with human PD2/PAF1 and ERK5 (human homologue of yeast Mpk1) demonstrate that this function is conserved in mammals as well (29). Human ERK5 is known to be a MAPK family member, one that plays a critical role in EGF (epidermal growth factor)-induced cell proliferation (30). Further, ERK5 transcriptional activity and signaling has been correlated to tumorigenesis of breast and prostate (31,32). Another study by Wu et al. highlights a novel role of human PAF1C as a ‘reader’ of the histone H3R17me2a mark (Figure 3B) (33). PAF1C is recruited to this specific histone modification mark by CARM1 (coactivator-associated arginine methyltransferase 1) to regulate downstream transcription of ERα (estrogen receptor α)-target genes such as pS2 that is found to be overexpressed in breast cancer (33). Interestingly, the recruitment of PAF1C to methylated histones is achieved through strong binding via PD2/PAF1 subunit. Therefore, the role of PD2/PAF1 as the chief interacting subunit of PAF1C in key signaling events associated with oncogenesis reemphasizes its importance in cancer pathogenesis.
PD2/PAF1 in organogenesis, and maintenance of stem cells and cancer stem cells
PAF1C is also implicated in the development of different species, from zebrafish to higher eukaryotes (34–36). It has been shown to regulate cardiac specification and heart morphogenesis in zebrafish (34,35). Even in mammals, components of PAF1C such as PD2/PAF1 and LEO1 appear to have roles in particular lineage specification (10,36). Silencing PD2/Paf1 in embryoid bodies has been shown to impair endodermal differentiation (10). Similarly, knockdown of LEO1 as well as PHD-finger protein 5a (Phf5a) blocks myogenic differentiation (36). Further, role of Phf5a in regulating myogenic differentiation appears to be dependent on PAF1C, as it stabilizes PAF1C on chromatin promoting myogenic programs. Indeed, loss of Phf5a led to a significant reduction in LEO1 occupancy at myogenic genes (36). These studies implicate the roles of PAF1C (or its components, PD2/PAF1 and LEO1) in regulating the genes involved in pluripotency and organismal development.
Using a genome-wide screen for key pluripotency genes such as Oct3/4, Ding et al. found PAF1C to be an important candidate for regulating stemness (37). Our study also shows that PD2/Paf1 heterozygous knockout in mouse embryonic stem cells affects maintenance of embryonic stem cells by downregulating Oct3/4, Sox2, and Nanog, critical “gate-keeper” and self-renewal genes highly expressed in early embryonic development for maintenance of pluripotency (10). Further, PD2/Paf1 plays an independent role in regulating self-renewal of mouse embryonic stem cells by interacting with Oct3/4 (Figure 3C) (10). From this perspective, the role of PD2/PAF1 in self-renewal is described below in light of its action on CSCs’ maintenance. CSCs are considered a small population of stem/progenitor or “cancer-initiating” cells residing within the tumor cell mass, drug resistant and capable of repopulating it. Of interest, PD2/PAF1 is elevated in pancreatic CSCs compared to non-CSCs, along with other well-known CSC markers such as OCT3/4 and SHH (14). More importantly, loss of PD2/PAF1 expression led to reduced viability of CSCs with a decrease in expression of multi-drug resistant genes and stem-cell markers (14). Further, we recently reported that PD2/PAF1 interacts with OCT3/4 to maintain self-renewal of ovarian CSCs, independently of PAF1C (7). Therefore, PD2/PAF1 emerges as a key molecule in maintenance of CSCs that contribute to tumor recurrence.
PD2/PAF1 in Acinar-to-Ductal Metaplasia (ADM) in pancreatic cancer
Based on the multi-faceted roles of PD2/PAF1 in PC cells, all intrinsically linked to its transcription regulatory function, we propose that PD2/PAF1 plays a unique role during PC pathogenesis. Indeed, PD2/Paf1 is involved in ADM, an early event contributing to PC initiation (38). We illustrate that PD2/Paf1 is absent in normal pancreatic ducts, but specifically present in metaplastic ducts (38). Of interest, cerulein-induced acinar-to-ductal trans-differentiation in mouse model is accompanied by loss of PD2/Paf1 expression, along with reduced acinar markers. Further, downregulation of PD2/Paf1 in pancreatic acinar cells leads to decrease in acinar marker genes, with a simultaneous increase in ductal marker expression, indicating a possible role of PD2/Paf1 in maintaining acinar cell lineage (Figure 3D). These findings are particularly intriguing in the light of earlier findings that demonstrated the role of several pancreas specific transcription factors, including Mist1, Sox9, and Hnf1a in ADM and PC development (38). Future studies will be directed towards delineating the exact mechanisms underlying the role of PD2/PAF1 in PC initiation.
Conclusion and Perspectives
As described above, PD2/PAF1 is a key molecular mastermind of major cellular pathways, and its deregulation is associated with cellular transformation. As a recent paradigm in the field of understanding tumor development, disease relapse, and chemoresistance, studies have begun to focus on the emerging role of CSCs as potent initiator cells, and PD2/PAF1 is upregulated in CSCs. The embryonic stem cell transcriptional and chromatin modifying networks are critical for self-renewal maintenance, and PD2/PAF1 is involved in both processes, hence determining the differentiation fate of embryonic stem cells. Moreover, CHD1, an ATP-dependent chromatin remodeling protein regulated by PD2/PAF1, is known to be important for maintaining open chromatin structure and maintenance of stem cells self-renewal and pluripotency (6). Additionally, Wdr5, a component of the histone methyltransferase complex, MLL1 that interacts with PD2/PAF1, is also known to mediate self-renewal and reprogramming via the stem cells core transcriptional network (39). Thus, PD2/PAF1 is involved in many important cellular events, particularly those related to self-renewal and pluripotency, and malfunction or misregulation of PD2/PAF1 is associated with cellular transformation. Our findings underlining the importance of PD2/PAF1 in maintenance of self-renewal of CSCs (7) implicate PD2/PAF1 as an attractive target for combating CSC-mediated tumor progression and recurrence. The recent findings demonstrating the “unholy nexus” of PD2/PAF1 and the MAPK pathway further highlights its oncogenic potential. Therefore, PD2/PAF1 lies at the heart of many important cellular events, and hence, serves different functions as a part of the larger network. PD2/PAF1 appears to be the “master regulator” that handles these multiple cellular chores simultaneously. Thus, PD2/PAF1 deregulation would tip the balance from normal cellular homeostasis towards oncogenic transformation. Naturally, an in-depth understanding of the detailed functions and modes of actions of PD2/PAF1 is crucial in the context of developing cancer therapeutics.
Acknowledgments
We would like to thank Dr. Adrian E. Koesters, Research Editor at UNMC, for her editorial contribution to this manuscript. The authors on this manuscript are, in parts, supported by the grants from the National Institutes of Health (RO1 CA210637, RO1CA206444, RO1 CA183459 R15GM088798, K22 CA175260 and UO1 CA185148) and Saswati Karmakar is supported by University of Nebraska Medical Center Graduate Studies Fellowship.
References
- 1.Chaudhary K, Deb S, Moniaux N, Ponnusamy MP, Batra SK. Human RNA polymerase II-associated factor complex: dysregulation in cancer. Oncogene. 2007;26:7499–507. doi: 10.1038/sj.onc.1210582. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Guermah M, Roeder RG. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell. 2010;140:491–503. doi: 10.1016/j.cell.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Sikes ML, Beyer AL, Schneider DA. The Paf1 complex is required for efficient transcription elongation by RNA polymerase I. Proc Natl Acad Sci U S A. 2009;106:2153–8. doi: 10.1073/pnas.0812939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marton HA, Desiderio S. The Paf1 complex promotes displacement of histones upon rapid induction of transcription by RNA polymerase II. BMC Mol Biol. 2008;9:4. doi: 10.1186/1471-2199-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squazzo SL, Costa PJ, Lindstrom DL, Kumer KE, Simic R, Jennings JL, et al. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 2002;21:1764–74. doi: 10.1093/emboj/21.7.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey P, Ponnusamy MP, Deb S, Batra SK. Human RNA polymerase II-association factor 1 (hPaf1/PD2) regulates histone methylation and chromatin remodeling in pancreatic cancer. PLoS One. 2011;6:e26926. doi: 10.1371/journal.pone.0026926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karmakar S, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, Sheinin YM, et al. hPaf1/PD2 interacts with OCT3/4 to promote self-renewal of ovarian cancer stem cells. Oncotarget. 2017;8:14806–20. doi: 10.18632/oncotarget.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moniaux N, Nemos C, Deb S, Zhu B, Dornreiter I, Hollingsworth MA, et al. The human RNA polymerase II-associated factor 1 (hPaf1): a new regulator of cell-cycle progression. PLoS One. 2009;4:e7077. doi: 10.1371/journal.pone.0007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moniaux N, Nemos C, Schmied BM, Chauhan SC, Deb S, Morikane K, et al. The human homologue of the RNA polymerase II-associated factor 1 (hPaf1), localized on the 19q13 amplicon, is associated with tumorigenesis. Oncogene. 2006;25:3247–57. doi: 10.1038/sj.onc.1209353. [DOI] [PubMed] [Google Scholar]
- 10.Ponnusamy MP, Deb S, Dey P, Chakraborty S, Rachagani S, Senapati S, et al. RNA polymerase II associated factor 1/PD2 maintains self-renewal by its interaction with Oct3/4 in mouse embryonic stem cells. Stem Cells. 2009;27:3001–11. doi: 10.1002/stem.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz AP, Deb S, Rachagani S, Dey P, Muniyan S, Lakshmanan I, et al. Overexpression of PD2 leads to increased tumorigenicity and metastasis in pancreatic ductal adenocarcinoma. Oncotarget. 2015 doi: 10.18632/oncotarget.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhi X, Giroux-Leprieur E, Wislez M, Hu M, Zhang Y, Shi H, et al. Human RNA polymerase II associated factor 1 complex promotes tumorigenesis by activating c-MYC transcription in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;465:685–90. doi: 10.1016/j.bbrc.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Muntean AG, Tan J, Sitwala K, Huang Y, Bronstein J, Connelly JA, et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 2010;17:609–21. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaz AP, Ponnusamy MP, Rachagani S, Dey P, Ganti AK, Batra SK. Novel role of pancreatic differentiation 2 in facilitating self-renewal and drug resistance of pancreatic cancer stem cells. Br J Cancer. 2014;111:486–96. doi: 10.1038/bjc.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaehning JA. The Paf1 complex: platform or player in RNA polymerase II transcription? Biochim Biophys Acta. 2010;1799:379–88. doi: 10.1016/j.bbagrm.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammad RM, Li Y, Muqbil I, Aboukameel A, Senapedis W, Baloglu E, et al. Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases. 2017 doi: 10.1080/21541248.2017.1329694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Yang W, Ni T, Tang Z, Nakadai T, Zhu J, et al. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science. 2015;350:1383–6. doi: 10.1126/science.aad2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu B, Mandal SS, Pham AD, Zheng Y, Erdjument-Bromage H, Batra SK, et al. The human PAF complex coordinates transcription with events downstream of RNA synthesis. Genes Dev. 2005;19:1668–73. doi: 10.1101/gad.1292105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen FX, Woodfin AR, Gardini A, Rickels RA, Marshall SA, Smith ER, et al. PAF1, a Molecular Regulator of Promoter-Proximal Pausing by RNA Polymerase II. Cell. 2015;162:1003–15. doi: 10.1016/j.cell.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaenicke LA, von EB, Carstensen A, Wolf E, Xu W, Greifenberg AK, et al. Ubiquitin-Dependent Turnover of MYC Antagonizes MYC/PAF1C Complex Accumulation to Drive Transcriptional Elongation. Mol Cell. 2016;61:54–67. doi: 10.1016/j.molcel.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Deb SPM, Senapati S, Dey P, Batra SK. Human PAF Complexes in Endocrine Tumors. 2008;3(5):557–65. doi: 10.1586/17446651.3.5.557. [DOI] [PubMed] [Google Scholar]
- 22.Wani R, Bharathi NS, Field J, Tsang AW, Furdui CM. Oxidation of Akt2 kinase promotes cell migration and regulates G1-S transition in the cell cycle. Cell Cycle. 2011;10:3263–8. doi: 10.4161/cc.10.19.17738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krogan NJ, Dover J, Wood A, Schneider J, Heidt J, Boateng MA, et al. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol Cell. 2003;11:721–9. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 24.Laribee RN, Krogan NJ, Xiao T, Shibata Y, Hughes TR, Greenblatt JF, et al. BUR kinase selectively regulates H3 K4 trimethylation and H2B ubiquitylation through recruitment of the PAF elongation complex. Curr Biol. 2005;15:1487–93. doi: 10.1016/j.cub.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Wood A, Schneider J, Dover J, Johnston M, Shilatifard A. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J Biol Chem. 2003;278:34739–42. doi: 10.1074/jbc.C300269200. [DOI] [PubMed] [Google Scholar]
- 26.Ng HH, Dole S, Struhl K. The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J Biol Chem. 2003;278:33625–8. doi: 10.1074/jbc.C300270200. [DOI] [PubMed] [Google Scholar]
- 27.Mulder KW, Brenkman AB, Inagaki A, van den Broek NJ, Timmers HT. Regulation of histone H3K4 tri-methylation and PAF complex recruitment by the Ccr4-Not complex. Nucleic Acids Res. 2007;35:2428–39. doi: 10.1093/nar/gkm175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims RJ, III, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem. 2005;280:41789–92. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KY, Levin DE. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell. 2011;144:745–56. doi: 10.1016/j.cell.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–6. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 31.Castro NE, Lange CA. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010;12:R60. doi: 10.1186/bcr2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCracken SR, Ramsay A, Heer R, Mathers ME, Jenkins BL, Edwards J, et al. Aberrant expression of extracellular signal-regulated kinase 5 in human prostate cancer. Oncogene. 2008;27:2978–88. doi: 10.1038/sj.onc.1210963. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Xu W. Histone H3R17me2a mark recruits human RNA polymerase-associated factor 1 complex to activate transcription. Proc Natl Acad Sci U S A. 2012;109:5675–80. doi: 10.1073/pnas.1114905109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenbacher AD, Nguyen CT, Cavanaugh AM, Huang J, Lu F, Chen JN. The PAF1 complex differentially regulates cardiomyocyte specification. Dev Biol. 2011;353:19–28. doi: 10.1016/j.ydbio.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen CT, Langenbacher A, Hsieh M, Chen JN. The PAF1 complex component Leo1 is essential for cardiac and neural crest development in zebrafish. Dev Biol. 2010;341:167–75. doi: 10.1016/j.ydbio.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strikoudis A, Lazaris C, Trimarchi T, Galvao Neto AL, Yang Y, Ntziachristos P, et al. Regulation of transcriptional elongation in pluripotency and cell differentiation by the PHD-finger protein Phf5a. Nat Cell Biol. 2016;18:1127–38. doi: 10.1038/ncb3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Paszkowski-Rogacz M, Nitzsche A, Slabicki MM, Heninger AK, de VI, et al. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell. 2009;4:403–15. doi: 10.1016/j.stem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Dey P, Rachagani S, Vaz AP, Ponnusamy MP, Batra SK. PD2/Paf1 depletion in pancreatic acinar cells promotes acinar-to-ductal metaplasia. Oncotarget. 2014;5:4480–91. doi: 10.18632/oncotarget.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–97. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]