Abstract
Introduction
Variability in individual response profiles to antiplatelet therapy, in particular clopidogrel, is a well-established phenomenon. Genetic variations of the cytochrome P450 (CYP) 2C19 enzyme, a key determinant in clopidogrel metabolism, have been associated with clopidogrel response profiles. Moreover, the presence of a CYP2C19 loss-of-function allele is associated with an increased risk of atherothrombotic events among clopidogrel-treated patients undergoing percutaneous coronary interventions (PCI), prompting studies evaluating the use of genetic tests to identify patients who may be potential candidates for alternative platelet P2Y12 receptor inhibiting therapies (prasugrel or ticagrelor).
Areas covered
The present manuscript provides an overview of genetic factors associated with response profiles to platelet P2Y12 receptor inhibitors and their clinical implications, as well as the most recent developments and future considerations on the role of genetic testing in patients undergoing PCI.
Expert Commentary
The availability of more user-friendly genetic tests has contributed towards the development of many ongoing clinical trials and personalized medicine programs for patients undergoing PCI. Results of pilot investigations have shown promising results, which however need to be confirmed in larger-scale studies to support the routine use of genetic testing as a strategy to personalize antiplatelet therapy and improve clinical outcomes.
Keywords: clopidogrel, P2Y12 receptor antagonist, genotype, pharmacogenomics, CYP2C19
1. Introduction
Therapeutic inhibition of platelet activation is essential for the management of ischemic cardiovascular disease [1]. The use of platelet adenosine diphosphate (ADP) P2Y12 receptor antagonists in addition to aspirin, also known as dual antiplatelet therapy (DAPT), has significantly contributed towards reduction in atherothrombotic events particularly in high-risk patients such as those with acute coronary syndromes (ACS) or undergoing percutaneous coronary interventions (PCI) [2–4]. Currently, three oral P2Y12 receptor antagonists (clopidogrel, prasugrel, and ticagrelor) are commonly used in clinical practice. Although prasugrel and ticagrelor are associated with more reliable pharmacological effects compared with clopidogrel, which translates into a greater reduction of atherothrombotic events in ACS patients, clopidogrel is the most broadly utilized P2Y12 receptor inhibitor [5,6]. Indeed, the reduced cost, ease of access, and reduced risk of bleeding complications associated with clopidogrel contribute to these observations. However, a plethora of investigations over the years has consistently shown a broad variability in interindividual response profiles among clopidogrel-treated subjects [7]. Most importantly, PCI patients with impaired clopidogrel-induced antiplatelet effects, also known as high on-treatment platelet reactivity (HPR), have an increased risk of ischemic events, including stent thrombosis [7,8]. There is also emerging evidence, albeit with conflicting data, that patients with enhanced clopidogrel-induced antiplatelet effects, also known as low on-treatment platelet reactivity (LPR), have an increased risk of bleeding complications [8,9].
Multiple mechanisms, including clinical, cellular and genetic factors, contribute to individual’s response profile to clopidogrel [7,8]. Among genetic factors, a number of genes coding for enzymes or receptors that may be involved with the pharmacokinetic (PK) or pharmacodynamic (PD) profiles of clopidogrel have been investigated. However, genetic variations of the hepatic cytochrome P450 (CYP) 2C19 enzyme, a key determinant in both metabolic steps of clopidogrel transformation into its active metabolite, has been consistently associated with interindividual variability in clopidogrel’s PK/ PD profile [10,11]. In particular, the presence of loss-of-function (LOF) alleles in the CYP2C19 gene is associated with reduced metabolism of clopidogrel and lower generation of its active metabolite, which in turn leads to reduced antiplatelet effects and consequently increases the risk atherothrombotic events [11,12]. These observations have prompted studies evaluating the use of genetic tests to identify patients with a CYP2C19 LOF allele who may be potential candidates for alternative platelet P2Y12 receptor inhibiting therapies, such as prasugrel or ticagrelor, the effects of which are not affected by this genotype [13]. The availability of more efficient genetic tests has also contributed towards the development of a number ongoing clinical trials and personalized medicine programs for patients undergoing PCI [14]. The present manuscript provides an overview of genetic factors associated with response profiles to platelet P2Y12 receptor inhibitors and their clinical implications, as well as the most recent developments and future considerations on the role of genetic testing in patients undergoing PCI.
2. Pharmacologic profiles and Clinical Outcomes of P2Y12 Receptor Inhibitors
2.1. Clopidogrel
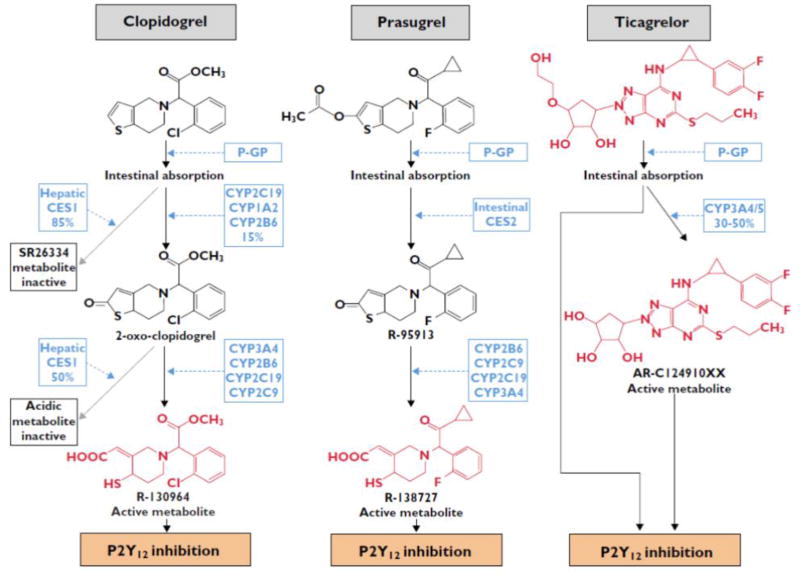
Clopidogrel is a second-generation thienopyridine, which has largely replaced ticlopidine, a first generation thienopyridine, because of its better safety profile [1,3]. Clopidogrel is an inactive form of pro-drug, which needs to undergo an oxidative process by the hepatic CYP system to become active. The hepatic conversion of clopidogrel to its active metabolite is a 2-step biotransformation process. Since 85% of clopidogrel pro-drug is inactivated by esterases in the blood, only15% of the pro-drug is available for transformation to the active metabolite (Figure 1) [7,15,16]. The active metabolite irreversibly inhibits the platelet P2Y12 receptor, thus exerting its antiplatelet effects for the life-span of the platelet. A number of trials conducted in high-risk patients with coronary artery disease (CAD), in particular those with ACS and/or undergoing PCI, have consistently shown that clopidogrel in addition to aspirin reduces both short- and long-term ischemic events and thrombotic complications [17–20]. For this reason, for nearly two decades, this DAPT regimen represented the standard of care in ACS and PCI patients. However, the observation that a considerable number of patients continue to experience recurrent ischemic events, including stent thrombosis, has prompted investigations to better understand the response profiles of clopidogrel, which have shown a broad variability in clopidogrel-induced antiplatelet effects [7,8]. Importantly, PCI patients with impaired clopidogrel-induced antiplatelet effects, or HPR, have an increased risk of ischemic events, including stent thrombosis, while those with enhanced clopidogrel induced antiplatelet effects, or LPR, may have an increased risk of bleeding complications [7–9]. These observations have prompted the development of newer generation oral P2Y12 receptor inhibitors, such as prasugrel and ticagrelor [1].
Figure 1. Metabolic profiles of three major P2Y12 receptor inhibitors.
Compounds with P2Y12-receptor inhibiting properties are in red.
CES, human carboxylesterase; CYP, cytochrome P450; P-GP, P-glycoprotein. Reproduced with permission from British Journal of Clinical Pharmacology (2014) [16]
2.2. Prasugrel
Prasugrel is a third-generation thienopyridine which, like clopidogrel, also is a pro-drug requiring hepatic metabolism to generate its active metabolite, which irreversibly inhibits the platelet P2Y12 receptor [1,3,21]. However, unlike clopidogrel, prasugrel is almost completely absorbed in the intestine and hydrolyzed into a thiolactone by the intestinal esterases. This thiolactone undergoes a single-step hepatic metabolism, primarily by CYP3A and CYP2B6, with a minor role of CYP2C19 and CYP2C9, to generate its active metabolite (Figure 1) [22]. Therefore, the metabolic conversion of prasugrel is more efficient than that of clopidogrel, providing higher drug bioavailability. In turn prasugrel has a more rapid onset of action, enhanced platelet inhibition, and less inter-individual variability in effects than with clopidogrel [23]. Its more potent irreversible platelet inhibitory effects also explains the slower offset of effects compared with clopidogrel [24]. These pharmacological properties contribute to the enhanced efficacy of prasugrel to reduce ischemic recurrences, including stent thrombosis, in high-risk ACS patients undergoing PCI, albeit at the expense of increased bleeding, including fatal bleeding [25]. In clinical trial participants with a prior cerebrovascular event, there was an increased risk of intracranial hemorrhage (ICH) and overall net harm, reason for which prasugrel is contraindicated in these patients [25]. The risk of bleeding was increased in patients of low body weight (≤60 kg) and old age (≥75 years), likely attributed to excess active metabolite formation (suggesting the need for lower doses in these patients) in whom the net effect of prasugrel was neutral [25–27].
2.3. Ticagrelor
Ticagrelor is a first in class cyclopentyltriazolopyrimidine which reversibly binds to the P2Y12 receptor at distinct site to that of ADP [28–30]. After intestinal absorption, ticagrelor directly binds (without the need for metabolism) to the P2Y12 receptor. However, 20–30% of the antiplatelet effects induced by ticagrelor derive from metabolites, mainly AR-C124910XX, generated by the hepatic CYP3A4 and CYP3A5 enzymes. Both the parent drug and the active metabolite have similar potency (Figure 1) [31,32]. These properties explain why ticagrelor has a more rapid onset of action, enhanced platelet inhibition, and less inter-individual variability in effects than with clopidogrel [33]. Its reversible binding property also explains the faster offset of effects compared with clopidogrel [33]. Overall, these pharmacological effects contribute to the enhanced efficacy of ticagrelor to reduce ischemic recurrences, including stent thrombosis, in ACS patients, irrespective of management (invasive or non-invasive) [34]. Moreover, it is the only P2Y12 receptor inhibitor which, compared with clopidogrel, also reduces cardiovascular mortality. These benefits have also been attributed to the off-target effects of ticagrelor, which does not occur for thienopyridines, represented by blockade of the equilibrative nucleoside transporter-1 (ENT-1) located on erythrocytes, which in turn leads to an increase in circulating adenosine plasma levels [35]. Although there were no differences in overall bleeding complications, there was an increase in spontaneous bleeding [34]. However, there was no subgroup identified to be at increased risk for bleeding complications with ticagrelor. The other most common side effect is represented by dyspnea, which has been attributed to ticagrelor’s effect on adenosine and is also the leading cause of drug discontinuation [34,36].
3. Genetic determinants of response to P2Y12 Receptor Inhibitors
The study of genetic polymorphisms has received a lot of attention over the past years in the field of cardiovascular disease. In particular, pharmacogenetics is the study of how genetic differences influence the variability in patients’ responses to drug [37]. Specific polymorphisms in genes which encode proteins or molecules associated with drug absorption, metabolism, transport, or eliciting therapeutic effects may potentially affect an individual’s response to the drug and thus explain drug effectiveness and safety profiles for the individual [38]. Therefore, the ultimate goal of a pharmacogenetic study is to identify specific genetic polymorphism(s) that are able to explain the variability in individual patient response to a given drug [37]. Identification of patients with specific genetic polymorphisms may be clinically important as these subjects may benefit from alternative therapies. This approach is the foundation for the concept of personalized treatment strategies. The ever-growing knowledge in the field of pharmacogenetics has indeed contributed the development of personalized medicine programs [39].
3.1. CYP2C19 gene and Clopidogrel Pharmacokinetics and Pharmacodynamics
A large number of drugs (such as clopidogrel, benzodiazepines, antidepressants, voriconazole, and some proton pump inhibitors) are metabolized by the CYP2C19 enzyme system in the liver [40]. The gene that codes the CYP2C19 enzyme is highly polymorphic, like many other CYP450 superfamily members [41]. Based on numerous studies including a genome-wide association study (GWAS), the CYP2C19 gene is known to be the most potent genetic determinant of interindividual variability in clopidogrel response [10–12,42–46]. It is located on chromosome 10 (10q24.1– q24.3), and over 30 gene alleles have been identified [41]. Among them, the CYP2C19*1 allele is the most prevalent and represents a normal activity allele. The CYP2C19*2 to *8 alleles are LOF alleles. Of these, CYP2C19*2 is the most frequently observed LOF allele [41]. There is a notable ethnic and racial difference in the frequency of CYP2C19 LOF alleles. For the East Asian population, the frequency of *2 and *3 is much higher than the European and African ancestry populations (Table 1) [47]. The allele frequency of *2 has been reported as about 15 % in Caucasians and Africans, and range between 25% and 35% in Asians [47–49]. The *3 through *8 alleles have a low frequency in the European and the African ancestry populations (less than 1%). The CYP2C19*3 allele is more common in East Asian populations (5–15%), and its effect on the clopidogrel response appeared greater than the CYP2C19*2 allele in an East Asian-based study [48,50]. The CYP2C19*17 allele results from a genetic variation in the gene promoter region associated with higher transcription rates of the gene, contributing to enhanced enzymatic activity and is thus called an increased or gain-of-function (GOF) allele [51,52]. The presence of *17 allele is significantly greater in African and European ancestry populations compared to Asian populations. The frequencies of CYP2C19 polymorphisms in major ethnic groups are presented in Table 1[47]. The distribution of CYP2C19 genotypes allows for individuals to be classified as follows: Ultrarapid metabolizers (UMs), Rapid metabolizers (RMs), normal metabolizers (NMs), Intermediate metabolizers (IMs), Poor metabolizers (PMs) (Table 2) [47,53]. PMs have 2 LOF alleles, and IM have one copy of a LOF allele. The *1/*1, *1/*17, and *17/*17 genotypes confer the NM, RM, and UM phenotypes, respectively. The prevalence of the PM phenotype is reported as 2–4% among African and Europeans ancestry populations, whereas it is 10% to 20% among East Asians [47,54].
Table 1.
Frequencies of CYP2C19 alleles in major ethnic groups.
| Allele | African | American | European | East Asian | South/Central Asian |
|---|---|---|---|---|---|
| *1 | 0.68 | 0.69 | 0.63 | 0.60 | 0.62 |
| *2 | 0.15 | 0.12 | 0.15 | 0.29 | 0.35 |
| *3 | 0.0052 | 0.00028 | 0.0042 | 0.089 | 0.024 |
| *17 | 0.16 | 0.18 | 0.21 | 0.027 | ND |
ND: not determined.
Among various reports of allele frequency, we present the value from Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C19 Genotype and Clopidogrel Therapy [47]
Table 2.
Five of CYP2C19 phenotypes in clopidogrel metabolism based on genotypes.
| Phenotype | Examples of diplotypes |
|---|---|
|
| |
| Ultrarapid metabolizer (UM) | *17/*17 |
| Increased enzyme activity compared to RM | |
| Rapid metabolizer (RM) | *1/*17 |
| Increased enzyme activity compared to NM but less than UM | |
| Normal metabolizer (NM) | *1/*1 |
| Fully functional enzyme activity | |
| Intermediate metabolizer (IM)† | *1/*2, *1/*3, *2/*17 |
| Decreased enzyme activity | |
| Poor metabolizer (PM) | *2/*2, *2/*3, *3/*3 or other combination of two LOF alleles (*2–*8) |
| Little to no enzyme activity | |
An individual carrying one functional allele (*1) plus one LOF allele (*2–*8) or one LOF allele (*2–*8) plus one increased-activity allele (*17). It seems that *17 allele is unable to completely compensate for reduced activity with the *2 allele, so *2/*17 is classified to IM.
Since there had been numerous reports that carriers of a LOF genotype (CYP2C19*2 and *3) have reduced capacity to produce active metabolites of clopidogrel, the Food and Drug Administration (FDA) requested that a dedicated study be performed to confirm these observations [55]. In this healthy subject study (n=40), PMs had lower conversion rate of clopidogrel to its active metabolite and HPR. This result led to the clopidogrel label change by drug regulating authorities, as described below.
In another study, carriers of a CYP2C19 LOF allele showed a 32.4% reduction in formation of the active metabolite of clopidogrel compared with non-carriers and a 9% absolute reduction of platelet aggregation (a relative reduction of approximately 25%) in response to clopidogrel [46]. Heterozygotes of CYP2C19 LOF alleles (e.g., *1/*2 and *1/*3) have clopidogrel response that lies between homozygotes for the *1 allele and LOF allele homozygotes or compound heterozygotes (e.g., *2/*2 and *2/*3) [45,46,56]. Carriers of the *17 allele have enhanced clopidogrel metabolism and with increased production of its active metabolite and greater clopidogrel-induced antiplatelet effects. In a meta-analysis, carriers of the CYP2C19*17 GOF allele showed lower prevalence of HPR than in non-carriers treated with standard dose clopidogrel [57].
3.2. Impact of CYP2C19 genotype on Clinical Outcomes
Numerous observational studies have shown an association between CYP2C19 LOF alleles and an increased risk of ischemic events [10–12,43,44,46]. However, this association has not been consistently observed across studies and may be attributed to the population under investigation. In a meta-analysis including 9 studies of 9,865 clopidogrel treated patients (54.5% with ACS and 91.3% with PCI), carriage of even one CYP2C19 LOF allele was associated with worse outcomes, particularly stent thrombosis [12]. Another large meta-analysis study, including 32 studies of 42,016 lower risk patients (e.g. patients with stable coronary disease, ACS managed medically, or atrial fibrillation) demonstrated the significant association between carriage of 1 or more CYP2C19 alleles and reduced clopidogrel responsiveness, however there was no significant association with adverse outcome when analyses were restricted to studies with 200 or more events (for exclusion of small-study bias) [58]. These conflicting results between two meta-analyses might be attributed to the baseline characteristics of the patients [59,60]. In fact, CYP2C19 LOF alleles have been shown to be clinically important mainly in high-risk subset (such as PCI) or the acute phase of myocardial infarction (MI), whereas in chronic stable patients or those with other indications like atrial fibrillation, genotype does not appear to be significant [61–63]. Recently, an intriguing result from a meta-analysis (including 24 studies of 36,076 participants) on CYP2C19 genotyping which specifically analyzed patients according to the indication for the use of clopidogrel (i.e., with or without PCI) and ethnic population (White versus Asian) was published [64]. In this analysis, the presence of a CYP2C19 LOF allele was associated with adverse outcomes only in patients using clopidogrel because of a PCI indication. This association was more modest (RR 1.20, 95% CI 1.10–1.31) among Whites undergoing PCI and stronger among Asians (RR 1.91, 95% CI 1.61–2.27).
On the contrary to the CYP2C19 LOF alleles, patients carrying the CYP2C19 GOF (i.e., *17) allele might be protected from ischemic recurrences but may also have a higher risk of bleeding due to the enhanced clopidogrel-induced antiplatelet effects [51,52,57,65]. Data from pooled analysis of six clinical studies showed that presence of the CYP2C19*17 GOF allele had a benefit against recurrent cardiovascular events in patients with CAD compared with non-carriers (OR 0.82, 95% CI 0.72–0.94, p = 0.005). Conversely, carriers of the *17 allele had an increased risk of bleeding complications (OR 1.25, 95% CI 1.07–1.47, p = 0.006) [57]. However, the data on the impact of the CYP2C19*17 allele on platelet function profiles and clinical outcomes, including bleeding risk, among clopidogrel treated patients are inconsistent [63,65,66].
Overall, the available evidence consistently supports that the presence of a CYP2C19 LOF allele contributes to HPR and an increased risk for adverse cardiovascular outcomes, including stent thrombosis among patients undergoing PCI [10–12,43,44,46].
3.3. CYP2C19 genotypes and Newer Generation P2Y12 Receptor Inhibitors
Although some of the CYP450 isoenzymes contributing to clopidogrel metabolism are also required for prasugrel metabolism, genetic polymorphisms of these enzymes have not shown to affect the PK and PD profiles of prasugrel [56,67–70]. Moreover, clinical outcomes of prasugrel-treated patients were not affected by CYP450 genetic polymorphisms [70,71]. Similarly, the pharmacological properties of ticagrelor and clinical outcomes of ticagrelor treated patients have not shown to be affected by CYP450 or other genetic polymorphisms [62,72,73].
3.4. Other candidate genes
A number of other candidate genes including ABCB1, Carboxylesterase 1 (CES 1), paraoxonase-1(PON1), CYP2C9, CYP3A4, P2Y12, and PlA genes have been proposed as genetic determinants of clopidogrel response and adverse clinical outcomes [37,56,71,74–79]. The absorption of thienopyridine is associated with an activity of the efflux pump P-glycoprotein, encoded by the ABCB1 gene. Increased activity of this pump system may decrease the absorption of clopidogrel in the intestine. Therefore, the ABCB1 polymorphism has been suggested to be correlated with clopidogrel intestinal efflux [71]. Carboxylesterase 1 is the main enzyme to convert the absorbed clopidogrel into an inactive form [80,81]. In one study, the presence of the CES1 143 E-allele led to an increase in clopidogrel active metabolite levels and enhanced platelet inhibition [74]. PON1 is associated with bioactivation of clopidogrel; PON1 catalyzes the conversion of 2-oxo-clopidogrel into active metabolite. There are some reports that Q192R polymorphism of PON1 contributes to reduced clopidogrel activity and increased risk of stent thrombosis in PCI patients [77]. The CYP2C9 and CYP3A4 enzymes are also involved in the clopidogrel metabolism, and thus genes encoding these enzymes have also been implicated in clopidogrel response [75,79]. Coexisting genetic variations of P2Y12 and CYP2C19 seemed to be associated with platelet activation in a small study [76]. The polymorphism of the Pl (A1/A2) gene-encoding glycoprotein (GP) IIIa also showed an association with clopidogrel response [78]. Although some studies have demonstrated the association between each polymorphism and clinical outcomes, most of data are inconsistent [11,62,82–87]. Importantly, GWAS showed that only CYP2C19 is associated with meaningful variability in clopidogrel response, suggesting that if other candidate genes that predict clopidogrel response exist, they likely have a much smaller impact [10].
4. Current Guidelines for CYP2C19 Genotyping and Assays for Bedside Genotyping
4.1. Current Guidelines for CYP2C19 genotyping
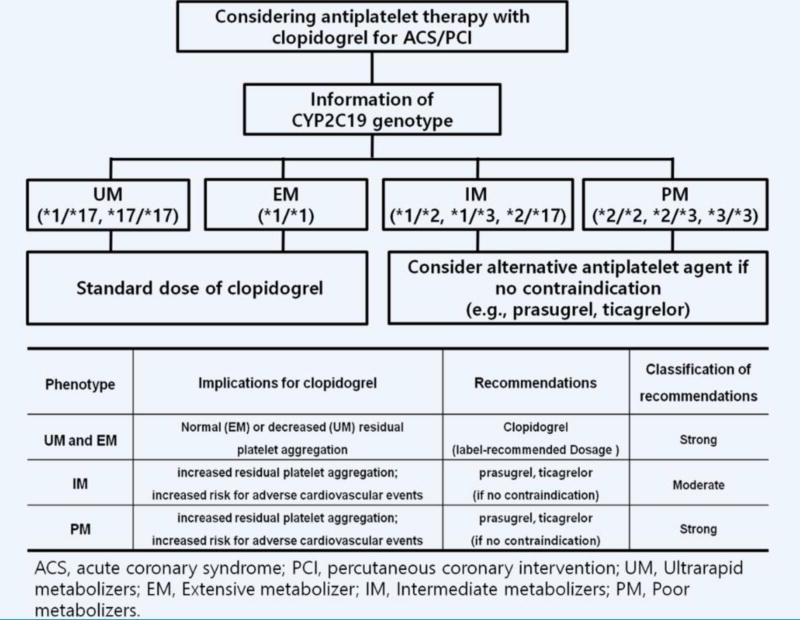
Data on the impact of CYP2C19 LOF alleles on the PK/PD profiles of clopidogrel and clinical outcomes have prompted drug regulating authorities including the FDA and the European Medicines Agency to issue a warning [88,89]. More specifically, they warn about the reduced effectiveness in patients who are PMs of clopidogrel and advise consideration of alternative antiplatelet agents [49]. Nevertheless, genotyping for CYP2C19 LOF alleles in high risk patients undergoing PCI is considered a Class IIb recommendation, and routine genotyping is not recommended (Class III) in the ACCF/AHA/SCAI guideline [2,90,91]. Similar to the ACCF/AHA/SCAI guideline, the ESC guidelines indicate that routine genetic testing is not recommended and should only be considered in selected patients treated with clopidogrel (Class IIb recommendation) [92,93]. Overall, the reason for this low level of recommendations derives from the lack of prospective randomized trial data demonstrating an impact on clinical outcomes with genotype-guided antiplatelet therapy. Moreover, it has been argued that the positive predictive value of a CYP2C19 LOF allele is low, and this polymorphism accounts for only 12% of clopidogrel response variability [94]. Ultimately, at the time of writing of the guidelines there were no user-friendly genetic tests that would allow for having results in the acute care setting, limiting the feasibility of applying results of genetic tests in real-world practice given that several days or weeks would be necessary prior to obtaining results [49]. The Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are available for clopidogrel use based on CYP2C19 genotype [47]. CPIC guidelines do not provide recommendations on whether pharmacogenetic testing should be conducted, but focus on how to apply genetic test results in clinical practice for optimal antiplatelet therapy. These guidelines recommend using alternative antiplatelet agents (e.g., ticagrelor or prasugrel) for CYP2C19 PMs and IMs in the absence of contraindications in ACS/PCI patients (Figure 2).
Figure 2. Algorithm for suggested clinical actions based on CYP2C19 genotype when considering treatment with clopidogrel for ACS patients undergoing PCI.
Recommendations from Clinical Pharmacogenetics Implementation Consortium [47]
4.2. Assays for Rapid CYP2C19 Genotyping
One of the major limitations to implementing genetic testing in clinical practice has been the availability of assays that allow for obtaining results in a timely fashion. With standard genetic assays, results may not be available for days or even weeks. In addition, many centers do not have the capability of conducting genetic testing, and thus, samples must be sent to off-site laboratories for testing. Having genetic test results available in a timely fashion is critical if test results are to be considered in the setting of PCI, where prompt use of a P2Y12 receptor inhibitor is necessary. Timing from clinical presentation to the cardiac catheterization laboratory has dramatically shortened over the years, particularly in ACS settings, and most patients undergo ad-hoc PCI procedures following diagnostic angiography [95]. Obtaining genetic test results after patients have been discharged is not practical as LOF carriers started on clopidogrel will not be optimally treated in the critical early weeks following PCI, and physicians must follow up with patients to switch to appropriate alternative therapy [96].
Recently, two rapid assays for CYP2C19 genetic testing have reached the market: SpartanRx™ (Spartan Bioscience, Ottawa, Canada) and the Verigene® System (Nanosphere, Northbrook, Illinois) (Figure 3) [14,97]. The SpartanRx is based on polymerase chain reaction and takes only one hour to obtain genetic information. The Spartan system is a user-friendly rapid genetic testing assay, which uses a buccal swab as a specimen, thus requiring minimal training. The feasibility of using this assay in real world clinical practice was demonstrated in patients (n=200) undergoing PCI for an ACS or stable angina in the point-of-care genetic testing for personalization of antiplatelet treatment (RAPID GENE) study [14]. The Verigene CYP2C19 test, an automated microarray-based assay, uses whole blood and takes 3 hours to provide results [98].
Figure 3. Rapid genetic assays for CYP2C19.
A. SpartanRx™ (Spartan Bioscience, Ottawa, Canada) and B. the Verigene® System (Nanosphere, Northbrook, Illinois)
5. Personalized Antiplatelet Treatment based on Genotype
5.1. Dosage adjustment of clopidogrel to overcome the CYP2C19 LOF allele
Several studies using a clopidogrel dose-escalation strategy to overcome HPR in the presence of a CYP2C19 LOF allele have been conducted. In healthy volunteers, clopidogrel dose escalation from 75mg to 150mg or 300mg per day was tested using platelet aggregation [99]. In a healthy volunteer study, the dose necessary to obtain similar inhibition of platelet aggregation as a standard 75mg/day dose in NMs was 300 mg/day in CYP2C19*2 homozygotes (PMs) and 150 mg/day in CYP2C19*2 heterozygotes (IMs). Therefore, a dose at least quadruple of the usual clopidogrel dose might be necessary to overcome the effect of the homozygous CYP2C19 LOF genotype [99]. Among patients with stable cardiovascular disease, tripling the dose of clopidogrel to 225 mg daily in CYP2C19*2 heterozygotes was shown to be required to reach similar levels of platelet reactivity as a 75 mg daily dose among non-carriers of a LOF allele. Importantly, among homozygotes for a LOF allele (PMs), a 300 mg/day dose failed to achieve the level of platelet inhibition observed with a 75 mg dose in non-carriers of a LOF allele [100]. In the Genotype Information and Functional Testing (GIFT) study (n=1,028), there was no significant PD effect with double-dose clopidogrel treatment (150mg daily) compared with a standard 75mg dosing regimen in among patients who were carriers of a CYP2C19 LOF allele and undergoing PCI [101]. Therefore, findings from dose escalation in healthy participants might not apply to patients of CAD. In a recently published meta-analysis, high-dose clopidogrel therapy was not shown to overcome the HPR rates in CYP2C19*2 carriers in patients undergoing PCI [102]. Overall, these findings indicate that switching to an alternative P2Y12 receptor inhibitor (i.e., prasugrel or ticagrelor) rather than increasing the clopidogrel dose should be considered for CYP2C19 LOF allele carriers.
5.2 Change to novel antiplatelet agent and adding another drug
Both ticagrelor and prasugrel have more potent PD effects compared with clopidogrel, and this superiority in PD has translated into better clinical outcomes in clinical studies of ACS patients [3,4]. The presence of CYP2C19 LOF alleles has not been shown to affect the PD effects of either prasugrel or ticagrelor, and more potent platelet inhibitory effects can be achieved by both drugs compared to clopidogrel in healthy volunteers and in CAD patients with CYP2C19 LOF alleles [56,67,72,103]. Most recently, prasugrel was shown to be superior to an escalated dosing strategy of clopidogrel in reducing HPR among patients with a ST-segment elevation myocardial infarction (STEMI) who had high risk genetic profiles identified using the SpartanRx system, further supporting the feasibility of performing genetic testing in real-world clinical practice including in the highest risk settings [104]. Although prasugrel and ticagrelor are deemed to have similar PD potency [105], it is unclear if they exert differential PD effects among carriers of CYP2C19 LOF alleles, which is currently being tested in patients undergoing non-emergent PCI (NCT 02065479). Overall, the novel antiplatelet agents may contribute to better clinical outcomes than clopidogrel in patients with CYP2C19 LOF alleles. Unfortunately, there are still limited data supporting improved clinical outcomes with genotype-based personalized antiplatelet therapy. Retrospective assessments of randomized controlled trial data suggest a benefit of using newer P2Y12 inhibitors in CYP2C19 LOF allele carriers. Specifically, the genetic sub-studies of the PLATO and TRITON-TIMI 38 trials concluded that genetic polymorphisms of CYP2C19 did not influence the efficacy of prasugrel or ticagrelor [62,70]. In an analysis of TRITON-TIMI 38, patients with a CYP2C19 LOF genotype were estimated to have a substantial reduction in the risk of the composite primary outcome with prasugrel as compared with clopidogrel [106]. However, these investigations were designed to demonstrate the efficacy of these drugs irrespective of the presence of LOF allele, underscoring the need for specifically designed studies investigating whether genotype based personalized antiplatelet therapy is associated with improved outcomes.
Cilostazol, an inhibitior of phosphodiesterase type 3 and indicated for symptomatic treatment of peripheral artery disease in United States, is a unique antiplatelet agent that exerts its effects through increasing the intracellular cAMP level [107]. Intriguingly, the addition of cilostazol to DAPT showed a reduction of HPR in patients with CYP2C19 LOF variants compared with high dose clopidogrel (150mg daily dose) [108,109]. Vorapaxar is new class of drug, which inhibited the action of thrombin in the protease activator receptors-1(PARs-1) [110]. The efficacy of this drug is demonstrated in the patients with prior MI or peripheral artery disease as an add-on therapy to standard DAPT [111]. However, the impact of adding vorapaxar among carriers of CYP2C19 LOF variants is unknown.
5.3 Clinical outcome studies of genotype based personalized medicine
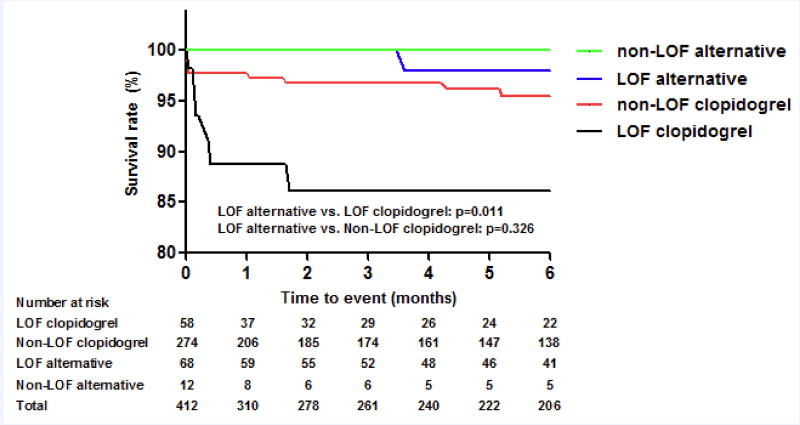
To date, data supporting the role of genotype based personalized antiplatelet therapy are limited and largely derived from registry data or small randomized studies, while there is no large randomized clinical trial demonstrating whether genotype based personalized antiplatelet therapy improve clinical outcomes or not [112–115]. A preliminary report from the University of Florida suggested improved outcomes with genotype based antiplatelet therapy. Among 412 patients who underwent PCI with genotyping, 126 (31%) had a LOF allele, and 68 (54%) of these received alternative antiplatelet therapy. On multivariable Cox regression analysis with propensity score adjustment, patients with a CYP2C19 LOF allele who were treated with alternative antiplatelet therapy (e.g. prasugrel or ticagrelor) had a significantly lower risk for MACE (defined as the composite of cardiovascular death, MI, stroke, or stent thrombosis) over the 6-month follow-up period compared to patients with an LOF allele who were treated with clopidogrel (p=0.035) (Figure 4) [116]. More recently, this study group expanded upon these observations in the context of a larger-scale multicenter investigation. This study was conducted as part of the NIH-funded Implementing GeNomics In pracTicE (IGNITE) Network [117]. In particular, among patients undergoing PCI and clinical CYP2C19 genotyping (n=1,815) at 7 U.S. institutions, the risk for MACE, defined as the composite of all-cause mortality, MI, or stroke, was compared between LOF allele carriers treated with clopidogrel and LOF allele carriers treated with alternative antiplatelet agent (ticagrelor, prasugrel). A total of 572 patients (31.5%) had a LOF allele, and 346 LOF allele carriers (60.5% of total carriers) received an alternative antiplatelet agent. The risk of MACE was significantly higher in LOF carriers treated with clopidogrel compared with the alternative treatment group (HR 2.3, 95% CI 1.2–4.5, p=0.015) [118]. With the recent availability of genetic tests providing results in a more timely fashion, as part of an extension of the University of Florida Health Personalized Medicine Program [119], funded under the IGNITE Network, there is an ongoing registry (NCT02724319) implementing CYP2C19-guided antiplatelet therapy after PCI, which genotyping conducted using the SpartanRx system.
Figure 4. Clinical outcomes of CYP2C19 LOF genotype-based antiplatelet therapy.
MACE free survival curve from multivariable Cox regression analysis with propensity score adjustment. Patients with CYP2C19 LOF alleles who were treated with alternative antiplatelet therapy (black line) had a significantly lower risk for MACE (major adverse cardiac events) compared to patients with LOF alleles who were treated with clopidogrel (blue line).
Currently, several ongoing studies are testing the efficacy of a genotype based approach to tailor personalize antiplatelet therapy (Table 3). The Genotyping Infarct Patients to Adjust and Normalize Thienopyridine Treatment (GIANT) trial (NCT 01134380) has completed patient recruitment. The GIANT trial is an observational case control study, including STEMI patients treated with primary PCI. The genetic information (CYP2C19*2 allele) of the patients tested after primary PCI is communicated to the treating physician. At the time of primary PCI, DAPT (Aspirin + Clopidogrel/Prasugrel) was used initially, and then the physician could change or adjust the regimen after the notification of genetic test (increase of the clopidogrel dosage, switch to clopidogrel or switch to prasugrel). Public reporting of the trial results are pending.
Table 3.
Large scale clinical outcome trials investigating genetic tests guided personalized antiplatelet therapy
| NCT number | Title | Status | Indication (number) |
Design | Test | Methods | Primary end point |
|---|---|---|---|---|---|---|---|
| NCT00995514 | Genotype Guided Comparison of Clopidogrel and Prasugrel Outcomes Study (GeCCO) | Terminated | ACS (14,600) | Observational Cohort | CYP2C19*2 | Prasugrel 10mg in LOF alleles vs. Clopidogrel 75mg control. | Composite of CV death, nonfatal MI, or non-fatal stroke at 6 months |
| NCT01452152 | Pharmacogenomics of Anti-platelet Intervention-2(PAPI-2) | Terminated | PCI (7,200) | Randomized | CYP2C19*2 or *3 | Genotype directed group: prasugrel 5–10 mg/day for IM and PM, Clopidogrel for other genotype. vs. Observational group: no Intervention and standard of care without genetic information | Composite of non-fatal MI, non-fatal stroke, ST and death secondary to any cardiovascular cause at 12 months |
| NCT01177592 | Thrombocyte Activity Reassessment and GEnoTyping for PCI (TARGET-PCI) | Terminated | PCI (1,500) | Randomized | CYP2C19*2 | CYP2C19*2 carriers: Prasugrel 60mg,vs. Others: clopidogrel 75mg | Composite of cardiovascular death, ischemic stroke, non-fatal myocardial infarction, urgent target vessel revascularization at 6 months |
| NCT01761786 | Cost-effectiveness of Genotype Guided Treatment With Antiplatelet Drugs in STEMI Patients: Optimization of Treatment (POPGenetics,) | Recruiting | Primary PCI (2,700) | Randomized | CYP2C19*2 or *3 | Wild type CYP2C19 allele: clopidogrel 75 mg. vs. CYP2C19*2 and *3: ticagrelor or prasugrel. | Composite of non-fatal MI, non-fatal stroke, CV death, and ST Pharmacoeconomics |
| NCT01134380 | Genotyping Infarct Patients to Adjust and Normalize Thienopyridine Treatment (GIANT) | Completed | Primary PCI (1,500) | Observational Case Control | CYP2C19*2 | Physician can change or adjust initial DAPT regimen after notification of result of genetic test (Increase of the clopidogrel dosage, Change drug to prasugrel or clopidogrel) | Composite of death, MI and ST at 12 months |
| NCT01742117 | Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI) | Recruiting | PCI (5,270) | Randomized | CYP2C19*2 or *3 | Wild type CYP2C19 allele: clopidogrel 75 mg. vs. CYP2C19*2 and *3: ticagrelor 90 mg. | Composite of non-fatal MI, non-fatal stroke, CV death, severe recurrent ischemia, and ST at 12 months |
| NCT02508116 | Assessment of Prospective CYP2C19 Genotype Guided Dosing of Anti-Platelet Therapy in Percutaneous Coronary Intervention (ADAPT) | Recruiting | PCI (700) | Randomized | CYP2C19*2 or *3 | The genotype guided arm: prasugrel or ticagrelor in LOF carrier and clopidogrel in non-carrier. vs. Control group: usual antiplatelet treatment. | Cost of medical service utilization. MACEs at 12 months |
LOF, loss-of-function; CV, cardiovascular; MI, myocardial infarction; IM, intermediate metabolizer; PM, poor metabolizer; DAPT, dual antiplatelet treatment; ST, stent thrombosis; MACEs, major adverse cardiovascular events
Indeed, the results of these observational studies are of interest, but warrant confirmation in randomized control studies which are currently ongoing. The Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI, NCT 01742117) is currently recruiting participants. Patients who undergo PCI are randomized to a conventional treatment arm (clopidogrel 75 mg once daily without prospective genotyping guidance) versus tailored therapy based on CYP2C19 genotype using SpartanRx (ticagrelor 90 mg twice daily for carriers of a CYP2C19 LOF allele, clopidogrel 75 mg once daily for non-carriers). The Assessment of Prospective CYP2C19 Genotype Guided Dosing of Anti-Platelet Therapy in Percutaneous Coronary Intervention (ADAPT, NCT 02508116) trial is also ongoing, and will test a similar concept using rapid genetic testing with SpartanRx™. The Patient Outcome after primary PCI (POPular) Genetics study (NCT01761786) is a prospective, randomized, controlled trial of 2,700 STEMI patients undergoing primary PCI investigating the efficacy, safety and cost-effectiveness of the CYP2C19 genotype guided antiplatelet treatment strategy, using prasugrel or ticagrelor in carriers of a CYP2C19 LOF allele and clopidogrel in non-carriers of a CYP2C19 LOF allele.
Unfortunately a number of clinical trials using genetic testing have terminated prematurely (Table 3). The GeCCO (NCT 00995514), PAPI-2 (NCT 01452152), TARGET-PCI (NCT 01177592) have been terminated prematurely because of administrative reasons, problems with the sponsor, or lack of financial support.
6. Conclusions
The use of DAPT composed of aspirin and a P2Y12 receptor inhibitor is the standard of care for patients undergoing PCI to prevent the risk of thrombotic events. Clopidogrel is the most broadly utilized P2Y12 receptor inhibitor. Despite the clinical benefits associated with the use of clopidogrel, numerous studies have shown that a considerable number of patients may have impaired clopidogrel-induced antiplatelet effects and persist with HPR leading to an increased risk of thrombotic complications. CYP2C19 LOF alleles have been associated with HPR and increased thrombotic event rates, prompting a number of investigations assessing the use of genetic tests to identify these patients who may be potential candidates for alternative platelet P2Y12 receptor inhibiting therapies (i.e., prasugrel and ticagrelor) not affected by these genotypes. The availability of more user-friendly genetic tests able to provide results in a timely fashion have indeed contributed towards the development of a number of ongoing clinical trials and personalized medicine programs for patients undergoing PCI. Findings from observational studies have thus far shown promising findings suggesting better outcomes with a personalized antiplatelet treatment approach using genetic testing to guide clinical decision making. However, results of large-scale randomized trials, currently ongoing, are needed to define whether genetic testing should be implemented routinely in clinical practice in patients undergoing PCI.
7. Expert commentary
7.1. Future directions and perspectives on personalized antiplatelet therapy
The well-established association between the presence of certain genotypes, HPR and increased risk of atherothrombotic events among clopidogrel-treated patients undergoing PCI as well as the encouraging preliminary clinical outcomes study results associated with the use of alternative therapies according to the results of genetic testing are indeed promising for the future personalizing antiplatelet treatment regimens [120]. Indeed, while many Institutions have already implemented the routine use of genetic testing to personalize antiplatelet therapy in PCI patients based on these observations, data from currently ongoing large scale randomized clinical trials are warranted to support their utilization. Practice guidelines, particularly in the field of cardiology, strongly rely on evidenced-based data and strategies that fall within high levels of recommendations derive from large scale randomized comparisons, which often need to be replicated in order to also obtain high level of evidence. Unfortunately, such data from large randomized controlled trials in the field of genetic testing in patients undergoing PCI are lacking. The use of genetic testing as a tool to incorporate into routine clinical practice is also challenged by the fact that a parallel line of research focused on personalizing antiplatelet therapy based on results of platelet function testing has provided thus far disappointing results [121–124]. In fact, although observational studies in high-risk PCI settings have shown that modification of antiplatelet therapy based on results of platelet function studies are associated with favorable outcomes, these findings have not been corroborated within a number of randomized clinical trials [125]. It may be argued that these trials were flawed by target population, antiplatelet medications, platelet function assay, HPR cut-off values, timing of testing, among other variables, which were not adequate to test for the study hypothesis [125]. Indeed, lessons learned from these studies have led to the development of perhaps more appropriately designed trials, such as the TROPICAL-ACS which will determine whether individualizing antiplatelet therapy based on platelet function tests remains an arena to nurture further research [126].
Genetic testing in PCI is still in its very earlier phases. In fact, while observational data have been thus far encouraging, results of large randomized clinical trials are still not available [116,118]. Moreover, the fact that more user-friendly genetic tests that can be used in daily clinical practice have not become available until recently may have hampered the evolution of this field of investigation. Therefore, the field of genetic testing in PCI has much room for future growth. Indeed, the use of genetic testing as a strategy to personalize antiplatelet therapy rather than platelet function testing has the advantage that while results of platelet function tests are subject to a lot of variability, which may thus lead to both false-negative and false-positive results, the genotype of an individual does not change. Moreover, the advantage of genotyping compared with platelet function testing is that results can be obtained prior to initiating treatment. Therefore, genetic testing becomes an attractive option to consider. In particular, the use of genetic testing would allow for identifying patients who have adequate clopidogrel metabolism and less likely to have HPR and atherothrombotic events. Importantly, such strategy would favor the use of a drug such as clopidogrel known to have less bleeding complications compared with prasugrel or ticagrelor. It is important to underscore that a bleeding complication has prognostic implications as detrimental as a recurrent atherothrombotic event [9,127]. Ultimately, there is an opportunity for cost savings given that clopidogrel is broadly available in a generic formulation.
7.1. Five-year view
In the upcoming years results of trials of testing personalized antiplatelet therapy approaches based on the results of genetic testing will become available (Table 3). Additional data from pragmatic studies, such as those from the IGNITE Network, are also expected. Moreover, user-friendly genetic testing assays will also become more broadly available. Evolution in such technology may also be paralleled by reduced cost of performing these tests. Indeed, the need for antiplatelet treatment strategies in PCI patients that are associated with improved efficacy and safety profiles still represents an area of unmet need. Therefore, amongst the various approaches currently being tested, such as testing new drugs, alternative combination therapies, and platelet function testing, the use of genetic tests falls within the viable options for the future pending results of ongoing randomized clinical trials.
Key issues.
Cytochrome P450 2C19 gene polymorphism contributes the wide variability in interindividual response to clopidogrel-induced antiplatelet effects.
Increasing clopidogrel dosing is overall ineffective in overcoming impaired clopidogrel-induced antiplatelet effects among carriers of CYP2C19 LOF alleles, underscoring the need for alternative drugs.
Prasugrel and ticagrelor are newer generation P2Y12 receptor inhibitors and their effects are not affected by CYP2C19 LOF alleles.
Findings from observational studies have shown promising findings suggesting better outcomes with a personalized antiplatelet treatment approach using genetic testing to guide clinical decision making.
Results of large-scale randomized trials, currently ongoing, are needed to define whether genetic testing should be implemented routinely in clinical practice in patients undergoing PCI.
Acknowledgments
Funding
There was no specific funding for this manuscript. Dr. Angiolillo is recipient of a grant from the Scott R. MacKenzie Foundation for the conduct of human genetic research. This work is supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01007269.
Dominick J. Angiolillo: Dr. Angiolillo reports receiving payments as an individual for: a) Consulting fee or honorarium from Amgen, Bayer, Biosensors, Sanofi, Eli Lilly, Daiichi-Sankyo, The Medicines Company, AstraZeneca, Merck, Chiesi, Pfizer, and PLx Pharma; b) Participation in review activities from Johnson & Johnson, and St. Jude Medical. Institutional payments for grants from Amgen, Biosensors, Glaxo-Smith-Kline, Eli Lilly, Daiichi-Sankyo, The Medicines Company, AstraZeneca, Janssen Pharmaceuticals, Inc., Osprey Medical, Inc., Novartis, CSL Behring, and Gilead.
Footnotes
Conflict of interest
Jae Youn Moon: has no conflict of interest to report.
Francesco Franchi: has no conflict of interest to report.
Fabiana Rollini: has no conflict of interest to report.
Jose R. Rivas Rios: has no conflict of interest to report.
Megha Kureti: has no conflict of interest to report.
Larisa Cavallari: has no conflict of interest to report.
References
- 1.Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs. 2012;72(16):2087–2116. doi: 10.2165/11640880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2016;68(10):1082. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 3.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nature reviews. Cardiology. 2015;12(1):30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 4.Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nature reviews. Cardiology. 2017 doi: 10.1038/nrcardio.2017.18. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood MW, Wiviott SD, Peng SA, et al. Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2014;3(2):e000849. doi: 10.1161/JAHA.114.000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W, Plent S, Prats J, Deliargyris EN. Trends in P2Y12 Inhibitor Use in Patients Referred for Invasive Evaluation of Coronary Artery Disease in Contemporary US Practice. The American journal of cardiology. 2016;117(9):1439–1443. doi: 10.1016/j.amjcard.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. Journal of the American College of Cardiology. 2007;49(14):1505–1516. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. Journal of the American College of Cardiology. 2013;62(24):2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 9.Rollini F, Tello-Montoliu A, Angiolillo DJ. Advances in platelet function testing assessing bleeding complications in patients with coronary artery disease. Platelets. 2012;23(7):537–551. doi: 10.3109/09537104.2012.704649. [DOI] [PubMed] [Google Scholar]
- 10*.Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. Jama. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. showed that CYP2C19*2 genotype was associated with diminished platelet response to clopidogrel treatment and poor cardiovascular outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. The New England journal of medicine. 2009;360(4):363–375. doi: 10.1056/NEJMoa0808227. showed that among AMI patients who were receiving clopidogrel, those carrying CYP2C19 loss-of-function alleles had a higher rate of subsequent cardiovascular events than those who were not. [DOI] [PubMed] [Google Scholar]
- 12**.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. Jama. 2010;304(16):1821–1830. doi: 10.1001/jama.2010.1543. showed that among PCI patients treated with clopidogrel, carriage of even one reduced-function CYP2C19 allele appears to be associated with a significantly increased risk of MACEs, particularly stent thrombosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallari LH, Obeng AO. Genetic Determinants of P2Y12 Inhibitors and Clinical Implications. Interv Cardiol Clin. 2017;6(1):141–149. doi: 10.1016/j.iccl.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379(9827):1705–1711. doi: 10.1016/S0140-6736(12)60161-5. demonstrated that point-of-care genetic testing after PCI can be done effectively at the bedside and treatment of identified CYP2C19*2 carriers with potent antiplatelets can reduce high on-treatment platelet reactivity. [DOI] [PubMed] [Google Scholar]
- 15.Savi P, Pereillo JM, Uzabiaga MF, et al. Identification and biological activity of the active metabolite of clopidogrel. Thrombosis and haemostasis. 2000;84(5):891–896. [PubMed] [Google Scholar]
- 16.Trenk D, Hochholzer W. Genetics of platelet inhibitor treatment. Br J Clin Pharmacol. 2014;77(4):642–653. doi: 10.1111/bcp.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. Jama. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. The New England journal of medicine. 2005;352(12):1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The New England journal of medicine. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25(4):357–374. doi: 10.1111/j.1527-3466.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 22.Rehmel JL, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(4):600–607. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116(25):2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 24.Price MJ, Walder JS, Baker BA, et al. Recovery of platelet function after discontinuation of prasugrel or clopidogrel maintenance dosing in aspirin-treated patients with stable coronary disease: the recovery trial. Journal of the American College of Cardiology. 2012;59(25):2338–2343. doi: 10.1016/j.jacc.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 26.Erlinge D, Ten Berg J, Foley D, et al. Reduction in platelet reactivity with prasugrel 5 mg in low-body-weight patients is noninferior to prasugrel 10 mg in higher-body-weight patients: results from the FEATHER trial. Journal of the American College of Cardiology. 2012;60(20):2032–2040. doi: 10.1016/j.jacc.2012.08.964. [DOI] [PubMed] [Google Scholar]
- 27.Erlinge D, Gurbel PA, James S, et al. Prasugrel 5 mg in the very elderly attenuates platelet inhibition but maintains noninferiority to prasugrel 10 mg in nonelderly patients: the GENERATIONS trial, a pharmacodynamic and pharmacokinetic study in stable coronary artery disease patients. Journal of the American College of Cardiology. 2013;62(7):577–583. doi: 10.1016/j.jacc.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro JL, Angiolillo DJ. New directions in antiplatelet therapy. Circ Cardiovasc Interv. 2012;5(3):433–445. doi: 10.1161/CIRCINTERVENTIONS.111.966176. [DOI] [PubMed] [Google Scholar]
- 29.Franchi F, Rollini F, Park Y, Angiolillo DJ. Novel Antiplatelet Agents: The Current State and What Is Coming Down the Pike. Prog Cardiovasc Dis. 2015;58(3):267–277. doi: 10.1016/j.pcad.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Capodanno D, Dharmashankar K, Angiolillo DJ. Mechanism of action and clinical development of ticagrelor, a novel platelet ADP P2Y12 receptor antagonist. Expert Rev Cardiovasc Ther. 2010;8(2):151–158. doi: 10.1586/erc.09.172. [DOI] [PubMed] [Google Scholar]
- 31.Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug metabolism and disposition: the biological fate of chemicals. 2010;38(9):1514–1521. doi: 10.1124/dmd.110.032250. [DOI] [PubMed] [Google Scholar]
- 32.VANG JJ, Nilsson L, Berntsson P, et al. Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. Journal of thrombosis and haemostasis : JTH. 2009;7(9):1556–1565. doi: 10.1111/j.1538-7836.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 33.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 34.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19(2):209–219. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 36.Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. Journal of the American College of Cardiology. 2014;63(23):2503–2509. doi: 10.1016/j.jacc.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 37.Marin F, Gonzalez-Conejero R, Capranzano P, Bass TA, Roldan V, Angiolillo DJ. Pharmacogenetics in cardiovascular antithrombotic therapy. Journal of the American College of Cardiology. 2009;54(12):1041–1057. doi: 10.1016/j.jacc.2009.04.084. [DOI] [PubMed] [Google Scholar]
- 38.Roses AD. Pharmacogenetics and future drug development and delivery. Lancet. 2000;355(9212):1358–1361. doi: 10.1016/S0140-6736(00)02126-7. [DOI] [PubMed] [Google Scholar]
- 39.Cavallari LH, Weitzel KW, Elsey AR, et al. University of Florida Health Personalized MedicineProgram. Pharmacogenomics. 2017 doi: 10.2217/pgs-2017-0028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates ER, Lau WC, Angiolillo DJ. Clopidogrel-drug interactions. Journal of the American College of Cardiology. 2011;57(11):1251–1263. doi: 10.1016/j.jacc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 41.Scott SA, Sangkuhl K, Shuldiner AR, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenetics and genomics. 2012;22(2):159–165. doi: 10.1097/FPC.0b013e32834d4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trenk D, Hochholzer W, Fromm MF, et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. Journal of the American College of Cardiology. 2008;51(20):1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 43.Sibbing D, Stegherr J, Latz W, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. European heart journal. 2009;30(8):916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 44.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373(9660):309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 45.Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 46.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. The New England journal of medicine. 2009;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 47.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clinical pharmacology and therapeutics. 2013;94(3):317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man M, Farmen M, Dumaual C, et al. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. Journal of clinical pharmacology. 2010;50(8):929–940. doi: 10.1177/0091270009355161. [DOI] [PubMed] [Google Scholar]
- 49.Holmes DR, Dehmer GJ, Kaul S, Jr, Leifer D, O'Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA "boxed warning": a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2010;56(4):321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Jeong YH, Abadilla KA, Tantry US, et al. Influence of CYP2C19*2 and *3 loss-of-function alleles on the pharmacodynamic effects of standard- and high-dose clopidogrel in East Asians undergoing percutaneous coronary intervention: the results of the ACCEL-DOUBLE-2N3 study. Journal of thrombosis and haemostasis : JTH. 2013;11(6):1194–1197. doi: 10.1111/jth.12200. [DOI] [PubMed] [Google Scholar]
- 51.Frere C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. Journal of thrombosis and haemostasis : JTH. 2009;7(8):1409–1411. doi: 10.1111/j.1538-7836.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- 52.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121(4):512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 53.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2017;19(2):215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong YH, Tantry US, Kim IS, et al. Effect of CYP2C19*2 and *3 loss-of-function alleles on platelet reactivity and adverse clinical events in East Asian acute myocardial infarction survivors treated with clopidogrel and aspirin. Circ Cardiovasc Interv. 2011;4(6):585–594. doi: 10.1161/CIRCINTERVENTIONS.111.962555. [DOI] [PubMed] [Google Scholar]
- 55.Simon T, Bhatt DL, Bergougnan L, et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clinical pharmacology and therapeutics. 2011;90(2):287–295. doi: 10.1038/clpt.2011.127. [DOI] [PubMed] [Google Scholar]
- 56.Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. Journal of thrombosis and haemostasis : JTH. 2007;5(12):2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. Journal of thrombosis and haemostasis : JTH. 2012;10(2):199–206. doi: 10.1111/j.1538-7836.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 58.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. Jama. 2011;306(24):2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 59.Shuldiner AR, Vesely MR, Fisch A. CYP2C19 genotype and cardiovascular events. Jama. 2012;307(14):1482. doi: 10.1001/jama.2012.443. author reply 1484–1485. [DOI] [PubMed] [Google Scholar]
- 60.Mega JL, Topol EJ, Sabatine MS. CYP2C19 genotype and cardiovascular events. Jama. 2012;307(14):1482–1483. doi: 10.1001/jama.2012.444. author reply 1484–1485. [DOI] [PubMed] [Google Scholar]
- 61.Siller-Matula JM, Trenk D, Schror K, et al. How to improve the concept of individualised antiplatelet therapy with P2Y12 receptor inhibitors--is an algorithm the answer? Thrombosis and haemostasis. 2015;113(1):37–52. doi: 10.1160/TH14-03-0238. [DOI] [PubMed] [Google Scholar]
- 62*.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376(9749):1320–1328. doi: 10.1016/S0140-6736(10)61274-3. showed that ticagrelor is a more efficacious treatment for ACS than clopidogrel, irrespective of CYP2C19 and ABCB1 polymorphisms. [DOI] [PubMed] [Google Scholar]
- 63.Pare G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. The New England journal of medicine. 2010;363(18):1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 64.Sorich MJ, Rowland A, McKinnon RA, Wiese MD. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circulation. Cardiovascular genetics. 2014;7(6):895–902. doi: 10.1161/CIRCGENETICS.114.000669. [DOI] [PubMed] [Google Scholar]
- 65.Tiroch KA, Sibbing D, Koch W, et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. American heart journal. 2010;160(3):506–512. doi: 10.1016/j.ahj.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 66.Sibbing D, Gebhard D, Koch W, et al. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. Journal of thrombosis and haemostasis : JTH. 2010;8(8):1685–1693. doi: 10.1111/j.1538-7836.2010.03921.x. [DOI] [PubMed] [Google Scholar]
- 67.Kelly RP, Close SL, Farid NA, et al. Pharmacokinetics and pharmacodynamics following maintenance doses of prasugrel and clopidogrel in Chinese carriers of CYP2C19 variants. Br J Clin Pharmacol. 2012;73(1):93–105. doi: 10.1111/j.1365-2125.2011.04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurbel PA, Bergmeijer TO, Tantry US, et al. The effect of CYP2C19 gene polymorphisms on the pharmacokinetics and pharmacodynamics of prasugrel 5-mg, prasugrel 10-mg and clopidogrel 75-mg in patients with coronary artery disease. Thrombosis and haemostasis. 2014;112(3):589–597. doi: 10.1160/TH13-10-0891. [DOI] [PubMed] [Google Scholar]
- 69.Braun OO, Angiolillo DJ, Ferreiro JL, et al. Enhanced active metabolite generation and platelet inhibition with prasugrel compared to clopidogrel regardless of genotype in thienopyridine metabolic pathways. Thrombosis and haemostasis. 2013;110(6):1223–1231. doi: 10.1160/TH13-03-0263. [DOI] [PubMed] [Google Scholar]
- 70.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 71*.Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1319. doi: 10.1016/S0140-6736(10)61273-1. Showed that individuals who have polymorphism of ABCB1 or CYP2C19 genotypes have reduced platelet inhibition and are at increased risk of recurrent ischemic events during clopidogrel treatment. These genetic variations were did not affect pharmacological effects or clinical outcomes in individuals treated with prasugrel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tantry US, Bliden KP, Wei C, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circulation. Cardiovascular genetics. 2010;3(6):556–566. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 73.Varenhorst C, Eriksson N, Johansson A, et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. European heart journal. 2015;36(29):1901–1912. doi: 10.1093/eurheartj/ehv116. [DOI] [PubMed] [Google Scholar]
- 74.Lewis JP, Horenstein RB, Ryan K, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenetics and genomics. 2013;23(1):1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harmsze A, van Werkum JW, Bouman HJ, et al. Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenetics and genomics. 2010;20(1):18–25. doi: 10.1097/FPC.0b013e328333dafe. [DOI] [PubMed] [Google Scholar]
- 76.Malek LA, Kisiel B, Spiewak M, et al. Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circulation journal : official journal of the Japanese Circulation Society. 2008;72(7):1165–1169. doi: 10.1253/circj.72.1165. [DOI] [PubMed] [Google Scholar]
- 77.Bouman HJ, Schomig E, van Werkum JW, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17(1):110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 78.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. PlA polymorphism and platelet reactivity following clopidogrel loading dose in patients undergoing coronary stent implantation. Blood Coagul Fibrinolysis. 2004;15(1):89–93. doi: 10.1097/00001721-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 79.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler Thromb Vasc Biol. 2006;26(8):1895–1900. doi: 10.1161/01.ATV.0000223867.25324.1a. [DOI] [PubMed] [Google Scholar]
- 80.Tang M, Mukundan M, Yang J, et al. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J Pharmacol Exp Ther. 2006;319(3):1467–1476. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- 81.Zhu HJ, Wang X, Gawronski BE, Brinda BJ, Angiolillo DJ, Markowitz JS. Carboxylesterase 1 as a determinant of clopidogrel metabolism and activation. J Pharmacol Exp Ther. 2013;344(3):665–672. doi: 10.1124/jpet.112.201640. [DOI] [PubMed] [Google Scholar]
- 82.Cayla G, Hulot JS, O'Connor SA, et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. Jama. 2011;306(16):1765–1774. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- 83.Hulot JS, Collet JP, Cayla G, et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ Cardiovasc Interv. 2011;4(5):422–428. doi: 10.1161/CIRCINTERVENTIONS.111.963025. [DOI] [PubMed] [Google Scholar]
- 84.Sibbing D, Koch W, Massberg S, et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. European heart journal. 2011;32(13):1605–1613. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 85.Trenk D, Hochholzer W, Fromm MF, et al. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circulation. Cardiovascular genetics. 2011;4(4):429–436. doi: 10.1161/CIRCGENETICS.111.960112. [DOI] [PubMed] [Google Scholar]
- 86.Jaitner J, Morath T, Byrne RA, et al. No association of ABCB1 C3435T genotype with clopidogrel response or risk of stent thrombosis in patients undergoing coronary stenting. Circ Cardiovasc Interv. 2012;5(1):82–88. S81–82. doi: 10.1161/CIRCINTERVENTIONS.111.965400. [DOI] [PubMed] [Google Scholar]
- 87.Floyd CN, Ferro A. The PlA1/A2 polymorphism of glycoprotein IIIa in relation to efficacy of antiplatelet drugs: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;77(3):446–457. doi: 10.1111/bcp.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.US Food and Drug Administration. [Accessed Febrary, 2017];Plavix: reduced effectiveness in patients who are poor metabolizers of the drug. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm.
- 89. [Accessed on March 2017];European Medicines Agency: summary of product characteristics (Plavix) Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000174/WC500042189.pdf.
- 90.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 91.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;64(24):e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 92.Authors/Task Force m. Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) European heart journal. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 93.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) European heart journal. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 94.Hochholzer W, Trenk D, Fromm MF, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. Journal of the American College of Cardiology. 2010;55(22):2427–2434. doi: 10.1016/j.jacc.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 95.Capodanno D, Angiolillo DJ. Pretreatment with antiplatelet drugs in invasively managed patients with coronary artery disease in the contemporary era: review of the evidence and practice guidelines. Circ Cardiovasc Interv. 2015;8(3):e002301. doi: 10.1161/CIRCINTERVENTIONS.114.002301. [DOI] [PubMed] [Google Scholar]
- 96.Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nature reviews. Cardiology. 2016;13(1):11–27. doi: 10.1038/nrcardio.2015.113. [DOI] [PubMed] [Google Scholar]
- 97.Saracini C, Vestrini A, Galora S, Armillis A, Abbate R, Giusti B. Pharmacogenetics of clopidogrel: comparison between a standard and a rapid genetic testing. Genet Test Mol Biomarkers. 2012;16(6):500–503. doi: 10.1089/gtmb.2011.0259. [DOI] [PubMed] [Google Scholar]
- 98.Chae H, Kim M, Koh YS, et al. Feasibility of a microarray-based point-of-care CYP2C19 genotyping test for predicting clopidogrel on-treatment platelet reactivity. Biomed Res Int. 2013;2013:154073. doi: 10.1155/2013/154073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horenstein RB, Madabushi R, Zineh I, et al. Effectiveness of clopidogrel dose escalation to normalize active metabolite exposure and antiplatelet effects in CYP2C19 poor metabolizers. Journal of clinical pharmacology. 2014;54(8):865–873. doi: 10.1002/jcph.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mega JL, Hochholzer W, Frelinger AL, 3rd, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306(20):2221–2228. doi: 10.1001/jama.2011.1703. [DOI] [PubMed] [Google Scholar]
- 101.Price MJ, Murray SS, Angiolillo DJ, et al. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. Journal of the American College of Cardiology. 2012;59(22):1928–1937. doi: 10.1016/j.jacc.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L, Yang J, Zhu X, et al. Effect of high-dose clopidogrel according to CYP2C19*2 genotype in patients undergoing percutaneous coronary intervention- a systematic review and meta-analysis. Thromb Res. 2015;135(3):449–458. doi: 10.1016/j.thromres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Alexopoulos D, Dimitropoulos G, Davlouros P, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC. Cardiovascular interventions. 2011;4(4):403–410. doi: 10.1016/j.jcin.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 104.So DY, Wells GA, McPherson R, et al. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST-elevation myocardial infarction: the RAPID STEMI study. Pharmacogenomics J. 2016;16(1):71–78. doi: 10.1038/tpj.2015.17. [DOI] [PubMed] [Google Scholar]
- 105.Rollini F, Franchi F, Cho JR, et al. A head-to-head pharmacodynamic comparison of prasugrel vs. ticagrelor after switching from clopidogrel in patients with coronary artery disease: results of a prospective randomized study. European heart journal. 2016;37(35):2722–2730. doi: 10.1093/eurheartj/ehv744. [DOI] [PubMed] [Google Scholar]
- 106.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. Journal of thrombosis and haemostasis : JTH. 2010;8(8):1678–1684. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 107.Goto S. Cilostazol: potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6(4):3–11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Hwang SJ, Jeong YH, Kim IS, et al. Cytochrome 2C19 polymorphism and response to adjunctive cilostazol versus high maintenance-dose clopidogrel in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3(5):450–459. doi: 10.1161/CIRCINTERVENTIONS.110.949859. [DOI] [PubMed] [Google Scholar]
- 109.Kim IS, Jeong YH, Park Y, et al. Platelet inhibition by adjunctive cilostazol versus high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype. JACC. Cardiovascular interventions. 2011;4(4):381–391. doi: 10.1016/j.jcin.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 110.Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. European heart journal. 2010;31(1):17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. The New England journal of medicine. 2012;366(15):1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 112.Shen DL, Wang B, Bai J, et al. Clinical Value of CYP2C19 Genetic Testing for Guiding the Antiplatelet Therapy in a Chinese Population. J Cardiovasc Pharmacol. 2016;67(3):232–236. doi: 10.1097/FJC.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 113.Xie X, Ma YT, Yang YN, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. 2013;168(4):3736–3740. doi: 10.1016/j.ijcard.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 114.Deiman BA, Tonino PA, Kouhestani K, et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Neth Heart J. 2016;24(10):589–599. doi: 10.1007/s12471-016-0873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanchez-Ramos J, Davila-Fajardo CL, Toledo Frias P, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289–295. doi: 10.1016/j.ijcard.2016.09.088. [DOI] [PubMed] [Google Scholar]
- 116.Cavallari LH, Magvanjav O, Anderson RD, et al. Clinical implementation of CYP2C19 genotype guided antiplatelet therapy reduces cardiovascular events after PCI. Circulation. 2015;132:A11802. [Google Scholar]
- 117.Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genomics. 2016;9:1. doi: 10.1186/s12920-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavallari LH, Denny JC, Lee CR, et al. Prospective Clinical Implementation of CYP2C19-Genotype Guided Antiplatelet Therapy After PCI: a Multi-Site Investigation of MACE Outcomes in a Real-World Setting. Circulation. 2016;134(25):e711–e712. [Google Scholar]
- 119.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. American journal of medical genetics. Part C, Seminars in medical genetics. 2014;166C(1):56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cavallari LH, Lee CR, Duarte JD, et al. Implementation of inpatient models of pharmacogenetics programs. Am J Health Syst Pharm. 2016;73(23):1944–1954. doi: 10.2146/ajhp150946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. Journal of the American College of Cardiology. 2012;59(24):2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 122.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. Jama. 2011;305(11):1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 123.Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388(10055):2015–2022. doi: 10.1016/S0140-6736(16)31323-X. [DOI] [PubMed] [Google Scholar]
- 124.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. The New England journal of medicine. 2012;367(22):2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 125.Franchi F, Rollini F, Cho JR, Ferrante E, Angiolillo DJ. Platelet function testing in contemporary clinical and interventional practice. Curr Treat Options Cardiovasc Med. 2014;16(5):300. doi: 10.1007/s11936-014-0300-y. [DOI] [PubMed] [Google Scholar]
- 126.Sibbing D, Aradi D, Jacobshagen C, et al. A randomised trial on platelet function-guided deescalation of antiplatelet treatment in ACS patients undergoing PCI. Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) Trial. Thromb Haemost. 2017;117(1):188–195. doi: 10.1160/TH16-07-0557. [DOI] [PubMed] [Google Scholar]
- 127.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]