Abstract
Objective
The mechanism underlying hot flashes is not well-understood, primarily because of complex relationships between and among hot flashes and their risk factors.
Methods
We explored those relationships using a Bayesian Network approach based on a 2006–2015 cohort study of hot flashes among 776 female residents, 45–54 years old, in the Baltimore area. Bayesian networks were fit for each outcome (current hot flashes, hot flashes before the end of the study, hot flash severity, hot flash frequency, and age at first hot flashes) separately and together with a list of risk factors (estrogen, progesterone, testosterone, body mass index and obesity, race, income level, education level, smoking history, drinking history, and activity level). Each fitting was conducted separately on all women and only perimenopausal women, at enrollment and 4 years after enrollment.
Results
Hormone levels, almost always interrelated, were the most common variable linked to hot flashes; hormone levels were sometimes related to body mass index, but were not directly related to any other risk factors. Smoking was also frequently associated with increased likelihood of severe symptoms, but not through an anti-estrogenic pathway. The age at first hot flashes was related only to race. All other factors were either not related to outcomes or were mediated entirely by race, hormone levels, or smoking.
Conclusions
These models can serve as a guide for design of studies into the causal network underlying hot flashes.
Keywords: hot flashes, Bayesian Network, epidemiology
Introduction
Hot flashes (HF), or vasomotor symptoms, are the most common menopausal symptom (1) and can last for extended periods of time (2,3). A number of potential lifestyle alterations have been suggested to manage vasomotor symptoms (4), but proper control is difficult due to the complex nature of their genesis. Clusters of factors have been identified as associated with HFs, including menopausal stage, hormones, race/ethnicity, body mass index (BMI), smoking, alcohol consumption, and physical activity (5). Essentially one mechanism has been suggested for these associations, primarily because these factors are assumed to cause alterations in estrogen (6), but these mechanisms are not easily studied due to the interactions between many of the potential factors. For instance, the association between African-American race and increased likelihood, severity, and frequency of HFs can be strong when considered alone (7), but is attenuated by controlling for obesity and estrogen (8), and the association with obesity is somewhat attenuated by controlling for estrogen (9), all of which suggest that estrogen levels are the proximate cause of HF. In addition, BMI has not been reliably associated with HFs across all menopause stages (10); high BMI has been associated with worse HFs during (9,11,12), but not after (11) menopause, and decreasing BMI may not be associated with easing of HFs (4). Although smoking is known to have anti-estrogenic effects (13), associations of smoking with HFs have been mixed (14–18) and the associations are not always attenuated by controlling for hormones (16), suggesting a different mechanism by which smoking is related to hot flashes. Even diet may be related to the experiencing of HFs (19). It has been suggested that study of this complex risk factor network would benefit from a more unified, multivariate approach (5), which can consider multiple factors within the same data set. However, the high level of multicollinearity between many of these factors has made the traditional multivariate approach untenable for these data, and the many interactions between factors have made simple linear models difficult to fit.
Bayesian network modeling (BN) is a data mining (or data-led machine learning) approach to understanding relationships (20) by representing these relationships as interaction networks, with each interaction made explicit through a statistically-based conditional probability. In this approach, all variables are represented by nodes, and all significant relationships are represented by directional arrows (or vectors). At each node, the probability distribution of the variable is defined by its relationships with parent nodes, which are the nodes from which all incoming vectors originate. In this way, the impact of a variable on an outcome may be direct, or it may be propagated through that variables effect on intervening variables.
Bayesian networks are considered most appropriate where relationships are complex or non-linear (21) or for dealing with uncertainty (22), both of which are highly applicable to epidemiologic questions (23), especially those, like hot flashes, with large, interacting variable sets. Bayesian networks can also incorporate expert understanding of relationships, allowing for a guided exploration of data (24). Bayesian networks have been applied to a number of health-related problems, including causes of child diarrhea in Pakistan (25), brain mapping in Alzheimer’s (26) and dementia (27) patients, diagnosis of breast cancer after mammography (28,29), diagnosis of early-stage Alzheimer’s disease (30), and risk of malignancy in ovarian cancer (31). Although BNs were developed as an explicitly causal approach, they have also been used to explore risk factors, such as exploring the role of risk factors in breast cancer invasiveness (32), and to predict outcomes, such as predicting the outcome of blast-related injury (33) and prediction of ectopic pregnancy (34). However, no studies to date have applied BNs to the question of menopausal symptoms. Thus, the objective of this study is to determine the shape of the potential risk factor network underlying HF experience by fitting BNs to data from a study of mid-life women. This may inform future hypothesis-driven research by highlighting interrelationships between risk factors that have not been detected using classical statistical techniques due to the ability of BN analyses to search networks broadly and without the bias inherent in manual model-building techniques.
Methods
Description of data
All participants gave written informed consent according to procedures approved by the University of Illinois and Johns Hopkins University Institutional Review Boards, which approved this research. The study design for the parent study is described in detail elsewhere [5]. Briefly, a cohort study of HFs among women 45–54 years of age was conducted starting in 2006 among residents of Baltimore and its surrounding counties. Women were recruited by mail, and were included if they were in the target age range, had intact ovaries and uteri, and were pre- or perimenopausal. Exclusion criteria consisted of pregnancy, a history of cancer, exogenous female hormone or herbal/plant substance, and no menstrual periods within the past year. Participants made a baseline clinic visit, which included measurement of height and weight to calculate BMI and completion of a detailed 26-page baseline survey. Participants completed a questionnaire annually after the baseline visit, repeating all previous questions about HFs, smoking, activity, and income, and BMI was calculated during the visit, with overweight/obese recorded as any BMI greater than or equal to 25. Both variables were included to determine if BMI was linearly related to outcomes, or if there was a threshold effect of BMI. Blood samples were collected at each scheduled clinic visit and stored until measurement of hormone levels as described below. A woman was considered perimenopausal if she experienced 1) her last menstrual period within the past year, but not within the past 3 months, or 2) her last menstrual period within the past 3 months and experienced 10 or fewer periods within the past year; postmenopausal women were those women who had not experienced a menstrual period within the past year. Follow-up was discontinued after 7 years, when the woman first reported hormone therapy use, an oophorectomy, or a cancer diagnosis, or at the year 4 visit for women determined to be postmenopausal. Women taking hormone therapy and herbal therapies were excluded from the study because the study was designed to focus on risk factors for natural hot flashes. Both hormone therapy and herbal therapies have been shown to treat hot flashes (35–41). Women with cancer were excluded from the study because many treatments for cancer (chemotherapies) destroy ovarian function and reduce estrogen levels (42–45), and this in turn increases the risk of hot flashes (5,6). Again, this exclusion was made because the study was designed to focus on the natural occurrence of hot flashes and not on medically-induced hot flashes. Recruitment and follow-up were completed in late June 2015.
During the baseline survey, women were asked if they had ever had HFs, with a description of HFs provided, how old they were when they first experienced HFs (age at first HF) and the severity and frequency of the majority of their HFs. During follow-up visits, women were asked if they had HFs in the last year and the severity and frequency of the majority of their HFs at the time of the visit. For severity, descriptions were: mild (sensation of heat without sweating), moderate (sensation of heat with sweating), or severe (sensation of heat with sweating that disrupts usual activity). For frequency, descriptions were: every hour, every 2–5 hours, every 6–11 hours, every 12–23 hours, 1–2 days per week, 3–4 days per week, 5–6 days per week, 2–3 days per month, 1 day per month, less than 1 day per month, or never; for purposes of this analysis, severity was dichotomized into “moderate or severe” versus “none or mild” and frequency was dichotomized into “at least weekly” versus “monthly or less”. Although self-report of HF was not validated in this study against an objective measurement, self-report of hot flashes has been accepted as a valid measure by both the National Institute on Aging and the FDA (46,47).
Serum samples extracted from the collected blood samples were used to measure estradiol, and progesterone levels in each sample using commercially available, previously validated enzyme-linked immunosorbent assay (ELISA) kits (DRG, Springfield, New Jersey, USA) [16–19]. The minimum detection limits and intra-assay coefficients of variation were as follows: estradiol 7 pg/ml, 3.3 ± 0.17%; testosterone 0.04 ng/ml, 2.2 ± 0.56%; and progesterone 0.1 ng/ml, 2.1 ± 0.65. The average inter-assay coefficient of variation for all assays was less than 5%. In the case of values lower than the detection limits for the assay, we used the limit of detection as the hormone value; of samples used for this analysis, 11/560 progesterone values were below the limit of detection, whereas no estrogen or testosterone levels were below the limit of detection. Each sample was measured in duplicate within the same assay. Progesterone, testosterone, and estradiol levels were log-transformed to meet normality assumptions.
Women were asked in the baseline visit to identify their race, annual family income, and education level. For this analysis, the race variable was dichotomized as white and non-white, income was dichotomized as “at least $50,000 annually” and “less than $50,000 annually”, and education level was dichotomized as “at least graduated college” and “did not graduate college”. At every visit, women were asked if they ever smoked, if they still smoked, and the number of packs of cigarettes smoked per year. Women were asked how their work compared to others physically, where 1 was much heavier and 5 was much lighter, and how their leisure activity compared to others, where 1 was much more and 5 was much less. A summary activity variable was computed as the sum of work and leisure activity levels. Women were also asked how frequently they sit at work, which was dichotomized for this analysis as “often or always” compared to “never, seldom, or sometimes”. Women were also asked if they consumed at least 12 alcoholic drinks per year, the average number of days per month on which they consumed alcohol, and the average number of alcoholic drinks consumed on days in which they consumed alcohol.
Only complete data were used; any individual with missing response data was dropped from the analysis and the number of individuals dropped was noted. For analysis of the full multivariable model, which included women with and without HFs, age at which HFs were first reported was set to be 99 for women not reporting HFs.
Two time-points were considered within the study: baseline (at the time of enrollment) and the midpoint (4 years after enrollment). For each time-point, models were fit based on current experience of HFs (presence/absence, severity, and frequency). Two other models were fit based on the full cohort study data: the risk of ever reporting HFs and the age at which HFs were first reported in women reporting HFs. A complete model, containing all outcomes, was also fit for the baseline time-point. All models were fit separately to data based on all women and to a subset of data including only perimenopausal women.
Description of model
A Bayesian Network model is one in which nodes indicate observed values (binary, count, or continuous) and arrows indicate connections or pathways between these values. The node that is the source of the arrow is referred to as the parent, while the node that the arrow is pointing to is referred to as the child. Each child node can have multiple parent nodes; we have limited the number of possible parent nodes per child node to 8 for this model fitting. The value of a child node is dependent on the value of all its parent nodes, and that relationship is similar to a multivariate statistical model (logistic regression for a binary child, poisson regression for a count child, and linear regression for a continuous child). This means that the full model, with all nodes included, is simply a multidimensional regression model. Fitting a BN model consists of identifying all possible configurations (parent-child combinations), computing the likelihood of each configuration given the observed data, and identifying the configuration with the maximum likelihood. In this sense, it is similar to traditional statistical model fitting in that the likelihood of the model given the data is maximized; the difference is in allowing a more complex relational structure.
Possible nodes for these models included levels of estradiol, progesterone, and testosterone at the current time-point, race (white vs. non-white), BMI (with the binary variable obesity as a subvariable, as it was calculated from BMI), smoking status (ever vs. never, with smoking frequency and current smoking as subvariables), alcohol consumption (more or less than 12 drinks/year, with frequency of alcohol consumption and amount of alcohol consumed as subvariables), activity levels (as a summary variable, with leisure activity and frequency of sitting as subvariables), annual income, and education level.
Model fitting process
For each outcome set, certain connections were retained in model construction as forced parent-child relationships. In all models, certain relationships were banned: no variables were parents for race; only race was a parent for education; HFs were not parents for hormone levels, BMI or obesity, income level, or education; hormone levels and BMI were not parents for smoking or alcohol consumption; and hormone levels were not parents of income or education. All other pathways were left as potential connections.
All models were fit using the “abn” package (48) in R version 3.0.3 (49) via Revolution R Enterprise 7.2.0 (©2014 Revolution Analytics, Inc.), which fits additive Bayesian networks. The number of parents allowed for any variable was limited to 8. The algorithm determines all possible network configurations with an uninformative prior for parent combinations, then fits each network configuration to the data and calculates the log marginal likelihood of all configurations to identify the optimal network configuration. Due to limitations in this software’s ability to fit only logistic, linear, and Poisson relationships, all categorical variables were dichotomized.
Best-fit models were plotted to include all parental nodes to the outcomes of interest. Vectors were plotted with the coefficient of the statistical relationship between the parent and child node. For categorical variables, these relationships are based on a logistic regression model. For continuous variables, these relationships are based on a linear regression model. For ordinal variables, these relationships are based on a Poisson regression model.
Results
Description of data
Of the 776 women enrolled in the study at baseline, 451 were available for analysis of current HFs (175 perimenopausal), 560 for ever having HFs (373 perimenopausal), 349 for age at first HF (294 perimenopausal), 439 for the frequency of HFs (167 perimenopausal), 448 for the severity of HFs (174 perimenopausal), and 436 for the full model (83 perimenopausal). The 164 women who had not experienced HFs were not included in the model for the age at first HF. At baseline, there was a significant univariate relationship between having experienced HFs in the past year and estrogen, progesterone, all smoking variables, and education level.
Of the 389 women remaining in the study at year 4, 117 were available for analysis of current HFs (53 perimenopausal), 114 for the frequency of HFs (51 perimenopausal), 118 for the severity of HFs (53 perimenopausal), and 71 for the full model (32 perimenopausal). Data were missing from 28 women on current HF status, from a further 241 women on activity level, and from a further single woman each on hormone levels, education, and income; women with missing data were demographically similar to those with full data available. The data used for fitting the models for all women are described in Tables 1 and 2. At year 4, there was a significant univariate relationship between experiencing HFs and leisure activity level.
Table 1.
Description of Continuous Variables Used in Fitting Bayesian Networks of Hot Flash Outcomes in Midlife Women. All variables were included using the Gaussian distribution. Variables in bold were univariately associated with the probability of having hot flashes prior to enrollment (P < 0.05).
| Variable | Year 1 | Year 4 | ||||
|---|---|---|---|---|---|---|
| Median | IQRb | P-valuec | Median | IQR | P-valuec | |
| Age at first hot flashes | 47 | 5 | NA | NA | NA | NA |
| Estrogen (pg/ml)a | 48 | 56 | 1.70e-8 | 45 | 45 | 0.0013 |
| Progesterone (ng/ml)a | 0.35 | 2.2 | 3.10e-8 | 1.3 | 3.3 | 0.037 |
| Testosterone (ng/ml)a | 0.29 | 0.25 | 0.062 | 0.46 | 0.31 | 0.18 |
| Body Mass Index | 26 | 9 | 0.28 | 30 | 7.7 | 0.97 |
| Packs smoked/year | 0 | 4.5 | 0.0098 | 0 | 0 | NA |
| Typical number of drinks when drinking alcohol | 1 | 1 | 0.47 | 1.3 | 1.4 | 0.028 |
| Number of days per month drinking alcohol | 4 | 9 | 0.89 | 6.2 | 8.4 | 0.32 |
Hormone values were log-transformed for model fitting
Inter-quartile range
Based on a logistic regression fitted against the response variable of having had hot flashes prior to enrollment using Year 1 data or in Year 4 using Year 4 data
Table 2.
Description of Categorical Variables Used in Fitting Bayesian Networks of Hot Flash Outcomes in Midlife Women. Variables with 2 levels were included using the Binomial distribution; those with more than 2 levels were included using the Poisson distribution. Variables in bold were significantly associated (P < 0.05) with the probability of having hot flashes in Year 1 or Year 4, while variables in italics were correlated with the probability of having hot flashes (P < 0.1).
| Variable | Level | Year 1 | Year 4 | ||||
|---|---|---|---|---|---|---|---|
| Number | % | P-valuea | Number | % | P-valuea | ||
| Had Hot Flashes prior to enrollment? | Yes | 245 | 45 | Ref | 83 | 71 | Ref |
| No | |||||||
| 305 | 55 | Ref | 34 | 29 | Ref | ||
| Ever have hot flashes? | Yes | 351 | 63 | Ref | 351 | 63 | Ref |
| No | 209 | 37 | Ref | 209 | 37 | Ref | |
| Race | White | 404 | 73 | 0.18 | 67 | 57 | 0.065 |
| Non-white | 146 | 27 | Ref | 50 | 43 | Ref | |
| Annual Family Income | ≥$50,000 | 444 | 81 | 0.55 | 84 | 72 | 0.28 |
| <$50,000 | 106 | 19 | Ref | 33 | 28 | Ref | |
| Education | College Degree | 370 | 67 | 0.004 | 79 | 68 | 0.99 |
| No Degree | 180 | 33 | Ref | 38 | 32 | Ref | |
| Obese (Body Mass Index≥25)? | Yes | 316 | 57 | 0.36 | 86 | 74 | 0.36 |
| No | 234 | 43 | Ref | 31 | 26 | Ref | |
| Ever Smoked? | Yes | 264 | 48 | 9.00E-4 | 57 | 49 | 0.15 |
| No | 286 | 52 | Ref | 60 | 51 | Ref | |
| Currently Smoke? | Yes | 58 | 11 | 0.047 | 11 | 9.4 | 0.41 |
| No | 492 | 89 | Ref | 106 | 91 | Ref | |
| At work, I sit | Never/Seldom/Sometimes | 319 | 58 | 0.38 | 76 | 65 | 0.7 |
| Often/Always | 231 | 42 | Ref | 41 | 35 | Ref | |
| At least 12 alcoholic drinks/year? | Yes | 443 | 81 | 0.35 | 73 | 62 | Ref |
| No | |||||||
| 107 | 19 | Ref | 44 | 38 | Ref | ||
| My work is physically, compared to others | Much heavier (5) | 14 | 12 | Ref | 4 | 3.4 | Ref |
| Heavier (4) | 74 | 63 | 0.54 | 15 | 13 | 0.59 | |
| As Heavy (3) | 192 | 160 | 0.47 | 29 | 25 | 0.97 | |
| Lighter (2) | 185 | 160 | 0.51 | 51 | 44 | 0.92 | |
| Much Lighter (1) | 85 | 73 | 0.64 | 18 | 15 | 0.75 | |
| My leisure physical activity level is, compared to others | Much more (5) | 62 | 53 | Ref | 8 | 6.8 | Ref |
| More (4) | 140 | 120 | 0.74 | 30 | 26 | 0.016 | |
| As much (3) | 175 | 150 | 0.67 | 45 | 38 | 0.1 | |
| Less (2) | 136 | 120 | 0.17 | 27 | 23 | 0.041 | |
| Much Less (1) | 37 | 32 | 0.98 | 7 | 6 | 0.83 | |
| Total activity levelb | 2 | 4 | 3.4 | Ref | 0 | 0 | Ref |
| 3 | 14 | 12 | 0.97 | 4 | 3.4 | Ref | |
| 4 | 46 | 39 | 0.97 | 6 | 5.1 | 0.6 | |
| 5 | 97 | 83 | 0.97 | 12 | 10 | 0.36 | |
| 6 | 133 | 110 | 0.97 | 36 | 31 | 0.24 | |
| 7 | 130 | 110 | 0.97 | 32 | 27 | 0.63 | |
| 8 | 79 | 68 | 0.97 | 20 | 17 | 0.23 | |
| 9 | 30 | 26 | 0.97 | 5 | 4.3 | 0.76 | |
| 10 | 17 | 15 | 0.97 | 2 | 1.7 | 1 | |
Based on a logistic regression fitted against the response variable of having had hot flashes prior to enrollment
Sum of leisure and work physical activity levels
Final Models
All final models are presented as the best fit model given the data as described; p-values are not provided as statistical significance is not relevant in the BN framework. Models can be interpreted as flow charts, with the outcome at any node impacted by the arrows pointing to it and the size of the impact indicated by the number accompanying the arrow.
The final model for having HFs were different by time period of the analysis and the menopause status of the subjects. In all women (Figure 1), baseline HFs were associated with low estrogen and progesterone levels, which were directly correlated, and low progesterone levels were associated with higher BMI, which was more likely in those who reported higher leisure activity levels and less likely in white women. By year 4 (see Figure B, Supplemental Digital Content 1) and in perimenopausal women at baseline (see Figure A, Supplemental Digital Content 1), the increased probability of HFs was significantly associated only with lower estrogen levels, which were themselves associated with lower progesterone levels at baseline and lower testosterone levels in year 4. In perimenopausal women at year 4 (see Figure C, Supplemental Digital Content 1), the model for HFs was more complicated and did not involve hormone levels; an increased probability of HFs was associated with currently smoking and less frequent sitting. The probability of current smoking was associated with a lower income and a lower education level (which were directly correlated), as well as being associated with more drinking.
Figure 1.
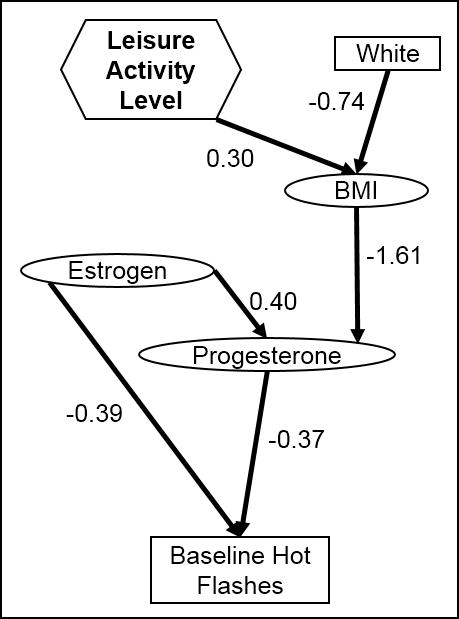
Final model structure for presence of hot flashes, based on all women at baseline in a cohort study of midlife women in the Baltimore area. Rectangles indicate categorical variables, while ovals indicate continuous variables and hexagons indicate ordinal variables.
The final model for age at first HF found, in both all women and perimenopausal women only, that race was the primary factor; white women were older at onset of HFs. In the final models for ever having HFs involving all women, estrogen levels at baseline were inversely correlated with having HFs during the study. In the model based on perimenopausal women only, ever having HFs during the study had no parent variables.
The final model for frequency of HFs in all women at baseline is shown in Figure 2. A higher frequency of HFs was associated with lower estrogen and progesterone levels, which were directly correlated. Low progesterone levels were associated with high BMI, which was associated with non-white women and higher leisure activity levels. In perimenopausal women only, the relationship between estrogen and hot flash frequency remained, but progesterone was not involved. In year 4, hot flash frequency had no parents in the final models.
Figure 2.
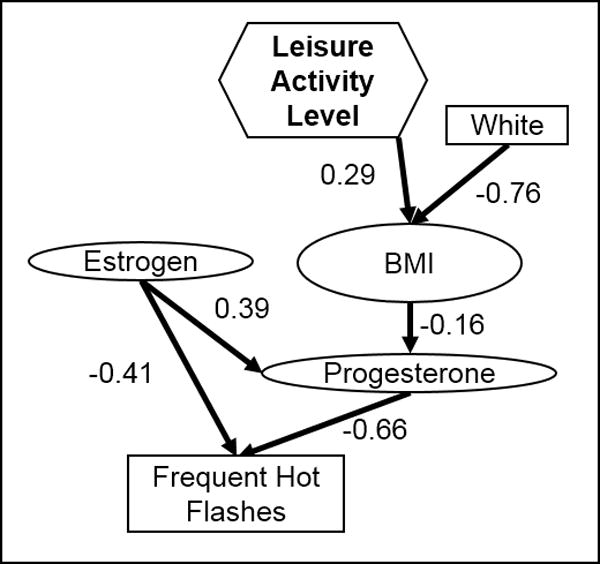
Final model structure for the frequency of hot flashes at baseline, based on all women in a cohort study of midlife women in the Baltimore area. Rectangles indicate categorical variables, while ovals indicate continuous variables.
The final model for severity of HFs in all women at baseline is shown in Figure 3. Severe HFs were associated with lower levels of progesterone and a history of smoking; progesterone was directly correlated with estrogen and testosterone and indirectly correlated with BMI, whereas smoking was less likely among those who attended college. Race was an indirect factor, with white women having a lower BMI and being more likely to attend college, which would lead to an overall lower severity in white women. Among perimenopausal women at baseline (see Figure A, Supplemental Digital Content 2), severe HFs were only linked to hormone levels. Among perimenopausal women in year 4 (see Figure B, Supplemental Digital Content 2), those who had ever smoked were more likely to have severe HFs; smoking was more likely in those with higher drinking levels, which were higher in white women. Among all women in year 4, no variables were significantly associated with HF severity.
Figure 3.
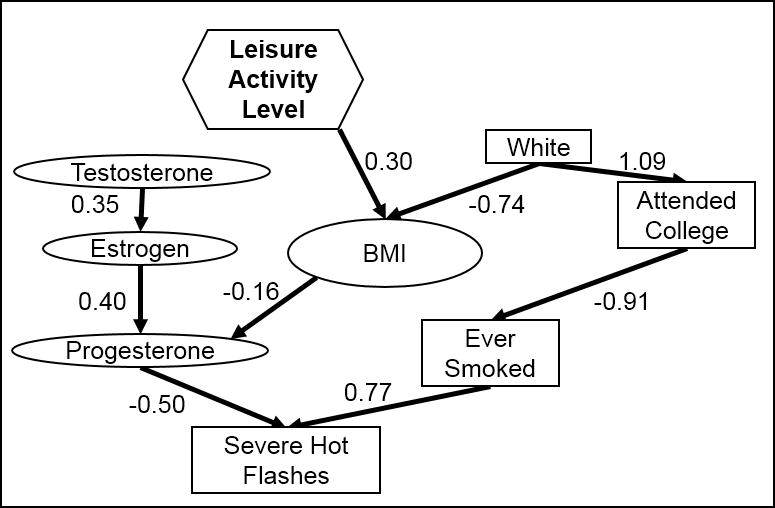
Final model structure for the moderate to severe hot flashes, based on all women at baseline in a cohort study of midlife women in the Baltimore area. Rectangles indicate categorical variables, while ovals indicate continuous variables and hexagons indicate ordinal variables.
When all outcomes were fit to all women at baseline (Figure 4), the strongest relationships were observed between the outcomes. Low estrogen and progesterone were associated with more frequent HFs, which was a parent of all other outcomes, and low progesterone was associated with a high BMI. High BMI was associated with more leisure activity and being non-white. White women were slightly older at first hot flashes. Severe hot flashes were associated with an increased likelihood of having smoked. When propagated through the model, white women and women with a low BMI would be expected to have less frequent HFs, a lower probability of HFs, less severe HFs, and a higher age at first HF, but most of those effects were mediated through endogenous hormone levels.
Figure 4.
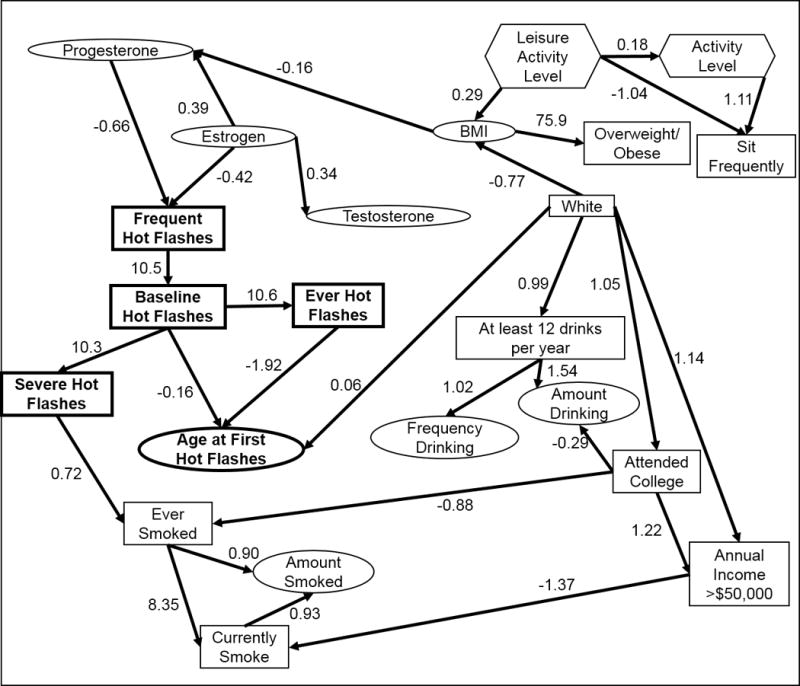
Final model structure for all outcomes at baseline, based on a cohort study of midlife women in the Baltimore area. Variables in bold were outcomes. Rectangles indicate categorical variables, while ovals indicate continuous variables and hexagons indicate ordinal variables.
The best-fit full model for perimenopausal women at baseline (see Figure, Supplemental Digital Content 3) had similar relationships in that the strongest relationships were observed between outcomes. Low estrogen was associated with ever having hot flashes, which was a parent to all other outcomes, while low progesterone was associated with having hot flashes at baseline. Beyond hormone levels, only race was considered a parent to the outcomes, with white women having a higher age at first hot flashes, which would propagate down to a lower probability of baseline hot flashes and therefore less severe and less frequent hot flashes. The best-fit full model for year 4 of the study, whether all women or perimenopausal only, had no factors as parents to HF outcomes.
Discussion
This study found that hormone levels, specifically estradiol and progesterone, were most consistently associated with HFs. The relationship between other risk factors and HFs was complex, with many interrelationships that could not be accounted for in simple regression modeling due to the high multicollinearity between risk factors. The primary factors directly related with HFs, besides hormone levels, were smoking and alcohol consumption.
There is strong evidence for the role of estrogen in HFs (6,50,51), upheld by these models. Although the association between BMI and HFs has been shown in previous studies to be mediated by hormone levels (11), our fitted models did not show this relationship. Estimates of the association between BMI and HFs have been mixed and may vary by menopause stage (4,6,9,10,52,53). Early analysis of perimenopausal women in this population found an association between BMI and HF severity and frequency (9,12), but a later analysis, before study termination, found no such relationship (10). In the current study, we found a relationship between obesity and HF severity only. It is possible that an increase in HF severity may confound studies of self-reported HFs, as women with very mild HFs may not recognize and report them as HFs. Adipose tissue and thus, BMI, may impact both hormonal and thermoregulatory function in multiple complex ways that may impact the occurrence of hot flashes.(5,12,54)
Smoking has been frequently associated with HF experience in previous studies (2,4,6,12,14,16,17) and was consistently linked to severity of HFs in this study. However, the relationship was not observed to be mediated by endogenous estrogen levels, the postulated mechanism for this relationship (13). This could indicate that other mechanisms are involved in the relationship, such as alteration of androgen levels or interference with the thermoregulatory pathways, which have been suggested elsewhere (14,16).
Race has been associated with HFs, but could be confounded by other factors, such as BMI (8,11,19) or other cultural factors (6). Our study found that race was the only parent to age at first HF, a consistent relationship across all models, but its relationship to other HF outcomes was indeed by way of other factors, as previous studies have suggested (8). This could explain why race was included in most final models, even though it did not have a significant univariate association with hot flashes; the effects of race on hot flash outcomes were primarily through its effect on BMI.
Several investigations have suggested that exercise may moderate the HF experience (15,55), although not with sufficient support for evidence-based treatment (4). Our study suggests that HFs may be decreased through the impact of activity on BMI and its relationship with education level. Our full models fail to uphold earlier observations that use of alcohol is associated with a decrease in risk of HFs (2,19), showing that the link is likely due to confounding with race or smoking. Likewise, education has previously been associated with HFs (7), as we consistently found across models. However, our findings show that the role of education could be mediated by smoking, as there is a strong association between education level and smoking.
Some relationships found in the fitted models may seem unusual if interpreted in a causal manner. For instance, the full models (Figure 4 and Figure, Supplementary Digital Content 3) showed a positive correlation between severe hot flashes and a history of smoking, indicating that women who had severe hot flashes in mid-life were more likely to have smoked at some time. Temporally, this is unlikely to be a causal relationship in the direction indicated; however, the relationship is understandable if causality is believed to be in the opposite direction. We chose to minimize the restrictions placed upon model fitting, which meant that unexpected relationships could occur, in order to limit the amount of investigator bias introduced to the model structure. In future causal investigations, we may wish to restrict relationships that are not temporally possible.
One caveat of these models is the smaller sample size, and therefore lower statistical power, in year 4 and perimenopausal groups. Hot flashes are most prevalent among perimenopausal women; thus, the perimenopausal women were the primary group of interest in this study (7). The more simplified models for perimenopausal women may have been the result of lower power or better sample selection to target at-risk individuals. If the former, only the strongest relationships would be detected; if the latter, any variables confounded by stage of menopause would have been removed. Either interpretation leads to the conclusion that the model fittings based on perimenopausal women are more likely to be stable, as the results are specific to the women at highest risk for HF.
It should also be noted that these results are limited to women who are not taking medication for hot flashes and who have not had ovarian or breast cancer prior to enrollment, and other co-morbidities were uncommon in this data set. No information was gathered about important life changes, which may cause stress and affect the way in which hot flashes were perceived (56,57), or medications for non-menopausal symptoms. While this is helpful in limiting the potential complications caused by medications or medically-induced menopause, it does limit the generalizability of the results to those in natural, untreated menopause. In addition, many of the factors included in the models were self-reported, which could lead to bias in the results. However, any biases in self-reporting during this study would also be present in clinical application of these findings, which increases the applicability of the findings. Likewise, the dichotomization of many variables is a limitation to this approach; however, we believe that the categories chosen represent reasonable divisions, and future analyses can explore the sensitivity of results to these categories.
Conclusions
This study presented many findings, but the underlying theme of the results is that HFs have a complex etiology, and that different aspects of HFs (i.e., severity as opposed to frequency) are affected at least in part by different risk factors. More studies are needed to understand the complex nature of HF symptoms and to advance the use of BN analysis for this purpose. However, we can conclude from these results that the effects of risk factors are not always mediated by estrogen levels, and that other biological mechanisms should be considered such as other hormones or toxicants, for example those associated with smoking. It is our hope that these models may inspire research designed to determine these biological mechanisms.
Supplementary Material
Supplemental Digital Content 1. Figure of final model structure for presence of hot flashes.
Supplemental Digital Content 2. Figure of final model structure for moderate to severe hot flashes.
Supplemental Digital Content 3. Figure of final model structure for all outcomes at baseline based on perimenopausal women only.
Acknowledgments
This work was supported by the National Institute for Aging at the National Institutes of Health, grant NIH R01AG18400 and National Institute of Environmental Health Sciences at the National Institutes of Health, grant NIH R01ES026956-01A1.
Footnotes
The authors have no conflicts to declare.
Contributor Information
Rebecca L. Smith, Department of Pathobiology, College of Veterinary Medicine, University of Illinois, Urbana, Illinois.
Lisa M. Gallicchio, Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland.
Jodi A. Flaws, Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois, Urbana, Illinois.
Reference List
- 1.Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE. AACE Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Menopause. Endocr Pract. 2011;17:1–25. doi: 10.4158/ep.17.s6.1. [DOI] [PubMed] [Google Scholar]
- 2.Smith RL, Gallicchio LM, Miller SR, Zacur HA, Flaws JA. Risk Factors for Extended Duration and Timing of Peak Severity of Hot Flashes. PLoS One [Internet] 2016;11(5):e0155079. doi: 10.1371/journal.pone.0155079. Available from: http://dx.plos.org/10.1371/journal.pone.0155079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose Sa, Gold EB, et al. Duration of Menopausal Vasomotor Symptoms Over the Menopause Transition. JAMA Intern Med [Internet] 2015;27157(4):531–9. doi: 10.1001/jamainternmed.2014.8063. Available from: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher TE, Chervenak JL. Lifestyle alterations for the amelioration of hot flashes. Maturitas [Internet] 2012;71(3):217–20. doi: 10.1016/j.maturitas.2011.12.006. Available from: http://dx.doi.org/10.1016/j.maturitas.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy middle-aged women. J Womens Health (Larchmt) 2010;19(10):1905–14. doi: 10.1089/jwh.2009.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteman MK, Staropoli CA, Benedict JC, Borgeest C, Flaws JA. Risk Factors for Hot Flashes in Midlife Women. J women’s Heal. 2003;12(5):459–72. doi: 10.1089/154099903766651586. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Colvin A, Avis NE, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller SR, Gallicchio LM, Lewis LM, Babus JK, Langenberg PW, Zacur HA, et al. Association between race and hot flashes in midlife women. Maturitas [Internet] 2006 Jun 20; doi: 10.1016/j.maturitas.2005.12.001. [cited 2015 Feb 6]; 54(3):260–9. Available from: http://www.sciencedirect.com/science/article/pii/S0378512205003725. [DOI] [PubMed]
- 9.Gallicchio LM, Visvanathan K, Miller SR, Babus JK, Lewis LM, Zacur HA, et al. Body mass, estrogen levels, and hot flashes in midlife women. Am J Obstet Gynecol [Internet] 2005 Oct; doi: 10.1016/j.ajog.2005.04.001. [cited 2015 Feb6]; 193(4):1353–60. Available from: http://www.sciencedirect.com/science/article/pii/S000293780500520X. [DOI] [PubMed]
- 10.Gallicchio LM, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Change in body mass index, weight, and hot flashes: a longitudinal analysis from the midlife women’s health study. J Women’s Heal [Internet] 2014 Mar; doi: 10.1089/jwh.2013.4526. [cited 2015 Jan 8]; 23(3)231–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24341351. [DOI] [PMC free article] [PubMed]
- 11.Santoro N, Sutton-Tyrrell K. The SWAN Song: Study of Women’s Health Across the Nation’s Recurring Themes. Obstet Gynecol Clin North Am. 2011;38(3):417–23. doi: 10.1016/j.ogc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–72. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 13.Tankó LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. 2004;11(1):104–9. doi: 10.1097/01.GME.0000079740.18541.DB. [DOI] [PubMed] [Google Scholar]
- 14.Gallicchio LM, Miller SR, Visvanathan K, Lewis LM, Babus JK, Zacur HA, et al. Cigarette smoking, estrogen levels, and hot flashes in midlife women. Maturitas [Internet] 2006 Jan 20; doi: 10.1016/j.maturitas.2005.03.007. [cited 2015 Feb 6];53(2)133–43. Available from: http://www.sciencedirect.com/science/article/pii/S0378512205000526. [DOI] [PubMed]
- 15.Moilanen J, Aalto AM, Hemminki E, Aro AR, Raitanen J, Luoto R. Prevalence of menopause symptoms and their association with lifestyle among Finnish middle-aged women. Maturitas [Internet] 2010;67(4):368–74. doi: 10.1016/j.maturitas.2010.08.007. Available from: http://dx.doi.org/10.1016/j.maturitas.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Cochran CJ, Gallicchio LM, Miller SR, Zacur H, Flaws JA. Cigarette Smoking, Androgen Levels, and Hot Flushes in Midlife Women. Obs gynecol. 2008;112(5):1037–44. doi: 10.1097/AOG.0b013e318189a8e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RL, Flaws JA, Gallicchio LM. Does quitting smoking decrease the risk of midlife hot flashes? A longitudinal analysis. Maturitas [Internet] 2015;82(1):123–7. doi: 10.1016/j.maturitas.2015.06.029. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0378512215007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenabi E, Poorolajal J. The association between hot flushes and smoking in midlife women: a meta-analysis. Climacteric [Internet] 2015;18(6):797–801. doi: 10.3109/13697137.2015.1080236. Available from: http://www.tandfonline.com/doi/full/10.3109/13697137.2015.1080236. [DOI] [PubMed] [Google Scholar]
- 19.Hyde Riley E, Inui TS, Kleinman K, Connelly MT. Differential association of modifiable health behaviors with hot flashes in perimenopausal and post menopausal women. J Gen Intern Med. 2004;19(7):740–6. doi: 10.1007/s11606-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L P. Bayesian analysis, pattern analysis, and data mining in health care. Curr Opin Crit Care [Internet] 2004;10(5):399–403. doi: 10.1097/01.ccx.0000141546.74590.d6. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-4744349442&partnerID=40&md5=396df5184ef69be25321d83ed630856f. [DOI] [PubMed] [Google Scholar]
- 21.Chen SH, Pollino CA. Good practice in Bayesian network modelling. Environ Model Softw [Internet] 2012;37:134–45. Available from: http://dx.doi.org/10.1016/j.envsoft.2012.03.012. [Google Scholar]
- 22.Lucas PJF, Van Der Gaag LC, Abu-Hanna A. Bayesian networks in biomedicine and health-care. Artif Intell Med. 2004;30(3):201–14. doi: 10.1016/j.artmed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Lewis F, Ward M. Improving epidemiologic data analyses through multivariate regression modelling. Emerg Themes Epidemiol [Internet] 2013;10(1):4. doi: 10.1186/1742-7622-10-4. Available from: http://www.ete-online.com/content/10/1/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadkarni S, Shenoy PP. A causal mapping approach to constructing Bayesian networks. Decis Support Syst. 2004;38(2):259–81. [Google Scholar]
- 25.Lewis FI, McCormick BJJ. Revealing the complexity of health determinants in resource-poor settings. Am J Epidemiol. 2012;176(11):1051–9. doi: 10.1093/aje/kws183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, et al. Altered default mode network connectivity in Alzheimer’s disease-A resting functional MRI and Bayesian network study. Hum Brain Mapp. 2011;32(11):1868–81. doi: 10.1002/hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burge J, Lane T, Link H, Qiu S, Clark VP. Discrete dynamic bayesian network analysis of fMRI data. Hum Brain Mapp. 2009;30(1):122–37. doi: 10.1002/hbm.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn CE, Roberts LM, Shaffer KA, Haddawy P. Construction of a Bayesian network for mammographic diagnosis of breast cancer. Comput Biol Med. 1997;27(1):19–29. doi: 10.1016/s0010-4825(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 29.Burnside ES, Rubin DL, Fine JP, Shachter RD, Sisney Ga, Leung WK. Bayesian network to predict breast cancer risk of mammographic microcalcifications and reduce number of benign biopsy results: initial experience. Radiology. 2006;240(3):666–73. doi: 10.1148/radiol.2403051096. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro P, Castro A, Pinheiro M. A Multicriteria Model Applied in the Diagnosis of Alzheimer’s Disease: A Bayesian Network. 11th IEEE Int Conf Comput Sci Eng. 2008:15–22. [Google Scholar]
- 31.Antal P, Verrelst H, Timmerman D, Moreau Y, Van Huffel S, De Moor B, et al. Bayesian networks in ovarian cancer diagnosis: potentials and limitations. Proc IEEE Symp Comput Med Syst [Internet] 2000:103–8. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-0033713172&partnerID=40&md5=d041c9c00969a37d9aed0336226fad6e.
- 32.Maskery S, Yonghong Z, Hai H, Shrivel C, Hooke J, Liebman M. Caffeine intake, race, and risk of invasive breast cancer lessons learned from data mining a clinical database. Proc - IEEE Symp Comput Med Syst. 2006;2006:714–8. [Google Scholar]
- 33.Toyinbo PA, Vanderploeg RD, Belanger HG, Spehar AM, Lapcevic WA, Scott SG. A Systems Science Approach to Understanding Polytrauma and Blast-Related Injury: Bayesian Network Model of Data From a Survey of the Florida National Guard. Am J Epidemiol [Internet] 2016;(2) doi: 10.1093/aje/kww074. Available from: http://aje.oxfordjournals.org/lookup/doi/10.1093/aje/kww074. [DOI] [PubMed]
- 34.Gevaert O, De Smet F, Timmerman D, Moreau Y, De Moor B. Predicting the prognosis of breast cancer by integrating clinical and microarray data with Bayesian networks. Bioinformatics [Internet] 2006 Jul 15; doi: 10.1093/bioinformatics/btl230. [cited 2014 Sep 3];22(14):e184–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16873470. [DOI] [PubMed]
- 35.Hill D, Crider M, Hill S. Hormone Therapy and Other Treatments for Symptoms of Menopause. Am Fam Physician. 2016;94(11):884–9. [PubMed] [Google Scholar]
- 36.Zhu X, Liew Y, Liu Z. Chinese herbal medicine for menopausal symptoms. Cochrane database Syst Rev. 2016;3:Cd009023. doi: 10.1002/14651858.CD009023.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaunitz A, Manson JE. Management of Menopausal Symptoms. Obstet Gynecol. 2015;126(4):859–76. doi: 10.1097/AOG.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginsburg E. Hot flashes-physiology, hormonal therapy, and alternative therapies. Obstet Gynecol Clin North Am. 1994;21(2):381–90. [PubMed] [Google Scholar]
- 39.Lee H, Choi J, Lee Y, Kil K, Lee M. Ginseng for managing menopausal woman’s health: A systematic review of double-blind, randomized, placebo-controlled trials. Medicine (Baltimore) 2016;95(38):e4914. doi: 10.1097/MD.0000000000004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghazanfarpour M, Saghedi R, Abdolahian S, Latifnejad Roudsari R. The efficacy of Iranian herbal medicines in alleviating hot flashes: A systematic review. Int J Reprod Biomed (Yazd, Iran) 2016;14(3):155–66. [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M, Lin C, Liu C. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric. 2015;18(2):260–9. doi: 10.3109/13697137.2014.966241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina J, Barton DL, Loprinzi C. Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf. 2005;28(5):401–16. doi: 10.2165/00002018-200528050-00004. [DOI] [PubMed] [Google Scholar]
- 43.Schover L. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26(5):753–8. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 44.Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Lansdale PL, Kinahan K, et al. Cancer survivors and infertility: a review of a new problem and novel answers. J Support Oncol [Internet] 2006;4(4):171–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16669459. [PubMed] [Google Scholar]
- 45.Marsden D, Hacker N. Fertility effects of cancer treatment. Aust Fam Physician. 2003;32(1–2):9–13. [PubMed] [Google Scholar]
- 46.Miller HG, Li RM. Measuring Hot Flashes: Summary of a National Institutes of Health Workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 47.Maki PM, Freeman EW, Greendale GA, Henderson VW, Newhouse Pa, Schmidt PJ, et al. Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause [Internet] 2010 Jul;17(4):815–22. doi: 10.1097/gme.0b013e3181d763d2. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042192-201017040-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis FI. abn: Data Modelling with Additive Bayesian Networks [Internet] 2014 Available from: http://cran.r-project.org/package=abn.
- 49.The R Development Core Team. Vol 0, R Foundation for Statistical Computing. Vienna, Austria: R; 2014. R : A Language and Environment for Statistical Computing [Internet] Available from: http://www.r-project.org. [Google Scholar]
- 50.McCoy N, Culter W, Davidson JM. Relationships among sexual behavior, hot flashes, and hormone levels in perimenopausal women. Arch Sex Behav [Internet] 1985;14(5):385–94. doi: 10.1007/BF01542000. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4062536. [DOI] [PubMed] [Google Scholar]
- 51.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women’s Health Study. J Womens Health (Larchmt) 2007;16(5):667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 52.Dhanoya T, Sievert LL, Muttukrishna S, Begum K, Sharmeen T, Kasim A, et al. Hot flushes and reproductive hormone levels during the menopausal transition. Maturitas [Internet] 2016;89(2016):43–51. doi: 10.1016/j.maturitas.2016.03.017. Available from: http://dx.doi.org/10.1016/j.maturitas.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Gold EB, Crawford SL, Shelton JF, Tepper PG, Crandall CJ, Greendale GA, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms. Menopause [Internet] 2017;24(1):9–26. doi: 10.1097/GME.0000000000000723. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00042192-201701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001 Feb;13:453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 55.Elavsky S, McAuley E. Personality, menopausal symptoms, and physical activity outcomes in middle-aged women. Pers Individ Dif [Internet] 2009;46(2):123–8. doi: 10.1016/j.paid.2008.09.014. Available from: http://dx.doi.org/10.1016/j.paid.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman EW, Sammel M, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12(3):258–66. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 57.Hunter M. Bio-psycho-socio-cultural perspectives on menopause. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):261–74. doi: 10.1016/j.bpobgyn.2006.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure of final model structure for presence of hot flashes.
Supplemental Digital Content 2. Figure of final model structure for moderate to severe hot flashes.
Supplemental Digital Content 3. Figure of final model structure for all outcomes at baseline based on perimenopausal women only.


