Abstract
Protein glycosylation is a posttranslational modification that affects more than half of all known proteins. Glycans covalently bound to biomolecules modulate their functions by both direct interactions, such as the recognition of glycan structures by binding partners, and indirect mechanisms that contribute to the control of protein conformation, stability, and turnover. The focus of this review is the discussion of aberrant glycosylation related to brain cancer. Altered sialylation and fucosylation of N- and O-glycans play a role in the development and progression of brain cancer. Additionally, aberrant O-glycan expression has been implicated in brain cancer. This review also addresses the clinical potential and applications of aberrant glycosylation for the detection and treatment of brain cancer. The viable roles glycans may play in the development of brain cancer therapeutics are addressed as well as cancer-glycoproteomics and personalized medicine. Glycoprotein alterations are considered as a hallmark of cancer while high expression in body fluids represents an opportunity for cancer assessment.
Keywords: brain cancer, posttranslational modification of proteins, glycosylation, central nervous system, aberrant glycosylation, bone marrow-derived human mesenchymal stem cells, glioblastoma, cancer stem cells, glioma stem cells, small cell lung carcinomas, human mucin family, carcinoembryonic antigen
Graphical abstract
1 Introduction
Protein glycosylation is both a co- and post-translational modification that affects more than half of all known proteins.1 Glycans covalently bound to biomolecules modulate their function by both direct interactions, such as the recognition of glycan structures by binding partners, and indirect mechanisms that contribute to the control of protein conformation, stability, and turnover. During, or shortly after, protein biosynthesis amino acids may be modified by the covalent attachment of a variety of functional groups. This type of protein modification is known as posttranslational modification (PTM). In the case of glycosylation, asparagine, serine, threonine, tryptophan,2 hydroxyproline,3 hydroxylysine3 and rarely tyrosine residues are modified by the attachment of carbohydrate moieties. Although there are over 200 PTMs characterized to date; glycosylation is unique in that it is a template free process that results in the addition of heterogeneous structures that cause a large mass shift (typically greater than 800 Da), relative to other PTMs. Glycosylation modifications are involved in several pathological and normal conditions including host–pathogen interaction, tumor invasion, cell infiltration as well as cell migration.4–6 Interactions and cell-cell adhesion are mediated by glycan moieties that are found on cellular membranes and secreted proteins.7–10
Protein glycosylation is generally classified as N- or O-linked. N-linked glycans are attached to asparagine residues that occur in the subsequence Asn-XXX-Ser/Thr, where XXX represents any amino acid except proline. While this is the norm, it should be noted that several studies have reported N-glycosylation sites at non-canonical protein consensus motifs such as Asn-XXX-Gly,11 Gln-GlyThr,12 and Asn-XXX-Cys.13 All N-linked glycans share a common carbohydrate sequence, Manα1–6(Manα1–3)Manβ1–4GlcNAcβ1–4GlcNAcβ1-Asn-X-Ser/Thr, that is referred to as the “core” structure. The multi-step process of N-glycan biosynthesis starts on the cytosolic surface of the endoplasmic reticulum (ER) membrane and is finalized in the Golgi complex.14 N-glycans found in humans are composed of GlcNAc, mannose, galactose, N-acetylgalactosamine (GalNAc), fucose, and N-acetylneuraminic acid (sialic acid) monosaccharide residues and are classified as either high mannose, complex or hybrid. High mannose structures have only mannose residues attached to the core structure, whereas complex structures have other monosaccharides. Hybrid structures possess characteristics of both high mannose and complex structures on different branches. Due to the diverse biological functions of sialic acid and fucose, glycans are also commonly classified as sialylated or fucosylated when these residues are present within the glycan structure.
With the exception of glycosaminoglycan biosynthesis, which represents an independent mechanism involving both the Golgi apparatus and the endoplasmic reticulum, O-glycan biosynthesis is a less complicated process than N-glycan biosynthesis. The process begins with direct covalent attachment of glycans to the serine, threonine or, less commonly, tyrosine hydroxyproline3 and hydroxylysine3 residues of polypeptide chains in the Golgi apparatus.15, 16 Despite the relative simplicity of their biosynthesis, O-glycan structures are still highly variable due to trimming and the addition of monosaccharides by various glycosyltransferases and glycosidases.17 A unique feature of O-glycosylation is the opportunity for attachment of glycans on consecutive and adjacent amino acids,18–23 a phenomenon not commonly observed in N-glycosylation perhaps due to steric hindrance.24 There are also a variety of other O-glycosylation forms documented, such as β-linked O-GlcNAc and α-linked O-fucose, β-linked O-xylose, α-linked O-mannose, α- or β-linked O-galactose, and α- or β-linked O-glucose glycans which were recently reviewed by Moremen et al.25 Additionally, while in-depth characterization of the mechanisms involved in the biosynthesis of all O-linked structures is beyond the scope of this review, those interested may refer to reviews of mucin-type glycan biosynthesis,26 glycosaminoglycan/proteoglycan biosynthesis27, 28 as well as a general review that describes all O-glycan biosynthesis by Wopereis et al.29
Cancer is characterized by aberrations in glycolipid and glycoprotein content; both at the structural and concentration levels.5, 6 Aberrant glycosylation has been associated with the mechanisms underlying tumorigenesis and metastasis.30 Several studies, which utilized experimental approaches that were correlative in nature have associated glycosylation and development of metastasis. For example, glycosylation staining of paraffin sections utilizing the helix pomatia (HPA) lectin, that binds to α-GalNAc residues of cellular structures, was employed to correlate the occurrence of metastasis to other parameters such as tumor size or histological stage.31, 32 Similarly, other approaches used glycosylation enrichment techniques such as Datura stramonium agglutinin (DSA)-sepharose column binding to show that metastasized carcinomas harbor elevated levels of glycosylation compared to normal or primary carcinomas.33 More recent studies, utilizing advanced techniques to dissect the stages of cancer metastasis, have been conducted providing more in depth information into pathological glycosylation mechanisms.34–37
Metastatic spreading is a multistep process that involves the ability of cancerous cells to escape normal tissue boundaries and detach from primary tumors. This process is coupled with degradation of the extracellular matrix (ECM) and invasion of the surrounding tissues or entry into the lymphatic/blood vessels to form metastatic lesions.38 Metastasis and invasion are regulated by alterations in the ECM39–42 impacting cell–cell interactions as well as structural changes in glycosylation that occur on cell surface components.43 Mechanistically, aberrant secretions of cell surface and /or secreted proteins by the malignant cells or surrounding adjacent cellular tissues is crucial for the metastatic process. These molecules include the ECM glycoproteins cytokines, growth factors, and cell surface proteins, and their altered glycosylation promotes self and contact-dependent interactions enabling propagating tumor cells to extravagate to remote tissues.44 A hallmark feature of several tumor cell-types is the upregulation of sialic acid sugars attached to glycolipids and glycoproteins.45
Gliomas represent one of the most prevalent primary brain tumors, which are difficult to treat due to their invasive characteristics.46 Owing to this characteristic feature, gliomas are able to invade normal tissues in a diverse and infiltrative manner compared to peripheral tumors that metastasize to the brain but are not able to penetrate the host nervous tissue, although they can colonize next to it.47, 48 Central nervous system (CNS) gliomas are able to interact with surface receptors which involve both the lectican family chondroitin sulfate proteoglycans and CD44 via binding to the hyaluronic acid-based ECM.49, 50
In the context of the invasiveness of gliomas, BEHAB/brevican protein, a CNS–specific lectican that is regulated in a spatiotemporal manner in the brain,51, 52 has been shown to be upregulated in conditions of glial cell motility, injury, and gliomas.51, 53, 54 It is of interest that being exposed to proteolytic cleavage, alternative splicing, differential glycosylation, and variable expression, in human gliomas, contributes to its progression and plays a key role in its capacity for invasiveness into naive, nervous tissues.55, 56 In an elegant study, Viapiano et al. assessed the role of BEHAB/brevican in gliomas; two novel specific isoforms were identified which were found to carry altered glycan structures due to differential glycosylation regulation. B/bsia and B/bΔg, isoforms were detected; B/bsia is an over-sialylated isoform that was identified in high- and low-grade gliomas, while B/ bΔg was shown to be an under-glycosylated isoform lacking the carbohydrates detected in the high-grade gliomas and absent in normal tissues.57 As they are absent in benign gliomas, this finding highlighted the role of modulated forms of glycosylation as diagnostic markers for glioma progression and as a putative aim for immunotherapy by targeting cell surface antigenicity.57
As previously mentioned, aberrant glycosylation has been closely linked to the development and progression of several cancers, including brain cancer. The primary focus of this review is to discuss the association of aberrant N- and O-glycosylation with human brain cancer, although some studies are utilizing murine and rat models will also be presented. This review also highlights and discusses the potential utility of glycans as biomarkers, and the role glycosylation plays in therapeutics.
2 Aberrant N-Linked Glycosylation in Brain Cancer
N-glycan changes commonly associated with cancer include increased terminal glycan sialylation,58, 59 and increased formation of extensively branched structures.60–62 The three glycosyltransferases, which are known to take part in the biosynthesis of cancer associated branched N-glycan structures, are β1,4-n-acetylglucosaminyltransferase (GnT-III), β1,6-N-acetylglucosaminyl transferase (GnT-V) and α1,6-fucosyltransferase (FUT8) (Fig. 1).63 The product of GnT-III is referred to as a bisecting GlcNAc linkage, where GnT-III catalyzes the addition of GlcNAc to a mannose residue linked through a β1,4-linkage. The introduction of the bisecting GlcNAc prevents further processing because this structure cannot act as a substrate for other glycosyltransferases.64 GnT-III is considered an essential enzyme in the biosynthetic pathway of N-glycans that inhibits metastasis.65
Figure 1.
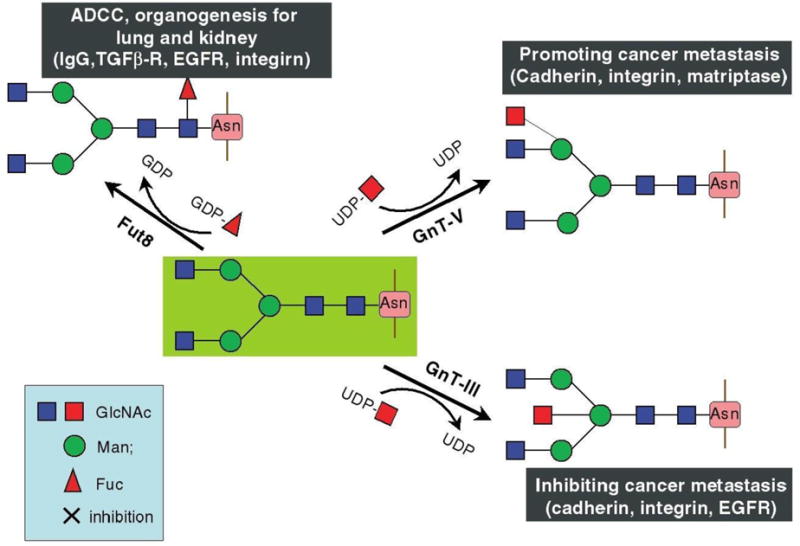
Glycosylation reactions catalyzed by the glycosyltransferases GnT-III and GnT-V, as well as by Fut8, and their biological functions. Reprinted from65, with permission.
The activity of GnT-V is in stark contrast to that of GnT-III, as the catalytic products of GnT-V are β1,6-GlcNAc branching structures known to play essential roles in tumor metastasis (Fig. 1).66, 67 GnT-V, which is encoded by the MGAT5 gene, exhibits increased expression in cancers of the colon, brain, and breast and is documented as decreasing cellular adhesion while promoting tumor invasion and metastasis.65, 68–70 Moreover, when studies were carried out with transplantable tumors in mice, the product of GnT-V was shown to contribute to both cancer growth and metastasis.68, 71 Glycans with β1,6-GlcNAc structures have also been demonstrated to be biomarkers for tumor progression in colon and breast cancer.61, 72 With regards to brain cancer specifically, when GnT-V was overexpressed in glioma cells changes in focal adhesions and increased tumor cell invasion was observed.69
Bone marrow-derived human mesenchymal stem cells (BM-hMSCs) have the intrinsic ability to seek out and engraft in tumors, including glioblastoma (GBM) of the brain. As a result, they have generated interest for use in cell-mediated delivery of therapeutic agents in GBM. When transcriptomic and glycomic profiling strategies were combined for the profiling of glioma stem cell xenografts, cells that did not attract BM-hMSCs were found to display increased truncated high mannose and sialylated N-glycan structures. Whereas cells that attracted BM-hMSCs exhibited N-glycan structures that terminated in either GlcNAc or galactose. The authors hypothesized that increased upregulation of sialic acid expression in cells that do not attract BM-hMSCs might facilitate a reduction in reactive oxygen species-mediated inflammation,73 an assertation supported by studies that indicate sialic acids act as antioxidants by scavenging free radicals.74–79
FUT8 catalysis results in the transfer of fucose residues from GDP-fucose to the sixth position on the inner GlcNAc residue of complex and hybrid glycoprotein N-linked glycans, a process referred to as core fucosylation (Fig. 1). FUT8 activity has been shown to be higher in brain tissues than in other non-diseased tissues, and it is the only core fucosyltransferase expressed in mammals.80 Core fucosylated glycoproteins have been shown to be altered during pathological states, such as hepatocellular carcinoma and cirrhosis of the liver,81, 82 and increased FUT8 expression was demonstrated to have a direct relationship with tumor size and lymph node metastasis in papillary cancer.83 Additionally, the removal of core fucose residues from Immunoglobulin G (IgG) 1 molecules was shown to increase antibody-dependent cell-mediated cytotoxicity (ADCC) by as much as 100-fold, indicating that core fucosylated structures are significantly involved in the ADCC process.84
In regard to the normal brain, Tsuchiya et al. characterized 46.4% of the total N-linked oligosaccharides in normal brain tissue. A2G2 was found expressed in normal brain. While, no tri- or tetra-antennary structures were expressed, unlike bi-antennary N-linked oligosaccharides that were observed most frequently. Oligomannose structures were also detected, with the largest proportion corresponding to M5A then M6B and M9A.85 Another study discerned the existence of O-glycosylation at the synaptosomes and identified three neuron specific proteins, involved in intracellular signaling, that carry glycomic modifications including collapsin response mediator protein-2 (CRMP-2), ubiquitin carboxyl hydrolase-L1 (UCH-L1) and β-synuclein. The presence of such O-GlcNAc modifications is reasonable since O-GlcNAc transferase (OGT), and N-acetyl- β-D-glucosaminidase (O-GlcNAcase) activity has been detected in the cytosol of nerve terminal regions.86
In one study by Zamze et al., it was shown that rodent rat brain proteins carry complex types of abundant N-linked oligosaccharides.87 These include neutral N-linked glycans comprising the “brain type” of N-glycosylation.88, 89 Interestingly, this analysis has been further investigated to assess other forms of N-linked oligosaccharides, including sialylated entities which were found to account for ~40% of the N-glycan pool.87 The remainder of the acidic structures included sulfate groups; in addition to, sialic acid (NeuAc). These altered acidic structures carry high relevance to neural tissue where they are involved in important functions such as neural plasticity90, 91; an excellent example of the involvement of such moieties are α(2-8)-linked polysialic acid and sulfoglucuronyl moieties which are present on the neural-cell-adhesion molecule (N-CAM), and the L2/HNK-1 epitope; respectively92, 93.
Recently, the concept of a hierarchy of cells within a tumor has been widely studied, and cancer stem cells (CSCs) are taking the lions share in this94. Glioma stem cells (GSCs) are found within GBMs and are considered among the main contributors for the initiation and perpetuation of these tumors.95 GSCs adopt the most undifferentiated profile and retain the ability of self-renewal and aberrant proliferation.96 A study by Boa et al. revealed that GSCs are able to defy ionizing radiation induced death through the activation of the DNA repair system.97 Thus, GSCs are resistant to the most exhaustive treatments, such as chemotherapy and radiotherapy, contributing to cancer recurrences.97, 98 During tumor re-initiation, GSCs undergo several molecular changes that are dependent on alterations in glycosylation. Of interest, the glycosylation status of cell surface glycoprotein epitopes has been associated with differentiation state, tumor transformation and progression.
2.1 Altered Sialylation Associated with Brain Cancer
Sialic acids are monosaccharides that are commonly observed as terminal residues of glycoprotein oligosaccharide chains. Sialic acids are involved in a number of biological processes, such as immune modulation and cell adhesion, and are known to bind proteins such as siglecs, selectins, and lectins.99, 100 Negatively charged sialic acid residues, on the cell surface, are capable of impacting tumorigenesis by decreasing cell adhesion, which may result in increased cancer cell motility and greater metastatic potential.101–104 Numerous studies have reported elevated glycoprotein sialic acid levels in malignant cells,105–107 and it has been suggested that the changes in sialic acid abundance take place early on in the process of tumorigenesis.108 Cell surface sialic acid epitopes have the ability to mask antigenic sites, preventing recognition of foreign or cancerous cells by the immune-defense system.101 An example of such masking is provided by the binding of tumor cell sialic acids to Siglec7 on NK cells, which prevents cancer cell immune recognition by NK T cells. Hudak et al. demonstrated this by inserting sialylated glycopolymers into the membrane of cancer cells, to emulate cancer associated sialylation, and showing that the ensuing localization of Siglecs to the activation site increases SHP-1 and SHP-2 phosphatase recruitment. This recruitment resulted in inhibition of the phosphorylation cascade, thus preventing NK cell activation (Fig. 2).109
Figure 2.
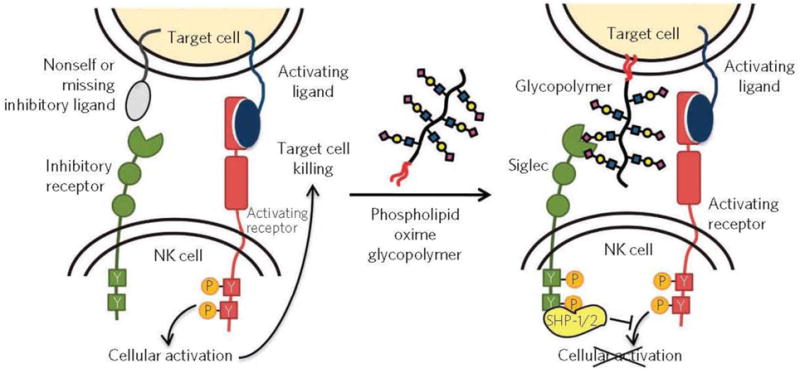
In the presence of activating ligands and absence of inhibitory ligands on the target cell, NK cells are activated to release cytotoxic effectors and cytokines. Coating cancer cells with sialylated glycopolymers by membrane insertion can emulate cancer associated glycosylation changes that engage the Siglec family of inhibitory receptors. Localization of Siglecs to the site of activation enhances SHP-1/2 phosphatase recruitment to halt the phosphorylation cascade before cellular activation. Reprinted from109, with permission.
A large number of glycomics and glycoproteomics studies have established a strong correlation between glycosylation and brain cancer. These studies have investigated the role of glycomic changes in the transformation and progression of neural cells. Several of these studies have shown that cancer transformation, progression, and metastasis are coupled with alterations in glycosylation phenotypes, as discussed later. Hu et al.110 evaluated the mechanisms by which MYCN gene amplification relates to neuroblastoma transformation. The study revealed that N-linked glycomic changes relate to the progression of these cells. The study was performed on two neuroblastoma cell lines: SY5Y, where MYCN was not amplified, and NLF which is a cell line that has elevated levels of the MYCN gene. Their results revealed that the more aggressive neuroblastoma cell line, NLF, had increased sialylation and expression of larger glycans compared to SY5Y. These cell lines demonstrated significant alterations in sixteen different glycans as detected by LC-MS/MS. Among the sialylated glycans that showed the most significant upregulation were HexNAc5Hex6NeuAc4, HexNAc5Hex6NeuAc3 and HexNAc6Hex7dHex1NeuAc2. Furthermore, SY5Y contained small glycans like HexNAc2Hex4 whereas NLF expressed larger glycans such as HexNAc4Hex5dHex1NeuAc2.110
Most of the proteins found in the sera of humans are glycosylated, and many of these glycosylated proteins contain sialylated structures. Alterations to sialylated glycan structures on serum glycoproteins are downstream of cancer induced changes in the behavior of glyco-enzymes, such as the sialyltransferase family of enzymes.63 As a result, a number of research groups have proposed the use of aberrant serum protein glycan sialylation as a cancer biomarker.111–115 A study involving the quantification of total serum sialic acid in healthy individuals versus lung cancer patients indicated that total serum sialic acid was significantly elevated in lung cancer patients when compared to healthy individuals, supporting the utility of serum glycoprotein sialylation as a cancer biomarker.116
Terminal N-glycan sialic acids can be attached via α2,3- or α2,6-linkages. α2,3-Linked sialic acids, and their associated sialyltransferase have been reported to be the only detectable form of sialic acid in patients with GBM.117, 118 Additionally, in vivo studies have indicated that α2,3- and α2,6-linked sialic acids promote opposing processes, with α2,3-linked sialic acid promoting tumor cell invasion and α2,6-linked sialic acid decreasing it. It was also reported that, along with reduced cancer cell invasion, α2,6-linked sialic acid was associated with changes in adhesion-mediated signaling and a reduction in tumor growth. Yamamoto et al. further postulated that because integrin-extracellular matrix interactions are critically involved in anchorage-dependent tumor growth, that ST6Gal1 expression, through glycosyltransferase gene delivery, may alter α3β1 integrin function in a manner useful for ortho-adhesion and signaling therapy for malignant brain tumors.70
The increased ST3GAL1 expression has been characterized as defining an invasive fraction of GBM cells with the capacity for self-renewal and a loss of its function has been shown to prolong survival in mouse models.119 It was also demonstrated, in the same study, that the transcriptomic program that is associated with ST3GAL1 is associated with poor prognosis in glioma patients and leads to higher tumor grades of the mesenchymal molecular classification.119 Additionally, hyper sialylated β1 integrin has been demonstrated to be endogenously expressed in human astrocytoma cell lines, and it has been suggested that sialylation may represent a mechanism for the regulation of β1 integrin signal transduction in glioma cells.120
When normal human astrocytes were transformed to mimic human brain tumor grades I-IV, the changes observed included elevated expression of α2,6-sialylation with a concomitant decrease in core 2 (GlcNAcβ1-6(Galβ1-3)GalNAcαSer/Thr) O-glycan biosynthesis.121 Furukawa et al. employed a multi-step human glioma model to unveil the glycosylation profiles and their contribution to tumor progression. The model consisted of consecutive introduction of hTERT, SV40ER, H-RasV12 and myrAKT, thereby transforming normal human astrocytes (NHA) in a way that mirrors the four stages (I – IV) of gliomas. Although it does not comprise the same complexity of brain cancer in vivo, this model allows for the modification assessment of N- and O-glycans along with glycosphingolipids (GSL) through the successive brain tumor stages121. The introduction of hTERT increases the life of NHAs; thus, establishing grade I glioma cells. At this stage, alterations in N-glycans were demonstrated by an elevation in paucimannose and monoantennary glycans as well as β1,6-branched glycans. In addition, an upregulation of the GSL GD3 was also identified. It was also shown that SV40ER immortalized the cells, that up to this stage were still benign, simulating grade II glioma cells. A decrease in total N-glycans and GSL occurs at this step. The N-glycans expressed are multi-fucosylated with an elevation in N-glycolylneuraminic acid (Neu5Gc) expression. The changes in GSL were displayed as a transition from ganglio-series GSLs to globo-series. Next, transformation and malignancy manifest in grades III and IV that are obtained by H-RasV12 and myrAKT induction, respectively. Of interest, Grade III phase cells exhibited an elevation in branched GlcNAc N-glycans and a downregulation of core 2 O-glycan biosynthesis. Moreover, transformed cells had higher levels of α2,6-sialylation. However, the fully transformed grade IV cells exhibited reduced levels of bisecting GlcNAc N-glycans and increased levels of fucosylated glycans. Furthermore, (neo) lacto-series GSLs were also upregulated. Thus, data from this study indicate that glycosylation modifications during glioma progression are transitory rather than stable.121
Polysialic acid (PSA) is a linear carbohydrate homopolymer composed of α2,8-linked sialic acids, that is primarily bound to N-CAM. N-CAM in most adult tissues lack PSA, while poly-sialylated N-CAM in embryonic tissues is abundant.122–124 Further, during the process of embryonic development, poly-sialylated N-CAM is localized specifically to migrating cell types.125, 126 The presence of large negatively charged PSA molecules on N-CAM regulate its adhesive properties, and a large body of research suggests that PSA regulates its function, as well.127 Despite the reduction of PSA expression in most adult tissues, some tumors have been shown to “re-express” PSA. Therefore, PSA is considered an oncodevelopmental antigen.
Human lung cancer cells, small cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC), as well as astrocytomas, are known to express PSA,128–130 and it is believed that either decreased N-CAM or a reduction in its adhesive properties, by PSA, result in increased cell migration and metastasis.127 PSA and N-CAM expressions correlate positively with both cell proliferation and metastatic potential, in SCLC.128, 131 Additionally, in NSCLC, PSA expression is found in 77% of stage IV tumors, whereas only 21% of stage I tumors are positive for PSA expression.129 This general trend is also observed in astrocytomas, where PSA expression is 10 times higher in world health organization grade III and IV tumors compared to grade II tumors.130
When the expression of PSA-NCAM, a neurodevelopmental protein, was examined in GBM it was found to be an adverse prognostic factor for overall survival and disease free survival. In the same study, PSA-NCAM expression was also found to correlate with olig2, which is a transcription factor required for gliomagenesis.132 In a different study, PSA-NCAM was found to be present in 46.3% of typical human pituitary tumors and 85% of human pituitary tumors regarded as highly aggressive, while being absent in the healthy pituitary gland, and PSA-NCAM expression was found to be strongly related to tumor invasion.133 Fibronectin and sialic levels have also been demonstrated to be significantly higher in human pituitary adenomas than in normal brain tissue, and infiltrative pituitary adenomas were found to contain higher levels than non-infiltrative adenomas.134 In a study conducted by the same authors, fibronectin, and sialic levels were also demonstrated to be significantly higher in human meningiomas and gliomas than in nondiseased control brain tissue, and grade III-IV gliomas were found to contain higher levels than grade I-II gliomas. Based on these findings, one can conclude that both sialic acid and PSA modifications have a high probability of involvement in the lung, brain, and other cancers.
2.2 Altered Fucosylation Associated with Brain Cancer
Fucosylation is a common modification that takes place when the deoxyhexose fucose is transferred from GDP to glycolipids, O-glycans, N-glycans or polypeptide chains. Fucosylation is known to be involved in a number of biological functions, such as ABO blood group determination (Fig. 3), host-microbe interactions, selectin dependent leukocyte-endothelial adhesion,135, 136 ontogenesis, including Notch receptor signaling, as well as several pathological conditions, such as atherosclerosis and cancer.137 An example of aberrant glycosylation in cancer involving fucosylated glycans is provided by the loss of A (GalNAcα1-3(Fucα1-2)Gal-) and B (Galα1-3(Fucα1-2)Gal-) blood group antigens, coupled with an increase in H (Fucα1-2Gal-) antigen and Lewis-Y (Fucα1-2Galβ1-4(Fucα1-3)GlcNAc-) antigen expression, in many tumors. Changes of this nature are of particular importance because they have been found to correlate with poor clinical prognosis.138–141 α-Fetoprotein provides an additional example, as its increased α1,6-fucosylation is used as a clinical diagnostic marker to distinguish between hepatocellular carcinoma and chronic liver disease.81
Figure 3.
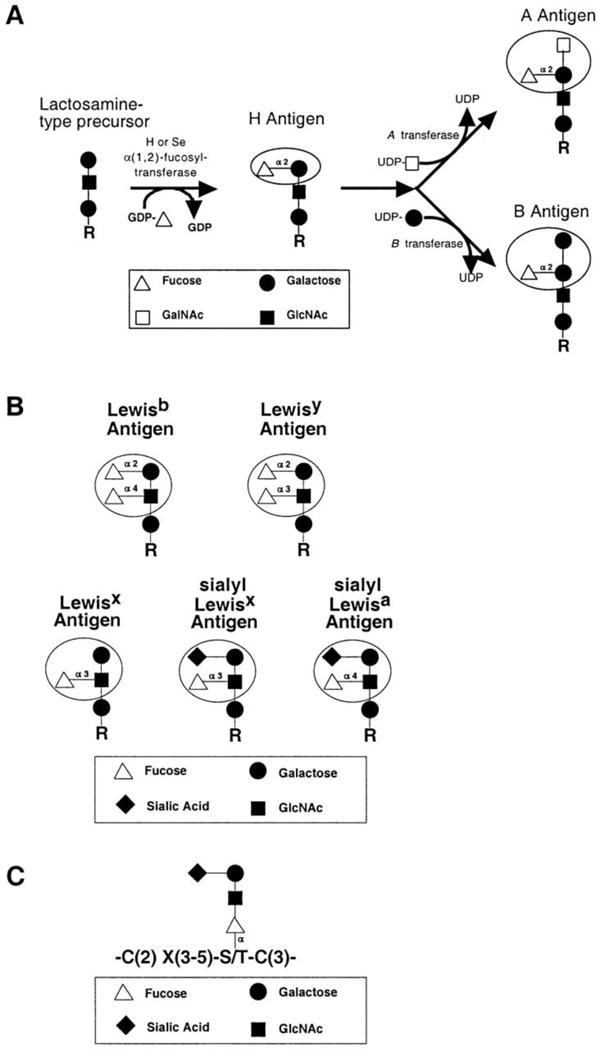
Structures of common fucosylated glycans. (A) Synthesis of ABO blood group antigens. The H and Se transferases are a pair of α(1,2)-fucosyltransferases that synthesize the H antigen in a variety of tissues. The ABO locus encodes a glycosyltransferase that further modifies the H antigen. The A allele at the ABO locus encodes an N-acteylgalactosaminyltransferase. The B allele encodes a galactosyltransferase that differs from the A transferase at four amino acid positions. The O allele at the ABO locus encodes a truncated, enzymatically inactive protein. (B) Lewis-related antigens. Circles indicate the immunodominant portion of each antigen. (C) A representative O-linked fucose glycan. Fucose modifies serines or threonine within the broad consensus site shown here, and in Table I. R indicates glycolipid and N- and O-linked glycoprotein precursors. Reprinted from137, with permission.
Sialyl Lewis-X (Siaα2-3Galβ1-4(Fucα1-3)GlcNAc-) and –A (Siaα2-3Galβ1-3(Fucα1-4)GlcNAc-) antigens (Fig. 3), glycan structures bound by the endothelial-leukocyte adhesion molecule E-selectin, are commonly expressed at higher levels in carcinoma cells.142–144 These higher expression levels are correlated with both advanced tumor grade and poor prognosis. Additionally, because sialyl Lewis-X and -A antigens function as ligands for selectin molecules, these glycans may facilitate hematogenous metastasis through direct binding of cancerous cells to P- and E-selectins present on the endothelium.138, 145 Additional potential scenarios where metastasis is facilitated by the fucose-containing structures sialyl Lewis-X and –A antigens include the formation of cellular thromboemboli by way of interaction with P-selectin146 and the blockage of tumor leukocyte infiltration through the secretion of inhibitors, that contain sialyl Lewis-X and -A antigen structures, of endothelial-leukocyte adhesion.138
The Lewis-X (Galβ1-4(Fucα1-3)GlcNAc-) antigen (Fig. 3), also known as stage-specific embryonic antigen 1 (SSEA-1) and CD15, is a fucose containing glycan proposed to be a marker for tumor-initiating cells/tumor stem cells among GBM cell populations.147 When SSEA-1+ cells were selected for from acutely isolated tumor cells from fresh GBM patient tumors they were found to be enriched in glioma tumor-initiating cells. Further, SSEA-1+ acutely isolated cells and cultured in vitro-established tumor-initiating cells were determined to exhibit, at least, a 100-fold tumorigenic enrichment in mouse xenograft models when compared to SSEA-1- cells and demonstrated capabilities for self-renewal and multi-lineage differentiation.147
Moreover, in a study that aimed to define the N-linked oligosaccharides present in GBM, Tsuchiya et al. identified 16 oligosaccharides from GBM tissue.85 They determined that major oligosaccharide structures were similar between the normal brain and GBM tissue. Interestingly, they reported an elevation in the levels of biantennary bigalactosylated sugar chains with one core fucosylation (A2G2F) moiety normally expressed in the brain at embryonic stages only. Owing to the core fucose in A2G2F, GBM cells were able to bind to lens culinaris agglutinin (LCA) lectin which subjected them to apoptosis, indicating the potential importance of A2G2F in targeted therapy.85
3. Aberrant O-Linked Glycosylation in Brain Cancer
Glycans covalently linked to the polypeptide chain of a protein by the hydroxyl group of serine or threonine are referred to as O-linked glycans. Mucin O-glycans are α-linked via GalNAc. Mucins are heavily O-glycosylated glycoproteins produced by the epithelia of most animals that come in both secreted and transmembrane varieties. They are integral to the formation of mucus barriers and are documented as being intimately involved in both inflammation and cancer (Fig. 4).148 Alternatively, when O-glycans are covalently bound through a β-linkage to a GlcNAc, the modification is known as O-GlcNAc. O-GlcNAcylation is comparable to protein phosphorylation in that it is a dynamic process where the PTM can be removed or added in response to changes in the local environment related to nutrients, hormones or stress.149 O-GlcNAcylation is one of the most abundant PTMs to occur in the cytoplasm and nucleus and is known to regulate a variety of essential biological processes, including metabolism, insulin recognition, cell proliferation, protein degradation, gene transcription and cell signaling pathways.63
Figure 4.
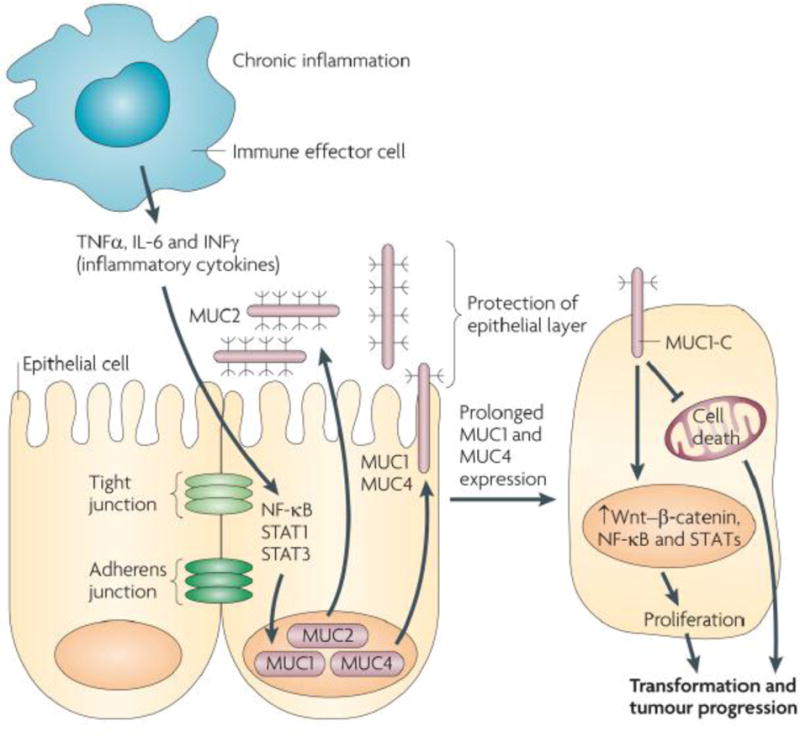
Mucins, chronic inflammation and cancer. In this proposed model of the association of mucins with chronic inflammation and cancer, the production of inflammatory cytokines by immune effector cells activates transcription factors, for example nuclear factor-κB (NF-κB), signal transducer and activator of transcription 1 (STAT1) and STAT3, in epithelial cells. In turn, these transcription factors upregulate mucin expression to enhance the mucous barrier and protect the epithelial layer. Mucin 2 (MUC2) limits the inflammatory response at the apical membrane and inhibits transformation. Upregulation of the MUC1 and MUC4 transmembrane mucins similarly contributes to the protective barrier and loss of polarity in the epithelial stress response. Activation of MUC1 is associated with targeting of the MUC1 C-terminal transmembrane subunit (MUC1-C) to the nucleus, where it promotes a gene programme for proliferation and survival. Targeting of MUC1-C to the mitochondria also blocks cell death to prevent loss of the epithelial barrier. However, with chronic inflammation and prolonged stimulation of this protective response, epithelial cells may become susceptible to the accumulation of genetic mutations that induce transformation in a setting with downregulation of pathways that would otherwise protect against oncogenic events. IL-6, interleukin-6; IFNγ, interferon-γ; TNFα, tumour necrosis factor-α. Reprinted from148, with permission.
3.1 O-GlcNAc Glycan Changes Associated with Brain Cancer
The process of O-GlcNAcylation is regulated by a pair of enzymes that catalyze the addition of GlcNAc, which is transferred from uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) and catalyzed by O-GlcNAc transferase (OGT), and the cleavage of GlcNAc, which is catalyzed by O-GlcNAcase (OGA). The O-GlcNAc modification is known to affect a variety of proteins involved in a number of diverse processes including insulin resistance (Akt, insulin receptor substrate 1),150 oncogene transcription (c-Myc, NF-κB),151, 152 tumor suppression (p53),153 hepatic gluconeogenesis (transducer of regulated cyclic adenosine monophosphate response element-binding protein 2),154 maintenance of neuronal health and prevention of Alzheimer’s disease (α-synuclein, amyloid precursor protein, tau, protein kinase A),155–159 regulation of mitosis and neuronal development (Cdh1),160 among other things.
The substrate for OGT, UDP-GlcNAc, is an end product of the hexosamine biosynthesis pathway that branches from glycolysis, directly linking cellular metabolism with O-GlcNAcylation (Fig. 5).161, 162 Additionally, nine out of the 10 enzymes required for glycolysis are potential substrates for OGT.163 Cancer cells produce energy by the non-oxidative breakdown of glucose, with tumors cells demonstrating glycolytic rates as high as 200 times that of normal tissues.164 O-GlcNAcylation serves as a nutrient and stress sensor that links the metabolic state of cells with a myriad of signaling pathways;165 therefore, it is plausible that glycosylation with O-GlcNAc may play a role in the regulation of glycolysis and the ensuing metabolic abnormalities observed in cancer cells. A study by Yi et al. supported this hypothesis by demonstrating that O-GlcNAcylation of phosphofructokinase 1 (PFK1) was induced by hypoxic conditions,166 which commonly occur in cancer cells. Further, glycosylation was shown to inhibit PFK1 activity and redirect glucose flux through the pentose phosphate pathway, resulting in a selective growth advantage for human NSCLC cells.166
Figure 5.
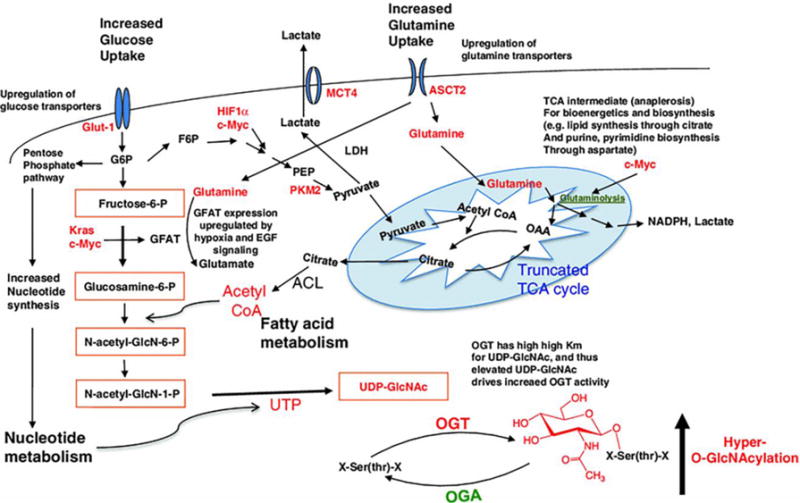
Cancer cell metabolic changes linked to hyper-O-GlcNAcylation. The hexosamine biosynthetic pathway (HBP) outlined in orange boxes integrates metabolic intermediates to generate the end-product UDP-GlcNAc. Glucose is transported into cells by glucose transporters such as Glut1 and then first phosphorylated by hexokinase to generate glucose-6-phosphate. Glucose-6-phosphate can be shunted into the PPP which produces nucleotides and NAPDH, or converted into fructose-6-phosphate. While most fructose-6-phosphate continues through glycolysis to produce pyruvate, some is directed into the HBP. GFAT, the HBP rate-limiting enzyme, irreversibly transfers the amino group from glutamine to fructose-6-phosphate, generating glucosamine-6-phosphate and glutamate. Glucosamine-6-phosphate is ultimately converted to UDP-GlcNAc, which is used by OGT to attach O-GlcNAc to hydroxyl groups of serine and/or threonine residues of cytosolic and nuclear proteins. O-GlcNAc is removed by OGA. Cancer cell metabolic changes including increased glucose uptake (due to “Warburg effect”) and increased glutamine uptake (along with elevated UTP and acetyl-CoA production) cooperate to maximize flux through the HBP. Oncogenes such as HIF1α, Kras, and c-Myc regulate cancer cell shifts to aerobic glycolysis and glutaminolysis, including upregulation of glucose and glutamine transporters and increased expression of GFAT. Additionally, the level of OGT is increased and the level of OGA is decreased. In sum, cancer cell metabolic reprogramming leads to increased HBP flux, elevated UDP-GlcNAc, and ultimately hyper-O-GlcNAcylation. Proteins and metabolic intermediates in red are increased in cancer cells. G6P: Glucose-6-phosphate; F6P: fructose-6-phosphate; FBP fructose 1,6-bisphosphate, PEP phosphoenolpyruvate, GFAT glutamine: fructose-6-phosphate amidotransferase, MCT4 monocarboxylate transporter, OAA oxaloacetate, PFK1 phosphofructokinase 1, PKM2 pyruvate kinase M2. Reprinted from161, with permission.
3.2 O-GalNAc Glycan Changes Associated with Brain Cancer
Members of the mucin family contain tandem repeat structures that have a high proportion of prolines, threonines, and serines, which are known as PTS domains. The PTS domains of mucins are heavily glycosylated with glycans attached through GalNAc residues O-linked to serine and threonine. Members of the human mucin family (MUC) are designated MUC1 through MUC21 and fall into secreted or transmembrane sub categories. Different glycosyltransferases synthesize the four most common mucin-type O-glycans which are the core1 or T-antigen (Galβ1-3GalNAcαSer/Thr), core2 (GlcNAcβ1-6(Galβ1-3)GalNAcαSer/Thr), core3 (GlcNAcβ1-3GalNAcαSer/Thr) and core 4 (GlcNAcβ1-6(GlcNAcβ1-3)GalNAcαSer/Thr) structures.
Abnormal patterns of mucin expression in the human respiratory mucosa have been found to be associated with lung carcinogenesis.167–169 An example of a secreted mucin playing a role in cancer progression is provided by MUC2, which has been shown to functionally suppress inflammation in the intestinal tract and inhibit the development of colorectal cancer.170, 171 When the expression of core Tn (GalNAcαSer/Thr), sialyl-Tn (Siaα2-6GalNAcαSer/Thr) and core 1) and terminal fucosylated and sialylated (Lewis antigens) glycans in different NSCLC lung tumors were compared, it was found that truncated structures were observed in both tumor types, but core and terminal structures were detected more frequently in adenocarcinoma than in squamous cell carcinoma, excepting Lewis-Y structures which are substantially expressed in both NSCLC types.172 This finding is interesting in that it implies that different combinations of glycosyltransferases are expressed in adenocarcinoma and squamous cell carcinoma. Further, it has been demonstrated that while most Lewis structures are expressed in NSCLC tumors, Lewis type 1 antigens are detected less frequently than Lewis type 2 antigens and Lewis-Y structures represent the most strongly expressed antigen.173, 174
MUC1 is a transmembrane mucin that is localized to the apical membrane of secretory epithelial cells under normal conditions.175 During the process of transformation when epithelial cells lose their polarity, MUC1 is known to exhibit high levels of expression on the entire surface of a diverse array of carcinoma cells. After being translated into a single polypeptide MUC1 splits, through auto proteolysis, into N-terminal (MUC1-N) and C-terminal (MUC1-C) subunits.148 Glycosylation of the tandem repeats present in MUC1-N have been shown to be altered in carcinomas, a change at least partially attributed to alterations in glycosyltransferase expression observed in tumors.176 Atypical glycosylation of MUC1 also has demonstrated involvement in cancer immunosurveillance.177
MUC1 is anchored to the cell surface when MUC1-N non-covalently associates with its corresponding transmembrane subunit, MUC1-C, forming a stable complex. MUC1-N, which is the mucin component of the complex, blocks both cell-extracellular and cell to cell interactions.178 When MUC1-N departs from the cellular surface, it is believed that MUC1-C remains, putatively functioning as a receptor interacting with a variety of signaling pathways associated with transformation and tumor growth.179 When the cellular distribution of MUC1 was examined, using immunohistochemistry, in patients with NSCLC, it was found that a depolarized cellular distribution of MUC1 was associated with modal metastasis, advanced pathological state, and poor prognosis.180–182 Additionally, immunohistochemical analyses also indicated that a high ratio of MUC1 to surfactant apoprotein A expression was strongly associated with poor outcomes in patients with small-sized lung adenocarcinoma.183 It should be noted that one study examining the expression of a novel carbohydrate-induced conformational tumor-associated MUC1 epitope, termed TA-MUC1, found it to be a favorable prognostic factor for NSCLC patients with lymph node metastases184, in contrast to many other findings.
MUC1 has also been proposed as a marker enabling researchers to follow the type II pneumocyte lineage through the process of lung carcinogenesis. It was reported that MUC1 expression was preserved in Type II alveolar pneumocytes, which serve as progenitor cells for neoplastic and normal epithelium during the repair of injury and cancer carcinogenesis, but was decreased when NSCLC cells reverted to more normal phenotypes.169 In addition to lung cancer, the MUC1 expression has also been shown to correlate with prognosis in ovarian cancer, where low expression of MUC1 was found to associate with early stage and good outcomes for patients with invasive tumors.185 In an indirect role, MUC1-induced tumorigenesis has been demonstrated to contribute to the regulation of genes highly associated with poor clinical outcomes in both lung and breast cancer patients.186, 187 Signal transducer and activator of transcription 3, better known as STAT3, is known to be abnormally activated in human lung cancer and was demonstrated to regulate expression of MUC1 mediating metastasis both in vitro and in vivo (Fig. 4).188 Additional findings have also indicated that NSCLC cells depend on MUC1-C for activation of the PI3K/Akt pathway and survival.189
The smoking of cigarettes accounts for 87% of lung cancer deaths and increases the risk for lung cancer 20-fold. Zhang et al. reported that cultured primary human bronchial epithelial cells treated with smoke-concentrated medium generated a novel 400 kDa glycoform of MUC1-N that differed from the 230 and 150 kDa glycoforms observed in untreated cells.190 Further, the smoke-induced shedding of glycosylated MUC1-N resulted in the exposure of the membrane spanning MUC1-C subunit, enabling it to serve as a putative receptor for the epidermal growth factor receptor (EGFR), proto-oncogene tyrosine-protein kinase Src and p120-catenin. The glycosylation of MUC1-C, induced by smoke exposure, modulated phosphorylation of MUC1-C tyrosine which was essential for interaction of MUC1-C with p120-catenin through bridging of Src-MUC1-C-galectin-3-EGFR signalosomes,190 a process upstream of the degradation of E-cadherin, reduction of cell-cell adhesion and the loss of cellular polarity that’s associated with the epithelial–mesenchymal transition and lung carcinogenesis. This body of work strongly suggests that aberrant O-glycosylation of MUC1 contributes to the pathogenesis of lung cancer.
The version of MUC1 associated with cancer is thought to be distinct from the normal protein. The malignant-associated form is reported to contain a lower percentage of O-glycosylated serine and threonine residues. Additionally, the O-glycans that are attached tend to be shorter and truncated forms relative to that of the normal MUC1.63, 191, 192 It has been proposed that MUC1 on cancerous cells may exhibit anti-adhesive effects, due to a combination of its net negative charge, stemming from sialic acid residues, and its ability to mask surrounding cell surface molecules inhibiting their interactions with adjacent binding partners.178 Examples of MUC1 exhibiting anti-adhesive effects have been provided by studies examining both integrin-193 and E-cadherin194 mediated cell matrix and cell-cell adhesion, respectively. An additional mechanism by with MUC1 and other glycoproteins may contribute to tumor growth was proposed by Paszek et al. who demonstrated that a bulky glycocalyx, as a result of the expression of bulky glycoproteins such as MUC1, may initiate metastasis by mechanically enhancing integrin-dependent growth factor signaling promoting growth and survival of tumor cells.195
MUC1 is a transmembrane protein expressed on epithelial cells that are characterized by aberrant O-glycosylation when in a cancerous state. These altered glycosylation patterns allow the exposure of the protein core which in turn facilitate the binding of tumor cells to other tissues; which is a major feature of metastasis196. MUC1 protein under-glycosylation is also associated with more aggressive stages. Therefore it represents a diagnostic marker.197 In addition, MUC1 associates with CIN85 protein forming a complex in tumor cells mediating cell migration and invasion.198 Finally, MUC1, MUC1-core, and Thomsen–Friedenreich (TF) proteins are expressed in mucinous tumors.199 On the other hand, galectins are galactoside-binding lectins involved in cell adhesion and growth regulation.200 Differential expression and function of the galectin family (elevated galectin-1 binding and downregulated galectin-3) have been correlated with lymph node metastasis.200 Binding to matrix metalloproteinase 9 (MMP-9) and laminin are among the roles of galectin-3. Upon decreased MMP-9 binding to ECM in tumor cells, the specific MMP cleaves the ECM-proteins leading to the activation of the metastatic process.201 The cancer-associated MUC1 protein serves as a ligand for galectin-3 mediated by the Thomsen–Friedenreich antigen (TF-Ag), and it promotes cancer cell adhesion to the endothelium, which initiates metastasis.202, 203
While the findings of Gordower et al., who demonstrated that galectin-3 expression significantly decreases in most tumor astrocytes from low to high grade,204 corroborates the aforementioned study it should be mentioned that conflicting evidence has been reported. In their study, Gordower et al. also observed some tumor cell clones, that were highly malignant, expressing galectin-3 at high levels.204 Additionally, it has been demonstrated that galectin-3 expression levels were significantly associated with astrocytic tumor grade,205 and a number of other studies have also reported that astrocytic tumors demonstrate high galectin-3 expression levels.206–210
In the interest of clarifying these conflicting findings Strik et al. implemented immunohistochemistry to pinpoint the cellular origin and degree of galectin-3 positivity in glioma samples.208 They demonstrated that galectin-3 expression was observed in B- and T-lymphocytes, endothelial cells, macrophages/microglial cells and neoplastic astrocytes and showed that tumor-infiltrating macrophages exerted considerable influence on galectin-3 positivity.208 Therefore, one can conclude that galectin-3 expression levels appear to be dependent on non-tumor cells such as endothelial cells or macrophages/microglial cells, which may explain, in part, the conflicting data that have been presented on the expression of galectin-3 in human gliomas.208, 211
The involvement of galectin-1 in cancer biology is more clear cut as it has been shown to have numerous functions that include involvement in cell migration, through interactions with integrins and the ECM,212 the formation of metastases,213 angiogenesis stimulation via ORP150 regulation,211 radio- and chemo- resistance through interactions with Ras214, 215 and modulation of p53 nuclear migration.214 Galectin-1 has also been shown to inhibit T-cell effector function through the promotion of apoptosis which has the effect of enabling tumor invasion of the immune system.216, 217 In light of the role galectin-1 plays in radio- and chemo-resistance, it is considered a therapeutic target and synthetic lactulose amines218 as well as galactomannan (Davanat®)219 have been used to bind and modulate its activity. The latter, Davanat®, has received approval for colorectal cancer treatment from the FDA and appears promising, but more research is required to determine its utility for GBM treatment.220
GBM is the most common primary brain tumor and results in the largest number of central nervous system tumor-related deaths. The expression of MUC4, a highly O-glycosylated protein, has been studied in a number of cancers.221–229 When MUC4 expression was examined in GBM cell lines, it was found to be overexpressed. Additionally, the capacity for GBM cell proliferation and invasion was dramatically increased by ectopic expression of MUC4. The expression of EGFR was found to be modulated by MUC4, and small interfering ribonucleic acid experiments targeting MUC4 and EGRF reduced proliferation and invasion, in both instances. Therefore, one can draw the conclusion that MUC4 expression plays a part in GBM proliferation and invasion, potentially through upregulation of EGFR.229
4. Clinical Potential and Applications of Aberrant Glycosylation for the Detection and Treatment of Brain Cancer
4.1 Identification of Glycan Biomarkers for Brain Cancer
It is well established that aberrant glycosylation commonly coincides with cancer and many instances of specific structures correlating with advanced disease states have been presented. Numerous studies, many of which are reviewed herein, indicate that aberrant glycosylation may contribute to the progression of cancer. Therefore, identification and characterization of glycan biomarkers have great potential for early diagnosis and favorable prognoses. This is particularly true in light of studies that demonstrate early detection of the brain, lung, and other cancers is correlated with improved prognoses and patient survival.230, 231 The importance of early detection, especially in cancers such as lung and brain where early disease is asymptomatic, is highlighted by Miller et al. who reviewed cancer treatment and survivorship statistics for 2016 and reported that the five year survival rate for lung cancer is 55% for cases detected early when the disease is localized to its place of origin, but only 4% when detected at an advanced stage.232 Brain cancer is similar to lung cancer in that by the time a glioma becomes symptomatic it is nearly always far too late in its clinical course for successful treatment.233 Therefore, if we hope to improve brain cancer prognoses, early detection is critically needed, and biomarker screening represents a minimally invasive option with much potential.
As was previously stated, over half of all proteins are glycosylated, and it is estimated that the majority of human serum are glycosylated.1 Changes in glycosylation reported to accompany pathological conditions range from minimal to significant and can be extremely specific to certain conditions, therefore it’s believed that the investigation of serum protein glycosylation provides a viable avenue for diagnosis and prognosis of many diseases.234, 235 Pathological states both acute and chronic in nature have been associated with changes in glycosylation236 and in the case of acute pathology changes may occur very early on.237, 238 IgGs are glycoproteins secreted by the adaptive immune system to impart long-term defense against antigens of previous exposure. IgGs exhibit significant diversity in the position and number of conserved N-linked glycosylation sites that are found on both the crystallizable fragments (Fc) and antigen-binding fragments (Fab) of the proteins. IgG glycans play multiple roles and are critical for IgG structure, binding events, solubility, conformation and function.239 Accordingly, IgG glycosylation status are viewed as potential biomarkers for specific diseases such as cancer. An example of this is provided by Chen et al. who reported a marked increase in IgG1 Fc-agalactosylation (a decrease in galactose) in lung cancer patients when 259 lung cancer patients were compared to 410 control individuals.240
Specific ablation of fucose and/or sialic acid of particular glycan chains can trigger ADCC, resulting in the death of cancer cells.241–243 The majority of the serological assays utilized for prognosis, diagnosis and surveying cancer development assess glycoconjugates including glycoproteins, proteoglycans, and glycosphingolipids, etc.4 One example is the mucin glycoprotein CA 125 assay, of ovarian cancer (detected in 80% of ovarian patients), where levels are correlated with regression and prognosis.244–246 Elevated levels of CA125 are detected in 50% of patients with stage I ovarian cancer.247 Currently, there are several glycolipid biomarkers found in tissues and biofluids, that are FDA approved, that are used in cancer diagnosis and prognosis, including CA27-29-Breast cancer, CEA Carcinoembryonic antigen-Colon cancer; CA19-9-Gastrointestinal cancer, etc. (please refer to Kim et al. for a detailed discussion248).
Cheray et al.95 examined two well-defined GBM cell lines, U87-MG and U251, at distinct phases: floating undifferentiated and adherent differentiated cells. The main focus of the study was to scrutinize the implication of changes in the glyco-genome expression during the differentiation of GSCs. They analyzed 559 glycosylation-related genes using TLDA and were able to identify eight chiefly upregulated genes in the GSC-enriched undifferentiated state in both cell lines. These genes comprised members of the glycosyltransferase family that are potentially involved in tumor aggression and invasion. In addition to glycosyl-hydrolases that are involved in tumor angiogenesis.95 As a result, the authors concluded that the identified genes might be considered as potential biomarkers for the characterization of the most aggressive and undifferentiated cells and provide new targets for GBM therapeutics.
Increased levels of expression of sialylated and fucosylated glycans have been associated with negative prognosis in a number of human cancers including breast, colorectal, gastric, urinary bladder carcinoma and pulmonary adenocarcinoma.249 The potential utility of fucosylation as a marker for cancer is reported by Kossowska et al.250 Elevated sialyl Lewis-X or –A, both of which are fucose-containing structures, expression has been observed in metastatic cancer cells and leads to selectin-mediated extravasation. The authors hypothesized that extensive fucosylation of the serum microenvironment might contribute to the loss of adhesion observed during the process of tumor formation. When the fucosylation of serum glycoproteins in SCLC and NSCLC patients was examined, fucosylated glycans were determined to be present in higher amounts in cancer patient sera when compared to healthy patient sera and differences in the degree of fucosylation were observed between SCLC and NSCLC patients. Therefore, the degree of fucosylation has been proposed to be a marker to estimate the glycosylation status of serum proteins in patients with cancer. Cluster analysis also pointed to the potential use of fucosylation status as a predictive factor for patient survival.250
As previously discussed, aberrant mucin O-glycosylation has been shown to accompany lung cancer, and the monitoring of mucins has been proposed to have prognostic value for individuals with lung cancer.180–183 MUC1 expression, in particular, has been demonstrated to correlate with poor prognoses in several different types of cancer.148 A study published in 2015, by Song et al., showed that the under-glycosylated form of MUC1 overexpressed in human malignant cancer cell lines (BT20, HT29, and LS174T) could be differentiated, using chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI), from the normally glycosylated form expressed in a benign human epithelial cell line (MCF10A) and a tumor cell line (U87) that did not express the under-glycosylated form of MUC1. Moreover, when tumor cells from the LS174T and U87 cell lines were implanted in the brains of mice, CEST MRI was able to differentiate between the two in vivo.251 These findings suggest that CEST MRI may represent a non-invasive method for the investigation of mucin glycosylation and the malignant transformation of brain tumors. In addition to MUC1, MUC4 has also been identified as a potential biomarker for diagnosis and prognosis of cancer. Examples of MUC4 overexpression, and often the loss of localization to the apical membrane, correlating with cancer include malignancies of the gall bladder,223 breast,226 pancreas,222, 252 ovaries,224, 225 lung,227, 228 and brain.229
The monitoring of glycopeptides is a potential strategy for the identification and utilization of cancer biomarkers, although enrichment procedures prior to analysis are necessary. A 2016 study by Zacharias et al. described a strategy where hydrophilic interaction liquid chromatography and electrostatic repulsion liquid chromatography enrichment techniques were used in a complementary fashion, with mass spectrometry, for the identification of glycopeptide biomarkers of cancer. The method was capable of distinguishing glycopeptides that were associated with brain cancer from those that were not when breast and brain cancer cell lines were examined.253
An additional candidate cancer biomarker is represented by sialylation which has reported involvement in a number of cancers,101–108 one such example is the increased expression of α2,3-linked glycoprotein sialylation on malignant gliomas.254 Sialic acids have been proposed as biomarkers for endocrinal cancers, including cancers of the thyroid, ovary, pancreas, thyroid, adrenal and pituitary gland, a topic reviewed by Ghosh in 2015.255 The use of the altered expression of sialic acids and or their linkages as a prognostic tool for human cancer is supported by in vivo findings that indicate, in mice, α2,6-linked sialic acids mediate tumor progression via β1 integrin.58 A study utilizing quantitative proteomic analyses revealed 35 cell surface sialoproteins that were overexpressed in human GBM tumors compared to normal human astrocytes.256 Investigations into the expression levels of lipid-associated sialoprotein (LSP) in the cerebrospinal fluid (CSF) of individuals with brain tumors revealed patients with newly diagnosed primary or metastatic brain tumors displayed on average 3.7 fold higher CSF LSP levels compared with CSF from tumor-free patients. Further, the CSF from progressive disease patients with brain tumors not responsive to treatment contained LSP at levels similar to the newly diagnosed patients while treatment-responsive patients exhibited CSF LSP levels comparable to non-diseased patients.257 Therefore, LSP levels in CSF appear to be a biomarker useful for the detection of brain malignancies and the assessment of treatment responsiveness.
A separate study examining total serum or plasma sialic acid and lipid bound sialic acid found that total sialic acid was significantly elevated in a variety of brain tumors, particularly in the microsomal fraction, and lipid bound sialic acid was found to significantly increased in the serum and tissue of patients with brain and thyroid tumors.258 The measurement of serum sialic acid concentration has also been demonstrated as a valid method for the differentiation between benign and malignant intracranial tumors.259 In a study examining the efficiency of biochemical tumor markers, patients with non-tumorous disease of the central nervous system, that coincide with brain tissue lesions, were described as having extremely high serum sialic acid levels, additionally while serum sialic acid levels in brain cancer patients were found to increase with increasing brain tumor malignancy the differences were not deemed statistically significant.260 However, when the sialic acid levels in CSF were examined in patients with brain tumors, CSF sialic acid concentration was found to be significantly increased in patients with malignant and semi-malignant brain tumors compared to CSF from healthy controls.261 The authors concluded that measurement of CSF sialic acid concentration represents a valid method for the diagnosis and assessment of brain tumor malignancy.261
4.2 Glycans and Development of Brain Cancer Therapeutics
4.2.1 Neurotherapeutics and Brain Neoplasms
A handful number of therapeutic interventions have been developed and tested in animal models of brain tumors though less on human subjects. Nevertheless, the variety of approaches utilized are promising especially when combination therapy is utilized as described below. Some researchers have used pharmacological agents while others employed microRNAs, viral vector delivery systems and nanotechnology (Table 1).
Table 1.
Therapeutic interventions in brain neoplasms and the observed outcomes.
| Therapy | Target | Outcome | +/− | Model | Ref. |
|---|---|---|---|---|---|
| Temozolomide + doxorubicin | P-gp | Increased survival | + | Rats | 263 |
| Palbociclib | CDK4/6 | Activity limited by P-gp | − | Mice | 264 |
| LIUS | P-gp and MDR1 | Apoptosis and CNS delivery | + | Rats and C6 cells | 266 |
| FGK45 | CD40 | Increased survival Decreased proliferation |
+ | Mice | 267 |
| γ9δ2 T cells | IFN γ | Increased cytotoxicity | + | GBM cells | 268 |
| miROPN | OPN | Less stem cell markers Less proliferation Increased survival |
+ | Mice | 270 |
| CAR-transduced T cells | PDPN | Inhibition of intracranial glioma xenografts growth | + | Mice | 273 |
| LRIG1 | Oncogenic receptor tyrosine kinases | Slowing of tumor growth. Apoptosis | + | Mice | 275 |
| VB-111 | Endothelial cells | Decreased tumor vasculature and growth | + | Rats | 276 |
| Decorin | EGFR, TGF- beta and p21 | Cellular differentiation Survival |
+ | Mice | 278 |
| HDAC6i | HDAC6 | Increased survival | + | Mice | 280 |
| oHSV | nectin-1 (CD111) | Increased cytotoxicity | + | Pediatric patients | 281 |
| HDACi + Delta24-RGD | HDAC and p16INK4/Rb pathway | Apoptosis and necrosis | + | Patient-derived GSCs | 282 |
| PDNCs | CNS delivery | Increased trans-BBB delivery and CNS retention time | + | Rats | 284 |
| Lf/FA/PLGA NPs | VEGF secretion | Less proliferation | + | U87MG cells | 285 |
| C-SLN | CNS delivery | Improved delivery and cytotoxicity | + | Mice | 286 |
| RDG | CNS delivery | Increased delivery and CNS retention | + | Mice | 287 |
| HA-paclitaxel nanoconjugate | Microtubules | Survival | + | Mice | 288 |
| VD3NPs combined with doxorubicin or epirobicin | Sphingomyelin pathway | Increase in drug resistance | − | C6 glioma cells | 290 |
+ indicates a favorable outcome.
−indicates an unfavorable outcome.
Given Temozolomide’s limited efficacy, which is used in chemotherapy for malignant gliomas, its combination with other agents might be beneficial.262 When combined with doxorubicin, temozolomide notably affected glioma-bearing rat survival via modulating P-glycoprotein (P-gp) transport activity when compared to treatment with a single agent.263 The importance of targeting P-gp lies in the fact that recently, de Gooijer et al. revealed that Palbociclib, an inhibitor of cyclin dependent kinase (CDK) 4/6, is a P-gp glycoprotein substrate restricting Palbociclib’s access to the CNS.264 Also, the brain tumor penetrance of two PI3K/mTOR kinase inhibitors, GDC-0980 and GNE-317, has been shown to be affected by the activity of P-gp.265 Given the recent evidence that low-intensity ultrasound (LIUS) may promote glioma cell apoptosis, Zhang et al. identified the underlying mechanism for this outcome characterized by the downregulation of P-gp expression levels along with multidrug resistance protein 1 (MDR1) orchestrated by the PI3K/Akt/NF-kappaB pathway.266 This finding suggests that LIUS can be utilized to enhance the sensitivity of glioma cells to therapy via downregulation of the P-gp glycoprotein.
Recently, patients with GBM were shown to exhibit good prognoses if elevated expression levels of CD40/CD40L were observed.267 The study revealed that the administration of FGK45 alone, an agonistic antibody for CD40, or in combination with OX86, an agonistic antibody for OX40, enhanced survival in GL261 and NSCL61 mouse glioma models. From a different perspective, in GBM patients, γ9δ2 T cells were found to be decreased when compared to healthy donors.268 Enriching these cells in vitro mediated cytotoxicity to GBM-derived cell lines along with decreased interferon (IFN) γ secretion, an effect that was abolished when γ9δ2 cells were removed.268 Overall, this suggests that CD40/CD40L may be utilized as a favorable prognostic marker with the promise of using the therapeutic potential of FGK45 in clinical applications.269 Friedmann-Morvinski et al. identified the Spp1 gene (secreted phosphoprotein 1) which encodes for the osteopontin (OPN) protein, a RGD-containing glycophosphoprotein with properties of both a cytokine and a chemokine. In GBM-bearing mice, inhibition of OPN, using miROPN, ceased the formation of neurospheres (stem cell markers showed decreased expression), altered neuron proliferation and prolonged mouse survival.270
In the interest of targeting glycoproteins in neuro-oncology as a novel approach, it was reported that podoplanin (PDPN), a type I transmembrane mucin-like glycoprotein, is overexpressed in mesenchymal GBM.271, 272 Using a lentiviral vector expressing a 3rd-generation chimeric antigen receptor (CAR), as a targeted PDPN-specific antibody, it was shown that systemic injection of CAR-transduced T cells into immunodeficient mice limited the survival of intracranial glioma xenografts.273 Also, LRIG1 (leucine-rich repeat and immunoglobulin domain-containing protein-1), a tumor suppressor gene, showed efficacy by acting as a negative regulator of the ErbB and Met receptors which are oncogenic tyrosine kinases receptors.274 Similarly, comparison of athymic nude mice injected with LRIG1-transfected RC-4B/C cells to those injected with normal pituitary tumor RC-4B/C cells showed that LRIG1-transfected tumors displayed significantly diminished tumor growth. This was mediated via inhibiting Ras/Raf/ERK as well as PI3K/Akt signaling pathways.275 Gruslova et al. developed a novel adenoviral gene therapy characterized by VB-111, a non-replicating Adenovirus 5 with a pro-apoptotic Fas-chimera A, targeting the tumor vasculature.276 Bioluminescence and MRI of U87MG-luc2-bearing rats showed that 4-week treatment with VB-111 significantly inhibited tumor growth, indicating its potential in the treatment of highly vascularized solid GBM tumors.
Along the same line, decorin targeting was assessed as a potential target. Decorin, a proteoglycan that mediates its anti-cancer activity using EGFR, transforming growth factor-beta (TGF-beta), and p21 was also investigated in brain tumors.277 Using decorin delivered into a mouse xenograft U87MG glioma tumor via an adeno-associated viral gene delivery system, cell growth was found to be inhibited with decorin by enhancing cellular differentiation and prolonging mouse survival.278 Additionally, employing a mass spectrometry-based approach was shown to help in identifying proteins involved in apoptosis, proliferation, resistance to chemotherapy, and fatty acid metabolism mediated by decorin overexpression.278, 279
On the level of epigenetics, the inhibition of histone deacetylase 6 (HDAC6i), either pharmacologically by valproic acid or using oncolytic engineered herpes simplex virus (oHSV) increased nucleus-targeted trafficking of post-entry oHSV.280 Nakashima et al. also observed improved survival in GSC-bearing mice treated with both oHSV and HDAC6i in comparison to oHSV treatment alone. Friedman et al. showed that pediatric medulloblastoma is an excellent target for oHSV virotherapy via viral adhesion to nectin-1 (CD111).281 The combinatorial treatment of HDACi such as the use of MS275, Valproic acid and Delta24-RGD, a genetically modified serotype-5 adenovirus, enhanced the anticancer activity in GBM stem-like cells.282
4.2.2 Glycosylation, Nanotechnology and Drug Delivery
On a different front, overcoming the tight constraints of the blood brain barrier (BBB) and the rapid removal of metabolites, drugs, and proteins from circulation constitute two major obstacles for CNS drug delivery. For this reason, nanotechnology has become instrumental for such treatment. For instance, Fang et al. developed a novel lactoferrin-based delivery system of dual-targeting magnetic polydiacetylene nanocarriers (PDNCs), which is found on the cell surface of GBMs.283, 284 Fang et al. showed an enhanced trans-BBB activity of the PDNCs along with improved retention time of traced lactoferrin. Furthermore, administrations of etoposide, which targets VEGF secretion, using cross-linked Lf and folic acid (FA) on poly(lactide-co-glycolide) (PLGA) nanoparticles (NPs), across the BBB revealed a two-fold increase in permeability.285 Further, Lf/FA/PLGA NPs were superior to FA/PLGA NPs in antiproliferative efficacy against U87MG cells, a human primary GBM cell line. In terms of diffusion capacity, solid lipid nanoparticles (SLN) may diffuse through the BBB due to their lipophilic nature. In addition, lactoferrin conjugated on the surface of SLN (C-SLN) increased the targeting potential for brain neoplasms and cytotoxicity.286
Wang et al. developed a PEGylated, RGD peptide modified, and disulfide cross-linked short polyethyleneimines (DSPEIs) functionalized gold nanorod (RDG) for shRNA delivery.287 Stable nanoparticles of shRNAs were formed by the RDG; thus, it is marked to defined human brain cancers (U-87 MG-GFP) via integrin-mediated endocytosis in U-87 MG-GFP tumor bearing BALB/c mice. Also, the high stability and long blood bioavailability, imparted by PEGylation, suggests a promising non-viral vector approach for effective tumor targeting and intra-tumor gene silencing.287 Interestingly, it has been shown that the administration of an ultra-small hyaluronic acid (HA) paclitaxel nanoconjugate leads to better survival levels, assessed in preclinical brain-breast metastases of cancer model, compared to controls.288 The passive trans-BBB diffusion of ultra-small molecules provides a sound option to optimize chemotherapeutic drug efficacy.
Given the anti-mitogenic impacts of vitamin D3 (VD3) on C6 glioma tumor cells,289 in an attempt to utilize the resistance-reducing capacity of combinatorial therapy, Maleklou et al. used VD3-loaded nanoparticles (VD3NPs) paradoxically revealing a significant increase in drug resistance once exogenous VD3NP-doxorubicin and VD3NP-epirobicin combinations were administered to C6 glioma cells.290 Taken together, in light of the roles glycoproteins play in brain cancers, it is of high importance to initiate the development of brain tumor therapies with glycobiology in mind. In particular, in the case of GBM, one of the most clinically devastating cancers, it is of the utmost necessity to treat CNS tumors utilizing drug delivery systems and neurotherapeutics, such as those that have been described herein. Moving forward, indubitably, further experimental studies and clinical interventions are needed to validate and implement the abovementioned interventions on human subjects.
4.2.3 Cancer Glycoproteomics and Personalized Medicine
Within the scope of recent advances in therapy against CNS cancers, personalized medicine has emerged as a concept and practice aimed at selecting and optimizing therapies based on cellular or genetic analyses. Glycoproteomics paved the way for employing a particular therapy on a subset of patients carrying a unique glycomic signature.291, 292 For instance, neo antigens can be identified via the addition of several of these glycosylation modifications; for example tumor-associated carbohydrate antigens (TACA), such as the dietary sialic acid Neu5Gc, which inserts on cell surfaces producing an immunogenic neo-TACA.293, 294 Additionally, it should be noted that several FDA-approved assays are used for the detection of cancer antigens; an excellent example is provided by the Truquant BR radioimmunoassay, which uses the monoclonal antibody B27.29 to quantify the MUC1 gene product CA 27.29.295
Recently, Thanabalasingham et al. identified a markedly lower glycan index in hepatocyte nuclear factor 1-α (HNF1A)-maturity-onset diabetes of the young than in controls or other diabetes subtypes.296 Mucins, carriers of cancer-associated O-glycans, showed a significant increase in expression in a variety of malignancies such as esophageal, colon, breast, gastric, bladder, pancreatic and ovarian cancers.297 Furthermore, in prostate cancer, α2,3 NeuAc levels were significantly increased whereas seminal fluid free PSA had more sialylation and core fucosylation.298 These findings make N- and O-glycans potential vaccination targets against the particular overexpressed glycans identified in certain individuals; thereby, highlighting the importance of glycosylation in personalized medicine. Furthermore, alterations in PTMs also occur at the level of the glycosyltransferases/glycosidases enzymes mediating the generated TACAs, such as UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase (ppGalNAc-T), alpha6-sialyl-transferase-I (ST6GalNAc-I), N-acetylgalactosaminyltransferase 6 (GALNT6), UDP-N-acetyl-D-glucosamine: N-acetylglucosamine transferase V (GlcNAcT-V) and α2-3 sialyltransferase I (ST3Gal-I).299–303 Specifically for alpha-fetoprotein (AFP-L3), increased fucosylation of AFP has been found to be due to the increased expression of α1-6 fucosyltransferase in hepatoma tissues304
Finally, given this brief overview of the pathological and regulatory roles of glycoprotein PTMs and the fact that unique cancer-associated-glycan profiles reflect the underlying genetic disruptions that are translated into distinctive glycosylation patterns, the novel development of methodologies that help in differentiating physiologic versus aberrant glycosylation patterns observed in cancer can be considered a promising strategy for shaping treatment and therapy. Personalized theranostics have the potential to be instrumental in the areas of vaccination, therapy, and diagnosis.
5. Concluding Remarks
In conclusion, alterations in glycoproteins represent a hallmark of cancer development and offer biomarker targets due to the presence of differential carbohydrate modifications. This, coupled with their heterogeneity and complexity of expression as well as their involvement in regulatory pathways render them as key markers worthy of investigation. The high expression of glycoproteins both in bodily fluids, such as serum and CSF, as well as on cell surfaces is a source of encouragement for researchers aiming to assess their roles in cancer-related and other pathological conditions.305
Acknowledgments
This work was supported by NIH grant (1R01GM112490-03) and CPRIT (RP130624).
Footnotes
Author Contributions
All Authors were involved in the writing and proofreading of the document. YM provided the article outline and materials to be covered. Each author was assigned specific sections of the manuscript while LV was responsible for the assembly and proofreading of the final document. LV was responsible for proof reading. YM was responsible for the final proofreading and submission of the manuscript.
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochimica et biophysica acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Ihara Y, Inai Y, Ikezaki M, Matsui ISL, Manabe S, Ito Y. C-Mannosylation: Modification on Tryptophan in Cellular Proteins. In: Taniguchi N, Endo T, Hart GW, Seeberger PH, Wong C-H, editors. Glycoscience: Biology and Medicine. Springer; Japan, Tokyo: 2015. pp. 1091–1099. [Google Scholar]
- 3.Song E, Mechref Y. LC-MS/MS identification of the O-glycosylation and hydroxylation of amino acid residues of collagen alpha-1 (II) chain from bovine cartilage. J Proteome Res. 2013;12:3599–3609. doi: 10.1021/pr400101t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. Journal of clinical pathology. 2010;63:322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer research. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 6.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Advances in cancer research. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Baum LG, Garner OB, Schaefer K, Lee B. Microbe-Host Interactions are Positively and Negatively Regulated by Galectin-Glycan Interactions. Frontiers in immunology. 2014;5:284. doi: 10.3389/fimmu.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W. Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase. The Journal of biological chemistry. 2013;288:18448–18457. doi: 10.1074/jbc.M113.464008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valliere-Douglass JF, Kodama P, Mujacic M, Brady LJ, Wang W, Wallace A, Yan B, Reddy P, Treuheit MJ, Balland A. Asparagine-linked oligosaccharides present on a non-consensus amino acid sequence in the CH1 domain of human antibodies. The Journal of biological chemistry. 2009;284:32493–32506. doi: 10.1074/jbc.M109.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valliere-Douglass JF, Eakin CM, Wallace A, Ketchem RR, Wang W, Treuheit MJ, Balland A. Glutamine-linked and non-consensus asparagine-linked oligosaccharides present in human recombinant antibodies define novel protein glycosylation motifs. The Journal of biological chemistry. 2010;285:16012–16022. doi: 10.1074/jbc.M109.096412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenthal MS, Davis KS, Formolo T, Kilpatrick LE, Phinney KW. Identification of Novel N-Glycosylation Sites at Noncanonical Protein Consensus Motifs. J Proteome Res. 2016;15:2087–2101. doi: 10.1021/acs.jproteome.5b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annual review of biochemistry. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 15.Ten Hagen KG, Fritz TA, Tabak LA. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1r–16r. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- 16.Clausen H, Bennett EP. A family of UDP-GalNAc: polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology. 1996;6:635–646. doi: 10.1093/glycob/6.6.635. [DOI] [PubMed] [Google Scholar]
- 17.Gill DJ, Clausen H, Bard F. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends in cell biology. 2011;21:149–158. doi: 10.1016/j.tcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Thanka Christlet TH, Veluraja K. Database analysis of O-glycosylation sites in proteins. Biophysical journal. 2001;80:952–960. doi: 10.1016/s0006-3495(01)76074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellermann J, Lottspeich F, Geiger R, Deutzmann R. Human urinary kallikrein–amino acid sequence and carbohydrate attachment sites. Protein sequences & data analysis. 1988;1:177–182. [PubMed] [Google Scholar]
- 20.Iwanaga S, Nishimura H, Kawabata S, Kisiel W, Hase S, Ikenaka T. A new trisaccharide sugar chain linked to a serine residue in the first EGF-like domain of clotting factors VII and IX and protein Z. Advances in experimental medicine and biology. 1990;281:121–131. doi: 10.1007/978-1-4615-3806-6_12. [DOI] [PubMed] [Google Scholar]
- 21.Chen R. Complete amino acid sequence and glycosylation sites of glycoprotein gp71A of Friend murine leukemia virus. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5788–5792. doi: 10.1073/pnas.79.19.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomita M, Marchesi VT. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens NW, Stetefeld J, Lattova E, Schweizer F. Contiguous O-galactosylation of 4(R)-hydroxy-l-proline residues forms very stable polyproline II helices. Journal of the American Chemical Society. 2010;132:5036–5042. doi: 10.1021/ja905724d. [DOI] [PubMed] [Google Scholar]
- 24.Gavel Y, von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein engineering. 1990;3:433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function, Nature reviews. Molecular cell biology. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran DT, Ten Hagen KG. Mucin-type O-Glycosylation during Development. The Journal of biological chemistry. 2013;288:6921–6929. doi: 10.1074/jbc.R112.418558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dick G, Akslen-Hoel LK, Grondahl F, Kjos I, Prydz K. Proteoglycan synthesis and Golgi organization in polarized epithelial cells. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2012;60:926–935. doi: 10.1369/0022155412461256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Progress in molecular biology and translational science. 2010;93:1–17. doi: 10.1016/S1877-1173(10)93001-9. [DOI] [PubMed] [Google Scholar]
- 29.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clinical chemistry. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 30.Couldrey C, Green JE. Metastases: the glycan connection. Breast cancer research : BCR. 2000;2:321–323. doi: 10.1186/bcr75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks SA, Carter TM. N-acetylgalactosamine, N-acetylglucosamine and sialic acid expression in primary breast cancers. Acta histochemica. 2001;103:37–51. doi: 10.1078/0065-1281-00576. [DOI] [PubMed] [Google Scholar]
- 32.Brooks SA, Leathem AJ. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet. 1991;338:71–74. doi: 10.1016/0140-6736(91)90071-v. [DOI] [PubMed] [Google Scholar]
- 33.Hiraizumi S, Takasaki S, Ohuchi N, Harada Y, Nose M, Mori S, Kobata A. Altered glycosylation of membrane glycoproteins associated with human mammary carcinoma. Japanese journal of cancer research : Gann. 1992;83:1063–1072. doi: 10.1111/j.1349-7006.1992.tb02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. Journal of molecular medicine. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 35.Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, Ryden L, Gallagher WM, O’Brien SL. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6421–6431. doi: 10.1158/1078-0432.CCR-06-0480. [DOI] [PubMed] [Google Scholar]
- 36.Duffy MJ, Shering S, Sherry F, McDermott E, O’Higgins N. CA 15-3: a prognostic marker in breast cancer. The International journal of biological markers. 2000;15:330–333. doi: 10.1177/172460080001500410. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Kemmner W, Grigull S, Schlag PM. Cell surface alpha 2–6 sialylation affects adhesion of breast carcinoma cells. Experimental cell research. 2002;276:101–110. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- 38.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 39.Fang W, Li H, Kong L, Niu G, Gao Q, Zhou K, Zheng J, Wu B. [Role of matrix metalloproteinases (MMPs) in tumor invasion and metastasis: serial studies on MMPs and TIMPs], Beijing da xue xue bao. Yi xue ban = Journal of Peking University. Health sciences. 2003;35:441–443. [PubMed] [Google Scholar]
- 40.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Seminars in cancer biology. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 41.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer metastasis reviews. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg GI, Eisen AZ. Extracellular matrix metalloproteinases in tumor invasion and metastasis. Cancer treatment and research. 1991;53:421–440. doi: 10.1007/978-1-4615-3940-7_20. [DOI] [PubMed] [Google Scholar]
- 43.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochimica et biophysica acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 44.Arnal-Estape A, Nguyen DX. Sweets for a bitter end: lung cancer cell-surface protein glycosylation mediates metastatic colonization. Cancer discovery. 2015;5:109–111. doi: 10.1158/2159-8290.CD-15-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bull C, Stoel MA, den Brok MH, Adema GJ. Sialic acids sweeten a tumor’s life. Cancer research. 2014;74:3199–3204. doi: 10.1158/0008-5472.CAN-14-0728. [DOI] [PubMed] [Google Scholar]
- 46.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear, nose, & throat journal. 2006;85:74. [PubMed] [Google Scholar]
- 47.Pilkington GJ. The paradox of neoplastic glial cell invasion of the brain and apparent metastatic failure. Anticancer research. 1997;17:4103–4105. [PubMed] [Google Scholar]
- 48.Subramanian A, Harris A, Piggott K, Shieff C, Bradford R. Metastasis to and from the central nervous system–the ‘relatively protected site’, The Lancet. Oncology. 2002;3:498–507. doi: 10.1016/s1470-2045(02)00819-7. [DOI] [PubMed] [Google Scholar]
- 49.Akiyama Y, Jung S, Salhia B, Lee S, Hubbard S, Taylor M, Mainprize T, Akaishi K, van Furth W, Rutka JT. Hyaluronate receptors mediating glioma cell migration and proliferation. Journal of neuro-oncology. 2001;53:115–127. doi: 10.1023/a:1012297132047. [DOI] [PubMed] [Google Scholar]
- 50.Goldbrunner RH, Bernstein JJ, Tonn JC. Cell-extracellular matrix interaction in glioma invasion. Acta neurochirurgica. 1999;141:295–305. doi: 10.1007/s007010050301. discussion 304–295. [DOI] [PubMed] [Google Scholar]
- 51.Gary SC, Kelly GM, Hockfield S. BEHAB/brevican: a brain-specific lectican implicated in gliomas and glial cell motility. Current opinion in neurobiology. 1998;8:576–581. doi: 10.1016/s0959-4388(98)80083-4. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cellular and molecular life sciences : CMLS. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gary SC, Zerillo CA, Chiang VL, Gaw JU, Gray G, Hockfield S. cDNA cloning, chromosomal localization, and expression analysis of human BEHAB/brevican, a brain specific proteoglycan regulated during cortical development and in glioma. Gene. 2000;256:139–147. doi: 10.1016/s0378-1119(00)00362-0. [DOI] [PubMed] [Google Scholar]
- 54.Jaworski DM, Kelly GM, Piepmeier JM, Hockfield S. BEHAB (brain enriched hyaluronan binding) is expressed in surgical samples of glioma and in intracranial grafts of invasive glioma cell lines. Cancer research. 1996;56:2293–2298. [PubMed] [Google Scholar]
- 55.Zhang H, Kelly G, Zerillo C, Jaworski DM, Hockfield S. Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion In vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:2370–2376. doi: 10.1523/JNEUROSCI.18-07-02370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nutt CL, Zerillo CA, Kelly GM, Hockfield S. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer research. 2001;61:7056–7059. [PubMed] [Google Scholar]
- 57.Viapiano MS, Bi WL, Piepmeier J, Hockfield S, Matthews RT. Novel tumor-specific isoforms of BEHAB/brevican identified in human malignant gliomas. Cancer research. 2005;65:6726–6733. doi: 10.1158/0008-5472.CAN-05-0585. [DOI] [PubMed] [Google Scholar]
- 58.Hedlund M, Ng E, Varki A, Varki NM. alpha 2-6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer research. 2008;68:388–394. doi: 10.1158/0008-5472.CAN-07-1340. [DOI] [PubMed] [Google Scholar]
- 59.Fogel M, Altevogt P, Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. The Journal of experimental medicine. 1983;157:371–376. doi: 10.1084/jem.157.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dennis JW, Waller CA, Schirrmacher V. Identification of asparagine-linked oligosaccharides involved in tumor cell adhesion to laminin and type IV collagen. The Journal of cell biology. 1984;99:1416–1423. doi: 10.1083/jcb.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science (New York, NY) 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 62.Asada M, Furukawa K, Segawa K, Endo T, Kobata A. Increased expression of highly branched N-glycans at cell surface is correlated with the malignant phenotypes of mouse tumor cells. Cancer research. 1997;57:1073–1080. [PubMed] [Google Scholar]
- 63.Lemjabbar-Alaoui H, McKinney A, Yang YW, Tran VM, Phillips JJ. Glycosylation alterations in lung and brain cancer. Advances in cancer research. 2015;126:305–344. doi: 10.1016/bs.acr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Advances in experimental medicine and biology. 1986;205:53–85. doi: 10.1007/978-1-4684-5209-9_2. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Gu J, Taniguchi N. Branched N-glycans regulate the biological functions of integrins and cadherins. The FEBS journal. 2008;275:1939–1948. doi: 10.1111/j.1742-4658.2008.06346.x. [DOI] [PubMed] [Google Scholar]
- 66.Cummings RD, Trowbridge IS, Kornfeld S. A mouse lymphoma cell line resistant to the leukoagglutinating lectin from Phaseolus vulgaris is deficient in UDP-GlcNAc: alpha-D-mannoside beta 1–6 N-acetylglucosaminyltransferase. The Journal of biological chemistry. 1982;257:13421–13427. [PubMed] [Google Scholar]
- 67.Shoreibah M, Perng GS, Adler B, Weinstein J, Basu R, Cupples R, Wen D, Browne JK, Buckhaults P, Fregien N, Pierce M. Isolation, characterization, and expression of a cDNA encoding N-acetylglucosaminyltransferase V. The Journal of biological chemistry. 1993;268:15381–15385. [PubMed] [Google Scholar]
- 68.Demetriou M, Nabi IR, Coppolino M, Dedhar S, Dennis JW. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. The Journal of cell biology. 1995;130:383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto H, Swoger J, Greene S, Saito T, Hurh J, Sweeley C, Leestma J, Mkrdichian E, Cerullo L, Nishikawa A, Ihara Y, Taniguchi N, Moskal JR. Beta1,6-N-acetylglucosamine-bearing N-glycans in human gliomas: implications for a role in regulating invasivity. Cancer research. 2000;60:134–142. [PubMed] [Google Scholar]
- 70.Yamamoto H, Oviedo A, Sweeley C, Saito T, Moskal JR. Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer research. 2001;61:6822–6829. [PubMed] [Google Scholar]
- 71.Seberger PJ, Chaney WG. Control of metastasis by Asn-linked, beta1-6 branched oligosaccharides in mouse mammary cancer cells. Glycobiology. 1999;9:235–241. doi: 10.1093/glycob/9.3.235. [DOI] [PubMed] [Google Scholar]
- 72.Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW. Beta 1–6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer research. 1991;51:718–723. [PubMed] [Google Scholar]
- 73.Wildburger NC, Zhou S, Zacharias LG, Kroes RA, Moskal JR, Schmidt M, Mirzaei P, Gumin J, Lang FF, Mechref Y, Nilsson CL. Integrated Transcriptomic and Glycomic Profiling of Glioma Stem Cell Xenografts. J Proteome Res. 2015;14:3932–3939. doi: 10.1021/acs.jproteome.5b00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gavella M, Garaj-Vrhovac V, Lipovac V, Antica M, Gajski G, Car N. Ganglioside GT1b protects human spermatozoa from hydrogen peroxide-induced DNA and membrane damage. International journal of andrology. 2010;33:536–544. doi: 10.1111/j.1365-2605.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- 75.Gavella M, Lipovac V. Protective effects of exogenous gangliosides on ROS-induced changes in human spermatozoa. Asian journal of andrology. 2013;15:375–381. doi: 10.1038/aja.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iijima R, Ichikawa T, Yamazaki M. Sialic acid attenuates the cytotoxicity of the lipid hydroperoxides HpODE and HpETE. Carbohydrate research. 2009;344:933–935. doi: 10.1016/j.carres.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 77.Iijima R, Takahashi H, Namme R, Ikegami S, Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS letters. 2004;561:163–166. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 78.Ogasawara Y, Namai T, Yoshino F, Lee MC, Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS letters. 2007;581:2473–2477. doi: 10.1016/j.febslet.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 79.Yang WS, Chang JW, Han NJ, Park SK. Darbepoetin alfa suppresses tumor necrosis factor-alpha-induced endothelin-1 production through antioxidant action in human aortic endothelial cells: role of sialic acid residues. Free radical biology & medicine. 2011;50:1242–1251. doi: 10.1016/j.freeradbiomed.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N. Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha1–>6fucosyltransferase. The Journal of biological chemistry. 1996;271:27810–27817. doi: 10.1074/jbc.271.44.27810. [DOI] [PubMed] [Google Scholar]
- 81.Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. The alpha 1-6-fucosyltransferase gene and its biological significance. Biochimica et biophysica acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- 82.Noda K, Miyoshi E, Gu J, Gao CX, Nakahara S, Kitada T, Honke K, Suzuki K, Yoshihara H, Yoshikawa K, Kawano K, Tonetti M, Kasahara A, Hori M, Hayashi N, Taniguchi N. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer research. 2003;63:6282–6289. [PubMed] [Google Scholar]
- 83.Ito Y, Miyauchi A, Yoshida H, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Taniguchi N, Matsuura N, Kuma K, Miyoshi E. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: its linkage to biological aggressiveness and anaplastic transformation. Cancer letters. 2003;200:167–172. doi: 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 84.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. The Journal of biological chemistry. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 85.Tsuchiya N, Yamanaka R, Yajima N, Homma J, Sano M, Komata T, Ikeda T, Fujimoto I, Takahashi H, Tanaka R, Ikenaka K. Isolation and characterization of an N-linked oligosaccharide that is increased in glioblastoma tissue and cell lines. International journal of oncology. 2005;27:1231–1239. [PubMed] [Google Scholar]
- 86.Cole RN, Hart GW. Cytosolic O-glycosylation is abundant in nerve terminals. Journal of neurochemistry. 2001;79:1080–1089. doi: 10.1046/j.1471-4159.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- 87.Zamze S, Harvey DJ, Chen YJ, Guile GR, Dwek RA, Wing DR. Sialylated N-glycans in adult rat brain tissue–a widespread distribution of disialylated antennae in complex and hybrid structures. European journal of biochemistry. 1998;258:243–270. doi: 10.1046/j.1432-1327.1998.2580243.x. [DOI] [PubMed] [Google Scholar]
- 88.Hoffmann A, Nimtz M, Wurster U, Conradt HS. Carbohydrate structures of beta-trace protein from human cerebrospinal fluid: evidence for "brain-type" N-glycosylation. Journal of neurochemistry. 1994;63:2185–2196. doi: 10.1046/j.1471-4159.1994.63062185.x. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann A, Nimtz M, Getzlaff R, Conradt HS. ‘Brain-type’ N-glycosylation of asialo-transferrin from human cerebrospinal fluid. FEBS letters. 1995;359:164–168. doi: 10.1016/0014-5793(95)00034-7. [DOI] [PubMed] [Google Scholar]
- 90.Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. The Journal of biological chemistry. 1992;267:9965–9971. [PubMed] [Google Scholar]
- 91.Regan CM, Fox GB. Polysialylation as a regulator of neural plasticity in rodent learning and aging. Neurochemical research. 1995;20:593–598. doi: 10.1007/BF01694541. [DOI] [PubMed] [Google Scholar]
- 92.Fryer HJ, Hockfield S. The role of polysialic acid and other carbohydrate polymers in neural structural plasticity. Current opinion in neurobiology. 1996;6:113–118. doi: 10.1016/s0959-4388(96)80016-x. [DOI] [PubMed] [Google Scholar]
- 93.Jungalwala FB. Expression and biological functions of sulfoglucuronyl glycolipids (SGGLs) in the nervous system–a review. Neurochemical research. 1994;19:945–957. doi: 10.1007/BF00968704. [DOI] [PubMed] [Google Scholar]
- 94.Yan K, Wu Q, Yan DH, Lee CH, Rahim N, Tritschler I, DeVecchio J, Kalady MF, Hjelmeland AB, Rich JN. Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy. Genes & development. 2014;28:1085–1100. doi: 10.1101/gad.235515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheray M, Petit D, Forestier L, Karayan-Tapon L, Maftah A, Jauberteau MO, Battu S, Gallet FP, Lalloue F. Glycosylation-related gene expression is linked to differentiation status in glioblastomas undifferentiated cells. Cancer letters. 2011;312:24–32. doi: 10.1016/j.canlet.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 96.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Experimental neurology. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 98.Bertrand J, Begaud-Grimaud G, Bessette B, Verdier M, Battu S, Jauberteau MO. Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. International journal of oncology. 2009;34:717–727. doi: 10.3892/ijo_00000198. [DOI] [PubMed] [Google Scholar]
- 99.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. International review of cytology. 1997;175:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system, Nature reviews. Immunology. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 101.Schauer R. Sialic acids and their role as biological masks. Trends in Biochemical Sciences. 1985;10(9):357–360. [Google Scholar]
- 102.Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Advances in carbohydrate chemistry and biochemistry. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- 103.Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Advances in cancer research. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- 104.Passaniti A, Hart GW. Cell surface sialylation and tumor metastasis Metastatic potential of B16 melanoma variants correlates with their relative numbers of specific penultimate oligosaccharide structures. The Journal of biological chemistry. 1988;263:7591–7603. [PubMed] [Google Scholar]
- 105.Holmes EH, Ostrander GK, Hakomori S. Biosynthesis of the sialyl-Lex determinant carried by type 2 chain glycosphingolipids (IV3NeuAcIII3FucnLc4, VI3NeuAcV3FucnLc6, and VI3NeuAcIII3V3Fuc2nLc6) in human lung carcinoma PC9 cells. The Journal of biological chemistry. 1986;261:3737–3743. [PubMed] [Google Scholar]
- 106.Vierbuchen MJ, Fruechtnicht W, Brackrock S, Krause KT, Zienkiewicz TJ. Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features, Cancer. 1995;76:727–735. doi: 10.1002/1097-0142(19950901)76:5<727::aid-cncr2820760504>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 107.Yogeeswaran G, Salk PL. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science (New York, NY) 1981;212:1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- 108.Gatchev O, Rastam L, Lindberg G, Gullberg B, Eklund GA, Tornberg S. Tumours of the central nervous system and serum sialic acid concentration in men and women. British journal of cancer. 1993;68:425–427. doi: 10.1038/bjc.1993.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature chemical biology. 2014;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu Y, Mayampurath A, Khan S, Cohen JK, Mechref Y, Volchenboum SL. N-linked glycan profiling in neuroblastoma cell lines. J Proteome Res. 2015;14:2074–2081. doi: 10.1021/pr5011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kakari S, Stringou E, Toumbis M, Ferderigos AS, Poulaki E, Chondros K, Dema A, Kotsovoulou V, Pavlidis N. Five tumor markers in lung cancer: significance of total and "lipid"-bound sialic acid. Anticancer research. 1991;11:2107–2110. [PubMed] [Google Scholar]
- 112.Shamberger RJ. Evaluation of water soluble and lipid soluble sialic acid levels as tumor markers. Anticancer research. 1986;6:717–720. [PubMed] [Google Scholar]
- 113.Polivkova J, Vosmikova K, Horak L. Utilization of determining lipid-bound sialic acid for the diagnosis and further prognosis of cancer. Neoplasma. 1992;39:233–236. [PubMed] [Google Scholar]
- 114.Patel PS, Raval GN, Rawal RM, Patel GH, Balar DB, Shah PM, Patel DD. Comparison between serum levels of carcinoembryonic antigen, sialic acid and phosphohexose isomerase in lung cancer. Neoplasma. 1995;42:271–274. [PubMed] [Google Scholar]
- 115.Patel PS, Raval GN, Rawal RR, Patel GH, Balar DB, Shah PM, Patel DD. Assessing benefits of combining biochemical and immunological markers in patients with lung carcinoma. Cancer letters. 1994;82:129–133. doi: 10.1016/0304-3835(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 116.Gökmen SS, Kazezoglu C, Tabakoglu E, Altiay G, Gungor O, Ture M. Serum total sialic acid levels in lung cancer patients of different histological types with and no extrapulmonary metastases. Turkish Journal of Biochemistsry. 2004;29(4):262–267. [Google Scholar]
- 117.Yamamoto H, Kaneko Y, Rebbaa A, Bremer EG, Moskal JR. alpha2,6-Sialyltransferase gene transfection into a human glioma cell line (U373 MG) results in decreased invasivity. Journal of neurochemistry. 1997;68:2566–2576. doi: 10.1046/j.1471-4159.1997.68062566.x. [DOI] [PubMed] [Google Scholar]
- 118.Kaneko Y, Yamamoto H, Kersey DS, Colley KJ, Leestma JE, Moskal JR. The expression of Gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2-6-linked sialoglycoconjugates in human brain tumors. Acta neuropathologica. 1996;91:284–292. doi: 10.1007/s004010050427. [DOI] [PubMed] [Google Scholar]
- 119.Chong YK, Sandanaraj E, Koh LW, Thangaveloo M, Tan MS, Koh GR, Toh TB, Lim GG, Holbrook JD, Kon OL, Nadarajah M, Ng I, Ng WH, Tan NS, Lim KL, Tang C, Ang BT. ST3GAL1-Associated Transcriptomic Program in Glioblastoma Tumor Growth, Invasion, and Prognosis. Journal of the National Cancer Institute. 2016:108. doi: 10.1093/jnci/djv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bartik P, Maglott A, Entlicher G, Vestweber D, Takeda K, Martin S, Dontenwill M. Detection of a hypersialylated beta1 integrin endogenously expressed in the human astrocytoma cell line A172. International journal of oncology. 2008;32:1021–1031. [PubMed] [Google Scholar]
- 121.Furukawa J, Tsuda M, Okada K, Kimura T, Piao J, Tanaka S, Shinohara Y. Comprehensive Glycomics of a Multistep Human Brain Tumor Model Reveals Specific Glycosylation Patterns Related to Malignancy. PloS one. 2015;10:e0128300. doi: 10.1371/journal.pone.0128300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Edelman GM. Cell adhesion and the molecular processes of morphogenesis. Annual review of biochemistry. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- 123.Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. The Journal of biological chemistry. 1982;257:11966–11970. [PubMed] [Google Scholar]
- 124.Rutishauser U, Acheson A, Hall AK, Mann DM, Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science (New York, NY) 1988;240:53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- 125.Fredette B, Rutishauser U, Landmesser L. Regulation and activity-dependence of N-cadherin, NCAM isoforms, and polysialic acid on chick myotubes during development. The Journal of cell biology. 1993;123:1867–1888. doi: 10.1083/jcb.123.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994;13:405–414. doi: 10.1016/0896-6273(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 127.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer research. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 128.Komminoth P, Roth J, Lackie PM, Bitter-Suermann D, Heitz PU. Polysialic acid of the neural cell adhesion molecule distinguishes small cell lung carcinoma from carcinoids. The American journal of pathology. 1991;139:297–304. [PMC free article] [PubMed] [Google Scholar]
- 129.Tanaka F, Otake Y, Nakagawa T, Kawano Y, Miyahara R, Li M, Yanagihara K, Nakayama J, Fujimoto I, Ikenaka K, Wada H. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer research. 2000;60:3072–3080. [PubMed] [Google Scholar]
- 130.Petridis AK, Wedderkopp H, Hugo HH, Maximilian Mehdorn H. Polysialic acid overexpression in malignant astrocytomas. Acta neurochirurgica. 2009;151:601–603. doi: 10.1007/s00701-009-0324-3. discussion 603–604. [DOI] [PubMed] [Google Scholar]
- 131.Scheidegger EP, Lackie PM, Papay J, Roth J. In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Laboratory investigation; a journal of technical methods and pathology. 1994;70:95–106. [PubMed] [Google Scholar]
- 132.Amoureux MC, Coulibaly B, Chinot O, Loundou A, Metellus P, Rougon G, Figarella-Branger D. Polysialic acid neural cell adhesion molecule (PSA-NCAM) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC cancer. 2010;10:91. doi: 10.1186/1471-2407-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trouillas J, Daniel L, Guigard MP, Tong S, Gouvernet J, Jouanneau E, Jan M, Perrin G, Fischer G, Tabarin A, Rougon G, Figarella-Branger D. Polysialylated neural cell adhesion molecules expressed in human pituitary tumors and related to extrasellar invasion. Journal of neurosurgery. 2003;98:1084–1093. doi: 10.3171/jns.2003.98.5.1084. [DOI] [PubMed] [Google Scholar]
- 134.Ozyurt E, Sonmez H, Suer S, Kokoglu E. The prognostic importance of fibronectin and sialic acid levels in human pituitary adenomas. Cancer letters. 1996;100:151–154. doi: 10.1016/0304-3835(95)04086-2. [DOI] [PubMed] [Google Scholar]
- 135.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 136.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiological reviews. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 137.Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41r–53r. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 138.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconjugate journal. 1997;14:569–576. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 139.Lee JS, Ro JY, Sahin AA, Hong WK, Brown BW, Mountain CF, Hittelman WN. Expression of blood-group antigen A–a favorable prognostic factor in non-small-cell lung cancer. The New England journal of medicine. 1991;324:1084–1090. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- 140.Miyake M, Taki T, Hitomi S, Hakomori S. Correlation of expression of H/Le(y)/Le(b) antigens with survival in patients with carcinoma of the lung. The New England journal of medicine. 1992;327:14–18. doi: 10.1056/NEJM199207023270103. [DOI] [PubMed] [Google Scholar]
- 141.Orntoft TF, Vestergaard EM. Clinical aspects of altered glycosylation of glycoproteins in cancer. Electrophoresis. 1999;20:362–371. doi: 10.1002/(SICI)1522-2683(19990201)20:2<362::AID-ELPS362>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 142.Sawada R, Tsuboi S, Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. The Journal of biological chemistry. 1994;269:1425–1431. [PubMed] [Google Scholar]
- 143.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer research. 1993;53:354–361. [PubMed] [Google Scholar]
- 144.Yamada N, Chung YS, Takatsuka S, Arimoto Y, Sawada T, Dohi T, Sowa M. Increased sialyl Lewis A expression and fucosyltransferase activity with acquisition of a high metastatic capacity in a colon cancer cell line. British journal of cancer. 1997;76:582–587. doi: 10.1038/bjc.1997.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconjugate journal. 1997;14:577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 146.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell stem cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kufe DW. Mucins in cancer: function, prognosis and therapy, Nature reviews. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 151.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. The Journal of biological chemistry. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 152.Ma Z, Chalkley RJ, Vosseller K. Hyper-O-GlcNAcylation activates NF-kappaB signaling through interplay with phosphorylation and acetylation. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M116.766568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nature cell biology. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 154.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science (New York, NY) 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- 155.Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:15120–15125. doi: 10.1073/pnas.1606899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gong CX, Liu F, Iqbal K. O-GlcNAcylation: A regulator of tau pathology and neurodegeneration. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016;12:1078–1089. doi: 10.1016/j.jalz.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 157.Wani WY, Chatham JC, Darley-Usmar V, McMahon LL, Zhang J. O-GlcNAcylation and neurodegeneration. Brain research bulletin. 2016 doi: 10.1016/j.brainresbull.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gatta E, Lefebvre T, Gaetani S, dos Santos M, Marrocco J, Mir AM, Cassano T, Maccari S, Nicoletti F, Mairesse J. Evidence for an imbalance between tau O-GlcNAcylation and phosphorylation in the hippocampus of a mouse model of Alzheimer’s disease. Pharmacological research. 2016;105:186–197. doi: 10.1016/j.phrs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 159.Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, Zhang L, Dai CL, Gu JH, Gong CX, Iqbal K, Liu F. O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging cell. 2016;15:455–464. doi: 10.1111/acel.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tian J, Geng Q, Ding Y, Liao J, Dong MQ, Xu X, Li J. O-GlcNAcylation Antagonizes Phosphorylation of CDH1 (CDC20 Homologue 1) The Journal of biological chemistry. 2016;291:12136–12144. doi: 10.1074/jbc.M116.717850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma Z, Vosseller K. O-GlcNAc in cancer biology. Amino acids. 2013;45:719–733. doi: 10.1007/s00726-013-1543-8. [DOI] [PubMed] [Google Scholar]
- 162.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annual review of biochemistry. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, Agnew BJ, Hsieh-Wilson LC. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. Journal of the American Chemical Society. 2008;130:11576–11577. doi: 10.1021/ja8030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alfarouk KO. Tumor metabolism, cancer cell transporters, and microenvironmental resistance. Journal of enzyme inhibition and medicinal chemistry. 2016;31:859–866. doi: 10.3109/14756366.2016.1140753. [DOI] [PubMed] [Google Scholar]
- 165.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 166.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science (New York, NY) 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, Aubert JP, Gosselin B, Porchet N. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. International journal of cancer. 2000;86:162–168. doi: 10.1002/(sici)1097-0215(20000415)86:2<162::aid-ijc3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 168.Lopez-Ferrer A, Curull V, Barranco C, Garrido M, Lloreta J, Real FX, de Bolos C. Mucins as differentiation markers in bronchial epithelium Squamous cell carcinoma and adenocarcinoma display similar expression patterns. American journal of respiratory cell and molecular biology. 2001;24:22–29. doi: 10.1165/ajrcmb.24.1.4294. [DOI] [PubMed] [Google Scholar]
- 169.Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, Szabo E. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer research. 1998;58:5582–5589. [PubMed] [Google Scholar]
- 170.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 171.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2, Science (New York, N.Y. ) 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 172.Lopez-Ferrer A, Barranco C, de Bolos C. Differences in the O-glycosylation patterns between lung squamous cell carcinoma and adenocarcinoma. American journal of clinical pathology. 2002;118:749–755. doi: 10.1309/LWP3-MFA8-8KX7-60YQ. [DOI] [PubMed] [Google Scholar]
- 173.Kawai T, Suzuki M, Kase K, Ozeki Y. Expression of carbohydrate antigens in human pulmonary adenocarcinoma. Cancer. 1993;72:1581–1587. doi: 10.1002/1097-0142(19930901)72:5<1581::aid-cncr2820720515>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 174.Longenecker BM, Rahman AF, Leigh JB, Purser RA, Greenberg AH, Willans DJ, Keller O, Petrik PK, Thay TY, Suresh MR, et al. Monoclonal antibody against a cryptic carbohydrate antigen of murine and human lymphocytes. I. Antigen expression in non-cryptic or unsubstituted form on certain murine lymphomas, on a spontaneous murine mammary carcinoma, and on several human adenocarcinomas, International journal of cancer. 1984;33:123–129. doi: 10.1002/ijc.2910330119. [DOI] [PubMed] [Google Scholar]
- 175.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 176.Ichige K, Perey L, Vogel CA, Buchegger F, Kufe D. Expression of the DF3-P epitope in human ovarian carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 1995;1:565–571. [PubMed] [Google Scholar]
- 177.Finn OJ. Journal of immunology. Vol. 181. Baltimore: Md: 2008. Immunological weapons acquired early in life win battles with cancer late in life; pp. 1589–1592. 1950. [DOI] [PubMed] [Google Scholar]
- 178.Ligtenberg MJ, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer research. 1992;52:2318–2324. [PubMed] [Google Scholar]
- 179.Kufe DW. Targeting the human MUC1 oncoprotein: a tale of two proteins. Cancer biology & therapy. 2008;7:81–84. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 180.Guddo F, Giatromanolaki A, Koukourakis MI, Reina C, Vignola AM, Chlouverakis G, Hilkens J, Gatter KC, Harris AL, Bonsignore G. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. Journal of clinical pathology. 1998;51:667–671. doi: 10.1136/jcp.51.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guddo F, Giatromanolaki A, Patriarca C, Hilkens J, Reina C, Alfano RM, Vignola AM, Koukourakis MI, Gambacorta M, Pruneri G, Coggi G, Bonsignore G. Depolarized expression of episialin (EMA, MUC1) in lung adenocarcinoma is associated with tumor progression. Anticancer research. 1998;18:1915–1920. [PubMed] [Google Scholar]
- 182.Nagai S, Takenaka K, Sonobe M, Ogawa E, Wada H, Tanaka F. A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2006;1:46–51. [PubMed] [Google Scholar]
- 183.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Yonezawa S. Combined status of MUC1 mucin and surfactant apoprotein A expression can predict the outcome of patients with small-size lung adenocarcinoma. Histopathology. 2004;44:147–155. doi: 10.1111/j.1365-2559.2004.01797.x. [DOI] [PubMed] [Google Scholar]
- 184.Kuemmel A, Single K, Bittinger F, Faldum A, Schmidt LH, Sebastian M, Micke P, Taube C, Buhl R, Wiewrodt R. TA-MUC1 epitope in non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2009;63:98–105. doi: 10.1016/j.lungcan.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, McGuckin MA. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. The Journal of pathology. 1997;183:311–317. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 186.Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, Weichselbaum RR. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer research. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.MacDermed DM, Khodarev NN, Pitroda SP, Edwards DC, Pelizzari CA, Huang L, Kufe DW, Weichselbaum RR. MUC1-associated proliferation signature predicts outcomes in lung adenocarcinoma patients. BMC medical genomics. 2010;3:16. doi: 10.1186/1755-8794-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao J, McConnell MJ, Yu B, Li J, Balko JM, Black EP, Johnson JO, Lloyd MC, Altiok S, Haura EB. MUC1 is a downstream target of STAT3 and regulates lung cancer cell survival and invasion. International journal of oncology. 2009;35:337–345. [PMC free article] [PubMed] [Google Scholar]
- 189.Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, Shimamura T, Shapiro GI, Supko J, Kharbanda S, Kufe D. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Molecular cancer therapeutics. 2011;10:806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang L, Gallup M, Zlock L, Chen YT, Finkbeiner WE, McNamara NA. Pivotal role of MUC1 glycosylation by cigarette smoke in modulating disruption of airway adherens junctions in vitro. The Journal of pathology. 2014;234:60–73. doi: 10.1002/path.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cao Y, Blohm D, Ghadimi BM, Stosiek P, Xing PX, Karsten U. Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterogeneous tumor-associated aberrations in glycosylation. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1997;45:1547–1557. doi: 10.1177/002215549704501111. [DOI] [PubMed] [Google Scholar]
- 192.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochimica et biophysica acta. 1999;1455:301–313. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 193.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. The Journal of cell biology. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Molecular biology of the cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, Rubashkin MG, Magbanua MJ, Thorn KS, Davidson MW, Rugo HS, Park JW, Hammer DA, Giannone G, Bertozzi CR, Weaver VM. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clinical & experimental metastasis. 2002;19:339–345. doi: 10.1023/a:1015590515957. [DOI] [PubMed] [Google Scholar]
- 197.Ghosh SK, Pantazopoulos P, Medarova Z, Moore A. Expression of underglycosylated MUC1 antigen in cancerous and adjacent normal breast tissues. Clinical breast cancer. 2013;13:109–118. doi: 10.1016/j.clbc.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cascio S, Farkas AM, Hughey RP, Finn OJ. Altered glycosylation of MUC1 influences its association with CIN85: the role of this novel complex in cancer cell invasion and migration. Oncotarget. 2013;4:1686–1697. doi: 10.18632/oncotarget.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gunkel L, Mylonas I, Richter DU, Makovitzky J. Immunohistochemical studies of mucinous mammary carcinomas and their metastases. Anticancer research. 2005;25:1755–1759. [PubMed] [Google Scholar]
- 200.Andre S, Kojima S, Yamazaki N, Fink C, Kaltner H, Kayser K, Gabius HJ. Galectins-1 and -3 and their ligands in tumor biology Non-uniform properties in cell-surface presentation and modulation of adhesion to matrix glycoproteins for various tumor cell lines, in biodistribution of free and liposome-bound galectins and in their expression by breast and colorectal carcinomas with/without metastatic propensity. Journal of cancer research and clinical oncology. 1999;125:461–474. doi: 10.1007/s004320050303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fry SA, Van den Steen PE, Royle L, Wormald MR, Leathem AJ, Opdenakker G, McDonnell JM, Dwek RA, Rudd PM. Cancer-associated glycoforms of gelatinase B exhibit a decreased level of binding to galectin-3. Biochemistry. 2006;45:15249–15258. doi: 10.1021/bi061254l. [DOI] [PubMed] [Google Scholar]
- 202.Simone G, Malara N, Trunzo V, Renne M, Perozziello G, Di Fabrizio E, Manz A. Galectin-3 coats the membrane of breast cells and makes a signature of tumours. Molecular bioSystems. 2014;10:258–265. doi: 10.1039/c3mb70359b. [DOI] [PubMed] [Google Scholar]
- 203.Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. The Journal of biological chemistry. 2007;282:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 204.Gordower L, Decaestecker C, Kacem Y, Lemmers A, Gusman J, Burchert M, Danguy A, Gabius H, Salmon I, Kiss R, Camby I. Galectin-3 and galectin-3-binding site expression in human adult astrocytic tumours and related angiogenesis. Neuropathol Appl Neurobiol. 1999;25:319–330. doi: 10.1046/j.1365-2990.1999.00192.x. [DOI] [PubMed] [Google Scholar]
- 205.Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776–787. [PubMed] [Google Scholar]
- 206.Kuklinski S, Pesheva P, Heimann C, Urschel S, Gloor S, Graeber S, Herzog V, Pietsch T, Wiestler OD, Probstmeier R. Expression pattern of galectin-3 in neural tumor cell lines. Journal of neuroscience research. 2000;60:45–57. doi: 10.1002/(SICI)1097-4547(20000401)60:1<45::AID-JNR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 207.Neder L, Marie SK, Carlotti CG, Jr, Gabbai AA, Rosemberg S, Malheiros SM, Siqueira RP, Oba-Shinjo SM, Uno M, Aguiar PH, Miura F, Chammas R, Colli BO, Silva WA, Jr, Zago MA. Galectin-3 as an immunohistochemical tool to distinguish pilocytic astrocytomas from diffuse astrocytomas, and glioblastomas from anaplastic oligodendrogliomas. Brain pathology (Zurich, Switzerland) 2004;14:399–405. doi: 10.1111/j.1750-3639.2004.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strik HM, Deininger MH, Frank B, Schluesener HJ, Meyermann R. Galectin-3: cellular distribution and correlation with WHO-grade in human gliomas. Journal of neuro-oncology. 2001;53:13–20. doi: 10.1023/a:1011874800612. [DOI] [PubMed] [Google Scholar]
- 209.Tews DS. Adhesive and invasive features in gliomas. Pathology, research and practice. 2000;196:701–711. doi: 10.1016/S0344-0338(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 210.Tews DS, Nissen A. Expression of adhesion factors and degrading proteins in primary and secondary glioblastomas and their precursor tumors. Invasion & metastasis. 1998;18:271–284. doi: 10.1159/000024520. [DOI] [PubMed] [Google Scholar]
- 211.Le Mercier M, Fortin S, Mathieu V, Kiss R, Lefranc F. Galectins and gliomas. Brain pathology (Zurich, Switzerland) 2010;20:17–27. doi: 10.1111/j.1750-3639.2009.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Camby I, Decaestecker C, Lefranc F, Kaltner H, Gabius HJ, Kiss R. Galectin-1 knocking down in human U87 glioblastoma cells alters their gene expression pattern. Biochemical and biophysical research communications. 2005;335:27–35. doi: 10.1016/j.bbrc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 213.Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends in immunology. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 214.Le Mercier M, Lefranc F, Mijatovic T, Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R, Mathieu V. Evidence of galectin-1 involvement in glioma chemoresistance. Toxicology and applied pharmacology. 2008;229:172–183. doi: 10.1016/j.taap.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 215.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20:7486–7493. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 216.Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ, Giaccia AJ, Koong AC. Galectin-1: a link between tumor hypoxia and tumor immune privilege. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer cell. 2004;5:241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 218.Rabinovich GA, Cumashi A, Bianco GA, Ciavardelli D, Iurisci I, D’Egidio M, Piccolo E, Tinari N, Nifantiev N, Iacobelli S. Synthetic lactulose amines: novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology. 2006;16:210–220. doi: 10.1093/glycob/cwj056. [DOI] [PubMed] [Google Scholar]
- 219.Miller MC, Klyosov A, Mayo KH. The alpha-galactomannan Davanat binds galectin-1 at a site different from the conventional galectin carbohydrate binding domain. Glycobiology. 2009;19:1034–1045. doi: 10.1093/glycob/cwp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug discovery today. 2015;20:899–905. doi: 10.1016/j.drudis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 221.Hanaoka J, Kontani K, Sawai S, Ichinose M, Tezuka N, Inoue S, Fujino S, Ohkubo I. Analysis of MUC4 mucin expression in lung carcinoma cells and its immunogenicity. Cancer. 2001;92:2148–2157. doi: 10.1002/1097-0142(20011015)92:8<2148::aid-cncr1557>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 222.Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T, Imai K, Yonezawa S. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. Journal of clinical pathology. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miyahara N, Shoda J, Ishige K, Kawamoto T, Ueda T, Taki R, Ohkohchi N, Hyodo I, Thomas MB, Krishnamurthy S, Carraway KL, Irimura T. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. European journal of cancer (Oxford, England : 1990) 2008;44:1048–1056. doi: 10.1016/j.ejca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 224.Giuntoli RL, 2nd, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer research. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 225.Davidson B, Baekelandt M, Shih Ie M. MUC4 is upregulated in ovarian carcinoma effusions and differentiates carcinoma cells from mesothelial cells. Diagnostic cytopathology. 2007;35:756–760. doi: 10.1002/dc.20771. [DOI] [PubMed] [Google Scholar]
- 226.Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 227.Kwon KY, Ro JY, Singhal N, Killen DE, Sienko A, Allen TC, Zander DS, Barrios R, Haque A, Cagle PT. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Archives of pathology & laboratory medicine. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]
- 228.Karg A, Dinc ZA, Basok O, Ucvet A. MUC4 expression and its relation to ErbB2 expression, apoptosis, proliferation, differentiation, and tumor stage in non-small cell lung cancer (NSCLC) Pathology, research and practice. 2006;202:577–583. doi: 10.1016/j.prp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 229.Li W, Wu C, Yao Y, Dong B, Wei Z, Lv X, Zhang J, Xu Y. MUC4 modulates human glioblastoma cell proliferation and invasion by upregulating EGFR expression. Neuroscience letters. 2014;566:82–87. doi: 10.1016/j.neulet.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 230.Matsuo S, Watanabe J, Mitsuya K, Hayashi N, Nakasu Y, Hayashi M. Brain metastasis in patients with metastatic breast cancer in the real world: a single-institution, retrospective review of 12-year follow-up. Breast cancer research and treatment. 2017;162:169–179. doi: 10.1007/s10549-017-4107-x. [DOI] [PubMed] [Google Scholar]
- 231.Sawada T, Kidowaki T, Sakamoto I, Hashida T, Matsumura T, Nakagawa M, Kusunoki T. Neuroblastoma Mass screening for early detection and its prognosis. Cancer. 1984;53:2731–2735. doi: 10.1002/1097-0142(19840615)53:12<2731::aid-cncr2820531232>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 232.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 233.Kelly PJ. Gliomas: Survival, origin and early detection. Surgical neurology international. 2010;1:96. doi: 10.4103/2152-7806.74243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gornik O, Lauc G. Glycosylation of serum proteins in inflammatory diseases. Disease markers. 2008;25:267–278. doi: 10.1155/2008/493289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hashimoto S, Asao T, Takahashi J, Yagihashi Y, Nishimura T, Saniabadi AR, Poland DC, van Dijk W, Kuwano H, Kochibe N, Yazawa S. alpha1-acid glycoprotein fucosylation as a marker of carcinoma progression and prognosis. Cancer. 2004;101:2825–2836. doi: 10.1002/cncr.20713. [DOI] [PubMed] [Google Scholar]
- 236.Higai K, Aoki Y, Azuma Y, Matsumoto K. Glycosylation of site-specific glycans of alpha1-acid glycoprotein and alterations in acute and chronic inflammation. Biochimica et biophysica acta. 2005;1725:128–135. doi: 10.1016/j.bbagen.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 237.Gornik O, Royle L, Harvey DJ, Radcliffe CM, Saldova R, Dwek RA, Rudd P, Lauc G. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology. 2007;17:1321–1332. doi: 10.1093/glycob/cwm106. [DOI] [PubMed] [Google Scholar]
- 238.Piagnerelli M, Boudjeltia KZ, Nuyens V, De Backer D, Su F, Wang Z, Vincent JL, Vanhaeverbeek M. Rapid alterations in transferrin sialylation during sepsis, Shock (Augusta, Ga. ) 2005;24:48–52. doi: 10.1097/01.shk.0000168524.20588.67. [DOI] [PubMed] [Google Scholar]
- 239.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual review of immunology. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 240.Chen G, Wang Y, Qin X, Li H, Guo Y, Wang Y, Liu H, Wang X, Song G, Li F, Li F, Guo S, Qiu L, Li Z. Change in IgG1 Fc N-linked glycosylation in human lung cancer: age- and sex-related diagnostic potential. Electrophoresis. 2013;34:2407–2416. doi: 10.1002/elps.201200455. [DOI] [PubMed] [Google Scholar]
- 241.Taniguchi N, Kizuka Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Advances in cancer research. 2015;126:11–51. doi: 10.1016/bs.acr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 242.Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert opinion on biological therapy. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- 243.Natsume A, Wakitani M, Yamane-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, Satoh M, Shitara K. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region. Journal of biochemistry. 2006;140:359–368. doi: 10.1093/jb/mvj157. [DOI] [PubMed] [Google Scholar]
- 244.Nustad K, Bast RC, Jr, Brien TJ, Nilsson O, Seguin P, Suresh MR, Saga T, Nozawa S, Bormer OP, de Bruijn HW, Nap M, Vitali A, Gadnell M, Clark J, Shigemasa K, Karlsson B, Kreutz FT, Jette D, Sakahara H, Endo K, Paus E, Warren D, Hammarstrom S, Kenemans P, Hilgers J. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop International Society for Oncodevelopmental Biology and Medicine. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 1996;17:196–219. doi: 10.1159/000217982. [DOI] [PubMed] [Google Scholar]
- 245.Pauler DK, Menon U, McIntosh M, Symecko HL, Skates SJ, Jacobs IJ. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:489–493. [PubMed] [Google Scholar]
- 246.Gostout BS, Brewer MA. Guidelines for referral of the patient with an adnexal mass. Clinical obstetrics and gynecology. 2006;49:448–458. doi: 10.1097/00003081-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 247.Zurawski VR, Jr, Orjaseter H, Andersen A, Jellum E. Elevated serum CA 125 levels prior to diagnosis of ovarian neoplasia: relevance for early detection of ovarian cancer. International journal of cancer. 1988;42:677–680. doi: 10.1002/ijc.2910420507. [DOI] [PubMed] [Google Scholar]
- 248.Kim EH, Misek DE. Glycoproteomics-based identification of cancer biomarkers. International journal of proteomics. 2011;1937;60 doi: 10.1155/2011/601937. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nangia-Makker P, Conklin J, Hogan V, Raz A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends in molecular medicine. 2002;8:187–192. doi: 10.1016/s1471-4914(02)02295-5. [DOI] [PubMed] [Google Scholar]
- 250.Kossowska B, Ferens-Sieczkowska M, Gancarz R, Passowicz-Muszynska E, Jankowska R. Fucosylation of serum glycoproteins in lung cancer patients. Clinical chemistry and laboratory medicine. 2005;43:361–369. doi: 10.1515/CCLM.2005.066. [DOI] [PubMed] [Google Scholar]
- 251.Song X, Airan RD, Arifin DR, Bar-Shir A, Kadayakkara DK, Liu G, Gilad AA, van Zijl PC, McMahon MT, Bulte JW. Label-free in vivo molecular imaging of underglycosylated mucin-1 expression in tumour cells. Nature communications. 2015;6:6719. doi: 10.1038/ncomms7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S. Mucin expression profile in pancreatic cancer and the precursor lesions. Journal of hepato-biliary-pancreatic surgery. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 253.Zacharias LG, Hartmann AK, Song E, Zhao J, Zhu R, Mirzaei P, Mechref Y. HILIC and ERLIC Enrichment of Glycopeptides Derived from Breast and Brain Cancer Cells. J Proteome Res. 2016;15:3624–3634. doi: 10.1021/acs.jproteome.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yamamoto H, Saito T, Kaneko Y, Kersey D, Yong VW, Bremer EG, Mkrdichian E, Cerullo L, Leestma J, Moskal JR. alpha2,3-sialyltransferase mRNA and alpha2,3-linked glycoprotein sialylation are increased in malignant gliomas. Brain research. 1997;755:175–179. doi: 10.1016/s0006-8993(97)00241-2. [DOI] [PubMed] [Google Scholar]
- 255.Ghosh S. Sialic acids: biomarkers in endocrinal cancers. Glycoconjugate journal. 2015;32:79–85. doi: 10.1007/s10719-015-9577-7. [DOI] [PubMed] [Google Scholar]
- 256.Autelitano F, Loyaux D, Roudieres S, Deon C, Guette F, Fabre P, Ping Q, Wang S, Auvergne R, Badarinarayana V, Smith M, Guillemot JC, Goldman SA, Natesan S, Ferrara P, August P. Identification of novel tumor-associated cell surface sialoglycoproteins in human glioblastoma tumors using quantitative proteomics. PloS one. 2014;9:e110316. doi: 10.1371/journal.pone.0110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Katopodis N, Glantz MJ, Kim L, Dafni U, Wu JK, Perides G. Lipid-associated sialoprotein in the cerebrospinal fluid: association with brain malignancies. Cancer. 2001;92:856–862. doi: 10.1002/1097-0142(20010815)92:4<856::aid-cncr1393>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 258.Kokoglu E, Sonmez H, Uslu E, Uslu I. Sialic acid levels in various types of cancer. Cancer biochemistry biophysics. 1992;13:57–64. [PubMed] [Google Scholar]
- 259.Marth E, Flaschka G, Stiegler S, Mose JR. Sialic acid as a marker for differentiation between benign and malignant intracranial tumors. Clinica chimica acta; international journal of clinical chemistry. 1988;176:251–257. doi: 10.1016/0009-8981(88)90184-2. [DOI] [PubMed] [Google Scholar]
- 260.Flaschka G, Marth E, Desoye G, Freidl W, Walzl M. Diagnostic value of biochemical tumor markers in brain tumors Review of the literature and own experience with serum analysis of sialic acid (NANA), carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) Zentralblatt fur Neurochirurgie. 1990;51:129–137. [PubMed] [Google Scholar]
- 261.Moroki K, Kuratsu J, Yoshioka S, Kohchi M, Uemura S, Matsukado Y, Ushio Y. Clinical significance of sialic acid in the CSF; in relation to the malignancy and leptomeningeal dissemination. No to shinkei = Brain and nerve. 1989;41:305–307. [PubMed] [Google Scholar]
- 262.Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, Heideman RL. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-oncology. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang R, Saito R, Shibahara I, Sugiyama S, Kanamori M, Sonoda Y, Tominaga T. Temozolomide reverses doxorubicin resistance by inhibiting P-glycoprotein in malignant glioma cells. Journal of neuro-oncology. 2016;126:235–242. doi: 10.1007/s11060-015-1968-x. [DOI] [PubMed] [Google Scholar]
- 264.de Gooijer MC, Zhang P, Thota N, Mayayo-Peralta I, Buil LC, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Investigational new drugs. 2015;33:1012–1019. doi: 10.1007/s10637-015-0266-y. [DOI] [PubMed] [Google Scholar]
- 265.Becker CM, Oberoi RK, McFarren SJ, Muldoon DM, Pafundi DH, Pokorny JL, Brinkmann DH, Ohlfest JR, Sarkaria JN, Largaespada DA, Elmquist WF. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro-oncology. 2015;17:1210–1219. doi: 10.1093/neuonc/nov081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang Z, Xu K, Bi Y, Yu G, Wang S, Qi X, Zhong H. Low intensity ultrasound promotes the sensitivity of rat brain glioma to Doxorubicin by down-regulating the expressions of p-glucoprotein and multidrug resistance protein 1 in vitro and in vivo. PloS one. 2013;8:e70685. doi: 10.1371/journal.pone.0070685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chonan M, Saito R, Shoji T, Shibahara I, Kanamori M, Sonoda Y, Watanabe M, Kikuchi T, Ishii N, Tominaga T. CD40/CD40L expression correlates with the survival of patients with glioblastomas and an augmentation in CD40 signaling enhances the efficacy of vaccinations against glioma models. Neuro-oncology. 2015;17:1453–1462. doi: 10.1093/neuonc/nov090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Marcu-Malina V, Garelick D, Peshes-Yeloz N, Wohl A, Zach L, Nagar M, Amariglio N, Besser MJ, Cohen ZR, Bank I. Peripheral blood-derived, gamma9delta2 t cell-enriched cell lines from glioblastoma multiforme patients exert anti-tumoral effects in vitro. Journal of biological regulators and homeostatic agents. 2016;30:17–30. [PubMed] [Google Scholar]
- 269.Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer immunology, immunotherapy : CII. 2014;63:847–857. doi: 10.1007/s00262-014-1561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Friedmann-Morvinski D, Bhargava V, Gupta S, Verma IM, Subramaniam S. Identification of therapeutic targets for glioblastoma by network analysis. Oncogene. 2016;35:608–620. doi: 10.1038/onc.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Motomura K, Natsume A, Watanabe R, Ito I, Kato Y, Momota H, Nishikawa R, Mishima K, Nakasu Y, Abe T, Namba H, Nakazato Y, Tashiro H, Takeuchi I, Mori T, Wakabayashi T. Immunohistochemical analysis-based proteomic subclassification of newly diagnosed glioblastomas. Cancer science. 2012;103:1871–1879. doi: 10.1111/j.1349-7006.2012.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas, Research N. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shiina S, Ohno M, Ohka F, Kuramitsu S, Yamamichi A, Kato A, Motomura K, Tanahashi K, Yamamoto T, Watanabe R, Ito I, Senga T, Hamaguchi M, Wakabayashi T, Kaneko MK, Kato Y, Chandramohan V, Bigner DD, Natsume A. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer immunology research. 2016;4:259–268. doi: 10.1158/2326-6066.CIR-15-0060. [DOI] [PubMed] [Google Scholar]
- 274.Rafidi H, Mercado F, 3rd, Astudillo M, Fry WH, Saldana M, Carraway KL, 3rd, Sweeney C. Leucine-rich repeat and immunoglobulin domain-containing protein-1 (Lrig1) negative regulatory action toward ErbB receptor tyrosine kinases is opposed by leucine-rich repeat and immunoglobulin domain-containing protein 3 (Lrig3) The Journal of biological chemistry. 2013;288:21593–21605. doi: 10.1074/jbc.M113.486050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang X, He XJ, Xu HQ, Chen ZW, Fan HH. Inhibition of subcutaneously implanted human pituitary tumor cells in nude mice by LRIG1. Genetics and molecular research : GMR. 2016:15. doi: 10.4238/gmr.15027681. [DOI] [PubMed] [Google Scholar]
- 276.Gruslova A, Cavazos DA, Miller JR, Breitbart E, Cohen YC, Bangio L, Yakov N, Soundararajan A, Floyd JR, Brenner AJ. VB-111: a novel anti-vascular therapeutic for glioblastoma multiforme. Journal of neuro-oncology. 2015;124:365–372. doi: 10.1007/s11060-015-1853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liang S, Xu JF, Cao WJ, Li HP, Hu CP. Human decorin regulates proliferation and migration of human lung cancer A549 cells. Chinese medical journal. 2013;126:4736–4741. [PubMed] [Google Scholar]
- 278.Ma HI, Hueng DY, Shui HA, Han JM, Wang CH, Lai YH, Cheng SY, Xiao X, Chen MT, Yang YP. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. International journal of molecular sciences. 2014;15:4393–4414. doi: 10.3390/ijms15034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sarkis GA, Mangaonkar MD, Moghieb A, Lelling B, Guertin M, Yadikar H, Yang Z, Kobeissy F, Wang KK. The Application of Proteomics to Traumatic Brain and Spinal Cord Injuries. Current neurology and neuroscience reports. 2017;17:23. doi: 10.1007/s11910-017-0736-z. [DOI] [PubMed] [Google Scholar]
- 280.Nakashima H, Kaufmann JK, Wang PY, Nguyen T, Speranza MC, Kasai K, Okemoto K, Otsuki A, Nakano I, Fernandez S, Goins WF, Grandi P, Glorioso JC, Lawler S, Cripe TP, Chiocca EA. Histone deacetylase 6 inhibition enhances oncolytic viral replication in glioma. The Journal of clinical investigation. 2015;125:4269–4280. doi: 10.1172/JCI80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, Xu H, Han X, Markert JM, Beierle EA, Gillespie GY. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro-oncology. 2016;18:227–235. doi: 10.1093/neuonc/nov123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Berghauser Pont LM, Kleijn A, Kloezeman JJ, van den Bossche W, Kaufmann JK, de Vrij J, Leenstra S, Dirven CM, Lamfers ML. The HDAC Inhibitors Scriptaid and LBH589 Combined with the Oncolytic Virus Delta24-RGD Exert Enhanced Anti-Tumor Efficacy in Patient-Derived Glioblastoma Cells. PloS one. 2015;10:e0127058. doi: 10.1371/journal.pone.0127058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism: clinical and experimental. 1987;36:892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 284.Fang JH, Chiu TL, Huang WC, Lai YH, Hu SH, Chen YY, Chen SY. Dual-Targeting Lactoferrin-Conjugated Polymerized Magnetic Polydiacetylene-Assembled Nanocarriers with Self-Responsive Fluorescence/Magnetic Resonance Imaging for In Vivo Brain Tumor Therapy. Advanced healthcare materials. 2016;5:688–695. doi: 10.1002/adhm.201500750. [DOI] [PubMed] [Google Scholar]
- 285.Kuo YC, Chen YC. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. International journal of pharmaceutics. 2015;479:138–149. doi: 10.1016/j.ijpharm.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 286.Singh I, Swami R, Pooja D, Jeengar MK, Khan W, Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. Journal of drug targeting. 2016;24:212–223. doi: 10.3109/1061186X.2015.1068320. [DOI] [PubMed] [Google Scholar]
- 287.Wang F, Zhang W, Shen Y, Huang Q, Zhou D, Guo S. Efficient RNA delivery by integrin-targeted glutathione responsive polyethyleneimine capped gold nanorods. Acta biomaterialia. 2015;23:136–146. doi: 10.1016/j.actbio.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 288.Mittapalli RK, Liu X, Adkins CE, Nounou MI, Bohn KA, Terrell TB, Qhattal HS, Geldenhuys WJ, Palmieri D, Steeg PS, Smith QR, Lockman PR. Paclitaxel-hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Molecular cancer therapeutics. 2013;12:2389–2399. doi: 10.1158/1535-7163.MCT-13-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kolter T, Sandhoff K. Sphingolipid metabolism diseases. Biochimica et biophysica acta. 2006;1758:2057–2079. doi: 10.1016/j.bbamem.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 290.Maleklou N, Allameh A, Kazemi B. Targeted delivery of vitamin D3-loaded nanoparticles to C6 glioma cell line increased resistance to doxorubicin, epirubicin, and docetaxel in vitro, In vitro cellular & developmental biology. Animal. 2016;52:989–1000. doi: 10.1007/s11626-016-0072-7. [DOI] [PubMed] [Google Scholar]
- 291.Kalia M. Personalized oncology: recent advances and future challenges. Metabolism: clinical and experimental. 2013;62(Suppl 1):S11–14. doi: 10.1016/j.metabol.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 292.Gonzalez de Castro D, Clarke PA, Al-Lazikani B, Workman P. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clinical pharmacology and therapeutics. 2013;93:252–259. doi: 10.1038/clpt.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Samraj AN, Laubli H, Varki N, Varki A. Involvement of a non-human sialic Acid in human cancer. Frontiers in oncology. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chan DW, Beveridge RA, Muss H, Fritsche HA, Hortobagyi G, Theriault R, Kiang D, Kennedy BJ, Evelegh M. Use of Truquant BR radioimmunoassay for early detection of breast cancer recurrence in patients with stage II and stage III disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2322–2328. doi: 10.1200/JCO.1997.15.6.2322. [DOI] [PubMed] [Google Scholar]
- 296.Thanabalasingham G, Huffman JE, Kattla JJ, Novokmet M, Rudan I, Gloyn AL, Hayward C, Adamczyk B, Reynolds RM, Muzinic A, Hassanali N, Pucic M, Bennett AJ, Essafi A, Polasek O, Mughal SA, Redzic I, Primorac D, Zgaga L, Kolcic I, Hansen T, Gasperikova D, Tjora E, Strachan MW, Nielsen T, Stanik J, Klimes I, Pedersen OB, Njolstad PR, Wild SH, Gyllensten U, Gornik O, Wilson JF, Hastie ND, Campbell H, McCarthy MI, Rudd PM, Owen KR, Lauc G, Wright AF. Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes. 2013;62:1329–1337. doi: 10.2337/db12-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Almeida A, Kolarich D. The promise of protein glycosylation for personalised medicine. Biochimica et biophysica acta. 2016:1583–1595. doi: 10.1016/j.bbagen.2016.03.012. 1860. [DOI] [PubMed] [Google Scholar]
- 298.Sarrats A, Saldova R, Comet J, O’Donoghue N, de Llorens R, Rudd PM, Peracaula R. Glycan characterization of PSA 2-DE subforms from serum and seminal plasma. Omics : a journal of integrative biology. 2010;14:465–474. doi: 10.1089/omi.2010.0050. [DOI] [PubMed] [Google Scholar]
- 299.Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, Pinto-de-Sousa J, Almeida R, Gartner F, Reis CA. Expression of UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57:79–86. doi: 10.1369/jhc.2008.952283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sellers TA, Huang Y, Cunningham J, Goode EL, Sutphen R, Vierkant RA, Kelemen LE, Fredericksen ZS, Liebow M, Pankratz VS, Hartmann LC, Myer J, Iversen ES, Jr, Schildkraut JM, Phelan C. Association of single nucleotide polymorphisms in glycosylation genes with risk of epithelial ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:397–404. doi: 10.1158/1055-9965.EPI-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Patani N, Jiang W, Mokbel K. Prognostic utility of glycosyltransferase expression in breast cancer. Cancer genomics & proteomics. 2008;5:333–340. [PubMed] [Google Scholar]
- 302.Handerson T, Camp R, Harigopal M, Rimm D, Pawelek J. Beta1,6-branched oligosaccharides are increased in lymph node metastases and predict poor outcome in breast carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2969–2973. doi: 10.1158/1078-0432.CCR-04-2211. [DOI] [PubMed] [Google Scholar]
- 303.Picco G, Julien S, Brockhausen I, Beatson R, Antonopoulos A, Haslam S, Mandel U, Dell A, Pinder S, Taylor-Papadimitriou J, Burchell J. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 2010;20:1241–1250. doi: 10.1093/glycob/cwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Noda K, Miyoshi E, Uozumi N, Gao CX, Suzuki K, Hayashi N, Hori M, Taniguchi N. High expression of alpha-1-6 fucosyltransferase during rat hepatocarcinogenesis. International journal of cancer. 1998;75:444–450. doi: 10.1002/(sici)1097-0215(19980130)75:3<444::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 305.Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M, Rahmoune H, Levin Y, Guest PC, Bahn S, Rudd PM. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J Proteome Res. 2010;9:4476–4489. doi: 10.1021/pr1002356. [DOI] [PubMed] [Google Scholar]


