Abstract
Older adults with type 2 diabetes (T2D) tend to have normal or greater areal bone mineral density (aBMD), as measured by DXA, than those who do not have diabetes (non-T2D). Yet, risk of fracture is higher in T2D, including 40%–50% increased hip fracture risk. We used HR-pQCT to investigate structural mechanisms underlying skeletal fragility in T2D. We compared cortical and trabecular bone microarchitecture, density, bone area, and strength in T2D and non-T2D. In secondary analyses we evaluated whether associations between T2D and bone measures differed according to prior fracture, sex, and obesity.
Participants included 1,069 members of the Framingham Study, who attended examinations 2005–2008 and underwent HR-pQCT scanning in 2012–2015. Mean age was 64 (±8) years (range, 40–87), and 12% (n=129) had T2D. After adjustment for age, sex, weight, and height, T2D had lower cortical vBMD (p<0.01), higher cortical porosity (p=0.02), and smaller cross-sectional area (p=0.04) at the tibia, but not radius. Trabecular indices were similar or more favorable in T2D than non-T2D. Associations between T2D and bone measures did not differ according to sex or obesity status (all interaction p>0.05), however associations did differ in those with a prior fracture and those with no history of fracture. Specifically, cortical vBMD at the tibia and cortical thickness at the radius were lower in T2D than non-T2D, but only among those individuals with a prior fracture. Cortical porosity at the radius was higher in T2D than non-T2D, but only among those who did not have a prior fracture.
Findings from this large, community-based study of older adults suggest that modest deterioration in cortical bone and reductions in bone area may characterize diabetic bone disease in older adults. Evaluation of these deficits as predictors of fracture in T2D is needed to develop prevention strategies in this rapidly increasing population of older adults.
Introduction
Osteoporotic fractures are a significant public health problem causing high morbidity, mortality, and health care costs. In the United States, 1 in 2 women and 1 in 4 men over age 50 will sustain a fragility fracture during their lifetime.(1) Risk of fracture is even greater in adults with type 2 diabetes (T2D),(2–12) including 40 to 50 percent increased risk of hip fracture,(4,5,9,13) the most serious of osteoporotic fractures. Notably, fracture risk is greater in older adults with T2D, even after accounting for T2D complications known to increase falls.(3,6,8,12) This increase in hip fracture risk among those with T2D is significant, because in the general population, 20% of patients with hip fracture die within one year. More than half never regain the ability to function independently.(14,15) Outcomes are even worse in T2D patients with hip fracture, including greater frequency of post-operative complications, longer length of stay in the hospital, higher in-hospital mortality, worse recovery in walking ability and physical function, greater frequency of pain and depression, and higher likelihood of permanent placement in a nursing home, compared to hip fracture patients without T2D.(16–22)
Over the next fifty years, the largest increase in T2D will be in those age 75 years and greater, with increases projected as high as 336% for this oldest age group.(23) Further, the public health impact of fracture in older adults with T2D will continue to increase due to the aging of the population and growing epidemic of T2D.(19) Thus, interventions to prevent fracture in older adults with T2D are urgently needed. However, a lack of understanding of the mechanisms of skeletal fragility in T2D hinders the ability to develop effective clinical strategies.
Despite the substantial fracture risk associated with T2D, clinicians may not recognize skeletal fragility as a diabetic complication, as individuals with T2D tend to have greater or normal areal bone mineral density, aBMD, as measured by DXA (dual-energy X-ray absorptiometry), than those who do not have diabetes (non-T2D).(13,24) In addition, T2D patients have high frequency of obesity, which is also associated with high aBMD and increased fracture risk at some skeletal sites.(25)
High resolution peripheral quantitative computed tomography (HR-pQCT) is a 3D imaging technology that evaluates volumetric bone density, microarchitecture, and geometry separately for cortical and trabecular compartments. Thus, HR-pQCT may provide additional insight to the mechanisms of bone fragility in patients with T2D. A few,(26–29) but not all(30,31) studies using HR-pQCT suggest that T2D is associated with deficits in cortical bone, but preserved trabecular indices. However, most prior studies used a case-control design, included small numbers of T2D patients, and were limited to women. In a small case-control study of postmenopausal women (n=19 T2D), Burghardt et al reported higher cortical pore volume and cortical porosity at the radius in T2D compared to controls.(26) Patsch and colleagues extended these findings to show, among T2D, those with prior fracture had higher pore volume and cortical porosity at the radius than those with no prior fracture. However, they did not report differences in bone microstructure by T2D status in those with or without prior fracture.(27) Findings from the SWAN cohort in African American women (n=22 T2D)(28) and the Hertfordshire Cohort in women and men (n=29 T2D)(29) support an association between unfavorable cortical bone microarchitecture and T2D. In contrast, in the largest study to date (n=99 T2D), Nilsson et al found no differences in cortical indices according to T2D status in women aged 75 to 80 years in the Gothenburg Study in Sweden.(32) Thus, it remains uncertain if differences in cortical bone density and microarchitecture or other skeletal parameters differ by T2D status in older adults.
We investigated the association between T2D and cortical and trabecular bone microarchitecture and density, as well as total bone density, area, and strength. We hypothesized that individuals with T2D would have lower cortical bone density and less favorable cortical microarchitecture than non-T2D. In secondary analyses, we examined whether the association between T2D and HR-pQCT bone outcomes differed by obesity status, sex, and history of prior fracture.
Methods
Study design and participants
Participants for this study included 1,069 members (606 women, 463 men) of the Framingham Offspring Cohort. The Framingham Offspring Cohort was established in 1971 with enrollment of 5,124 adult children and spouses of members of the original cohort of the Framingham Heart Study, which was enrolled in 1948.(33,34) The original cohort was based on a population-based sample of two-thirds of the residents living in the town of Framingham, MA.(35) Participants were predominantly Caucasian reflecting the race distribution of the source population. Every four years, members of the Offspring Cohort have attended clinic visits that included physician administered interviews, physical examinations, and laboratory tests.
The current study included those participants who attended clinic visits in 2005 to 2008, considered the baseline visit for this analysis, and completed HR-pQCT scanning and bone densitometry at the subsequent follow-up visit in 2012 to 2015. Information on T2D status and covariates was obtained at the baseline visit (2005 to 2008). This prospective design was used to ensure that T2D onset preceded evaluation of skeletal outcomes, performed an average of seven years after the baseline visit. Participants provided written informed consent, and the Institutional Review Board for Human Research at Boston University and Hebrew SeniorLife approved the study.
High-resolution peripheral quantitative computed tomography (HR-pQCT)
Volumetric bone density and bone microarchitecture were assessed at the ultradistal tibia and ultradistal radius using HR-pQCT (XtremeCT, SCANCO Medical AG, Brüttisellen, Switzerland).(36,37) Scans were acquired with a nominal isotropic voxel size of 82 μm3. The non-dominant forearm and right leg were scanned, unless a participant reported previous extremity fracture or had metal in the region of the scan, then the contralateral extremity was examined (forearm, n=53 or 5%; tibia, n=171 or 16%). Antero-posterior scout views were used to place a reference line on the distal tibial and radial joint surfaces, as previously described.(36,37) The scan region (110 slices) was 9 mm in length and offset proximally to the reference line by 22.5 mm for the tibia and 9.5 mm for the radius. Scans were evaluated for motion artifacts and repeated if significant movement occurred.(38) Scanning of a quality control phantom, containing rods of hydroxyapatite (densities of 0, 100, 200, 400 and 800 mg HA/cm3), was performed daily to monitor longitudinal stability of the system. Each scan was graded using a 5-point movement artifact scale (1=none, 2=minor, 3=moderate, 4=severe, and 5=extreme).(38–40) For density measures, scans with movement artifact rated grades 1 to 4 were retained, but for microarchitectural measures, only grades 1 to 3 were retained.(38–40)
We used the standard analysis program (Scanco software version V6.0) to assess bone cross-sectional area, total density, trabecular density and trabecular microarchitecture. Cortical densities, thickness, and porosity were evaluated using a semi-automated cortical bone segmentation technique.(39,41) We focused on the following indices for bone outcomes: trabecular volumetric bone mineral density (Tb.BMD, mgHA/cm3), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp, mm), standard deviation of trabecular separation (Tb.Sp.SD, mm), total (integral) volumetric bone mineral density (Tt.BMD, mgHA/cm3), and total cross-sectional area (Tt.CSA, mm2). The cortical analysis algorithm was used to evaluate cortical volumetric bone mineral density (Ct.BMD, mgHA/cm3), cortical porosity (Ct.Po, %), cortical thickness (Ct.Th, mm), cortical area fraction (Ct.Ar/Tt.Ar, %), and cortical tissue mineral density (Ct.TMD, mgHA/cm3). Linear micro finite element analysis (FEA, Numerics88 Solutions Inc) was performed to estimate failure load (N: Newtons), as previously described.(42) Briefly, axial compression conditions were applied with 1% apparent strain, and a tissue modulus of 6.829 GPa and Poisson’s ratio of 0.3.
Clinical examinations
We used information collected by physical examinations, laboratory testing, and questionnaires administered at clinic visits in 2005–2008. Hemoglobin A1C (%) and glucose (mg/dl) levels mIU/ml) were measured from blood samples drawn after an 8-hour fast using standard methods (Roche Diagnostics). T2D was defined as individuals with fasting plasma glucose levels greater than 125 mg/dl (7.0 mmol/dl) or on treatment with insulin or oral hypoglycemic agents. Medical histories were reviewed to rule out individuals with Type 1 diabetes.
Height, to the nearest one quarter inch, and weight, to the nearest half pound, were measured using a stadiometer and balance beam scale, respectively. Body mass index was calculated as weight (kilograms) divided by the square of height (meters). Information on age, smoking, physical activity (Framingham Physical Activity Index)(43), alcohol consumption, prior fracture during adulthood (excluding skull, fingers, toes and fractures due to trauma), medication use (bisphosphonates, estrogen replacement therapy, calcium and vitamin D supplements), and in women, menopause (cessation of periods for one year or more) was obtained by physician-administered interviews using standardized questionnaires.
Statistical analysis
We used analysis of covariance (ANCOVA) to compare least-squares means (± 95% confidence intervals, CI) for bone parameters (separately for the radius and tibia) by T2D status, adjusted for age (years), sex, weight (pounds), and height (inches). We provided results for bone parameters in their original units to show distributions in the scale on which each was measured. In addition, we standardized each bone parameter to a mean of 0 and a standard deviation (SD) of 1 to show mean differences across bone parameters that are independent of the original unit of measurement.
In secondary analyses, we examined the association between T2D and HR-pQCT bone outcomes stratified by history of prior fracture, obesity, and sex, and tested for interaction. We considered BMI>30 kg/m2 as obese, as per the definition used by the World Health Organization.(44) We retained weight as an adjustment variable to control for residual confounding by weight within obesity groups. In order to compare our findings with prior work, we examined prior fracture as a main effect, stratifying by T2D status.(27)
We performed two sensitivity analyses. To investigate the role of duration of T2D, we considered a 4-level ordinal variable categorized by duration of T2D as greater than 10 years, 6 to 10 years, less than 5 years, and 0 years (no T2D), and we performed tests for trend in the association with each bone measure. These cut-points were selected to assure adequate numbers of individuals in each group for analysis. We repeated all the analyses excluding T2D taking thiazolidinediones (n=18). Results remained the same, so results are based on the full sample. We used p<0.05 as the level of statistical significance. Analyses were performed using PC-SAS (version 9.4; SAS Institute, Cary, NC).
Results
Baseline characteristics
At baseline for the current study, mean age of participants was 64 (±8) years and ranged from 40 to 87 years. Mean duration of follow-up time from the baseline visit to the follow-up visit with assessment of bone outcomes was 7.4 (±0.8) years. Mean BMI was 28 kg/m2, and 31% were obese (Table 1). Eight percent were current smokers. One-third of individuals reported a prior fracture. Twelve percent of participants (n=129) were diagnosed with T2D, including 54 women and 75 men. Among those with T2D, 48% (n=62) did not use any T2D medications, 33% (n=43) used biguanides, 29% (n=37) used sulfonylureas, 14% (n=18) used thiazolidinediones, and 6% (n=8) used insulin. Forty percent (n=51) of T2D were diagnosed in the past 5 years, 36% (n=46) in the past 6 to 10 years, and 24% (n=32) in the past 10 or more years (including 12% (n=16) in past 11 to 15 years, 6% (n=8) in past 16 to 19 years, 5% (n=7) in past 20 to 25 years, and 1% (n=1) diagnosed 34 years ago). Individuals with T2D were older, heavier, taller, and less physically active than non-T2D. Participants with T2D were less likely to have used bisphosphonates and calcium/vitamin D supplements compared to non-T2D. In contrast, menopause status and estrogen use in women and frequency of prior fracture and smoking did not differ by T2D status.
Table 1.
Baseline characteristics in total sample and according to diabetes status, Framingham Offspring Cohort, 2005–2008*
| Total | Diabetes (T2D) | No Diabetes (Non-T2D) | p** | |
|---|---|---|---|---|
| N=1,069 | N=129 | N=940 | ||
| Mean ± SD or Percent (N) | ||||
| Age, yr | 64 ± 8 | 66 ± 7 | 64 ± 8 | <0.01 |
| Women | 56% (606) | 42% (54) | 59% (552) | <0.01 |
| Height, in | 66 ± 4 | 67 ± 4 | 66 ± 4 | <0.01 |
| Weight, lbs | 174 ± 38 | 200 ± 41 | 172 ± 36 | <0.01 |
| Body mass index, kg/m2 | 28 ± 5 | 32 ± 6 | 28 ± 5 | <0.01 |
| Obese (BMI>30 kg/m2) | 31% (331) | 56% (72) | 28% (259) | <0.01 |
| Alcohol consumption, oz/wk | 2.3 ± 3.9 | 1.2 ± 2.0 | 2.5 ± 4.0 | <0.01 |
| Physical activity index (range, 26–66) | 36 ± 5 | 34 ± 5 | 36 ± 5 | <0.01 |
| Current smoking | 8% (87) | 10% (13) | 8% (74) | 0.40 |
| Post-menopausal, women | 96% (581) | 100% (54) | 95% (527) | 0.11 |
| Natural menopause, women | 73% (446) | 72% (39) | 74% (407) | 0.98 |
| Current estrogen use, women | 10% (60) | 6% (3) | 10% (57) | 0.26 |
| Prior fracture | 33% (349) | 33% (42) | 33% (307) | 0.96 |
| Current bisphosphonate use | 13% (137) | 4% (5) | 14% (132) | <0.01 |
| Current calcium/vitamin D supplement use | 24% (259) | 11% (14) | 26% (245) | <0.01 |
| Fasting plasma glucose, mg/100ml | 105 ± 19 | 141 ± 32 | 99 ± 9 | <0.01 |
| Hemoglobin A1C | 5.7 ± 0.5 | 6.6 ± 0.9 | 5.5 ± 0.2 | <0.01 |
| Serum creatinine, mg/dL | 0.89 ± 0.21 | 0.96 ± 0.28 | 0.88 ± 0.20 | <0.01 |
| Diabetes medication*** | -- | -- | -- | -- |
| None | 48% (62) | |||
| Biguanides | -- | 33% (43) | -- | -- |
| Sulfonylureas | -- | 29% (37) | -- | -- |
| Thiazolidinediones | -- | 14% (18) | -- | -- |
| Insulin | -- | 6% (8) | -- | -- |
| Diabetes duration, years | -- | 7.3 ± 7.2 | -- | -- |
| ≤ 5 | -- | 40% (51) | -- | -- |
| 6–10 | -- | 36% (46) | -- | -- |
| > 10 | -- | 24% (32) | -- | -- |
Unadjusted values are presented.
p-value for difference between diabetes and non-diabetes
Individual medications are not mutually exclusive.
Comparison of HR-pQCT parameters by T2D status
Comparison of adjusted means for bone measures between T2D and non-T2D are shown in original units in Table 2 and SD units in Figure 1. Individuals with T2D had lower cortical vBMD (p<0.01) and higher cortical porosity at the tibia (p=0.02), but not the radius (Ct.BMD, p=0.69; Ct.Po, p=0.22), than non-T2D (Table 2 and Figure 1). Cortical thickness, cortical area fraction, and cortical tissue mineral density at the tibia and radius did not differ by T2D status. Trabecular indices (Tb.BMD, Tb.N, Tb.Th, Tb.Sp, and Tb.Sp.SD) and total BMD (Tt.BMD) at the tibia and radius did not differ by T2D status or were somewhat more favorable in T2D than non-T2D (for example, tibia Tb.BMD: 181.17 vs. 174.73 g/cm3; p=0.06). T2D had smaller cross-sectional area (Tt.CSA) at the tibia (759.30 vs. 777.85 mm2, p=0.04), but the difference in cross-sectional area was only borderline significant at the radius (298.58 vs. 307.10 mm2, p=0.06). T2D tended to have lower FEA-estimated failure load at the tibia (6288 vs. 6452, p=0.07) and radius (2451 vs. 2526, p=0.10) than non-T2D, however, the differences were not statistically significant.
Table 2.
Comparison of HR-pQCT indices and DXA bone mineral density, adjusted for age, sex, weight, and height according to diabetes status
| Diabetes (T2D) | No Diabetes (Non-T2D) | ||||
|---|---|---|---|---|---|
| N=129 | N=940 | ||||
| Mean | SE | Mean | SE | p | |
| HR-pQCT Measures, Tibia | |||||
| Cortical BMD (mg/cm3) | 794.78 | 6.20 | 812.42 | 2.23 | <0.01 |
| Cortical porosity (%) | 10.70 | 0.27 | 9.98 | 0.10 | 0.02 |
| Cortical thickness (mm) | 1.1685 | 0.0214 | 1.1903 | 0.0077 | 0.34 |
| Cortical area fraction (%) | 16.66 | 0.35 | 16.54 | 0.12 | 0.73 |
| Cortical tissue mineral density (mg/cm3) | 973.20 | 3.98 | 979.85 | 1.43 | 0.11 |
| Trabecular BMD (mg/cm3) | 181.17 | 3.29 | 174.73 | 1.18 | 0.06 |
| Trabecular number (1/mm) | 2.12 | 0.02 | 2.07 | 0.01 | 0.09 |
| Trabecular thickness (mm) | 0.0716 | 0.0011 | 0.0703 | 0.0004 | 0.29 |
| Trabecular separation (mm) | 0.4184 | 0.0087 | 0.4315 | 0.0031 | 0.16 |
| SD trabecular separation (mm) | 0.1908 | 0.0090 | 0.2006 | 0.0032 | 0.31 |
| Total BMD (mg/cm3) | 286.88 | 4.65 | 283.59 | 1.68 | 0.50 |
| Cross-sectional area (mm2) | 759.30 | 8.74 | 777.85 | 3.15 | 0.04 |
| FEA failure load (N) | 6288.1 | 87.3 | 6452.5 | 31.2 | 0.07 |
| HR-pQCT Measures, Radius | |||||
| Cortical BMD (mg/cm3) | 842.57 | 6.60 | 845.38 | 2.38 | 0.69 |
| Cortical porosity (%) | 4.12 | 0.15 | 3.91 | 0.05 | 0.22 |
| Cortical thickness (mm) | 0.8679 | 0.0177 | 0.8726 | 0.0063 | 0.80 |
| Cortical area fraction (%) | 20.63 | 0.48 | 20.51 | 00.17 | 0.81 |
| Cortical tissue mineral density (mg/cm3) | 1016.29 | 4.01 | 1016.95 | 1.44 | 0.87 |
| Trabecular BMD (mg/cm3) | 163.59 | 3.55 | 163.53 | 1.28 | 0.98 |
| Trabecular number (1/mm) | 2.04 | 0.03 | 2.05 | 0.01 | 0.80 |
| Trabecular thickness (mm) | 0.0668 | 0.0011 | 0.0663 | 0.0003 | 0.69 |
| Trabecular separation (mm) | 0.4536 | 0.0151 | 0.4460 | 0.0054 | 0.63 |
| SD trabecular separation (mm) | 0.2165 | 0.0163 | 0.2131 | 0.0058 | 0.84 |
| Total BMD (mg/cm3) | 311.59 | 5.91 | 311.38 | 2.13 | 0.97 |
| Cross-sectional area (mm2) | 298.58 | 4.36 | 307.10 | 1.57 | 0.06 |
| FEA failure load (N) | 2451.4 | 43.05 | 2526.6 | 15.3 | 0.10 |
| DXA, Femoral Neck | |||||
| Areal BMD, mg/cm2 | 0.8918 | 0.0111 | 0.9070 | 0.0040 | 0.20 |
Figure 1.
Comparison of HR-pQCT parameters by T2D status, stratified by history of fracture
The association between T2D and tibial cortical vBMD, radius cortical porosity, and radius cortical thickness differed among persons with a prior fracture and persons who did not have a history of fracture; interaction, p<0.05 (Figure 2). Specifically cortical vBMD at the tibia and cortical thickness at the radius were lower in T2D than non-T2D, but only among those with a history of fracture. Cortical porosity at the radius was higher in T2D, but only among those who did not have a prior fracture.
Figure 2.
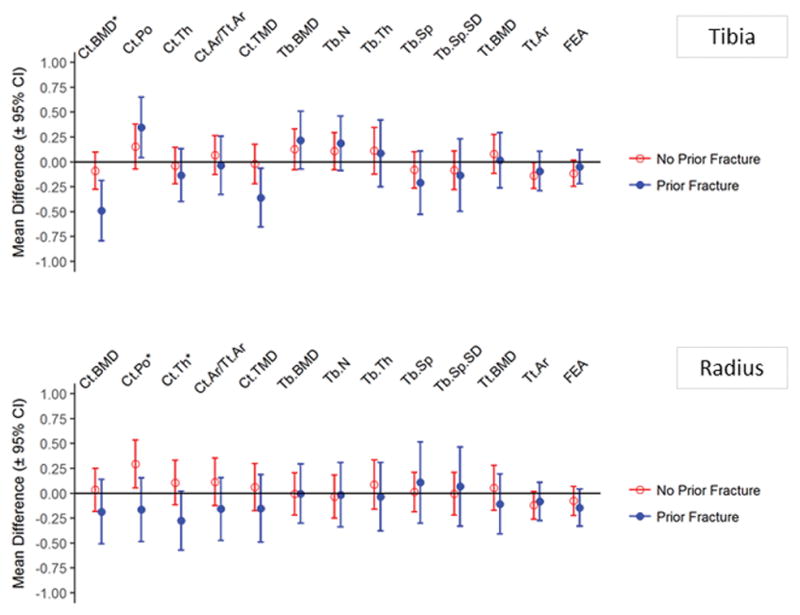
Comparison of HR-pQCT parameters by history of fracture, stratified by T2D status
To compare our findings with prior work, we compared bone measures between individuals with prior fracture and those with no history of fracture, according to T2D status (Supplementary Table 1). Among T2D, prior fracture was associated with worse cortical indices (lower tibia cortical vBMD, lower tibia cortical tissue mineral density, lower radius cortical thickness), except radius cortical porosity which was lower in those with a history of fracture. Among T2D, prior fracture was not associated with trabecular indices at the tibia or radius.
In contrast, among non-T2D, prior fracture was associated with worse trabecular indices at both the tibia and radius (lower trabecular vBMD, lower trabecular number, higher trabecular separation, higher trabecular separation SD; trabecular thickness at radius), and with lower cortical vBMD and cortical tissue mineral density at the tibia (Supplementary Table 1). Prior fracture was associated with lower FEA failure load at the radius in T2D, and prior fracture was associated with lower FEA failure load at both the radius and tibia in non-T2D. Notably, the sample sizes for these comparisons were smaller for the group of individuals with T2D (42 with prior fracture versus 87 with no history of fracture) than the group of non-T2D (307 with prior fracture versus 633 with no history of fracture).
Comparison of HR-pQCT parameters by T2D status, stratified by obesity and sex
The associations between T2D and tibia and radius bone measures did not differ by obesity status (Supplementary Figure 1) or sex (Supplementary Figure 2), all tests for interaction, p>0.05. Finally, we found evidence for a trend between increasing duration of T2D (non-T2D, T2D ≤5 years, T2D 6–10 years, T2D >10 years) and decreasing tibia cortical density (812.45, 778.33, 810.83, 791.68, respectively; trend p=0.05), increasing tibial cortical porosity (9.98%, 10.20%, 11.04%, 10.88%; trend p<0.01), and increasing tibial trabecular number (2.07, 2.04, 2.17, 2.17; trend p=0.02). We observed no trends between duration of T2D and any other tibial or radius parameters.
Discussion
In this large, community-based study of older adults, we found that individuals with T2D had modest deficits in cortical bone density and microarchitecture at the tibia, as well as decreased cross-sectional area at the tibia, compared to those who did not have T2D. In contrast, trabecular indices did not differ by T2D status. These findings suggest that modest deterioration in cortical bone and lower cross-sectional area may characterize diabetic bone disease at the weight bearing skeleton in older adults.
Our results are consistent with previous studies that reported lower cortical bone density and higher cortical porosity in post-menopausal women with T2D,(26,28) and in women with T2D and prior fracture.(27) However, our study extends these previous findings by including a large, community-based population of women and men. The case-control studies conducted by Burghardt,(26) Patsch,(27) and co-workers included small, clinically-based samples of postmenopausal women. Findings from the Hertfordshire Cohort(29) and the SWAN (Study of Women’s Health Across the Nation) Cohort(28) are consistent with our study. Notably, the number of subjects with T2D was also small in these cohort studies: 11 women and 18 men in the Hertfordshire Cohort(29) and 22 women in SWAN.(28)
In contrast to prior work,(27) we found that decreased cortical density at the tibia and increased cortical porosity at the tibia differentiated between T2D with prior fracture and non-T2D with prior fracture. Further, there was a suggestion of a trend between decreasing cortical density and increasing cortical porosity at the tibia with increasing duration of T2D. However, T2D duration was not associated with deficits in any other bone parameters. The Gothenburg Study(32) reported no differences in cortical indices by T2D status in a cross-sectional study of women in a population-based cohort (n=99 T2D). Differences in results between our studies may be attributable in part to differences in characteristics of participants or to differences in study designs. Our prospective study included both women and men who ranged in age from 40 to 87 (mean 64 years), whereas the cross-sectional Gothenburg Study included only women who were 75 to 80 years old (mean 77 years). In addition, 25% of the women with T2D were newly diagnosed at the time of HR-pQCT assessments in the Gothenburg Study. The relatively short duration of disease may have obscured potential differences in bone measures between T2D and non-T2D. The Gothenburg Study used a more proximal measuring site, which may be more reliable than the more distal site used in the present study. The risk of misplacing the endosteal contour is greater more distally, especially in older individuals who may have thin cortices.
The reduction in cross-sectional area observed in older adults with T2D in our study has not been previously reported in studies using HR-pQCT. In the MrOS study, Petit et al(45) found men with T2D had smaller bone area at the distal tibia and radius, evaluated by pQCT, compared to men without T2D. Reasons for detrimental impact of T2D on bone cross-sectional area are not readily apparent. Decreased cross-sectional area has been observed with hypogonadism in men with T2D and with insulin resistance in non-diabetic women.(46) Smaller cross-sectional area could indicate failure in adaptive responses to increased demands from mechanical loading or metabolic or other disturbances. Nevertheless, deficits in bone area may contribute to high fracture risk in T2D, despite normal or high levels of BMD.
We observed deficits in cortical bone and reduced cross-sectional area predominately in the tibia; however, results were similar (but not statistically significant) for the radius. The stronger association between T2D and skeletal deficits observed at the tibia may be due to a greater tendency for slight movement in radius versus tibia scans, which may make it more difficult to detect subtle differences in bone microarchitecture at the radius site. Alternatively, the site-specificity may implicate a role for vascular disease, causing greater damage to peripheral blood flow in the distal lower extremities. Both animal and human studies have documented the presence of skeletal microangiopathy and vascular calcification in the setting of T2D,(47–49) and there is evidence that vascular dysfunction may have adverse effects on skeletal health. (50–54)
Results from the current investigation, together with findings from prior studies, provide compelling evidence that T2D is characterized by moderately increased cortical porosity and decreased cross-sectional area. The critical question this study cannot address is whether these deficits explain the increased fracture risk observed in older adults with T2D. The differences in cortical bone density and microarchitecture observed in our study are likely not large enough to fully explain the increased risk of fracture observed in T2D. For example, multivariable-adjusted mean values for tibial cortical porosity were 11% in T2D versus 10% in non-T2D, representing a difference of 0.22 SD units. Instead, cortical deficits shown in the present study as well as others may be a biomarker for other mechanisms responsible for skeletal fragility in T2D, such as accumulation of advanced glycation end products (AGEs) or microvascular dysfunction, which we were not able to consider in this study. Alternatively, it is also possible that the differences in cortical porosity between T2D and non-T2D were underestimated in our study, since standard HR-pQCT analyses cannot detect cortical pores less than 100 microns in diameter. However, the impact of small pores on whole bone strength remains to be elucidated.(55,56)
One potential limitation of our study protocol was the acquisition of the HR-pQCT scans at a fixed distance from the articular joint surface in all subjects, rather than at location that is relative to the individual’s limb length. Use of a fixed scan location has been the standard in the field, but may obscure differences in bone morphology when comparing individuals with differing limb lengths, even after statistical adjustment for height and weight.(57,58) In our study, individuals with T2D were slightly taller than those without T2D, which could have led to their scan region being relatively more distal than those without T2D. The cross-sectional area is one of the outcomes that is most influenced by the scan region,(57) with CSA increasing distally. Thus, potential bias introduced by using a fixed scan region does not explain the lower CSA seen in T2D.
A second limitation of our study is that a single HR-pQCT scan does not allow characterization of changes over time in bone microarchitecture in persons with T2D. Accordingly, the maintenance of trabecular bone in T2D could be due to preferential trabecularization of the endocortical cortex in T2D vs non-T2D. However, this is unlikely given the similar cortical thickness and cortical area fraction in T2D and non-T2D. Nonetheless, longitudinal evaluation of bone microstructure is required to describe the evolution of bone microarchitecture changes in T2D.
As an observational study, this investigation was not able to definitively determine that T2D causes cortical deficits and smaller bone area, while preserving trabecular bone. However, our study provides important evidence to support this hypothesis. A notable strength of our study was that we included a large, community-based population of women and men from a non-clinical population. However, participants are predominantly Caucasian, as the Framingham Study is a population-based cohort established in 1948 in Framingham, Massachusetts.(35) Thus, our findings may not be fully generalizable to populations with different race, ethnicity, and socio-economic characteristics. Importantly, the paradox of high fracture risk with high aBMD in T2D is observed in African Americans and other race/ethnic groups.(59) In addition, T2D was associated with deficits in cortical (but not trabecular) bone in a study of African Americans,(28) results consistent with our findings. While our sample size of 129 participants with T2D is modest, with few exceptions,(32) prior studies included 20 to 30 individuals per comparison group. Nevertheless, our study may have had limited power to detect associations, particularly in stratified analysis.
The Framingham Study is a well-characterized cohort, and we were able to control for important confounders. However, as in all observational studies, there is potential for residual confounding. Finally, we defined T2D status based on fasting glucose levels and were able to confirm exclusion of T1D. It is possible that cohort members may have developed T2D over the follow-up time. However, this misclassification of T2D status would have resulted in an underestimation of associations between T2D and HR-pQCT indices. We were also able to determine that our findings were not due to use of T2D medications associated with bone loss, as excluding individuals taking thiazolidinediones did not change results.
In conclusion, T2D may be deleterious to cortical bone density, cortical microarchitecture, and bone area, but not trabecular bone. Prospective studies are highly needed to determine if these deficits, undetectable by DXA, predict fracture risk in older adults with T2D. If so, this would support the development of interventions that target the maintenance of cortical bone and bone area for fracture prevention in patients with T2D. Given the exponential growth projected for both T2D and osteoporosis, diabetic bone disease will increasingly become a major clinical and public health problem. Development of preventive strategies, including effective imaging modalities for screening, is urgently needed since current clinical guidelines do not adequately identify T2D patients at risk of fracture.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR061445 and the National Heart, Lung and Blood Institute’s Framingham Heart Study under Contract No.N01-HC-25195, HHSN268201500001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by Friends of Hebrew SeniorLife and a research grant from the Investigator Initiated Studies Program of Merck Sharp & Dohme. The opinions expressed in this work are those of the authors and do not necessarily represent those of Merck.
Authors’ roles: Study design: EJS, LAC, DPK, and MLB. Study conduct: EJS, XZ, KEB, DPK, SKB, MLB. Data collection: KEB, DPK, MLB. Data analysis: EJS, SD, LAC, and XZ. Data interpretation: All authors. Drafting manuscript: EJS and MLB. Revising manuscript content: SD, LAC, C-TL, SKB, RRM, KEB, DPK, and MLB. Approving final version of manuscript: All authors. EJS takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
All authors state that they have no conflicts of interest. DPK received grant support from Merck Sharp & Dohme in the form of an investigator initiated research grant but retained full independence in use and reporting of data generated from this funding.
References
- 1.Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671–85. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed LA, Joakimsen RM, Berntsen GK, Fonnebo V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporos Int. 2006;17(4):495–500. doi: 10.1007/s00198-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 3.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404–10. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 4.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes Care. 2006;29(7):1573–8. doi: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23(8):1334–42. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165(14):1612–7. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292–9. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 8.de Liefde I, van der Klift M, de Laet CE, et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005 doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 9.Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag Health Survey. Diabetologia. 1999;42(8):920–5. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 10.Jones KB, Maiers-Yelden KA, Marsh JL, et al. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87(4):489–95. doi: 10.1302/0301-620X.87B4.15724. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AV, Sellmeyer DE. Women, type 2 diabetes, and fracture risk. Curr Diab Rep. 2004;4(5):364–9. doi: 10.1007/s11892-004-0039-z. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–8. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 14.Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Survival experience of aged hip fracture patients. Am J Public Health. 1989;79(3):274–8. doi: 10.2105/ajph.79.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJd. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137(9):1001–5. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Lu YW, Lan L, et al. Impact of diabetes on the prognosis of hip fracture: a cohort study in the Chinese population. Chin Med J (Engl) 2013;126(5):813–8. [PubMed] [Google Scholar]
- 17.Pan HH, Li CY, Chen PC, et al. Contributions of diabetic macro-vascular complications and hip fracture to depression onset in elderly patients with diabetes: an 8-year population-based follow-up study. J Psychosom Res. 2012;73(3):180–4. doi: 10.1016/j.jpsychores.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Mizrahi EH, Fleissig Y, Arad M, Adunsky A. Functional outcome of elderly hip fracture patients: does diabetes matter? Arch Gerontol Geriatr. 2006;43(2):165–73. doi: 10.1016/j.archger.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Reistetter TA, Graham JE, Deutsch A, et al. Diabetes comorbidity and age influence rehabilitation outcomes after hip fracture. Diabetes Care. 2011;34(6):1375–7. doi: 10.2337/dc10-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey A, Aharonoff GB, Zuckerman JD, Koval KJ. The effects of diabetes on outcome after hip fracture. Bull Hosp Jt Dis. 2000;59(2):94–8. [PubMed] [Google Scholar]
- 21.Huang YF, Shyu YI, Liang J, et al. Diabetes and health outcomes among older Taiwanese with hip fracture. Rejuvenation Res. 2012;15(5):476–82. doi: 10.1089/rej.2011.1308. [DOI] [PubMed] [Google Scholar]
- 22.Ekstrom W, Al-Ani AN, Saaf M, et al. Health related quality of life, reoperation rate and function in patients with diabetes mellitus and hip fracture--a 2 year follow-up study. Injury. 2013;44(6):769–75. doi: 10.1016/j.injury.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Boyle JP, Honeycutt AA, Narayan KMV, et al. Projection of Diabetes Burden Through 2050: Impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24(11):1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compston JE, Flahive J, Hosmer DW, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW) J Bone Miner Res. 2014;29(2):487–93. doi: 10.1002/jbmr.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–55. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–24. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu EW, Putman MS, Derrico N, et al. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26(2):673–9. doi: 10.1007/s00198-014-2927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paccou J, Ward KA, Jameson KA, et al. Bone Microarchitecture in Men and Women with Diabetes: The Importance of Cortical Porosity. Calcif Tissue Int. 2015 doi: 10.1007/s00223-015-0100-8. [DOI] [PubMed] [Google Scholar]
- 30.Shu A, Yin MT, Stein E, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23(2):635–41. doi: 10.1007/s00198-011-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr JN, Drake MT, Amin S, et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–95. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson AG, Sundh D, Johansson L, et al. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res. 2016 doi: 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- 33.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4(4):518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 34.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 35.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Pub Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 37.Rozental TD, Deschamps LN, Taylor A, et al. Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am. 2013;95(7):633–42. doi: 10.2106/JBJS.L.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50(1):111–8. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sode M, Burghardt AJ, Kazakia GJ, Link TM, Majumdar S. Regional variations of gender-specific and age-related differences in trabecular bone structure of the distal radius and tibia. Bone. 2010;46(6):1652–60. doi: 10.1016/j.bone.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int. 2013;24(5):1733–40. doi: 10.1007/s00198-012-2160-1. [DOI] [PubMed] [Google Scholar]
- 43.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;112(4):820–5. doi: 10.1016/0002-8703(86)90480-1. [DOI] [PubMed] [Google Scholar]
- 44.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 45.Petit MA, Paudel ML, Taylor BC, et al. Bone mass and strength in older men with type 2 diabetes: the Osteoporotic Fractures in Men Study. J Bone Miner Res. 2010;25(2):285–91. doi: 10.1359/jbmr.090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association Between Insulin Resistance and Bone Structure in Nondiabetic Postmenopausal Women. J Clin Endocrinol Metab. 2016;101(8):3114–22. doi: 10.1210/jc.2016-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oikawa H, Hayashi K, Maesawa C, Masuda T, Sobue K. Expression profiles of nestin in vascular smooth muscle cells in vivo and in vitro. Exp Cell Res. 2010;316(6):940–50. doi: 10.1016/j.yexcr.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Spinetti G, Cordella D, Fortunato O, et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA-155/FOXO3a signaling pathway. Circ Res. 2013;112(3):510–22. doi: 10.1161/CIRCRESAHA.112.300598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadini GP. Is bone marrow another target of diabetic complications? Eur J Clin Invest. 2011;41(4):457–63. doi: 10.1111/j.1365-2362.2010.02417.x. [DOI] [PubMed] [Google Scholar]
- 50.Shanbhogue VV, Hansen S, Frost M, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016;174(2):115–24. doi: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- 51.Kiel DP, Kauppila L, Wilson PWF, Cupples LA. Bone loss and the progression of lumbar aortic calcification. Journal of Bone and Mineral Research. 1997;12:174–174. [Google Scholar]
- 52.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. Journal of Bone and Mineral Research. 2008;23(1):95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 53.Chan JJ, Cupples LA, Kiel DP, et al. QCT Volumetric Bone Mineral Density and Vascular and Valvular Calcification: The Framingham Study. J Bone Miner Res. 2015;30(10):1767–74. doi: 10.1002/jbmr.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19(8):1161–6. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 55.Bala Y, Zebaze R, Seeman E. Role of cortical bone in bone fragility. Curr Opin Rheumatol. 2015;27(4):406–13. doi: 10.1097/BOR.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 56.Jorgenson BL, Buie HR, McErlain DD, Sandino C, Boyd SK. A comparison of methods for in vivo assessment of cortical porosity in the human appendicular skeleton. Bone. 2015;73:167–75. doi: 10.1016/j.bone.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 57.Boyd SK. Site-specific variation of bone micro-architecture in the distal radius and tibia. J Clin Densitom. 2008;11(3):424–30. doi: 10.1016/j.jocd.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Shanbhogue VV, Hansen S, Halekoh U, Brixen K. Use of Relative vs Fixed Offset Distance to Define Region of Interest at the Distal Radius and Tibia in High-Resolution Peripheral Quantitative Computed Tomography. J Clin Densitom. 2015;18(2):217–25. doi: 10.1016/j.jocd.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone. 2016;82:9–15. doi: 10.1016/j.bone.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



