Abstract
Objective
Research on sources of variation in adolescent’s gonadal hormone levels is limited. We sought to decompose individual differences in adolescent testosterone, estradiol and pubertal status, into genetic and environmental components.
Design
A sample of male and female adolescent twins from the greater Austin and Houston areas provided salivary samples, with a subset of participants providing longitudinal data at two waves.
Participants
The sample included 902 adolescent twins, 49% female, ages 13–20 years (M = 15.91) from the Texas Twin Project. Thirty-seven percent (37%) of twin pairs were monozygotic; 30% were same-sex dizygotic pairs; and 33% were opposite-sex dizygotic pairs.
Measurements
Saliva samples were assayed for testosterone and estradiol using chemiluminscense-immunoassays. Pubertal status was assessed using self-report. Biometric decompositions were performed using multivariate quantitative genetic models.
Results
Genetic factors contributed substantially to variation in testosterone in males and females in the follicular phase of their menstrual cycle (h2 = 60% and 51%, respectively). Estradiol was also genetically influenced in both sexes, but was predominately influenced by non-shared environmental factors. The correlation between testosterone and estradiol was mediated by a combination of genetic and environmental influences for males and females. Genetic and environmental influences on hormonal concentrations were only weakly correlated with self-reported pubertal status, particularly for females.
Conclusions
Between-person variability in adolescent gonadal hormones and their inter-relationship reflects both genetic and environmental processes, with both testosterone and estradiol containing sizeable heritable components.
Keywords: testosterone, estradiol, heritability, adolescence, twin models
Activation of the hypothalamic-pituitary-gonadal axis (HPG) during puberty results in the increased biosynthesis of androgens and estrogens; however, research is lacking on the sources of between-person variation in hormone concentrations. In particular, the extent to which individual differences in hormones reflect genetic differences between people, or are rather a biomarker of variation in environmental experience, is unclear. Supporting the role of genetic variation, genome-wide association studies have identified single-nucleotide polymorphisms in the sex hormone-binding globulin (SHBG) locus that affect circulating testosterone levels in adults.1,2 At the same time, environmental influences on hormonal levels may also be sizable, as observational and experimental research has established that hormones respond to psychological3 and physiological stress.4
Previous Quantitative Genetic Research
Quantitative genetic designs use genetic similarities between relatives, most commonly twins, to estimate the extent to which variation in phenotypes is explained by environmental and genetic factors. Although genes relevant to hormone biosynthesis may give rise to an average hormone level across participants, quantitative genetic studies only examine variation about the average. That is, the mean level of testosterone could be due to a constrained genetic architecture, with differences about this average entirely a reflection of environmental input. Prior twin studies suggest the heritability of testosterone levels varies across the lifespan (reviewed in Table 1). Three out of five studies conducted with adolescent samples indicated higher heritability of testosterone in males than in females, although no sex differences and larger heritability in females than in males have also been reported. However, estimates vary widely. A number of factors—hormonal phenotype, time of collection, included covariates, age range, and sample size—may have contributed to the heterogeneity in prior results.
Table 1.
Overview of prior studies examining heritability of testosterone or estradiol
| Testosterone | Estradiol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Citation | Sample Type | Samples | Time of Collection | Sample Size | Covariates | Age Range (years) | male h2 | female h2 | male h2 | female h2 |
|
|
|
|
||||||||
| Bogaert et al. (2008)21 | serum | 1 | Before 1000 | 274 male sibling pairs | BMI; age | 30–39 | 65%; 54% | - | 44%; 52% | - |
| Caramaschi et al. (2012)22 | saliva | 1 | 0805–1215 | 301 twin pairs | none | 4–8 months | 0% | 0% | - | - |
| Harden et al. (2014)5 | saliva | 1 | 0900–1500 | 117 twin pairs | age, age2 | 14–19 | 55% | 12% | - | - |
| Harris et al. (1998)23 | plasma | 1 | 0830–1030 | 160 twin pairs | none | 14–21 | 66% | 41% | - | - |
| Hoekstra et al. (2006)7 | saliva | 2 | Before lunch | 183 twin pairs | none | 12 | 52% | 52% | - | - |
| Hong et al. (2001)24 | plasma | 2 days | Morning | 200 families; 794 individuals | age, age2, age3 | 17–40 | 91% | 65% | - | - |
| Koenis et al. (2013)6 | saliva (T)urine (E) | 2 days | After waking | Wave 1: 202 twin pairs | none | Wave 1: 9 | 64% | 70% | 72% | 2% |
| Wave 2: 163 twin pairs | none | Wave 2: 12 | 78% | 51% | 45% | 54% | ||||
| Kuijper et al. (2007)25 | plasma | 1 | Before 1100 | 94 twins and 34 brothers | none | 15–68 | 56% | - | - | - |
| Meikle et al. (1986)26 | plasma | 1 | 0800–0930 | 160 male twin pairs | none | 20–60 | 26% | - | 76% | - |
| Ring et al. (2005)27 | plasma | 1 | 0800–1000 | 266 male twin pairs | BMI; age | 59–70 | 57% | - | 25% | - |
| Sakai et al. (1991)28 | serum | 1 | At birth | 116 twin pairs | none | at birth | 0% | 0% | 68% | 68% |
| Travison et al. (2014)29 | serum | 1 | Morning | 1066 father/son, 1284 male sibling pairs | age | Mean = 49 | 40% | - | 30% | - |
| Van Hulle et al. (2015)30 | saliva | 2 days | Waking + 30 | 332 twin pairs | time since waking | 12–16 | 13% | 63% | - | - |
Note. Heritability estimates in bolded italics indicate estimates of heritability for free (as opposed to total) hormone levels in plasma.
Only two studies to date have estimated the magnitude of genetic influence of estradiol in females, and these studies focused exclusively on pre-menarcheal girls. The absence of twin research in a post-menarcheal sample is likely due to methodological challenges in controlling for variation in menstrual cycle phase in large samples. Our study will present the first quantitative genetic decomposition of variability in estradiol in post-menarcheal females. We begin to address the role of menstrual cycle variation by comparing biometric estimates between all females and a restricted sub-sample of non-contracepting females in the follicular phase of their menstrual cycle.
Twin designs are also able to estimate the magnitude of genetic and environmental contributions to the correlation between testosterone and estradiol, which is expected to be high given overlapping biosynthetic pathways (Figure S1), and between pubertal status and gonadal hormones. Prior findings suggest that the phenotypic correlation between pubertal development and testosterone is moderate, with values reported between ~.2 and ~.5 for both sexes.5–8 A slightly larger association (~.4 to ~.7) has been described for estradiol and female pubertal development.6,8 Twin studies indicate that the association between pubertal status and gonadal hormones reflects the influence of overlapping genetic causes.5–7 In addition to understanding the underlying genetic component of phenotypic correlations, examining pubertal status and hormones together clarifies whether important genetic or environmental components of the pubertal process are missed when using only readily observable secondary sex characteristics.
Goals of Present Study
In the present study, we estimate sex-specific genetic and environmental contributions to individual differences in testosterone and estradiol. In line with prior findings, we hypothesized there would be a stronger genetic influence on testosterone in males, and near equivalent estimates of heritability for estradiol. The correlation between pubertal status and gonadal hormones was also parsed into genetic and environmental components. We predicted that this association would be genetically mediated in both sexes. After examining associations with age, hormones and pubertal status were residualized for age in order to examine variation in these outcomes relative to same age peers.
Method
Participants
Twins were identified using public school rosters from Austin and Houston area high schools. Five participants were excluded for reported endocrine problems. Participants ranged in age from 13.5 to 20.1 years (M = 15.91, SD = 1.39). The final sample consisted of N = 902 individuals (49% female) from 443 unique families enrolled in the Texas Twin Project.9 Ninety-three of these individuals provided data on two occasions, eleven individuals within a twin pair were missing hormonal observations, and one individual was missing data for the repeat visit only for a total of i = 984 testosterone data points. Of the 984 individuals, 6 were missing pubertal status scores and 17 were missing estradiol due to non-detectable levels. One family had quadruplets and 2 families had repeat triplets who contributed six pairwise contributions, 12 families contained triplets contributing three pairwise contributions, and 44 families were repeat twins contributing two pairwise comparisons1 for a total of 526 twin pairs (194 monozygotic [MZ] pairs [94 male, 100 female] and 332 dizygotic [DZ] pairs [98 male, 74 female, and 160 opposite-sex]). Fifty-seven percent (57%) of participants were non-Hispanic White, 20% were Hispanic/Latino, 13% were African American, and 10% were another race/ethnicity. Of the participating families, 31.5% reported receiving some form of public assistance, including food stamps, since the twins’ birth.
Measures
Zygosity
Opposite-sex twin pairs were classified as DZ. The zygosity of same-sex twin pairs was assessed using responses to a survey concerning the twin’s physical similarities (e.g., facial appearance) and the frequency that they are mistaken for one another. Parents, two research assistants, and each twin completed the survey. Scores on the measure were entered into a latent class analysis (LCA) that was used to obtain the above zygosity classifications. LCA using parent-report for young twins on the same survey has been found to accurately determine zygosity ~93% of the time, as validated by genotyping.10
Hormones
A saliva sample collected via passive-drool was assayed to determine testosterone and estradiol concentrations. Participants were instructed to avoid eating or drinking anything for the 2 hr prior to beginning the experiment, to avoid flossing the morning of the experiment, and to avoid smoking 4 hr prior to coming in. Participants provided salivary samples into a 2-ml vial after completing consent forms. Samples were collected at one of three appointment times: 09:00–10:00h (29% of participants), 12:00–13:00h (51% of participants) or 14:00–15:00h (20% of participants). Immediately following collection, saliva samples were frozen on-site at ≤ − 30°C prior to being shipped on dry ice within 12 months of collection to Dr. Clemens Kirschbaum’s laboratory at the Technical University of Dresden for analyses. Commercially available chemiluminscense-immunoassays (IBL International, Hamburg, Germany) were used to measure testosterone and estradiol concentrations. The lower limit of sensitivity for the assays were 0.3 pg/mL for estradiol and 1.8 pg/mL for testosterone; extremely high values were estimated from standard curves. The intra-assay and inter-assay coefficients of variation were < 8% and < 11%, respectively, for both testosterone and estradiol.
When possible, female adolescents were brought into the lab within the first 14 days of their menstrual cycle (day 0 = first day of menstruation). The average length of the follicular phase (including menses) in adult women is 16.5 days.11 In addition, total cycle lengths are typically longer in adolescents.12 Thus, using the cut-off of 14 days from start of menses, it is reasonable to assume that the majority of adolescent females will be within the follicular phase of their menstrual cycle. Sixty-eight participants were within the menstruating phase (days 0–5), 177 were in the late follicular phase (days 6–14), 135 were in the luteal phase (days 15–35), 23 participants reported an irregular cycle characterized by more than 2 months since last visible bleeding, 24 female participants had not begun menstruation, 10 were unsure of their last day of menstruation, and 45 female participants reported current use of hormonal contraceptives. Analyses were conducted both using and omitting participants outside of the follicular phase, on contraceptives, or not currently menstruating. Removing these participants resulted in a dataset that included 66 MZ female twins [30 full pairs], 49 DZ female twins [19 full pairs], and 102 full opposite-sex DZ pairs.
Pubertal Status
Pubertal status was assessed using the Pubertal Development Scale (PDS).13 All participants rated growth in height, growth of body hair, and skin changes on a 4-point scale (1 = Not Yet Begun to Change, 4 = Finished Changing). In addition to these three items, male participants rated growth of facial hair and deepening of voice on the same 4-point scale. Female participants also rated growth of breasts and whether they had begun to menstruate. The menstruation item was coded to be consistent with the 4-point scale (1 = No, 4 = Yes). Scores were taken as the average across the five items. Internal consistency for the current sample was good for both males (Cronbach’s α = .83) and females (α =.75). The distribution of scores on the PDS by age and sex are depicted in Figure S2.
Results
Table 2 provides descriptive statistics, including the observed range of hormone levels. Testosterone and estradiol were both positively skewed, and hormonal measurements were log-transformed to approximate normal distributions more closely and then standardized. All outcomes were residualized for sex-specific effects of body mass index and race/ethnicity (see Supplement for effects). Hormonal outcomes were additionally residualized for analytic batch, to control for random variation in the assays across years, and time since waking, to control for diurnal variation in hormone levels.2 Finally, outliers were replaced, for males and females separately, using a winsorizing procedure that replaced extreme values by the highest observed scores within 3 standard deviations of the sex-specific sample mean. This involved replacing three female and six male outliers for testosterone, three female and two male outliers for estradiol, and one male and eleven female outliers for pubertal status. All variables were standardized within sex to have a mean of 0 and standard deviation of 1.
Table 2.
Phenotypic correlations between testosterone, estradiol, pubertal status, and age
| Phenotypic Correlations | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male | Female | ||||||
|
|
|
||||||
| Testosterone | Estradiol | Pubertal Status | Testosterone | Estradiol | Pubertal Status | ||
| Testosterone | - | .42 (.33, .52) | .25 (.17, .34) | - | .52 (.44, .60) | .03 (−.07, .13) | |
| Estradiol | .43 (.35, .52) | - | .07 (−.04, .18) | .52 (.44, .60) | - | .07 (−.03, .16) | |
| Pubertal Status | .40 (.31, .49) | .12 (.01, .23) | - | .06 (−.03, .16) | .12 (.02, .21) | - | |
| Age | .36 (.29, .44) | .09 (.−01, .18) | .52 (.46, .58) | .09 (−.03, .20) | .13 (.04, .22) | .42 (.34, .50) | |
| M (SD) | 100.60 (72.12) | 4.37 (4.10) | 2.94 (0.57) | 31.99 (27.34) | 4.45 (3.01) | 3.45 (0.48) | |
| Range | 3.94 – 616.25 | 0.30 – 39.60 | 1.0 – 4.0 | 2.10 – 199.42 | 0.30 – 22.71 | 1.0 – 4.0 | |
|
| |||||||
| Cross-Twin Correlations | |||||||
|
| |||||||
| Monozygotic | Dizygotic | ||||||
|
|
|
||||||
| Male | Female-Full | Female-Restricted | Male | Female-Full | Female-Restricted | Opposite-Sex | |
|
| |||||||
| Within-Trait (Univariate) Twin Correlations | |||||||
|
| |||||||
| Testosterone (T) | .68 (.58, .78) | .56 (.42, .70) | .57 (.35, .80) | .43 (.25, .61) | .52 (.36, .67) | .38 (−.04, .81) | .17 (−.01, .36) |
| Estradiol (E) | .59 (.42, .76) | .30 (.08, .51) | .41 (.14, .68) | .30 (.13, .48) | .23 (.05, .40) | .38 (−.10, .86) | .25 (.06, .45) |
| Pubertal Status (P) | .40 (.23, .58) | .29 (.05, .54) | .61 (.46, .76) | .11 (−.08, .30) | .34 (.13, .55) | .44 (−.23, 1.10) | −.04 (−.19, .12) |
|
| |||||||
| Cross-Trait (Bivariate) Twin Correlations | |||||||
|
| |||||||
| r(T-E) | .21 (.04, .38) | .18 (.01, .35) | .33 (.10, .56) | .18 (.02, .34) | .31 (.15, .47) | .29 (−.24, .82) | .22 (.08, .36) |
| r(T-P) | .25 (.08, .42) | .07 (−.10, .23) | .06 (−.24, .37) | .10 (−.06, .25) | .07 (−.09, .23) | −.26 (−.86, .34) | −.01 (−.16, .14) |
| r(E-P) | −.03 (−.22, .16) | .05 (−.11, .20) | .19 (−.05, .43) | .14 (−.01, .28) | .09 (−.12, .29) | −.35 (−.72, .02) | −.04 (−.17, .09) |
Note. All outcomes were residualized for race and BMI. Hormonal outcomes were also residualized for time since waking and analytic batch. Female-restricted participants were off hormonal contraceptives, and in the follicular phase of their menstrual cycle. The point estimates for opposite-sex twins were equivalent using the female-restricted sample (results not presented). Phenotypic correlations on the upper diagonal, and cross-twin correlations, were calculated for outcomes additionally residualized for age and age2. Means, standard deviations, and ranges reflect untransformed values for interpretive purposes. 95% confidence intervals are given in parentheses, and are corrected for the dependency between within-family observations.
Phenotypic and Cross-Twin Correlations
The correlation between estradiol and testosterone concentrations was moderate for males (.43, 95% CI: .35, .52) and females (.52, 95% CI: .44, .60), and unaffected when controlling for age (Table 2). The correlation between pubertal status and testosterone was moderate for males (.40, 95% CI: .31, .49) and minimal for females (.06, 95% CI: −.03, .16). Controlling for age, the partial correlation between pubertal status and testosterone was reduced for males. The correlation between pubertal status and estradiol was minimal for males and females. All remaining analyses controlled for sex-specific effects of age and age2 (see Supplement and Figure S3 for effects).
Higher MZ than DZ cross-twin, within-trait correlations (e.g., twin 1’s testosterone correlated with twin 2’s testosterone) indicate genetic effects. The pattern of twin correlations indicated that testosterone, estradiol, and pubertal status were all heritable in males. Conversely, MZ and DZ correlations were approximately equal in females for testosterone, estradiol, and pubertal status, indicating that the heritability of these outcomes was negligible for females. Cross-twin correlations were also calculated for non-contracepting, post-menarcheal females in the follicular phase of their menstrual cycle. Twin correlations for the restricted female sample revealed increased heritability in testosterone and a higher MZ correlation for pubertal status relative to the full female sample (Table 2).
Cross-twin, cross-trait associations (e.g., twin 1’s testosterone correlated with twin 2’s estradiol), are also reported in Table 2. Higher MZ than DZ cross-twin, cross-trait, correlations indicate that phenotypic correlations are driven by genetic effects. The pattern of these correlations indicated that the correlation between testosterone and estradiol was largely environmental for males and females, but may additionally be described by a small genetic component for males and the restricted female sample. The cross-trait correlation between pubertal status and testosterone indicated genetic effects for males, while the remaining cross-trait correlations with pubertal status were minimal.
Twin Model Specification
Three-group (MZ, same-sex DZ, opposite-sex DZ) quantitative genetic models were fit to the data to determine variance attributable to additive genetic (A), shared environmental (C) and non-shared environmental factors, including error variance, unique to each twin (E). A bivariate Cholesky decomposition was fit for both sexes in which estradiol was regressed on testosterone ACE components. In addition, we fit a univariate twin model to pubertal status. Two types of sex differences were estimated in these models. In a qualitative sex-differences model, the correlation between the A (rA) factors in each twin pair is freely estimated for opposite-sex DZ pairs, rather than fixed to 0.5. This allows for different sets of genes to influence the outcomes for males and females. Conversely, a quantitative sex-differences model allows the magnitude of each ACE component’s influence to differ across sexes. Quantitative sex-differences are estimated in three-group models by including interactions between each ACE component and the sex of the participant.
Model specification was informed by the pattern of twin correlations (see Supplement for details). The primary results reported here are from models that allowed for quantitative sex differences in all variables and in the cross-paths (e.g., estradiol regressed on ATestosterone), and also allowed qualitative sex differences in testosterone and pubertal status. These models were estimated using both the full and restricted sample of females. In a final model, we estimate a bivariate Cholesky between pubertal status and testosterone. This model was only fit for same-sex male twins as the phenotypic correlation between testosterone and pubertal status was minimal for females. For the same reason, associations between estradiol and pubertal status were not examined for either sex.
Model Results
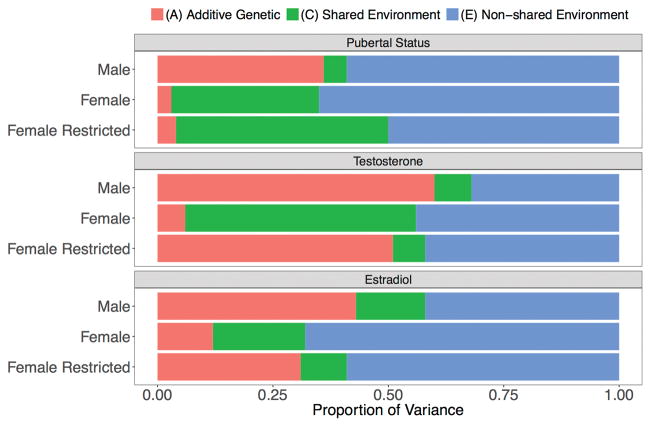
Parameter estimates are summarized in Table 3. To illustrate these results, Figure 1 shows the proportions of total variance in pubertal status, testosterone, and estradiol that are attributable to additive genetic, shared environmental, and non-shared environmental differences. Pubertal status was primarily due to non-shared environment in all groups. The remaining variance in pubertal status was largely heritable in males (h2 = 36%) relative to minimal additive genetic influences for the full (h2 = 3%) and restricted female sample (h2 = 4%). When all females were included in analyses, the heritability of gonadal hormone concentrations was higher in males (testosterone: h2 = 60%; estradiol: h2 = 44%) than in females (testosterone: h2 = 6%; estradiol: h2 = 12%). Female-specific heritability estimates for hormones increased when analyses were restricted to a subset of non-contracepting, menstruating females in the follicular phase (testosterone: h2 = 51%; estradiol: h2 = 31%). The remaining variance in testosterone for the full female sample was largely due to shared environment. This suggests heterogeneity within the full female sample did not decrease heritability due to increased error variance, as this would have resulted in higher non-shared environmental variance components. As the majority of females excluded in the restricted sample were in the luteal phase, this particular phase may be characterized by higher levels of shared environmental input.
Table 3.
Proportion of variance explained in quantitative and qualitative sex differences model
| Males | Females-Full Sample | Females-Restricted | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| h2 | c2 | e2 | h2 | c2 | e2 | h2 | c2 | e2 | |
| Pubertal Status | .36 (.13)** | .05 (.09) | .59 (.09)*** | .03 (.07) | .32 (.09)*** | .65 (.09)*** | .04 (.09) | .46 (.12)*** | .50 (.10)*** |
|
| |||||||||
| Testosterone | .60 (.10)*** | .08 (.07) | .32 (.05)*** | .06 (.10) | .50 (.10)*** | .44 (.06)*** | .51 (.21)* | .07 (.18) | .42 (.11)*** |
| Testosterone → Estradiol | .05 (.05) | .15 (.08) | .08 (.03)* | .09 (.11) | .20 (.09)* | .18 (.05)** | .25 (.13) | < .01 (.05) | .10 (.07) |
| Model Implied Correlations | rA = .32 (.14)* | - | rE = .44 (.07)*** | rA = .87 (.48) | - | rE = .51 (.08)*** | rA = .90 (.30)** | - | rE = .41 (.14)** |
| Estradiol Unique | .39 (.11)*** | .00 (.00) | .34 (.06)*** | .03 (.10) | .00 (.00) | .50 (.07)*** | .06 (.20) | .09 (.29) | .49 (.10)*** |
Note. The model allowed for quantitative sex differences in all outcomes and cross-paths and qualitative differences in pubertal status. Variance due to shared environmental effects in pubertal status was estimated at 0 and was subsequently fixed to 0. Female-restricted participants were off hormonal contraceptives, and in the follicular phase of their menstrual cycle. Standard errors are shown in parentheses. All variables were residualized for sex-specific effects of age, age2, BMI, and race; hormonal outcomes were residualized for time since waking and analytic batch. Model implied correlations are not shown for parameters estimated at < .01.
significantly different than zero at p < .001;
p < .01;
p < .05
Figure 1.
Total proportion of variance in each outcome explained by genetic (A) and environmental (C or E) factors. Estimates for testosterone and estradiol are from a bivariate model that allowed for quantitative sex differences in all paths and qualitative sex differences in testosterone. Pubertal status results are from a univariate model that allowed for quantitative and qualitative sex differences. Female-restricted participants were off hormonal contraceptives, and in the follicular phase of their menstrual cycle.
In addition to estimating the sex-specific heritabilities, the models also capitalized on the inclusion of opposite-sex DZ twins in order to estimate the extent to which the same set of genetic variants influenced testosterone and pubertal status. When including all female participants, genetic influences on testosterone (rA = .15, SE = .28) and pubertal status (rA = .14, SE = 1.09) were minimally correlated between opposite-sex DZ twins. Using the restricted female sample, opposite-sex DZ correlations were estimated above 1 for pubertal status and testosterone and subsequently fixed to 0.5. This is likely due to the large sample size needed to accurately estimate this correlation.
The total variance in estradiol can be further split into components unique of, versus shared with, testosterone. Unstandardized estimates by sex are provided in Figure S3. The largest cross-paths between testosterone and estradiol in the full female sample were shared and non-shared environmental, explaining 20% and 18% of the total variance, respectively. Similarly, the largest cross-path for males was shared environmental (15%). These were both in contrast to a large additive genetic cross-path for the restricted female sample (25%). The bivariate model of pubertal status and testosterone in males (Table 4) indicated that additive genetic and shared environmental predictors of pubertal status explained the largest portion of total variance in testosterone (18% and 19%, respectively). Results based on model comparisons were largely consistent with the results described above (Table S1 and S2).
Table 4.
Bivariate model of pubertal status and testosterone in males only
| h2 | c2 | e2 | |
|---|---|---|---|
| Pubertal Status | .42 (.09)*** | .01 (.03) | .57 (.09)*** |
| Pubertal Status → Testosterone | .18 (.15) | .19 (.16) | .01 (.01) |
| Model Implied Correlations | rA = .61 (.28)* | - | rE = .13 (.11) |
| Testosterone Unique | .29 (.23) | .00 (.00) | .33 (.06)*** |
Note. Standard errors are shown in parentheses. All variables were residualized for sex-specific effects of age, age2, BMI, and race; testosterone was residualized for time since waking and analytic batch. Model implied correlations are not shown for parameters estimated at < .01.
significantly different than zero at p < .001;
p < .01;
p < .05
Discussion
The current paper examined genetic and environmental determinants of testosterone, estradiol, and pubertal status in a diverse sample of adolescent twins. The heritability of all phenotypes was higher in males and in a more homogenous set of female participants than in the full female sample. Although testosterone and estradiol are both associated with a host of socially important adolescent outcomes (e.g., externalizing behaviors16,17), it is unclear whether these associations reflect hormonal mediation of environmental impacts on behavior, genetically-based factors, or some combination of the two. A major advantage of this research is that estimates of hormone heritability can both supplement the interpretation of prior hormone-behavior association findings and guide the selection of future outcomes to associate with hormonal predictors. If a behavioral outcome is determined by high levels of environmental input, but testosterone is largely heritable, the explanatory power of testosterone is likely to be quite small. That is, genetic variance, by definition, is unable to predict environmental variance.
Variability in heritability estimates across female samples that were defined by different exclusion criteria tentatively suggests that, as hormone concentrations change across the menstrual cycle, genetic contributions to hormone levels also vary. Consistent with this idea, previous research has found that genetic influences on hormone-related eating phenotypes (e.g., emotional eating) shift across the menstrual cycle in late adolescent and adult women.18 Research using dense longitudinal measurement of hormones across the menstrual cycle, coupled with biological indicators of menstrual phase,19 is necessary to evaluate this topic further.
Clinicians currently have limited evidence to guide interpretation of adolescent hormone levels. For female hormone levels, potentially varying genetic inputs across the menstrual cycle indicates that single hormone samples obtained for one menstrual phase may only be useful for evaluating the developmental trajectories within that phase. More generally, we find that hormone levels reflect both genetic and environmental variation for both sexes. If these environments oscillate, multiple samples may be necessary to gain a comprehensive understanding of the individual’s hormonal development. Future research should look to identify measurable environments that predict environmental variance in adolescent hormones. This would allow for intervention strategies that seek first to modify these environmental targets for individuals deviating from developmental curves.
A large proportion of variation in estradiol resulted from non-shared environmental influences in males and in both samples of females. This finding might be interpreted as a reflection of measurement error. However, within adult females, significant differences in average estradiol levels have been reported across separate menstrual cycles only a few months apart.20 These large within-person deviations are suggestive of high levels of individual-specific environmental input. It, therefore, appears reasonable to expect differences in estradiol levels even between identical twins who provide samples at the same time. Hormonal discordance due to individual-specific environmental input offers a possible biological explanation for disparate, hormonally-influenced, behaviors across identical twins.
Limitations
Participants provided single hormone measurements that may reflect a combination of transient and stable hormonal variation. In addition, self-reported pubertal development may be unreliable, and future research should look to obtain multiple indicators within the same sample (e.g., physician rated Tanner stage). Participants in the current study were characterized by a relatively wide age range, and results may have aggregated across disparate developmental processes. In addition, females that were towards the later stages of pubertal development may have attenuated phenotypic associations between puberty and estradiol. Although the current study represents the largest genetically informed sample to examine hormone levels in adolescence, the sample size is still small for detecting qualitative sex differences.
Conclusions
This study is the first to examine the heritability of gonadal hormones in menstruating females, and the largest twin sample to date to report quantitative genetic decompositions of estradiol and testosterone variation. Gonadal hormones are increasingly linked to human behavior, but it is currently unclear whether this correlation is driven by genetic or environmental pathways. The present findings, therefore, make an important contribution to the growing literature on determinants of hormone concentrations.
Supplementary Material
Acknowledgments
Funding: This study was supported by Grants 1-R21-AA020588 and 1-R21-AA023322 from the National Institute on Alcohol Abuse and Alcoholism. Drs. Paige Harden and Elliot Tucker-Drob are Faculty Research Associates of the Population Research Center at the University of Texas at Austin, which is supported by a center grant from the National Institute of Child Health and Human Development (5-R24-HD042849).
Footnotes
Twin models in Mplus only allow for a pair of individuals to be entered into the model, which requires triplets be entered as three separate twin pairs and quadruplets as six separate pairs. The complex sampling option was used to correct standard errors for the dependency between quadruplet and triplet pairs, and for repeat participants in the phenotypic models. In addition, the weighting option was used to correct for individual members of triplet and quadruplet groups that were entered into the model more than once.
Time since waking— computed as the minutes between waking that morning and the time of saliva collection—significantly predicted male testosterone (β = −.11, SE = .05, p = .02) but was not significant for female testosterone (β = −.06, SE = .05, p = .20). In addition, time since waking significantly predicted female estradiol (β = −.13, SE = .05, p =.004) but not male estradiol (β = −.03, SE = .05, p =.49). This is in line with findings that adolescent testosterone decreases throughout the day14 and adolescent estradiol decreases for females who recently completed puberty.15
Conflict of interest: Nothing to disclose.
References
- 1.Jin G, Sun J, Kim ST, et al. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Human Molecular Genetics. 2012;2:5222–5228. doi: 10.1093/hmg/dds361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohlsson C, Wallaschofski H, Lunetta KL, et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genetics. 2011;27:e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennartsson AK, Kushnir MM, Bergquist J, et al. Sex steroid levels temporarily increase in response to acute psychosocial stress in healthy men and women. International Journal of Psychophysiology. 2012;84:246–53. doi: 10.1016/j.ijpsycho.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman JR, Falk B, Radom-Isaac S, et al. The effect of environmental temperature on testosterone and cortisol responses to high intensity, intermittent exercise in humans. European Journal of Applied Physiology. 1996;75:83–87. doi: 10.1007/s004210050130. [DOI] [PubMed] [Google Scholar]
- 5.Harden KP, Kretsch N, Tackett JL, et al. Genetic and environmental influences on testosterone in adolescents: Evidence for sex differences. Developmental Psychobiology. 2014;56:1278–1289. doi: 10.1002/dev.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenis MMG, Brouwer RM, van Baal GCM, et al. Longitudinal Study of Hormonal and Physical Development in Young Twins. The Journal of Clinical Endocrinology & Metabolism. 2013;98:518–527. doi: 10.1210/jc.2012-3361. [DOI] [PubMed] [Google Scholar]
- 7.Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-Year-Old twins and its relation to pubertal development. Twin Research and Human Genetics. 2006;9:558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- 8.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harden KP, Tucker-Drob EM, Tackett JL. The Texas Twin Project. Twin Research and Human Genetics. 2013;16:385–390. doi: 10.1017/thg.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rietveld M, van der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Research. 2012;3:134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- 11.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2006;35:376–84. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics. Menstruation in girls and adolescents: Using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 13.Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 14.Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology. 2003;15:431–449. [PubMed] [Google Scholar]
- 15.Norjavaara E. Diurnal rhythm of 17 beta-estradiol secretion throughout pubertal development in healthy girls: evaluation by a sensitive radioimmunoassay. The Journal of Clinical Endocrinology & Metabolism. 1996;81:4095–4102. doi: 10.1210/jcem.81.11.8923866. [DOI] [PubMed] [Google Scholar]
- 16.Reardon KW, Herzhoff K, Tackett JL. Adolescent personality as risk and resiliency in the testosterone-externalizing association. Journal of Research on Adolescence. 2015;26:390–402. doi: 10.1111/jora.12198. [DOI] [PubMed] [Google Scholar]
- 17.Tackett JL, Reardon KW, Herzhoff K, et al. Estradiol and cortisol interactions in youth externalizing psychopathology. Psychoneuroendocrinology. 2015;55:146–53. doi: 10.1016/j.psyneuen.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Klump KL, Hildebrandt BA, O’Connor SM. Changes in genetic risk for emotional eating across the menstrual cycle: a longitudinal study. Psychological Medicine. 2015;45:3227–3237. doi: 10.1017/S0033291715001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen AM, McRae-Clark AL, Carlson S, et al. Determining menstrual phase in human biobehavioral research: A review with recommendations. Experimental and Clinical Psychopharmacology. 2016;24:1–11. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gann PH, Giovanazzi S, Van Horn L, et al. Saliva as a medium for investigating intra- and interindividual differences in sex hormone levels in premenopausal women. Cancer Epidemiology Biomarkers, and Prevention. 2001;10:59–64. [PubMed] [Google Scholar]
- 21.Bogaert V, Taes Y, Konings P, et al. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clinical Endocrinology. 2008;69:129–35. doi: 10.1111/j.1365-2265.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 22.Caramaschi D, Booij L, Petitclerc A, et al. Genetic and environmental contributions to saliva testosterone levels in male and female infant twins. Psychoneuroendocrinology. 2012;37:1954–1959. doi: 10.1016/j.psyneuen.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behavior Genetics. 1998;28:165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- 24.Hong Y, Gagnon J, Rice T, et al. Familial resemblance for free androgens and androgen glucuronides in sedentary black and white individuals: the HERITAGE Family Study. Journal of Endocrinology. 2001;170:485–92. doi: 10.1677/joe.0.1700485. [DOI] [PubMed] [Google Scholar]
- 25.Kuijper EAM, Lambalk CB, Boomsma DI, et al. Heritability of reproductive hormones in adult male twins. Human Reproduction. 2007;22:2153–2159. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- 26.Meikle AW, Bishop DT, Stringham JD, et al. Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metabolism. 1986;35:1090–1095. doi: 10.1016/0026-0495(86)90020-x. [DOI] [PubMed] [Google Scholar]
- 27.Ring HZ, Lessov CN, Reed T, et al. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. The Journal of Clinical Endocrinology & Metabolism. 2005;90:3653–3658. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- 28.Sakai LM, Baker LA, Jacklin CN, et al. Sex steroids at birth: Genetic and environmental variation and covariation. Developmental Psychobiology. 1991;24:559–570. doi: 10.1002/dev.420240804. [DOI] [PubMed] [Google Scholar]
- 29.Travison TG, Zhuang WV, Lunetta KL, et al. The heritability of circulating testosterone, oestradiol, oestrone and sex hormone binding globulin concentrations in men: the Framingham Heart Study. Clinical Endocrinology. 2014;80:277–82. doi: 10.1111/cen.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Hulle CA, Moore MN, Shirtcliff EA, et al. Genetic and environmental contributions to covariation between DHEA and testosterone in adolescent twins. Behavior Genetics. 2015;45:324–340. doi: 10.1007/s10519-015-9709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.