Abstract
Background
Esophageal dysfunction and gastroesophageal reflux disease (GERD) are common among patients with systemic sclerosis (SSc). Although high-dose proton pump inhibitors (PPIs) typically normalize esophageal acid exposure, the effectiveness of PPI therapy has not been systematically studied in SSc patients. The aim of this study was to characterize reflux in SSc patients on high-dose PPI using esophageal pH-impedance testing.
Methods
In this case-controlled retrospective analysis, 38 patients fulfilling 2013 American College of Rheumatology SSc criteria who underwent esophageal pH-impedance testing on twice-daily PPI between 1/2014-3/2017 at a tertiary referral center were compared with a control-cohort of 38 non-SSc patients matched for PPI formulation and dose, hiatal hernia size, age and gender. Patient clinical characteristics, including endoscopy and high-resolution manometry findings, were assessed via chart review.
Key Results
On pH-impedance, SSc patients had higher acid exposure times (AETs) than controls. 61% of the SSc patients and 18% of the control patients had a total AET ≥ 4.5% (p<0.001). SSc patients also had significantly longer acid exposure times, longer median bolus clearance, and lower nocturnal impedance values.
Conclusions & Inferences
Abnormal esophageal acid exposure despite high-dose PPI therapy was common among patients with SSc. The lack of increased reflux episodes in the SSc patients, as well as longer bolus clearance times and lower nocturnal impedance, supports ineffective clearance as the potential mechanism. SSc patients may require adjunctive therapies to PPIs to control acid reflux.
Keywords: acid suppression, esophageal dysfunction, proton pump inhibition, reflux disease, scleroderma, systemic sclerosis
Graphical abstract
Introduction
Patients with systemic sclerosis (SSc) have dysfunction of multiple organ systems including the skin, vasculature and gastrointestinal tract. Up to 80% of patients with SSc have esophageal involvement.1 The most common esophageal disorder in SSc patients is gastroesophageal reflux disease (GERD).2 Complications from esophageal reflux can be serious and include bleeding, stricture formation, Barrett's esophagus and adenocarcinoma.2 In patients with SSc, uncontrolled acid reflux may contribute to the progression of interstitial lung disease.3, 4 Furthermore, reflux severity may predict survival status in SSc patients awaiting lung transplant.5
Several pathophysiologic mechanisms contribute to acid reflux in SSc patients. Patients may develop distal esophageal smooth muscle atrophy and fibrosis, which can subsequently cause decreased lower esophageal sphincter (LES) pressure and loss of esophageal peristalsis.6 These physiologic changes can lead to increased esophageal acid exposure. The combination of hypotensive LES and distal esophageal aperistalsis is often referred to as scleroderma esophagus, although these changes are not specific to SSc disease, and SSc patients can have a range of motility abnormalities on high resolution manometry (HRM) testing.7, 8
Despite the known risks of GERD in SSc patients, optimal treatment regimens for these patients have not been standardized.2, 6, 9 Many patients are prescribed a proton pump inhibitor (PPI) to suppress gastric acid only when they endorse symptoms of GERD, although esophageal involvement in SSc is often asymptomatic.10 Other patients start low-dose PPI, with the dose up-titrated based on symptoms. There are no large-scale randomized controlled trials of PPI use in SSc patients, and the PPI response rate of SSc patients is still unknown.11 Given the multiple pathophysiologic processes in SSc that contribute to excess acid exposure, patients may benefit from empiric high-dose PPI therapy. However, recent concerns regarding adverse effects associated with long term PPI therapy posed by observational studies, including kidney disease, fracture risk, and dementia, prompt further research regarding the effectiveness of PPIs in SSc patients.12, 13
Thirty percent of non-SSc patients with typical GERD symptoms taking a daily PPI have abnormal esophageal acid exposure, but only 7% will have persistently abnormal distal esophageal acid exposure on twice-daily (BID) PPI.14 This suggests that PPI has a dose-dependent effect and that reflux-like symptoms while on high-dose PPI are more likely related to esophageal hypersensitivity or a functional esophageal syndrome than breakthrough acid reflux.14, 15 To date, there have been no studies that assess the effect of PPI therapy on esophageal acid exposure in SSc patients on high-dose PPI. The aim of this case controlled retrospective analysis was to characterize the extent and degree of reflux in SSc patients on high-dose PPI compared to non-SSc matched controls.
Materials and Methods
Patients
The study protocol was approved by the Northwestern University Institutional Review Board. Adult SSc patients and a matched control group of patients (“controls”) who underwent combined pH-impedance testing on BID PPI at the discretion of the treating physician between January 2014 and March 2017 were retrospectively identified from the Esophageal Center at Northwestern Motility Laboratory Registry. Electronic health records were reviewed to ensure that SSc patients fulfilled the 2013 American College of Rheumatology (ACR) SSc criteria16 and that control subjects lacked a diagnosis of autoimmune disease. Patients with a hiatal hernia > 5 cm, prior foregut surgery, or who were post-lung transplant were excluded.
Matching was initially performed based on formulation and dose of PPI. If an exact match by formulation and dose was not available, a best-available match was obtained based on PPI-potency, with the following PPIs considered most to least potent: rabeprazole, dexlansoprazole, esomeprazole, omeprazole, lansoprazole, pantoprazole.17, 18 After matching for PPI dose and formation, patients were subsequently matched by size of hiatal hernia, age, and gender. All authors had access to study data and reviewed and approved the manuscript.
Combined pH-impedance
A 24-hour ambulatory impedance-pH study was performed on all patients (Sandhill Scientific, Inc., Highlands Ranch, CO, USA). A catheter containing 2 antimony pH electrodes (at 5 cm above the esophagogastric junction (EGJ) and 10 cm below the EGJ) and six impedance recording channels (at 3, 5, 7, 9, 15 and 17 cm from the EGJ) was used. The pH electrodes were calibrated externally with buffers of pH 4.01 and 7.01. During the study, the esophageal pH electrode was positioned 5 cm above the superior margin of the LES, as determined by esophageal manometry. The catheter was then secured in position by taping it to the nose. During the study, patients kept a diary of events, noting the time of meals, sleep and symptoms that they experienced. Patients also indicated events on the recording system by means of an event button. Data were analyzed and summarized by computer.
The pH-impedance recordings were analyzed using dedicated software (“Bioview Analysis”, version 5.5.5.1, Sandhill Scientific, Inc.). The pH-impedance tracings were manually reviewed to confirm reflux episodes defined by a retrograde 50% drop in impedance values. Nadir pH of each liquid and mixed reflux event was measured. Liquid and mixed reflux events were then classified as acidic when the pH dropped below 4, as weakly acidic when the nadir pH was between 4 and 7, and as weakly alkaline when nadir pH was above 7. Acid exposure time (AET) was calculated as total time in the distal esophagus at pH <4 (including only pH drops associated with reflux events detected with impedance) divided by recording duration and expressed in percent. Other recorded parameters included longest acid exposure time (measured in minutes), median bolus clearance time (seconds) and DeMeester score.19 The total number of acid, weakly acid, and non-acid reflux events was also noted. Mean nocturnal baseline impedance (MNBI) (measured in ohms) were measured at 1, 2, and 3 hours after sleep onset with the mean value of the three measures recorded.20
Patient Clinical Characteristics
Chart review was performed to record demographic information, patient-reported upper gastrointestinal symptoms (including dysphagia, heartburn and regurgitation), acid-suppression therapy, and results of upper endoscopy and high-resolution esophageal manometry testing. For SSc patients, chart review was also utilized to examine SSc-related clinical characteristics such as extent of disease (diffuse vs limited disease). Most patients completed the GERD-Q self-assessment questionnaire and the Brief Esophageal Dysphagia Questionnaire (BEDQ) at the time of pH-impedance testing.21, 22
Statistical Analysis
Descriptive statistics for all continuous and ordinal measures were presented as median and interquartile range (IQR), unless otherwise stated. Groups were compared using the Mann-Whitney U test for continuous variables and Χ2 for dichotomous and categorical variables. Analyses assumed a 5% level of statistical significance.
Results
Patients characteristics
Sixty SSc patients were initially identified from the esophageal registry. Eleven were excluded after determining they were not actually taking BID PPI at time of pH-impedance testing. Six were excluded as they did not meet 2013 ACR Criteria for SSc. Four were excluded for being post-surgery (2 Nissen fundoplications, 2 lung transplants). One was excluded for having a hiatal hernia > 5 cm.
Baseline characteristics of the remaining 38 SSc patients and 38 matched controls are summarized in Table 1. Of note, there were no significant differences between the SSc patients and controls on baseline characteristics except for female gender, which was significantly more frequent in the SSc patients. Of the SSc patients, 26 had limited cutaneous disease whereas 12 had diffuse cutaneous disease. Symptom questionnaire scores were similar between SSc and control patients: GERD-Q scores (median [IQR]: SSc patients 8 [7 – 11], controls 10 [7 – 12], p=0.28) and BEDQ scores (median [IQR]: SSc patients 7 [1 – 12], controls 2 [0 – 11], p=0.36). Of the 36 SSc patients who underwent endoscopy, 2 patients had Candida esophagitis, 2 had a stricture, 2 had reflux esophagitis (1 LA Grade A, 1 LA Grade C), and 2 had biopsy confirmed Barrett's esophagus. 28 control patients underwent endoscopy, with 1 case of Candida esophagitis, 4 patients with strictures, 2 patients with reflux esophagitis (1 LA Grade D, 1 without LA classification), and 2 patients with biopsy confirmed Barrett's esophagus.
Table 1. Patient characteristics.
BMI – body mass index. IQR – interquartile range. PPI – proton pump inhibitor. SD – standard deviation. SSc – systemic sclerosis.
| SSc | Control | p-value | |
|---|---|---|---|
|
| |||
| n | 38 | 38 | |
|
| |||
| Age, years, mean+/-SD | 52 +/- 9 | 53 +/- 13 | 0.903 |
|
| |||
| Gender (F/M) | 35/3 | 21/17 | <0.001 |
|
| |||
| Ethnicity, n (%) | 0.430 | ||
| Not Hispanic or Latino | 26 (68) | 29 (76) | |
| Asian | 0 (0) | 1 (3) | |
| Hispanic or Latino | 7 (18) | 3 (8) | |
| Unknown | 5 (13) | 5 (13) | |
|
| |||
| BMI, median (IQR) | 24 (20 – 29) | 27 (23 – 30) | 0.097 |
|
| |||
| PPI/dose, n (%) | |||
| Rabeprazole 20mg BID | 2 (5) | 2 (5) | |
| Dexlansoprazole 60mg BID | 6 (16) | 5 (13) | |
| Esomeprazole 40mg BID | 9 (24)1 | 10 (26) | |
| Esomeprazole 20mg BID | 1 (3) | 1(3)3 | |
| Omeprazole 40mg BID | 6 (16) | 6 (16) | |
| Omeprazole 20mg BID | 5 (13) | 5 (13) | |
| Lansoprazole 30mg BID | 1 (3) | 1 (3) | |
| Pantoprazole 40mg BID | 7 (18)2 | 7 (18) | |
| Pantoprazole 20mg BID | 1 (3) | 1 (3)4 | |
|
| |||
| Hiatal hernia, n (%) | 0.967 | ||
| None | 21 (55) | 22 (58) | |
| 1-2 cm | 12 (32) | 11 (29) | |
| 3-4 cm | 5 (13) | 5 (13) | |
|
| |||
| Indication for reflux monitoring, n (%) | 0.226 | ||
| Typical reflux symptoms | 23 (61) | 15 (40) | |
| Extraesophageal symptoms | 11 (29) | 19 (50) | |
| Other symptoms | 2 (5) | 3 (8) | |
| Pre-lung transplant evaluation | 2 (5) | 1 (3) | |
One SSc patient was on esomeprazole 60mg BID.
One SSc patient was on pantoprazole 40mg TID.
Matched control was on esomeprazole 40mg DAILY.
Matched control was on pantoprazole 40mg daily.
Combined pH-impedance characteristics
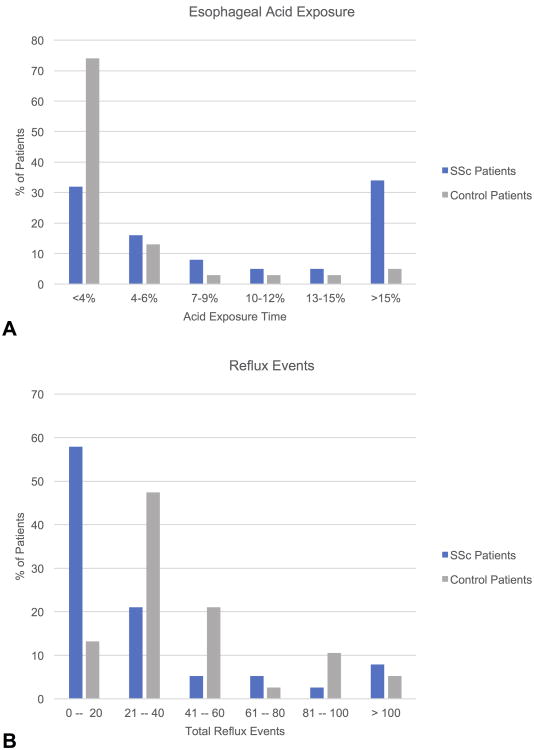
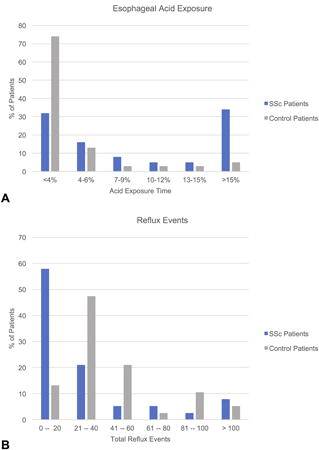
A detailed description of the pH-impedance findings in SSc patients and controls is found in Table 2. SSc patients had significantly higher total AET (%) compared to controls (Figure 1A). 23/38 (61%) SSc patients, compared to 7/38 (18%) controls, had a total AET ≥ 4.5% (p<0.001). 21/28 (55%) SSc patients, compared to 6/38 (16%) control patients, had a total AET > 6% (p<0.001). In contrast, the controls had higher total reflux events than the SSc patients (Figure 1B), and this finding held true for both acid and weakly acid reflux events. SSc patients had higher acid exposure than controls regardless of hiatal hernia size. AETs were 6.5 (1.3 – 18.7) for SSc patients without a hiatal hernia, 12.9 (3.5 – 22.8) for SSc patients with a 1-2 cm hernia, and 3.9 (2.1 – 26.2) for patients with a 3-4 cm hernia. In contrast, AETs were 1.5 (0.5 – 3.8) for controls without a hernia, 1.8 (0.7 – 4.6) for controls with a 1-2 cm hernia, and 2.4 (0.2 – 5.0) for controls with a 3-4 cm hernia. Mean nocturnal baseline impedance was significantly lower in SSc patients compared to controls.
Table 2. Combined pH-Impedance findings.
Data presented as median (interquartile range) unless otherwise stated. SSc – systemic sclerosis.
| SSc | Control | p-value | |
|---|---|---|---|
|
| |||
| n | 38 | 38 | |
|
| |||
| Acid exposure time, % | |||
| Upright | 5.9 (1.8 – 20.9) | 1.9 (0.6 – 4.6) | 0.003 |
| Supine | 1.7 (0 – 13.2) | 0.1 (0 – 1.1) | 0.013 |
| Total | 6.8 (2.4 – 18.7) | 1.8 (0.5 – 3.8) | <0.001 |
|
| |||
| Longest Acid exposure, minutes | |||
| Upright | 14.0 (7.1 – 40.2) | 4.7 (2.2 – 8.2) | 0.001 |
| Supine | 11.4 (0 – 41.1) | 0.7 (0 – 4.8) | 0.017 |
| Total | 32.8 (10.9 – 85.7) | 6.0 (2.5 – 20.4) | <0.001 |
|
| |||
| Median bolus clearance, seconds | |||
| Upright | 21.0 (11.3 – 28.0) | 13.0 (10.8 – 18.3) | 0.015 |
| Supine | 14.5 (0 – 30.5) | 11.0 (7.0 – 21.5) | 0.885 |
| Total | 20.5 (11.5 – 28.8) | 13.0 (10.0 – 19.0) | 0.021 |
|
| |||
| DeMeester Score | 27.3 (10.0 – 65.7) | 6.0 (1.8 – 13.9) | <0.001 |
|
| |||
| Acid reflux events | |||
| Upright | 1 (0 – 9) | 5 (1 – 21) | 0.005 |
| Supine | 0 (0 – 1) | 1 (0 – 4) | 0.125 |
| Total | 2 (0 – 10) | 8 (2 – 22) | 0.011 |
|
| |||
| Weakly acid reflux events | |||
| Upright | 7 (1 – 16) | 17 (10 – 28) | 0.003 |
| Supine | 2 (0 – 5) | 2 (1 – 6) | 0.452 |
| Total | 10 (2 – 20) | 25 (12 – 36) | 0.002 |
|
| |||
| Non-acid reflux events | |||
| Upright | 0 (0 – 1) | 0 (0 – 3) | 0.172 |
| Supine | 0 (0 – 0) | 0 (0 – 0) | 0.536 |
| Total | 0 (0 – 1) | 0 (0 – 3) | 0.117 |
|
| |||
| Total reflux events | 13 (6 – 34) | 37 (26 – 54) | 0.002 |
|
| |||
| Mean nocturnal baseline impedance, ohms | 492 (257 – 915) | 2783 (1050 – 3275) | <0.001 |
Figure 1. Esophageal acid exposure and reflux events.
A. Distribution of esophageal acid exposure times among systemic sclerosis (SSc) patients and control patients. B. Distribution of total reflux events among SSc patients and control patients.
Manometric characteristics
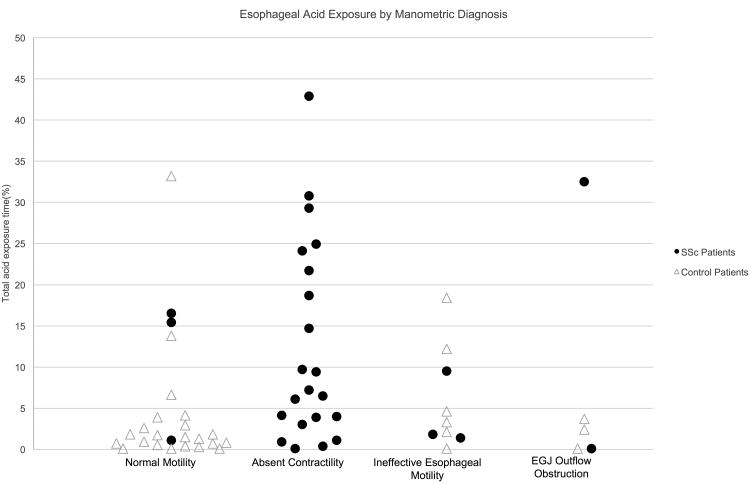
Esophageal manometry was performed in 31 SSc patients and 32 controls. Details on the manometric findings are found in Table 3. 72% of patients with SSc had absent contractility on manometry, whereas no control patients had this finding. Additionally, 72% of control patients had normal motility, compared to only 10% of SSc patients. Figure 2 shows the association between motility diagnosis and AET for SSc patients and controls.
Table 3. Manometric characteristics.
Data presented as median (IQR) unless otherwise stated. EGJ – esophagogastric junction. IRP – integrated relaxation pressure. SSc – systemic sclerosis.
| SSc | Control | p-value | |
|---|---|---|---|
|
| |||
| n | 31 | 32 | |
|
| |||
| EGJ Morphology, n (%) | 0.09 | ||
| Type I | 24 (77) | 18 (56) | |
| Type II | 5 (16) | 13 (41) | |
| Type III | 2 (7) | 1 (3) | |
|
| |||
| EGJ end expiratory pressure, mmHg | 7 (4 – 13) | 14 (5 – 24) | 0.03 |
|
| |||
| EGJ-contractile integral, mmHg-cm | 33 (17 – 48) | 47 (34 – 75) | 0.023 |
|
| |||
| Median IRP, mmHg | 7 (5 – 11) | 8 (5 – 12) | 0.48 |
|
| |||
| Motility diagnosis based on Chicago Classification, n (%) | <0.001 | ||
| Normal motility | 3 (10) | 23 (72) | |
| Absent contractility | 22 (71) | 0 (0) | |
| Ineffective esophageal motility | 3 (10) | 6 (19) | |
| Achalasia | 1 (3) | 0 (0) | |
| EGJ Outflow Obstruction | 2 (7) | 3 (9) | |
Figure 2. Association between manometric diagnosis and acid exposure times.
Breakdown of total esophageal acid time by motility diagnosis in both systemic sclerosis (SSc) and control patients. There was one additional SSc patient with an achalasia motility pattern and an acid exposure time of 1.3% not represented on the figure. EGJ – esophagogastric junction.
SSc patients had lower basal EGJ pressures, as measured at end expiration and with the EGJ-contractile integral, than controls. 22 (71%) SSc patients had a hypotensive EGJ, defined as EGJ end-expiratory pressure < 10 mmHg. Eighteen (58%) of these patients had the typical findings of “scleroderma esophagus,” defined as hypotensive EGJ with absent contractility. Patients with the “scleroderma” pattern had a median AET of 12.1% (IQR 4.1 – 24.3).
Discussion
GERD is a common and serious problem in patients with SSc. To our knowledge, this is the first study to assess and characterize the extent of esophageal reflux on high-dose PPI therapy in SSc patients using combined pH-impedance testing. We found that despite high-dose PPI, 61% of SSc patients had an AET ≥ 4.5% and 55% of SSc patients had an AET > 6%. These AET values were significantly higher than in a matched-control cohort of patients also on high-dose PPI. These AET values were also much higher than in historical cohorts of GERD patients, in which only 7% have persistent abnormal esophageal exposure on ambulatory 24-hour pH testing while on BID PPI.14 Compared to controls, SSc patients were also found to have longer acid exposure time, longer median bolus clearance time, and lower MNBI. However, the control group of patients had significantly more individual acid reflux events than the SSc patients.
Our findings are consistent with the existing literature on reflux abnormalities in SSc patients. A study published in 1989 by Zaninotto et al. described abnormal esophageal acid exposure on 24-hour pH testing in 11/13 (85%) SSc patients.23 More recently, a 2015 study by Arif et al. evaluated reflux parameters via ambulatory 24-hour pH testing in 41 SSc patients, and found that 80.5% had abnormal results, with a mean AET of 8.5%.24 However, the study lacked impedance-pH metry, which is the gold standard for the assessment of gastroesophageal reflux disease.24 Furthermore, all patients were studied off PPI therapy. Given that many SSc patients are on a PPI for their upper GI symptoms, and some experts recommend all SSc patients be on PPI, the findings have limited generalizability.6
The differences in reflux parameters between the SSc patients and controls suggest that the underlying mechanism of reflux may be distinct between the two groups. The longer acid exposure time and bolus clearance time in SSc patients, as well as lower nocturnal impedance, are likely secondary to esophageal stasis, as supported by the higher frequencies of absent contractility observed on HRM. In fact, results from our center demonstrate that SSc patients have a dilated esophagus on axial chest high resolution computed tomography, and that esophageal diameter is positively correlated with radiographic ILD and negatively correlated with pulmonary function.25 The controls had higher individual reflux events, which speaks to a different mechanism of reflux in this population. Our data also suggest that additional aspiration precautions should be considered in SSc patients.
Increased esophageal acid exposure in SSc patients despite high-dose PPI therapy has several diagnostic and treatment implications. Among SSc patients with persistent reflux symptoms despite PPI use, physicians should have a low threshold to escalate therapy to high-dose and high potency PPIs and utilize esophageal pH-impedance testing. Based on pH-impedance results, adjunctive therapies, such as pro-motility agents or antacids, may need to be considered. A recent study by Foocharoen et al. investigated the role of add on therapy with either domperidone or algycon in SSc patients.26 In this study, 88/151 (59.4%) of all enrolled SSc patients had a partial response to omeprazole 20mg BID, defined as less than 50% improvement in symptoms from baseline after 4 weeks of therapy. Patients were randomly assigned to take omeprazole plus either domperidone or algycon for an additional 4 weeks. Only 13% of patients in the domperidone group and 22% of patients in the algycon groups did not respond to the additional therapy. Although this study looked only at symptomatic endpoints rather than pH-impedance testing, the findings suggest that there may be a role for these additional therapies in SSc patients. Pro-motility agents may improve LES tone and gastric emptying, thus improving reflux control, although the effects on esophageal contractility may wane with progressive smooth muscle atrophy and fibrosis.2
This study has several limitations. First, the design was retrospective in nature. Therefore, patients underwent diagnostic testing at the discretion of the referring provider, which could introduce bias in the cohort of included patients. Although we attempted to control for reflux-related risk factors by utilizing a matched-control group, there were factors that we could not match for given the sample size of our pH-impedance registry. Given the nature of the SSc population and the underlying pathophysiology, there were inherent differences in gender and motility disorders between groups. Additionally, although BMI could not formally be matched, between group comparisons showed no significant differences and a trend observed toward higher BMI in the control population would only potentially increase risk of reflux.
In conclusion, this retrospective case controlled study of patients with SSc undergoing combined pH-impedance testing suggests that SSc patients have significantly worse esophageal acid reflux control on high-dose PPI when compared to a similarly matched population of patients. The mechanism may relate to esophageal stasis, perhaps worsened esophageal dysmotility and hypotony of the LES. Practitioners should consider aggressive PPI therapy in these patients given the known risks of poorly controlled acid reflux in this population. Adjunct therapies to PPI, including antacids and pro-motility agents, should also be considered, although further research is needed to clarify their role. Prospective studies investigating the dose response to PPI therapy in SSc patients are also needed.
Key Points.
Esophageal dysfunction and reflux are common in patients with systemic sclerosis (SSc) yet the effectiveness of proton pump inhibitor (PPI) therapy has not been systematically studied in this population. This study evaluated esophageal reflux in SSc patients on high-dose PPIs using esophageal pH testing.
This study demonstrated that SSc patients have significant esophageal acid exposure despite high-dose PPI therapy, which may be related to ineffective esophageal clearance.
SSc patients may require adjunctive therapies to PPIs to control their acid reflux.
Acknowledgments
EKS and DAC contributed to study concept and design, data acquisition, data analysis, interpretation of data, and drafting of the manuscript; MH and DMB contributed to study concept, data analysis and interpretation, and revised the manuscript critically; MC, ADH, and SF contributed to data acquisition; JEP contributed to interpretation of the data, and revised the manuscript critically. All authors approved the final version.
Funding: This work was supported by R01 DK092217 (JEP), and from the Public Health Service National Institutes of Health grants K23 AR059763 (MH), L30 AR054311 (MH) and Scleroderma Research Foundation Grants (MH)
Abbreviations
- ACR
American College of Rheumatology
- AET
Acid exposure time
- BID
Twice daily
- EGJ
Esophagogastric junction
- GERD
Gastroesophageal reflux disease
- HRM
High resolution manometry
- LES
Lower esophageal sphincter
- MNBI
Mean nocturnal baseline impedance
- PPI
Proton pump inhibitor
- SSc
Systemic sclerosis
Footnotes
Disclosures: John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking). Monique Hinchcliff: Sanofi (Consultant). Darren M. Brenner: consultant/advisor and on the speakers bureaus of Allergan, Ironwood, Salix, Astra Zeneca, Daiichi Sankyo, Synergy Pharmaceuticals and the GI Health Foundation. Emily K. Stern, Dustin A. Carlson, Sophia Falmagne, Aileen Hoffmann, Mary A. Carns: none
References
- 1.Thonhofer R, Siegel C, Trummer M, Graninger W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatol Int. 2012;32:165–168. doi: 10.1007/s00296-010-1595-y. [DOI] [PubMed] [Google Scholar]
- 2.Nagaraja V, McMahan ZH, Getzug T, Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015;1:82–105. doi: 10.1007/s40674-014-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179:408–413. doi: 10.1164/rccm.200808-1359OC. [DOI] [PubMed] [Google Scholar]
- 4.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40:241–249. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Fisichella PM, Reder NP, Gagermeier J, Kovacs EJ. Usefulness of pH monitoring in predicting the survival status of patients with scleroderma awaiting lung transplantation. J Surg Res. 2014;189:232–237. doi: 10.1016/j.jss.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyger G, Baron M. Systemic Sclerosis: Gastrointestinal Disease and Its Management. Rheum Dis Clin North Am. 2015;41:459–473. doi: 10.1016/j.rdc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Crowell MD, Umar SB, Griffing WL, DiBaise JK, Lacy BE, Vela MF. Esophageal Motor Abnormalities in Patients With Scleroderma: Heterogeneity, Risk Factors, and Effects on Quality of Life. Clin Gastroenterol Hepatol. 2016;15:207–213. doi: 10.1016/j.cgh.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel JN, Carlson DA, Hinchcliff M, et al. The association between systemic sclerosis disease manifestations and esophageal high-resolution manometry parameters. Neurogastroenterol Motil. 2016;28:1157–1165. doi: 10.1111/nmo.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson DA, Hinchcliff M, Pandolfino JE. Advances in the evaluation and management of esophageal disease of systemic sclerosis. Curr Rheumatol Rep. 2015;17:475. doi: 10.1007/s11926-014-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntoumazios SK, Voulgari PV, Potsis K, Koutis E, Tsifetaki N, Assimakopoulos DA. Esophageal involvement in scleroderma: gastroesophageal reflux, the common problem. Semin Arthritis Rheum. 2006;36:173–181. doi: 10.1016/j.semarthrit.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Hansi N, Thoua N, Carulli M, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol. 2014;32:S-214–221. [PubMed] [Google Scholar]
- 12.Kia L, Kahrilas PJ. Therapy: Risks associated with chronic PPI use - signal or noise? Nat Rev Gastroenterol Hepatol. 2016;13:253–254. doi: 10.1038/nrgastro.2016.44. [DOI] [PubMed] [Google Scholar]
- 13.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol. 2005;100:283–289. doi: 10.1111/j.1572-0241.2005.41210.x. [DOI] [PubMed] [Google Scholar]
- 15.Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Esophageal Disorders. Gastroenterology. 2016;150:1368–1379. doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19–31. doi: 10.1007/s00228-008-0576-5. [DOI] [PubMed] [Google Scholar]
- 18.Kukulka M, Eisenberg C, Nudurupati S. Comparator pH study to evaluate the single-dose pharmacodynamics of dual delayed-release dexlansoprazole 60 mg and delayed-release esomeprazole 40 mg. Clin Exp Gastroenterol. 2011;4:213–220. doi: 10.2147/CEG.S24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson LF, DeMeester TR. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol. 1986;8 Suppl 1:52–58. doi: 10.1097/00004836-198606001-00008. [DOI] [PubMed] [Google Scholar]
- 20.Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the Post-reflux Swallow-induced Peristaltic Wave Index and Nocturnal Baseline Impedance Parameters Increase the Diagnostic Yield of Impedance-pH Monitoring of Patients With Reflux Disease. Clin Gastroenterol Hepatol. 2016;14:40–46. doi: 10.1016/j.cgh.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 22.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil. 2016;28:1854–1860. doi: 10.1111/nmo.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaninotto G, Peserico A, Costantini M, et al. Oesophageal motility and lower oesophageal sphincter competence in progressive systemic sclerosis and localized scleroderma. Scand J Gastroenterol. 1989;24:95–102. doi: 10.3109/00365528909092245. [DOI] [PubMed] [Google Scholar]
- 24.Arif T, Masood Q, Singh J, Hassan I. Assessment of esophageal involvement in systemic sclerosis and morphea (localized scleroderma) by clinical, endoscopic, manometric and pH metric features: a prospective comparative hospital based study. BMC Gastroenterol. 2015;15:24. doi: 10.1186/s12876-015-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson C, Agrawal R, Lee J, et al. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin Arthritis Rheum. 2016;46:109–114. doi: 10.1016/j.semarthrit.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foocharoen C, Chunlertrith K, Mairiang P, et al. Effectiveness of add-on therapy with domperidone vs alginic acid in proton pump inhibitor partial response gastro-oesophageal reflux disease in systemic sclerosis: randomized placebo-controlled trial. Rheumatology. 2017;56:214–222. doi: 10.1093/rheumatology/kew216. [DOI] [PubMed] [Google Scholar]