Abstract
Motherhood is a period of intense behavioral and brain activity. However, we know less about the neural and molecular mechanisms associated with the demands of fatherhood. Here, we report the results of two experiments designed to track changes in behavior and brain activation associated with fatherhood in male threespined stickleback fish (Gasterosteus aculeatus), a species in which fathers are the sole providers of parental care. In experiment 1, we tested whether males’ behavioral reactions to different social stimuli depends on parental status, i.e. whether they were providing parental care. Parental males visited their nest more in response to social stimuli compared to nonparental males. Rates of courtship behavior were high in non-parental males but low in parental males. In experiment 2, we used a quantitative in situ hybridization method to compare the expression of an immediate early gene (Egr-1) across the breeding cycle–from establishing a territory to caring for offspring. Egr-1 expression peaked when the activities associated with fatherhood were greatest (when they were providing care to fry), and then returned to baseline levels once offspring were independent. The medial dorsal telencephalon (basolateral amygdala), lateral part of dorsal telencephalon (hippocampus) and anterior tuberal nucleus (ventral medial hypothalamus) exhibited high levels of Egr-1 expression during the breeding cycle. These results help to define the neural circuitry associated with fatherhood in fishes, and are consistent with the hypothesis that fatherhood–like motherhood–is a period of intense behavioral and neural activity.
Keywords: fatherhood, immediate early gene, sociogenomics, sticklebacks, social behavior network
INTRODUCTION
Motherhood is a period of intense behavioral and neural activation. Decades of studies have started to reveal the structure and organization of the maternal brain (Hillerer et al., 2014; Kinsley and Amory-Meyer, 2011; Lambert, 2012; Rilling and Young, 2014), the brain areas that are activated during mothering (Rocchetti et al., 2014) and the neural control of maternal care (Dulac et al., 2014). However, we know less about the neural mechanisms underlying the transition to fatherhood (Kentner et al., 2010).
Fishes are particularly good subjects for studying the neuroendocrine mechanisms involved in fathering because they exhibit tremendous diversity in reproductive mode, and paternal care is relatively common in fishes compared to other vertebrates (Smith and Wootton, 2016). Recent studies have begun to describe the dramatic neuroendocrine changes that accompany the transition to fatherhood in fishes, e.g. (DeAngelis and Rhodes, 2016; Pradhan et al., 2014; Stiver et al., 2015), and have suggested that isotocin and arginine vasotocin, like their mammalian homologs arginine vasopressin and oxytocin, are involved in regulating paternal behavior in fishes (Kleszczynska et al., 2012; O'Connell et al., 2012; Ripley and Foran, 2010). GnRH and the distribution of GnRH neurons in key brain areas such as the preoptic area of the hypothalamus are also key players that orchestrate reproductive behavior in fishes, e.g. (Burmeister et al., 2005; Scaggiante et al., 2004, 2006; Tubert et al., 2012), reviewed in (Chen and Fernald, 2008; Fernald, 2012; Maruska and Fernald, 2011).
Threespined stickleback fish are especially good models for studying fathering because male sticklebacks are the sole providers of parental care that is necessary for offspring survival, and their paternal behavior has been well studied in the field and in the lab. Male sticklebacks undergo dramatic changes in behavior and physiology during the reproductive cycle (Wootton, 1976, 1984), which is photoperiod-dependent (Hellqvist et al., 2008). For example, as day length increases, males become aggressive, defend territories and construct nests. Only upon completing their nest do males start to court females and display courtship behaviors such as the conspicuous zig zag dance. Males also advertise their parental abilities during courtship, e.g. by fanning, even when they do not have eggs in their nest (Candolin, 1997). After spawning, males provide parental care for the eggs in the form of territory defense and fanning. After the eggs hatch, certain paternal behaviors make an abrupt appearance: fathers become very active, chasing and retrieving their free-swimming fry (Stein and Bell, 2012). Males continue to defend their newly-hatched and vulnerable fry from predators. Fathers and their fry are intimately associated during this period, with many opportunities for sensory, especially tactile, interactions. Parenting is an energetically demanding period, yet it is necessary for reproductive success (Smith and Wootton, 1999). Interestingly, threespine sticklebacks exhibit greater sexual dimorphism in brain size than any other vertebrate (Kotrschal et al. 2012) and in sticklebacks populations in which males do not provide care, the sexual dimorphism in brain size is reversed (Samuk et al. 2014). These results are consistent with the hypothesis that the male stickleback brain has evolved in response to the cognitive demands of parenting (Kotrschal et al. 2012).
The reproductive cycle in male sticklebacks is marked by dramatic neuroendocrine changes. For example, GnRH and gonadotropins (Andersson et al., 1995; Hellqvist et al., 2004; Shao et al., 2015) as well as androgens (Hoffmann et al., 2008) change as males move through the breeding cycle, from establishing a territory to caring for offspring. In particular, levels of 11 keto-testosterone (11KT), a potent androgen, are high during the territorial and courtship phase but then drop when males are providing care (Mayer et al., 2004; Pall et al., 2005; Pall et al., 2002b). However, the drop in 11KT is not responsible for the increase in care (Pall et al., 2002a). Instead, other studies point to arginine vasotocin (AVT) (Kleszczynska et al., 2012) and prolactin as important players during the parental phase (Rall et al., 2004).
Here, we report the results of two experiments designed to track changes in behavior and neural immediate early gene expression (IEG) as male sticklebacks become fathers. In experiment 1, we compare behavior toward conspecifics and a model predator between parental and nonparental males. In experiment 2, we use in situ hybridization to track changes in the expression of an IEG (Egr-1) across different stages of the breeding cycle. IEG expression has been used to reveal brain areas important for behavior (Clayton, 2000; Fernald, 2012; Hillerer et al., 2014; Hofmann, 2010; Robinson et al., 2008), including those involved in fathering in rodent models (e.g. prairie voles (Northcutt and Lonstein, 2009), California mice (de Jong et al., 2009; Lambert et al., 2011). IEG expression has also been used to track changes in brain activation in fishes (Burmeister et al., 2005; Butler and Maruska, 2016; Desjardins et al., 2015; Desjardins and Fernald, 2010; Desjardins et al., 2010; Harvey-Girard et al., 2010; Kress and Wullimann, 2012; Lau et al., 2011; Loveland and Fernald, 2017; Maruska et al., 2013a; Maruska et al., 2013b; O'Connell et al., 2012; O'Connell et al., 2013; Rajan et al., 2011; Yaeger et al., 2014). We focus on Egr-1 expression in brain areas involved in the social behavior network (Goodson, 2005; Newman, 1999; O'Connell and Hofmann, 2011), a linked set of brain nuclei important for social behavior in vertebrates. We use a whole mount, quantitative in situ hybridization protocol that has been validated in several species and tissues (Bacharach et al., 2016; Long, 2016; McNeill and Robinson, 2015; Stapel et al., 2016; Tantirigama et al., 2016).
METHODS
Animals
The three-spined sticklebacks were collected as juveniles from Putah Creek, California. Freshwater sticklebacks typically reproduce at one year of age, and breed several times during the spring-summer. Fish were maintained in the laboratory in 104L tanks at approximately 16 C under 8:16h light/dark photoperiod until they became sexually mature. The water was filtered through particulate, UV, biological and charcoal filters. The fish were fed ad libitum with a mixture of bloodworms, brine shrimp and mysis shrimp daily.
Once nuptial coloration was observed, male sticklebacks were switched to a 16:8h light/dark photoperiod at 20 C, measured for length and housed individually in 9.5L (36 x 21 x 18 cm) tanks with a refuge, an open plastic box (13 x 13 x 3 cm) filled with sand, and algae for nest building. Prior to the experiment, males were randomly assigned to one of five breeding stage treatments (territorial, courtship, tending eggs, tending fry, and post-fry). To induce spawning, females were added to the tanks 24 hours after the male crept through his nest. If spawning did not occur, another female was introduced into the tank 12–24 hours later. Males were observed every day to assure that they were providing parental care (fanning nest, hovering near nest, oxygenating the eggs). The experiment was carried out during summer 2011.
Experiment 1
In order to examine changes in behavior that occur as males become fathers, we compared the behavioral reaction of nonparental and parental males to three different stimuli: a male stickleback, a female stickleback and a model predator. Nonparental males were measured during the courtship stage (24 hours after the male crept through his nest) and parental males were measured during the tending fry stage (three days after hatching).
In order to measure their behavioral reaction to a female stickleback, males were presented with a gravid female (potential mate) in a clear round bottom flask for 10 minutes. Five different gravid females were used as stimuli. To measure their behavioral reaction to a male stickleback, males were presented with a reproductive male (potential rival) in a clear round bottom flask for 10 minutes. Five different reproductive and nuptially-colored males were used as stimuli. To measure their behavioral reaction to a model predator, males were confronted with a model bird predator. The beak of a great blue heron (a predator that occurs in this population) was plunged into the tank every minute for 10 minutes. We recorded the number of zig zags (a conspicuous courtship behavior) and visits to the nest during each ten-minute observation period. Different individuals were measured in each condition, with the following final sample sizes: nonparental: female stickleback, n=9; male stickleback, n=6; model bird predator, n=7; parental: female stickleback, n=7; male stickleback, n=5; model bird predator, n=6.
Experiment 2
Prior to the experiment, males were randomly assigned to one of five conditions. Males assigned to the territorial stage were sampled after the fish started but not yet completed a nest. Males assigned to the courtship stage were sampled within 24 hours after creeping through the nest, a conspicuous behavior that marks the transition into the courtship stage. Males assigned to the tending eggs stage were removed three days after fertilization. Males assigned to the tending fry treatment were sacrificed three days after the fry hatched, when levels of parental behavior are high (Stein and Bell, 2012). Males assigned to the post-fry treatment were transferred to a new tank seven days after the fry hatched, when males typically begin to defend new territories, and were sacrificed twenty-four hours later, after males had recovered from handling but had not yet started a new nest.
Males were sacrificed via decapitation between 1000–1400. The head was removed from the body just behind the operculum. The muscles at the base of the skull along with the skull were removed using rongeurs (FST, Foster City, CA, USA). The eyes were detached from the optic nerve using fine inverted scissors (FST). The brain (minus pituitary) was then placed in 4% paraformaldahyde (Sigma Aldrich, St Louis, MO, USA) made in phosphate buffered saline (PBS: Fisher Scientific, Fair Lawn, NJ, USA).
Twenty-four hours later, all brains were cleaned of dura, excess fibers that were attached to the brain after it was removed from the skull and miscellaneous tissues using a stereomicroscope (Leica Microsystems Inc., Buffalo Grove, IL, USA) to view the brain. Fine forceps and fine inverted scissors were used for removal of all excess. Once the brains were clean they were placed in 100% methanol stored at −20°C until being processed through the in situ hybridization protocol.
The final sample sizes in each condition were: territorial (n=9), courtship (n=8), tending eggs (n=6), tending fry (n=4) and post-fry (n=4). Fewer males were sampled in the later stages because not all males mated and/or successfully reared eggs or fry.
In situ hybridization
To quantify Egr-1 expression, we modified an mRNA in situ hybridization protocol developed for insects to analyze differential expression in whole mount brains (McNeil and Robinson 2015). This method combines whole mount protocols that use bright field microscopy with a fluorescence microscopy protocol (Raj and Tyagi, 2010; Raj et al., 2008). Stellaris® (Biosearch Technologies, Petaluma, CA, USA) mRNA in situ hybridization probe sets comprised 48 DNA sequences, where each 20bp sequence was attached to a single Quasar fluorophore. Probes are highly specific because probe sequences designed in the antisense direction bind in series along the targeted mRNA transcript. We designed antisense probe sets against Egr-1 using an online tool available through BioSearch (Supplementary Table 1).
Brains were prepared for hybridization through sequential rehydration from 100% methanol into PBS (Fisher) with Triton-X (Promega, Madison, WI, USA). Next, brains were treated with 2μg/ml proteinase K (Invitrogen, Carlsbad, CA, USA) for 20 minutes. Proteinase K was used to increase signal that may have been lost due to paraformaldahyde exposure. Brains were then placed in 2mg/ml glycine for 20 minutes to stop the proteinase K reaction. The brains were then refixed in 4% paraformaldehyde (Sigma Aldrich) for 20 minutes and treated with 2mg/ml glycine plus 75mM ammonium acetate. Next, brains were incubated in prehybridization solution containing 2X Saline sodium citrate buffer (SCC) (Fisher), 15% formamide (Fisher), 1% blocking solution (Roche, Indianapolis, IN), 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) (Fisher), 0.1% Triton-X (Fisher), 1mg/ml yeast tRNA (Promega), 5mM EDTA, 100mg/ml Dextran Sulfate (Fisher) and 1X Denhardt’s solution (Fisher) at 37°C for four hours. Brains were incubated overnight at 37°C in fresh prehybridization solution with Stellaris probes (table S1) tagged with Quasar 570 (BioSearch) at a concentration of 1:100.
Brains were put through sequential stringency (15% formamide, 2X SCC, 0.1% Triton-X 100, 0.5% CHAPS) washes to remove excess probe. Washes were diluted with 2X SCC ending in a 1:3 dilution of wash to SCC. After the stringency washes, the brains were gradually dehydrated into 100% methanol and cleared for imaging with 100% methyl salicylate (Sigma Aldrich). The brains were then covered to prevent photobleaching. After dehydration the brains were submersed in a 1mM solution of DAPI dissolved in methanol.
Imaging
The whole mount brains were processed on am automated LSM 710, confocal microscope (Carl Zeiss Microimaging Inc, Jena, Germany; NY, USA) from the dorsal to ventral side. Brains were focused on the microscope and then 12 tile images (4x3) were taken to allow for the entire brain to be analyzed. The overall thickness of the brain was also determined and then 7μm sections were imaged by exciting fluorophores with a 555nm laser line. The tiles and sections were compiled together to give a complete image of the whole brain. Pictures were taken and processed using software provided by Zeiss (Zeiss, 2010).
Analysis
Images were analyzed using NIH software, ImageJ. Whole brain images were first used to measure thickness and then key anatomical landmarks were identified, such as the anterior and posterior commissures. The two commissures were used to determine the location of brain areas. There is no brain atlas for the stickleback brain, therefore brain atlases from other teleost species as well as previous immunohistochemical studies in sticklebacks were used to locate putative brain areas within the social behavior network (Burmeister et al., 2009; Cerda-Reverter et al., 2008; Cerda-Reverter et al., 2001a, b; Ekstrom, 1994; Ekstrom et al., 1995; Ekstrom et al., 1992; Ekstrom et al., 1985; Ekstrom and Ohlin, 1995; Ekstrom et al., 1986; Ekstrom and Van Veen, 1984; Honkanen and Ekstrom, 1991; O'Connell and Hofmann, 2011; Peter RE et al., 1975; Wulliman, 1996).
For example, before scanning each brain for Egr-1 and DAPI, brains were measured on the z-plane to determine initial whole brain thickness. All brains were scanned on the confocal microscope in the dorsal to ventral direction to ensure similar patterns of expression across all scans. Brain section images were taken every 7μm. Before a brain area of interest was located, a second measurement of thickness was taken. The number of images were counted from first visualization of DAPI until no signal was present. The number of images was then multipled by 7 to give a total μm thickness of the brain and compared to the initial number. If the measurements were incorrect the landmarks were used to confirm the correct location. When a brain area such as the anterior tuberal nucleus/ventral medial hypothalamus (aTn/VMH) was located by determining distance from the anterior commissures and overall thickness of the brain compared to published brain atlases (Burmeister et al., 2009; Cerda-Reverter et al., 2008; Cerda-Reverter et al., 2001a, b; Ekstrom, 1994; Ekstrom et al., 1995; Ekstrom et al., 1992; Ekström et al., 2001; Ekstrom et al., 1985; Ekstrom and Ohlin, 1995; Ekstrom et al., 1986; Ekstrom and Van Veen, 1984; Honkanen and Ekstrom, 1991; O'Connell and Hofmann, 2011; Peter RE et al., 1975; Wulliman, 1996) and an additional landmark, the ventricle, was used to confirm the correct location. Approximately 2mm from when the DAPI signal was first observed, the aTn/VMH can be observed surrounding the ventricle. Once the correct section was located within the z-plane mean grey value (MGV) measurements for Egr-1 expression were taken. A total of 35μm of tissue was averaged to ensure a relative MGV for each brain area like the aTn/VMH.
Egr-1 expression was measured in brain areas important for the social behavior network (the fish name is indicated first followed by the putative mammalian homolog): ventral tuberal/anterior hypothalamus (VT/AH), medial dorsal telencephalon/basolateral amygdala (Dm/AMY), ventral pallium/bed nucleus of the stria terminalis_medial amygdala (VP/BNST), lateral part of dorsal telencephalon/hippocampus (DI/HC), ventral part of the ventral telencephalon/lateral septum (Vv/LS), dorsal part of the ventral telencephalon/nucleus accumbens (Vd/NA), periaqueductal gray (PAG), preoptic area parvocellular (POAp), anterior tuberal nucleus/ventral medial hypothalamus (aTn/VMH) and posterior tuberculum/ventral tegmental area (TPp/VTA) (Figure 1). We also measured Egr-1 expression in brainstem (located behind the cerebellum where cell bodies were present) as a control brain area that is unlikely to be involved in fathering.
Figure 1.
(a) Schematic horizontal drawings of brain areas measured. Images start at the dorsal part of the brain moving ventrally, with the anterior part of the brain on the left and the posterior on the right. Brain areas measured are highlighted in gray. The fish name is indicated first followed by the putative mammalian homolog: anterior tuberal nucleus/ventral medial hypothalamus (aTn/VMH), brain stem (BS), dorsal part of the ventral telencephalon/nucleus accumbens (Vd/NA), lateral part of dorsal telencephalon/hippocampus (DI/HC), medial dorsal telencephalon/basolateral amygdala (Dm/AMY), periaqueductal gray (PAG), posterior tuberculum/ventral tegmental area (TPp/VTA), preoptic area parvocellular (POAp), ventral pallium/bed nucleus of the stria terminalis_medial amygdala (Vs/BNST), ventral part of the ventral telencephalon/lateral septum (Vv/LS) and ventral tuberal/anterior hypothalamus (vTn/AH). (b) Photomicrographs of Egr-1 staining show the location of each brain area sampled.
Once a brain area was located, it was measured for mean gray value (MGV), a measurement of optical density (Ferreira and Rasband, 2011). MGV is calculated as MGV of pixels in region of interest divided by the number of pixels. To control for fluorescent signal background, a second MGV (control) was also taken in an area on the section where no staining occurred (i.e. ventricle). The second MGV was subtracted from the first to account for possible bleed-through of signal between sections. Because the optical sections were only 7μm thick, there was a chance that a key part of an area might have been missed in a single section. Therefore, the same brain area was measured in five consecutive optical sections and the average of the five sections was computed for each area. In situ hybridization, imaging and analysis of the samples were carried out blind with respect to treatment and processed in a random order between October and April 2012.
To evaluate the effectiveness of the in situ protocol for detecting differences in brain activation, Egr-1 expression was compared in brains taken during day vs night using both the in situ protocol and qPCR (whole brain). Egr-1 expression was higher during the day according to both the in situ protocol (Supplementary Figure 1) and qPCR (Supplementary Figure 2). Further inspection of the ISH brains showed higher expression in specific brain areas associated with visual processing (Supplementary Figure 1b). Further validation studies compared a sense to antisense probe (Supplementary Figure 3), a probe from a different species (Supplementary Figure 4) and used a Northern blot (Supplementary Figure 5) to confirm that the probes for Egr-1 were specific.
Data analysis
We used generalized linear models (Poisson distribution) to test for the effect of stimulus (male, female, predator), parental status (nonparental, parental) and their interaction on zig zags (plus 1 to account for zeros) and number of visits to the nest followed by post-hoc tests based on marginal meals. We report the range of Cohen’s d estimates for significant pairwise comparisons. General linear models were used to test for differences in Egr-1 expression across breeding stages within each brain area. Posthoc comparisons were made with the LSD test. Within each brain region we estimated Cohen’s d between each stage relative to the tending fry stage. Model fit was assessed by visual inspection of the residuals. Figures show controlled mean gray value (MGV) ± one standard error of the mean (S.E.M.). Statistical analyses were carried out in SPSS version 24.
All procedures were carried out under IACUC approval by the University of Illinois Urbana-Champaign IACUC (#12118) and conform to NIH standards for animal welfare. Fish were collected under collecting permit # SC-3310 to AMB from California Fish and Game.
RESULTS
Experiment 1
Males adjusted their courtship behavior depending on their stage in the breeding cycle. Nonparental males exhibited high rates of courtship behavior (the zig zag display) toward a female stickleback, but courtship behavior was almost entirely absent in parental males (Figure 2a, Stimulus Wald Chi-Square = 61.3, P<0.0001, Parental status Wald Chi-Square = 7.1, P=0.008, Stimulus x Parental status Wald-Chi-Square = 22.2, P<0.0001, n=40; Cohen’s d pairwise estimates ranged from 1.24–1.29). Rates of nest visitation also depended on parental status (Figure 2b, Stimulus Wald Chi-Square = 5.5, P=0.064), Parental status Wald Chi-Square = 16.7, P<0.0001, Stimulus x Parental status Wald Chi-Square = 2.8, P=0.25, n=40; Cohen’s d pairwise estimates ranged from 0.89–1.2). Overall, parental males visited the nest more compared to nonparental males, and rates of nest visitation were particularly high when males were presented with a female stickleback and a model predator.
Figure 2.
Rates of behavior toward social stimuli in nonparental and parental males. (a) Zig zag displays during the 10-min exposure to either a female stickleback, a male stickleback or a model predator. (b) Number of trips to the nest during the 10-min exposure to either a female stickleback, a male stickleback or a model predator. Different letters indicate statistically significant differences between brain areas according to post-hoc tests.
Experiment 2
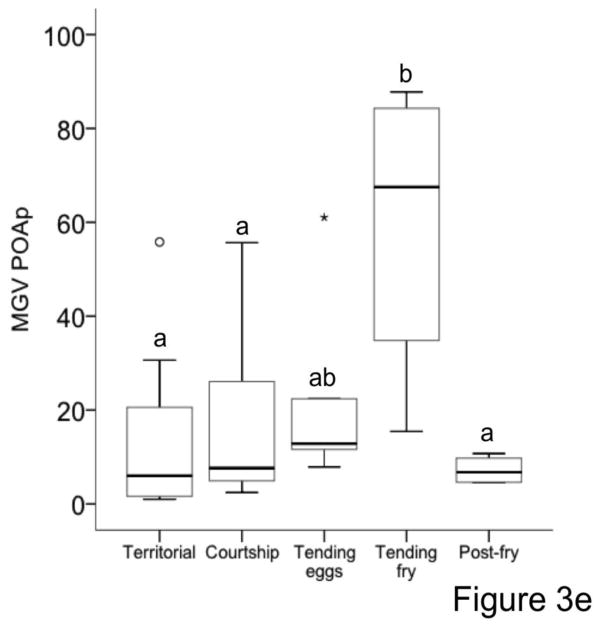
Egr-1 expression was consistently highest while males were tending fry compared to the other stages (Figure 3). Cohen’d d effect size estimates comparing each stage to the tending fry stage ranged from 0.26–4.58. This result was confirmed by visual inspection of individual brain regions (example in Supplementary figure 6). The expression patterns within each brain region suggest that in general, Egr-1 expression increased as males proceeded through the breeding stages but then dropped to levels comparable to those at the beginning of the breeding cycle, i.e. the territorial stage, after parenting (Figure 3).
Figure 3.
Egr-1 expression across breeding stages within each brain area. (a) anterior tuberal nucleus/ventral medial hypothalamus (vTn/VMH), (b) lateral part of dorsal telencephalon/hippocampus (DI/HC), (c) medial dorsal telencephalon/basolateral amygdala (Dm/AMY), (d) periaqueductal gray (PAG), (e) preoptic area parvocellular (POAp), (f) posterior tuberculum/ventral tegmental area (TPp/VTA), (g) dorsal part of the ventral telencephalon/nucleus accumbens (Vd/NA), (h) ventral pallium/bed nucleus of the stria terminalis_medial amygdala (Vs/BNST) (i) ventral tuberal/anterior hypothalamus (vTn/AH) and (j) ventral part of the ventral telencephalon/lateral septum (Vv/LS). Different letters indicate statistically significant differences between brain areas according to post-hoc tests.
In contrast, Egr-1 expression in a brain area outside the social behavior network (brain stem) did not differ across stages (F4,26=1.63, P=0.129, Supplementary Figure 7). Moreover, there were no differences in DAPI staining in the optic tectum (where penetration was greatest) across stages (F1,26=0.16, P=0.958). The Egr-1 ISH and DAPI staining were performed on the same tissue and scanned on the microscope at the same time with two different channels for DAPI and the Egr-1 fluorescent tag, 461 and 567 respectively. These results suggest that the observed differences in were in fact due to Egr-1 expression and not due other confounds, e.g. batch differences between runs, bleed-through of staining, etc. To assess the specificity of the probe, we also compared Egr-1 expression between day and night in medial dorsal telencephalon/basolateral amygdala and lateral part of dorsal telencephalon/hippocampus, two areas not likely to be sensitive to daylight. Neither comparison was statistically significant (medial dorsal telencephalon/basolateral amygdala: t16=1.567, p=0.137, lateral part of dorsal telencephalon/hippocampus: t16=0.404, p=0.179, Supplementary Figure 1c).
Overall, levels of Egr-1 expression were particularly high in the lateral part of dorsal telencephalon/hippocampus, with some subtle differences between different brain areas across stages (Figure 3). For example, during the territorial stage, Egr-1 expression also tended to be high in the anterior tuberal nucleus/ventral medial hypothalamus. Once males entered the courtship stage, Egr-1 expression tended to increase in the ventral pallium/bed nucleus of the stria terminalis medial amygdala and dorsal part of the ventral telencephalon/nucleus accumbens. When males were tending eggs, expression tended to increase in all measured brain areas except ventral part of the ventral telencephalon/lateral septum, and then expression in all brain areas increased dramatically while males were caring for fry. When males were no longer caring for offspring (i.e. during the post-fry stage), Egr-1 expression dropped, although it tended to remain high in the lateral part of dorsal telencephalon/hippocampus, medial dorsal telencephalon/basolateral amygdala and anterior tuberal nucleus/ventral medial hypothalamus relative to other brain areas (Figure 3).
We did not detect any differences in body size among males assigned to the different breeding stages (standard length F1,24=0.75, P=0.57).
DISCUSSION
Becoming a parent involves dramatic changes in brain, physiology and behavior. Detailed studies of the transition to motherhood have shown that hormonal changes during pregnancy and parturition cause structural remodeling of the brain, which is involved in mothers’ attraction to and responsiveness to her offspring (Pereira and Ferreira, 2016). Importantly, similar neural circuitry and systems are recruited in the service of fathering and in adoptive parents. Indeed, while fathers do not typically undergo pregnancy and birth (but see, for example “pseudopregnancy” in seahorses (Roth et al., 2012) and crop milk production in birds (Shetty et al., 1991)), fathers do often undergo changes that serve the same function, namely to successfully rear offspring, e.g. building a nest, defending the nest, providing care, responding to the changing needs of their offspring, etc. Indeed, functional MRI studies in humans show that similar brain areas are activated in mothers and fathers while they care for their offspring (Abraham et al., 2014; Rilling, 2013). However, we know less about the neural mechanisms associated with fatherhood in particular. Most of the animal models for fatherhood exhibit biparental care (e.g. African striped mice (Schradin et al., 2013), cichlids (O'Connell et al., 2012), California mice (Perea-Rodriguez et al., 2015), dart frogs (Schulte and Summers, 2017), degus (Gos et al., 2014), hamsters (Brooks et al., 2005)), and in biparental systems it can be difficult to tease out the effects of joint-caregiving from fatherhood per se (but see (DeAngelis et al., 2017) and (O'Connell et al., 2012)).
Results of experiment 1 suggest that males fine-tune their behavioral reactions to social stimuli depending on their parental status. Previous studies have suggested that although male sticklebacks will mate multiply within a given breeding attempt, they usually stop courting females once they have eggs in their nest (Kraak et al., 1999). Our behavioral results are consistent with this observation: males finely tuned their conspicuous courtship behavior depending on their parental status–only nonparental males performed zig zags. We also found that males modulated their nest-directed activities depending on their parental status and the immediate social context: parental males visited their nests more than nonparental males, and rates of nest visitation were particularly high when they were presented with a female stickleback and a model predator. Both female sticklebacks and birds are known to be nest predators (Bellesisles et al., 1990; Bellesisles and Fitzgerald, 1993; Defraipont et al., 1992; Fitzgerald, 1991, 1992; Fitzgerald et al., 1992; Foster, 1988; Largiader et al., 2001) therefore increased nest visitation rate in the presence of a female stickleback and a model bird predator could reflect increased nest defense.
The results from Experiment 2 suggest that dramatic changes in brain IEG expression accompany the transition between the nonparental and parental phases. Egr-1 expression increased as males started to provide care, and was especially high after the eggs hatched. This result parallels a study of biparental California mice, where exposing fathers to pup stimuli increased IEG expression in key brain areas that are involved in processing emotional stimuli such as the lateral habenula, caudal dorsal raphe nucleus (de Jong et al., 2010). We infer that the increase in Egr-1 expression in this study reflects the growing behavioral demands of caring for active offspring. For example, once the eggs hatch, fathers constantly engage in reciprocal behavioral interactions with their fry, and fry provide multiple types of sensory information. Fathering fry is particularly demanding as fathers must adjust their caregiving to match the physiological and behavioral needs of their free-swimming offspring.
It is also noteworthy that Egr-1 expression in fathers’ brains returned to ‘baseline’, pre-reproductive levels after they successfully reproduced. This result is consistent with studies of maternal care which have shown that brain IEG expression increases during care, and drops when offspring are absent (reviewed in (Stack and Numan, 2000)). The fact that Egr-1 expression dropped after breeding suggests that the high levels of Egr-1 expression observed while males were caring for offspring does not simply reflect the effects of season, maturation, age or experience. This pattern is intriguing given that male sticklebacks typically breed more than once during the breeding season, and there is widespread evidence that parenting experience permanently changes the brain and behavior of mothers (‘once a mother always a mother’, reviewed in (Stolzenberg and Champagne, 2016)). Further investigations of changes in neural IEG expression as a function of parenting experience in male sticklebacks is a promising future direction.
In experiment 2, Egr-1 expression changed over a relatively long, approximately 10 day period. These results are consistent with the literature showing that IEG expression can vary over both relatively short (minutes) and long (days) timescales. Other studies have documented similar constitutively different levels of IEG expression between relatively long-lasting states, e.g. between times of day (Kornhauser et al., 1996; Moffatt et al., 1995; Prosser et al., 1994) and stages of estrus (Lloyd et al., 1994; Nappi et al., 1997; Slade and Carter, 2000). Our results suggests that our in situ protocol is capable of tracking these quantitative changes in gene expression.
Egr-1 expression in particular brain areas
One somewhat surprising result from this study is that Egr-1 was expressed in many nodes within the social behavior network, rather than just a few. One possible explanation for this finding is that paternal care in sticklebacks involves elements not only of providing care, but also of aggression toward intruders and predators, and mating (because males continue to court females after they mate). We speculate that there was IEG expression in so many nodes of the social behavior network in male sticklebacks during the reproductive cycle because their behavior during this period is multidimensional. Indeed, it is possible that some of the changes in Egr-1 expression could be due to changes in activity or swimming, rather than attributable to the effects of parenting per say.
That being said, IEG expression was especially high in certain nodes within the social behavior network. For example, our results suggest that the lateral part of dorsal telencephalon/hippocampus had high levels of IEG expression in male sticklebacks throughout the breeding cycle. High levels of IEG expression in the lateral part of dorsal telencephalon/hippocampus could reflect the importance of cognitive processes, especially spatial cognition, for territorial males. Learning and memory are especially important for territorial animals (Stamps and Krishnan, 2001), which need to learn the spatial boundaries of their territory and engage in repeated interactions with their neighbors as they learn the boundaries of their territory and how to detect and repel intruders (Peeke, 1969; Peeke and Veno, 1973). In rodents, both mothers (Kinsley and Lambert, 2006; Levy et al., 2011; Pawluski et al., 2016; Ruscio et al., 2008) and fathers (Glasper et al., 2011; Lambert et al., 2011) undergo changes in hippocampal-mediated plasticity. A promising research direction is to examine the effect of experience as a parent on neurogenesis, hippocampal plasticity and performance on cognitive tasks in male sticklebacks.
The preoptic area is often associated with maternal, paternal and biparental behaviors in vertebrates (Alger et al., 2009; Bales and Saltzman, 2016; Buntin et al., 2006; de Jong et al., 2009; Gammie, 2005; Lee and Brown, 2002, 2007; Numan, 1974, 1986). For example, the preoptic bed nuclei of the stria terminalis is involved in the switch from infanticidal to paternal behavior (Tsuneoka et al., 2015) and lesions to the POA disrupted paternal behavior (Akther et al., 2014) in male mice. Surprisingly, IEG expression in the POAp was not as high as in other brain areas in this study. Instead, two other brain areas–the anterior tuberal nucleus (ventral medial hypothalamus) and medial dorsal telencephalon (basolateral amygdala)–exhibited relatively higher levels of Egr-1 expression during the breeding cycle in this study, especially when fry were present. The VMH has been implicated with maternal care in both birds (Pawlisch et al., 2012) and mammals (Cameron et al., 2011; Kinsley and Lambert, 2006). For example, the VMH is rich in steroid receptors in vertebrates (O'Connell and Hofmann, 2011), was activated in female starlings once they had a nest (Pawlisch et al., 2012), and inhibits maternal behavior in rats (Bridges et al., 1999). The VMH projects directly to the dorsal telencephalon/hippocampus in teleosts (O'Connell and Hofmann, 2011) therefore we speculate that the increased IEG expression in the VMH and hippocampus observed in this study reflects their coordinated regulation of parental and territorial behavior in sticklebacks. Further investigation into the types of cells showing Egr-1 expression will provide more information as to the role of both the VMH and hippocampus in the behavioral shifts necessary for coordinating care and defense.
The amygdala has also been linked to paternal behavior in mammals (reviewed in (Bales and Saltzman, 2016)). Both the PAG and VTA have shown increases in IEG expression in mothers (Gaffori and Le Moal, 1979; Lonstein and Stern, 1997), similar to the increases observed in this study of fathers. While we detected Egr-1 expression in the lateral septum, its expression was not quite as high as expected based on other studies. For example, cfos expression was higher in the lateral septum of fathers vs non-fathers in cichlids (O'Connell et al., 2012), and the lateral septum shows high levels of peptides such as vasopressin in fathering California mice (Bester-Meredith et al., 1999). It is possible that these differences reflect different demands of care-giving in the different species, e.g. huddling and grooming versus fanning and territory defense. Altogether, our results suggest that dramatic changes in behavior as male sticklebacks become fathers is accompanied by changes in neural gene expression in the brain. These results help to define the neural circuitry of fatherhood in fishes.
Supplementary Material
Highlights.
Becoming a father is a transformative event
Male stickleback fish are solely responsive for caring for their offspring
Males’ brains and behavior change as they become fathers
Acknowledgments
We thank David Clayton, Justin Rhodes and Andy Suarez for comments, Matt McNeil for technical help, the Robinson lab for access to the qPCR machine, Core Facilities at the Carl Woese Institute for Genomic Biology and Mayandi Sivaguru. We thank three anonymous reviewers for providing helpful comments on the manuscript. Support was provided by NIH GM082937 and NSF 1121980.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Molly Kent, Program in Neuroscience, University of Illinois, Urbana Champaign. Present address: University of Richmond.
Alison M. Bell, School of Integrative Biology, Program in Neuroscience, Program in Ecology, Evolution and Conservation, Institute for Genomic Biology, University of Illinois, Urbana Champaign
REFERENCES CITED
- Abraham E, Hendler T, Shapira-Lichter I, Kanat-Maymon Y, Zagoory-Sharon O, Feldman R. Father's brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9792–9797. doi: 10.1073/pnas.1402569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akther S, Fakhrul A, Higashida H. Effects of electrical lesions of the medial preoptic area and the ventral pallidum on mate-dependent paternal behavior in mice. Neuroscience Letters. 2014;570:21–25. doi: 10.1016/j.neulet.2014.03.078. [DOI] [PubMed] [Google Scholar]
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male european starlings. The European Journal of Neuroscience. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson E, Bogerd J, Borg B, Sharp PJ, Sherwood NM, Goos HJT. Characterization and localization of gonadotropin-releasing-hormone in the brain and pituitary of the 3-spined stickleback, Gasterosteus aculeatus. Cell and Tissue Research. 1995;279:485–493. [Google Scholar]
- Bacharach E, Mishra N, Briese T, Zody MC, Kembou Tsofack JE, Zamostiano R, Berkowitz A, Ng J, Nitido A, Corvelo A, Toussaint NC, Abel Nielsen SC, Hornig M, Del Pozo J, Bloom T, Ferguson H, Eldar A, Lipkin WI. Characterization of a novel orthomyxo-like virus causing mass die-offs of tilapia. MBio. 2016;7:e00431–00416. doi: 10.1128/mBio.00431-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Hormones and Behavior. 2016;77:249–259. doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesisles JC, Cloutier D, Fitzgerald GJ. Female cannibalism and male courtship tactics in threespine sticklebacks. Behavioral Ecology and Sociobiology. 1990;26:363–368. [Google Scholar]
- Bellesisles JC, Fitzgerald GJ. A fitness advantage of cannibalism in female sticklebacks. Ethology Ecology & Evolution. 1993;5:187–191. [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Hormones and Behavior. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: Inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. J Neuroendocrinol. 1999;11:259–266. doi: 10.1046/j.1365-2826.1999.00322.x. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Vella ET, Wynne-Edwards KE. Dopamine agonist treatment before and after the birth reduces prolactin concentration but does not impair paternal responsiveness in djungarian hamsters Phodopus campbelli. Hormones and Behavior. 2005;47:358–366. doi: 10.1016/j.yhbeh.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buntin L, Berghman LR, Buntin JD. Patterns of fos-like immunoreactivity in the brains of parent ring doves (Streptopelia risoria) given tactile and nontactile exposure to their young. Behavioral Neuroscience. 2006;120:651–664. doi: 10.1037/0735-7044.120.3.651. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biology. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Munshi RG, Fernald RD. Cytoarchitecture of a cichlid fish telencephalon. Brain, Behavior and Evolution. 2009;74:110–120. doi: 10.1159/000235613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Maruska KP. The mechanosensory lateral line system mediates activation of socially-relevant brain regions during territorial interactions. Frontiers in Behavioral Neuroscience. 2016:10. doi: 10.3389/fnbeh.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Soehngen E, Meaney MJ. Variation in maternal care influences ventromedial hypothalamus activation in the rat. J Neuroendocrinol. 2011;23:393–400. doi: 10.1111/j.1365-2826.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- Candolin U. Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behavioral Ecology and Sociobiology. 1997;41:81–87. [Google Scholar]
- Cerda-Reverter JM, Muriach B, Zanuy S, Munoz-Cueto JA. A cytoarchitectonic study of the brain of a perciform species, the sea bass (Dicentrarchus labrax): The midbrain and hindbrain. Acta Histochemica. 2008;110:433–450. doi: 10.1016/j.acthis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Zanuy S, Munoz-Cueto JA. Cytoarchitectonic study of the brain of a perciform species, the sea bass (Dicentrarchus labrax). I. The telencephalon. Journal of Morphology. 2001a;247:217–228. doi: 10.1002/1097-4687(200103)247:3<217::AID-JMOR1013>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Zanuy S, Munoz-Cueto JA. Cytoarchitectonic study of the brain of a perciform species, the sea bass (Dicentrarchus labrax). II. The diencephalon. Journal of Morphology. 2001b;247:229–251. doi: 10.1002/1097-4687(200103)247:3<229::AID-JMOR1014>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Chen CC, Fernald RD. Gnrh and gnrh receptors: Distribution, function and evolution. Journal of Fish Biology. 2008;73:1099–1120. [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiology of Learning and Memory. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: Neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Hormones and Behavior. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- DeAngelis R, Gogola J, Dodd L, Rhodes JS. Opposite effects of nonapeptide antagonists on paternal behavior in the teleost fish amphiprion ocellaris. Hormones and Behavior. 2017;90:113–119. doi: 10.1016/j.yhbeh.2017.02.013. [DOI] [PubMed] [Google Scholar]
- DeAngelis RS, Rhodes JS. Sex differences in steroid hormones and parental effort across the breeding cycle in Amphiprion ocellaris. Copeia. 2016;104:586–593. [Google Scholar]
- Defraipont M, Fitzgerald GJ, Guderley H. Femme fatale – the case of the threespine stickleback. Ethology. 1992;91:147–152. [Google Scholar]
- Desjardins JK, Becker L, Fernald RD. The effect of observers on behavior and the brain during aggressive encounters. Behavioural Brain Research. 2015;292:174–183. doi: 10.1016/j.bbr.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Fernald RD. What do fish make of mirror images? Biology Letters. 2010;6:744–747. doi: 10.1098/rsbl.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Klausner JQ, Fernald RD. Female genomic response to mate information. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21176–21180. doi: 10.1073/pnas.1010442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O'Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom P. Developmental changes in the brain-stem serotonergic nuclei of teleost fish and neural plasticity. Cellular and Molecular Neurobiology. 1994;14:381–393. doi: 10.1007/BF02088718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom P, Holmqvist BI, Panula P. Histamine-immunoreactive neurons in the brain of the teleost Gasterosteus aculeatus l. Correlation with hypothalamic tyrosine hydroxylase- and serotonin-immunoreactive neurons. Journal of Chemical Neuroanatomy. 1995;8:75–85. doi: 10.1016/0891-0618(94)00030-w. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Honkanen T, Borg B. Development of tyrosine hydroxylase-, dopamine- and dopamine beta-hydroxylase-immunoreactive neurons in a teleost, the three-spined stickleback. Journal of Chemical Neuroanatomy. 1992;5:481–501. doi: 10.1016/0891-0618(92)90004-a. [DOI] [PubMed] [Google Scholar]
- Ekström P, Johnsson C, Ohlin L. Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. Journal of Comparative Neurology. 2001;436:92–110. [PubMed] [Google Scholar]
- Ekstrom P, Nyberg L, van Veen T. Ontogenetic development of serotoninergic neurons in the brain of a teleost, the three-spined stickleback. An immunohistochemical analysis. Brain Research. 1985;349:209–224. doi: 10.1016/0165-3806(85)90145-2. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Ohlin LM. Ontogeny of gaba-immunoreactive neurons in the central nervous system in a teleost, Gasterosteus aculeatus l. Journal of Chemical Neuroanatomy. 1995;9:271–288. doi: 10.1016/0891-0618(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Reschke M, Steinbusch H, van Veen T. Distribution of noradrenaline in the brain of the teleost Gasterosteus aculeatus l.: An immunohistochemical analysis. The Journal of Comparative Neurology. 1986;254:297–313. doi: 10.1002/cne.902540304. [DOI] [PubMed] [Google Scholar]
- Ekstrom P, Van Veen T. Distribution of 5-hydroxytryptamine (serotonin) in the brain of the teleost Gasterosteus aculeatus l. The Journal of Comparative Neurology. 1984;226:307–320. doi: 10.1002/cne.902260302. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Social control of the brain. In: Hyman SE, editor. Annual Review of Neuroscience. Vol. 35. 2012. pp. 133–151. [DOI] [PubMed] [Google Scholar]
- Ferreira T, Rasband W. ImageJ user guide (ij 1.45r) National Institutes of Health; 2011. pp. 112–125. [Google Scholar]
- Fitzgerald GJ. The role of cannibalism in the reproductive ecology of the threespine stickleback. Ethology. 1991;89:177–194. [Google Scholar]
- Fitzgerald GJ. Egg cannibalism by sticklebacks - spite or selfishness. Behavioral Ecology and Sociobiology. 1992;30:201–206. [Google Scholar]
- Fitzgerald GJ, Whoriskey FG, Morrissette J, Harding M. Habitat scale, female cannibalism and male reproductive success in 3-spined sticklebacks (Gasterosteus aculeatus) Behavioral Ecology. 1992;3:141–147. [Google Scholar]
- Foster SA. Diversionary displays of paternal stickleback -- defenses against cannibalistic groups. Behavioral Ecology and Sociobiology. 1988;22:335–340. [Google Scholar]
- Gaffori O, Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiology and Behavior. 1979;23:317–323. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behavioral and Cognitive Neuroscience Reviews. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Glasper ER, Kozorovitskiy Y, Pavlic A, Gould E. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. The Journal of Comparative Neurology. 2011;519:2271–2281. doi: 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gos T, Schulkin J, Gos A, Bock J, Poeggel G, Braun K. Paternal deprivation affects the functional maturation of corticotropin-releasing hormone (crh)- and calbindin-d28k-expressing neurons in the bed nucleus of the stria terminalis (bnst) of the biparental Octodon degus. Brain Structure & Function. 2014;219:1983–1990. doi: 10.1007/s00429-013-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Girard E, Tweedle J, Ironstone J, Cuddy M, Ellis W, Maler L. Long-term recognition memory of individual conspecifics is associated with telencephalic expression of egr-1 in the electric fish Apteronotus leptorhynchus. Journal of Comparative Neurology. 2010;518:2666–2692. doi: 10.1002/cne.22358. [DOI] [PubMed] [Google Scholar]
- Hellqvist A, Bornestaf C, Borg B, Schmitz M. Cloning and sequencing of the fsh-beta and lh beta-subunit in the three-spined stickleback, Gasterosteus aculeatus, and effects of photoperiod and temperature on lh-beta and fsh-beta mrna expression. General and Comparative Endocrinology. 2004;135:167–174. doi: 10.1016/j.ygcen.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hellqvist A, Schmitz M, Borg B. Effects of castration and androgen-treatment on the expression of fsh-beta and lh-beta in the three-spine stickleback, Gasterosteus aculeatus - feedback differences mediating the photoperiodic maturation response? General and Comparative Endocrinology. 2008;158:178–182. doi: 10.1016/j.ygcen.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: An organ with peripartal plasticity. Neural Plasticity. 2014;2014:574159. doi: 10.1155/2014/574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Osterman A, Mayer I, Borg B. 11-ketotestosterone is not responsible for the entire testicular effect on male reproductive behaviour in the threespine stickleback. Behaviour. 2008;145:509–525. [Google Scholar]
- Hofmann HA. The neuroendocrine action potential. Hormones and Behavior. 2010;58:555–562. doi: 10.1016/j.yhbeh.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Honkanen T, Ekstrom P. An immunocytochemical study of the development of the olfactory system in the three-spined stickleback (Gasterosteus aculeatus l., teleostei) Anatomy and Embryology. 1991;184:469–477. doi: 10.1007/BF01236053. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Abizaid A, Bielajew C. Modeling dad: Animal models of paternal behavior. Neuroscience Biobehavioral Reviews. 2010;34:438–451. doi: 10.1016/j.neubiorev.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Amory-Meyer E. Why the maternal brain? Journal of Neuroendocrinology. 2011;23:974–983. doi: 10.1111/j.1365-2826.2011.02194.x. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. The maternal brain. Scientific American. 2006;294:72–79. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]
- Kleszczynska A, Sokolowska E, Kulczykowska E. Variation in brain arginine vasotocin (avt) and isotocin (it) levels with reproductive stage and social status in males of three-spined stickleback (Gasterosteus aculeatus) General and Comparative Endocrinology. 2012;175:290–296. doi: 10.1016/j.ygcen.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate-early genes, and circadian rhythms. Behavior Genetics. 1996;26:221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- Kraak SBM, Bakker TCM, Mundwiler B. Correlates of the duration of the egg collecting phase in the three-spined stickleback. Journal of Fish Biology. 1999;54:1038–1049. [Google Scholar]
- Kress S, Wullimann MF. Correlated basal expression of immediate early gene egr1 and tyrosine hydroxylase in zebrafish brain and downregulation in olfactory bulb after transitory olfactory deprivation. Journal of Chemical Neuroanatomy. 2012;46:51–66. doi: 10.1016/j.jchemneu.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Lambert KG. The parental brain: Transformations and adaptations. Physiology & Behavior. 2012;107:792–800. doi: 10.1016/j.physbeh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Franssen CL, Bardi M, Hampton JE, Hainley L, Karsner S, Tu EB, Hyer MM, Crockett A, Baranova A, Ferguson T, Ferguson T, Kinsley CH. Characteristic neurobiological patterns differentiate paternal responsiveness in two peromyscus species. Brain, Behavior and Evolution. 2011;77:159–175. doi: 10.1159/000326054. [DOI] [PubMed] [Google Scholar]
- Largiader CR, Fries V, Bakker TCM. Genetic analysis of sneaking and egg-thievery in a natural population of the three-spined stickleback (Gasterosteus aculeatus l.) Heredity. 2001;86:459–468. doi: 10.1046/j.1365-2540.2001.00850.x. [DOI] [PubMed] [Google Scholar]
- Lau BYB, Mathur P, Gould GG, Guo S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2581–2586. doi: 10.1073/pnas.1018275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female california mice (Peromyscus californicus) Behavioral Neuroscience. 2002;116:968–975. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in california mice (Peromyscus californicus) Physiology and Behavior. 2007;92:617–628. doi: 10.1016/j.physbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Levy F, Gheusi G, Keller M. Plasticity of the parental brain: A case for neurogenesis. Journal of Neuroendocrinology. 2011;23:984–993. doi: 10.1111/j.1365-2826.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- Long X, Colonell J, Wong A, Singer R, Lionnet T. Quantitative mrna imaging throughout the entire drosophila brain. BioRxiv. 2016 doi: 10.1101/096388. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: Effects of perioral, rooting, and suckling stimuli from pups. Hormones and Behavior. 1997;32:155–166. doi: 10.1006/hbeh.1997.1416. [DOI] [PubMed] [Google Scholar]
- Loveland JL, Fernald RD. Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behavioural Brain Research. 2017;317:188–203. doi: 10.1016/j.bbr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Becker L, Neboori A, Fernald RD. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. Journal of Experimental Biology. 2013a;216:3656–3666. doi: 10.1242/jeb.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology. 2011;26:412–423. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Zhang A, Neboori A, Fernald RD. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an african cichlid fish. Journal of Neuroendocrinology. 2013b;25:145–157. doi: 10.1111/j.1365-2826.2012.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer I, Borg B, Pall M. Hormonal control of male reproductive behaviour in fishes: A stickleback perspective. Behaviour. 2004;141:1499–1510. [Google Scholar]
- McNeill MS, Robinson GE. Voxel-based analysis of the immediate early gene, c-jun, in the honey bee brain after a sucrose stimulus. Insect Molecular Biology. 2015;24:377–390. doi: 10.1111/imb.12165. [DOI] [PubMed] [Google Scholar]
- Moffatt CA, Ball GF, Nelson RJ. The effects of photoperiod on olfactory c-fos expression in prairie voles, Microtus ochrogaster. Brain Research. 1995;677:82–88. doi: 10.1016/0006-8993(95)00125-a. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Bonneau MJ, Rivest S. Influence of the estrous cycle on c-fos and crh gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology. 1997;65:29–46. doi: 10.1159/000127162. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1974;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M. The role of the medial preoptic area in the regulation of maternal behavior in the rat. Annals of the New York Academy of Sciences. 1986;474:226–233. doi: 10.1111/j.1749-6632.1986.tb28014.x. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. The Journal of Comparative Neurology. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Matthews BJ, Hofmann HA. Isotocin regulates paternal care in a monogamous cichlid fish. Hormones and Behavior. 2012;61:725–733. doi: 10.1016/j.yhbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Rigney MM, Dykstra DW, Hofmann HA. Neuroendocrine mechanisms underlying sensory integration of social signals. Journal of Neuroendocrinology. 2013;25:644–654. doi: 10.1111/jne.12045. [DOI] [PubMed] [Google Scholar]
- Pall MK, Hellqvist A, Schmitz M, Olsson PE, Mayer I, Borg B. Changes in reproductive physiology and behaviour over the nesting cycle in male three-spined sticklebacks. Journal of Fish Biology. 2005;66:1400–1410. [Google Scholar]
- Pall MK, Mayer I, Borg B. Androgen and behavior in the male three-spined stickleback, Gasterosteus aculeatus i. - changes in 11-ketotestosterone levels during the nesting cycle. Hormones and Behavior. 2002a;41:377–383. doi: 10.1006/hbeh.2002.1777. [DOI] [PubMed] [Google Scholar]
- Pall MK, Mayer I, Borg B. Androgen and behavior in the male three-spined stickleback, Gasterosteus aculeatus ii. Castration and 11-ketoandrostenedione effects on courtship and parental care during the nesting cycle. Hormones and Behavior. 2002b;42:337–344. doi: 10.1006/hbeh.2002.1820. [DOI] [PubMed] [Google Scholar]
- Pawlisch BA, Kelm-Nelson CA, Stevenson SA, Riters LV. Behavioral indices of breeding readiness in female european starlings correlate with immunolabeling for catecholamine markers in brain areas involved in sexual motivation. General and Comparative Endocrinology. 2012;179:359–368. doi: 10.1016/j.ygcen.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lambert KG, Kinsley CH. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Hormones and Behavior. 2016;77:86–97. doi: 10.1016/j.yhbeh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Peeke HVS. Habituation of conspecific aggression in the three-spined stickleback (Gasterosteus aculeatus l.) Behaviour. 1969;35:137–156. [Google Scholar]
- Peeke HVS, Veno A. Stimulus specificity of habituated aggression in the stickleback (Gasterosteus aculeatus) Behavioral Biology. 1973:427–432. doi: 10.1016/s0091-6773(73)80083-5. [DOI] [PubMed] [Google Scholar]
- Perea-Rodriguez JP, Takahashi EY, Amador TM, Hao RC, Saltzman W, Trainor BC. Effects of reproductive experience on central expression of progesterone, oestrogen alpha, oxytocin and vasopressin receptor mrna in male california mice (Peromyscus californicus) Journal of Neuroendocrinology. 2015;27:245–252. doi: 10.1111/jne.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Neuroanatomical and neurochemical basis of parenting: Dynamic coordination of motivational, affective and cognitive processes. Hormones and Behavior. 2016;77:72–85. doi: 10.1016/j.yhbeh.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Peter RE, Macey MJ, VEG A stereotaxic atlas and technique for forbrain neclei of the killifish, Fundulus heteroclitus. The Journal of Comparative Neurology. 1975;159:103–127. doi: 10.1002/cne.901590107. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Solomon-Lane TK, Willis MC, Grober MS. A mechanism for rapid neurosteroidal regulation of parenting behaviour. Proceedings of the Royal Society B-Biological Sciences. 2014:281. doi: 10.1098/rspb.2014.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Macdonald ES, Heller HC. C-fos mrna in the suprachiasmatic nuclei in vitro shows a circadian rhythm and responds to a serotonergic agonist. Brain Research Molecular Brain Research. 1994;25:151–156. doi: 10.1016/0169-328x(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Raj A, Tyagi S. Detection of individual endogenous rna transcripts in situ using multiple singly labeled probes. Methods in Enzymology. 2010;472:365–386. doi: 10.1016/S0076-6879(10)72004-8. [DOI] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mrna molecules using multiple singly labeled probes. Nature Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan KE, Ganesh A, Dharaneedharan S, Radhakrishnan K. Spatial learning-induced egr-1 expression in telencephalon of gold fish Carassius auratus. Fish Physiology and Biochemistry. 2011;37:153–159. doi: 10.1007/s10695-010-9425-4. [DOI] [PubMed] [Google Scholar]
- Rall MK, Liljander M, Borg B. Prolactin diminishes courtship behaviour and stimulates fanning in nesting male three-spined sticklebacks, Gasterosteus aculeatus. Behaviour. 2004;141:1511–1519. [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley JL, Foran CM. Quantification of whole brain arginine vasotocin for two syngnathus pipefishes: Elevated concentrations correlated with paternal brooding. Fish Physiology and Biochemistry. 2010;36:867–874. doi: 10.1007/s10695-009-9361-3. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetti M, Radua J, Paloyelis Y, Xenaki LA, Frascarelli M, Caverzasi E, Politi P, Fusar-Poli P. Neurofunctional maps of the 'maternal brain' and the effects of oxytocin: A multimodal voxel-based meta-analysis. Psychiatry and Clinical Neurosciences. 2014;68:733–751. doi: 10.1111/pcn.12185. [DOI] [PubMed] [Google Scholar]
- Roth O, Klein V, Beemelmanns A, Scharsack JP, Reusch TBH. Male pregnancy and biparental immune priming. American Naturalist. 2012;180:802–814. doi: 10.1086/668081. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behavioural Brain Research. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaggiante M, Grober MS, Lorenzi V, Rasotto MB. Changes along the male reproductive axis in response to social context in a gonochoristic gobiid, Zosterisessor ophiocephalus (teleostei, gobiidae), with alternative mating tactics. Hormones and Behavior. 2004;46:607–617. doi: 10.1016/j.yhbeh.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Scaggiante M, Grober MS, Lorenzi V, Rasotto MB. Variability of gnrh secretion in two goby species with socially controlled alternative male mating tactics. Hormones and Behavior. 2006;50:107–117. doi: 10.1016/j.yhbeh.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Schradin C, Kenkel W, Krackow S, Carter CS. Staying put or leaving home: Endocrine, neuroendocrine and behavioral consequences in male African striped mice. Hormones and Behavior. 2013;63:136–143. doi: 10.1016/j.yhbeh.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Schulte LM, Summers K. Searching for hormonal facilitators: Are vasotocin and mesotocin involved in parental care behaviors in poison frogs? Physiology & Behavior. 2017;174:74–82. doi: 10.1016/j.physbeh.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Shao YT, Tseng YC, Chang CH, Yan HY, Hwang PP, Borg B. Gnrh mrna levels in male three-spined sticklebacks, Gasterosteus aculeatus, under different reproductive conditions. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2015;180:6–17. doi: 10.1016/j.cbpa.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Shetty S, Jacob RT, Shenoy KB, Hegde SN. Patterns of breeding behavior in the domestic pigeon. Bird Behaviour. 1991;9:14–19. [Google Scholar]
- Slade JP, Carter DA. Cyclical expression of egr-1/ngfi-a in the rat anterior pituitary: A molecular signal for ovulation? J Neuroendocrinol. 2000;12:671–676. doi: 10.1046/j.1365-2826.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- Smith C, Wootton RJ. Parental energy expenditure of the male three-spined stickleback. Journal of Fish Biology. 1999;54:1132–1136. [Google Scholar]
- Smith C, Wootton RJ. The remarkable reproductive diversity of teleost fishes. Fish Fish. 2016;17:1208–1215. [Google Scholar]
- Stack EC, Numan M. The temporal course of expression of c-fos and fos b within the medial preoptic area and other brain regions of postpartum female rats during prolonged mother--young interactions. Behavioral Neuroscience. 2000;114:609–622. doi: 10.1037//0735-7044.114.3.609. [DOI] [PubMed] [Google Scholar]
- Stamps Judy A, Krishnan VV. How territorial animals compete for divisible space: A learning – based model with unequal competitors. The American Naturalist. 2001;157:154–169. doi: 10.1086/318634. [DOI] [PubMed] [Google Scholar]
- Stapel LC, Lombardot B, Broaddus C, Kainmueller D, Jug F, Myers EW, Vastenhouw NL. Automated detection and quantification of single rnas at cellular resolution in zebrafish embryos. Development (Cambridge, England) 2016;143:540–546. doi: 10.1242/dev.128918. [DOI] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Consistent individual differences in fathering in threespined stickleback. Current Zoology. 2012;58:45–52. doi: 10.1093/czoolo/58.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Paternal programming in sticklebacks. Animal Behaviour. 2014;95:165–171. doi: 10.1016/j.anbehav.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Consistent individual differences in paternal behavior: A field study of threespine stickleback. Behavioral Ecology and Sociobiology. 2015;69:227–236. doi: 10.1007/s00265-014-1835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Trapp RM, Bell AM. Do reproduction and parenting influence personality traits? Insights from threespine stickleback. Animal Behaviour. 2016;112:247–254. doi: 10.1016/j.anbehav.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver KA, Harris RM, Townsend JP, Hofmann HA, Alonzo SH. Neural gene expression profiles and androgen levels underlie alternative reproductive tactics in the ocellated wrasse, Symphodus ocellatus. Ethology. 2015;121:152–167. [Google Scholar]
- Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Hormones and Behavior. 2016;77:204–210. doi: 10.1016/j.yhbeh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Tantirigama ML, Oswald MJ, Clare AJ, Wicky HE, Day RC, Hughes SM, Empson RM. Fezf2 expression in layer 5 projection neurons of mature mouse motor cortex. The Journal of Comparative Neurology. 2016;524:829–845. doi: 10.1002/cne.23875. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, Yu LMY, Odaka Y, Shinozuka K, McHugh TJ, Kuroda KO. Distinct preoptic-bst nuclei dissociate paternal and infanticidal behavior in mice. Embo Journal. 2015;34:2652–2670. doi: 10.15252/embj.201591942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubert C, Lo Nostro F, Villafane V, Pandolfi M. Aggressive behavior and reproductive physiology in females of the social cichlid fish Cichlasoma dimerus. Physiology & Behavior. 2012;106:193–200. doi: 10.1016/j.physbeh.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Wootton RJ. The Biology of the Sticklebacks. Academic Press; London ; New York: 1976. [Google Scholar]
- Wootton RJ. A Functional Biology of Sticklebacks. University of California Press; Berkeley: 1984. [Google Scholar]
- Wulliman MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhauser; Basel: 1996. [Google Scholar]
- Yaeger C, Ros AM, Cross V, Deangelis RS, Stobaugh DJ, Rhodes JS. Blockade of arginine vasotocin signaling reduces aggressive behavior and c-fos expression in the preoptic area and periventricular nucleus of the posterior tuburculum in male Amphiprion ocellaris. Neuroscience. 2014;267:205–218. doi: 10.1016/j.neuroscience.2014.02.045. [DOI] [PubMed] [Google Scholar]
- Zeiss. Zen imaging software. Carl Zeiss Microimaging Inc; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.