Abstract
Background
Steroid-resistant nephrotic syndrome (SRNS) is the second most frequent cause of end-stage renal disease (ESRD) among patients manifesting <25 years of age. We performed mutation analysis using a high-throughput PCR-based microfluidic technology in 24 single-gene causes of SRNS in a cohort of 72 families, who presented with SRNS before the age of 25 years.
Methods
Within an 18-month interval, we obtained DNA samples, pedigree information, and clinical information from 77 consecutive children with SRNS from 72 different families seen at Boston Children’s Hospital (BCH). Mutation analysis was completed by combining high-throughput multiplex PCR with next-generation sequencing. We analyzed the sequences of 18 recessive and 6 dominant genes of SRNS in all 72 families for disease-causing variants.
Results
We identified the disease causing mutation in 8 of 72 (11.1%) families. Mutations were detected in the 6 genes: NPHS1 (2/72), WT1 (2/72), and in NPHS2, MYO1E, TRPC6, and INF2.
Median age of onset was 4.1 years in patients without a mutation (range 0.5–18.8), and 3.2 years in those where the causative mutation was detected (range 0.1–14.3). Mutations in dominant genes presented with median onset of 4.5 years (range 3.2–14.3). Mutations in recessive genes presented with median onset of 0.5 years (range 0.1–3.2).
Conclusion
Our molecular genetic diagnostic study identified underlying monogenic causes of steroid-resistant nephrotic syndrome in ~11% of patients with SRNS using a cost-effective technique. We delineated some of the therapeutic, diagnostic, and prognostic implications. Our study confirms that genetic testing is indicated in pediatric patients with SRNS.
Keywords: nephrotic syndrome, monogenic, multiplex PCR
INTRODUCTION
Steroid-resistant nephrotic syndrome (SRNS) is the second most frequent cause of end-stage renal disease (ESRD) in individuals under <25 years of age [1]. It is clinically defined by proteinuria (>3.5 g/day in adults or 40 mg/m2/hr in children), hypoalbuminemia, and edema. SRNS frequently progresses to ESRD. The hallmark histologic feature on kidney biopsy is focal segmental glomerulosclerosis (FSGS). FSGS recurs in approximately 11–50 % of patients who undergo renal transplantation [1–3]. SRNS has very limited treatment options [4] and in the United States, the prevalence of chronic kidney disease (CKD) has been rising in the last few decades [5]. The underlying pathogenesis of SRNS remains elusive, and thus treatment options have largely remained unchanged for the last 50 years. However, the discovery of >30 monogenic genes, that if mutated, cause SRNS has highlighted the central role of the glomerular podocyte in the pathogenesis of SRNS [4, 6, 7]. Study of the mechanisms that are dysregulated in monogenic forms of SRNS has also revealed insights into potential treatment modalities [8–10]. Our laboratory recently published an international study demonstrating that 29.5 % of cases of SRNS had causative monogenic mutations in one of 27 SRNS genes. This has recently been confirmed by other groups [11, 12]. However, there has been a paucity of data from primarily outbred populations. Most children in the United States that have SRNS are from a non-consanguineous union. We therefore studied the prevalence of monogenic causes of nephrotic syndrome (NS) in our patient cohort at Boston Chidren’s Hospital (BCH). During the study interval of 18 months, we examined all patients that met SRNS clinical criteria for 24 of the most common monogenic causes of NS (Supplementary Table 1). We employed a high-throughput panel-based exon-targeted PCR approach combined with next-generation sequencing that we previously established [13–16]. Our data demonstrate prevalence rates that are similar to the outbred populations seen in larger cohort studies [11, 13, 14]. In regards to clinical management, our findings emphasize the need for molecular genetic diagnostics in children and young adults with SRNS.
METHODS AND STUDY DESIGN
Study population
Between May, 2013 and October, 2014 (18 months) we obtained genomic DNA from blood or saliva samples, pedigree information, and clinical information from a consecutive cohort at Boston Children’s Hospital (BCH). The cohort consisted of 77 individuals (72 families) that were recruited following informed consent (www.renalgenes.org) (Table 1). All recruited patients were diagnosed by pediatric nephrologists using standard clinical and histologic criteria for SRNS as previously described [17]. Inclusion criteria included onset of symptoms before 25 years of age AND a clinical diagnosis of SRNS (e.g. proteinuria, hypoalbuminemia, edema) OR nephrotic range proteinuria with kidney histology of FSGS or diffuse mesangial sclerosis.
Table 1.
Demographic data of probands from 72 families with SRNS in whom panel sequencing was performed and evaluated for 24 known SRNS genes.
| Causative gene detected | No detection of the causative gene | Dominant gene detected | Recessive gene detected | Total | |
|---|---|---|---|---|---|
| Male (sex) | 4 | 37 | 3 | 0 | 41 |
| Female (sex) | 4 | 27 | 1 | 4 | 31 |
| Median age of SRNS Onset [years] (range) | 3.2 (0.1–14.3) | 4.1 (0.5–18.8) | 4.5 (3.2–14.3) | 0.5 (0.1–3.2) | 3.5 (0.1–18.8) |
| Parental consanguinity | 2/8 (25%) | 5/64 (7.8%) | 0/4 (0%) | 2/4 (50%) | 7/72 (9.7%) |
SRNS = steroid resistant nephrotic syndrome
Selection of genes for panel sequencing
24 of the 27 genes that were previously examined in our large international study [14] were included in the panel (Supplementary Table 1). We chose the most commonly mutated of the 27 genes found in patients with nephrotic syndrome and excluded the following three very rare causes of SRNS: SCARB2, MEFV, and NEIL1. We chose to exclude these genes because of limitations to multiplex PCR technique [15, 16]. We chose to include CUBN as a phenocopy of nephrotic syndrome as it was discovered in patients with high grade proteinuria and/or SRNS [10]. A list of genes, their mode of inheritance, and references is given in (Supplementary Table 1).
High-throughput targeted exon sequencing
We evaluated the recruited samples by using a high-throughput multiplex PCR approach combined with next-generation sequencing for mutation analysis as previously described [15, 16]. We applied 13-fold primer multiplexing allowing PCR-based amplification of 609 amplicons in 24 genes for all 72 families simultaneously as developed previously [13–16]. Our analysis included 18 recessive SRNS genes and 6 dominant SRNS genes.
In a second PCR we indexed all 77 patient derived PCR products uniquely with genetic barcodes. Unidirectional sequencing (150 bases) was performed on 1 lane of a HiSeq 2500 [13–16].
Data analysis
Bioinformatic analysis was performed using CLC Genomics Workbench™ software, and all variants called were confirmed via Sanger sequencing including parental DNA for segregation analysis if available. Criteria for variant calling was applied as previously described [14].
Mutation confirmation
If requested on the consent form, primary clinicians were notified of a result from research based sequencing, codified by mutated genes and exon only. If desired by the family, referral to genetic counseling as well as CLIA-certified specific mutation confirmation (SMC) was pursued before any clinical decision making was performed regarding the genetic findings.
RESULTS
We performed high-throughput targeted exon sequencing with Fluidigm™ technology combined with next-generation sequencing in 77 individuals from 72 families as previously described [14–16]. We then performed bioinformatic mutation analysis in each variant called and isolated disease-causing mutations in 24 monogenic causes of SRNS [14].
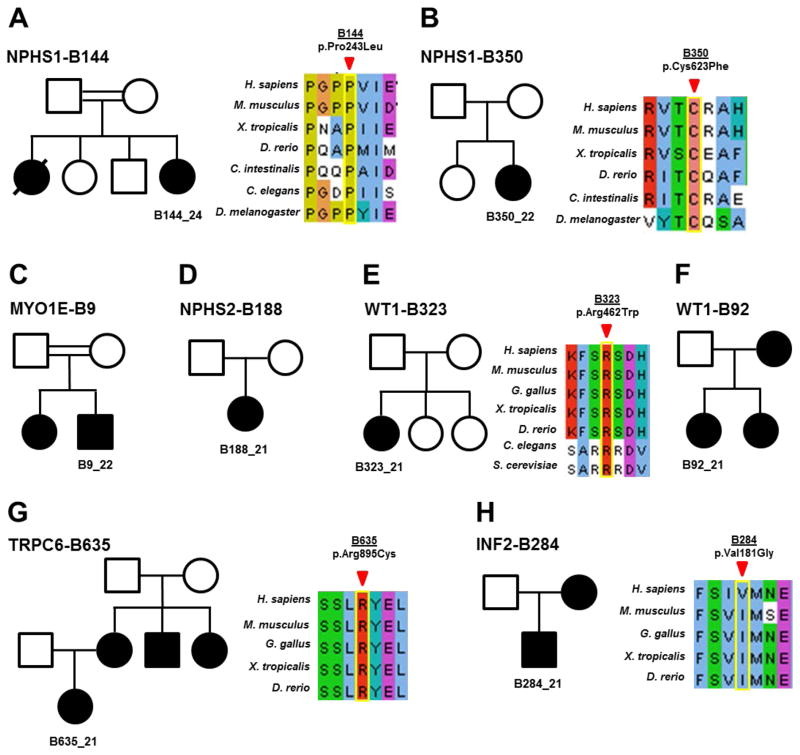
In this single center study we identified the disease-causing mutation in 8 of 72 (11.1 %) families with SRNS manifesting before 25 years of life (Table 2). Mutations were detected in 6 of the 24 genes examined, including NPHS1 (2/72), WT1 (2/72), with single families with mutations in NPHS2, MYO1E, TRPC6 and INF2 (Table 2). Clinical details of each family and gene are discussed below.
Table 2. Genotypes and phenotypes from patients with SRNS, in whom the causative mutation was identified in one of 24 known monogenic SRNS genes.
Patients are listed in order of age of onset (see Figure 2).
| Patient | Gene | Nucleotide alteration |
Amino acid alteration |
Sex (karyot- ype) |
MOI (zygosity) |
Amino acid conservation to species |
ExAC** | Ethnicity | Age of onset (years) |
Consan- guinity |
Initial biopsy |
Variant Class*** |
Reference (PubMed ID) |
Clinical consequences of finding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B144_24 | NPHS1 | c.728C>T | p.P243L | F | AR (Hom) | D. melanogaster | - | Arabic | 0.1 | Yes | ND | Likely Pathogenic | novel | Genetic Counseling; recurrence risk in Tx reduced |
| B350_22 | NPHS1 | c.1868G>T | p.C623F | F | AR (Hom) | D. rerio | 3/0/119,848 | Caucasian | 0.3 | No | DMS | Pathogenic | 9915943; 11726550 | Genetic Counseling; recurrence risk in Tx reduced |
| B9_22 | MYO1E | c.141C>G | p.Y47* | M | AR (Hom) | NA | - | Arabic | 0.8 | Yes | ND | Pathogenic | 23595123 | Genetic Counseling; Recurrence risk in Tx reduced |
| B188_21 | NPHS2 | c.855-56AA>del | p.R286Tfs *17 | F | AR (Hom) | NA | 8/0/115,938 | Hispanic | 3.2 | No | MCNS | Pathogenic | 10742096 | Genetic Counseling; recurrence risk in Tx reduced |
| B323_21 | WT1 | c.1384C>T | p.R462W | F (46,XY) | AD (het) | S. cerevisiae | - | Caucasian | 3.2 | No | FSGS | Pathogenic | 1655284 | Genetic Counseling; recurrence risk in Tx reduced; screening for GB |
| B92_21 | WT1 | c.1432+4C> T | IVS9 C-T +4 KTS | F (46,XY) | AD (het) | NA | - | Caucasian | 4.0 | No | FSGS | Pathogenic | 9398852 | Genetic Counseling; recurrence risk in Tx reduced |
| B635_21 | TRPC6 | c.2683C>T | p.R895C | F | AD (het) | D. rerio | - | Indian | 5.2 | No | ND | Pathogenic | 15924139 | potentially CNI sensitive; recurrence risk in Tx reduced |
| B284_21 | INF2 | c.542T>G | p.V181G | M | AD (het) | H. sapiens | - | Caucasian | 14.3 | No | FSGS | Pathogenic | 23014460 | Genetic Counseling; recurrence risk in Tx reduced |
All probands from each of the families in whom the causative gene was identified are listed above. AR, autosomal recessive; AD, autosomal dominant; CNI, calcineurin inhibitor; DMS, diffuse mesangial sclerosis; F, female sex; fs, frameshift; FSGS; focal segmental glomerulosclerosis; GB, gonadoblastoma; het, heterozygous; Hom, homozygous; KTS, KTS splice site at junction of exon/intron 9; M, male sex; MOI, mode of inheritance; NA, not available; ND, not done; Tx, transplant;
truncating;
ExAC frequencies reported heterozygotes/homozygotes/total alleles;
-, not present in ExAC.
Variants classified as per ACMG guidelines [34]
Median age of onset was 3.5 years in all individuals (range 0.1–18.8), 4.1 years in those in which no mutation was found (range 0.5–18.8), and 3.2 years in those where the causative gene was detected (range 0.1–14.3). Mutations in dominant genes presented at a later age, with a median onset of 4.5 years (range 3.2–14.3). Mutations in recessive genes presented with a median onset of 0.5 years (range 0.1–3.2) (Table 1). As shown in Figure 2, patients with mutations in recessive genes presented at an earlier age and invariably progressed to ESRD. Patients with mutations in dominant genes presented later in life and had a more protracted/indolent course towards ESRD.
Figure 2. Time intervals between manifestation of steroid-resistant nephrotic syndrome (SRNS) and start of renal replacement therapy.
Time intervals are shown for each patient and are arranged from earliest to latest in age of onset, from bottom to top. If the patient has not progressed to end-stage renal disease, this is indicated by a dotted line.
B144-Family B144 represents a case of congenital nephrotic syndrome that manifested in the first 3 weeks of life with swelling and hypoalbuminemia. There is a strong family history of consanguinity in the parents (2nd cousins with multiple intermarriages). There is also a family history of a previous infant death secondary to complications of congenital nephrotic syndrome (Figure 1a). The proband underwent living related donor transplantation from her father at 1.5 years of age. On investigation, B144_24 had a novel missense allele in the podocyte gene NPHS1 (c.728C>T, p.P243L, homozygous). The altered amino acid residue, proline, is conserved to D. melanogaster and is not present in healthy control cohorts of the ExAC browser (60,706 individual exomes) (Table 2) [18].
Figure 1. Pedigree structure and CLUSTAL alignment of evolutionary conservation in eight families, in whom the steroid-resistant nephrotic syndrome (SRNS)-causing mutation was identified.
(a–h) Pedigrees are shown with proband identified in the pedigree structure. Segregation of genetic variants was examined if affected sibling or parental DNA was available. In (c, d, and f) no CLUSTAL alignment was created as the mutation resulted in a truncating, frameshift mutation, or splice site (intronic), respectively.
B350-The proband in family B350 was initially diagnosed at an outside institution with congenital nephrotic syndrome at 3 months of life, as is typical for NPHS1 mutations. Although there is no history of consanguinity, the proband is from a rural part of the country. Her family’s remote origin may explain the homozygous presentation of the mutated recessive allele due to a founder effect (Figure 1b). B350_21 had a renal biopsy that demonstrated diffuse mesangial sclerosis, the hallmark histologic finding of congenital nephrotic syndrome. B350_21 underwent nephrectomy at 9 months of age and received a living donor kidney transplantation at 2 years of age. Genetic analysis revealed a known missense mutation in NPHS1 (c.1868G>T, p.C623F, homozygous) [19]. The altered amino acid, cysteine, is conserved through D. rerio (Table 2).
In both cases above, no steroid treatment was ever initiated, because symptoms were consistent with congenital nephrotic syndrome. Both patients presented to our center for transplant evaluation as their clinical conditions required nephrectomy and initiation of dialysis prior to transplantation. Post-transplantation prognosis is favorable in confirmed NPHS1 mutations as the risk of recurrence of disease is low [20, 21] (Table 2). After CLIA confirmation, both families were offered genetic counseling for future progeny.
B9-In this consanguineous family, there were two affected siblings (Figure 1c). The proband B9_22, was found to have a previously known truncating mutation in the gene MYO1E, that was also confirmed in the sister B9_21 (c.141C>G, p.Y47*, homozygous) (Table 2). The proband presented with CKD and progressed to ESRD while being evaluated at BCH. The family returned to the Middle East prior to completing workup for transplantation. No genetic counseling could be initiated.
B188-For the non-consanguineous family B188 (Figure 1d), the proband (-21) presented at age 3 with nephrotic-range proteinuria. She was refractory to steroid therapy and progressed to ESRD after failing oral cyclophosphamide and cyclosporine therapy. B188_21 underwent two biopsies at age 3 and 3.5 that demonstrated minimal change and FSGS, respectively (Table 2). As a result, B188 underwent extensive treatment pre-transplant for her FSGS with placement of a plasmapheresis catheter and weekly pheresis sessions in an attempt to prevent recurrence. She underwent a deceased donor renal transplantation in 2009 and has since only had one episode of acute cellular rejection in 2014 secondary to non-adherence. Molecular genetics performed in this study revealed a previously published mutation (Table 2) in NPHS2 (c.855-56AA>del, p.R286T*fs17, homozygous) several years after her pre-transplant treatment and transplantation had already occurred. For B188, the mutated allele is reported to be present heterozygously as a small fraction of individuals in ExAC (Table 2). The presence of this rare deleterious polymorphism in the general population makes the presentation of a homozygous recessive mutation in a non-consanguineous family plausible.
B323-B323_21 is an example of an isolated case of FSGS and proteinuria. The proband has no other family history or extra-renal manifestations (Figure 1e). A percutaneous renal biopsy was performed at age 3 after an initial diagnosis of proteinuria, and demonstrated FSGS (Table 2). She has since been maintained on ACE inhibitor therapy with normal serum creatinine levels. Molecular diagnostics demonstrated a mutation in WT1 (c.1384C>T, p.R462W, heterozygous), with the allele being a well characterized missense mutation that is seen classically in Denys-Drash syndrome. However, her biopsy and clinical presentation is much more akin to Frasier Syndrome [22, 23]. There is evidence to suggest that Denys-Drash and Frasier syndrome are on a spectrum of disease, and that specific alleles can be very heterogeneous in their clinical presentation [24]. B323_21 was prepubertal at the time of genetic diagnosis and was referred to a multi-disciplinary clinic where she met with urology and endocrinology after her karyotype returned as 46 XY. Family B323 also received additional genetic counseling for the proband’s 46 XY karyotype. To confirm if B323_21 has a de novo mutation, both her parents are currently in the process of obtaining genetic confirmation of the mutated allele. Ultimately, B323_21 underwent a pelvic ultrasound that demonstrated streak gonads. Given the risk for malignant transformation into gonadoblastoma in patients with Frasier syndrome, she underwent surgical removal of her streak gonads and is currently on oral hormone replacement therapy. The overall risk for Wilms Tumor is increased in patients with this allele, but B323_21 has yet to manifest any symptoms of Wilms Tumor by 12 years of age and is not currently scheduled for any surveillance imaging as she is considered low risk at this age [23].
B92-The proband (-21) in family B92 demonstrated a well described mutation in WT1 (c.1432+4C>T, IVS9 ds C-T KTS, heterozygous) a gene implicated in both Denys-Drash and Frasier syndrome [23]. There is a family history of FSGS as the mother (-12) and sister (-22) both are affected (Figure 1f). B92_21 presented as a young child with nephrotic syndrome and was diagnosed with FSGS at age 4 (Table 2). She underwent bilateral nephrectomy and began hemodialysis before being transplanted at 14 years of age. Interestingly, an underlying genetic diagnosis for B92_21 was considered only after a workup for primary amenorrhea was initiated. B92_21 had a karyotype that demonstrated 46 XY. Given the strong family history of FSGS, molecular diagnostics was performed for B92_21. The same mutation was also confirmed in her mother, B92_12, who received a living donor transplant in 1991 for progression of her isolated FSGS and her sister, B92_22, who has FSGS and CKD. The molecular genetic findings revealed a heterozygous mutation in the KTS splice site of intron 9 in WT1 and confirmed the clinical diagnosis of Frasier syndrome (constellation of FSGS, ambiguous genitalia, streak gonads, and risk for gonadoblastoma). Although patients with Frasier Syndrome are not at a high risk for developing Wilms Tumor [22, 23], B92_21 had already undergone bilateral nephrectomies as part of her pre-transplantation treatment course.
B635- Family B635 is a case demonstrating a dominant form of FSGS with a mutation in TRPC6 (c.2683C>T, p.R895C, heterozygous). B635_21 currently is being medically managed with ACE inhibitor monotherapy to control her proteinuria. The allele has been previously characterized as a known cause for autosomal dominant FSGS via a gain of function mechanism of the calcium ion channel with subsequent downstream effects of NFAT-mediated transcription [9, 25, 26]. The proband has multiple affected family members (Figure 1g), including her mother who has had FSGS, progression to ESRD, and is status post renal transplantation. B635 also has had two maternal aunt/uncles that required dialysis and transplantation in the fourth decade of life. Unfortunately, as the family immigrated into the United States, we did not have access to the grandparents for genetic information or clinical data. The proband’s mother responded to a calcineurin inhibitor for about 5 years before relapsing with proteinuria and eventual progression to ESRD. No clinical action has occurred following identification of the causative mutation, as the proband has turned 18 years old after consent was initially obtained and is now contemplating about whether she is interested to re-consent for CLIA confirmation before obtaining genetic counseling.
B284-This family presented with a prominent family history of kidney disease and the proband presented at age 14 with proteinuria and CKD. B284_21 had a medical history notable for a diagnosis of Charcot-Marie-Tooth (CMT) characterized by sensorimotor polyneuropathy and foot drop that had required several orthopedic surgeries. B284_21’s renal biopsy demonstrated FSGS with advanced global and segmental sclerosis with tubular atrophy (Table 2). He eventually progressed to ESRD within 12 months and received a deceased donor renal transplant at age 15. His mother also had ESRD and was transplanted in 1981 (Figure 1h). Molecular genetic diagnostics of B284_21 demonstrated a published mutation in INF2 (c.542T>G, p.V181G, heterozygous). The amino acid change, although not very well conserved, represents a significant change from a non-polar to a polar amino acid. INF2 protein is important in actin regulation and mutations are known to cause FSGS and Charcot-Marie-Tooth [27, 28]. As the proband was transplanted at a time before molecular diagnostics was widely available, he is now in the process of obtaining CLIA confirmation of his mutation and obtaining genetic counseling for family planning.
Lastly, in Figure 3 we present a flowchart in which we describe the process of how research samples were collected, studied, and findings were reported back to clinicians. Thereafter, specific mutation confirmation (SMC) was performed via a CLIA lab to confirm the research-based finding prior to initiating any clinical decision making.
Figure 3.
Flowchart on recruitment, mutation analysis, feedback of findings, confirmation of mutation, and realizing clinical action
DISCUSSION
In the 72 families studied, we demonstrate that autosomal recessive genes in patients present at an earlier onset (Figure 2) and that in older patients the underlying genetic cause is more likely autosomal dominant and have a more indolent course in progressing to ESRD. Although the numbers in our study are too small to demonstrate statistical significance, this is a finding that is borne out in larger cohort studies [11, 14].
Of the 8 families in whom the underlying genetic mutation was identified, there were two consanguineous cases from foreign countries and one out of state referral. As a quaternary care facility, BCH receives many referrals from outside institutions and countries. Therefore, the results of this study are reflective of patient cohorts seen in pediatric referral centers, most of which are tertiary or quaternary care centers. Nevertheless, our molecular diagnosis rate of 11.1 % (8/72 families) in our study cohort is lower than previously published. In Sadowski et al., the published mutation identification rate was 29.5 % (526/1783) families [14]. The higher rate of detection of the causal mutation in that study is most likely due to the larger percentage of families that are of consanguineous union, as our current study population is mostly outbred. As shown in supplementary figure 6 [14], in referral centers where the consanguinity is ~70 %, up to 45 % of families had a causative mutation identified. As a corollary, referral centers in which the established rate of consanguinity is almost zero, have a much lower rate of mutation identification (13.7 % in Los Angeles) [14]. The correlation curve as proposed in the Sadowski paper would predict a rate of mutation detection of 18.8 % for our referral center, where we have 9.7 % (7/72) of consanguineous families. The discrepancy in the actual and predicted mutation detection rate may be in part due to the small sample size in this study (72 families). As molecular diagnostics (such as WES) becomes more feasible and widespread, further studies would be warranted to establish the prevalence of monogenic disease in referral centers such as BCH.
Even within our small sample size, this method of genetic investigation revealed actionable results in several of the families that had an identified molecular diagnosis. In family B323, there were consequences for counseling and management for this family. After a genetic diagnosis was made for B323_21, gonadal dysgenesis was identified and the proband had streak gonad removal to prevent development of gonadoblastoma. The family was also referred to genetic counseling prior to any procedure.
In the case of B635, the TRPC6 allele she harbors is a well-studied mutation. There is recent functional data that supports the possible role of calcineurin inhibitor therapy in the treatment of patients with TRPC6 mutations [9]. In the published human podocyte cell culture model, the R895C mutation in B635 leads to NFAT-mediated activated transcription via constitutive channel overactivity. Inhibition of the pathway with the calcineurin inhibitor cyclosporine A abrogated mutated TRPC6-mediated NFAT activation [9]. It has been reported that patients with monogenic causes of SRNS typically do not respond to intensified immunosuppressive therapy [20, 29]. The fact that B635’s affected mother responded to cyclosporine therapy for several years is very interesting given the previously published functional data. Although not borne out in direct study, this loose association makes potential therapy of TRPC6 mutations with calcineurin inhibitors a worthy avenue to pursue.
The experience from this study also makes the need for referral to genetic counseling very clear. Approximately half of the molecularly solved cases had no overt “familial” component on clinical history (family history, multiple generations, or other siblings affected) (see Figure 1). Once the genetic diagnosis has been made, genetic counseling is a valuable resource to both the physician and the family that can be utilized to provide information regarding family planning, prognosis, and counseling for ethical/social dilemmas that are outside the scope of the practicing subspecialist.
Finally, as shown in the results, although genetic diagnosis may not alter clinical outcome, it may change management by allowing providers to avoid unnecessary treatments/tests. In the case of FSGS in B188, if the monogenic NPHS2 mutation had been identified earlier, she may have been able to avoid plasmapheresis and catheter placement for FSGS transplant protocol. The knowledge that patients with a monogenic form of FSGS have a reduced rate of recurrence could have spared her from multiple procedures and therapies aimed to reduce FSGS recurrence [20, 30].
As demonstrated in case B350, the genetic cause of congenital nephrotic syndrome correlates poorly with renal biopsy. This case adds to the mounting evidence that genetic testing is often more useful in understanding the underlying cause of congenital nephrotic syndrome than percutaneous renal biopsy [31–33].
Newer technologies are rapidly becoming cost-effective and feasible to allow further genetic study in patients: Whole exome sequencing (WES) and the method of trio analysis can generate more in-depth genetic data than panel sequencing allows. In the era of precision medicine, the goal of every tertiary care center should offer genetic diagnostics as standard of care when seeing patients with kidney diseases such as SRNS.
Supplementary Material
Supplementary Table 1. Alphabetic list of monogenic SRNS genes in panel sequencing.
Acknowledgments
We thank the physicians and the participating families for their contribution. F.H. is the William E. Harmon Professor of Pediatrics. This research was supported by grants from the National Institutes of Health (DK076683 to FH). WT is supported by the NIH T32 Training grant (DK007726) as well as the ASN Benjamin J. Lipps Research Fellowship Award (FP01014311). EW is supported by Leopoldina Fellowship Program, German National Academy of Sciences Leopoldina (LPDS 2015-07).
Footnotes
Compliance with ethical standards
Approval was obtained from the Institutional Review Board (IRB) on human subjects’ research of the Boston Children’s Hospital. The cohort consisted of 77 individuals (72 families) that were recruited following informed consent
DISCLOSURES
F.H. receives royalties from Claritas Genomics and is a cofounder of Goldfinch Bio.
None of the other authors have competing financial interests to disclose.
References
- 1.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 2.Cheong HI, Han HW, Park HW, Ha IS, Han KS, Lee HS, Kim SJ, Choi Y. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78–81. doi: 10.1093/ndt/15.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Senggutuvan P, Cameron JS, Hartley RB, Rigden S, Chantler C, Haycock G, Williams DG, Ogg C, Koffman G. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: analysis of incidence and risk factors in 59 allografts. Pediatr Nephrol. 1990;4:21–28. doi: 10.1007/BF00858431. [DOI] [PubMed] [Google Scholar]
- 4.Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12:133–146. doi: 10.1038/nrneph.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 7.Lovric S, Ashraf S, Tan W, Hildebrandt F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant. 2015;3:1802–1813. doi: 10.1093/ndt/gfv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V, Killen PD, Raphael Y, Ashraf S, Ovunc B, Schoeb DS, McLaughlin HM, Airik R, Vlangos CN, Gbadegesin R, Hinkes B, Saisawat P, Trevisson E, Doimo M, Casarin A, Pertegato V, Giorgi G, Prokisch H, Rotig A, Nurnberg G, Becker C, Wang S, Ozaltin F, Topaloglu R, Bakkaloglu A, Bakkaloglu SA, Muller D, Beissert A, Mir S, Berdeli A, Varpizen S, Zenker M, Matejas V, Santos-Ocana C, Navas P, Kusakabe T, Kispert A, Akman S, Soliman NA, Krick S, Mundel P, Reiser J, Nurnberg P, Clarke CF, Wiggins RC, Faul C, Hildebrandt F. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlondorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol. 2009;296:C558–569. doi: 10.1152/ajpcell.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, Fathy HM, Schoeb D, Chernin G, Lyons RH, Yilmaz E, Hildebrandt F. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol. 2011;22:1815–1820. doi: 10.1681/ASN.2011040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglio S, Provenzano A, Mazzinghi B, Becherucci F, Giunti L, Sansavini G, Ravaglia F, Roperto RM, Farsetti S, Benetti E, Rotondi M, Murer L, Lazzeri E, Lasagni L, Materassi M, Romagnani P. Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J Am Soc Nephrol. 2015;26:230–236. doi: 10.1681/ASN.2013111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Ozkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Ozcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. J Am Soc Nephrol. 2015;10:592–600. doi: 10.2215/CJN.06260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovric S, Fang H, Vega-Warner V, Sadowski CE, Gee HY, Halbritter J, Ashraf S, Saisawat P, Soliman NA, Kari JA, Otto EA, Hildebrandt F. Rapid detection of monogenic causes of childhood-onset steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2014;9:1109–1116. doi: 10.2215/CJN.09010813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbritter J, Diaz K, Chaki M, Porath JD, Tarrier B, Fu C, Innis JL, Allen SJ, Lyons RH, Stefanidis CJ, Omran H, Soliman NA, Otto EA. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet. 2012;49:756–767. doi: 10.1136/jmedgenet-2012-100973. [DOI] [PubMed] [Google Scholar]
- 16.Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013;132:865–884. doi: 10.1007/s00439-013-1297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 18.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999;64:51–61. doi: 10.1086/302182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–732. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 21.Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, Niaudet P, Antignac C. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher V, Scharer K, Wuhl E, Altrogge H, Bonzel KE, Guschmann M, Neuhaus TJ, Pollastro RM, Kuwertz-Broking E, Bulla M, Tondera AM, Mundel P, Helmchen U, Waldherr R, Weirich A, Royer-Pokora B. Spectrum of early onset nephrotic syndrome associated with WT1 missense mutations. Kidney Int. 1998;53:1594–1600. doi: 10.1046/j.1523-1755.1998.00948.x. [DOI] [PubMed] [Google Scholar]
- 23.Chernin G, Vega-Warner V, Schoeb DS, Heeringa SF, Ovunc B, Saisawat P, Cleper R, Ozaltin F, Hildebrandt F. Genotype/phenotype correlation in nephrotic syndrome caused by WT1 mutations. Clin J Am Soc Nephrol. 2010;5:1655–1662. doi: 10.2215/CJN.09351209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTaggart SJ, Algar E, Chow CW, Powell HR, Jones CL. Clinical spectrum of Denys-Drash and Frasier syndrome. Pediatr Nephrol. 2001;16:335–339. doi: 10.1007/s004670000541. [DOI] [PubMed] [Google Scholar]
- 25.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiluiza D, Krishna S, Schumacher VA, Schlondorff J. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2) J Biol Chem. 2013;288:18407–18420. doi: 10.1074/jbc.M113.463059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42:72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Huynh Cong E, Arrondel C, Tete MJ, Montjean R, Richard L, Karras A, Pouteil-Noble C, Balafrej L, Bonnardeaux A, Canaud G, Charasse C, Dantal J, Deschenes G, Deteix P, Dubourg O, Petiot P, Pouthier D, Leguern E, Guiochon-Mantel A, Broutin I, Gubler MC, Saunier S, Ronco P, Vallat JM, Alonso MA, Antignac C, Mollet G. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med. 2011;365:2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- 29.Buscher AK, Kranz B, Buscher R, Hildebrandt F, Dworniczak B, Pennekamp P, Kuwertz-Broking E, Wingen AM, John U, Kemper M, Monnens L, Hoyer PF, Weber S, Konrad M. Immunosuppression and renal outcome in congenital and pediatric steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2010;5:2075–2084. doi: 10.2215/CJN.01190210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungraithmayr TC, Hofer K, Cochat P, Chernin G, Cortina G, Fargue S, Grimm P, Knueppel T, Kowarsch A, Neuhaus T, Pagel P, Pfeiffer KP, Schafer F, Schonermarck U, Seeman T, Toenshoff B, Weber S, Winn MP, Zschocke J, Zimmerhackl LB. Screening for NPHS2 mutations may help predict FSGS recurrence after transplantation. J Am Soc Nephrol. 2011;22:579–585. doi: 10.1681/ASN.2010010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kari JA, Montini G, Bockenhauer D, Brennan E, Rees L, Trompeter RS, Tullus K, Van’t Hoff W, Waters A, Ashton E, Lench N, Sebire NJ, Marks SD. Clinico-pathological correlations of congenital and infantile nephrotic syndrome over twenty years. Pediatr Nephrol. 2014;29:2173–2180. doi: 10.1007/s00467-014-2856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heeringa SF, Vlangos CN, Chernin G, Hinkes B, Gbadegesin R, Liu J, Hoskins BE, Ozaltin F, Hildebrandt F. Thirteen novel NPHS1 mutations in a large cohort of children with congenital nephrotic syndrome. Nephrol Dial Transplant. 2008;23:3527–3533. doi: 10.1093/ndt/gfn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoeb DS, Chernin G, Heeringa SF, Matejas V, Held S, Vega-Warner V, Bockenhauer D, Vlangos CN, Moorani KN, Neuhaus TJ, Kari JA, MacDonald J, Saisawat P, Ashraf S, Ovunc B, Zenker M, Hildebrandt F. Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS) Nephrol Dial Transplant. 2010;25:2970–2976. doi: 10.1093/ndt/gfq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Alphabetic list of monogenic SRNS genes in panel sequencing.