Abstract
Dual processing streams in sensory systems have been postulated for a long time. Much experimental evidence has been accumulated from behavioral, neuropsychological, electrophysiological, neuroanatomical and neuroimaging work supporting the existence of largely segregated cortical pathways in both vision and audition. More recently, debate has returned to the question of overlap between these pathways and whether there aren’t really more than two processing streams. The present piece defends the dual-system view. Focusing on the functions of the dorsal stream in the auditory and language system I try to reconcile the various models of Where, How and When into one coherent concept of sensorimotor integration. This framework incorporates principles of internal models in feedback control systems and is applicable to the visual system as well.
Keywords: Dual pathways, dorsal stream, ventral stream, audition, language
1. Introduction
The concept of dual processing streams in sensory cortical systems has been around for over three decades in the visual system (Ungerleider & Mishkin, 1982; Goodale & Milner, 1992) and for nearly two decades in the auditory system (Rauschecker, 1998a; Rauschecker & Tian, 2000). Yet, people are still debating their functions as well as the question whether there are not, in fact, more than two processing streams, especially in the auditory system (Kaas & Hackett, 2000). The present piece defends the dual-system view against more complex models with various substreams and branches (e.g. Kravitz et al., 2011). Focusing on the functions of the auditory dorsal stream it tries to reconcile the various models of a Where-, How-, or When-stream into one coherent concept and attempts to generalize it into a single model for sensorimotor control, in which spatial and temporal aspects are fully integrated. The idea of internal models of the outside world (including our own body) is of paramount importance and can be applied to both vision and audition.
2. Dual visual pathways
Even before Ungerleider & Mishkin (1982), various authors proposed dual visual functions related to ‘what’ and ‘where’. Trevarthen (1968), Schneider (1969), and Diamond & Hall (1969) advocated a view in which the midbrain superior colliculus (SC) and ‘the’ visual cortex supported ambient (spatial) and focal (object) vision, respectively. Weiskrantz (1978), based on studies of human patients with lesions in different cortical regions, suggested that visual information could reach the cortex via two largely independent routes: the classical geniculo-striate pathway and an extra-geniculo-striate pathway including the SC and pulvinar.
The later suggestion that two cortical visual pathways formed the basis for (a) visual object identification and (b) visual space perception was based on studies of monkeys that were trained in different behavioral tasks involving short-term memory (Mishkin et al., 1983). Knowledge of these tasks was eliminated by lesioning the ventral temporal or posterior parietal cortex, respectively. Related studies were undertaken in humans with natural lesions caused by strokes (Mesulam, 1998) and led to the universal conclusion that the ventral pathway did indeed play a major role in object perception. The role of the dorsal pathway, however, while containing an important spatial component, remained mysterious and controversial.
The most prominent opposition against a purely spatial role of the dorsal visual stream came from studies by Goodale and Milner (1992), who found that patients with lesions of the ventral visual stream but an intact dorsal stream could still perform acts of (‘unconscious’) visual perception using visuomotor strategies, for instance during reaching and grasping. This led to the concept of the dorsal stream as an “action” stream, and hence, the dorsal pathway mutated from a ‘where’-pathway to something like a ‘how’-pathway.
3. Dual auditory pathways
The debate about segregated processing streams and their functions continued when higher auditory cortical functions in nonhuman primates began to be explored neurophysiologically in the late 1990s (Rauschecker et al., 1995; Rauschecker, 1997, 1998a,b; Rauschecker & Tian, 2000; see Figure 1). Single-unit studies in nonprimary areas of auditory cortex in anesthetized monkeys found selective responses to species-specific vocalizations in anterior (ventral) regions (area AL) of the lateral auditory belt (Rauschecker et al., 1995; Tian et al., 2001). By contrast, clear evidence for spatial tuning was found in posterior (dorsal) regions (area CL) of the auditory belt (Tian et al., 2001). Concomitant neuroanatomical studies using fluorescent tracers demonstrated that direct projections exist from anterior belt to ventrolateral prefrontal cortex (VLPFC) and from caudal belt to dorsolateral prefrontal cortex (DLPFC; Romanski et al., 1999), where neural substrates of object and spatial working memory, respectively, had been found in the visual system (Wilson et al., 1993; Goldman-Rakic, 1995). The concept of an auditory ventral stream for the identification or recognition of auditory ‘objects’ was accepted quite rapidly (Binder et al., 2000; Scott et al., 2000; Griffiths & Warren, 2004). Later studies in awake behaving monkeys using neurophysiological techniques (Woods et al., 2006) and functional MRI (Ortiz-Rios et al., 2017) also confirmed the special role of posterior-dorsal cortical areas in auditory spatial processing.
Figure 1. Proto-version (A) and most recent instantiation (B) of dual-stream model of auditory processing in the human brain.

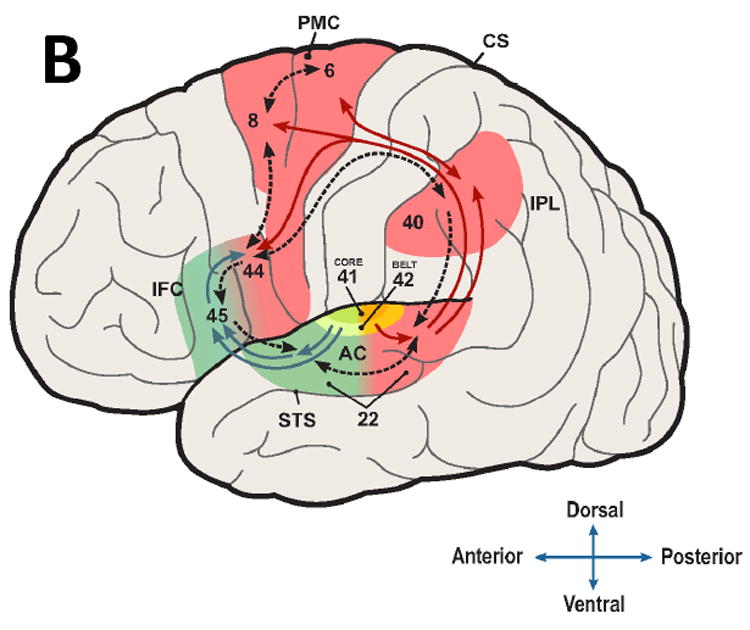
(A) from Rauschecker (2011), which was based on an earlier model of the macaque brain (Rauschecker, 1998b; Rauschecker & Tian, 2000); (B) from Rauschecker (2015) and Bornkessel-Schlesewsky et al. (2015b). In both cases, the ventral stream is shown in green, the dorsal stream in red. Compared to the ‘upgraded’, current version (B), the protoversion (A) contains fewer reciprocal feedback connections (dashed lines), and premotor cortex (PMC) is not contiguous with Broca’s area in the inferior frontal cortex (IFC). In the current version, BA44 constitutes the target region of the dorsal stream, and BA45 is the endpoint of the ventral stream, with both regions interacting as indicated by the region with yellow shading and the reciprocal connection between them.
Other abbreviations (Brodmann area [BA] in parentheses): CS central sulcus, PMC (6, 8), IPL inferior parietal lobule (40), AC auditory cortex (41, 42) IFC inferior frontal cortex (44, 45), STS superior temporal sulcus (22).
3.1. Spatial and temporal processing in the auditory dorsal stream
Functional imaging and stroke studies in humans were also consistent with the participation of posterior superior temporal and parietal cortex in spatial hearing (Alain et al., 2001; Maeder et al., 2001; Warren et al., 2002; Arnott et al., 2004; Ahveninen et al., 2006; Deouell et al., 2007; Spierer et al., 2009; Zundorf et al., 2016). Transcranial magnetic stimulation studies further corroborated the participation of posterior temporal and parietal cortices in spatial hearing (Lewald et al. 2002; At et al. 2011). Some of these and other studies also demonstrated a participation of DLPFC in spatial hearing (Weeks et al. 2000; Zatorre et al. 2002; Lewald et al. 2008), thus completing the extent of the auditory dorsal stream in humans to the same regions that were identified in the monkey. Therefore, the concept of a ‘where’- pathway that comprises posterior-dorsal and dorsolateral prefrontal regions of the cerebral cortex in both monkeys and humans was upheld for the auditory modality as well (see Rauschecker, 2007, 2015, for further review).
Spatial processing in both the visual and auditory modalities includes the processing of motion-in-space as the first derivative of space over time, thus adding a temporal component. Visual motion areas, like the middle temporal region (MT or V5), are therefore often subsumed within the visual dorsal stream. Areas of the auditory dorsal stream in the human inferior parietal cortex that participate in the processing of space are activated even more strongly by auditory motion-in-space than by localized stationary sounds (Griffiths et al., 1998; Krumbholz et al., 2005).
Some neurophysiological studies in macaques have emphasized the higher temporal resolution of neurons in the dorsal pathway, especially in the auditory system (Camalier et al., 2012; Kusmierek & Rauschecker, 2014) and, therefore, expanded its role to include time processing. Coming from a quite different field (musical rhythm processing in humans), other authors have emphasized the superior temporal resolution of the dorsal stream as well and went as far as postulating the existence of a dorsal ‘when’-pathway (e.g. Schubotz et al., 2003). Therefore, preferential processing of temporal aspects of audition (‘when’) has become a further important aspect (besides space) for the consideration of function of the auditory dorsal stream. So, the question might be asked if there are separate ‘where’ and ‘when’ pathways or streamlets within the auditory dorsal stream.
4. Dual pathways in speech and language
Then came studies of speech and language, which picked up on the idea of dual processing pathways in the auditory cortex (Hickok & Poeppel, 2004). Perception and recognition of auditory ‘objects’ in the ventral stream, as established by the earlier studies in monkeys and humans (Rauschecker & Tian, 2000; Binder et al., 2000; Scott et al., 2000; Griffiths & Warren, 2004; Scott, 2005), was quite readily adopted by neurolinguistics, because phonemes and words could easily be considered auditory objects in this framework as well (DeWitt & Rauschecker, 2012) and could be envisioned to communicate with a lexical-semantic network.
The dorsal pathway’s function in space processing, by contrast, posed a problem for neurolinguists, many of whom thought it had to be refuted in order to make room for a role in syntax and grammar instead, and it was touted as a possible species difference. It was soon pointed out by Rauschecker & Scott (2009), however, that a conceptually expanded role of the dorsal stream could easily accommodate both space and speech. The unifying concept was that of an ‘internal model’ for sensorimotor integration and control (Rauschecker, 2011; see Figure 1), related to Gibson’s (1977) affordance model (Grush, 2004). Affordances, i.e. the necessity for motor systems to be tuned to sensory demands and vice versa, have also been mentioned in the context of visual reaching and grasping (Rizzolatti & Craighero, 2004), but similar models can be applied with equal justification to spoken speech (Wilson et al., 2004).
Sensorimotor systems frequently form a closed loop, in which sensory signals feed back to the motor structures that produce them (‘reafference’; von Holst & Mittelstaedt, 1950). In addition, motor structures may send out a signal (‘corollary discharge’; Sperry, 1950; or ‘efference copy’; von Holst & Mittelstaedt, 1950) that informs the sensory processing machinery about an impending movement, so the sensory structures can take that signal into account (see Sommer & Wurtz, 2008, for review).
Proprioceptive information undoubtedly plays an important role for these feedback processes in the human brain as well, and studies with brain-machine interfaces demonstrate the significance of a continuous stream of rich tactile/proprioceptive feedback (Donati et al., 2016; Hochberg et al., 2012). Humanoid robots are only just beginning to incorporate these fundamental principles, making robots more human than ever before (Cheng, 2014).
5. The auditory dorsal stream as an internal model for sensorimotor control
Thus we are converging on a unified function of the dorsal stream as an ‘internal model’ of the outside world (including our own body), which incorporates important elements of a control circuit (Wolpert et al., 1995; Kawato, 1999). Depending on one’s vantage point, a ‘forward model’ is implemented in one direction and an ‘inverse model’ in the opposite direction. Importantly, this circuit model may be applied not only to the auditory modality, for which it was first proposed (Rauschecker, 2011). We can now return to the visual modality and apply the concept of an internal model to that as well. Vision is not a purely visual process, but is spatio-temporal too: Due to the ubiquitous presence of eye movements, vision requires the stitching or papering together of snapshots of visual scenes to generate a unified and continuous percept. The same need arises as a consequence of head (and whole-body) movements during scene analysis in both the visual and auditory modalities. Therefore, the conversion of sensorimotor sequences into a unified experience may be one of the generalized functions of the dorsal stream and, as Milner and Goodale (2008) have pointed out, it all – amazingly! - happens in real time.
Two crucial features are required for this sensorimotor model of the dorsal stream: 1) Working memory (Baddeley, 1992), which is capable of keeping the activity of neuronal assemblies alive for finite periods of time (Miller et al., 1996), and 2) a sequencing mechanism that preserves the temporal order of sensorimotor events. By combining these two features an essential mechanism is created, which not only supports mundane functions like eye movements and the stabilization of the retinal image, but also higher cognitive functions like language (both signed and spoken) as well as music.
One of the central problems that the dorsal stream has to solve then becomes the coding and processing of ordered sequences and the implementation of sequence memory in sensory perception (Micheyl et al., 2005; Cusack, 2005; Leaver et al., 2009; Artchakov et al., 2012; Rauschecker, 2014). In a certain sense, this view comes close to the ‘action’ concept of Goodale & Milner (1992), but it provides an even more unified view across sensory modalities and into sensorimotor behavior as a whole.
6. Alternative models of the dorsal pathway
6.1. Multiple branches of the dorsal stream?
The age-old question whether two pathways are sufficient to describe sensory (or sensorimotor) systems then becomes one of “lumping versus splitting”. Modified dual-pathway models have sprung up that are postulating streamlets or substreams. Recently, Kravitz et al. (2011), for instance, presented a model of the visual dorsal stream wherein three different functions (spatial working memory, visually guided actions, and spatial navigation) are performed by anatomically distinct branches. All three branches originate from parietal cortex. Thus, when these authors talk about “three pathways emerging from the dorsal stream”, they define the dorsal stream as a limited occipito-parietal network, which does not include frontal regions. While this may conform to the very first definition of the dorsal stream on the basis of lesions in monkey parietal cortex (Ungerleider & Mishkin, 1982; Mishkin et al., 1983), it does not take into account later anatomical tracer studies reporting direct projections from visual cortical regions to prefrontal cortex (PFC) (Goldman-Rakic, 1995; Ungerleider, 1995).
The same is true for the definition of auditory dorsal and ventral streams: direct anatomical pathways have been identified with tracers injected into physiologically identified areas AL, ML and CL in the lateral belt surrounding primary core areas (Romanski et al., 1999). These early (secondary) auditory areas project directly and differentially to distinct regions of prefrontal cortex (VLPFC and DLPFC) with little overlap via segregated anatomical routes and are, therefore, justly termed segregated (ventral and dorsal) pathways. Together with the functional identification of the originating belt areas (AL and CL) as object- versus space-selective regions (Tian et al., 2001), the two anatomically segregated pathways can also be thought of as functionally segregated streams.
These anatomical results of direct projections from functionally distinct early sensory areas to prefrontal regions have, therefore, become the defining feature of both ventral and dorsal pathways. In that sense a “trifurcation” of the dorsal pathway (Kravitz et al., 2011) does not exist. Parieto-prefrontal and parietopremotor projections would only be considered indirect pathways (or side branches) by comparison with the main, direct dorsal stream from dorsal-sensory to prefrontal areas.
6.2. Multiple dorsal streams for language?
In auditory and language processing of humans, sub-streams of the dorsal pathway (‘ventral-dorsal’ and ‘dorsal-dorsal’) have been postulated on the basis of noninvasive structural anatomical techniques (Saur et al., 2008; Friederici, 2009; Dick et al., 2014). These distinctions were made on the basis of the observation that some fiber bundles of the dorsal pathway are split into separate macroanatomical fasciculi, which mature at different stages of development. Whether this says anything about their respective functions, however, remains unclear. Furthermore, diffusion tensor imaging (DTI) techniques, which are used for noninvasive human neuroanatomy, are notoriously difficult, and their inability to distinguish crossing fibers from parallel fiber bundles is well known (Frey et al., 2008).
Other macroanatomical differences between humans and monkeys, such as the presumed presence or absence of a direct dorsal pathway (“arcuate fascicle”) from posterior auditory cortex to inferior frontal cortex, have been used to argue for the presence or absence of language (Skeide & Friederici, 2015). When these conclusions are based on techniques with vastly different resolution (anatomical tracers versus DTI), then the argument becomes truly tenuous (Bornkessel-Schlesewsky et al., 2015a).
It seems more likely, as Darwin (1871) suggested, that spoken language has evolved gradually on several fronts as a “multicomponent” system, whose individual components have exploded by comparison with nonhuman primates, rather than a single, unique step function: First, an increased ability of auditory neurons in the ventral stream to resolve more and more refined acoustic elements and use them for the identification of distinct auditory objects; and second, a refined ability of dorsal-stream networks to establish sensorimotor models of actions representing speech and language. This includes the ability to control a much larger number of articulators, thus leading to a much greater vocal repertoire. A third component facilitating the evolution of language may have been the development of enhanced working memory capacities (Fritz et al., 2005), supporting the functions of a phonological-articulatory loop (Baddeley et al., 1998), which ultimately enabled recursion and complex syntactic structure. As has been argued previously (Rauschecker, 2012; Bornkessel et al., 2015b), frontal cortex in and around “Broca’s area”, where ventral and dorsal pathways converge and interact (Figure 1), may therefore carry the ultimate key to understanding language evolution.
7. Conclusions
In summary, understanding the function of the dorsal pathway, in both vision and audition, may well turn out to be one of the central problems for understanding human behavior. By enabling us to predict the sensory consequences of our actions, automatically and in real time, this system goes way beyond simple terminologies of ‘where’, ‘when’, or ‘how’. Instead, it combines elements of all three of these perspectives in the interest of sensorimotor coupling in space-time, in order to organize and produce coordinated behavior. This comprises behaviors as seemingly diverse as the programming of eye movements in space and time and the processing of audiomotor sequences in speech.
Figure 2. Dual streams or multiple streams?

(A) A relatively recent dual-pathway model of the visual cortical system (Kravitz et al., 2011). The model posits that the dorsal stream trifurcates at the level of the posterior parietal cortex (PP) and splits into three sub-streams that project, respectively, to the prefrontal, premotor, and medial temporal lobe. (B) The standard dual-pathway model of the auditory cortical system shows direct projections from the anterolateral and caudolateral belt fields AL and CL to segregated prefrontal regions (VLPFC and DLPFC; modified from Romanski et al., 1999). Besides these direct projections (shown as solid lines), both processing streams also contain indirect projections to prefrontal cortex (shown as dashed lines). The dorsal stream projects to DLPFC via the inferior parietal lobule (IPL) and premotor cortex (PMC); the ventral stream’s indirect route leads polysynaptically to VLPFC via the rostral parabelt (RPB) and the anterior superior temporal (AST) region. Whether direct and indirect projections carry different functions is currently unknown. The important feature of this model (compared to the model of Kravitz et al.) is that both processing streams begin in early sensory areas and target prefrontal cortex as their ultimate endpoint. Feedback projections are not shown here (but see Rauschecker & Scott, 2009).
Acknowledgments
The research summarized here was supported by the National Science Foundation [grant numbers BCS-0519127 and OISE-0730255] and the National Institutes of Health [grant numbers R01DC03489, R01NS052494, and R01DC014989]. The manuscript was prepared with partial support from the Technische Universitat Munchen – Institute for Advanced Study, funded by the German Excellence Initiative and the European Union Seventh Framework Programme under grant agreement n° 291763 (JPR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ungerleider LG, Mishkin M. Two visual systems. In: Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge: MIT Press; 1982. [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998a;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci U S A. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen CB. Two mechanisms of vision in primates. Psychol Forsch. 1968;31(4):299–348. doi: 10.1007/BF00422717. [DOI] [PubMed] [Google Scholar]
- Schneider GE. Two visual systems. Science. 1969;163(3870):895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Hall WC. Evolution of neocortex. Science. 1969;164(3877):251–262. doi: 10.1126/science.164.3877.251. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Some aspects of visual capacity in monkeys and man following striate cortex lesions. Arch Ital Biol. 1978;116(3-4):318–323. [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983;6(10):414–417. [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–4. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Processing of complex sounds in the auditory cortex of cat, monkey and man. Acta Otolaryngologica (Stockh) 1997;532(Suppl):34–38. doi: 10.3109/00016489709126142. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998b;8:516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FAW, Scalaidhe SP, Goldman-Rakic PS. Dissociation of Object and Spatial Processing Domains in Primate Prefrontal Cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. What is an auditory object? Nat Rev Neurosci. 2004;5(11):887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. J Neurophysiol. 2006;96:3323–3337. doi: 10.1152/jn.00392.2006. [DOI] [PubMed] [Google Scholar]
- Ortiz-Rios M, Azevedo FAC, Kuśmierek P, Balla DZ, Munk MH, Keliris GA, Logothetis NK, Rauschecker JP. Opponent signals and posterior specialization represent sound location in macaque auditory cortex. Neuron. 2017;93(4):971–983. e4. doi: 10.1016/j.neuron.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. ‘What’ and ‘where’ in the human auditory system. Proc Natl Acad Sci U S A. 2001;98:12301–12306. doi: 10.1073/pnas.211209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder PP, Meuli RA, Adriani M, Bellmann A, Fornari E, Thiran JP, Pittet A, Clarke S. Distinct pathways involved in sound recognition and localization: a human fMRI study. Neuroimage. 2001;14:802–816. doi: 10.1006/nimg.2001.0888. [DOI] [PubMed] [Google Scholar]
- Warren JD, Zielinski BA, Green GGR, Rauschecker JP, Griffiths TD. Perception of sound-source motion by the human brain. Neuron. 2002;34:139–148. doi: 10.1016/s0896-6273(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Arnott SR, Binns MA, Grady CL, Alain C. Assessing the auditory dualpathway model in humans. Neuroimage. 2004;22:401–408. doi: 10.1016/j.neuroimage.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Jääskeläinen IP, Raij T, Bonmassar G, Devore S, Hämäläinen M, Levänen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103:14608–14613. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deouell LY, Heller AS, Malach R, D’Esposito M, Knight RT. Cerebral Responses to Change in Spatial Location of Unattended Sounds. Neuron. 2007;55:985–996. doi: 10.1016/j.neuron.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Spierer L, Bellmann-Thiran A, Maeder P, Murray MM, Clarke S. Hemispheric competence for auditory spatial representation. Brain. 2009;132:1953–1966. doi: 10.1093/brain/awp127. [DOI] [PubMed] [Google Scholar]
- Zündorf IC, Lewald J, Karnath H. Testing the dual-pathway model for auditory processing in human cortex. Neuroimage. 2016;124:672–681. doi: 10.1016/j.neuroimage.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Lewald J, Foltys H, Töpper R. Role of the posterior parietal cortex in spatial hearing. J Neurosci. 2002;22(3):RC207. doi: 10.1523/JNEUROSCI.22-03-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- At A, Spierer L, Clarke S. The role of the right parietal cortex in sound localization: A chronometric single pulse transcranial magnetic stimulation study. Neuropsychologia. 2011;49(9):2794–2797. doi: 10.1016/j.neuropsychologia.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20(7):2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Bouffard M, Ahad P, Belin P. Where is ‘where’ in the human auditory cortex? Nat Neurosci. 2002;5:905–909. doi: 10.1038/nn904. [DOI] [PubMed] [Google Scholar]
- Lewald J, Riederer KAJ, Lentz T, Meister IG. Processing of sound location in human cortex. Eur J Neurosci. 2008;27(5):1261–1270. doi: 10.1111/j.1460-9568.2008.06094.x. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of auditory space: pathways and plasticity. In: Mast F, Jancke L, editors. Spatial Processing in Navigation, Imagery, and Perception. Springer-Verlag; New York: 2007. pp. 389–410. [Google Scholar]
- Rauschecker JP. Auditory and visual cortex of primates: a comparison of two sensory systems. Eur J Neurosci. 2015;41(5):579–585. doi: 10.1111/ejn.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Rees G, Rees A, Green GG, Witton C, Rowe D, Büchel C, Turner R, Frackowiak RS. Right parietal cortex is involved in the perception of sound movement in humans. Nat Neurosci. 1998;1(1):74–79. doi: 10.1038/276. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Schonwiesner M, von Cramon DY, Rubsamen R, Shah NJ, Zilles K, Fink GR. Representation of interaural temporal information from left and right auditory space in the human planum temporale and inferior parietal lobe. Cereb Cortex. 2005;15(3):317–324. doi: 10.1093/cercor/bhh133. [DOI] [PubMed] [Google Scholar]
- Camalier CR, D’Angelo WR, Sterbing-D’Angelo SJ, de la Mothe LA, Hackett TA. Neural latencies across auditory cortex of macaque support a dorsal stream supramodal timing advantage in primates. Proc Natl Acad Sci U S A. 2012;109:18168–18173. doi: 10.1073/pnas.1206387109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmierek P, Rauschecker JP. Selectivity for space and time in early areas of the auditory dorsal stream in the rhesus monkey. J Neurophysiol. 2014;111(8):1671–1685. doi: 10.1152/jn.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY, Lohmann G. Auditory what, where, and when: a sensory somatotopy in lateral premotor cortex. Neuroimage. 2003;20:173–185. doi: 10.1016/s1053-8119(03)00218-0. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Scott SK. Auditory processing--speech, space and auditory objects. Curr Opin Neurobiol. 2005;15(2):197–201. doi: 10.1016/j.conb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA. 2012;109(8):E505–514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. An expanded role for the dorsal auditory pathway in sensorimotor integration and control. Hear Res. 2011;271:16–25. doi: 10.1016/j.heares.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. The theory of affordances. In: Shaw R, Bransford J, editors. Perceiving, acting, and knowing: Toward an ecological psychology. Hillsdale, NJ: Erlbaum; 1977. pp. 67–82. [Google Scholar]
- Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 2004;27:377–96. doi: 10.1017/s0140525x04000093. discussion 396-442. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7(7):701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Von Holst E, Mittelstaedt H. Das Reafferenzprinzip (Wechselwirkungen zwischen Zentralnervensystem und Peripherie) Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, Augusto PB, Tripodi S, Pires CG, Pereira GA, Brasil FL, Gallo S, Lin AA, Takigami AK, Aratanha MA, Joshi S, Bleuler H, Cheng G, Rudolph A, Nicolelis MAL. Long-Term Training with a Brain-Machine Interface-Based Gait Protocol Induces Partial Neurological Recovery in Paraplegic Patients. Sci Rep. 2016;79(6):N13–N14. doi: 10.1038/srep30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neutrally controlled robotic arm. Nature. 2012;485(7398):372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G. Frontiers in Neuroengineering. CRC Press; 2014. Humanoid Robotics and Neuroscience: Science, Engineering and Society. [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Tian B, Carlyon RP, Rauschecker JP. Perceptual organization of sound sequences in the auditory cortex of awake macaques. Neuron. 2005;48:139–48. doi: 10.1016/j.neuron.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Cusack R. The intraparietal sulcus and perceptual organization. J Cogn Neurosci. 2005;17:641–651. doi: 10.1162/0898929053467541. [DOI] [PubMed] [Google Scholar]
- Leaver AM, Van Lare JE, Zielinski BA, Halpern A, Rauschecker JP. Brain activation during anticipation of sound sequences. J Neurosci. 2009;29(8):2477–2485. doi: 10.1523/JNEUROSCI.4921-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artchakov D, Ortiz M, Kusmierek P, Cui D, VanMeter I, Jääskeläinen I, Sams M, Rauschecker JP. Representation of sound sequences in the auditory dorsal stream after sensorimotor learning in the rhesus monkey. Soc Neurosci Abstr 368.04 2012 [Google Scholar]
- Rauschecker JP. Is there a tape recorder in your head? How the brain stores and retrieves musical melodies. Front Syst Neurosci. 2014;8:149. doi: 10.3389/fnsys.2014.0014928. published online: 28 August 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms for memory. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The language connectome: new pathways, new concepts. Neuroscientist. 2014;20(5):453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. Journal of Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide MA, Friederici AD. Response to Bornkessel-Schlesewsky et al.--towards a nonhuman primate model of language? Trends in Cognitive Sciences. 2015;19(9):483. doi: 10.1016/j.tics.2015.05.011. Epub 2015 Jun 18. [DOI] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP. Response to Skeide and Friederici: The myth of the uniquely human ‘direct’ dorsal pathway. Trends in Cognitive Sciences. 2015a Jun 16; doi: 10.1016/j.tics.2015.05.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Descent of Man and Selection in Relation to Sex. First ed. London: John Murray; 1871. [Google Scholar]
- Fritz J, Mishkin M, Saunders RC. In search of an auditory engram. Proc Natl Acad Sci U S A. 2005;102(26):9359–9364. doi: 10.1073/pnas.0503998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Gathercole SE, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Ventral and dorsal streams in the evolution of speech and language. Front Evol Neurosci. 2012;4:7. doi: 10.3389/fnevo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP. Neurobiological roots of language in primate audition: common computational properties. Trends in Cognitive Sciences. 2015b;19(3):142–150. doi: 10.1016/j.tics.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


