Abstract
Background
Neurosteroids like alphaxalone are potent anxiolytics, anticonvulsants, amnestics, and sedative-hypnotics, effects linked to enhancement of GABAA receptor gating in the central nervous system. Data locating neurosteroid binding sites on synaptic αβγ GABAA receptors are sparse and inconsistent. Some evidence points to outer transmembrane β+–α − interfacial pockets, near sites that bind the anesthetics etomidate and propofol. Other evidence suggests that steroids bind more intracellularly in β+–α − interfaces.
Methods
We created 12 single-residue β3 cysteine mutations: β3T262C and β3T266C in β3-M2; and β3M283C, β3Y284C, β3M286C, β3G287C, β3F289C, β3V290C, β3F293C, β3L297C, β3E298C, and β3F301C in β3-M3 helices. We co-expressed α1 and γ2L with each mutant β3 subunit in Xenopus oocytes and electrophysiologically tested each mutant for covalent sulfhydryl modification by the water soluble reagent para-chloromercuribenzenesulfonate. We then assessed whether receptor-bound alphaxalone, etomidate, or propofol blocked cysteine modification, implying steric hindrance.
Results
Eleven mutant β3 subunits, when co-expressed with α1 and γ2L, formed functional channels that displayed varied sensitivities to the three anesthetics. Exposure to para-chloromercuribenzenesulfonate produced irreversible functional changes in ten mutant receptors. Protection by alphaxalone was observed in receptors with β3V290C, β3F293C, β3L297C, or β3F301C mutations. Both etomidate and propofol protected receptors with β3M286C or β3V290C mutations. Etomidate also protected β3F289C. In α1β3γ2L structural homology models, all these protected residues are located in transmembrane β+–α − interfaces.
Conclusions
Alphaxalone binds in transmembrane β+–α − pockets of synaptic GABAA receptors that are adjacent and intracellular to sites for the potent anesthetics etomidate and propofol.
Keywords: Ligand-gated ion channel, glycine receptor, GABA, GluCl, general anesthetic, etomidate, propofol, neurosteroid, neuroactive steroid, cysteine mutation, modification, photolabel, electrophysiology, allosteric modulator, allosteric agonist
Introduction
Neurosteroids (neuroactive steroids), including the general anesthetic alphaxalone (ALX), allopregnanolone, and tetrahydro-deoxycorticosterone, are potent rapid-acting anxiolytics, anticonvulsants, amnestics, and sedative-hypnotics 1. These effects are linked to enhanced gating of γ-aminobutyric acid type A (GABAA) receptors, the main inhibitory neurotransmitter receptors in mammalian brain and major molecular targets for the general anesthetics propofol (PRO) and etomidate (ETO) 2,3. Typical synaptic GABAA receptors consist of 2α, 2β, and 1γ subunits arranged βαβαγ counterclockwise, viewed from the extracellular space 4. Each GABAA subunit contains an N-terminal extracellular domain and a transmembrane domain with four alpha helices: M1 to M4. Five M2 helices surround a receptor’s central chloride channel, while M1 and M3 helices form an intermediate ring between M2s and M4 helices. Subunit interfaces are designated β+–α − (two per receptor), α+–β −, γ+–β −, and α+–γ −, where ‘+’ corresponds to the M3 face and ‘−‘ is the M1 face.
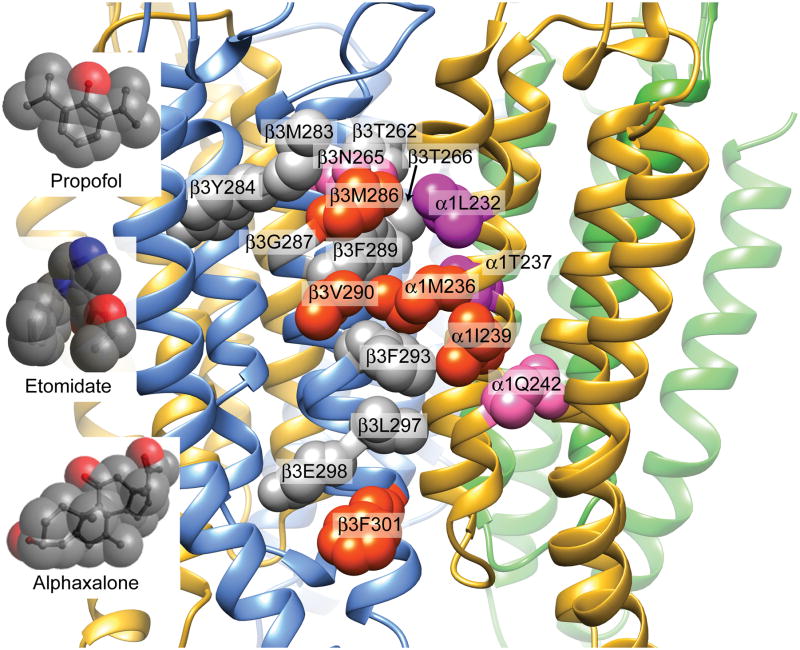
Data locating neurosteroid sites on GABAA receptors are sparse and inconsistent (Table 1 5–22). Pharmacokinetic studies indicate that neurosteroids reach GABAA receptors via membrane lipids 23. Mutations in α1-M1 at α1M236, α1T237, and α1I239 reduce neurosteroid sensitivity 5,13. These residues map to outer transmembrane β+–α − clefts in homology models based on glutamate-gated chloride (GluCl) channels from Caenorhabditis elegans 24 (Fig 1 25) and are identified by photolabeling and substituted cysteine modification-protection (SCAMP) studies as contacts for ETO and PRO (Table 1) 26. Ivermectin binds to outer transmembrane inter-subunit pockets on GluCl 24 and triiodothyronine displaces both ivermectin and allopregnanolone from homologous GABAA receptor sites, including the ETO/PRO sites 27. Thus, neurosteroids may act through the outer transmembrane β+–α − pockets where ETO and PRO bind.
Table 1.
Evidence of Neurosteroid and Anesthetic Contacts in β+–α − Transmembrane Interfaces of GABAA receptors
| Residue | Receptor | Mutant Effects a | Photolabels b | SCAMP c |
|---|---|---|---|---|
| α1L232 | α1β3γ2L | ETO, PRO 5 | — | ETO 5,6 |
|
| ||||
| α1M236 | α1β3γ2L | ETO, PRO, ALX 5,7 | Azi-ETO 8 | ETO, PRO 5,6,9 |
| α1β3 | Azi-ETO 10 | |||
| α1β3 | TDBzl-ETO 11 | |||
| α1β3 | Azi-Pm 12 | |||
|
| ||||
| α1T237 | α1β2γ2L | Neurosteroids 13 | ||
| α1β3γ2L | — | ETO 6 | ||
|
| ||||
| α1I239 | α1β2γ2L | Neurosteroids 13 | ||
| α1β3γ2L | — | — 6, d | ||
| α1β3 | Azi-Pm 12 | |||
|
| ||||
| α1Q242 | α1β2γ2L | Neurosteroids 13,14 | ||
| α1β3γ2L | — | — 6, d | ||
|
| ||||
| β3N265 | α1β3γ2L | ETO, PRO 15–18 | — | ETO, PRO 9 |
|
| ||||
| β3M286 | α1β2/3γ2 | ETO, PRO 7,18,19 | Azi-ETO 8,10 | ETO, PRO 20,21 |
| α1β3 | TDBzl-ETO 11 | |||
| α1β3 | Azi-Pm 12 | |||
|
| ||||
| β3F289 | α1β3γ2L | (ETO) 8, b | ||
|
| ||||
| β3V290 | α1β3 | TDBzl-ETO 11 | ||
|
| ||||
| β3F301 | β3 | 6-AziP 22 | ||
ETO = etomidate; PRO = propofol; ALX = alphaxalone; Azi-ETO = azi-etomidate; TD-Bzl-ETO = p-trifluoromethyldiaziryl-phenyl-etomidate; Azi-Pm = m-azi-propofol; 6-AziP = 6-azi-pregnanolone; neurosteroids are allopregnanolone (ALLOP) and tetrahydro-deoxycorticosterone (THDOC). — Indicates negative results.
Drugs displaying reduced enhancement of submaximal GABA responses in mutant receptors are listed. Not all loci have been tested with ETO, PRO, and ALX. Negative effects of α1M236 and βM286 mutations on ALX sensitivity have been reported.5,18
Direct or indirect (indicated by parentheses) photolabeling evidence is included. Specifically, β3F289 photolabeling by m-trifluoromethyl-mephobarbital is inhibited by etomidate.
SCAMP is Substituted Cysteine Modification-Protection. Drugs demonstrating protection are listed. Not all loci have been tested with ETO, PRO, and ALX. Negative results have been reported for PRO at α1L232C and ALX at α1L232C, α1M236C and β3M286C.5,6,21
Application of a cysteine modifying reagent (p-chloromercuribenzenesulfonate) to α1I239C and α1Q242C did not alter function, precluding protection studies.
Fig 1. General anesthetic contacts within the GABAA receptor β+–α − transmembrane cleft.
The transmembrane domain of a α1β3γ2L structural homology model based on GluCl (pdb 4COF) is depicted 25. Subunit peptide backbones are shown as ribbons (α1 = yellow; β3 = blue; γ2L = green), with sidechains of interest (see Table 1) shown in space-filling mode and labeled. Amino acid sidechains on β3-M3 and α1-M1 that are directly photolabeled by analogs of one or more study anesthetics are colored orange-red. Anesthetic contact sidechains that have previously been identified using substituted cysteine modification-protection are colored purple. Other β3-M2 and β3-M3 sidechains that line the β+–α– cleft and three sidechains predicted to face the β3 intra-subunit helix bundle pocket (Y284, G287, and E298), are colored gray. The location of α1Q242 (pink) is also shown. Inserts display the molecular space-filling structures of propofol, etomidate, and alphaxalone, approximately scaled to the receptor model. Hydrogens have been hidden for clarity.
Other evidence indicates that neurosteroid sites are separate from ETO and PRO sites. Neurosteroids synergize with ETO and its derivatives when co-applied to GABAA receptors 28,29. Previous SCAMP experiments find no ALX interactions at several ETO and PRO contacts in outer transmembrane β+–α − clefts or other homologous pockets in α1β3γ2L receptors 5,21. Other evidence points to inner transmembrane β+–α − neurosteroid sites. Mutations in inner α1-M1 at α1Q242 reduce neurosteroid sensitivity 13,14. The photolabel (3α,5β)-6-azi-pregnanolone (6-AziP) incorporates in inner β3-M3 at β3F301, but this study used β3 homomeric receptors 22. Finally, β2Y284 mutations also impair neurosteroid effects13. This residue’s location in β3 crystals 30 and homology models (Fig 1) suggests neurosteroid sites within β3 intra-subunit helix bundles.
To test whether ALX binds in β+–α − transmembrane clefts and compare ALX sites to those for ETO and PRO, we used SCAMP to assess drug contacts on β3-M2 and β3-M3 helices in α1β3γ2L receptors. Using the structure of β3 homomeric receptors30 and our GluCl-based structural homology model 25 (Fig 1), we selected residues spanning most of the β3-M3 helix, from β3M283 (outer) to β3F301 (inner), most facing the β+–α − interface and several facing the intra-subunit β3 helix pocket. Our results suggest that ALX contacts β3-M3 at β+–α − interfacial residues that are adjacent and intracellular to those for PRO and ETO.
Materials and Methods
Animals
Oocytes were harvested from female Xenopus laevis frogs in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal use in this study was approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (protocol #2005N000051). Frogs were housed and maintained in a veterinarian-supervised facility and anesthetized in tricaine during oocyte collection. All efforts were made to minimize suffering.
Materials
Alphaxalone was purchased from Tocris Bioscience (Bristol, UK) and propofol (2,6-diisopropylphenol) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Both were stored as 10 mM solutions in DMSO and diluted in electrophysiology buffer for experiments. R-Etomidate was purchased from Hospira, Inc (Lake Forest, IL, USA) as a 2 mg/ml (~8.2 mM) solution in 35% propylene glycol:water and diluted in electrophysiology buffer for experiments. We have previously shown that DMSO and propylene glycol at the dilutions used during electrophysiology experiments produce no effects on GABAA receptor function 25. R-mTFD-MPAB (R-allyl-m-trifluoromethyl-mephobarbital) 31 was a gift from Prof. Karol Bruzik, PhD (Dept. of Medicinal Chemistry and Pharmacognosy, University of Illinois, Chicago, USA). It was stored as a 100 mM solution in DMSO and diluted in electrophysiology buffer for experiments. Para-chloromercuribenzenesulfonic acid sodium salt (pCMBS) was purchased from Toronto Research Chemicals (Toronto, ON, Canada). Fresh pCMBS stock solutions in electrophysiology buffer were prepared on the day of use, and kept on ice until final dilution. γ-Aminobutyric acid (GABA), picrotoxin, salts, and buffers were purchased from Sigma-Aldrich.
GABAA Receptor Expression in Xenopus Oocytes
Oocytes were prepared for use as previously described 5. Complementary DNAs encoding human α1, β3, and γ2L GABAA receptor subunits in pCDNA3.1 expression vectors (Thermo Fisher Scientific, Waltham, MA, USA) were used. Cysteine mutations were introduced into β3 by site-directed mutagenesis using QuikChange kits (Agilent Technologies, Santa Clara, CA, USA). After sequencing several clones through the entire coding region, one clone for each mutant was chosen for further use. Messenger RNAs were synthesized on linearized DNA templates using mMessage mMachine kits (Thermo Fisher), purified, and combined at ratios of 1α:1β:5γ (final concentration 1 ng/nl in RNAase-free water). Oocytes were injected with ~50 ng mRNA mix and incubated in ND96 buffer (in mM: 96 NaCl, 2 KCl, 1 CaCl2, 0.8 MgCl2, 10 HEPES, pH 7.5) supplemented with ciprofloxacin (2 mg/ml) and amikacin (100 μg/ml) at 17 °C for 48 to 72 hours before electrophysiological studies.
Two Electrode Voltage-Clamp Electrophysiology
Electrophysiological experiments were performed in ND96 buffer at 21 to 23 °C as previously described 5. Oocytes were placed in a 30 μl custom flow-cell, impaled with borosilicate glass micro-electrodes filled with 3 M KCl (resistance < 1 MΩ), then voltage-clamped at −50 mV (model OC-725C, Warner Instruments, Hamden CT, USA). Superfusion solutions in ND96 were controlled by electrical valves (VC-8, Warner Instruments) and delivered at a rate of 2–3 ml/min from glass reservoir syringes via PTFE tubing and a PTFE micro-manifold (MP-8, Warner Instruments). Specialized software and a digital input/output interface (pClamp 8.0 and Digidata 1322, both from Molecular Devices, Sunnyvale, CA) were used to coordinate delivery of solutions and recordings. Current signals were filtered at 1 kHz, digitized at 100 Hz, and stored on a computer disk for offline analysis.
GABA concentration-responses, spontaneous receptor activity, and GABA efficacy
Each mutant receptor was initially characterized to establish its sensitivity to GABA, maximal GABA efficacy, and whether it was spontaneously active. Voltage-clamped oocytes were exposed to GABA solutions (range 0.1 μM to 10 mM) for 10 to 20 s, followed by 5 minute ND96 wash. Normalization sweeps at the maximum GABA concentration for the specific receptor (>10 x EC50; 1–10 mM) were recorded every second or third experiment. At least 3 oocytes from two different frogs were used for each concentration-response.
Spontaneous activation of GABAA receptors (in the absence of GABA or anesthetics) was assessed by applying 2 mM picrotoxin to voltage-clamped oocytes. Reversible outward currents during picrotoxin application represent closure of spontaneously active channels. Spontaneous activity was normalized to maximal GABA-elicited current in the same cell (n ≥ 3 cells).
Maximal GABA efficacy for each receptor was estimated by comparing peak currents elicited with maximal GABA (1–10 mM) to currents elicited with high GABA supplemented with either 2.5 to 5 μM ALX or 3.2 to 6.4 μM ETO, depending on the receptor’s drug sensitivity (see below). Agonist efficacy was calculated by normalizing maximal GABA responses to GABA + anesthetic responses in the same cell, assuming the latter represents 100% activation (n ≥ 3 cells).
GABA EC5 enhancement
Each mutant was also characterized for sensitivity to ETO, PRO, and ALX. Voltage-clamped oocytes expressing GABAA receptors were repetitively exposed for 20 s to GABA EC5 (eliciting ~ 5% of maximal GABA response) separated by 5 min wash until three stable responses (varying by less than 5%) were sequentially recorded. The oocyte was then exposed for 30 s to anesthetic, followed by 20 s exposure to a solution containing GABA EC5 combined with anesthetic at 2 x EC50 for loss-of-righting-reflexes (LoRR) in tadpoles: 2.5 μM alphaxalone 32, 3.2 μM etomidate 33, or 5 μM propofol 34. For each receptor type and three anesthetics, multiple measurements of current response to GABA EC5 and GABA EC5 + anesthetic were obtained in at least four oocytes from two different frogs. EC5 enhancement (mean ± sem; n ≥ 4) was calculated from the set of individual oocyte ratios of currents measured with anesthetic present to EC5 GABA alone.
Substituted Cysteine Modification and Protection (SCAMP)
SCAMP studies followed the approach we have described previously 5,21. In each mutant receptor, functional effects and rates of cysteine modification were assessed electrophysiologically after applications of pCMBS either alone or together with maximally activating GABA (1 to 10 mM). Before and after pCMBS exposures, voltage clamped Xenopus oocytes expressing mutant receptors were exposed to first GABA EC5 (low) and then a maximally activating GABA concentration (high; 1–10 mM). After 5 minute wash, oocytes were exposed for 10 to 20 s to pCMBS (1 μM to 1 mM), a water-soluble sulfhydryl modifying reagent, either alone or co-applied with maximal GABA (1 to 10 mM). PCMBS exposure was followed by a 3–5 minute wash in ND96. Electrophysiological responses to low and high GABA were then re-tested to assess any irreversible changes in receptor function produced by pCMBS modification (in most cases an increase in the ratio of low versus high GABA-induced peak currents). By testing a range of pCMBS concentrations this way, we identified conditions resulting in maximal modification effects and those appropriate for studying modification rates.
To measure apparent modification rates, pCMBS exposure conditions (concentration x time) were chosen that produced about 10% of the maximal modification effect per cycle. In nearly all mutants, higher pCMBS concentrations were needed to irreversibly affect receptors when applied alone than when co-applied with GABA. Voltage-clamped oocytes were first repeatedly tested for responses to both low and high GABA, then washed for 5 min in ND96, to confirm that the response ratio was stable (< 5% variation) before pCMBS exposure. Oocytes were then exposed for 5 to 10 s to pCMBS (with or without GABA), followed by 5 minute wash, and re-testing for low and high GABA responses. At least three cycles of pCMBS exposure/wash/low:high GABA response testing were performed on each oocyte used for rate analysis. The series of modification cycles under the selected conditions typically produced less than 50% of the maximal modification effect. A final modification cycle was performed using 10 x pCMBS concentration for 20 s to fully modify receptors, and subsequent electrophysiological response was assessed as the maximal modification effect.
Protection experiments were performed in the presence of maximally activating GABA, as previously described 5, so control modification conditions were pCMBS + GABA. Oocytes were exposed to anesthetic for 30 s followed by exposure to a solution of pCMBS + GABA + anesthetic. Post-modification wash and response tests were identical to control modification conditions (i.e. usually with no anesthetic present, but see below). Anesthetic concentrations used in initial protection studies were chosen to maximize site occupancy, while enabling washout within 5 minutes (10 μM ETO; 20 μM PRO; and 10 μM ALX). In receptors with β3F289, β3F293C, and β3L297C mutations, higher concentrations of anesthetics (50 μM ETO, 100 μM PRO, or 50 μM ALX) were also used in protection experiments. Under these conditions, anesthetic washout between pCMBS exposure and testing for modification effects was extremely slow. We therefore used an alternative approach to low GABA responses, measuring direct activation by anesthetics alone (50 μM ETO, 100 μM PRO, or 50 μM ALX), normalized to high GABA responses. At least two anesthetics were tested in the same manner, to test for drug-specific interactions. In the case of receptors with β3V290C mutations, we tested for allosteric effects (i.e. whether all anesthetics similarly affect pCMBS modification), by including SCAMP studies with 10 μM mTFD-MPAB, a barbiturate hypnotic that acts through GABAA receptor sites outside the β+–α − interfaces 8,31. For each cysteine mutant, at least 5 oocytes were studied in control modification experiments and at least 4 oocytes were studied in each set of anesthetic protection experiments. Group sample sizes of 5 per group were based both on prior experience and a power analysis performed as previously described 5, using a one-tail t-test with α = 0.017 (adjusted for three drug comparisons to each control).
Data analysis and statistics
Results in text and figures are mean ± sem unless otherwise indicated.
GABA concentration-responses
Digitized GABA concentration-response data was corrected for baseline leak currents and digitally filtered (10 Hz low-pass, Bessel function) using Clampfit 9.0 software (Molecular Devices). Peak currents were normalized to control (maximal currents), and combined GABA data from multiple cells (n ≥ 3) was fitted with logistic equations using Prism 5.02 (GraphPad Software Inc, La Jolla, CA):
| Eq. 1 |
where EC50 is the half-maximal activating GABA concentration, and nH is the Hill slope. Mean GABA EC50 and 95% confidence interval are reported. To assess whether mutations altered GABA EC50 relative to wild-type, we performed sum-of-squares F-tests in Graphpad Prism 5.02, using p < 0.0045 as a statistical significance threshold (the Bonferroni correction for p<0.05 with 11 comparisons).
Functional characteristics of mutant receptors
To test whether mutations altered spontaneous activity and/or GABA efficacy from wild-type values we used one-way ANOVA with post-hoc Dunnett’s tests (in Prism 5.02). To test whether mutations affected receptor sensitivities to ETO, PRO, or ALX, EC5 enhancement data for the three equi-potent anesthetic concentrations in wild-type and all functional cysteine mutants was tabulated and analyzed with two-way ANOVA and Bonferroni posttests for wild-type vs. mutation for each anesthetic (Prism 5.02).
SCAMP
Inferences regarding contact between receptor-bound anesthetics and substituted cysteine sidechains were made when an anesthetic inhibited pCMBS modification selectively, with at least one other anesthetic failing to inhibit modification. Apparent pCMBS modification rates were calculated from data for individual oocytes expressing cysteine mutants. Either normalized maximal GABA responses (for α1β3T262Cγ2L) or normalized low:high GABA response ratios (all other mutants) were plotted against cumulative pCMBS exposure (M×s) and fitted by linear least squares with y-axis intercepts fixed at 1.0. The linear slope, under conditions of partial modification, is presumed to be proportional to the bimolecular reaction rate between pCMBS and the substituted cysteine sulfhydryl.
For α1β3T262Cγ2L data, apparent modification rates were calculated as the absolute values of the negative fitted slopes. Absolute slopes less than 10 M−1s−1 (the lower limit of detection) were assigned a rate of 10 M−1s−1 for statistical analysis. To identify anesthetics that either accelerated or inhibited modification of each substituted cysteine, apparent rates from control and anesthetic protection studies for that mutant were log transformed, tabulated, and compared using one-way ANOVA (Prism 5.02) with p < 0.05 as a significance threshold.
Results
Functional characteristics of β3 cysteine mutants
Based on both crystallographic data for β3 homomeric GABAA receptors (PDB 4COF) 30 and our α1β3γ2L structural homology model based on GluCl bound to ivermectin (PDB 3RHW; Fig 1) 25,26, we identified nine β3-M2 and M3 helix residues facing the β+–α − cleft: T262, T266, M283, M286, F289, V290, F293, L297, and F301. We created mutant β3 cDNAs encoding cysteine substitutions at these positions, as well as at Y284, G287, and E298, which are predicted to instead face the intra-subunit β3 helix-bundle pocket. Wild-type and mutant β3 subunits were co-expressed with wild-type α1 and γ2L subunits in Xenopus oocytes and functionally characterized using two-microelectrode voltage clamp electrophysiology. No GABA-activated currents were detected when β3 subunits with Y284C mutations were co-expressed with α1 and γ2L, consistent with a prior report 35. All the other mutations produced GABA-sensitive ion channels with sufficient oocyte currents elicited by 1–10 mM GABA (≥ 0.5 μA at −50 mV) for further experiments. Table 2 summarizes GABA EC50, spontaneous activation, apparent maximal GABA efficacy, and the effect of pCMBS application in these mutant receptors, in comparison to wild-type α1β3γ2L. Six mutations (β3T266C, β3M286C, β3G287C, β3F293C, β3L297C, and β3E298C) significantly increased GABA EC50 and one (β3F289C) reduced GABA EC50 approximately five-fold. Four mutant receptors characterized by increased GABA EC50 also exhibited significantly reduced GABA efficacy (β3M286C, β3F293C, β3L297C, and β3E298C). Like other mutations that sensitize receptors to GABA 7,36, β3F289C was associated with both high GABA efficacy and measurable spontaneous activation. Our observations were also consistent with previous studies of β2M286C, β2G287C, and β2F289C mutations 20,21,35,37,38.
Table 2.
Functional and Pharmacological Characteristics of α1β3γ2L GABAA Receptors with β3 Cysteine Substitutions
| Receptor Type | GABA EC50(μM) [95% CI] (n) | GABA Efficacy mean ± se (n) | Spont. Activation mean ± se (n) | Maximal Effect of pCMBS Modification (range) |
|---|---|---|---|---|
| α1β3γ2L | 31 [23 to 41] (8) | 0.88 ± 0.025 (5) | < 0.005 (5) | No effect |
| α1β3T262Cγ2L | 21 [18 to 25] (4) | 0.93 ± 0.03 (4) | < 0.005 (3) | Reduce max current (95–99%) |
| α1β3T266Cγ2L | 143 [130 to 157] (6) *** | 0.88 + 0.02 (4) | < 0.005 (3) | ↑ lo/hi GABA response (10 to 13-fold) |
| α1β3M283Cγ2L | 46 [43 to 50] (3) | 0.92 ± 0.023 (3) | < 0.005 (3) | No effect |
| α1β3M286Cγ2L | 148 [122 to 180] (3) *** | 0.65 ± 0.023 (3) ** | < 0.005 (3) | ↑ lo/hi GABA response (5.8 to 7.8-fold) |
| α1β3G287Cγ2L | 78 [67 to 92] (4) ** | 0.96 ± 0.034 (4) * | < 0.005 (3) | ↑ lo/hi GABA response (2.3 to 3.5-fold) |
| α1β3F289Cγ2L | 5.9 [5.3 to 6.7] (4) *** | 0.99 ± 0.02 (4) ** | 0.034 ± 0.016* (4) | ↑ lo/hi GABA response (2.8 to 4.0-fold) |
| α1β3V290Cγ2L | 36 [32 to 41] (4) | 0.92 ± 0.03 (4) | < 0.005 (3) | ↑ lo/hi GABA response (2.3 to 3.1-fold) |
| α1β3F293Cγ2L | 181 [143 to 229] (3) *** | 0.16 ± 0.018 (3) *** | < 0.005 (3) | ↑ lo/hi GABA response (2.6 to 4.3-fold) |
| α1β3L297Cγ2L | 114 [104 to 125] (4) *** | 0.54 ± 0.07 (5) *** | < 0.005 (4) | ↑ lo/hi GABA response (5.5 to 7.2-fold) |
| α1β3E298Cγ2L | 103 [88 to 112] (4) *** | 0.63 ± 0.07 (3) *** | < 0.005 (3) | ↑ lo/hi GABA response (3.5 to 5.5-fold) |
| α1β3F301Cγ2L | 34 [30 to 39] (4) | 0.93 ± 0.04 (4) | < 0.005 (4) | ↑ lo/hi GABA response (3.8 to 4.9-fold) |
Differs from wild-type at:
p < 0.0045,
p < 0.0005,
p < 0.0001
Anesthetic sensitivities of cysteine mutants
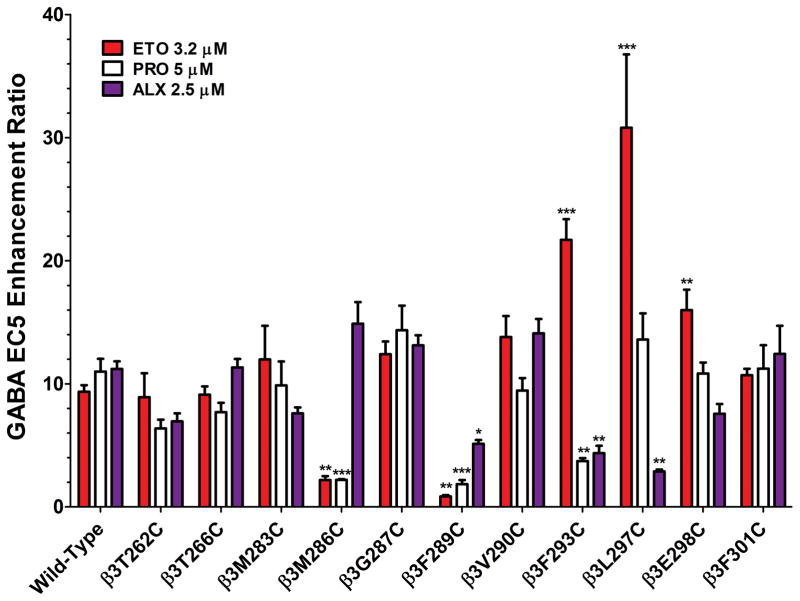
GABAA receptor mutations may alter anesthetic modulation, which can in turn affect the conditions appropriate for SCAMP tests for drug contacts. We therefore characterized each mutant receptor’s sensitivity to ETO, PRO, and ALX by measuring anesthetic enhancement of activation by EC5 GABA. Results are summarized in Fig. 2. Drug solutions of 3.2 μM ETO, 5 μM PRO, and 2.5 μM ALX are all twice the EC50 for tadpole loss-of-righting reflexes and also similarly enhance the gating of wild-type α1β3γ2L GABAA receptors activated with EC5 GABA 5 (Fig 2). Compared to wild-type, two mutations, β3M286C and β3F289C reduced EC5 enhancement by 3.2 μMETO, while β3F293C, β3L297C, and β3E298C increased EC5 enhancement by ETO. EC5 enhancement by 5 μM PRO was also reduced by β3M286C and β3F289C, as well as by β3F293C. EC5 enhancement by 2.5 μM ALX was reduced by β3F289C, β3F293C, and β3L297C.
Fig 2. Modulation of wild-type vs. cysteine-substituted GABAA receptors by etomidate, propofol, and alphaxalone.
Each bar represents mean ± sem results (n ≥ 4) of experiments quantifying the anesthetic enhancement of GABA EC5 responses in wild-type and 11 cysteine substituted mutants. The drug concentrations are each 2 x EC50 in tadpole loss of righting reflexes assays and similarly modulate wild-type receptor currents: 3.2 μM etomidate (ETO, red); 5 μM propofol (PRO, white); and 2.5 μM alphaxalone (ALX, purple). Of note, EC5 GABA concentrations were established in comparison with maximal GABA responses. Thus, in mutants where maximal GABA efficacy is low (Table 2), enhancements ratios greater than 20 are possible. Statistically significant differences from wild-type results are indicated by * p < 0.05, ** p < 0.01, or *** p < 0.001.
Effects of pCMBS on cysteine mutant function
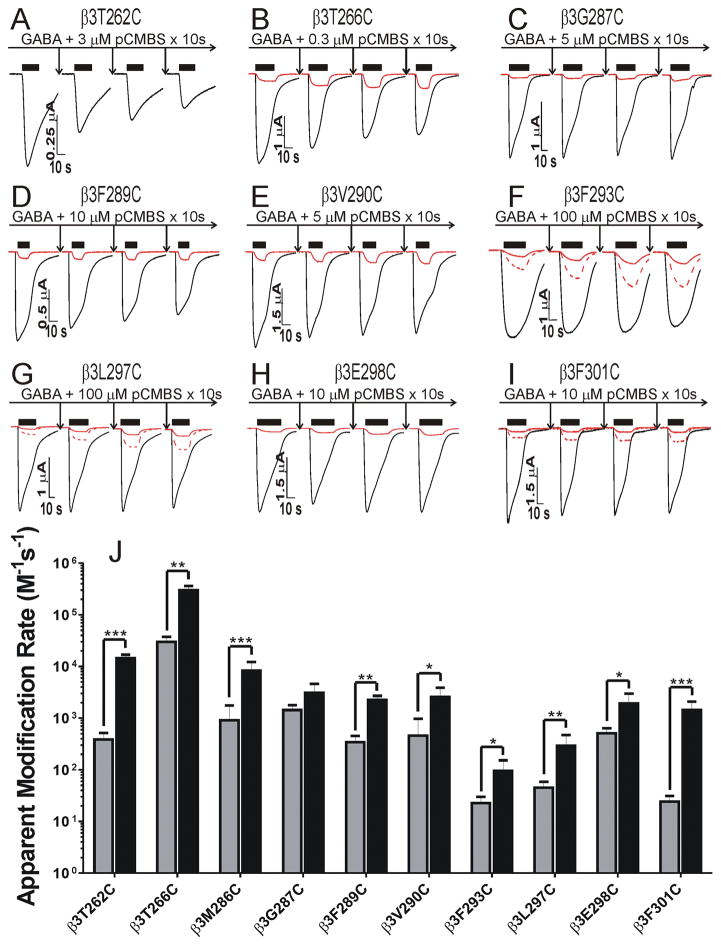
To establish conditions for SCAMP experiments, we examined the effects of pCMBS exposure, both alone and co-applied with GABA, in each of the cysteine mutants. Wild-type α1β3γ2L receptors were unaffected by pCMBS exposure at 1 mM for 60 s (n = 4). In all but one (β3M283C) of the functional cysteine-substituted mutant receptors we studied, exposure to pCMBS alone or with maximally-activating GABA concentrations induced consistent irreversible functional changes that significantly differed from repeated baseline GABA responses prior to pCMBS exposure (Fig 3A–I; Table 2). In α1β3T262Cγ2L receptors, pCMBS exposure similarly reduced activation by both low and high GABA (Fig 3A). In the other mutant receptors, pCMBS exposure enhanced GABA sensitivity, increasing low:high response ratios in the range of 2-fold to 13-fold (Table 2). With the exception of β3G287C, modification in the presence of GABA required lower pCMBS concentrations than without GABA at all substituted cysteines, resulting in faster apparent modification rates (Fig 3J). Results in α1β3M286Cγ2L receptors (currents not shown in Fig 3) were consistent with earlier studies of α1β2M286Cγ2L 20,21.
Fig 3. Effects of pCMBS exposure on cysteine-substituted GABAA receptors in the absence and presence of GABA.
Panels A through I, each labeled with the relevant cysteine mutant, show current traces from an oocyte stimulated with either EC5 GABA (red) or maximal GABA (black) before and after three cycles of pCMBS + GABA exposure and ND96 wash. Panel A omits EC5 traces, which diminished in parallel with high GABA responses. EC5 traces in panels F, G, and I are duplicated at 3 x magnitude (red dashed lines) to better illustrate the effects of pCMBS modification. Specific modification conditions are indicated in each panel. GABA exposure periods are indicated by black bars over traces. Panel J summarizes the apparent rates of receptor modification (average ± sem) in the absence (gray bars) and presence of GABA (black bars). Corresponding examples of rate analyses are shown in Fig 4A through I. With the exception of β3G287C, GABA significantly accelerated the apparent modification rates. * p < 0.05, ** p < 0.01, or *** p < 0.001
Anesthetic protection (SCAMP) with ETO, PRO, and ALX
We previously have shown that SCAMP reliably identifies anesthetic contacts when drugs significantly and selectively inhibit pCMBS modification 5. Thus, apparent initial rates of cysteine modification in control conditions (pCMBS + GABA) were compared to rates in the presence of added ALX, ETO, or PRO in each of the modifiable mutant receptors. We chose control pCMBS modification conditions in the presence of maximally activating GABA because, a) GABA enhances anesthetic binding and thus site occupancy, b) GABA accelerates pCMBS modification (Fig 3J), and c) GABA helps to establish similar mixtures of functional receptor states in both control modification and protection experiments 6,21. Initial protection conditions included 10 μM ETO, 20 μM PRO, or 10 μM ALX along with GABA and pCMBS. In some mutant receptors that displayed low apparent affinity for anesthetics, we also used five-fold higher protecting anesthetic concentrations. In these cases, we used equivalent high concentrations of at least one other anesthetic to test for drug-specific protection.
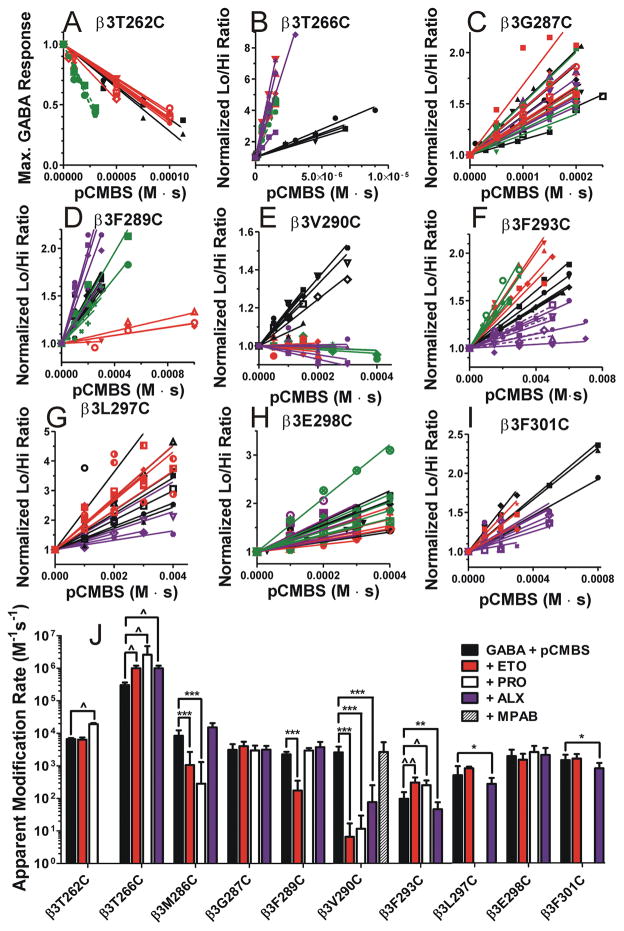
Normalized modification data and rate analyses for nine mutations are shown in Fig 4 and summarized in Fig 4J 21. The apparent rate of modification of α1β3T262Cγ2L receptors (Fig 4A) was unaffected by ETO (red symbols and lines), but accelerated by PRO (green symbols and lines). Modification of α1β3T266Cγ2L receptors (Fig 4B) was accelerated by all three anesthetics, suggesting an allosteric effect. β3M286C protection was fully consistent with previous SCAMP studies of α1β2M286Cγ2L receptors, showing that both ETO and PRO block modification, while ALX weakly accelerates pCMBS modification (summarized in Fig 4J) 20,21. Modification of α1β3G287Cγ2L receptors (Fig 4C) was unaffected by the three anesthetics. Modification of α1β3F289Cγ2L receptors was weakly blocked by 10 μM ETO, unaffected by 20 μM PRO, and accelerated by 10 μM ALX (data not shown). Because this mutant was insensitive to anesthetics (Fig 2), we also tested 50 μM ETO, which inhibited the apparent rate of β3F289C modification over ten-fold, while neither 100 μM PRO nor 50 μM ALX inhibited modification (Fig 4D). Modification of α1β3V290Cγ2L receptors (Fig 4E) was strongly blocked by 10 μM ETO, 20 μM PRO, and 10 μM ALX. To test whether β3V290C modification was allosterically inhibited by anesthetics that do not bind in β+–α − sites, we also tested the effect of 10 μM mTFD-MPAB, a potent barbiturate that selectively binds to GABAA receptor α+–β − and γ+–β − transmembrane interfaces 8. Modification of receptors with β3V290C mutations was unaffected by 8 μM mTFD-MPAB (Fig 4J), indicating that inhibition of modification by ETO, PRO, and ALX was likely steric rather than allosteric.
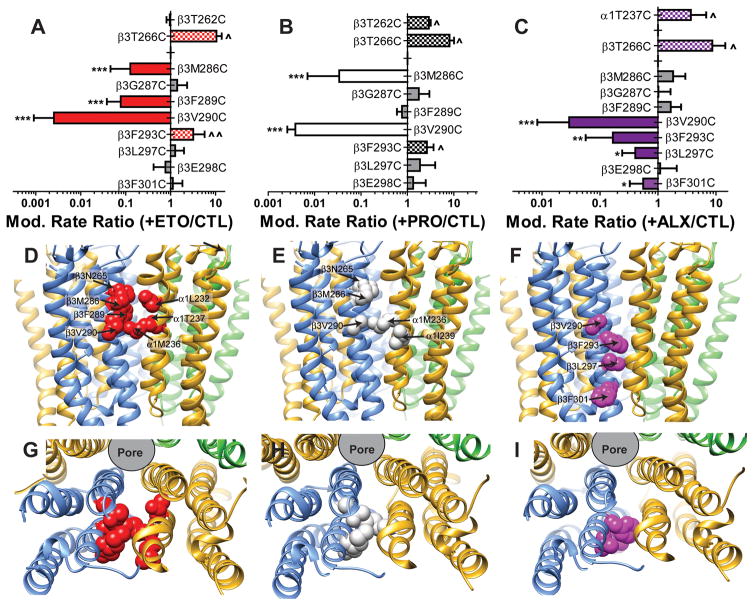
Fig 4. Anesthetic protection of substituted cysteine mutant GABAA receptors.
Panels A through I, labeled by mutation, show individual oocyte data and linear fits for control modification (GABA + pCMBS; black symbols and lines), and modification in the presence of etomidate (red symbols and lines), propofol (green symbols and lines), and alphaxalone (purple symbols and lines) results. Corresponding example current traces for control modification are shown in Fig. 3. Anesthetic concentrations were 10 μM etomidate, 20 μM propofol, and 10 μM alphaxalone, except for β3F289C, β3F293C, and β3L297C where five-fold higher concentrations were used. Data for β3M286C is not shown, because we have previously reported similar results 21. Panel J summarizes mean ± SD rates (fitted linear slopes) for all 10 ten cysteine-substituted mutants on a logarithmic scale. Results for 8 μM mTFD-MPAB effects on β3V290C modification (n =6) are included. Negative slopes for β3T262C and β3V290C were inverted for rate comparisons. Two-way ANOVA analysis was used to assess whether addition of anesthetics significantly altered the apparent rates of modification relative to control conditions with GABA + pCMBS in each mutant. Protection is inferred in cases where addition of anesthetics significantly reduced modification rates. * indicates significantly reduced modification rate, while ^ indicates significantly increased modification rate: * or ^, p < 0.05; ** or ^^, p < 0.01; ***, p < 0.001.
Modification of α1β3F293Cγ2L receptors was accelerated by ETO and PRO, but unaffected by 10 μM ALX. Increasing ALX to 20 μM (Fig 4F, dashed purple lines) or 50 μM (solid purple lines) resulted in significantly reduced rates of β3F293C modification in comparison to 50 μM ETO and 100 μM PRO (Fig 4F). Modification of α1β3L297Cγ2L receptors was unaffected by low concentrations of ETO, PRO or ALX (not shown). Because α1β3L297Cγ2L is relatively insensitive to ALX (Fig 2), we performed additional SCAMP experiments with 50 μM ALX vs. 50 μM ETO in this mutant, revealing inhibition by ALX, but not ETO (Fig 4G). Modification of α1β3E298Cγ2L receptors (Fig 4H) was unaffected by any of the anesthetics. Modification of α1β3F301Cγ2L receptors (Fig 4I) was weakly but significantly blocked by 10 to 20 μM ALX and unaffected by 10 to 20 μM ETO.
On the opposite face of the transmembrane β+–α − cleft, Hosie et al13 identified mutant effects on neurosteroid sensitivity at three residues in α1-M1: α1T237, α1I239, and α1Q242 (Table 1). We have previously reported that receptors with both α1I239C and α1Q242C mutations are unaffected by pCMBS, precluding SCAMP studies 6. To supplement our studies of β3-M2 and β3-M3 residues, we used SCAMP to test whether ALX protects the cysteine substitution at α1T237. No inhibition of pCMBS modification rates in α1T237Cβ3γ2L receptors by 10 μM ALX was observed (data not shown), whereas 10 μM ETO inhibited modification, in agreement with previous results 6.
Discussion
Major Findings
Our aims in this study were to assess hypothesized ALX contacts with β3 sidechains that face transmembrane β+–α − clefts in α1β3γ2L GABAA receptors, and to compare these with ETO and PRO contacts. Using electrophysiology, we studied ten mutant receptors with single cysteine-substitutions in β3-M2 or β3-M3 helices, in which the sulfhydryl modifier pCMBS produced irreversible functional changes. Based on drug-specific inhibition of pCMBS modification, we infer a number of anesthetic contact residues: ETO binds near β3M286, β3F289, and β3V290 (Fig 5A); PRO binds near β3M286 and β3V290 (Fig 5B); and ALX binds near β3V290, β3F293, β3L297, and β3F301 (Fig 5C). Mapping these residues onto our α1β3γ2L structural model (Figs 5D–I) suggests that all three anesthetics bind in transmembrane β+–α − inter-subunit clefts, with overlapping ETO and PRO sites extending from the middle of β3-M3 (near β3V290) extracellularly (Figs 5D and E), and the ALX site extending from β3V290 intracellularly (Fig 5F).
Fig 5. Summary of substituted cysteine modification and protection results by anesthetic drug.
The top row of panels summarizes the ratio of modification rates (mean ± sd) in the presence vs. absence of anesthetic for each drug at the mutations used in the current study. Cases where no significant change was observed are indicated by gray bars. Cases where anesthetics caused significant slowing of modification are identified by solid colored bars and those where anesthetics produced significant acceleration of modification are identified by checked bars with the same coloring scheme (etomidate = ETO, red; propofol = PRO, white; alphaxalone = ALX, purple). Contact between anesthetics and sidechains is inferred in cases where modification is inhibited. Significance is annotated as described for figure 4J. The middle row of panels depicts the transmembrane domain backbone ribbon structure of our α1β3γ2L homology model as viewed from the side. Subunit color coding is the same as in Fig 1. Contact residues, based on both photolabeling and substituted cysteine modification-protection studies, are identified for each drug in separate panels as colored and labeled space-filling models. The bottom set of panels depict the same models and contact sidechains viewed from the extracellular space, with the extracellular domains removed.
Alphaxalone and Neurosteroids Bind to Inner Transmembrane β+–α − Sites
Single-point mutations that affect neurosteroid sensitivity in heteromeric mammalian GABAA receptors (Table 1) are found throughout the transmembrane β+–α − cleft. Our SCAMP results for ALX provide evidence of contact with four inner β3-M3 residues facing the β+–α − interface. The strongest prior evidence for an inner transmembrane β+–α − neurosteroid site is β3F301 photolabeling with 6-AziP 22, but the use of homomeric β3 receptors and failure to test if neurosteroids block 6-AziP labeling make it far weaker than studies in heteromeric receptors using photolabeling derivatives of ETO and PRO 26. Mutations at both α1I239 and α1Q242, located opposite β3F293 in our structural model (Fig 1), impair receptor sensitivity to neurosteroids 13,14,28 and α1Q242C confers insensitivity to ALX, but not to ETO (our unpublished data). The lack of pCMBS-induced effects in receptors with α1I239C and α1Q242C mutations 6 precludes SCAMP tests, and contrasts with our current findings in inner β3-M3 mutants. Other indirect support for inner transmembrane neurosteroid sites include evidence that a membrane-impermeant steroid positively modulates GABAA receptors only when applied intracellularly 23. Docking calculations using the β3 homomeric GABAA receptor structure 30 also locate pregnanolone and allopregnanolone sites near both β3F301 and β3L297 39.
Previous functional, SCAMP, and photolabeling evidence (Table 1) all locate ETO and PRO sites in outer transmembrane β+–α − clefts. In comparing ALX contacts in β3-M3 with those for ETO and PRO, we found that, with the exception of β3V290C, ALX contacts were mutually exclusive with PRO or ETO contacts. We also recently reported that ETO contacts α1L232, and that both ETO and PRO contact α1M236, while ALX contacts neither 5. Altogether, our current results indicate that ALX binds in inner transmembrane β+–α − cleft sites abutting outer transmembrane ETO/PRO sites, with possible contact of outer and inner sites near β3V290.
Neurosteroids enhance GABAA receptor photolabeling by ETO derivatives 28 and neurosteroid-ETO combinations synergize in both enhancing GABAA receptor gating and anesthetizing animals 29. An allosteric mechanism for this synergy through mutual coupling of sites to channel gating is suggested by our observations that both ETO and PRO accelerate pCMBS modification of β3F293C in the ALX sites, while ALX accelerates pCMBS modification at β3M286C in the ETO/PRO sites. Direct contact between neurosteroids and ETO in abutting sites could also mutually enhance drug binding, contributing to functional synergy.
Propofol and Etomidate Bind to Outer Transmembrane β+–α − Sites
Our current results extend the map of PRO and ETO contacts on the β+ aspect of the outer β+–α − sites (Table 1; Fig 5). Functional and SCAMP results with β3M286C echoed previous studies of β2M286C 20,21. We identified two additional ETO contact residues, β3F289 and β3V290, while PRO protects β3V290C but not β3F289C. Thus, the β+–α − sites for PRO and ETO overlap, agreeing with previous SCAMP and photolabel competition results (Table 1) 5,8,12. Interestingly, despite evidence that PRO and ETO might contact β2/3N265 on the M2 helix (Table 1), we found no evidence of contact at β3T262 or β3T266 that also abut β+–α − interfaces in structural models (Fig 1).
Mutant Functional Effects Reflect Allosteric Linkages, Not Drug-Receptor Contacts
The functional effects of both cysteine-substitution and pCMBS modification provide insight into allosteric linkages and aqueous accessibility at the residues we studied. Spanning from M286 to E298, most β3-M3 cysteine mutations altered GABA EC50 and/or GABA efficacy (Table 2), indicating that this region is coupled to ion channel gating. Similar observations were made in a series of α1-M1 cysteine-substitutions 6. Cysteine mutants throughout β3-M3 were also accessible to pCMBS, indicating an aqueous pathway extending intracellularly to at least β3F301, and echoing similar findings on the β1-M2 helix 40.
Mutant functional analyses underlie many of the hypotheses we have tested (Table 1) and it is tempting to infer drug contacts from the altered anesthetic sensitivities of cysteine mutants (Fig 2). However, we recently compared SCAMP with tryptophan mutant drug sensitivity for two photolabeled residues and four anesthetics, finding perfect agreement between SCAMP and photolabeling, but poor concordance with mutant drug sensitivities 5. There are multiple other examples of SCAMP identifying anesthetic contacts in GABAA receptors that weren’t photolabeled 5,6,26,41, but only one published report of SCAMP disagreeing with photolabeling 25.
SCAMP Conditionally Reflects Drug-Receptor Contacts
Our SCAMP approach requires functional heterologous receptor expression, quantifiably consistent cysteine modification effects, and drug occupation of a large fraction of sites 26. Even under these conditions, we cannot formally rule out allosteric effects in SCAMP experiments. However, allosteric mechanisms should strongly link the functional effects of different anesthetics to inhibition of modification in relevant mutants. Comparing Figs 2 and 4J, such correlations are absent at many positions where modification was inhibited: F289, V290, F293, and F301. Moreover, drug specificity was demonstrable at every protected cysteine (Fig 4J). Thus, our SCAMP results are more compatible with a steric than an allosteric mechanism for inhibiting pCMBS modification. Inferences of steric interactions between receptor-bound drugs and substituted cysteines are strengthened when protection is concentration-dependent and profound. ALX protection at β3F293C, β3L297C, and β3F301C was relatively weak compared to results for ETO, PRO, and mTFD-MPAB at some of their outer transmembrane contacts 5,6,21. For β3F293C and β3L297C, this is attributable to low ALX affinity (see below). The β3F301C sidechain may be located at the periphery of the steroid site, limiting ALX protection at this position.
In three mutant receptors, α1β3F289Cγ2L, α1β3F293Cγ2L, and α1β3L297Cγ2L, high anesthetic concentrations demonstrated concentration-dependent block of pCMBS modification. In these mutants, weak EC5 enhancement (Fig 2) indicated weak drug binding based on the Monod-Wyman-Changeux allosteric principle that positive gating modulation reflects the relative affinity of ligands for active (open) vs. inactive (closed) receptors. Thus, weak EC5 enhancement relative to wild-type implies reduced drug affinity for GABA-activated receptors and a need for high drug concentrations to occupy most binding sites. In addition, α1β3F293Cγ2L receptors were characterized by low GABA efficacy, with maximal GABA activating only about 16% of these receptors (Table 2) under control modification conditions. With addition of ETO or PRO, the fraction of activated and desensitized receptors increased, allosterically accelerating β3F293C modification (Fig 4J). Adding ALX to high GABA likely produced two opposing effects on α1β3F293Cγ2L modification: increased activation/desensitization that accelerates modification, and steric protection that inhibits modification. In initial experiments, 10 μM ALX produced approximate balance in these opposing effects, while higher ALX concentrations resulted in overall slowing of modification.
Intra-Subunit Pockets
Crystallographic studies of pentameric ligand-gated ion channels reveal that small anesthetics and alcohols can occupy both inter-subunit and intra-subunit transmembrane pockets 42–44. In this study, we examined two mutations, β3G287C and β3E298C, that are predicted to face the β3 intra-subunit helix bundle pocket, in both outer and inner regions of β3-M3 (Fig 1). While we observed altered GABA sensitivity as evidence of pCMBS access and modification in these mutants, no anesthetic protection was observed (Figs 4 and 5) arguing against the presence of positively modulating anesthetic sites in β3 intra-subunit pockets.
Conclusions and Significance
Endogenous and synthetic neurosteroids are potent neuromodulators with broad therapeutic potential. Our current SCAMP studies locate positively modulating ALX sites on α1β3γ2L GABAA receptors in inner transmembrane β+–α − inter-subunit clefts. These neurosteroid sites are adjacent to outer transmembrane β+–α − sites where ETO and PRO act, suggesting both direct and indirect mechanisms for cooperativity between neurosteroids and ETO 28,29. Two other outer transmembrane inter-subunit sites, in α+–β − and γ+–β − clefts, bind PRO and barbiturates 5,8. No ligands have yet been identified for the transmembrane α+–γ − cleft and the inner transmembrane portions of α+–β − and γ+–β − interfaces, but membrane lipids probably modulate ion channel activity by interacting with transmembrane inter-subunit clefts 45. In summary, large portions of the five transmembrane inter-subunit clefts in α1β3γ2L GABAA receptors are allosterically coupled to ion channel gating. Sub-regions of these clefts form sites for hydrophobic modulators that in several cases, including that of neurosteroids, display remarkable drug selectivity. Structural variations in these inter-subunit interfaces also contribute to subtype-selective GABAA receptor pharmacology.
Summary Statement.
Substituted cysteine modification-protection indicates that the neurosteroid anesthetic alphaxalone contacts α1β3γ2L GABAA receptors within transmembrane β+–α− inter-subunit sites that are adjacent and intracellular to previously mapped sites for the potent anesthetics etomidate and propofol.
Acknowledgments
Funding: This work was supported by grants (GM089745 and GM058448) from the National Institutes of Health, Bethesda, MD, USA.
We thank Youssef Jounaidi, PhD (Instructor, Dept. of Anesthesia Critical Care & Pain Medicine, Massachusetts General Hospital, Boston, MA, USA) for his help with molecular biology. Prof. Karol Bruzik, PhD (Dept of Medicinal Chemistry and Pharmacognosy, University of Illinois, Chicago, USA) provided mTFD-MPAB. Keith W. Miller (Professor, Dept. of Anesthesia Critical Care & Pain Medicine, Massachusetts General Hospital, Boston, MA, USA) and Jonathan B. Cohen (Professor of Neurobiology, Harvard Medical School, Boston, MA, USA) provided helpful comments on the manuscript.
Footnotes
Conflict of interest: The authors have no conflicts of interest related to this work.
References
- 1.Porcu P, Barron AM, Frye CA, Walf AA, Yang SY, He XY, Morrow AL, Panzica GC, Melcangi RC. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J Neuroendocrinol. 2016;28:12351. doi: 10.1111/jne.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen RW, Sieghart W. GABA(A) receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287:40224–31. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–5. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 5.Nourmahnad A, Stern AT, Hotta M, Stewart DS, Ziemba AM, Szabo A, Forman SA. Tryptophan and Cysteine Mutations in M1 Helices of α1β3γ2L γ-Aminobutyric Acid Type A Receptors Indicate Distinct Intersubunit Sites for Four Intravenous Anesthetics and One Orphan Site. Anesthesiology. 2016;125:1144–58. doi: 10.1097/ALN.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart DS, Hotta M, Li GD, Desai R, Chiara DC, Olsen RW, Forman SA. Cysteine Substitutions Define Etomidate Binding and Gating Linkages in the alpha-M1 Domain of gamma-Aminobutyric Acid Type A (GABAA) Receptors. J Biol Chem. 2013;288:30373–30386. doi: 10.1074/jbc.M113.494583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart DS, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–1695. doi: 10.1124/mol.108.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic binding sites in the transmembrane domain of the human alpha1beta3gamma2 GABAA receptor. J Biol Chem. 2013;288:19343–19357. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart DS, Pierce DW, Hotta M, Stern AT, Forman SA. Mutations at Beta N265 in Gamma-Aminobutyric Acid Type A Receptors Alter Both Binding and Efficacy of Potent Anesthetics. PLoS One. 2014 Oct 27;9(10):e111470. doi: 10.1371/journal.pone.0111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayakar SS, Zhou X, Chiara DC, Dostalova Z, Savechenkov PY, Bruzik KS, Dailey WP, Miller KW, Eckenhoff RG, Cohen JB. Multiple Propofol Binding Sites in a gamma-Aminobutyric Acid Type A Receptor (GABAAR) Identified Using a Photoreactive Propofol Analog. J Biol Chem. 2014;289:456–68. doi: 10.1074/jbc.M114.581728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 14.Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha-1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai R, Rüsch D, Forman SA. Gamma-amino butyric acid type A receptor mutations at beta2N265 alter etomidate efficacy while preserving basal and agonist-dependent activity. Anesthesiology. 2009;111:774–84. doi: 10.1097/ALN.0b013e3181b55fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldifassi MC, Baur R, Sigel E. Functional sites involved in modulation of the GABA receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology. 2016;105:207–214. doi: 10.1016/j.neuropharm.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Siegwart R, Krahenbuhl K, Lambert S, Rudolph U. Mutational analysis of molecular requirements for the actions of general anaesthetics at the gamma-aminobutyric acidA receptor subtype, alpha1beta2gamma2. BMC pharmacology. 2003;3:13. doi: 10.1186/1471-2210-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasowski MD, Nishikawa K, Nikolaeva N, Lin A, Harrison NL. Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001;41:952–64. doi: 10.1016/s0028-3908(01)00141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 21.Stewart DS, Hotta M, Desai R, Forman SA. State-Dependent Etomidate Occupancy of Its Allosteric Agonist Sites Measured in a Cysteine-Substituted GABAA Receptor. Mol Pharmacol. 2013;83:1200–8. doi: 10.1124/mol.112.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the beta3 subunit of the GABA(A) receptor. Mol Pharmacol. 2012;82:408–19. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–13. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern AT, Forman SA. A Cysteine Substitution Probes beta3H267 Interactions with Propofol and Other Potent Anesthetics in alpha1beta3gamma2L gamma-Aminobutyric Acid Type A Receptors. Anesthesiology. 2016;124:89–100. doi: 10.1097/ALN.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman SA, Miller KW. Mapping General Anesthetic Sites in Heteromeric gamma-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes. Anesth Analg. 2016;123:1263–1273. doi: 10.1213/ANE.0000000000001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westergard T, Salari R, Martin JV, Brannigan G. Interactions of L-3,5,3′-Triiodothyronine, Allopregnanolone, and Ivermectin with the GABAA Receptor: Evidence for Overlapping Intersubunit Binding Modes. PLoS One. 2015;10:e0139072. doi: 10.1371/journal.pone.0139072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li GD, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–5. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Bracamontes JR, Manion BD, Mennerick S, Steinbach JH, Evers AS, Akk G. The neurosteroid 5beta-pregnan-3alpha-ol-20-one enhances actions of etomidate as a positive allosteric modulator of alpha1beta2gamma2L GABAA receptors. Br J Pharmacol. 2014;171:5446–57. doi: 10.1111/bph.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–5. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savechenkov PY, Zhang X, Chiara DC, Stewart DS, Ge R, Zhou X, Raines DE, Cohen JB, Forman SA, Miller KW, Bruzik KS. Allyl m-Trifluoromethyldiazirine Mephobarbital: An Unusually Potent Enantioselective and Photoreactive Barbiturate General Anesthetic. J Med Chem. 2012;55:6554–65. doi: 10.1021/jm300631e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandyopadhyaya AK, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 15. A comparative study of the anesthetic and GABAergic actions of alphaxalone, Delta16-alphaxalone and their corresponding 17-carbonitrile analogues. Bioorg Med Chem Lett. 2010;20:6680–4. doi: 10.1016/j.bmcl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Husain SS, Stewart D, Desai R, Hamouda AK, Li SG, Kelly E, Dostalova Z, Zhou X, Cotten JF, Raines DE, Olsen RW, Cohen JB, Forman SA, Miller KW. p-Trifluoromethyldiazirinyl-etomidate: a potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels. J Med Chem. 2010;53:6432–44. doi: 10.1021/jm100498u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonner PH, Poppers DM, Miller KW. The general anesthetic potency of propofol and its dependence on hydrostatic pressure. Anesthesiology. 1992;77:926–931. doi: 10.1097/00000542-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Borghese CM, Hicks JA, Lapid DJ, Trudell JR, Harris RA. GABA(A) receptor transmembrane amino acids are critical for alcohol action: disulfide cross-linking and alkyl methanethiosulfonate labeling reveal relative location of binding sites. J Neurochem. 2014;128:363–75. doi: 10.1111/jnc.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y, Weiss DS. Allosteric activation mechanism of the alpha1beta2gamma2 gamma-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys J. 1999;77:2542–51. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bali M, Akabas MH. Gating-induced Conformational Rearrangement of the gamma-Aminobutyric Acid Type A Receptor beta-alpha Subunit Interface in the Membrane-spanning Domain. J Biol Chem. 2012;287:27762–70. doi: 10.1074/jbc.M112.363341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bali M, Jansen M, Akabas MH. GABA-induced intersubunit conformational movement in the GABAA receptor alpha1M1-beta2M3 transmembrane subunit interface: Experimental basis for homology modeling of an intravenous anesthetic binding site. J Neurosci. 2009;29:3083–92. doi: 10.1523/JNEUROSCI.6090-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez LD, Estrin DA. Exploring the molecular basis of neurosteroid binding to the beta3 homopentameric GABAA receptor. J Steroid Biochem Mol Biol. 2015;154:159–67. doi: 10.1016/j.jsbmb.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Goren EN, Reeves DC, Akabas MH. Loose protein packing around the extracellular half of the GABA(A) receptor beta1 subunit M2 channel-lining segment. J Biol Chem. 2004;279:11198–205. doi: 10.1074/jbc.M314050200. [DOI] [PubMed] [Google Scholar]
- 41.Woll KA, Dailey WP, Brannigan G, Eckenhoff RG. Shedding Light on Anesthetic Mechanisms: Application of Photoaffinity Ligands. Anesth Analg. 2016;123:1253–1262. doi: 10.1213/ANE.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–31. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 43.Spurny R, Billen B, Howard RJ, Brams M, Debaveye S, Price KL, Weston DA, Strelkov SV, Tytgat J, Bertrand S, Bertrand D, Lummis SC, Ulens C. Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC) J Biol Chem. 2013;288:8355–64. doi: 10.1074/jbc.M112.424507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Althoff T, Hibbs RE, Banerjee S, Gouaux E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature. 2014;512:333–7. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]