Abstract
Background
Pregnancy loss can have physical and psychological consequences for women and their families. Though a previous study described an increase in the risk of self-reported pregnancy loss from 1970 to 2000, more recent examinations from population-based data of US women are lacking.
Methods
We used data from the 1995, 2002, 2006–2010, 2011–2015 National Survey of Family Growth on self-reported pregnancy loss (miscarriage, stillbirth, ectopic pregnancy) among US women (15–44 years) who reported at least one pregnancy conceived during 1990–2011 that did not result in induced termination (n = 20 012 women; n = 42 526 pregnancies). Trends in the risk of self-reported pregnancy loss and early pregnancy loss (<12 weeks) were estimated, separately, by year of pregnancy conception (limited to 1990–2011 to ensure a sufficient sample of pregnancies for each year and maternal age group) using log-Binomial and Poisson models, adjusted for maternal- and pregnancy-related factors.
Results
Among all self-reported pregnancies, excluding induced terminations, the risk of pregnancy loss was 19.7% and early pregnancy loss was 13.5% during 1990–2011. Risk of pregnancy loss increased by a relative 2% (rate ratio [RR] 1.02, 95% confidence interval [CI] 1.01, 1.02) per year in unadjusted models and 1% per year (RR 1.01, 95% CI 1.00, 1.02) during 1990–2011, after adjustment for maternal characteristics and pregnancy-related factors. In general, trends were similar for early pregnancy loss.
Conclusion
From 1990 to 2011, risk of self-reported pregnancy loss increased among US women. Further work is needed to better understand the drivers of this increase in reported pregnancy loss in the US.
Keywords: miscarriage, spontaneous abortion, fetal loss, stillbirth
Pregnancy loss has been associated with a host of adverse physical and psychological sequelae, including the experience of grief, depression, and anxiety.1–8 In addition, women who experience a pregnancy loss often report dissatisfaction with clinical care following a loss, including lack of care that focused on their emotional well-being.9 It has been estimated that between 10 and 28 per cent of all recognized pregnancies end in pregnancy loss.9–14
An earlier study examined trends in the risk of self-reported pregnancy loss between 1970 and 2000 among women aged 13–25 years in the US.15 They described an increasing trend of 1%–2% per year in pregnancy losses occurring before 8 weeks’ gestation, a weaker (<1% per year) increasing trend in losses occurring at 8–12 weeks’ gestation, but no changes in the risk of losses occurring beyond 12 weeks. The authors speculated that increased use of more accurate home-pregnancy tests might account for this increasing trend, with women better able to identify early miscarriages that would otherwise be unrecognized. However, a recent analysis using NSFG data by Branum and Ahrens16 found that mean gestational age at time of pregnancy awareness did not change during 1990–2012, suggesting that earlier awareness or detection is unlikely to account for increasing in trends in loss for this time period.
Recent trends in reported pregnancy loss have not been described, particularly for the full age range of reproductive women. Furthermore, factors related to the risk of pregnancy loss, such as maternal age and health-related characteristics,9,17–20 have shifted over time since 2000. Subsequently, we examined trends in self-reported pregnancy loss and early pregnancy loss (<12 weeks) and estimated associations between various maternal characteristics and pregnancy loss for over 42 000 pregnancies occurring during 1990–2011, as reported in the NSFG.
Methods
Study participants
We analysed data on pregnancies reported by 20 012 women from the 1995, 2002, 2006–2010, and 2011–2015 NSFG, a nationally representative, in-person, household survey of the US population ages 15–44 years that uses a complex, multistage, probability design to select participants. Female response rates for these survey periods ranged from 71% to 80%.21 We used data from the pregnancy file (pregnancy-level data), which contains detailed information on all reported pregnancies in a woman’s history up to the time of the interview. The pregnancy file was then merged with the female respondent file (respondent-level data) to obtain additional woman-level characteristics. Pregnancies were the unit of analysis. Although the NSFG has added and removed questions over time, questions about pregnancy history have remained the same since 1995.
Study variables
Details on pregnancy occurrence, duration, and outcome were asked about all pregnancies completed by the time of interview. Pregnancy occurrence was captured by asking about the month and year the pregnancy began. Pregnancy duration was assessed by asking the respondent how many months or weeks pregnant she was when the pregnancy ended. Pregnancy outcome was assessed by asking the respondent how the pregnancy ended: miscarriage, stillbirth, abortion, ectopic or tubal pregnancy, livebirth by caesarean, vaginal delivery, or refused/don’t know. We collapsed livebirths as a single category and miscarriages, stillbirths, and ectopic or tubal pregnancies were all considered pregnancy losses, regardless of the gestational age at the time of the loss. This was because these pregnancy outcomes captured all nonterminated pregnancies that did not result in a livebirth. Women were not given definitions of each of these outcomes (e.g. miscarriage was not defined based on a certain gestational age); however, because women were also asked for the pregnancy duration, we were able to classify pregnancy losses according to when they occurred. Early pregnancy losses, defined as losses prior to 12 weeks’ gestation, were a subset of all pregnancy losses.
The following maternal and pregnancy-related factors were available from the NSFG across survey periods: maternal age at the time of conception, race/ethnicity, educational attainment at interview, poverty-income ratio at interview, marital status at end of pregnancy, ever smoked cigarettes (at least 100 cigarettes in lifetime), pregnancy intendedness at conception, gravidity at conception, and ever received medical help to become pregnant at the time of interview.
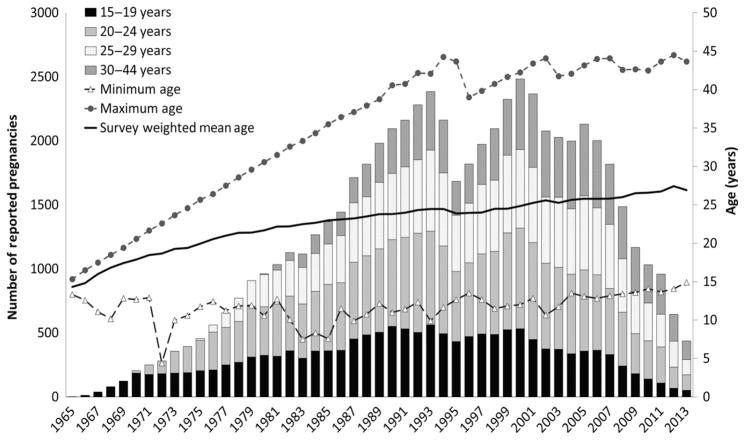
Maternal age at time of pregnancy conception was categorised into 4 groups: 15–19, 20–24, 25–29, and 30–44 years. Although maternal age ≥35 years (“advanced maternal age”) is often used as a factor in determining prenatal screening protocols, such as amniocentesis testing, and measuring clinical infertility, this group of women had the fewest number of pregnancies reported per calendar year (Figure 1); therefore, they were combined with the next oldest maternal age group, aged 30–34, in order to produce stable estimates of trends by maternal age.
Figure 1.
Unweighted number of reported pregnancies by maternal age group and calendar year of conception, NSFG 1995, 2002, 2006–2010, 2011–2015. At least 100 pregnancies were reported for each age group during 1990–2011. Minimum, maximum, and survey-weighted mean age at conception are presented on the secondary axis.
Exclusions
We excluded pregnancies that resulted in induced terminations (n = 8890) and on-going pregnancies at the time of the interview (n = 1783), because the proportion of these pregnancies that would have resulted in pregnancy loss had the pregnancy proceeded or been completed, respectively, was unknown. Furthermore, we excluded pregnancies conceived in years with fewer than 100 conceptions reported per maternal age category (20 699 pregnancies conceived prior to 1990 or after 2011, Figure 1) to control for confounding by maternal age, due to the truncation of age at the time of the interview (15–44 years). We also excluded pregnancies occurring at younger than 15 years of age (420 pregnancies) because of the small number of pregnancies among this age group.
The data included a 22-year span of time covering pregnancies conceived during the following years for each survey period: 1990–1995 (1995 NSFG, n = 4455), 1990–2002 (2002 NSFG, n = 7951), 1990–2010 (2006–2010 NSFG, n = 15 604), and 1990–2011 (2011–2015 NSFG, n = 14 516). This resulted in 42 526 pregnancies for analysis (Figure S1).
Statistical analysis
Descriptive statistics
The percentage of all non-terminated pregnancies resulting in pregnancy loss and early pregnancy losses were calculated, overall, and for each survey period and maternal and pregnancy-related factor included in the analysis. Descriptive statistics and differences in these estimates among levels of each maternal and pregnancy-related characteristic were assessed using risk ratios and 95% confidence intervals obtained from predicted margins using unadjusted logistic regression models where the group with the lowest reported percentage of pregnancies resulting in loss served as the reference group.
Trends
Log-Binomial regression was used to assess whether the risks of reported pregnancy loss and early pregnancy loss, separately, increased from 1990 to 2011. Pregnancy loss was modelled as a function of calendar year of conception. Non-linear trends were assessed by adding a quadratic term for calendar year of conception as a covariate to the models. Restricted cubic spline models were also implemented as a more flexible alternative to explore potential non-linear patterns. Graphical results from these models appeared largely similar to the findings from the linear and quadratic models, and are thus not presented here (see Supporting Information).
We ran log-Binomial models that were adjusted for factors potentially related to risk of reported loss, based on prior literature,9,17,18,22–24 including race/ethnicity, educational attainment, poverty-income ratio, marital status, ever smoked cigarettes, gravidity, and ever received medical help to become pregnant (Table S1). All models also include maternal age as a covariate, as retrospective time trend data from the NSFG are confounded by maternal age (i.e. mean, minimum, and maximum maternal age during pregnancy conception year increase over time up to the year of the interview; Figure 1) due to the truncation of age at the time of the interview (15–44 years). Because some adjusted log-Binomial models failed to converge, log-Poisson models were used for all adjusted analyses. The same set of models was repeated with early pregnancy loss as the outcome. Unadjusted and adjusted models were fit for all women combined and then stratified by maternal age group due to the differential risks of loss by maternal age.19 These age-stratified models also included maternal age (in years) as a continuous covariate to further adjust for any residual confounding by age within the broader maternal age group strata.
Sensitivity analysis
We conducted several sensitivity analyses. First, similar to a prior study,19 we restricted the analysis to pregnancies that were not unwanted at the time of conception (84% of non-terminated pregnancies) to address concerns about the possible misclassification of induced terminations as pregnancy losses over time; by including only intended or mistimed pregnancies, we assumed there was a lower possibility of pregnancy outcome misclassification. Second, we expanded the analytic cohort to include all completed pregnancies, including induced terminations, as some studies have suggested estimates of reported pregnancy loss should include induced abortion in the denominator.25 Third, we stratified the analysis by how recently the pregnancy occurred (within 2 years of interview vs. more than 2 years) to see if trends over time were different for recently reported pregnancies compared with those not recently reported. Finally, we also fit a set of adjusted models including a covariate indicating whether the respondent had a prior history of pregnancy loss given that a history of loss has been associated with higher risk of loss in subsequent pregnancies.9,18,26
All analyses were conducted with SAS 9.3 (SAS Institute, Cary, North Carolina), SUDAAN 11.0 (RTI International, Research Triangle Park, North Carolina), and Stata 13 SE (StataCorp LP, College Station, TX) and accounted for the complex survey design and sample weights of the NSFG.
Results
During 1990–2011, the percentage of pregnancies (excluding induced terminations) that resulted in pregnancy loss (Table 1) was 19.7% and the percentage that resulted in early pregnancy loss was 13.5% (Table S2). The percentages of pregnancy loss and early pregnancy loss were higher among women aged 30–44 at time of conception vs. those aged 20–24, among non-Hispanic black and non-Hispanic white compared with Hispanic women, and for unwanted and mistimed pregnancies vs. those that were intended. Other differences can be seen in Table 1 and Table S2. Risk of pregnancy loss among nonterminated pregnancies was 17.8% in 1990 (95% CI 15.4, 20.1) and 23.3% (95% CI 19.2, 27.4) in 2011; corresponding risks for all completed pregnancies (including terminated pregnancies) were 15.6% (95% CI 13.5, 17.7) and 21.9% (95% CI 18.0, 25.9), respectively.
Table 1.
Per cent of pregnanciesa in the United States resulting in pregnancy loss by maternal characteristics and pregnancy-related factors, NSFG survey years: 1995, 2002, 2006–2010, 2011–2015
| Unweighted n | Pooled | 1995 | 2002 | 2006—2010 | 2011—2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women: 20 012 | 3200 | 3967 | 6746 | 6099 | ||||||||
| Pregnancies: 42 526 | 4455 | 7951 | 15 604 | 14 516 | ||||||||
|
|
|
|
|
|
||||||||
| % | SE | RR (95% CI) | % | SE | % | SE | % | SE | % | SE | ||
| Overall pregnancy loss (%) | 42 526 | 19.7 | 0.4 | 20.9 | 0.9 | 20.1 | 0.7 | 18.9 | 0.6 | 19.4 | 0.5 | |
| Age at pregnancy conception | ||||||||||||
| 15–19 | 8846 | 18.4 | 0.7 | 1.07 (0.98, 1.16) | 18.0 | 2.1 | 21.3 | 1.6 | 19.9 | 1.5 | 16.9 | 1.0 |
| 20–24 | 13 265 | 18.0 | 0.6 | 1.00 (Reference) | 18.1 | 1.6 | 17.4 | 1.0 | 17.0 | 0.9 | 18.4 | 0.9 |
| 25–29 | 11 127 | 17.7 | 0.6 | 0.98 (0.91, 1.06) | 18.9 | 1.2 | 17.1 | 0.9 | 15.6 | 0.8 | 19.0 | 1.1 |
| 30–44 | 9288 | 25.1 | 0.7 | 1.43 (1.32, 1.54) | 26.5 | 1.5 | 26.9 | 1.5 | 24.2 | 1.2 | 24.1 | 1.3 |
| Hispanic origin and race | ||||||||||||
| Hispanic or Latina | 11 300 | 15.0 | 0.7 | 1.00 (Reference) | 16.6 | 1.6 | 16.4 | 1.0 | 13.6 | 0.9 | 15.3 | 1.1 |
| Non-Hispanic white | 19 373 | 21.3 | 0.5 | 1.42 (1.30, 1.55) | 21.4 | 1.1 | 22.4 | 1.0 | 20.6 | 0.9 | 21.2 | 0.7 |
| Non-Hispanic black | 9772 | 20.7 | 1.1 | 1.36 (1.22, 1.51) | 23.2 | 2.3 | 20.6 | 1.3 | 19.4 | 1.2 | 20.6 | 1.6 |
| Non-Hispanic other | 2081 | 18.3 | 1.5 | 1.23 (1.04, 1.45) | 20.3 | 3.3 | 18.2 | 3.4 | 19.3 | 2.4 | 17.4 | 2.2 |
| Education (at time of interview) | ||||||||||||
| No high school diploma or GED | 9 919 | 17.8 | 0.8 | 1.00 (Reference) | 19.3 | 1.8 | 21.5 | 1.8 | 17.9 | 1.4 | 16.2 | 1.5 |
| High school diploma or GED | 13 258 | 18.7 | 0.6 | 1.03 (0.93, 1.14) | 21.0 | 1.4 | 19.3 | 1.3 | 19.0 | 1.1 | 17.9 | 0.9 |
| Some college, no bachelor’s degree | 11 780 | 20.8 | 0.6 | 1.12 (1.01, 1.24) | 21.3 | 1.4 | 21.5 | 1.1 | 19.8 | 0.8 | 20.5 | 1.1 |
| Bachelor’s degree | 5329 | 21.2 | 1.0 | 1.12 (0.99, 1.28) | 20.6 | 2.1 | 20.3 | 1.8 | 18.7 | 1.5 | 22.9 | 1.9 |
| Master’s degree or higher | 2240 | 21.4 | 1.4 | 1.18 (1.01, 1.38) | 25.7 | 3.4 | 26.5 | 4.7 | 18.3 | 2.1 | 21.2 | 1.7 |
| Percentage of poverty level (at time of interview) | ||||||||||||
| Less than 100% | 15 286 | 17.0 | 0.8 | 1.00 (Reference) | 15.5 | 1.8 | 18.0 | 1.1 | 17.6 | 1.2 | 16.7 | 1.2 |
| 100%—299% | 17 343 | 19.0 | 0.4 | 1.10 (1.01, 1.20) | 19.6 | 1.2 | 19.1 | 1.1 | 18.3 | 0.7 | 19.1 | 0.7 |
| 300%—399% | 4785 | 19.6 | 1.0 | 1.14 (1.01, 1.29) | 21.9 | 2.6 | 20.9 | 1.8 | 18.1 | 1.5 | 19.7 | 2.0 |
| 400% or more | 5112 | 26.6 | 1.0 | 1.57 (1.43, 1.74) | 27.7 | 1.7 | 29.6 | 2.0 | 26.6 | 2.1 | 25.3 | 1.5 |
| Marital status at pregnancy end | ||||||||||||
| Married | 21 379 | 17.6 | 0.5 | 1.00 (Reference) | 18.4 | 0.9 | 17.7 | 0.9 | 16.1 | 0.8 | 18.1 | 0.7 |
| Widowed, divorced, separated | 2910 | 30.6 | 1.7 | 1.83 (1.61, 2.08) | 32.5 | 2.7 | 37.7 | 4.6 | 30.8 | 2.3 | 26.8 | 2.4 |
| Never married | 18 236 | 21.2 | 0.6 | 1.25 (1.16, 1.34) | 24.9 | 1.8 | 23.0 | 1.1 | 21.7 | 1.0 | 20.2 | 1.0 |
| Ever smoked cigarettesb | ||||||||||||
| Yes | 16 643 | 22.2 | 0.6 | 1.25 (1.17, 1.33) | 22.7 | 1.2 | 23.5 | 1.0 | 23.0 | 1.1 | 20.9 | 0.9 |
| No | 25 643 | 18.0 | 0.4 | 1.00 (Reference) | 19.5 | 1.1 | 19.1 | 1.0 | 16.1 | 0.6 | 18.4 | 0.7 |
| Intendedness of pregnancy at conception | ||||||||||||
| Intended | 24 674 | 17.9 | 0.4 | 1.00 (Reference) | 18.5 | 0.9 | 18.4 | 1.0 | 16.9 | 0.7 | 18.0 | 0.6 |
| Unwanted | 10 965 | 23.5 | 0.7 | 1.32 (1.23, 1.42) | 24.2 | 1.6 | 25.2 | 2.1 | 21.6 | 1.1 | 23.9 | 1.1 |
| Mistimed | 6883 | 21.2 | 0.8 | 1.25 (1.15, 1.36) | 29.6 | 2.7 | 25.3 | 1.6 | 23.0 | 1.5 | 18.1 | 1.2 |
| Gravidity | ||||||||||||
| First pregnancy | 14 158 | 18.5 | 0.5 | 1.00 (Reference) | 19.0 | 1.3 | 18.5 | 1.0 | 17.2 | 0.8 | 18.8 | 0.8 |
| Not first pregnancy | 28 368 | 20.3 | 0.4 | 1.12 (1.05, 1.19) | 21.7 | 1.0 | 21.9 | 0.9 | 19.6 | 0.8 | 19.8 | 0.6 |
| Any medical help to become pregnant everc | ||||||||||||
| Yes | 3904 | 33.4 | 1.1 | 1.85 (1.71, 2.02) | 39.8 | 5.2 | 34.1 | 1.9 | 32.1 | 2.0 | 33.2 | 1.8 |
| No | 38 232 | 17.9 | 0.4 | 1.00 (Reference) | 18.8 | 0.9 | 19.1 | 0.7 | 17.0 | 0.7 | 17.7 | 0.6 |
RR, risk ratio; CI, confidence interval.
Missing values for the following number of observations: marital status (n = 1), ever smoked cigarettes (n = 240), intendedness of pregnancy at conception (n = 4), and any medical help to become pregnant ever (n = 390).
Only includes livebirths and pregnancy losses (excludes induced terminations and current pregnancies). Pregnancy loss includes: miscarriage, stillbirth and ectopic pregnancies, regardless of gestational age at time of loss.
Excluding induced terminations. Years of pregnancies included from each survey: 1990–1995 (1995 NSFG, n = 4455), 1990–2002 (2002 NSFG, n = 7951), 1990–2010 (2006–2010 NSFG, n = 15 604), and 1990–2011 (2011–2015 NSFG, n = 14 516).
Ever smoked at least 100 cigarettes in lifetime.
Ever received medical help to become pregnant in lifetime.
Pregnancy loss
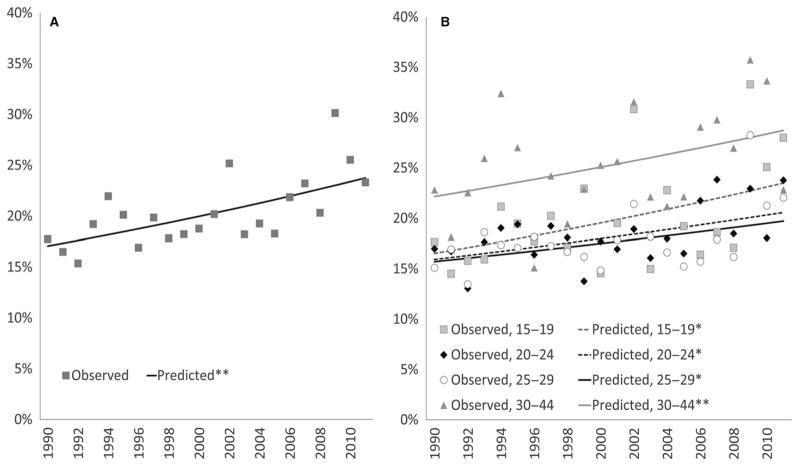
The percentage of non-terminated pregnancies resulting in pregnancy loss increased over time by approximately 2% (relatively) per year from 1990 to 2011, which was attenuated to approximately 1% after adjustment for maternal factors (Table 2, Figure 2A). In analyses stratified by maternal age (at conception) group, the percentage of non-terminated pregnancies ending in loss increased among all age groups by 1%–2% per year in both unadjusted and adjusted models (Table 2, Figure 2B, and Figure S3). No quadratic time trends were observed for pregnancy loss overall.
Table 2.
Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception (1990–2011)a: NSFG 1995, 2002, 2006–2010, 2011–2015 (n = 42 526)
| Unadjusted per yearb | Adjusted per yearc | |
|---|---|---|
| Risk ratio (95% CI) | Risk ratio (95% CI) | |
| All pregnancy loss | ||
| All ages | 1.02 (1.01, 1.02) | 1.01 (1.01, 1.02) |
| 15–19 | 1.02 (1.00, 1.03) | 1.02 (1.01, 1.03) |
| 20–24 | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| 25–29 | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| 30–44 | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Early pregnancy loss | ||
| All ages | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.02) |
| 15–19 | 1.02 (1.00, 1.03) | 1.02 (1.01, 1.04) |
| 20–24 | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| 25–29 | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.03) |
| 30–44 | 1.02 (1.01, 1.03) | 1.02 (1.00, 1.03) |
Pregnancies reported in the 1995, 2002, 2006–2010, and 2011–2015 NSFG spanning the time period 1990–2011; excluding induced terminations.
Unadjusted risk ratio estimated using log-Binomial regression.
Adjusted risk ratio estimated using Poisson regression (log-Binomial models failed to converge). Covariates included: year end (continuous), any medical help getting pregnancy (reference = no), ever smoked 100 cigarettes (reference = no), poverty category (reference =< 1.00), marital status at conception (reference = married), educational category (reference = no high school/General Educational Development), race/ethnicity (reference = non-Hispanic white), pregnancy order (continuous), and age at conception (continuous).
Figure 2.
Risk of pregnancy loss in the United States (a) and by maternal age (b) for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-Binomial models). See Table 2 for per year risk ratio estimates and confidence intervals.
Early pregnancy loss
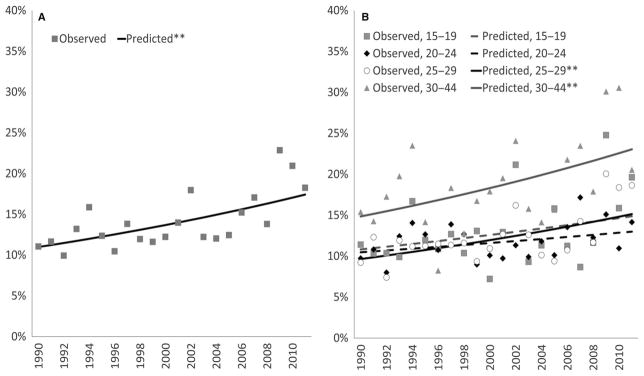
The risk of reported early pregnancy loss increased by a relative 2% annually from 1990 to 2011, both before and after adjustment for maternal factors (Table 2 and Figure 3A). In unadjusted models stratified by maternal age group, the percentage of non-terminated pregnancies resulting in early pregnancy loss increased by 1%–2% annually (Figure S4). In adjusted analyses, increases of 2% annually for early loss were observed among all age groups except for women aged 20–24 (increases of 1%; Table 2 and Figure 3B). For early pregnancy loss, a quadratic trend was observed overall, but not for any age group (Figure S2).
Figure 3.
Risk of early pregnancy loss in the United States (a) and by maternal age (b) for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-Binomial models). See Table 2 for per year risk ratio estimates and confidence intervals.
Sensitivity analyses
Findings of a relative increase of 1%–2% per year in the percentage of non-terminated pregnancies resulting in pregnancy loss were observed after excluding unwanted pregnancies, providing evidence that the trends in pregnancy loss we found may not be due to an artefact of increasing misclassification of induced terminations as pregnancy losses during 1990–2011 (Table S3). Findings were also similar when we included induced terminations in the denominator (Table S4). Patterns were also similar when models were stratified by how recently the pregnancy occurred (e.g. pregnancies completed within 2 years of the interview vs. completed two or more years before the interview); however, the estimates had wider confidence intervals (Table S5). Finally, results did not change when a history of prior pregnancy loss was included as a covariate in adjusted models.
Comment
Main findings
In this analysis of over 42 000 non-terminated U.S. pregnancies spanning a 22-year period we found that the percentage of self-reported pregnancy loss increased by a relative 1%–2% each year in both unadjusted and adjusted models. Similar findings were seen for early losses (2% increases per year in both unadjusted and adjusted models). We also found that several maternal characteristics and pregnancy-related factors were associated with higher percentages of pregnancies resulting in pregnancy loss and early pregnancy loss, including, among other factors, older maternal age and having an unwanted or mistimed pregnancy.
Interpretation
Although the literature on trends in risk of pregnancy loss is relatively scant, our results are comparable to at least one other analysis that used NSFG data.15 In their analysis of pregnancies reported in the 1988, 1995 and 2002 NSFG, Lang and Nuevo-Chiquero15 found that the risk of pregnancy loss increased by a relative 1%–2% per year between 1970 (12%) and 2000 (15%) among women ages 13–25. Early pregnancy loss, <8 weeks’ gestation, increased by 1.2%–1.6% per year, while no changes over time were observed for losses occurring at 12 weeks or more.15 This previous study only examined women ages 13–25 at conception, as this was the only group that had consistent representation across calendar years included in their analysis; but this group also has among the lowest risks for pregnancy loss, and the study did not account for the complex survey design of the NSFG, which may have led to inappropriately small standard errors. Despite the differences in methodology, analytic sample, and calendar years included, the results reported here are consistent with that prior analysis for the common age groups examined15 and are similarly robust to various exclusion/inclusion criteria, model specifications, and sensitivity analyses, suggesting the small but significant upward trend in risk of pregnancy loss has continued through 2011.
In describing their findings, Lang & Nuevo-Chiquero15 conclude that the apparent increase in risk of pregnancy loss was most likely due to improvements in pregnancy tests which would have increased awareness of early pregnancy and thus led to increased self-reporting of pregnancy losses. A recent analysis using NSFG data found that mean gestational age at time of pregnancy awareness did not change during 1990–2012.16 We also conducted a post-hoc analysis, and found that the geometric mean gestational age at the time of pregnancy awareness among pregnancy losses decreased during 1990–2011 by 0.14 days per year (95% CI −0.29, 0.00); however, this decrease was slightly attenuated after adjustment for maternal age (0.13 days per year, 95% CI −0.28, 0.02), suggesting that earlier pregnancy awareness of losses over time may, in part, be due to changing demographics. A decrease in gestational age at time of pregnancy awareness was not observed for other pregnancy outcomes. Regardless, adding gestational age at the time of pregnancy awareness to our multivariable model did not change estimates for trends in loss.
There are other explanations for the increase in self-reported pregnancy loss we observed in our analysis. For example, it is possible that pregnancy losses may have been less likely to be reported in the 1990’s compared with later years because of increasing trends in intended pregnancy, as women intending to become pregnant might be more likely to recognize an early pregnancy loss. However, while recent studies suggest that the proportion of intended pregnancies increased from 2008–2011,27 it actually decreased during 2001–2008.28 In addition, our sensitivity analysis excluding unwanted pregnancies resulted in similar estimates of relative increases in loss over the study period (albeit, measuring pregnancy intentions is complex29 and it is possible that alternative ways of operationalizing pregnancy intention might have led to different results). Another explanation for our findings may be that feelings of shame or guilt after a loss1,30,31 have decreased over time, potentially contributing to more accurate reporting; yet there is little evidence that reporting accuracy for pregnancy loss has changed over time.
While we accounted for various maternal characteristics and pregnancy-related factors related to the risk of pregnancy loss that may have changed over the study period (e.g. maternal age), adjusting or stratifying for these factors did not completely attenuate the increasing risk of pregnancy loss we observed during 1990–2011, though RR estimates were smaller in magnitude in some cases. Results also may be due in part to temporal shifts in maternal characteristics and pregnancy-related factors that were not included in this analysis such as maternal health status and behaviours at the time of pregnancy,9,18,20,24 many of which are not captured in the NSFG (e.g. body mass index, alcohol/drug use, dietary intake, physical activity, presence or history of infections, history of induced abortion, stress and mental health factors, pre-pregnancy contraceptive history), paternal characteristics, as well as environmental exposures.17,32
It is also possible that some induced terminations may have been reported as pregnancy losses due to stigma associated with intentionally terminating a pregnancy.1,30,33 However, for misreporting of induced terminations as pregnancy losses to have accounted for our findings, the degree of this misreporting would have had to have increased over the study period. In addition, our results were also robust to the inclusion of induced terminations in the denominator.
Finally, we explored the possibility that our findings could be due to excluding on-going pregnancies from the analysis, as these pregnancies would most likely end in a livebirth had we followed them up. However, in sensitivity analyses examining pregnancies conceived 2 or more years prior to the interview (for which all pregnancy outcomes were known) relative increases of 1%–2% per year were still observed for pregnancy losses and early losses (Table S5).
Strengths of the study
The strengths of this study include the use of a large sample of pregnancies among a nationally representative sample of U.S. women of childbearing age, with detailed information on maternal characteristics, timing of pregnancy awareness and pregnancy outcomes. Our analysis examining how risk of reported pregnancy loss changed across 22 years provides the only national population-based trend analysis on this topic using recently available data from the NSFG. In addition, our sensitivity analyses suggest that findings are robust to a variety of different model specifications and inclusion/exclusion criteria. Additionally, we expand on prior studies looking only at younger maternal age groups15 by including women up to 44 years of age at the time of conception. We considered the potential confounding by maternal age due to the age truncation of the sample, and selected only those years (i.e. 1990–2011) where there were a sufficient number of pregnancies within each of the age strata. Finally, our estimates of self-reported pregnancy loss and early pregnancy loss are within the range of estimates provided by previous studies,9–14 but are higher than some estimates based on spontaneous abortions requiring hospital admission,34 presumably reflecting that many pregnancy losses are not captured in hospital records.
Limitations of the data
Our study was not without limitations. First, self-reported pregnancy loss is conditional on the self-awareness of the pregnancy, and as such, is not an estimate of the total risk of loss. Prior studies that have prospectively followed women prior to conception or from very early pregnancy using urinary hCG to assess pregnancy have reported higher estimates of total risk of loss overall and different patterns in the total risk of loss, at least by maternal race and Hispanic origin,22,23 compared with studies of self-reported pregnancy loss. These discrepancies suggest that trends in the total risk of loss may not necessarily parallel the upward trend in self-reported loss observed here and in prior studies, and that the characteristics associated with total pregnancy loss may be different.15 Second, the restrictions imposed on the study years and maternal age groups, along with the inclusion of maternal age in the age-stratified models, may have resulted in overadjustment for age; however, RR estimates were very similar in unadjusted and adjusted analyses. Finally, some early pregnancy losses may have been misclassified, as the accuracy of reported gestational age of pregnancy losses has not been validated. The direction of this misclassification is unknown; thus, early pregnancy losses could have been over or underestimated.
Conclusions
Over the last two decades, women in the US report that about 20% of non-terminated pregnancies resulted in loss, with percentages varying by several maternal characteristics and pregnancy-related factors. Findings suggest that the risk of reported pregnancy loss has increased by a relative 2% per year in unadjusted models and 1% in models adjusted for maternal age, race/Hispanic origin, socio-economic factors, and other health-related factors. These results confirm that pregnancy loss is a common experience shared by many US women who become pregnant, and suggest that the risk of loss may have increased over time. Further work is needed to better understand the drivers of this increase in reported pregnancy loss in the US.
Supplementary Material
Figure S1. Unweighted number of pregnancies conceived in each calendar year that resulted in livebirth or pregnancy loss by survey period, NSFG 1995, 2002, 2006–2010, 2011–2015 (n = 42 526).
Figure S2. Risk of pregnancy loss in the United States by maternal age for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-binomial models). Separate plots by maternal age group.
Figure S3. Risk of early pregnancy loss in the United States by maternal age for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-binomial models). Separate plots by maternal age group.
Figure S4. Graphs of significant quadratic trend for the percentage reporting early losses (<12 weeks gestation) in the United States, all ages.
Table S1. Maternal characteristics and pregnancy-related factors by year of pregnancy conception in the United States, NSFG survey years: 1995, 2002, 2006–2010, 2011–2015.
Table S2. Per cent of pregnancies in the United States resulting in early pregnancy loss (<12 weeks) by maternal characteristics and pregnancy-related factors, NSFG 1995, 2002, 2006–2010, 2011–2015.
Table S3. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Excluding unwanted pregnancies (n = 35 639).
Table S4. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Including pregnancies that ended in induced termination (n = 47 701).
Table S5. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Stratified by recency of reported pregnancy, those completed within 2 years of the interview date (n = 6061) and those completed 2 or more years before the interview date (n = 36 465).
Acknowledgments
We thank Anjani Chandra and Hanyu Ni, for their input and advice on this manuscript. This work was presented at the 27th annual meeting of the Society of Pediatric and Perinatal Epidemiologic Research (SPER) in Denver, Colorado, on June 15–17, 2015. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, Centers for Disease Control and Prevention or the Office of Population Affairs, Office of the Assistant Secretary for Health.
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstetrics & Gynecology. 2015;125:1313–1320. doi: 10.1097/AOG.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brier N. Anxiety after miscarriage: a review of the empirical literature and implications for clinical practice. Birth. 2004;31:138–142. doi: 10.1111/j.0730-7659.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 3.Cumming GP, Klein S, Bolsover D, Lee AJ, Alexander DA, Maclean M, et al. The emotional burden of miscarriage for women and their partners: trajectories of anxiety and depression over 13 months. BJOG: An International Journal of Obstetrics & Gynaecology. 2007;114:1138–1145. doi: 10.1111/j.1471-0528.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 4.Radford EJ, Hughes M. Women’s experiences of early miscarriage: implications for nursing care. Journal of Clinical Nursing. 2015;24:1457–1465. doi: 10.1111/jocn.12781. [DOI] [PubMed] [Google Scholar]
- 5.Carter D, Misri S, Tomfohr L. Psychologic aspects of early pregnancy loss. Clinical Obstetrics & Gynecology. 2007;50:154–165. doi: 10.1097/GRF.0b013e31802f1d28. [DOI] [PubMed] [Google Scholar]
- 6.Kolte AM, van Oppenraaij RH, Quenby S, Farquharson RG, Stephenson M, Goddijn M, et al. Non-visualized pregnancy losses are prognostically important for unexplained recurrent miscarriage. Human Reproduction. 2014;29:931–937. doi: 10.1093/humrep/deu042. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy FP, Khashan AS, North RA, Rahma MB, Walker JJ, Baker PN, et al. Pregnancy loss managed by cervical dilatation and curettage increases the risk of spontaneous preterm birth. Human Reproduction. 2013;28:3197–3206. doi: 10.1093/humrep/det332. [DOI] [PubMed] [Google Scholar]
- 8.Lemmers M, Verschoor MA, Hooker AB, Opmeer BC, Limpens J, Huirne JA, et al. Dilatation and curettage increases the risk of subsequent preterm birth: a systematic review and meta-analysis. Human Reproduction. 2016;31:34–45. doi: 10.1093/humrep/dev274. [DOI] [PubMed] [Google Scholar]
- 9.Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG: An International Journal of Obstetrics & Gynaecology. 2007;114:170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 11.Kline J, Stein Z, Susser M. Conception to Birth: Epidemiology of Prenatal Development. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 12.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Sapra KJ, Schisterman EF, Lynch CD, Maisog JM, Grantz KL, et al. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: the LIFE Study. Fertility and Sterility. 2016;106:180–188. doi: 10.1016/j.fertnstert.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM, et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Human Reproduction. 2016;31:657–665. doi: 10.1093/humrep/dev329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang K, Nuevo-Chiquero A. Trends in self-reported spontaneous abortions: 1970–2000. Demography. 2012;49:989–1009. doi: 10.1007/s13524-012-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branum AM, Ahrens KA. Trends in timing of pregnancy awareness among US women. Maternal and Child Health Journal. 2016;21:715–726. doi: 10.1007/s10995-016-2155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reproductive Biomedicine Online. 2008;17:101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 18.Buss L, Tolstrup J, Munk C, Bergholt T, Ottesen B, Gronbaek M, et al. Spontaneous abortion: a prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstetricia et Gynecologica Scandinavica. 2006;85:467–475. doi: 10.1080/00016340500494887. [DOI] [PubMed] [Google Scholar]
- 19.Nybo AA, Wohlfahrt J, Christens P, Olsen J, Melbye M. Is maternal age an independent risk factor for fetal loss? The Western Journal of Medicine. 2000;173:331. doi: 10.1136/ewjm.173.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu G, Wu Y, Yang L, Yuan L, Guo H, Zhang F, et al. Risk factors for early miscarriage among Chinese: a hospital-based case-control study. Fertility and Sterility. 2014;101:1663–1670. doi: 10.1016/j.fertnstert.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 21.National Survey of Family Growth. [last accessed September 2015];Questionnaires, Datasets and Related Documentation. http://www.cdc.gov/nchs/nsfg.htm [updated May 2015.
- 22.Harb HM, Al-rshoud F, Dhillon R, Harb M, Coomarasamy A. Ethnicity and miscarriage: a large prospective observational study and meta-analysis. Fertility and Sterility. 2014;102:e81. [Google Scholar]
- 23.Mukherjee S, Velez Edwards DR, Baird DD, Savitz DA, Hartmann KE. Risk of miscarriage among black women and white women in a U.S. Prospective Cohort Study. American Journal of Epidemiology. 2013;177:1271–1278. doi: 10.1093/aje/kws393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risch HA, Weiss NS, Clarke EA, Miller AB. Risk factors for spontaneous abortion and its recurrence. American Journal of Epidemiology. 1988;128:420–430. doi: 10.1093/oxfordjournals.aje.a114982. [DOI] [PubMed] [Google Scholar]
- 25.Eskild A, Vatten LJ, Nesheim BI, Vangen S. The estimated risk of miscarriage should be corrected for induced abortion rates. Acta Obstetricia et Gynecologica Scandinavica. 2009;88:569–574. doi: 10.1080/00016340902814567. [DOI] [PubMed] [Google Scholar]
- 26.Ahrens KA, Rossen LM, Branum AM. Pregnancy loss history at first parity and selected adverse pregnancy outcomes. Annals of Epidemiology. 2016;26:474–481. e479. doi: 10.1016/j.annepidem.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. New England Journal of Medicine. 2016;374:843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. American Journal of Public Health. 2014;104(Suppl 1):S43–S48. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mumford SL, Sapra KJ, King RB, Louis JF, Buck Louis GM. Pregnancy intentions-a complex construct and call for new measures. Fertility and Sterility. 2016;106:1453–1462. doi: 10.1016/j.fertnstert.2016.07.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones RK, Kost K. Underreporting of induced and spontaneous abortion in the United States: an analysis of the 2002 National Survey of Family Growth. Studies in Family Planning. 2007;38:187–197. doi: 10.1111/j.1728-4465.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 31.Fu H, Darroch JE, Henshaw SK, Kolb E. Measuring the extent of abortion underreporting in the 1995 National Survey of Family Growth. Family Planning Perspectives. 1998;30:128–133. 138. [PubMed] [Google Scholar]
- 32.Krieg SA, Shahine LK, Lathi RB. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertility and Sterility. 2016;106:941–947. doi: 10.1016/j.fertnstert.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Shellenberg KM, Moore AM, Bankole A, Juarez F, Omideyi AK, Palomino N, et al. Social stigma and disclosure about induced abortion: results from an exploratory study. Global Public Health. 2011;6(Suppl 1):S111–S125. doi: 10.1080/17441692.2011.594072. [DOI] [PubMed] [Google Scholar]
- 34.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Unweighted number of pregnancies conceived in each calendar year that resulted in livebirth or pregnancy loss by survey period, NSFG 1995, 2002, 2006–2010, 2011–2015 (n = 42 526).
Figure S2. Risk of pregnancy loss in the United States by maternal age for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-binomial models). Separate plots by maternal age group.
Figure S3. Risk of early pregnancy loss in the United States by maternal age for conception years 1990–2011: NSFG 1995, 2002, 2006–2010, 2011–2015 (observed and predicted from unadjusted log-binomial models). Separate plots by maternal age group.
Figure S4. Graphs of significant quadratic trend for the percentage reporting early losses (<12 weeks gestation) in the United States, all ages.
Table S1. Maternal characteristics and pregnancy-related factors by year of pregnancy conception in the United States, NSFG survey years: 1995, 2002, 2006–2010, 2011–2015.
Table S2. Per cent of pregnancies in the United States resulting in early pregnancy loss (<12 weeks) by maternal characteristics and pregnancy-related factors, NSFG 1995, 2002, 2006–2010, 2011–2015.
Table S3. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Excluding unwanted pregnancies (n = 35 639).
Table S4. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Including pregnancies that ended in induced termination (n = 47 701).
Table S5. Linear trends in pregnancy loss and early pregnancy loss in the United States by calendar year and age at pregnancy conception, NSFG 1995, 2002, 2006–2010, 2011–2015. Stratified by recency of reported pregnancy, those completed within 2 years of the interview date (n = 6061) and those completed 2 or more years before the interview date (n = 36 465).