Key Clinical Message
A sputum test is noninvasive and simple. It contributed to correct diagnosis of a patient with severe acute respiratory failure. We again point out the usefulness of sputum cytodiagnosis for differentiating severe pneumonia.
Keywords: acute lymphoblastic leukemia, acyclovir, herpes simplex virus pneumonia, sputum cytodiagnosis
Introduction
We have often encountered patients with hematological malignancies who develop pulmonary complications. While the most frequent cause is infection, complications can also arise due to treatment (radiation/chemotherapy)‐associated toxicity, tumor cell infiltration, and hemorrhage. Furthermore, it is common for multiple causes to contribute to the onset of pulmonary complications in these patients 1.
Moreover, pulmonary complications often become severe quite rapidly in patients with hematological malignancies due to factors such as myelosuppression and reduced immune function, leading to a fatal outcome in some cases. Therefore, early diagnosis and appropriate intervention for the etiological factors are important. While bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) are useful for differential diagnosis of pulmonary diseases, these procedures are more invasive in patients with hematological malignancies due to an elevated risk of infection and/or hemorrhage. It can also be difficult to perform such procedures because of rapid deterioration of respiratory function.
We report a patient with rapidly progressive pneumonia after chemotherapy for acute lymphoblastic leukemia (ALL). Herpes simplex virus (HSV) pneumonia was identified by sputum cytodiagnosis, and treatment with acyclovir (ACV) was successful in salvaging the patient.
Case Report
A 57‐year‐old man was referred to our hospital with a bleeding tendency. The results of bone marrow aspiration suggested ALL, and prednisolone (PSL) therapy was started on the day of admission. After evaluation of the disease type, remission induction therapy was initiated as soon as possible. Febrile neutropenia‐related septic shock occurred 9 days after admission. Treatment with a broad‐spectrum antibiotic resulted in prompt improvement, and the patient's temperature was normalized. However, the patient complained of painful dry lips from day 12, and his respiratory status showed rapid deterioration on day 15 (Fig. 1). Chest computed tomography (CT) revealed ground‐glass opacities in the bilateral lung fields (Fig. 2). Respiratory failure and hypoxemia became worse on the evening of day 16. Various examinations were conducted for differential diagnosis of conditions causing diffuse ground‐glass opacities, but there were no significant changes of serological markers for interstitial lung disease (SP‐A: 50.9 ng/mL, SP‐D: 310 ng/mL, and KL‐6: 149 U/mL) or fungal infection (β‐D glucan <0.5 pg/mL, Candida antigen: negative, and Aspergillus galactomannan antigen: 0.1). Examination of representative viral antibodies indicated previous infection (anti‐HSV‐IgM 0.26, anti‐HSV‐IgG 41.6, anti‐HZV‐IgM 0.20, anti‐HZV‐IgG 14.3, anti‐CMV‐IgM 0.44, anti‐CMV‐IgG 1.29), and CMV antigenemia was negative. Cytodiagnosis was performed using a sputum sample obtained on the morning of day 16, revealing multinucleated cells with ground‐glass‐like nuclei on the same day (Fig. 3). This finding suggested that HSV pneumonia was the etiology of the patient's rapidly progressive respiratory failure, so administration of ACV was started immediately. Noninvasive positive pressure ventilation was also performed for respiratory support, and the patient gradually improved over 1 week (Fig. 1). Subsequently, polymerase chain reaction (PCR) analysis of sputum identified HSV type 1 and PCR of serum confirmed a high HSV‐DNA level (1.3 × 104 copies/mL). On day 28, repeat chest CT confirmed that there was marked improvement of the interstitial changes (Fig. 4). Oxygen administration was ceased. Subsequently, he recovered from myelosuppression, and bone marrow analysis confirmed hematological remission.
Figure 1.
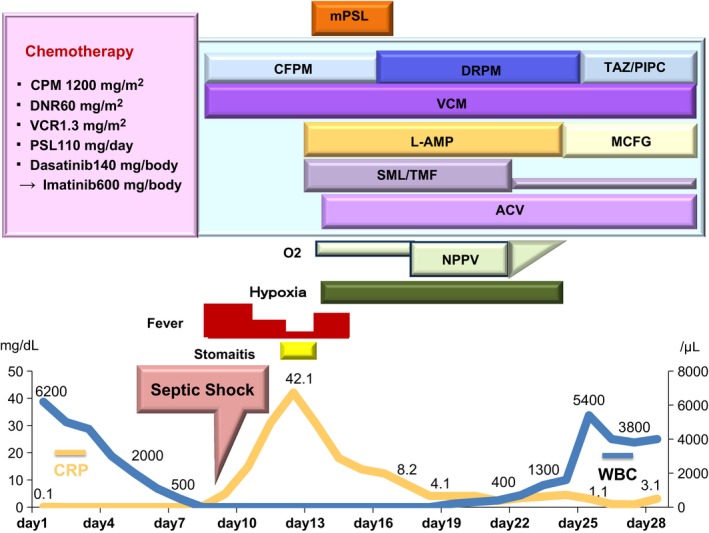
Clinical course from before and to after the onset of HSV pneumonia.
Figure 2.
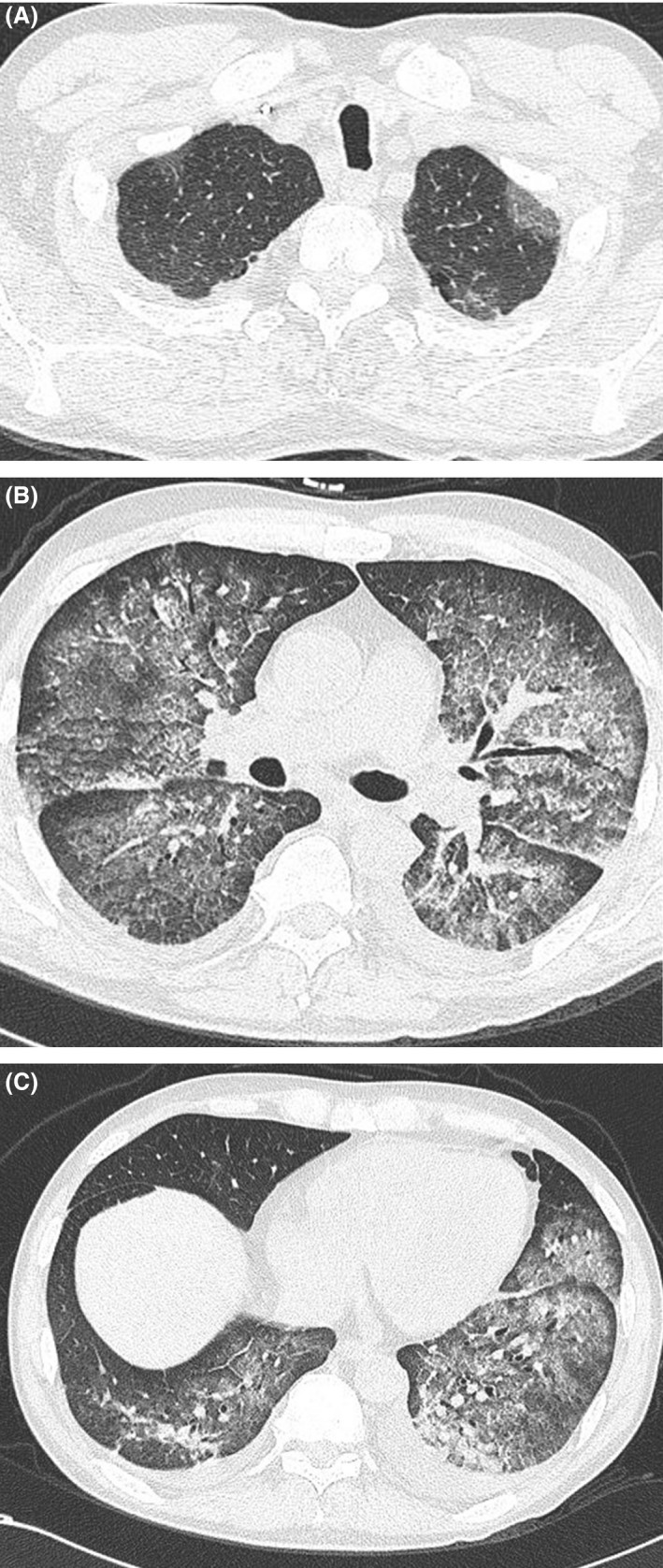
Chest CT scan on Day 15. Although the margins of the lungs are relatively maintained, bilateral diffuse ground‐glass opacities are observed from apex to base.
Figure 3.
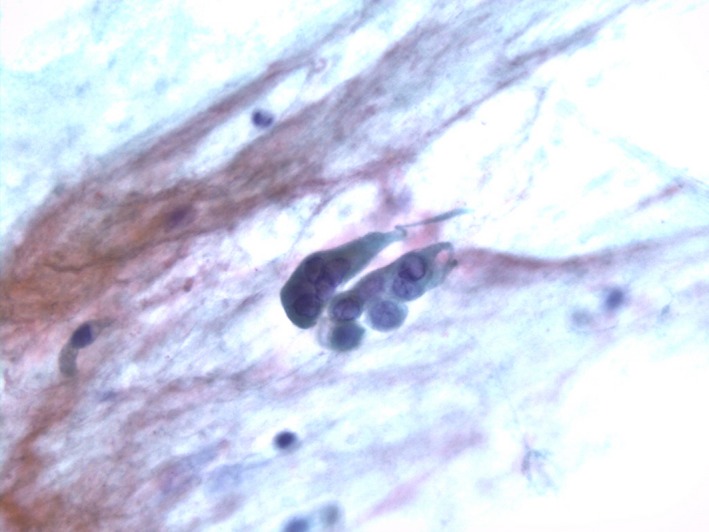
Multinuclear cells with ground‐glass‐like nuclei can be seen, which are characteristic of HSV infection.
Figure 4.
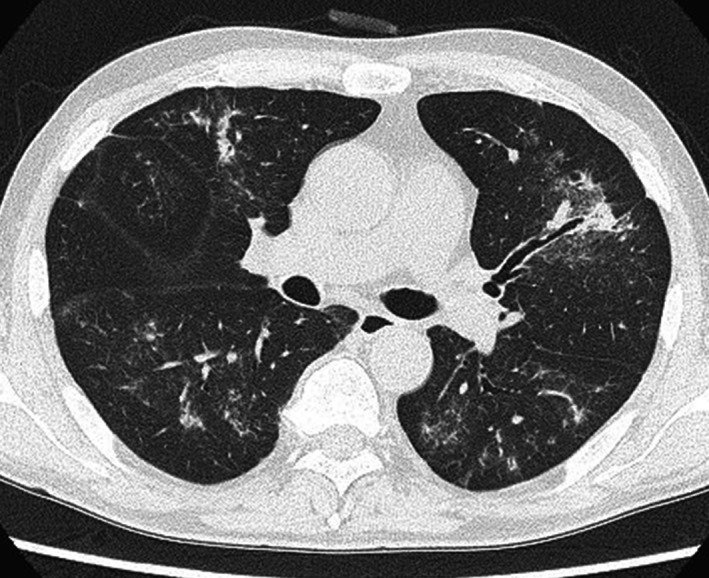
Chest CT scan on Day 28. There is marked improvement of the bilateral diffuse ground‐glass opacities.
Discussion
Herpes viruses are double‐helical DNA viruses 2, 3. Among them, HSV 4, varicella‐zoster virus (VZV) and cytomegalovirus (CMV) are causes of pneumonia in humans. HSV infects the host's neural ganglion cells and generally lies dormant 5, 6. It can be reactivated by stress or if the host develops immunosuppression 2, 3, 7, 8, 9, causing infection of various organs such as the skin, brain, and esophagus 10, 11. Respiratory infection may lead to tracheobronchitis or pneumonia 9, 12, 13. In the present patient, the HSV antibody titer was consistent with previous infection. After the start of remission induction therapy for ALL, the latent virus may have been reactivated due to immunosuppression, leading to development of HSV pneumonia. HSV is classified into two types (HSV‐I and HSV‐II) based on its antigenicity profile 8, 14, 15, 16. HSV‐I is frequently involved in pulmonary disease 9, 12, 13. It may be transmitted in the saliva 17, 18, 19 and causes lesions from the oral to pharyngeal regions 20, 21 with the potential for progression to respiratory tract infection 14, 22. In the present patient, stomatitis was advanced before exacerbation of respiratory dysfunction and HSV‐I was identified in the sputum by PCR.
For diagnosis of HSV pneumonia, it is initially necessary to isolate the virus. However, the virus may be inactivated by neutralizing antibodies produced by the host from 1 week after the onset of infection, resulting in a marked decrease in the isolation rate. Therefore, isolation of the virus by cell culture of pharyngeal fluid or BAL specimens needs to be performed early after the onset of infection 23. However, HSV is sometimes isolated from the airways or urine in the absence of active infection, so its significance should be evaluated carefully 2, 9, 19, 24.
In patients with HSV pneumonia, focal or diffuse ulceration of the tracheobronchial mucosa occurs 22. Furthermore, necrotic bronchopneumonia is observed in patients with severe disease, and the features of diffuse interstitial pneumonia are noted in some cases. HSV‐infected cells with intranuclear inclusion bodies (owl's eyes), which are smaller than those of CMV‐infected cells, and mononuclear to multinucleated ground‐glass‐like nuclei are observed at the periphery of the mucosal ulcers. The intranuclear inclusion bodies are basophilic in the initial phase of infection and become eosinophilic with a halo in the late phase 19, 25. In addition, proliferating type II alveolar epithelial cells fuse to form large multinucleated cells 26. In the present patient, HSV‐infected cells were identified by sputum cytodiagnosis. However, when HSV‐infected cells are detected in respiratory tract specimens like sputum, it is difficult to evaluate whether the source is an upper airway lesion (with influx of cells into the lower airway) or an actual lower airway infection from the pathological findings alone. In this patient, we confirmed a high level of HSV DNA in the serum before initiating ACV administration, and there was a good response to ACV therapy 27, 28.
To obtain more accurate information for the diagnosis of HSV pneumonia, it might be important to perform bronchoscopy‐guided TBLB, which provides pathological information about the lung parenchyma. However, the sample volume collected by TBLB is often quite small 29, and the risk of this examination is higher in patients with hematological malignancies. Also, it is difficult to perform TBLB if rapid deterioration of the respiratory status occurs, as in the present case. In fact, it has been reported that antemortem diagnosis of HSV pneumonia is difficult in most cases 6, 10, 22, 26, 30. On the other hand, a sputum test is noninvasive and simple. In our critically ill patient with severe acute respiratory failure, sputum cytodiagnosis contributed to identifying the correct etiology and allowed rapid initiation of appropriate treatment. This case emphasizes the usefulness of sputum cytodiagnosis for making a differential diagnosis of severe pneumonia.
Authorship
YS: contributed to follow the patient. MM: contributed to the sputum cytodiagnosis of the patient. MY: supervised the evaluation of the management of the patient as well as wrote the manuscript in its entirety.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgments
The authors would like to thank Dr. Yuji Kikuchi, Dr. Tsuyoshi Takahashi at Department of Hematology, and Dr. Kino Hiroshi at Department of Pneumology, and laboratory technicians at testing department, Mitsui Memorial Hospital.
Clinical Case Reports 2018; 6(1): 165–169
References
- 1. Tenholder, M. F. , and Hooper R. G.. 1980. Pulmonary infiltrates in leukemia. Chest 78:468–473. [DOI] [PubMed] [Google Scholar]
- 2. Corey, L. , and Spear P. G.. 1986. Infections with herpes simplex viruses (1). N. Engl. J. Med. 314:686–691. [DOI] [PubMed] [Google Scholar]
- 3. Schuller, D. , Spessert C., Fraser V. J., and Goodenberger D. M.. 1993. Herpes simplex virus from respiratory tract secretions: epidemiology, clinical characteristics, and outcome in immunocompromised and nonimmunocompromised hosts. Am. J. Med. 94:29–33. [DOI] [PubMed] [Google Scholar]
- 4. Morgan, H. R. , and Finland M.. 1949. Isolation of herpes virus from a case of atypical pneumonia and erythema multiforme exudativum with studies of four additional cases. Am. J. Med. Sci. 217:92–95. [DOI] [PubMed] [Google Scholar]
- 5. Warren, K. G. , Brown S. M., Wroblewska Z., Gilden D., Koprowski H., and Subak‐Sharpe J.. 1978. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N. Engl. J. Med. 298:1068–1069. [DOI] [PubMed] [Google Scholar]
- 6. Warren, K. G. , Devlin M., Gilden D. H., Wroblewska Z., Brown S. M., Subak‐Sharpe J., et al. 1977. Isolation of Herpes simplex virus from human trigeminal ganglia, including ganglia from one patient with multiple sclerosis. Lancet 2:637–639. [DOI] [PubMed] [Google Scholar]
- 7. Chang, T. W. 1971. Recurrent viral infection (reinfection). N. Engl. J. Med. 284:765–773. [DOI] [PubMed] [Google Scholar]
- 8. Gasparetto, E. L. , Escuissato D. L., Inoue C., Marchiori E., and Muller N. L.. 2005. Herpes simplex virus type 2 pneumonia after bone marrow transplantation: high‐resolution CT findings in 3 patients. J. Thorac. Imaging 20:71–73. [DOI] [PubMed] [Google Scholar]
- 9. Bruynseels, P. , Jorens P. G., Demey H. E., Goossens H., Pattyn S. R., Elseviers M. M., et al. 2003. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet 362:1536–1541. [DOI] [PubMed] [Google Scholar]
- 10. Buss, D. H. , and Scharyj M.. 1979. Herpesvirus infection of the esophagus and other visceral organs in adults. Incidence and clinical significance. Am. J. Med. 66:457–462. [DOI] [PubMed] [Google Scholar]
- 11. Lightdale, C. J. , Wolf D. J., Marcucci R. A., and Salyer W. R.. 1977. Herpetic esophagitis in patients with cancer: ante mortem diagnosis by brush cytology. Cancer 39:223–226. [DOI] [PubMed] [Google Scholar]
- 12. Sherry, M. K. , Klainer A. S., Wolff M., and Gerhard H.. 1988. Herpetic tracheobronchitis. Ann. Intern. Med. 109:229–233. [DOI] [PubMed] [Google Scholar]
- 13. Prellner, T. , Flamholc L., Haidl S., Lindholm K., and Widell A.. 1992. Herpes simplex virus–the most frequently isolated pathogen in the lungs of patients with severe respiratory distress. Scand. J. Infect. Dis. 24:283–292. [DOI] [PubMed] [Google Scholar]
- 14. Whitley, R. J. , and Roizman B.. 2001. Herpes simplex virus infections. Lancet 357:1513–1518. [DOI] [PubMed] [Google Scholar]
- 15. Nahmias, A. J. , and Roizman B.. 1973. Infection with herpes‐simplex viruses 1 and 2. 3. N. Engl. J. Med. 289:781–789. [DOI] [PubMed] [Google Scholar]
- 16. Aquino, S. L. , Dunagan D. P., Chiles C., and Haponik E. F.. 1998. Herpes simplex virus 1 pneumonia: patterns on CT scans and conventional chest radiographs. J. Comput. Assist. Tomogr. 22:795–800. [DOI] [PubMed] [Google Scholar]
- 17. Stern, H. , Elek S. D., Millar D. M., and Anderson H. F.. 1959. Herpetic whitlow, a form of cross‐infection in hospitals. Lancet 2:871–874. [DOI] [PubMed] [Google Scholar]
- 18. Lindgren, K. M. , Douglas R. G. Jr, and Couch R. B.. 1968. Significance of Herpesvirus hominis in respiratory secretions of man. N. Engl. J. Med. 278:517–523. [DOI] [PubMed] [Google Scholar]
- 19. Graham, B. S. , and Snell J. D. Jr. 1983. Herpes simplex virus infection of the adult lower respiratory tract. Medicine 62:384–393. [DOI] [PubMed] [Google Scholar]
- 20. Bader, C. , Crumpacker C. S., Schnipper L. E., Ransil B., Clark J. E., Arndt K., et al. 1978. The natural history of recurrent facial‐oral infection with herpes simplex virus. J. Infect. Dis. 138:897–905. [DOI] [PubMed] [Google Scholar]
- 21. Lafferty, W. E. , Coombs R. W., Benedetti J., Critchlow C., and Corey L.. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444–1449. [DOI] [PubMed] [Google Scholar]
- 22. Ramsey, P. G. , Fife K. H., Hackman R. C., Meyers J. D., and Corey L.. 1982. Herpes simplex virus pneumonia: clinical, virologic, and pathologic features in 20 patients. Ann. Intern. Med. 97:813–820. [DOI] [PubMed] [Google Scholar]
- 23. Ustacelebi, S. 2001. Diagnosis of herpes simplex virus infections. J. Clin. Virol. 21:255–259. [DOI] [PubMed] [Google Scholar]
- 24. Crombach, W. H. , Beckers E. A., and Claessens W. L.. 1992. Clinical relevance of herpes simplex virus in the throat of elderly with Salmonella enteritidis gastroenteritis. Eur. J. Med. 1:244–245. [PubMed] [Google Scholar]
- 25. Tuxen, D. V. , Cade J. F., McDonald M. I., Buchanan M. R., Clark R. J., and Pain M. C.. 1982. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 126:416–419. [DOI] [PubMed] [Google Scholar]
- 26. Nash, G. , and Foley F. D.. 1970. Herpetic infection of the middle and lower respiratory tract. Am. J. Clin. Pathol. 54:857–863. [DOI] [PubMed] [Google Scholar]
- 27. Hirsch, M. S. , and Schooley R. T.. 1983. Drug therapy. Treatment of herpesvirus infections. N. Engl. J. Med. 309:1034–1039. [DOI] [PubMed] [Google Scholar]
- 28. Hirsch, M. S. , and Swartz M. N.. 1980. Drug therapy: antiviral agents (second of two parts). N. Engl. J. Med. 302:949–953. [DOI] [PubMed] [Google Scholar]
- 29. Simoons‐Smit, A. M. , Kraan E. M., Beishuizen A., Strack van Schijndel R. J., and Vandenbroucke‐Grauls C. M.. 2006. Herpes simplex virus type 1 and respiratory disease in critically‐ill patients: real pathogen or innocent bystander? Clin. Microbiol. Infect. 12:1050–1059. [DOI] [PubMed] [Google Scholar]
- 30. Nash, G. 1972. Necrotizing tracheobronchitis and bronchopneumonia consistent with herpetic infection. Hum. Pathol. 3:283–291. [DOI] [PubMed] [Google Scholar]


