Abstract
Objective
Medically refractory chronic rhinosinusitis (CRS) can be managed with appropriate continued medical therapy (CMT) or surgery followed by CMT. Patients who initially elect CMT, and do not experience adequate symptom resolution, may ‘cross-over’ to endoscopic sinus surgery (ESS). Our objective was to identify patient covariates associated with this subset of patients who elect this change in treatment modality.
Study Design
Retrospective analysis of a prospective, multi-center cohort of adult patients with CRS enrolled between March, 2011 and June, 2015 in academic, tertiary referral clinics.
Methods
Subjects who initially elected CMT were followed up to 18-months, provided a comprehensive medical history, and completed the 22-item SinoNasal Outcome Test (SNOT-22) at baseline and during 6-month follow-up intervals. Hazard regression modeling was used to identify covariates associated with elective change in treatment modality.
Results
179 subjects were followed for an average 15.1 (SD±4.6) months. Subjects who elected ESS (55/179) had significantly worse average endoscopy scores and reported worse SNOT-22 sleep dysfunction scores at baseline (p≤0.026). For each single increasing (worsening) point of Lund-Kennedy endoscopy score, the hazard ratio (HR) of cross-over increased by ~6%. Similarly, for every point of worsening in baseline SNOT-22 total score, the hazard of treatment cross-over increased by ~2%. After covariate adjustment, only baseline SNOT-22 sleep dysfunction scores were associated with an increased risk of treatment cross over (HR=1.07; 95%CI:1.02–1.11; p=0.003).
Conclusion
Baseline total SNOT-22 and endoscopy scores are associated with treatment crossover, but reported sleep dysfunction is the only significant independent predictor of treatment crossover.
MeSH Key Words: Sinusitis, Chronic Disease, Endoscopy, Therapeutics, Outcome Assessment (Health Care)
INTRODUCTION
Patients with chronic rhinosinusitis (CRS) who do not experience adequate disease-specific symptom control will, at some point, decide whether to continue with treatment consisting of appropriate medical therapies or elect endoscopic sinus surgery (ESS) followed by continued medical therapy (CMT). This decision results from a physician-patient dialogue on the frequency and magnitude of risks and benefits surrounding ESS as a subsequent treatment option. Fortunately, the prevalence and clinical significance of symptom improvement following ESS has been well characterized.1,2 Greater postoperative improvements are reported, on average, by a majority of patients electing ESS when compared to patients electing CMT alone.2,3 Patients electing CMT experience relatively stable but higher symptom burden on average, except for those patient subgroups who continue to experience increasing symptom burden and eventually ‘cross-over’ to ESS as a subsequent treatment option.2
An improved understanding of both the prevalence and incidence of the escalation of care towards ESS would help patients and otolaryngologists more efficiently navigate the choices and expectations between treatments for CRS. Prior investigation has found that patients that elect ESS for medically recalcitrant CRS at baseline differ only in their symptom burden in the psychological and sleep dysfunction domains when compared to patients that elect CMT alone.4,5 Identification of clinical factors which predispose or protect patients from progression to ESS would further aid in patient counseling. The objective of this investigation was to determine the prevalence, timing, and potential baseline clinical risk factors associated with electing a change in treatment modality from CMT to ESS in patients with medically recalcitrant chronic rhinosinusitis that initially elect CMT alone. We hypothesized that the sleep and psychological burden of disease would associate with increased risk of progression to ESS.
MATERIALS and METHODS
Patient Population
This investigation retrospectively analyzed data from an observational, prospectively collected (March, 2011 and June, 2015), multi-institutional, non-randomized cohort study of treatment outcomes surrounding CRS.3,5,6 Study participants included English speaking adult subjects (≥18 years old) presenting for evaluation and management at four academic, rhinology practices in North America including: Oregon Health & Science University (OHSU, Portland, OR), the Medical University of South Carolina (Charleston, SC), Stanford University (Palo Alto, CA), and the University of Calgary (Calgary, Alberta, Canada). Upon enrollment, subjects voluntarily elected CMT after extensive patient counseling. Continued medical therapy was defined for patients without nasal polyposis (CRSsNP) to include: saline irrigation, intranasal corticosteroid sprays, and a short course of oral antibiotics as recommended as the minimal initial treatment, with oral corticosteroids as an option. For patients with nasal polyposis (CRSwNP): saline irrigation, intranasal corticosteroid sprays, and a short course of oral corticosteroids are recommended as a minimal initial treatment, with oral antibiotics as an option.7 Subjects were observed through the standard of care up to 18-months and were unrestricted to cross-over to ESS at any time during the observational period. An Institutional Review Board at each enrollment location provided oversight and annual review of all investigational protocols and informed consent.
Inclusion and Exclusion Criteria
All subjects met diagnostic criteria for CRS based on current Rhinosinusitis Treatment Guidelines7–9 and had not achieved adequate symptom control from previous medical therapy. Prior medical therapy was defined as either broad-spectrum and/or culture-directed antibiotics as well as at least one trial of oral and topical steroid therapy prescribed within the previous year. Clinical subtypes of CRS included patients with aspirin sensitivity, cystic fibrosis-associated disease, or primary comorbid ciliary dyskinesia. Study participants were evaluated at ~6-month intervals during routine, physician-directed clinical appointments per the standard of care.
Clinical Data Collection
Study participants were asked to provide a comprehensive medical and social history. Additional measures of disease severity, collected as part of the standard of care, were used simultaneously as investigational data. High resolution computed tomography (CT) of the sinuses was obtained, without contrast, to assess disease severity using 1.0mm. contiguous images in the axial plane. Bilateral opacification was quantified by the enrolling physician in accordance with Lund-Mackay staging (range: 0–24).10 Post-treatment CT images were not collected, unless required by the standard of care, due to elevated radiation exposure.
Sinonasal regions were also evaluated using rigid, fiberoptic endoscopes (Karl Storz, Tuttlingen, Germany) during both baseline and follow-up evaluations. Bilateral endoscopic examinations were quantified by each enrolling physician using Lund-Kennedy staging (range: 0–20).11 Higher total scores on both staging systems represent worse overall disease severity.
Olfactory function was also measured using the Brief Smell Identification Test (BSIT; Sensonics, Inc., Haddon Heights, NJ). The BSIT is a validated 12-item, cross-cultural, non-invasive test of olfactory detection utilizing microencapsulated odorant strips with one correct identification response and 3 distractor options in a “forced choice” format. The BSIT is designed for “scratch-and-sniff” identification using pencil strikes for activation. Higher BSIT total scores (range: 0–12) reflect a better sense of smell. Total BSIT scores ≥ 9 can be clinically interpreted as “normal olfaction” for healthy subjects of all ages using gender adjusted, adult, normative data. Lower BSIT scores (≤ 8) can be clinically interpreted as either some degree of olfactory dysfunction or potential malingering,12 however, alternative interpretations of threshold scores for the BSIT have been suggested for patients with CRS.13
Disease Severity Measures
The primary outcome measure was the prevalence of subjects electing to cross-over from CMT to ESS as the subsequent treatment option during the observational period. Secondary aims included evaluating the post-treatment interval change in mean Lund-Kennedy endoscopy scores, mean BSIT scores, as well as mean scores of the 22-item SinoNasal Outcome Test (SNOT-22) survey for both treatment groups. These scores were also evaluated for their ability to predict treatment cross-over. Follow-up interval change time periods were defined as the time difference between the date of enrollment to the date of ESS for the cross over cohort and the difference between the date of enrollment and date of last available follow-up for the CMT cohort.
The SNOT-22 is a validated instrument developed to quantify sinonasal symptom severity across various disease states (©2006, Washington University, St. Louis, MO).14 Symptom severity is measured using Likert-scale choices (range: 0 – 5) while higher summarized scores on the SNOT-22 suggest worse overall patient functioning and/or symptom severity (total range: 0–110). Previous factor analysis has also identified 5 subdomains including: rhinologic symptoms (range: 0–30), extra-nasal rhinologic symptoms (range: 0–15), ear/facial symptoms (range: 0–25), psychological dysfunction (range: 0–35), and sleep dysfunction (range: 0–25; Table 1).15 Previously defined criteria has also identified symptom severity, as measured by a SNOT-22 total score of ≥ 20 as a threshold for appropriately offered ESS.16–18
Table 1.
Survey items for individual domains of the SNOT-22
| SNOT-22 Domains: | Survey Items: | Score Range: |
|---|---|---|
| Rhinologic Symptoms | #1, #2, #3, #6, #21, #22 | 0–30 |
| Extra-Nasal Rhinologic Symptoms | #4, #5, #6 | 0–15 |
| Ear/Facial Symptoms | #2, #7, #8, #9, #10 | 0–25 |
| Psychological Dysfunction | #14, #15, #16, #17, #18, #19, #20 | 0–35 |
| Sleep Dysfunction | #11, #12, #13, #14, #15 | 0–25 |
SNOT-22, 22-item Sinonasal Outcome Test
Data Management and Statistical Analysis
All protected health information was eliminated and clinical data was safeguarded using unique study identification number assignment. Data was securely transferred to OHSU from each enrollment site for manual entry into a relational database (Access, Microsoft Corp, Redmond, WA). All statistical analyses were finalized using commercial software (SPSS version 24.0, IBM Corp, Armonk, NY). Study data was first evaluated descriptively and data normality assumptions were confirmed for all ordinal or continuous measures. Means [±standard deviations] are reported where appropriate. Clinical characteristics were compared between treatment modalities using with Mann-Whitney U or chi-square (χ2) testing. Within-subject, post-treatment improvement over time was evaluated using Wilcoxon signed rank testing and compared between treatment modalities using two-tailed independent sample t-testing or Mann-Whitney U tests, were appropriate. Last available follow-up was used to define post-treatment scores due to historic durability in all mean outcome measures between 6 and 18 months in this cohort.19,20 Kaplan-Meier curve estimations were used to determine one-minus cumulative survival proportion, defined as experiencing the event of treatment modality crossover to ESS. Multivariate hazard ratio (HR) regression modeling was completed to identify risk factors significantly associated with treatment cross over. Univariate models were completed to screen covariates at the 0.200 level of significance for final model inclusion. Final models were conducted using forward selection and backwards elimination (p<0.050) in a manual, stepwise process. Unadjusted and adjusted HR values are reported with 95% confidence intervals (CIs) with corresponding type-I error (p-values).
RESULTS
Final Study Cohort and Baseline Characteristics
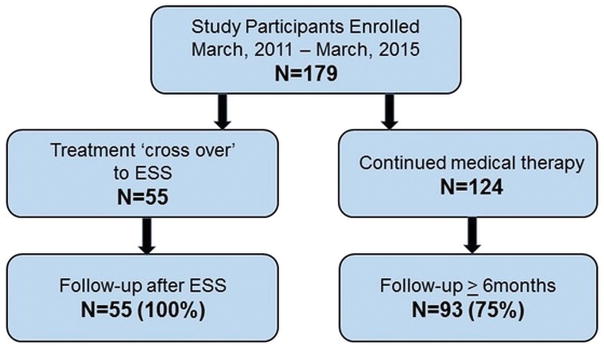
A total of 179 study participants provide informed consent, met inclusion criteria, and completed all baseline study requirements (Figure 1). A total of 124 (69%) study participants elected to remain on CMT for the duration of the observational period while 55 (31%) subjects elected ESS within 18-months. An earlier analysis of a subset of this cohort (n=117) was published previously.21 Post-treatment follow-up was available for a total of 148 (83%) participants. The average follow-up for the CMT cohort was 15.1[±4.6] months compared to the overall 5.5[±4.8] months for those who crossed over to ESS. Cross-over occurred across the entire follow-up period with 67% (37/55) crossing over in the first 6 months, 22% (12/55) crossing-over in the second 6 months and 11% (6/55) crossing over the in the final 6 months. Baseline characteristics between the two cohorts were further compared across demographic factors, secondary age, gender, comorbid conditions, socioeconomic data, clinical data scores, SNOT-22 scores, and time of onset of symptoms related to CRS (Table 2). Average duration of symptoms months since CRS on-set were not significantly different between participants electing CMT and treatment cross over to ESS (69.4±74.6 vs 103.1±142.8, p=0.718). No significant differences in baseline characteristics between treatment groups were discovered with the exception of average SNOT-22 sleep dysfunction domain scores.
Figure 1.
Flow diagram for final cohort. ESS, endoscopic sinus surgery.
Table 2.
Comparison of baseline characteristics for study participants electing continued medical therapy or crossed over to endoscopic sinus surgery (n=179)
| Continued medical therapy (n=124) | Treatment cross-over to ESS (n=55) | ||||
|---|---|---|---|---|---|
| Characteristics: | Mean [±SD] | N (%) | Mean [±SD] | N (%) | p-value |
| Duration of symptoms (months) | 69.4 [±74.6] | 103.1 [±142.8] | 0.718 | ||
| Age (years) | 51.6 [±14.3] | 49.1 [±16.8] | 0.305 | ||
| Females | 70 (57%) | 27 (49%) | 0.362 | ||
| White/Caucasian | 103 (83%) | 47 (86%) | 0.689 | ||
| African American | 13 (11%) | 6 (11%) | 0.932 | ||
| Asian | 3 (2%) | 2 (4%) | >0.999 | ||
| Hispanic/Latino | 1 (1%) | 1 (2%) | 0.521 | ||
| Educational attainment (years) | 15.5 [±3.1] | 15.5 [±2.5] | 0.961 | ||
| Household income: | 0.916 | ||||
| $0–$25,000 | 16 (13%) | 6 (11%) | ---- | ||
| $26,000–$50,000 | 27 (22%) | 16 (29%) | ---- | ||
| $51,000–$75,000 | 18 (15%) | 8 (15%) | ---- | ||
| $76,000–$100,000 | 25 (20%) | 11 (20%) | ---- | ||
| $100,000+ | 32 (13%) | 13 (24%) | ---- | ||
| Medical insurance type: | 0.776 | ||||
| Employer provided | 68 (55%) | 29 (53%) | ---- | ||
| Medicare | 32 (26%) | 13 (24%) | ---- | ||
| Medicaid | 5 (4%) | 1 (2%) | ---- | ||
| State assisted program | 1 (1%) | 0 (0%) | ---- | ||
| Private | 9 (7%) | 8 (15%) | ---- | ||
| VA benefit/Tricare | 1 (1%) | 0 (0%) | ---- | ||
| Canadian Medicare | 7 (6%) | 3 (6%) | ---- | ||
| Asthma | 49 (40%) | 16 (29%) | 0.181 | ||
| Nasal polyposis | 47 (38%) | 22 (40%) | 0.790 | ||
| Septal deviation | 20 (16%) | 12 (22%) | 0.359 | ||
| Turbinate hypertrophy | 7 (6%) | 3 (6%) | 0.959 | ||
| Allergy | 55 (44%) | 22 (40%) | 0.587 | ||
| AERD | 15 (12%) | 4 (7%) | 0.334 | ||
| COPD | 9 (7%) | 1 (2%) | 0.179 | ||
| Depression (self-reported) | 13 (11%) | 7 (13%) | 0.660 | ||
| Current tobacco use/smoking | 4 (3%) | 1 (2%) | >0.999 | ||
| Current alcohol use | 62 (50%) | 26 (47%) | 0.749 | ||
| Previous sinus surgery | 72 (58%) | 40 (73%) | 0.061 | ||
| Ciliary dyskinesia/CF | 4 (3%) | 2 (4%) | >0.999 | ||
| Diabetes mellitus (Type I/II) | 13 (11%) | 2 (4%) | 0.154 | ||
| Corticosteroid dependency | 18 (15%) | 5 (9%) | 0.317 | ||
| Computed tomography score | 12.4 [±6.2] | 13.2 [±6.3] | 0.418 | ||
| Endoscopy score | 6.1 [±4.1] | 7.6 [±4.5] | 0.026 | ||
| BSIT olfactory score | 8.3 [±3.6] | 8.8 [±3.2] | 0.663 | ||
| Normal olfaction (BSIT ≥ 9) | 72 (63%) | 32 (63%) | 0.987 | ||
| SNOT-22 total score: | 44.1 [±19.6] | 48.8 [±20.0] | 0.145 | ||
| Rhinologic symptom score | 14.7 [±6.2] | 16.0 [±6.1] | 0.197 | ||
| Extra-nasal rhinologic score | 7.6 [±3.4] | 7.7 [±3.6] | 0.809 | ||
| Ear/facial symptom score | 7.9 [±5.4] | 7.8 [±5.5] | 0.905 | ||
| Psychological dysfunction score | 12.2 [±7.9] | 14.2 [±8.4] | 0.135 | ||
| Sleep dysfunction score | 10.9 [±6.7] | 13.6 [±6.4] | 0.015 | ||
| Low-SNOT-22 total score(< 20)18 | 16 (13%) | 4 (7%) | 0.270 | ||
ESS, endoscopic sinus surgery; N, sample size; SD, standard deviation; VA, Veteran’s Administration; AERD, aspirin exacerbated respiratory disease; COPD, chronic obstructive pulmonary disease; CF, cystic fibrosis; BSIT, Brief Smell Identification Test; SNOT-22, 22-item SinoNasal Outcome Test.
Post-treatment Improvement in Disease Severity Measures
Average post-treatment changes in disease severity measures were compared between both treatment groups (Table 3). Significantly greater mean improvements in total endoscopy scores were found for study participants electing to cross-over to ESS. Similarly, greater significant improvement in SNOT-22 total scores, as well as rhinologic, psychological dysfunction, and sleep dysfunction subdomain scores, were reported by those patients electing to cross over to ESS, compared to those electing continued medical therapy. For patients with follow-up, the prevalence of normal olfaction remained similar (p=0.383) from 63% in the CMT group at baseline to 69% after treatment. Similarly, the prevalence of normal olfaction remained similar (p>0.999) from 64% in the cross-over group at baseline to 64% after ESS in patients with follow-up.
Table 3.
Average post-treatment score changes in secondary outcomes between for study participants electing continued medical therapy or cross-over to endoscopic sinus surgery
| Continued medical therapy | Treatment cross-over to ESS | Between group significance | |
|---|---|---|---|
| Secondary Outcomes: | Mean [±SD] | Mean [±SD] | p-value |
| Endoscopy score | −2.4 [±4.0]* | −4.3 [±4.4]* | 0.047 |
| BSIT olfactory score | 0.7 [±3.3] | −0.1 [±3.0] | 0.328 |
| SNOT-22 total score: | −5.0 [±20.4]* | −15.5 [±23.1]* | 0.013 |
| Rhinologic symptom score | −2.4 [±7.4]* | −5.7 [±8.0]* | 0.013 |
| Extra-nasal rhinologic score | −1.1 [±4.0]* | −1.9 [±4.1]* | 0.252 |
| Ear/facial symptom score | −0.8 [±4.8] | −1.9 [±5.4]* | 0.269 |
| Psychological dysfunction score | −1.1 [±7.3] | −4.7 [±8.0]* | 0.019 |
| Sleep dysfunction score | −0.8 [±7.1] | −5.1 [±6.2]* | 0.001 |
ESS, endoscopic sinus surgery; SD, standard deviation; BSIT, Brief Smell Identification Test; SNOT-22, 22-item SinoNasal Outcome Test.
indicates significant within-subject improvement over time (p<0.050) as determined by Wilcoxon signed rank testing.
Low SNOT-22 score (<20) has been suggested as a cutoff below which ESS cannot reliably deliver clinically meaningful improvement16 For patients with follow-up, the prevalence of low SNOT-22 total scores (<20) significantly improved from 13% to 29% (p=0.004) in patients electing CMT. Similarly, the prevalence of low SNOT-22 total scores improved even more greatly in the cross-over group from 7% to 39% (p<0.001) after ESS.
Hazard Regression Estimations for Treatment Crossover
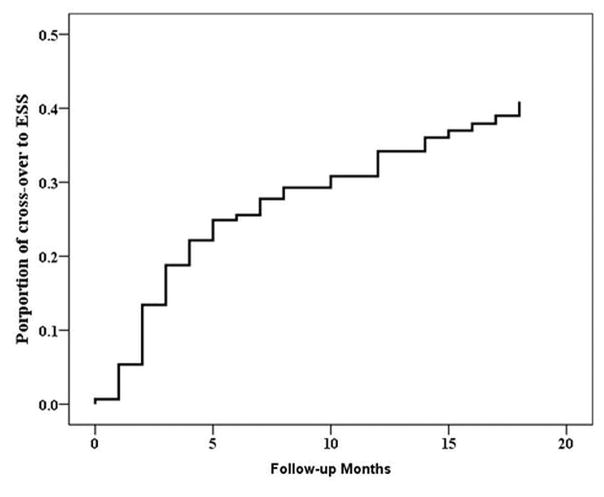
All cofactors listed in Table 2 were screened for univariate significance using Cox’s regression modeling to identify HR values associated with treatment cross-over from CMT during the total observed follow-up time. From all patients who initially elected CMT, the unadjusted HR of treatment cross-over to ESS for patients with a history of previous ESS was found to be 0.55 times (~50% less) than patients without a history of ESS (p=0.048). Additionally, the unadjusted effect of baseline endoscopy scores was found to be clinically meaningful (p=0.057); for each single increasing (worsening) point of endoscopy score, the hazard of treatment cross-over during the follow-up period increased by ~6%. Similarly, for every single point increase in baseline SNOT-22 total score, the hazard of cross-over increased by ~2% during follow-up; largely a product of significant hazard ratio increases associated with unadjusted SNOT-22 psychological subdomain scores (~4%; p=0.017) and sleep dysfunction subdomain scores (~7%; p=0.003). Overall, Kaplan-Meier survival curve estimates for the prevalence of patients electing ESS are provided in Figure 2.
Figure 2.
Kaplan-Meier estimates for treatment modality cross-over from continued medical therapy to endoscopic sinus surgery (ESS) for entire cohort with follow-up (n=149).
Multivariate Cox’s regression modeling was conducted with inclusion of all significant unadjusted covariates. After statistical exclusion criteria, the only cofactor remaining in the final model was the SNOT-22 sleep dysfunction subdomain scale. All other remaining cofactors were excluded at the 0.050 alpha level with substantial collinearity between all other disease severity measures. In fact, multivariate modeling found that previous sinus surgery was no longer significantly associated with treatment cross-over (p=0.105) if baseline sleep dysfunction scores were included in the final regression model. Overall, patients with worse baseline sleep dysfunction scores were significantly associated (χ2=11.38; p=0.023) with increasing prevalence of changing treatment modality to include ESS (Table 4).
Table 4.
The frequency of treatment cross-over across sleep dysfunction score category
| Sleep dysfunction scores: | Treatment modality cross-over |
|---|---|
| Category: | N (%) |
| 0–5 (n=37) | 7 (19%) |
| 6–10 (n=38) | 7 (18%) |
| 11–15 (n=51) | 17 (33%) |
| 16–20 (n=39) | 19 (49%) |
| 21–25 (n=14) | 5 (36%) |
DISCUSSION
Patients undergoing treatment for medically recalcitrant CRS will eventually decide between CMT and ESS. This decision is in part facilitated by a transparent discussion of the risks and benefits of both treatment options with their physician. Surgical outcomes have been thoroughly investigated and have historically identified that only a history of prior surgery is associated with lesser improvement after ESS.1 Unfortunately there is minimal reported data regarding failure rates of CMT to date. The present study seeks to elucidate the prevalence, likelihood and characteristics associated with patient groups who elect to escalate symptom management of CRS using ESS. Approximately 30% of patients who initially elected CMT went onto escalate care to involve ESS in this unique cohort. After adjustment for significant cofactors, only baseline sleep dysfunction subdomain scales on the SNOT-22 were significantly associated with a change in treatment modality, with an ~7% increasing risk of cross-over to ESS for every one point increase in baseline sleep domain dysfunction scale. This would suggest that for patients undergoing CMT, the greater the sleep dysfunction, the higher increased likelihood that they will eventually elect surgical intervention in the future.
Identification of sleep dysfunction as the only predictive baseline characteristic is consistent with a prior analysis of a subset of patients within this cohort electing medical versus surgical therapy at the time of enrollment.4,5 Patients initially electing ESS over CMT had comparable sinonasal symptoms (ie, rhinologic, extra-nasal rhinologic and ear/facial subdomains) to patients electing medical therapy, but carried a greater burden of symptoms in the psychological and sleep subdomains. The present study reinforces these findings in that worse baseline sleep symptoms are predictive of eventually electing sinus surgery in this patient group. Although initially disconcerting that subjects were electing ESS for psychological and sleep dysfunction, outcomes data have shown significant improvement in these subdomains after surgery, and there is emerging evidence on the mechanisms supporting the link between central nervous system dysfunction and chronic inflammation.22 Further establishing the underlying mechanisms linking sinonasal symptoms and general-health related outcomes may identify important targets for medical therapies.
This study is part of a continual effort to identify and clarify symptoms that patients prioritize and are motivated by to change treatment modalities over time. Understanding medical outcomes for CRS is critical for surgeons in order to facilitate patients who are navigating these treatment decisions. The vast majority of ESS is performed for a relative indication, and as such, patient-specific expectations for improvement are required for therapeutic decision-making. Yet this cohort demonstrates that patients with medically recalcitrant CRS that elect CMT improve only a minority of the time. An earlier analysis of the present cohort found that patients initially electing medical therapy, 32/117 (27%) subjects achieved a clinically significant improvement and an additional 28/117 (24%) maintained symptoms.21 The remaining subjects 60/117 (51%) either experienced worsening symptoms (n=18) or elected ESS (n=42). These findings were reinforced in a different cohort study of patients awaiting surgery (~7 months on average) in Canada23 which found during that wait time that patients experience on average an 8.5 point worsening in the SNOT-22 and an additional 3.6 mean lost work days. The current available evidence illustrates that the majority of patients with medically recalcitrant CRS continue to worsen the majority of the time.
There are some important limitations of the present study important to keep in context with its findings. Although there is a statistically significant difference between the sleep subdomains of the SNOT-22, definitions for what constitutes clinically meaningful differences have yet to be clarified for this population. No formal investigation into what constitutes an MCID for SNOT-22 subdomains has been undertaken to date, but based on a change of a half-standard deviation of baseline (1/2 SD of baseline sleep domain of crossovers ≥ 3.2, Table 2)24 a mean improvement of at least 5.1 after ESS is a clinically meaningful improvement by one distribution-based method. Further investigation into MCID of subdomains would help to further clarify if post-treatment differences in sleep dysfunction scores is discernible by patients across a larger cohort and with a variety of methodologies. Additional investigations using validated sleep instruments would also validate that sleep dysfunction and a patients’ decision to pursue ESS versus CMT are associated. Similarly, use of sleep-specific instruments across more frequent follow-up time points in the CMT cohort might allow for a wider range of patient responses with pre-defined categories of severity as well as a better understanding of the trajectory of sleep impairment and how that change impacts the decision to crossover. Regardless, it may be that sleep-related symptoms worsened between the time of enrollment and crossover to ESS. SNOT-22 evaluations were not administered at time of crossover for this investigation. Given the observational design there is concern for sources of unmeasured confounding as well. One concern about studying treatment selection, and in particular study of patients that cross-over to ESS, is that duration of symptoms and the temporality of symptom recurrence and/or worsening may be an important confounding cofactor. We did investigate duration of symptoms (data available in only 29 patients) and found that patients who did cross-over to ESS reported longer duration of symptoms, however not to a statistical threshold (Table 2; p=0.718), nor was symptom duration a significant, independent predictor of cross-over in hazard regression modeling. Further data collection would be required to clarify how symptom duration influences treatment modality selection in patients with CRS. Finally, although the present study reports an average follow-up of 15.1[±4.6] months, we cannot extrapolate the hazard ratios outside of this time range.
CONCLUSIONS
An understanding of both medical and surgical outcomes of CRS is necessary in order to facilitate an informed selection of either treatment modality. The present study clarifies the baseline characteristics that associate with eventually electing ESS. For each single increasing (worsening) point of endoscopy score, the hazard of treatment cross-over during the follow-up period increased by ~6%. Similarly, for every single point worsening in baseline SNOT-22 total score, the hazard of cross-over increased by ~2%. However, after multivariate regression modeling, only the baseline sleep dysfunction domain associated with treatment crossover. For every 1-point worsening in the baseline sleep domain there is a ~7% relative risk increase in crossover on the final adjusted model (HR=1.07; 95% CI:1.02–1.11;p=0.003). These findings may help patients and surgeons identify surgical candidates in a more timely fashion.
Acknowledgments
Financial Disclosures: Timothy L. Smith, Jess C. Mace, Vijay R. Ramakrishnan, and Jeremiah A. Alt are supported by a grant for this investigation from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (R01 DC005805; PI: TL Smith). Public clinical trial registration (www.clinicaltrials.gov) IDs: NCT01332136 and NCT02720653. This funding organization did not contribute to the design or conduct of this study; collection, management, analysis, or interpretation of the data; preparation, review, approval or decision to submit this manuscript for publication. Adam S. DeConde is a consultant for IntersectENT, (Menlo Park, CA). Adam S. DeConde is a consultant for Stryker Endoscopy (San Jose, CA) and Olympus. Jeremiah A. Alt and Vijay R. Ramakrishnan are consultants for Medtronic (Jacksonville, FL), none of which are affiliated with this investigation.
Footnotes
Potential Conflicts of Interest: None
References
- 1.Smith TL, Litvack JR, Hwang PH, et al. Determinants of Outcomes of Sinus Surgery: A Multi-Institutional Prospective Cohort Study. Otolaryngol Head Neck Surg. 2010;142(1):55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TL, Kern R, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. Int Forum Allergy Rhinol. 2013;3(1):4–9. doi: 10.1002/alr.21065. [DOI] [PubMed] [Google Scholar]
- 3.DeConde AS, Mace JC, Alt JA, Soler ZM, Orlandi RR, Smith TL. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2015;5(1):36–45. doi: 10.1002/alr.21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–6. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeConde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–9. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele TO, Rudmik L, Mace JC, DeConde AS, Alt JA, Smith TL. Patient-centered decision making: the role of the baseline SNOT-22 in predicting outcomes for medical management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(6):590–6. doi: 10.1002/alr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld RM, Andes D, Neil B, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical Practice Guideline (Update) Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg. 2015;152(4):598–609. doi: 10.1177/0194599815574247. [DOI] [PubMed] [Google Scholar]
- 10.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–4. [PubMed] [Google Scholar]
- 11.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 12.Doty RL, Mishra A. Olfaction and Its Alteration by Nasal Obstruction, Rhinitis, and Rhinosinusitis. Laryngoscope. 2001;111(3):409–23. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Rassi E, Mace JC, Steele TO, Alt JA, Soler ZM, Fu R, et al. Sensitivity analysis and diagnostic accuracy of the Brief Smell Identification Test in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(3):287–92. doi: 10.1002/alr.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 15.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140(8):712–9. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudmik L, Soler ZM, Hopkins C, et al. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: a RAND/UCLA appropriateness study. Int Forum Allergy Rhinol. 2016;6(6):557–67. doi: 10.1002/alr.21769. [DOI] [PubMed] [Google Scholar]
- 17.Levy JM, Mace JC, Rudmik L, Soler ZM, Smith TL. Low 22-item sinonasal outcome test scores in chronic rhinosinusitis: Why do patients seek treatment? Laryngoscope. 2017;127(1):22–8. doi: 10.1002/lary.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudmik L, Soler ZM, Mace JC, DeConde AS, Schlosser RJ, Smith TL. Using preoperative SNOT-22 score to inform patient decision for Endoscopic sinus surgery. Laryngoscope. 2015;125(7):1517–22. doi: 10.1002/lary.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy JM, Mace JC, Sansoni ER, Soler ZM, Smith TL. Longitudinal improvement and stability of olfactory function in the evaluation of surgical management for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(11):1188–1195. doi: 10.1002/alr.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deconde AS, Mace JC, Alt JA, Rudmik L, Soler ZM, Smith TL. Longitudinal improvement and stability of the SNOT-22 survey in the evaluation of surgical management for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(3):233–239. doi: 10.1002/alr.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steele TO, Rudmik L, Mace JC, DeConde AS, Alt JA, Smith TL. Patient-centered decision making: the role of the baseline SNOT-22 in predicting outcomes for medical management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(6):590–6. doi: 10.1002/alr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alt JA, Smith TL. Chronic rhinosinusitis and sleep: a contemporary review. Int Forum Allergy Rhinol. 2013;3(11):941–9. doi: 10.1002/alr.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(1):34–8. doi: 10.1002/alr.21238. [DOI] [PubMed] [Google Scholar]
- 24.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]