Abstract
Background
PhytoSERM, a preparation of genistein, daidzein, and S-equol, has an 83-fold selective affinity for estrogen receptor (ER) β and may promote neuronal survival and estrogenic mechanisms in the brain without exerting feminizing activity in the periphery.
Objective
To assess the safety, tolerability and single-dose pharmacokinetics of the phytoSERM formulation in peri- and postmenopausal women.
Methods
Eighteen women aged 45–60 years from a 12-week clinical trial evaluating cognitive performance and vasomotor symptoms were randomly assigned to placebo, 50mg, or 100mg phytoSERM treatment groups. Plasma levels of the 3 parent phytoestrogens and their metabolites were measured before and at 2, 4, 6, 8 and 24 hours after ingestion by isotope dilution HPLC electrospray ionization tandem mass spectrometry.
Results
Plasma concentrations of genistein, daidzein and S-equol peaked at 9, 6 and 4 h, respectively for the 50mg dose, and at 6, 6 and 5 h, respectively for the 100 mg dose. The maximum concentration (Cmax) and area under the curve (AUC) for the 3 parent compounds were greater in the 100 mg dose group indicating a dose-dependent change in concentration with the phytoSERM treatment. No adverse events were elicited.
Conclusion
A single-dose oral administration of the phytoSERM formulation was well tolerated and did not elicit any adverse events. It was rapidly absorbed, reached high plasma concentrations, and showed a linear dose- concentration response in its pharmacokinetics. These findings are consistent with previously reported parameters for each parent compound (Clinicaltrials.gov NCT02221622).
Keywords: phytoestrogens, pharmacokinetics, menopause, peri-menopause, cognitive impairment, estrogen receptor-beta
INTRODUCTION
The search for a safe approach to promote estrogenic signaling in the brain, without eliciting adverse effects,1–3 has focused on the development of tissue-selective estrogen receptor (ER) modulators (SERMs). In recent years, selective estrogen receptor β (ERβ) targeting has been attempted in the development of therapies for a range of conditions including cognitive impairment and menopausal symptoms. There are plausible mechanisms by which ERβ stimulation could lead to improved cognition, feelings of well-being, reduced risk for cognitive impairment, and improved vasomotor symptoms.4–9
We sought to develop a rationally designed formulation composed of selected ERβ-selective phytoestrogens (phytoSERMs) that could provide greater cognitive or vasomotor effect than various nutraceuticals that are blended with both ERα and ERβ modulators. The phytoSERM formulation exhibits an 83-fold selectivity for ERβ over ERα, and induces synergistic rather than antagonistic effects on various neuronal outcomes.10 The formulation enhances ERβ-mediated responses by combining S-equol, genistein, and daidzein in equal parts. Equol in the phytoSERM formulation serves the purpose of moderating the potential influences of inter-individual differences in the metabolism of daidzein by intestinal bacteria.
Various studies have reported on the pharmacokinetics of the individual components of the phytoSERM formulation or a combination of daidzein and genistein only.11–14 Similar to steroidal estrogens, isoflavones undergo enterohepatic circulation and appear in the bile soon after oral administration. The typical biphasic appearance of isoflavone metabolites in plasma following ingestion suggests that they are initially absorbed in the duodenum, and proximal jejunum, and then the colon.15–17 We expected the pharmacokinetics of daidzein and genistein in this combination to be similar to each of these isoflavones alone. We expected also a prolonged apparent elimination half-life with S-equol since about 25–30% of participants likely will convert daidzein to equol by intestinal bacteria18 which should start to appear in plasma at 6 to 8 hours after daidzein intake.
We report here on the single-dose pharmacokinetics, acute tolerability, and adverse events of the phytoSERM formulation in peri- and postmenopausal women. Such knowledge is important in determining the characteristics and optimal doses of this phytoSERM formulation and serves as a stepping stone for the design of future clinical trials aimed at assessing its safety and effectiveness for cognitive and functional improvement.
METHODS
Study product and doses
Study products included 50 mg, 100 mg of phytoSERM-, or placebo- containing film-coated tablets composed of equal amounts of genistein, dadzein, and S-equol, or placebo. Thus the 50 mg tablet contained 16.7 mg of each of genistein, daidzein, and S-equol; and the 100 mg tablet contained 33.3 mg of each constituent.
The phytoSERM combination and identically-appearing and tasting placebo tablets were manufactured to cGMP standards and supplied by MeriCal, Inc, Vista, California. Each active supplement raw material and excipient was catalogued, tested for identification using FTIR instrumentation, and verified for potency by Certificate of Analysis. All ingredients were weighed and blended according to a detailed formulation sheet, and compressed into tablet form (oval; 8.5mm×15.5mm).
Dosage selection was based on preclinical experiments,10,19,20 past studies of isoflavones individually and combined, and the dosage used in various soy extract products that are marketed as food supplements regarding safety and chronic exposure which are generally between 50mg and 100mg of isoflavones.6,21,22
Study design
The first 18 participants of 71 randomized into a dose-ranging, double-blinded, placebo-controlled 12-week clinical trial evaluating cognitive performance and vasomotor symptoms (Clinicaltrials.gov NCT 01723917) were included in this single-dose, 24-hour pharmacokinetics study. Six women were included in each of the three treatment groups (Table 1). Eligible participants were generally healthy peri- to postmenopausal women, ages 45 to 60 years, with intact uteri and ovaries, whose last natural menstrual cycle completed from at least 2 months to less than 4 years prior to screening. Participants had at least one cognitive complaint and one vasomotor-related symptom based on the Memory Function Questionnaire and Greene Climacteric Scale.23,24
Table 1.
Participant demographics
| Placebo | 50 mg | 100 mg | P-value | |
|---|---|---|---|---|
|
| ||||
| N | 6 | 6 | 6 | |
|
| ||||
| Age, y mean (SD) | 54.0 (2.2) | 51.7 (2.9) | 55.3 (3.8) | 0.14* |
|
| ||||
| BMI, kg/m,2 mean (SD), | 26.3 (8.1) | 27.9 (4.9) | 28.3 (3.5) | 0.81* |
|
| ||||
| Years since last menstrual period, mean (SD) | 3.2 (1.3) | 1.5 (0.9) | 2.1 (1.6) | 0.11* |
|
| ||||
| Race, n (%) | ||||
| Asian | 0 | 1 (16.7) | 2 (33.3) | 0.62** |
| African American | 1 (16.7) | 1 (16.7) | 1 (16.7) | |
| White | 5 (83.3) | 3 (50.0) | 2 (33.3) | |
| Unknown | 0 | 1 (16.7) | 1 (16.7) | |
|
| ||||
| Hispanic, n (%) | ||||
| Yes | 1 (16.7) | 1 (16.77) | 2 (33.3) | 0.72** |
| No | 5 (83.3) | 5 (83.3) | 4 (66.7) | |
ANOVA,
Fisher’s exact test
For this pharmacokinetic study, participants were asked to abstain from food containing soy for at least two days prior to the baseline visit and their taking the first tablet. Participants swallowed 1 tablet in the morning, in the presence of the study staff. Blood samples were obtained before and 2, 4, 6, 8, and 24 hours after administration of the first dose. Blood was obtained by venipuncture and collected into 6 ml lithium-heparin Vacutainer tubes. Plasma was collected after centrifugation at 1000×g for 20 min at 4°C. Aliquots were catalogued and then stored at −80°C in a designated laboratory at the USC School of Pharmacy before being shipped for analysis. All study participants were monitored and observed for any adverse events or complaints during the first 8 hours and at 24 hours after the single tablet. Safety assessments included vital signs and direct observation of any new signs or symptoms emerging during this time interval. Participants then continued in the randomized trial taking a tablet of study medication per day for 12 weeks.
Plasma phytoestrogen assays and pharmacokinetic analysis
Plasma concentrations of genistein, dihydrogenistein (DHGE), daidzein, dihydrodaidzein (DHDE), O-demethylangolensin (DMA), and equol were analyzed by a previously established method using isotope dilution high performance liquid chromatography (HPLC) electrospray ionization (negative mode) tandem mass spectrometry at the University of Hawaii Cancer Center.25 Limits of quantitation (LOQ) for all analytes were 5nM for genistein, DHGE, daidzein, DHDE and DMA, and 20nM for equol. All 108 samples (18 participants with 6 samples each) were processed in one batch to limit variability. Between-day coefficients of variation ranged between 4–18% for all analytes, while intraday variation was no more than half of that.
Statistical analysis
Characteristics of the study sample were summarized and compared between the three dose groups using ANOVA for continuous variables and Fisher’s exact test for categorical variables. Using the subject-level time-concentration data for each analyte, pharmacokinetic parameters were calculated for each subject: Cmax (peak plasma concentration), tmax (time at peak plasma concentration), AUC (area under the time-concentration curve) and t1/2 (terminal elimination half-life). AUC was calculated over 0–24 hours using the trapezoidal rule to estimate the AUC between sampling times in the terminal part of the curve. The t1/2 parameter was estimated as ln(2) divided by the elimination rate; the elimination rate was calculated as the linear regression slope of log concentration versus sampling time fit over the last 3 sampling times (6, 8, 24 hours). The PK parameters were summarized and compared between the 50 mg phyto-β-SERM, 100 mg phyto-β-SERM, and placebo groups using non-parametric Kruskal-Wallis tests; p-values for post hoc pairwise comparisons were adjusted for multiplicity using a Hochberg correction. All analyses used the PK and other statistical modules of STATA Version 13 software ((StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
RESULTS
Demographics of study participants
Eighteen women were enrolled in the PK study and compliance was 100%. The three groups had similar characteristics (Table 1). The overall mean age was 53 years, and the overall mean BMI was 26.15. The majority was of non-Hispanic ethnicity (78%) and the number of years since their last menstrual period ranged from means 1.5 to 3.2 per group. Demographics of the individual dose groups are described in Table 1.
Plasma genistein, daidzein, and equol pharmacokinetics
A summary of the pharmacokinetic data is presented in Table 2. The plasma concentrations for the 3 parent isoflavones after a single oral administration of the phytoSERM tablet in both the 50mg and 100mg cohorts are shown in Figure 1A and 2B, respectively and as log-linear plots in Figures 1B and 2B. The 50 mg dose of the phytoSERM preparation yielded (mean ± SD) a Cmax of 570 ±143 nM, a Tmax of 9 ± 3 hours, and an elimination phase half-life of 7.5 ± 1.1 hours for genistein; a Cmax of 622 ±128 nM, a Tmax of 6 ± 1 hour, and an elimination phase half-life of 8.1 ± 1.2 hours for daidzein; and a Cmax of 781.2 ±123 nM, a Tmax of 4.3±1 hour, and an elimination phase half-life of 8.9±2.1 hours for equol.
Table 2.
Plasma pharmacokinetics determined after a single dose of placebo, 50 mg or 100 mg phytoSERM tablets
| Placebo* | 50 mg | 100 mg | P-value | Placebo vs 50 mg |
50 mg vs 100 mg |
Placebo vs 100 mg |
|
|---|---|---|---|---|---|---|---|
| N | 6 | 6 | 6 | ||||
| Genistein (mean ± SEM, nM) | 4.1 ± 0.5 | 282.2 ± 44.7 | 673.3 ± 83.4 | ||||
| tmax (h) | 17.3 ± 4.3 | 9.0 ± 3.0 | 6.0 ± 0.7 | 0.17 | 0.31 | 0.61 | 0.21 |
| Cmax (nM) | 8.2 ± 2.0 | 570.4 ± 142.6 | 1147.3 ± 167.7 | 0.0013 | 0.008 | 0.037 | 0.008 |
| AUC (nM · h) | 138.1 ± 47.1 | 5248.4 ± 800.7 | 16747.6 ± 6292.3 | 0.0013 | 0.008 | 0.037 | 0.008 |
| t1/2 (h) | 20.5 ± 11.4 | 7.5 ± 1.1 | 14.5 ± 4.1 | 0.32 | 0.29 | 0.29 | 032 |
| Dihydrogenistein (mean ± SEM, nM) | 2.5 ± 0.00 | 26.4 ± 7.8 | 26.1 ± 8.3 | ||||
| tmax (h) | 24.0 ± 0.00 | 10.7 ± 2.7 | 15.3 ± 3.9 | 0.073 | 0.015 | 0.86 | 0.12 |
| Cmax (nM) | 2.5 ± 0.00 | 83.6 ± 31.3 | 79.3 ± 32.6 | 0.019 | 0.015 | 0.81 | 0.015 |
| AUC (nM · h) | 60.0 ± 0.00 | 1848.4 ± 736.8 | 682.0 ± 455.7 | 0.042 | 0.022 | 0.23 | 0.22 |
| t1/2 (h)** | . | 9.6 ± 2.6 | 12.3 ± 7.7 | 0.88 | . | 0.88 | . |
| Daidzein (mean ± SEM, nM) | 9.1 ± 2.1 | 311.6 ± 45.2 | 612.2 ± 83.7 | ||||
| tmax (h) | 8.7 ± 4.9 | 6.0 ± 0.5 | 6.3 ± 0.6 | 0.57 | 0.65 | 0.65 | 0.65 |
| Cmax (nM) | 26.8 ± 9.6 | 622.8 ± 127.7 | 1146.1 ± 176.6 | 0.001 | 0.008 | 0.025 | 0.008 |
| AUC (nM · h) | 260.0 ± 92.9 | 6629.3 ± 1432.4 | 14683.5 ± 2763.9 | 0.0015 | 0.008 | 0.055 | 0.008 |
| t1/2 (h) | 36.6 ± 14.5 | 8.1 ± 1.2 | 13.1 ± 4.5 | 0.097 | 0.08 | 0.71 | 0.24 |
| Dihydrodaidzein (mean ± SEM, nM) | 2.8 ± 0.3 | 34.8 ± 7.3 | 55.1 ± 15.4 | ||||
| tmax (h) | 24.0 ± 0.0 | 13.3 ± 3.4 | 18.0 ± 3.8 | 0.21 | 0.06 | 0.73 | 0.28 |
| Cmax (nM) | 4.5 ± 2.0 | 107.9 ± 22.6 | 162.3 ± 46.4 | 0.008 | 0.008 | 0.26 | 0.026 |
| AUC (nM · h) | 71.0 ± 11.0 | 2749.0 ± 654.0 | 2020.1 ± 955.5 | 0.026 | 0.008 | 0.42 | 0.29 |
| t1/2 (h) | . | 24.6 ± 14.4 | 3.8 ± 0.8 | 0.08 | 0.08 | . | |
| O-demethylangolensin (mean ± SEM, nM) | 3.4 ± 0.9 | 11.0 ± 5.0 | 41.6 ± 14.9 | ||||
| tmax (h) | 24.0 ± 0.00 | 21.3 ± 2.7 | 18.7 ± 1.4 | 0.62 | 0.52 | 0.52 | 0.41 |
| Cmax (nM) | 8.2 ± 5.7 | 41.8 ± 28.4 | 125.5 ± 63.7 | 0.009 | 0.07 | 0.07 | 0.014 |
| AUC (nM · h) | 91.8 ± 91.8 | 389.4 ± 176.8 | 2085.4 ± 1362.1 | 0.007 | 0.055 | 0.055 | 0.014 |
| t1/2 (h)** | . | . | 55.2 ± 42.1 | . | . | . | . |
| Equol (mean ± SEM, nM) | 10.0 ± 0.0 | 395.6 ± 55.2 | 986.4 ± 128.3 | ||||
| tmax (h) | 24.0 ± 0.0 | 4.3 ± 0.6 | 4.7 ± 0.8 | 0.003 | 0.004 | 0.86 | 0.004 |
| Cmax (nM) | 10.0 ± 0.0 | 781.2 ± 123.3 | 1928.2 ± 172.1 | <0.001 | 0.004 | 0.004 | 0.004 |
| AUC (nM · h) | 240.0 ± 0.0 | 6510.6 ± 1494.9 | 17567.4 ± 4248.9 | 0.0013 | 0.004 | 0.037 | 0.004 |
| t1/2 (h) | . | 8.8 ± 2.1 | 7.1 ± 1.2 | 0.86 | 0.86 | . | |
Values are means ± SEMs; p-values for overall group comparisons used Kruskal-Wallis test; pairwise comparisons used Wilcoxon rank sum tests, with Hochberg corrections for multiple comparisons. Abbreviations: tmax: time to reach maximum concentration, Cmax: maximum plasma concentration, AUC: total area under the plasma concentration-time curve, t1/2: terminal elimination half-life
For the placebo group the PK parameters values may reflect background dietary intake of soy products
t1/2 of metabolites may be driven by formation rate and not elimination processes.
Figure 1.


(A) Plasma appearance and disappearance curves for daidzein, genistein, and equol in healthy peri- and post-menopausal women after single oral intake of 50 mg of phytoSERM.
(B) Log/linear plots of plasma concentrations of daidzein, genistein, and equol after a single oral dose of 50 mg phytoSERM combination illustrating the charateristic linear slope seen in first-order elimination kinetics. Values are means ± SE, n=18
Figure 2.
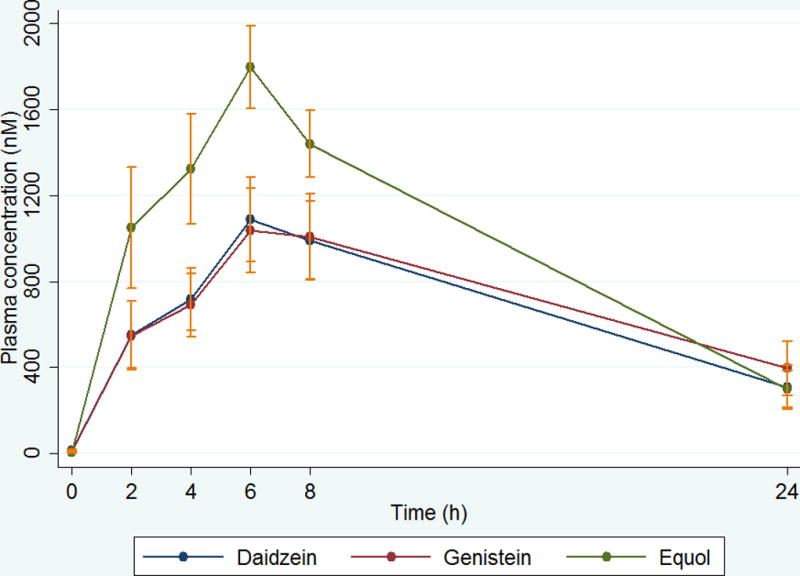

(A) Plasma appearance and disappearance curves for daidzein, genistein, and equol in healthy peri- and post-menopausal women after single oral dose of 100 mg of phytoSERM.
(B) Log/linear plots of plasma concentrations of daidzein, genistein, and equol after a single oral dose of 100 mg phytoSERM combination illustrating the charateristic linear slope seen in first-order elimination kinetics. Values are means ± SE, n=18
There was a dose-dependent difference in the plasma concentrations for the 3 parent compounds. The areas under the curve (AUC) for genistein, daidzein and equol were significantly greater in the 100 mg dose (all p<0.025) indicating a dose-proportional change in concentration with the phytoSERM treatment. The time to reach maximum concentration (Tmax) varied among the 3 constituents from 4 to 9 hours and was not dose-dependent. Elimination half-life for all 3 phytoestrogens did not significantly differ between the 50 mg and 100 mg doses. The log-linear plots of plasma concentration time curves during the 24-hour sampling period for the 3 parent compounds of both the 50 mg and 100 mg doses exhibited a linear slope for the terminal portion of the curve demonstrating that elimination follows first-order kinetics (Figures 1B and 2B).
We provided the plasma appearance and disappearance curves for specific phytoestrogens for each participant in each cohort (placebo, 50mg dose and 100mg dose) in the supplemental digital content. These data show both the inter-individual heterogeneity of the pharmacokinetics of the phytoSERM formulation, and the clear dose-related plasma level differences in the parent compounds (see Supplemental Digital Content 1).
Safety
No treatment emergent adverse events or complaints were observed or elicited after the single dose oral administration of the phytoSERM combination during the 24-hour study period. No signs and symptoms of any adverse events were reported by the 18 participants during the 24-hour period.
DISCUSSION
Widely marketed dietary phytoestrogen supplements contain genistein and daidzein alone or in combination but do not contain s-equol, which makes this phytoSERM formulation unique. Genistein and daidzein occur natively in soy products but s-equol is exclusively produced through the metabolism of daidzein by intestinal microbiota following the intake of daidzein or daidzein-containing products including soy.11 Wide variations exist across populations in the ability to produce equol from daidzein; about 25–35% of Western adults have an equol-producing phenotype compared to 50–65% of Asian populations.26–28 Adding equol to this phytoSERM preparation is a strategy to minimize such individual variations and to ensure an effect in both phenotypes. The development of the phytoSERM combination is based on a foundation of pre-clinical science indicating that the equimolar combination of genistein, daidzein, and s-equol can function as an efficacious phytoestrogen combination to selectively target ERβ.10,20
The phytoSERM combination showed a clear dose proportional increase in concentration exposures in healthy peri- and post-menopausal women with cognitive complaints. After oral administration, genistein, daidzein and equol rapidly appeared in plasma and reached maximum concentrations between 4 and 6 h after ingestion. In contrast to previous studies that reported concentrations of genistein being consistently higher than those of daidzein,12,15,29 plasma concentrations of genistein were about the same as those of daidzein in our study. Equol, however, showed higher concentrations at each time point than either genistein or daidzein. This was probably due to a two-fold exposure to S-equol, primarily by its presence in the oral formulation, and secondarily by its formation through the gut bacteria from dihydrodaidzein in a minority of participants.
Plasma concentration appearance and disappearance curves of genistein, daidzein and s-equol are similar to those of previous studies.11,12,15,25,30 The elimination half-life of approximately 8 hours for all 3 components in the 50 mg cohort is also consistent with other reports. 11,12 The elimination half-life for genistein and daidzein in the 100 mg cohort, however, was about twice as long, 14.5 and 13.1 h, respectively (the t1/2 for equol may be confounded by daidzein metabolism in the gut). The near tripling of the AUC with the 100mg genistein high dose and the decrease in the dihydrogenistein and dihydrodaidzein metabolites suggests that CYP 1A2, required for metabolism of genistein and daidzein is saturated at this dose.
As described previously,31 the plasma equol concentration appearance and disappearance curve suggested that it had a much higher bioavailability and a slower elimination than genistein and daidzein. Equol was absorbed more rapidly at both doses than genistein and daidzein, showing a tmax of about 4.5 hours compared to about 9 h and 6 h for genistein and daidzein respectively. At 2 hours the plasma concentration of S-equol was about twice that of genistein and daidzein. Such rapid appearance of S-equol in plasma is consistent with previous reports.11,32 Pharmacokinetic parameters for s-equol were similar to the ones reported in previous studies 11,22,33,34 wherein dosages were used that ranged from 10 – 30 mg and showed tmax ranges of 1 – 6 hours, a t1/2 range of 4.9–8.2 hours and Cmax ranging widely from 991±129 to 4,953±747 nM/L. Differences in tmax and Cmax values between the PhytoSERM combination and other studies may be accounted for by differences among the formulations and whether they were administered with or without meals.
CONCLUSION
The phytoSERM combination was well-tolerated, appeared without adverse effects, and exhibited a favorable pharmacokinetic profile. After single oral administration of 50 and 100mg tablets of the formulation the phytoestrogens genistein, daidzein and S-equol were rapidly absorbed, reached detectable plasma concentrations, and showed a dose proportional increase in concentration exposures in their pharmacokinetics. The formulation may prove to be advantageous for several peri- and post-menopausal conditions.
Supplementary Material
Supplemental Figure 1. Individual plasma appearance and disappearance curves for daidzein (DE).
Supplemental Figure 2. Individual plasma appearance and disappearance curves for: dihydrogenistein (DHGE).
Supplemental Figure 3. Individual plasma appearance and disappearance curves for genistein (GE).
Supplemental Figure 4. Individual plasma appearance and disappearance curves for dihydrodaidzein (DHDE).
Supplemental Figure 5. Individual plasma appearance and disappearance curves for O-demethylangolensin (DMA).
Supplemental Figure 6. Individual plasma appearance and disappearance curves for equol (EQ).
Acknowledgments
Sources of funding: Funding for this work was provided by the National Institute on Aging through, NIH R01 AG033288 and NIH P50 AG05142. Coral Street Partners facilitated sourcing and synthesis of PhytoSERM components.
Footnotes
Conflicts of interest: None reported.
The SDC contains the following figures of plasma appearance and disappearance curves for specific phytoestrogens for each participant in each cohort (placebo, 50mg dose and 100mg dose).
References
- 1.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. Jama. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. The New England journal of medicine. 2007;356:1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Brinton RD. Estrogen receptor beta as a therapeutic target for promotion of neurogenesis and prevention of neurodegeneration. Drug Development Research. 2006;66:103–17. [Google Scholar]
- 5.Zhao L, Brinton RD. In search of estrogen alternatives for the brain. In: Hogervorst E, Henderson VW, Gibbs RB, Brinton RD, editors. Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge, UK: Cambridge University Press; 2009. pp. 93–100. [Google Scholar]
- 6.Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert review of neurotherapeutics. 2007;7:1549–64. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, Brinton RD. Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. Journal of medicinal chemistry. 2005;48:3463–6. doi: 10.1021/jm0490538. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150:770–83. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- 11.Setchell KD, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(−)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–43. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 12.Setchell KD, Faughnan MS, Avades T, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–9. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 13.Metzner JE, Frank T, Kunz I, Burger D, Riegger C. Study on the pharmacokinetics of synthetic genistein after multiple oral intake in post-menopausal women. Arzneimittelforschung. 2009;59:513–20. doi: 10.1055/s-0031-1296435. [DOI] [PubMed] [Google Scholar]
- 14.Bloedon LT, Jeffcoat AR, Lopaczynski W, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76:1126–37. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 15.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 16.Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. The British journal of nutrition. 2003;89:S45–58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 17.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutrition and cancer. 2004;50:141–54. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 18.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–62S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Mao Z, Chen S, Schneider LS, Brinton RD. Early Intervention with an Estrogen Receptor beta-Selective Phytoestrogenic Formulation Prolongs Survival, Improves Spatial Recognition Memory, and Slows Progression of Amyloid Pathology in a Female Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2013;37:403–19. doi: 10.3233/JAD-122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Mao Z, Schneider LS, Brinton RD. Estrogen receptor beta-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause. 2011;18:1131–42. doi: 10.1097/gme.0b013e3182175b66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–37. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–90. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 24.Greene JG. Constructing a standard climacteric scale. Maturitas. 1998;29:25–31. doi: 10.1016/s0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 25.Franke AA, Halm BM, Kakazu K, Li X, Custer LJ. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Test Anal. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–93. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 27.Akaza H, Miyanaga N, Takashima N, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Japanese journal of clinical oncology. 2004;34:86–9. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 28.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. Journal of epidemiology / Japan Epidemiological Association. 2000;10:127–35. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 29.Franke AA, Lai JF, Halm BM. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Archives of Biochemistry and Biophysics. 2014;559:24–8. doi: 10.1016/j.abb.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setchell KD, Brzezinski A, Brown NM, et al. Pharmacokinetics of a slow-release formulation of soybean isoflavones in healthy postmenopausal women. Journal of agricultural and food chemistry. 2005;53:1938–44. doi: 10.1021/jf0488099. [DOI] [PubMed] [Google Scholar]
- 31.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363S–8S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson RL, Greiwe JS, Desai PB, Schwen RJ. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor -agonist being developed for the treatment of menopausal symptoms. Menopause. 18:185–93. [PubMed] [Google Scholar]
- 33.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 34.Setchell KD, Clerici C, Lephart ED, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Individual plasma appearance and disappearance curves for daidzein (DE).
Supplemental Figure 2. Individual plasma appearance and disappearance curves for: dihydrogenistein (DHGE).
Supplemental Figure 3. Individual plasma appearance and disappearance curves for genistein (GE).
Supplemental Figure 4. Individual plasma appearance and disappearance curves for dihydrodaidzein (DHDE).
Supplemental Figure 5. Individual plasma appearance and disappearance curves for O-demethylangolensin (DMA).
Supplemental Figure 6. Individual plasma appearance and disappearance curves for equol (EQ).


