Key Clinical Message
Esophageal inflammatory myofibroblastic tumors (IMT) are extremely rare, and the understanding on the clinical presentation is limited. IMT of esophagus should be considered as a differential diagnosis in the context of unexplained upper gastrointestinal bleeding and rapidly progressing dysphagia in young patients.
Keywords: Esophageal inflammatory myofibroblastic tumors, hematemesis and melaena, upper gastrointestinal bleeding
Introduction
Inflammatory myofibroblastic tumors (IMT), also known as plasma cell granuloma or fibroxanthomas, are mesenchymal tumors 1. It occurs mostly in young adults and has a slight male preponderance (female to male‐1:1.4) 1. Although IMTs are generally benign, they are classified as intermediate tumors because of a rare possibility of recurrence. It is composed of multiple myofibroblastic spindle cells with an inflammatory infiltrate of lymphocytes, plasma cells, and eosinophils 1. It can occur anywhere in the body and are most commonly known to occur in the lungs. Other rare sites are brain, orbit, spinal meninges, breast, thyroid gland, heart, trachea, spleen, kidneys, liver, stomach, colon, ampulla of Vater, omentum, and retroperitoneal structures 2, 3. The occurrence of IMT in the esophagus is extremely rare, and only few cases have been reported 1, 2. We report a 33‐year‐old man who presented with hematemesis and melaena and was diagnosed to have a rapidly enlarging esophageal IMT, which was successfully managed with surgery.
Case Report
A 33‐year‐old otherwise healthy man presented with an episode of hematemesis and melena. He had no history of swallowing difficulty or weight loss, and the physical examination was unremarkable. He was evaluated with upper and lower gastrointestinal endoscopy which did not reveal any significant findings except few gastric fundal erosions and a small mucosal bulge in the midesophagus with intact overlying mucosa.
Three weeks later, he developed progressive dysphagia. The upper gastrointestinal endoscopy was repeated, and it showed a significant mucosal bulge with intact overlying mucosa obstructing the lumen along the upper half of the esophagus. CT (computed tomography) scan of the neck, thorax, and abdomen revealed a well‐defined, longitudinally oriented, nonenhancing lesion extending from the level of C7 to T9 (Fig. 1). Endoscopic ultrasound examination found a well‐demarcated submucosal tumor, and the histological analysis of the endoscopic ultrasound‐guided fine needle aspiration cytology (FNAC) specimen showed the presence of necrotic tissue only and therefore did not help in the diagnosis.
Figure 1.
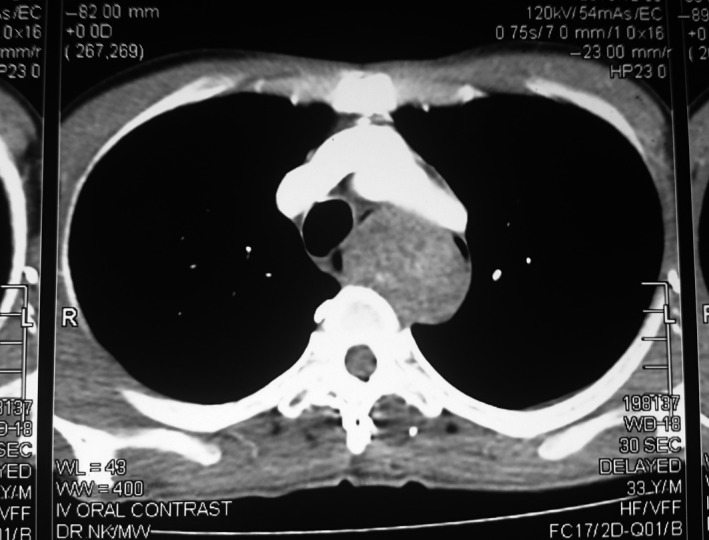
CT scan showing a well‐defined, longitudinally oriented, nonenhancing lesion displacing the lumen of the esophagus.
The patient was prepared for a total esophagectomy or excision of the tumor through cervical esophagotomy after a multidisciplinary team discussion involving cardiothoracic, radiology, pathology, and oncology teams. The patient was placed in reverse Trendelenburg position with the neck rotated to the right. An incision along the anterior border of the left sternomastoid muscle was made, and the investing layer of deep fascia was incised. Esophagus was identified and mobilized circumferentially with blunt dissection while preserving the recurrent laryngeal nerve. A transverse esophagotomy was made proximal to the lesion. The overlying mucosa was normal. A benign‐looking pedunculated polyp (length‐ 7 cm, width‐ 1 cm at the base and 3 cm at the tip) with a short pedicle was noted in the cervical and upper part of the thoracic esophagus. The lesion was located in the submucosal layer with its pedicle blending with the muscular coat and was easily dissected. Excision of the polypoidal lesion with 1 cm margin was carried out. Esophagotomy was closed transversely. As the imaging and operative findings were strongly in favor of a benign lesion, we did not proceed with an esophagectomy.
The histological analysis of the resected specimen revealed a spindle cell tumor overlined by stratified squamous epithelium, and the resection margin was free of tumor. There were scattered lymphocytes, plasma cells, and mast cells in the stroma. Immunohistochemical markers including CD 117, SMA, and desmin were negative, and Ki 67 proliferative index was 5%. Thus, the morphological and immunohistochemical features were in favor of esophageal IMT (Fig. 2).
Figure 2.
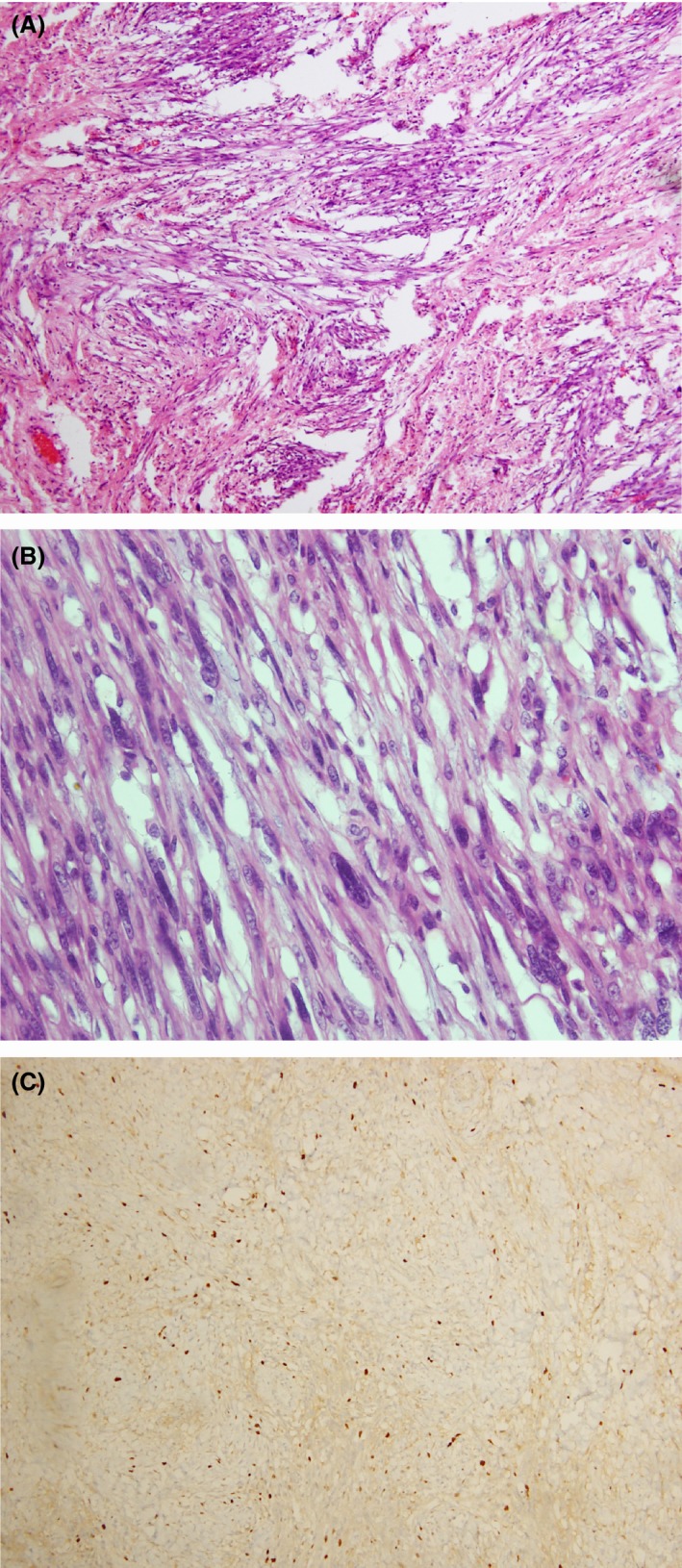
(A–C) Histology of inflammatory myofibroblastic tumor of esophagus. (A) H&E staining viewed under x 10 showing aggregate of spindle‐shaped cells with inflammatory infiltrate. (B) H&E staining viewed under x 40 showing spindle‐shaped cells with elongated mildly hyperchromatic nuclei and pale cytoplasm. (C) Immunohistochemical analysis showing Ki 67 proliferative index of 5%.
Postoperatively, the patient developed a transient left unilateral vocal cord palsy which improved with speech therapy. Oncological advice was to follow up with an upper GI endoscopy after 6 months and thereafter annually. After a follow‐up period of 20 months, patient is symptom free and there was no endoscopic evidence of recurrence.
Discussion
Inflammatory myofibroblastic tumors is defined as a lesion composed of multiple myofibroblastic spindle cells with associated inflammatory infiltrate of lymphocytes, plasma cells, and eosinophils. It is known to occur primarily in the viscera and soft tissue of children and young adults 1. They are now considered as intermediate‐grade tumor with a potential for recurrence 4. The etiology of IMT is controversial and thought to be an aberrant response to tissue injury 4. The commonly identified causes of IMTs include Epstein–Barr virus, human herpes virus eight, reflux, trauma, and overexpression of interleukin 6 4.
Inflammatory myofibroblastic tumors occurring in esophagus is extremely rare, and only few cases have been reported in literature 3. Dousek et al. 3 reported a similar case in a 13‐year‐old boy with near‐complete obstruction of the esophagus requiring radical surgery. Several associations of IMT of esophagus were reported which were chemotherapy, esophageal variceal sclerotherapy, gastroesophageal reflux, trauma, coexisting malignant tumor, and stem cell transplantation 3. However, there was no sufficient evidence to suggest a definite causative agent. The previously reported cases presented with a long‐standing history of dysphagia 2, 3, 4, 5, 6. Chen and colleagues reported a 55‐year‐old woman who presented with progressive dysphagia for 1 year and was found to have a large IMT of the esophagus 7. However, in our patient, the initial presentation was hematemesis and melaena, which is a rare clinical manifestation of IMT of the esophagus. Livolsi et al. 5 reported a 45‐year‐old woman who presented with hematemesis and melaena and was later diagnosed to have an IMT of the esophagus. However, she had esophageal varices secondary to cirrhosis which was the likely cause for the presentation, and the tumor was probably an incidental finding. In the present case, the patient subsequently developed progressive dysphagia over a few weeks and was found to have a significant tumor obstructing the lumen. This is different to previously reported cases where a gradual progression of symptoms was noted 2, 3, 4, 5, 6.
Diagnostic difficulties in relation to esophageal IMT have been reported in literature, and most tumors were diagnosed following resection. Endoscopic and radiological examinations can identify the lesion, but precise taxonomic diagnosis is often confusing 3. Endoscopic ultrasound is the investigation of choice which usually shows a hypoechoic lesion in the muscularis propria. However, the findings were correlated with histopathology in only 77% of cases 8. Accurate preoperative tissue biopsy is often difficult due to the intramural submucosal location and the necessity for examination of multiple areas of the tumor 3.
In a discussion of a case report, Privette et al. 6 recommended that partial or total esophagectomy, should be the treatment of choice for large (>2.5 cm) or obstructing esophageal IMTs. Limited procedures, such as enucleation via esophagotomy and endoscopic excision, were also reported 2, 9. If the resection of IMT is complete, adjuvant therapy is not needed but close follow‐up would be necessary3.
Conclusion
In conclusion, esophageal IMT is extremely rare and can vary greatly in terms of clinical presentation. We reported a 33‐year‐old man who presented with hematemesis and melaena with rapidly progressive difficulty in swallowing. Thus, IMT of the esophagus should be considered as a differential diagnosis in the context of unexplained upper gastrointestinal bleeding and rapidly progressing dysphagia in young patients. Complete surgical resection and routine follow‐up are necessary for successful management.
Consent
Informed written consent was obtained from the patient for publication prior to collecting information.
Authorship
UJ, RPB, and DMSH: contributed to collection of information and wrote the manuscript. DNS: contributed to writing and approved the final manuscript.
Conflict of Interest
Authors declare that they have no conflict of interests.
Acknowledgments
The authors wish to thank Prof. Preethika Angunawela, Department of Pathology, Faculty of Medicine, University of Colombo, Sri Lanka, for the histology images.
Clinical Case Reports 2018; 6(1): 82–85
References
- 1. Coffin, C. M. , Watterson J., Priest J. R., and Dehner L. P.. 1995. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am. J. Surg. Pathol. 19:859–872. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Ruiz, M. , Gonzalez‐Ibarra F., Diaz‐Becerril L., and Sanchez‐Mora C.. 2013. Inflammatory myofibroblastic tumor of the esophagus treated by endoscopy. Dis. Esophagus 26:323–326. [DOI] [PubMed] [Google Scholar]
- 3. Dousek, R. , Tuma J., Planka L., Husek K., Sterba J., and Penka I.. 2015. Inflammatory myofibroblastic tumor of the esophagus in childhood: a case report and a review of the literature. J. Pediatr. Hematol. Oncol. 37:e121–e124. [DOI] [PubMed] [Google Scholar]
- 4. Khakural, P. , Sapkota R., Shrestha U. K., and Sayami P.. 2015. Successful surgical management of a rare esophageal inflammatory myofibroblastic tumour: a case report. J. Cardiothorac. Surg. 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LiVolsi, V. A. , and Perzin K. H.. 1975. Inflammatory pseudotumors (inflammatory fibrous polyps) of the esophagus. Am. J. Dig. Dis. 20:475–481. [DOI] [PubMed] [Google Scholar]
- 6. Privette, A. , Fisk P., Leavitt B., Cooper K., and McCahill L.. 2008. Inflammatory myofibroblastic tumor presenting with esophageal obstruction and an inflammatory syndrome. Ann. Thorac. Surg. 86:1364–1367. [DOI] [PubMed] [Google Scholar]
- 7. Chen, Y. , Tang Y., Li H., Zhang P., Cui Y., Zhang H., et al. 2010. Inflammatory myofibroblastic tumor of the esophagus. Ann. Thorac. Surg. 89:607–610. [DOI] [PubMed] [Google Scholar]
- 8. Gan, S. I. , Rajan E., Adler D. G., Baron T. H., Anderson M. A., Cash B. D., et al. 2007. Role of EUS. Gastrointest. Endosc. 66:425–434. [DOI] [PubMed] [Google Scholar]
- 9. Goldin, S. B. , Osborne D., Paidas C., Iannello J., Gilbert‐Barness E., Karl R., et al. 2007. Inflammatory myofibroblastic tumor of the midesophagus. Fetal Pediatr. Pathol. 26:243–254. [DOI] [PubMed] [Google Scholar]


