Abstract
Background
Immunomodulatory therapies, including CTLA-4 and PD-1 inhibitors, provide a directed attack against cancer cells by preventing T cell deactivation. However, these drugs also prevent the down-regulation of auto-reactive T cells, resulting in immune-related adverse events (IRAEs). Reports show a varied incidence of endocrine IRAEs, ranging from 0–63%.
Objective
To describe the frequency and clinical characteristics of endocrine IRAEs in patients taking cancer immunomodulatory therapies.
Design
Retrospective cohort study.
Patients
388 patients aged ≥18 years who were prescribed ipilimumab, nivolumab, and/or pembrolizumab between 2009–2016 at our institution.
Measurements
Biochemical criteria were used to define endocrine IRAEs, including thyroid, pituitary, pancreas, and adrenal dysfunction, following use of immunomodulatory therapies.
Results
50 endocrine IRAEs occurred in our cohort, corresponding to a rate of 12.9%. The most common endocrine IRAEs were thyroid dysfunction (11.1%), with a lower incidence of pituitary dysfunction (1.8% of patients).
Conclusions
Over 12% of patients receiving ipilimumab, nivolumab, and/or pembrolizumab in our study sample developed an endocrine IRAE. Patients who undergo treatment with immunomodulatory therapies should be monitored for the development of endocrine IRAEs.
Keywords: Thyroiditis, hypopituitarism, hyperthyroidism
Introduction
The field of cancer immunotherapy has seen many exciting developments recently, from the approval of new agents to the expansion of approved indications for existing agents. Research in the last decade has focused on a variety of ways to modulate the immune system to treat cancer.(1) Checkpoint inhibitors, which prevent T cell deactivation, have been particularly successful. However, by down-regulating a host’s immune system control mechanisms, novel side effects can occur that are distinct from the adverse effects associated with conventional chemotherapy. These events, known as immune-related adverse events (IRAEs), are thought to be secondary to the effects of autoimmunity induced by lack of deactivation of auto-reactive T cells.(1)
Two classes of checkpoint inhibitor are currently in use: the first, CTLA-4 inhibitors, blocks the interaction between CTLA-4 on the surface of T cells and B7 on antigen-presenting cells, which physiologically leads to down-regulation of T cell activation. CTLA-4 inhibitors thereby suppress what is usually a checkpoint along the pathway of T cell activation that weeds out auto-reactive cells.(2) The second class, PD-1 inhibitors, targets the interaction between the PD-1 receptor on activated T cells and its ligand, PD-L1, which is expressed by tumor cells.(2) Binding of PD-1 by PD-L1 when the two cells interact within the tumor microenvironment inhibits the T cell response.
The advent of checkpoint inhibitors was in March 2011, when ipilimumab, a CTLA-4 inhibitor, became the first member of the class to be FDA-approved for any indication. At the time, it became the first drug to improve overall survival in patients with metastatic melanoma. Endocrine IRAEs such as hyperthyroidism, hypothyroidism, adrenal insufficiency, and hypophysitis were observed in several trials.(1)
Another class of immunomodulatory agents, PD-1 inhibitors, have been increasingly utilized, most notably pembrolizumab and nivolumab. The KEYNOTE-002 trial demonstrated pembrolizumab’s effectiveness in ipilimumab-refractory melanoma.(3) Later, the KEYNOTE-006 trial demonstrated the superiority of PD-1 inhibitors over ipilimumab, including improved progression-free survival and overall decreased mortality in advanced melanoma.(4) The combination of nivolumab and ipilimumab has also been utilized successfully,(5) with a concomitantly higher risk of adverse reactions compared to monotherapy.(6) Although noted to have a decreased overall rate of adverse events compared to CTLA-4 inhibitors, endocrinopathies were still observed during treatment with single agent pembrolizumab or nivolumab, including a higher incidence of hypothyroidism.(3,7) In one series of 17 patients taking pembrolizumab, hypothyroidism (35%) and thyrotoxicosis (63%) were the most common endocrine reactions reported.(7)
Checkpoint inhibitors are currently being used for a myriad of different cancers, including Merkel cell carcinoma,(8) renal cell carcinoma,(9) non-small cell lung cancer,(10,11) and squamous cell carcinoma of the head and neck.(12) They are also being studied for effectiveness in patients with other solid organ cancers.(3) Although previous existing literature has focused on immunomodulatory therapies in patients with metastatic melanoma, the present study includes patients prescribed these drugs for any malignant indication at an urban academic tertiary care institution, with several years of follow up. Our goal is to provide a retrospective report of identified endocrine IRAEs associated with these increasingly utilized immunomodulatory therapies in a large cohort that includes patients with several years of follow up.
Methods
We performed a retrospective analysis of all patients ≥18 years old who were prescribed ipilimumab, nivolumab, and/or pembrolizumab at the University of California Los Angeles (UCLA) Health center from 2009–2016. Available laboratory and radiographic results were used to evaluate the incidence of primary thyroid dysfunction (hyperthyroidism and hypothyroidism), hypopituitarism, primary adrenalitis, and pancreatitis following the administration of one of these immune-related medications. Events were identified retrospectively by compiling available laboratory data of all patients who were prescribed an immunomodulatory therapy at our institution during the study inclusion time period, which corresponds with the availability of data from the electronic medical record (EMR). A protocol was not in place to identify patients with immune-related adverse events (IRAEs) in real time, as this was a retrospective chart review.
Patients were screened for potential IRAEs by serum concentrations of the following: adrenocorticotropin-releasing hormone (ACTH), cortisol, thyroid stimulating hormone (TSH), free thyroxine (fT4), free thyroxine index (FTI), free triiodothyronine (fT3), total triiodothyronine (TT3), luteinizing hormone (LH), follicle-stimulating hormone (FSH), free and total testosterone, insulin-derived growth factor (IGF-1), thyroid stimulating immunoglobulin (TSI), anti-thyroglobulin antibody (anti-Tg), anti-thyroperoxidase antibody (anti-TPO), adrenal antibody, immunoglobulin G subclass 4 (IgG4), insulin, C-peptide, anti-nuclear antibody (ANA), and rheumatoid factor (RF). Amylase and lipase were not included to define pancreatitis to prevent the inclusion of patients who had an episode of pancreatitis that was not related to immunomodulatory therapy.
Patients were included in the cohort on the basis of meeting inclusion criteria for one or more groups as follows: Overt hyperthyroidism defined as TSH <0.3μIU/mL and fT4 >20.59pmol/L; subclinical hyperthyroidism defined as TSH <0.3μIU/mL and [fT4 10.29–20.59pmol/L or FTI >135.15nmol/L or TT3 >2.85nmol/L or fT3 >2.49pmol/L]; subclinical hypothyroidism defined as TSH >4.7μIU/mL and [fT4 3.86–20.59pmol/L or FTI <57.92nmol/L or TT3 <1.31nmol/L or fT3 <2.49pmol/L]; overt hypothyroidism defined as TSH >4.7μIU/mL and [fT4 <3.86pmol/L or FTI <57.92nmol/L or TT3 <1.31nmol/L or fT3 <2.49pmol/L]; central adrenal insufficiency defined as ACTH <1.32pmol/L and cortisol <165.53nmol/L for males or ACTH <0.88pmol/L and cortisol <165.53nmol/L for females; central hypothyroidism defined as TSH of any value and [fT4 <3.86pmol/L or FTI <57.92nmol/L or TT3 <1.31nmol/L or fT3 <2.49pmol/L]; secondary hypogonadism defined as FSH <1.6IU/L or LH <2IU/L for males or FSH <1IU/L or LH <1IU/L for females; growth hormone deficiency defined as IGF-1 <6.55nmol/L; primary adrenalitis defined as ACTH >12.98pmol/L and cortisol <220.70nmol/L for males and ACTH >10.56pmol/L and cortisol <220.70nmol/L for females; pancreatitis defined as IgG4 >0.86g/L or insulin <20.84pmol/L or C-peptide <0.36nmol/L or ANA >1:40 or RF >25. Diabetes insipidus was not assessed for in our sample. Patients were included in the cohort if they had been prescribed a checkpoint inhibitor and had subsequently labs that fit into one of the above categories.
The charts of these patients were then manually reviewed to record any available results of endocrine-related radiographic studies and to evaluate patients’ clinical courses, when available to verify the presence of an endocrine IRAE. Cases where the patient had an underlying endocrine disorder (such as hypothyroidism), or who had laboratory values assessed for an unrelated reason, were not characterized as having experienced an endocrine IRAEs for the purposes of this study. Upon review, patients who received treatment were reclassified as clinical hypothyroidism or hyperthyroidism instead of subclinical hypothyroidism or hyperthyroidism regardless of lab values. In cases where imaging was performed and was possibly indicative of an endocrine IRAE, correlation with biochemical results was performed where possible. Imaging studies were included when relevant but were not used as sole method of diagnosis of an endocrine IRAE. Finally, pertinent negative findings were included where present if imaging was specifically ordered for evaluation of a potential endocrinopathy. Stable lesions observed on imaging studies that predated the initiation of therapy were not included. Mean time to event was calculated as the number of days between the date of drug administration and the date of abnormal laboratory tests. All analyses performed were retrospective in nature. The study was approved by the UCLA Institutional Review Board.
Results
The study sample consisted of 388 patients (mean age: 62.7 ± 14.9 years, 56.4% men, 71.7% white) (Table 1). 388 patients had measurements of thyroid data, 369 had measurements of pituitary data, 38 had measurements of adrenal data, and 4 had measurements of pancreatic data. A total of 50 endocrine IRAEs were observed, representing 12.9% of the study population (Table 2, Figure 1). The mean time to event for all patients was 17.85 days with a standard deviation of 32.25 days.
Table 1.
Subject Demographics (n=388)
| Demographic | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 219 (56.4%) |
| Ethnicity | |
| Not Hispanic or Latino | 329 (84.8%) |
| Hispanic or Latino | 23 (5.9%) |
| Other | 36 (9.3%) |
| Race | |
| White or Caucasian | 278 (71.7%) |
| Asian | 43 (11.1%) |
| Black | 8 (2.1%) |
| Other | 59 (15.2%) |
The row “Other” for the ethnicity data includes the responses “Unknown” and “Patient refused.” The row “Hispanic or Latino” for the ethnicity data includes the responses “Hispanic or Latino,” “Mexican/Mexican-American,” and “Hispanic/Spanish origin.” The row “Other” for the race data includes the responses “Other,” “Unknown,” “Patient refused,” “American Indian,” and “Multiple races.”
Table 2.
Rates of Endocrine IRAEs Following Immunomodulatory Therapy
| Description of event | n | Percentage of total sample | Percentage requiring treatment | Mean time to event (days ± SD) | Serum biochemical results (mean ± SD) |
|---|---|---|---|---|---|
| Thyroid | |||||
| Overt hyperthyroidism | 9 | 2.3% | 0% | 8.88 ± 16.04 | TSH: 0.13 ± 0.08 μIU/mL Free T4: 362.519 ± 102.2 pmol/L Free T3: 0.57 ± 1.47 nmol/L Total T3: 2.20 ± 0.37 nmol/L |
| Subclinical hyperthyroidism | 3 | 0.8% | 0% | 0.67 ± 0.58 | TSH: 0.42 ± 0.58 μIU/mL Free T4: 19.05 ± 7.98 pmol/L Free T3: 3.64 ± 1.21 nmol/L |
| Overt hypothyroidism | 10 | 2.6% | 90% (9/10) | 4.89 ± 9.71 | TSH: 14.04 ± 24.67 μIU/mL Free T4: 51.61 ± 170.41 pmol/L Free T3: 2.61 ± 1.55 pmol/L Anti-thyroglobulin antibody (1 patient): <0.9 IU/mL Anti-thyroperoxidase antibody (1 patient): 5.6 IU/mL |
| Subclinical hypothyroidism | 21 | 5.4% | 0% | 30.3 ± 44.61 | TSH: 5.18 ± 3.46 μIU/mL Free T4: 14.29 ± 2.70 pmol/L Free T3: 0.75 ± 0.95 pmol/L Anti-thyroglobulin antibodies: 9.5% (11.95 ± 2.90 IU/mL) |
| Total Thyroid Events | 43 | 11.1% | 20.9% (9/43) | 17.63 ± 34.21 | -- |
| Pituitary | |||||
| Hypophysitis | 7 | 1.8% | 57.1% (4/7) | 16.71 ± 14.75 | TSH: 2.69 ± 4.1 μIU/mL Free T4: 13.89 ± 52.77 pmol/L Free T3: 2.01 ± 1.43 nmol/L Total T3: 1.08 ± 0.48 nmol/L LH: 4.5 ± 1.98 IU/L Total testosterone: 4.16± 4.93 nmol/L |
| Cohort total | 50 | 12.9% | 26% | 17.85 ± 32.25 | -- |
Figures in the “number” column may not add up to the totals due to exclusion of duplicates within groups (ie, overlap of patients with radiographic and biochemical findings or patients who had multiple events). Incidence rates are calculated as percentage of total study population (388 patients). Reference ranges: Anti-thyroglobulin antibody: <4 IU/mL; Anti-thyroperoxidase antibody: <20IU/mL; Free T3 index: 1.20–2.49nmol/L; Free T4: 10.29–20.59pmol/L; LH (adult female): 2–91IU/L; LH (adult male): 2–12IU/L; TSH: 0.3–4.7μIU/mL; Total T3: 1.31–2.85nmol/L; Total testosterone: 6.94–34.7nmol/l.
Figure 1.
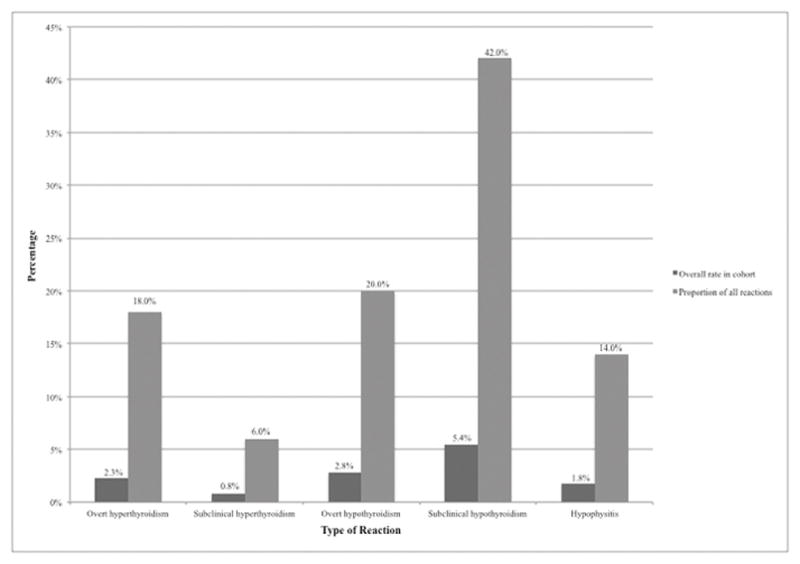
Incidence Rates of Endocrine IRAEs Following Immunomodulatory Therapy
Values are expressed as percentage of the total patient population (n=388), and as percentage of the total number of patients experiencing events (n=50).
Chart review was performed of patients who were found to have abnormal laboratory values indicative of a thyroid IRAE to evaluate the course of the IRAEs, where available. Ten patients developed overt hypothyroidism and received treatment with levothyroxine except one patient who passed away two months after the event. Four patients were previously hypothyroid and one had a prior diagnosis of Graves’ disease. In general, patients did not undergo further investigation, other than repeat checks of TSH and fT4 levels after starting or increasing levothyroxine with subsequent dose adjustments as appropriate. One patient underwent thyroid ultrasound, which revealed a small nodule without suspicious features, but passed away before his next dose of immunomodulatory therapy was scheduled. An additional patient underwent a PET-CT for the purpose of disease staging that revealed increased FDG uptake suggestive of thyroidits after initiation of their immunomodulatory therapy. Patients did not have their immunomodulatory therapy discontinued as a result of the IRAE. The mean serum thyroid stimulating hormone (TSH) of patients with hypothyroidism was 14.04 ± 24.6 μIU/mL and their mean free thyroxine (fT4) was 51.61 ± 170.41 pmol/L. One patient had anti-thyroperoxidase antibody (anti-TPO) and anti-thyroglobulin antibody (anti-Tg) drawn, both of which were within normal limits. The mean time to event was 4.89 ± 9.71 days.
Twenty-one patients developed subclinical hypothyroidism and were not treated with thyroid hormone replacement therapy. Six patients demonstrated eventual normalization of TSH on repeat investigation. Four patients passed away within three months of the abnormal lab values. Eleven patients either did not have labs repeated or information on their course was not available in the chart. Their mean serum TSH was 5.18 ± 3.46 μIU/mL with a mean serum free T4 of 14.29 ± 2.70 pmol/L. The mean time to event was 30.3 ± 44.61 days. 9.9% of these patients were found to have anti-thyroglobulin antibodies.
Nine patients experienced overt hyperthyroidism, including seven who were hypothyroid at baseline before developing temporary thyrotoxicosis. In all cases, the dose of levothyroxine was unchanged prior to the noted reaction. In all but two of these cases, levothyroxine was held or reduced until TSH normalized. Patients generally reverted to their baseline hypothyroid state and resumed levothyroxine therapy after the acute reaction resolved; two remained hyperthyroid but passed away within a few months of the reaction. One patient underwent a bedside thyroid ultrasound study which was unrevealing, and later reverted to hypothyroidism within a year, with an increased levothyroxine requirement thereafter. Otherwise, no additional workup was performed in any patients, including thyroid scans, with the exception of repeat lab checks. Immunomodulatory therapies were not held in any patient due to these reactions. Two of those nine patients were euthyroid prior to the event; one became hypothyroid after resolution of the acute reaction and the other did not have labs rechecked to verify thyroid status. Overall, patients with overt hyperthyroidism had a mean serum TSH of 0.13 ± 0.08 μIU/mL with a mean serum free T4 of 362.52 ± 102.2 pmol/L. The mean time to event was 8.88 days ± 16.04 days.
Three patients developed subclinical hyperthyroidism and did not undergo any interventions or workup. Their mean serum TSH was 0.42 ± 0.58 μIU/mL with a mean serum free T4 of 19.05 ± 7.98 pmol/L. The mean time to event was 0.67 ± 0.58 days.
In total, a thyroid-related biochemical and/or radiographic thyroid abnormality was noted in 43 patients (11.1% of the cohort) following initiation of a checkpoint inhibitor medication. The overall mean time to event for thyroid events was 8.88 days with a standard deviation of 16.04 days.
Seven patients were found to have hypophysitis. Four of these patients were treated with levothyroxine and systemic steroids and two were also treated with testosterone. One patient had a history of prostate cancer so was not treated with testosterone to maintain suppression of his prostate-specific antigen (PSA). In addition, two of the seven patients underwent imaging studies, which were unremarkable for pituitary abnormalities. Immunomodulatory therapies were not discontinued in these patients for these reactions, although two patients did experience reactions of diarrhea and lower extremity edema that required cessation of therapy. A total of seven patients (1.8%) had evidence of hypopituitarism following administration of a checkpoint inhibitor, with a mean time to event of 16.71 ± 14.75 days.
No patients were found to have evidence of primary pancreatic or adrenal IRAEs.
Discussion
This study describes a 12.9% prevalence of endocrine abnormalities associated with the use of ipilimumab, nivolumab, and/or pemrolizumab for any indication at a large, urban tertiary-care academic medical center. We noted a predominance of thyroid-related abnormalities, which comprised 43 of the 50 observed IRAEs (86%). Further, 2.6% of patients experienced overt hypothyroidism, 2.3% experienced overt hyperthyroidism, and 5.4% experienced subclinical hypothyroidism. 1.8% of patients experienced hypophysitis. Of the nine patients who experienced hyperthyroidism, seven were previously hypothyroid. This alternation between hypo- and hyperthyroid states can be potentially explained by a preexisting autoimmune thyroid disease making a patient more susceptible to developing a hyperthyroid state from additional inflammation (similar to a condition known as Hashi-toxicosis).(13)
A prior analysis of 238 patients in a clinical trial of ipilimumab noted hypophysitis in 8% of patients, hypothyroidism in 6%, and primary adrenal dysfunction in 0.8%.(14) A review by Corsello et al found that the rate of hypophysitis varied from 0% to 17% in recent studies of CTLA-4 inhibitors, with larger studies usually not exceeding an incidence of 5%.(1) Thyroiditis occurred even less frequently in their sample, with incidence rates ranging from 0 to 4%.
PD-1 inhibitors are believed to be associated with a slightly higher risk of IRAEs than CTLA-4 inhibitors, particularly for thyroid events. The KEYNOTE-006 trial noted hypothyroidism in 18.8% of patients taking pembrolizumab, compared to just 2% of patients taking ipilimumab.(4) Further, a trial of pembrolizumab in 655 patients with melanoma reported an incidence rate of 10.9% of thyroid reactions, including hypothyroidism, hyperthyroidism, and thyroiditis and 0.9% of pituitary insufficiency.(15) Regarding nivolumab, hypothyroidism has been reported in 5–6% of patients, while hypophysitis is noted in about 1%.(16) A study of 190 patients in the United Kingdom reported that 29.5% of patients treated with ipilimumab, a PD-1 inhibitor, or a combination of the two developed any type of thyroid abnormality; incidences ranged from 23% in patients treated with ipilimumab alone to 39% in patients treated with a PD-1 inhibitor alone to 50% of patients who received both therapies.(6)
Primary adrenal and pancreatic endocrinopathies due to immunomodulatory therapies have been uncommonly reported in the literature; similarly, none were found amount our cohort. Recent reports of adrenal insufficiency due to ipilimumab have ranged from 1–10%, although it is not always clear if primary adrenal insufficiency is clearly differentiated from central adrenal insufficiency in these studies.(1) A meta-analysis of 191 reports found that pancreatitis occurred in 0.9% of 234 patients treated with ipilimumab, and that primary adrenal insufficiency occurred in 1 case (0.4%).(17)
The present study did not describe any cases of adrenal and pancreatic IRAEs, which is slightly lower than or potentially consistent with the low rates of such events described in existing literature. This may in part be due to the retrospective nature of the study, without a proactive evaluation of patients for symptoms that would be in line with these reactions. The rates of overt pituitary and thyroid IRAEs, including hypothyroidism and thyrotoxicosis, fall within the ranges of previously reported findings. These findings indicate that previous reports of the frequency of endocrine IRAEs are likely reflective of the actual incidence, and could provide realistic estimates of side effect profiles when counseling patients. In addition, we report a high incidence of subclinical hypothyroidism (5.4%), which is usually not documented in other reports.
This study is strengthened by the inclusion of a large cohort, as well as by our differentiation between primary and central causes of adrenal and thyroid dysfunction, which at times is difficult to distinguish in the existing literature.
The major limitation of this study is its retrospective nature. Patients were not identified or followed prospectively, which made it difficult to determine the presence or severity of symptoms that may have prompted screening due to availability of documentation. In particular, screening of thyroid function is performed routinely, but screening for other IRAEs including of the pituitary, pancreas, and adrenal glands is usually prompted only by patient symptoms. There may therefore be a lower rate of pituitary, pancreatic, and adrenal IRAEs in our cohort than would be reported in a prospective study. Data on length of follow-up or discontinuation of immunomodulatory therapy were excluded because they were not always clearly noted in patient charts. Additionally, the retrospective nature of this study does not account for patients with preexisting endocrine abnormalities that were only diagnosed after initiation of immunomodulatory therapy.
Other limitations include limitation of cohort size due to additional laboratory data and imaging studies that patients may have had obtained but that are not contained within our institution’s data repository. Further, attributing reactions to particular drugs is complicated by the fact that some patients were prescribed more than one immunomodulatory therapy over the course of their treatment. In addition, it is difficult to assign a causal relationship between particular immunomodulatory therapy and an IRAE in a retrospective study, therefore this data is also not reported.
The incidence, severity, and duration of endocrine IRAEs vary, to the extent that some patients may be discontinued from their immunotherapy. Information about discontinuation of therapy was included when available; none of the overt thyroid reactions or pituitary reactions in our cohort specifically necessitated discontinuation of immunotherapy. However, the vast majority of IRAEs can be managed by correction of the endocrine dysfunction and/or monitoring of patient symptoms and laboratory values. Given the multidisciplinary nature of care required to achieve optimal outcomes, we urge for close coordination between oncologists and endocrinologists in the management of these complex patients.
In summary, our findings indicate that subclinical endocrine IRAEs may be more common than previously described. We recommend that clinicians be vigilant for signs and symptoms of such reactions when treating patients with immunomodulatory therapies, and have a low threshold for initiating biochemical testing.
Acknowledgments
A.R. has received honoraria from Merck for consulting. B.C. serves on the Advisory Board for Merck, Lilly, BMS, Genentech, Immunocore, and Eisai, and is on the Speakers Bureau for Janssen and Genentech. G.C. participates in the Merck Speakers Bureau.
Financial Disclosure: This work was supported by a grant from the National Institutes of Health (K23HD068552 to AML).
References
- 1.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. J Clin Endocrinol Metab. 4. Vol. 98. Endocrine Society Chevy Chase; MD: 2013. Apr 7, Endocrine side effects induced by immune checkpoint inhibitors; pp. 1361–75. [DOI] [PubMed] [Google Scholar]
- 2.Daud A. Current and Emerging Perspectives on Immunotherapy for Melanoma. Semin Oncol. 2015 Dec;42( Suppl 3):S3–11. doi: 10.1053/j.seminoncol.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Lancet Oncol. 8. Vol. 16. Elsevier; 2015. Aug, Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial; pp. 908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. N Engl J Med. 26. Vol. 372. Massachusetts Medical Society; 2015. Jun 25, Pembrolizumab versus Ipilimumab in Advanced Melanoma; pp. 2521–32. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013 Jul 11;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid Abnormalities following the use of CTLA-4 and PD-1 Inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2016 Dec 27; doi: 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 7.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab. 2016 Nov;101(11):4431–9. doi: 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016 Jun 30;374(26):2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zibelman M, Ghatalia P, Geynisman DM, Plimack ER. Checkpoint inhibitors for renal cell carcinoma: current landscape and future directions. Immunotherapy. 2016 Jun;8(7):785–98. doi: 10.2217/imt-2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. N Engl J Med. 19. Vol. 375. Massachusetts Medical Society; 2016. Nov 10, Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer; pp. 1823–33. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. N Engl J Med. 17. Vol. 373. Massachusetts Medical Society; 2015. Oct 22, Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer; pp. 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. N Engl J Med. 19. Vol. 375. Massachusetts Medical Society; 2016. Nov 10, Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck; pp. 1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander I, Eckhart H, Hahn G, Strobel D. Hashitoxicosis – Three Cases and a Review of the Literature. Eur Endocrinol. 2008;4:70. [Google Scholar]
- 14.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014 Apr;21(2):371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016 Apr 19;315(15):1600–9. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 16.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol. 2014 Mar 3;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]


