Abstract
Background
Individual-level simulation models are valuable tools for comparing the impact of clinical or public health interventions on population health and cost outcomes over time. However, a key challenge is ensuring that outcome estimates correctly reflect real-world impacts. Calibration to targets obtained from randomized trials may be insufficient if trials do not exist for populations, time periods, or interventions of interest. Observational data can provide a wider range of calibration targets, but requires methods to adjust for treatment-confounder feedback. We propose the use of the parametric g-formula to estimate calibration targets and present a case-study to demonstrate its application.
Methods
We used the parametric g-formula applied to data from the HIV-CAUSAL Collaboration to estimate calibration targets for 7-year risks of CDC-defined AIDS and/or death (AIDS/death) under 3 treatment initiation strategies. We compared these targets to projections from the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model for treatment-naïve individuals presenting to care in 1996–1999, 2000–2002, or 2003 or onwards.
Results
The parametric g-formula estimated a decreased risk of AIDS/death over time and with earlier treatment. The uncalibrated CEPAC model successfully reproduced targets obtained via the g-formula for baseline 1996–1999, but over-estimated calibration targets in contemporary populations and failed to reproduce time trends in AIDS/death risk. Calibration to g-formula targets improved CEPAC model fit for contemporary populations.
Conclusion
Individual-level simulation models are developed based on best available information about disease processes in one or more populations of interest, but these processes can change over time or between populations. The parametric g-formula provides a method for using observational data to obtain valid calibration targets and enables updating of simulation model inputs when randomized trials are not available.
Keywords: Agent-based model, calibration, HIV, g-formula
Introduction
Simulation models, such as agent-based, microsimulation, or individual-level models, are often used to estimate the population impact of implementing public health interventions or clinical treatment strategies. Individual-level simulation models estimate (counterfactual) outcome distributions under alternative policy or treatment strategies, and therefore answer causal questions of the form: What would the outcome distribution in a population be if a particular strategy had been implemented?
An individual-level simulation model is defined by parameters that describe the relationships between treatments, outcomes, and other variables.(1) A key practical challenge to creating these models is that these parameters cannot be directly estimated from the population of interest. Otherwise, the outcome distributions could be estimated directly from the data using causal inference techniques rather than via simulation. Instead, model parameters typically need to be selected from a variety of sources and thus calibration procedures are required before the model can be applied to the population of interest.(2–5)
Calibration is the process of comparing the simulation model results to outcome distributions estimated using another analytic method, and has two steps. First, investigators must identify appropriate benchmarks as calibration targets. Second, they must ensure that the simulation model reproduces these targets in the population of interest.(4) A large literature is devoted to the second step, including methods for searching the input parameter space and determining goodness of fit.(6–9) Here, we focus on the first step, for which two broad types of calibration targets may be of interest.
One possible target is the observed outcome distribution in the population of interest. This can be estimated via a randomized trial or using observational data.(5) However, replicating the observed outcomes requires knowing the distribution of treatment strategies in the population so that these can be included in the analysis. This information may be difficult to obtain except from randomized trials, where it is fixed by design. Because of this, randomized trials are often seen as the gold-standard for obtaining calibration targets, but there are several limitations to relying solely on randomized trials. First, a given trial typically compares only a limited number of interventions, and the particular strategies of interest for a model may not be included. Second, trials are often not available in the population for which the simulation model is being calibrated, or may have been conducted in a highly specific subset of the population and thus of limited generalizability.
A second possible target is the counterfactual outcome distribution under a single treatment strategy selected from among the strategies present in the population. If sufficient observational data are available from the population of interest, this distribution can be validly estimated using causal inference techniques, such as the parametric g-formula, which is a generalization of standardization to settings with time-varying treatment and confounders (10, 11) and similar in many aspects to individual-level simulation models.(12)
Here, we propose the application of the parametric g-formula to obtain a range of calibration targets for individual-level simulation models when longitudinal data (on treatments, outcomes, and confounders) is available in a population of interest. As a case study, we apply the parametric g-formula to observational data to estimate calibration targets for the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model. We consider the question of when to initiate antiretroviral therapy (ART) for people living with HIV.
The structure of this paper is as follows. We first describe the scenario of interest, the observational dataset, and the parametric g-formula. We then describe the estimation of calibration targets, the CEPAC model, and the calibration process. The calibration process is presented in two stages: (I) estimation of the calibration targets via the parametric g-formula and assessment of original parameterization fit, and (II) update of model parameters to improve fit. We conclude with a discussion of the assumptions required for the parametric g-formula to validly estimate the true counterfactual outcome distributions.
Methods
Scenario
We consider 3 treatment initiation strategies for people living with HIV in Europe and North America: (a) immediate ART initiation at diagnosis of HIV infection (currently recommended in the US); (b) early ART initiation when CD4 count falls below 500 cells/μl; and (c) late ART initiation when CD4 count falls below 350 cells/μl. ART was defined as a regimen of antiretroviral drugs including at least two nucleoside reverse transcriptase inhibitors (NRTIs) and either one or more protease inhibitors (PI) or boosted PIs, one non-nucleoside reverse transcriptase inhibitor (NNRTI), one integrase inhibitor (INSTI), one entry or fusion inhibitor, or three or more NRTIs.
Until relatively recently, it was debated whether the benefits of early treatment initiation might be offset by an increased risk of side effects and resistance that limits future treatment options.(13–15) As of 2017, these strategies have been assessed in both observational cohorts and randomized trials, and there is consensus that early initiation is preferable. We use these strategies here as simple demonstration targets,(16–18) but the same approach described here could be applied to a wider range of strategies or to more complex strategies, such as scheduled treatment interruption plans or treatment switching strategies, provided data exist.
We considered two types of outcomes: death and a combined outcome of death or AIDS (defined as first diagnosis of an AIDS-defining opportunistic illness (19)). For each outcome, we estimated two calibration targets: the 7-year outcome risk and the 7-year survival curve under each treatment initiation strategy.
Data source
We used data from the HIV-CAUSAL Collaboration (20, 21) to estimate our calibration targets. This collaboration combines data from prospective cohorts of HIV-positive individuals in Brazil, Canada, France, Greece, Netherlands, Spain, Switzerland, UK, and USA. We excluded data from Brazil from our analyses because a separate parameterization of the CEPAC model exists for Brazil. The estimates presented here are based on data pooled in December 2015.
Our analyses included all HIV-positive individuals in the HIV-CAUSAL Collaboration aged 18 years or older, who were ART-naïve, did not have AIDS, were not pregnant, and had a CD4 cell count and HIV RNA measurement within the past three months of baseline. The start of follow-up for each individual was defined as the first month in which all eligibility criteria were met during three baseline time periods: 1996–1999, 2000–2002, and 2003 and onwards.
Each individual was followed until the earliest of death; censoring, defined as 12 months after the last recorded CD4 cell count and HIV RNA measurements or pregnancy (if known); or the study-specific administrative end of follow-up between February 2010 and March 2013. Unlike in a previous application of the parametric g-formula to estimate the impact of treatment initiation on survival in these data,(17) we did not require that baseline occur within 6 months of HIV diagnosis because date of diagnosis is not used in CEPAC.
Parametric g-formula
We used the parametric g-formula to estimate our calibration targets adjusting for time-fixed and time-varying confounders.(10, 11, 22) The implementation of the parametric g-formula involves two steps: (I) parametric estimation of the joint distribution of covariates and outcome over time; (II) simulation of the counterfactual outcome distribution under different treatment strategies.(22) A brief description of each step follows. For more details we refer readers to previous applications of the parametric g-formula. (17, 23–25)
Step I: Parametric estimation of the joint distribution of the data
As in previous analyses (16–18), we considered the following covariates: baseline CD4 count in cells/μl (<50, 50–99, 100–199, 200–349, 350–499, ≥500), HIV RNA log copies per mL (<4, 4–5, >5), sex, race, geographical origin (Europe and the USA, sub-Saharan Africa, rest of the world, unknown), transmission group (heterosexual, homosexual or bisexual, injection drug user, and other/unknown), age in years (<35, 35–50, >50), calendar year (<2000, 2000–04, 2005–10, 2011–13), and cohort; and time-varying CD4 cell count, HIV RNA, and AIDS (when not an outcome). Importantly, these time-varying confounders are also affected by prior treatment history leading to treatment-confounder feedback.
We next fit parametric regression models to the HIV-CAUSAL data to estimate the joint distribution of the time-varying covariates, treatment initiation, and mortality over time. We used logistic regression to model the time-varying indicators for death, AIDS, and treatment initiation, and linear regression to model the natural logarithms of HIV RNA and CD4 cell count. All models included the two most recent values of the time-varying covariates, as well as time since last CD4 count and HIV RNA measurements, and all baseline covariates. Models for CD4 count and HIV RNA also included product terms for the number of months since ART initiation.
Step II: Estimation of the counterfactual outcome distribution under several treatment strategies
For each treatment strategy, we used the parametric models fit in Step I to generate a cohort of the same size as the original data via Monte-Carlo simulation. At each time point, we assigned treatment according to the treatment strategy. We then calculated the outcome distribution in the simulated data.
We used non-parametric bootstraps with 500 samples to obtain 95% confidence intervals. For each bootstrap, we took a random sample of the HIV-CAUSAL data with replacement and repeated the full process of Steps I –II. We then calculated the 95% confidence interval based on the 2.5th and 97.5th percentiles of the bootstrap outcome estimates. All analyses were conducted using the GFORMULA macro(26) in SAS 9.4.
As a sensitivity analysis, we estimated the outcome distributions under no intervention. That is, we assigned treatment at each month based on the estimated conditional probability of treatment observed in the HIV-CAUSAL data from Step II.
CEPAC Model
We calibrated the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model. The CEPAC model is a large, well-established individual-level simulation model that has been used to assess cost-effectiveness of a range of clinical and public health policies aimed at reducing the burden of disease and improving survival among people living with HIV(27–29). Simulated individuals in CEPAC exist in one of three health states: chronic infection, acute opportunistic infections (OIs), or death. Time-varying covariates, including CD4 count, HIV RNA level, age, and sex, govern transitions between health states. There are three distinct probabilities that govern mortality each month: the probability of chronic AIDS-related mortality, the probability of acute OI-related mortality, and the probability of non-AIDS-related mortality. Full specification of the model is provided on the CEPAC website (30) and elsewhere (28, 31).
The CEPAC model was originally designed using data from the Multicenter AIDS Cohort Study (MACS) —a cohort study of HIV-positive men which began enrollment in 1984.(32) CEPAC was originally calibrated to data from cohorts of people living with HIV in the US; the prior calibration is described in detail elsewhere.(33) However, changes in available treatments, testing strategies, and demographics of people living with HIV over time require that the model be periodically recalibrated to ensure it continues to provide valid estimates of survival and AIDS progression in contemporary settings. Here, we compared the estimated outcome distributions from CEPAC to calibration targets obtained using the parametric g-formula applied to HIV-CAUSAL data in the three baseline time periods. Because more recent cohort data more accurately reflect the current clinical course of HIV disease and treatment, we aimed for the recalibrated model to better fit the data from 2003 onwards.
Calibration
We simulated each treatment strategy and time period in CEPAC using 105 individuals. For each simulated population, we used the observed baseline distributions of CD4 count (mean and standard deviation), HIV RNA strata (% within strata), age (mean and standard deviation), gender (% male), and transmission risk group from individuals in HIV-CAUSAL who met the inclusion criteria within that baseline time period (Table 1, Appendix 1).
Table 1.
Baseline characteristics of eligible individuals in the HIV-CAUSAL Collaboration by time period for variables used in CEPAC*
| Jan 1 1996 to Dec 31 1999 | Jan 1 2000 to Dec 31 2002 | On or after Jan 1 2003 | |||||
|---|---|---|---|---|---|---|---|
| N = 28,269 | % | N = 25,433 | % | N = 78,093 | % | ||
| CD4 cell count, cells/μl | <50 | 1,519 | 5.4 | 1,375 | 5.4 | 3,847 | 4.9 |
| 50–100 | 1,172 | 4.1 | 1,109 | 4.4 | 3,279 | 4.2 | |
| 100–200 | 2,979 | 10.5 | 2,645 | 10.4 | 8,067 | 10.3 | |
| 200–350 | 6,295 | 22.3 | 5,678 | 22.3 | 18,069 | 23.1 | |
| 350–500 | 6,612 | 23.4 | 5,922 | 23.3 | 18,661 | 23.9 | |
| >500 | 9,692 | 34.3 | 8,704 | 34.2 | 26,170 | 33.5 | |
| Mean (SE) | 426.5 (267.9) | — | 426.3 (266.9) | — | 421.2 (256.8) | — | |
| HIV RNA, copies/mL | >100,000 | 6,584 | 23.3 | 6,117 | 24.1 | 22,324 | 28.6 |
| 30,000–100,000 | 5,958 | 21.1 | 5,775 | 22.7 | 19,824 | 25.4 | |
| 10,000–30,000 | 5,363 | 19.0 | 4,774 | 18.8 | 14,991 | 19.2 | |
| 3,000–10,000 | 4,438 | 15.7 | 4,075 | 16.0 | 10,835 | 13.9 | |
| 500–3,000 | 3,812 | 13.5 | 3,326 | 13.1 | 7,777 | 10.0 | |
| 20–500 | 2,105 | 7.4 | 1,337 | 5.3 | 2,263 | 2.9 | |
| 0–20 | 9 | 0.0 | 29 | 0.1 | 79 | 0.1 | |
| Sex | Men | 21,361 | 75.6 | 18,803 | 73.9 | 62,498 | 80.0 |
| Women | 6,908 | 24.4 | 6,630 | 26.1 | 15,595 | 20.0 | |
| Age, years | Mean (SE) | 36.9 (9.4) | — | 37.9 (10.0) | — | 37.9 (10.9) | — |
| Transmission group | Heterosexual | 8,401 | 29.7 | 8,953 | 35.2 | 24,673 | 31.6 |
| Homosexual or bisexual | 9,197 | 32.5 | 8,280 | 32.6 | 37,434 | 47.9 | |
| Injection drug user | 5,132 | 18.2 | 3,053 | 12.0 | 3,361 | 4.3 | |
| Other/unknown | 5,539 | 19.6 | 5,147 | 20.2 | 12,625 | 16.2 | |
CEPAC: Cost-Effectiveness of Preventing AIDS Complications model; OIs: opportunistic infections
We used a previously published set of CEPAC parameter values for modeling AIDS-related mortality, OI incidence, and changes to CD4 count and HIV RNA over time (Table 2).(28, 32, 34–36) Sex-stratified lifetables for the probability of non-AIDS-related mortality were obtained from the Eurostat website.(37) Key parameter values are given in Table 2. For more detail on these parameters, their sources, and how they govern transitions between health states in CEPAC see Appendix 2.
Table 2.
| Mean monthly CD4 count decrease (cells/μl), by HIV | CD4 > 500 cells/μl | CD4: 350 – 499 cells/μl | CD4: 200 – 299 cells/μl | CD4: 100 – 199 cells/μl | CD4: 50 – 99 cells/μl | CD4 < 50 cells/μl |
|---|---|---|---|---|---|---|
| RNA stratum, copies/mL | ||||||
| >100,000 | 9.54 | 7.94 | 6.34 | 4.66 | 3.30 | 1.37 |
| 30,000–100,000 | 9.54 | 7.94 | 6.34 | 4.66 | 3.30 | 1.37 |
| 10,000–30,000 | 6.85 | 5.70 | 4.55 | 3.34 | 2.37 | 0.98 |
| 3,000–10,000 | 5.87 | 4.88 | 3.90 | 2.87 | 2.03 | 0.84 |
| 500–3,000 | 4.77 | 3.97 | 3.17 | 2.33 | 1.65 | 0.69 |
| 20–500 | 2.45 | 2.03 | 1.63 | 1.19 | 0.85 | 0.35 |
| 0–20 | 2.45 | 2.03 | 1.63 | 1.19 | 0.85 | 0.35 |
|
| ||||||
| Monthly risk of opportunistic infections (OIs), per 10,000 | CD4 > 500 cells/μl | CD4: 350 – 499 cells/μl | CD4: 200 – 299 cells/μl | CD4: 100 – 199 cells/μl | CD4: 50 – 99 cells/μl | CD4 < 50 cells/μl |
| Pneumocystis pneumonia | 4.0 | 8.0 | 16.0 | 61.8 | 110.0 | 83.6 |
| Mycobacterium avium complex | 1.0 | 3.0 | 3.0 | 12.0 | 26.0 | 46.9 |
| Toxoplasmosis | 0.5 | 1.0 | 2.0 | 3.0 | 10.0 | 7.0 |
| Cytomegalovirus | 1.0 | 3.0 | 7.0 | 13.0 | 31.0 | 81.7 |
| Fungal infections | 1.0 | 3.0 | 3.0 | 15.0 | 25.0 | 31.9 |
| All other OIs | 6.0 | 12.0 | 27.0 | 66.8 | 114.3 | 115.0 |
|
| ||||||
| Monthly risk for acute OI-related death, among those with OI, % | Pneumocystis pneumonia | Mycobacterium avium complex | Toxoplasmosis | Cytomegalovirus | Fungal infections | All other OIs |
| 3.5 | 4.5 | 18.2 | 4.8 | 3.6 | 4.3 | |
|
| ||||||
| Efficacy of antiretroviral therapy | Viral suppression after 24 weeks, % | Monthly risk of late failure after initial viral suppression, % | On-treatment multiplier for OI incidence | On-treatment multiplier for chronic AIDS-related mortality | ||
| 86.0** | 0.5** | 1.0 | 1.0 | |||
CEPAC: Cost Effectiveness of Preventing AIDS Complications model; OIs: opportunistic infections
Parameter values for original CEPAC model before calibration
Values represent mean risk; individual’s risk of viral suppression and late failure depends on probability of treatment response
We focused our calibration on two parameters, selected because their initial values had been determined based on discussions with clinicians and other experts in the field, without a substantial empirical evidence base. Specifically, we assessed the on-treatment multiplier for OI incidence and the on-treatment multiplier for chronic AIDS-related mortality. In the original CEPAC calibration, both multipliers were set to 1.0. We performed a grid search by varying the on-treatment multipliers jointly between 1 and 0 by 0.2 units for a total of 36 calibration runs. We chose a simple grid search, rather than more formal search procedures such as Nelder Mead, because our goal was chiefly to demonstrate the method of obtaining calibration targets, rather than performing a full calibration of CEPAC.
We assessed the fit of the 7-year mortality and combined AIDS or mortality risks from the calibration runs in comparison to the values of the calibration targets estimated using the parametric g-formula applied to HIV-CAUSAL. Fit was determined based on the number of treatment strategies and outcomes for which the CEPAC risk estimates were within the 95% confidence intervals for the g-formula risks estimates. We also visually inspected survival and AIDS-free survival curves produced by the calibration runs of CEPAC and those obtained via the g-formula.
As sensitivity analyses, we compared the distributions of mean CD4 count and HIV RNA over time estimated using CEPAC and the parametric g-formula (Appendix 3), and assessed the impact on the CEPAC model results of varying a range of additional input parameters (Appendix 4).
Results
Stage I: Comparison of the g-formula estimates with the original CEPAC model results
The HIV-CAUSAL Collaboration included 28,269 eligible individuals in 1996–1999, 25,433 in 2000–2002, and 78,093 in 2003 and onwards. Baseline characteristics are shown in Table 1. The median duration of follow-up was 65 months (IQR: 27–151) in 1996–1999, 64 months (IQR: 27–126) in 2000–2002, and 36 months (IQR: 18–66) in 2003 and onwards. 63% of participants initiated ART during follow-up when baseline was between 1996 and 1999, 61% when baseline was between 2000 and 2002, and 65% when baseline was in 2003 or later.
Table 3 compares the observed risks from HIV-CAUSAL with those estimated via the g-formula under no intervention on treatment, and indicates that the g-formula provides a good fit with the observational data. Additional sensitivity results for covariates are presented in Figures A2–A5. The g-formula estimates for AIDS onset are somewhat further from the observational data in the 1996–1999 baseline. This may be due to differences in confounders early in the HIV epidemic, and differences in the subset of individuals infected with HIV presenting to care. A similar process could be used to assess the calibrated CEPAC model, if the distribution of treatment initiation strategies in HIV-CAUSAL were known, by taking a weighted average of CEPAC results over the treatment strategies followed in the data.
Table 3.
Observed and estimated 7 year risk in each baseline cohort under no intervention, HIV-CAUSAL Collaboration
| Baseline period | Outcome | Observed risk at 7 years, % | Estimated risk at 7 years, % (95% CI)** |
|---|---|---|---|
| Jan 1, 1996 – Dec 31, 1999 | Mortality | 9.5 | 9.3 (8.8, 9.9) |
| AIDS or death | 16.2 | 16.6 (16.0, 17.3) | |
| Jan 1, 2000 – Dec 31, 2002 | Mortality | 8.3 | 8.2 (7.8, 8.7) |
| AIDS or death | 15.4 | 16.0 (15.4, 16.7) | |
| Jan 1, 2003 onwards | Mortality | 4.6 | 4.4 (4.2, 4.7) |
| AIDS or death | 9.5 | 9.5 (9.2, 9.8) |
HIV-CAUSAL estimates based on the parametric g-formula adjusted for measured time-varying confounders (CD4 count, HIV-RNA and AIDS) and baseline characteristics (calendar period and age of HIV diagnosis, risk group, gender, geographical origin, ethnicity and cohort). CI: confidence interval
The calibration targets obtained from the g-formula under the three interventions of interest are reported in Table 4. Using the g-formula, the estimated 7-year risk of mortality under immediate ART initiation was 8.0% (95% CI: 7.5, 8.5) for baseline in 1996–1999, 6.9% (95% CI: 6.4, 7.4) for baseline in 2000–2002, and 4.1% (95% CI: 3.9, 4.4) for baseline in 2003 and onwards (Table 4). The estimated 7-year risk of AIDS or death under immediate ART initiation was 14.0% (95% CI: 13.4, 14.6) in 1996–1999, 12.6% (95% CI: 12.0, 13.3) in 2000–2002, and 7.9% (95% CI: 7.6, 8.3) in 2003 and onwards (Table 4). Earlier treatment initiation resulted in decreased risks for both outcomes, in all time periods.
Table 4.
Risk of all-cause mortality and combined AIDS or death outcome in each baseline cohort by ART initiation strategy estimated using the parametric g-formula and the CEPAC model.
| All-cause mortality | AIDS or mortality | ||||||
|---|---|---|---|---|---|---|---|
| CEPAC* | Parametric g-formula** | CEPAC* | Parametric g-formula** | ||||
|
|
|||||||
| Baseline period | ART initiation strategy (CD4/μl) | Original† | Calibrated‡ | Risk, % (95% CI) | Original† | Calibrated‡ | Risk, % (95% CI) |
| Jan 1, 1996 –Dec 31, 1999 | Immediate universal | 7.9 | 5.1 | 8.0 (7.5, 8.5) | 19.8 | 8.9 | 14.0 (13.4, 14.6) |
| CD4 <500 | 8.0 | 5.5 | 8.2 (7.8, 8.7) | 20.0 | 10.7 | 14.5 (13.9, 15.1) | |
| CD4 <350 | 8.5 | 6.2 | 8.7 (8.3, 9.2) | 22.6 | 14.1 | 15.5 (15.0, 16.1) | |
| Jan 1, 2000 –Dec 31, 2002 | Immediate universal | 7.8 | 5.0 | 6.9 (6.4, 7.4) | 19.8 | 8.8 | 12.6 (12.0, 13.3) |
| CD4 <500 | 7.9 | 5.4 | 7.1 (6.6, 7.6) | 20.0 | 10.6 | 13.1 (12.5, 13.8) | |
| CD4 <350 | 8.4 | 6.1 | 7.5 (7.1, 8.0) | 22.5 | 14.0 | 14.2 (13.6, 14.9) | |
| Jan 1, 2003 onwards | Immediate universal | 7.0 | 4.2 | 4.1 (3.9, 4.4) | 19.0 | 8.0 | 7.9 (7.6, 8.3) |
| CD4 <500 | 7.0 | 4.6 | 4.2 (3.9, 4.4) | 19.2 | 9.7 | 8.3 (8.0, 8.6) | |
| CD4 <350 | 7.6 | 5.2 | 4.3 (4.1, 4.5) | 21.8 | 13.1 | 9.1 (8.8, 9.4) | |
CEPAC: Cost-Effectiveness of Preventing AIDS Complications model; OIs: opportunistic infections
HIV-CAUSAL estimates based on the parametric g-formula adjusted for measured time-varying confounders (CD4 count, HIV-RNA and AIDS) and baseline characteristics (calendar period and age of HIV diagnosis, risk group, gender, geographical origin, ethnicity and cohort). 95% CI: confidence interval
Original CEPAC settings: opportunistic infection (OI) incidence on-treatment multiplier and chronic AIDS-related mortality on-treatment multiplier both equal to 1.0
Calibrated CEPAC settings: opportunistic infection (OI) incidence on-treatment multiplier equal to 0.2 and chronic AIDS-related mortality on-treatment multiplier equal to 0.1.
The original CEPAC parameterization (using 1.0 for each of the on-treatment multipliers) resulted in 7-year mortality risk estimates that were within the 95% confidence intervals of the g-formula estimates under all treatment strategies for 1996–1999, but not for the 2000–2002 or 2003 and onwards (Table 3, original CEPAC column). The CEPAC estimates of the risk of combined AIDS/death outcome were higher than the g-formula estimates in all time periods. Appendix Figures A1 and A2 display the estimated survival and AIDS-free survival curves obtained via the g-formula and the original CEPAC model.
Stage II: Update of the simulation model parameters via calibration to g-formula targets
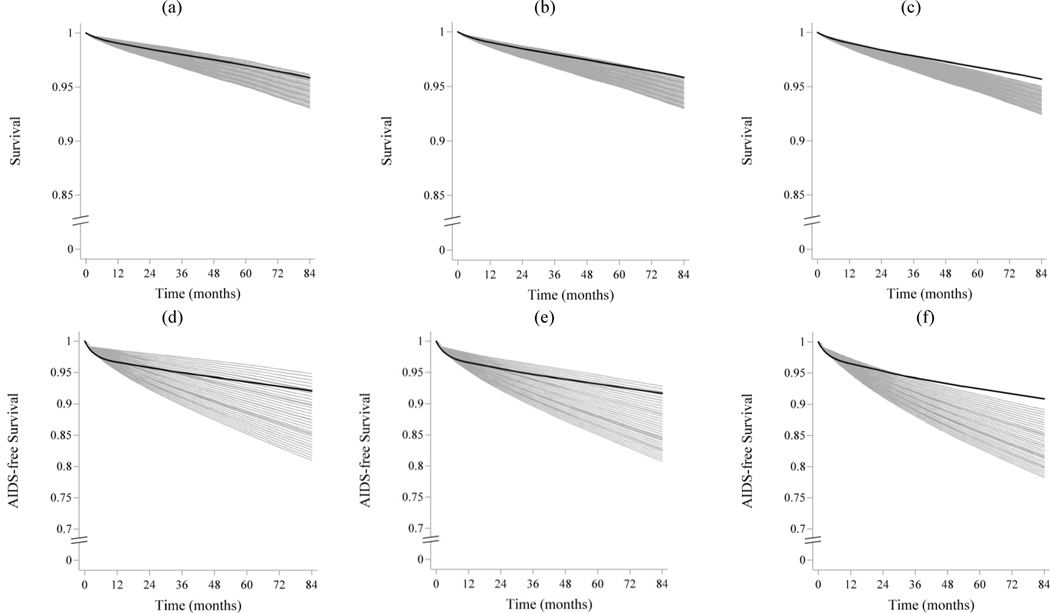
Figure 1 displays the survival and AIDS-free survival curves from all 36 CEPAC calibration runs compared to the g-formula estimates from 2003 and onwards (the CEPAC estimates are shown in grey and the observational estimates are shown in black). For the strategies of immediate initiation and initiation at CD4 <500, multiple CEPAC calibration parameter sets returned estimated survival curves that fit well with the observational estimates for both outcomes, although survival and AIDS-free survival curves were somewhat steeper in the first 6 to 12 months of follow-up when using the g-formula (Figure 1). In contrast, for initiation at CD4 <350, all CEPAC calibration parameter sets underestimated both survival and AIDS-free survival compared with the observational data (Figure 1).
Figure 1.
Survival and AIDS-free survival over follow-up estimated via the parametric g-formula applied to HIV-CAUSAL data and each of 36 CEPAC calibration runs, varying on-treatment multipliers for opportunistic infection incidence and chronic AIDS-related mortality from 0 to 1 by 0.2.
CEPAC calibration runs (grey);, parametric g-formula estimates (black). All runs have baseline on or after Jan 1, 2003. Survival (a–c), AIDS-free survival (d–f). Immediate universal treatment initiation(a,d); Initiation at CD4 <500 cells/μl(b,e); and Initiation at CD4 <350 cells/μl(c,f). CEPAC: Cost-Effectiveness of Preventing AIDS Complications model.
Figure 2 shows the 7-year mortality and combined AIDS or death risks in the 36 CEPAC calibration runs. The top left corner of each sub-figure represents the 7-year risk when the on-treatment multipliers for both OI incidence and chronic AIDS-mortality were set to 0, for a given treatment strategy. This is equivalent to a situation in which individuals on ART are not at risk for these outcomes. The bottom right corner of each sub-figure represents the scenario where both multipliers are set to 1, which would occur if the probability of OIs or chronic AIDS-related mortality was the same regardless of treatment status. In each sub-figure, the location in the heat map which is closest to the 7-year risk estimated from the observational data is indicated by the black box; the area of the heat map which produces estimates within the 95% confidence intervals estimated from the observational data is enclosed in grey boxes.
Figure 2.
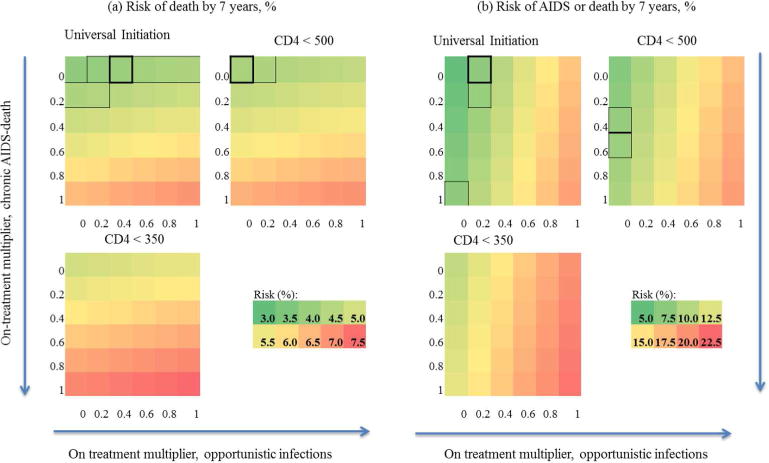
7-year mortality (a) and combined mortality/AIDS risk (b) from CEPAC calibration runs for baseline on or after Jan 1, 2003, varying on-ART multipliers for chronic AIDS-related mortality and opportunistic infections from 0 to 1 by 0.2, stratified by treatment strategy.
Scales for each outcome shown below the strategy ‘CD4 < 500’. On-treatment multiplier for chronic AIDS-related mortality increases from 0 to 1 down y-axis, on-treatment multiplier for opportunistic infections increases from 0 to 1 across x-axis, following direction of arrows. Black boxes indicate closest matchs to parametric g-formula estimates when baseline is on or after Jan 1, 2003; grey boxes indicate 95% confidence interval for parametric g-formula using 500 bootstrap samples. For the strategy ‘treat at CD4 < 350’, no CEPAC runs resulted in risk estimates within the g-formula 95% confidence intervals for either outcome. CEPAC: Cost-Effectiveness of Preventing AIDS Complications model.
The g-formula mortality risk at 7 years was reproducible for immediate initiation and initiation at CD4 <500 when the multiplier for chronic AIDS-related mortality was close to zero and the multiplier for OI incidence was at or below 0.2 (Figure 2). For initiation at CD4 <500 cells/μl the best fitting on-treatment multiplier for chronic AIDS-related mortality changed to between 0.4 and 0.6. For initiation at CD4 <350, all calibration runs returned estimates greater than the upper bound of the 95% confidence intervals from the g-formula estimates for both outcomes.
Based on the 36 calibration runs, we fine-tuned the on-treatment parameter values by applying a range of multipliers to the incidence of opportunistic infections and chronic AIDS-related mortality in CEPAC for simulated patients on ART and projected overall mortality and composite outcome of AIDS or death using this grid of possible multipliers. We compared these projections to those generated by parametric g-formula estimates from HIV-CAUSAL. Subsequently, we validated the newly calibrated CEPAC projections to mortality estimates from the COHERE cohort.(38) This fine-tuning resulted in final calibrated parameter values of 0.2 for OI incidence and 0.1 for chronic AIDS-related mortality. We used these values to run CEPAC in all three time periods and present the risk estimates obtained from this calibrated model under the three treatment initiation strategies in Table 3.
The mortality risk and the combined AIDS or mortality risk estimates from the calibrated CEPAC model were very close to the corresponding observational estimates for 2003 and onwards under immediate treatment initiation and under initiation at CD4 counts above 500 cells/μl, but over-estimated the observational estimates under initiation below 350 cells/μl (Table 3, calibrated column). Further calibration using the approach described here could be performed to improve model performance for delayed treatment initiation strategies. Here, we omit this calibration, since our goal is to demonstrate the process of using the g-formula for calibration rather than present a full calibration of CEPAC (calibrated CEPAC column).
Discussion
We have described a step-by-step procedure to identify counterfactual calibration targets from observational data for use in calibrating an individual-level simulation model. To do so, we estimated risks under several treatment strategies in an observational study (HIV-CAUSAL Collaboration) using the parametric g-formula, identified model (CEPAC) input parameters for which empirical data did not exist, and modified values of these parameters to match the counterfactual outcome estimates.
The parametric g-formula allows us to estimate the outcome distributions under any treatment strategy of interest followed by at least some individuals in the data. Unlike traditional regression approaches, the parametric g-formula is able to provide unbiased outcome estimates even in the presence of treatment-confounder feedback – that is, even when time-varying confounders are themselves affected by prior treatment.(11, 12, 22, 39) A similar approach could be used to estimate counterfactual calibration targets under other types of interventions, such as treatment interruption, monitoring of treatment efficacy, or treatment switching, and is particularly useful when complex interventions which depend on the evolution of time-varying markers of disease progression are of interest. For example, the g-formula could be used to obtain calibration targets for a cardiovascular risk model under a range of statin use strategies. Finally, even when trials with appropriate interventions exist, non-adherence and loss to follow-up may make intention-to-treat estimates insufficient. In these cases, obtaining appropriate calibration targets may require adjustment for time-varying non-adherence or analysis of intermediate outcomes, and can benefit from the use of methods such as the parametric g-formula. (10, 11, 22, 40, 41)
Our case study was based on the HIV-CAUSAL Collaboration, a consortium of observational cohorts that collect longitudinal data on treatments, confounders, and outcomes. The validity of this calibration procedure relies critically on the validity of the parametric g-formula estimates. The parametric g-formula requires the assumptions of no unmeasured treatment-outcome confounders and no misspecification of the parametric models for treatment, confounders, and outcomes. Although unmeasured confounding is always possible in observational studies, we adjusted our models for the main clinical indications for treatment initiation during the study period such as CD4 count, HIV RNA, and AIDS diagnosis, as well as for other potential confounders such as age, sex, geographic origin, and mode of transmission. We also assessed the potential for model misspecification in our parametric g-formula estimates by estimating the outcome distributions under the observed distribution of treatment initiation strategies in HIV-CAUSAL, and found good agreement between the data and our model results.
In our analysis, survival and AIDS-free survival both increased substantially between the three baseline time periods when using the g-formula. This trend for improved survival of people living with HIV has been observed elsewhere. (42) The original CEPAC parameterization resulted in estimates that fit well with the g-formula estimates from 1996-1999, but not with those from most recent periods and CEPAC does not currently model changes in parameters by calendar time. After calibration, the CEPAC model fit for the 2003 onwards baseline period was greatly improved especially under immediate treatment initiation and initiation at CD4 counts below 500 cells/μl.
The validity of the calibration also relies on the assumption that the population used for calibration experiences the same treatment effects and outcome risks as the target population for model inference. In our case, a majority of HIV-CAUSAL participants live in Europe, while the CEPAC-US model has generally been used to estimate outcomes in the United States. Differences in access to, and cascades of, care, underlying mortality risks, or infectious disease transmission between these regions may explain some of the differences we observed for the strategy of initiating treatment at CD4 counts below 350 cells/μl in recent years.(42) Individuals who delayed treatment initiation in the post-2003 period are likely to have presented to care later in the disease process, and those presenting late may differ between US and European populations. Therefore, estimates of calibration targets based on HIV-CAUSAL for this strategy may be less appropriate to compare with US-specific CEPAC estimates.
However, comparison of our calibrated CEPAC results with previous studies suggests that, for individuals who are retained in care and on treatment, mortality risk may not differ substantially between American and European populations. A study of a highly adherent cohort of people living with HIV in the USA found a projected mean life expectancy of 69.3 years from the time of seroconversion,(43) whereas we estimate a mean life expectancy of 67.6 years using the newly calibrated CEPAC model. The calibrated CEPAC model also matched previously published AIDS-related death rates from the COHERE collaboration within a margin of 5 deaths per 10,000 people over a 2.7 year period. Five-year survival estimates from the calibrated CEPAC model were within 2% of previously published HIV-CAUSAL estimates for the strategy of immediate treatment initiation.(20)
Here, we used a simplified process for varying and assessing parameter fit – a grid search of parameter space, and a comparison with 95% confidence intervals of the calibration targets. More sophisticated search techniques and methods of assessing goodness of fit between model outputs and calibration targets exist and could result in further improvements in the calibration.(8, 44–48) In addition, in order to focus on the process of obtaining a range of counterfactual calibration targets, we did not perform a full calibration to fit the model results for all outcomes. However, we were able to greatly improve the fit of the CEPAC model to the strategy of immediate treatment initiation for the period of 2003 onwards, which is the strategy currently recommended for people living with HIV in the US and will therefore likely serve as a comparison strategy for future analyses.(49)
In summary, the original CEPAC model performed well when compared to calibration targets estimated from data starting in 1996–1999 – the time period for which the model was developed – but recalibration of CEPAC parameters was required to obtain a fit with targets estimated from 2003 onwards. This highlights the fact that populations can change over time such that periodic recalibration of individual-level simulation models may be needed to ensure that these models remain relevant over time. Similarly, recalibration of simulation models may be needed when the target of inference changes to a new population. Calibration must thus be an ongoing process to keep simulation models up-to-date and relevant for the population, or populations, of interest. However, randomized controlled trials will likely not be available for all populations or time periods of interest. The parametric g-formula can provide a solution by increasing the availability of calibration targets for these models.
Acknowledgments
We are indebted to the contributors to the HIV-CAUSAL Collaboration (Appendix 5). We thank Michael Girouard, Moses Flash, Mingshu Huang, and Ethan Borre for their support and advice on the CEPAC model. We also thank Drs Roger Logan, Lauren Cain, and Ellen Caniglia for their technical assistance, and Drs Marc Lipsitch, Andrew Philips, and Caroline Sabin for helpful comments to an earlier version of the manuscript.
Funding: Supported by NIH grants R01-AI073127, R01-AI042006, K01-HL123349, and K01-DA042687.
Financial support for this study was provided in part by grants from the NIH. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
The project described was supported by Grant Numbers R01AI073127 and R01AI042006 from the National Institute of Allergy and Infectious Diseases; Grant Number K01HL123349 from the National Heart, Lung, and Blood Institute; and Grant Number K01DA042687 from the National Institute on Drug Abuse. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Appendix
1. HIV-CAUSAL Collaboration detailed baseline characteristics
The main text (Table 1) provides a summary of relevant HIV-CAUSAL cohort baseline covariates necessary for parameterizing the CEPAC runs. We provide additional information on the cohort profiles in Appendix Tables A1, A2, A3, and A4 to provide further context to our results.
2. CEPAC input parameter sources
The main text (Table 2) provides the value of key CEPAC input parameters. We provide additional information on the source of these parameters here to provide further context to our results (Appendix Table A5).
Life table data were obtained based on average values for France, Greece, Netherlands, Spain, Switzerland, and the UK. For 1996–1999, we used lifetables for the period 1996–2013; for 2000–2002, we used 2000–2013; and for 2003 and onwards, 2003–2013. This parameter is independent of treatment status.
Among individuals not on ART, CD4 cell counts decline each month (34, 35), and HIV RNA remains at an individual-specific set point. The monthly probabilities of opportunistic infections (OIs), chronic AIDS-related mortality, and acute OI-related mortality vary over time based on current CD4 cell count in a given month. When not on ART, these probabilities were obtained from the Multi-center AIDS Cohort Study (MACS). (32)
Use of ART affects simulated individuals through three main mechanisms: reducing in HIV RNA, increasing CD4 cell count, and lowering the probabilities of OIs and of mortality. The overall probability of virologic suppression (HIV RNA reduced to lowest stratum) within 6 months of treatment initiation is 86.0%.(36) To model heterogeneity of response to ART, each individual is assigned a probability of treatment response, a measure of treatment adherence, which is multiplied by the overall probability of virologic suppression to determine the individual’s final probability of suppression. CD4 cell count increases by a mean of 88 cells/μl in the first two months after treatment initiation, and a mean of 4 cells/μl in the following months (36, 50). This CD4 cell count increase reduces the probability of OI incidence, acute OI-related mortality, and chronic AIDS-related mortality, since these probabilities are CD4-dependent. In addition, the probabilities of chronic AIDS-related mortality, and OI incidence can be further adjusted by ‘on treatment’ multipliers stratified by CD4 cell count and OI type (Pneumocystis pneumonia, Mycobacterium avium complex, Toxoplasmosis, Cytomegalovirus, fungal infections, other OIs).
Individuals who achieve virologic suppression have a monthly probability of treatment failure, meaning that treatment no longer decreases the individual’s HIV RNA or increases their CD4 count. Individuals who fail treatment will experience stable, or declining CD4 counts, and rising HIV RNA. At their next modeled clinic visit, these individuals can be detected as failing and changed to a new ART regimen. There are 6 lines of ART available in the model all with the same efficacy; individuals who fail all 6 lines remained on the last failed ART regimen.
3. Survival and AIDS-free survival distributions by antiretroviral therapy initiation strategy and baseline time period
Figures A1 and A2 show the survival and AIDS-free survival estimates over time for each baseline time period and antiretroviral therapy initiation strategy, estimated using the parametric g-formula applied to HIV-CAUSAL data and using the original CEPAC parameterization.
4. Mean of study variables over time observed in HIV-CAUSAL and estimated using the parametric g-formula
Figures A3, A4, and A5 compare the means of treatment and study covariates over time estimated using the parametric g-formula under the strategy “start treatment with the observed conditional probability from the data” with the corresponding means observed in HIV-CAUSAL. Though this comparison can detect model misspecification in the parametric g formula, the lack of differences does not guarantee no model misspecification under other strategies. If the distribution of treatment initiation strategies followed by individuals in HIV-CAUSAL were known, a similar comparison could be made between CEPAC estimates of treatment and covariates over time and the observed means. However, parameterizing treatment to match the observed conditional probability distributions in a simulation model may often not be feasible.
5. Progression of CD4 count and viral load
Some differences in survival and AIDS onset estimates between CEPAC and the g-formula may be related to differences in progression of CD4 count and HIV RNA over time. Figure A6 shows the estimated mean CD4 cell count and mean HIV RNA levels from the g-formula applied to HIV-CAUSAL, and from CEPAC for the 2003 baseline, by treatment initiation strategy, among individuals remaining alive at each month over follow-up. The CEPAC runs shown here were obtained from the original parameterization, where the treatment multipliers for OIs and for chronic AIDS-related mortality were both set to 1. Mean CD4 counts improved over the three time periods and were higher when treatment was initiated earlier for both CEPAC and the parametric g-formula. Mean CD4 counts improved more rapidly and reached much higher levels by the end of follow-up in CEPAC than with the parametric g-formula. Mean HIV RNA level decreased over the three time periods and with earlier treatment initiation. HIV RNA levels were more variable in the parametric g-formula estimates than in CEPAC, especially in the earlier baseline periods.
6. Additional parameters assessed in calibration
We first varied a range of parameters to identify those to which the CEPAC results were most sensitive. Specifically, we varied the 1-month probabilities of OI incidence and chronic AIDS-related mortality when not on-treatment, the on-treatment multipliers for OI incidence and chronic AIDS-related mortality, the highest CD4 count stratum at which OIs and/or chronic AIDS-related mortality could occur while on treatment, the probability of acute OI-related mortality (on and off treatment), the probability of treatment response, and the probability of treatment failure after suppression. To assess the importance of specific baseline characteristics, we also varied the distributions for baseline CD4 cell counts and HIV RNA strata, and assessed using transmission risk group distributions and sex-stratified lifetables from the USA only (28).
Changes to the on-treatment multipliers for OIs and chronic AIDS-related mortality, distribution of probability of response, and probability of treatment failure were the most effective in reducing the discrepancies between the CEPAC and observational estimates. We selected the on-treatment multipliers for our main calibration example since empirical data regarding the appropriate value of these parameters did not exist.
7. Contributors to the HIV-CAUSAL Collaboration
AMACS
Steering Committee: Antoniadou A., Chrysos G., Daikos G., Gargalianos Kakolyris P., Gogos HA., Katsarou O., Kordossis T., Lazanas M., Nikolaidis P., Panos G., Paparizos V., Paraskevis D., Sambatakou H., Skoutelis A., Touloumi G. (Chair). Coordinating Center: Department of Hygiene, Epidemiology and Medical Statistics, Athens University Medical School, Greece (Touloumi G., Pantazis N., Vourli G., Gountas I., Gioukari V.)
Participating Centers: 4th Dept of Internal Medicine, Athens Medical School, Attikon University Hospital (Antoniadou A, Papadopoulos A, Petrikkos G); Infectious Disease Unit, “Tzaneio” General Hospital of Pireaus (Chrysos G, Paraskeva D, Hatziastros P); 1st Dept of Propedeutic Medicine, Athens University, Medical School “Laikon” General Hopsital (Daikos G, Psichogiou M); 1st Dept of Medicine, Infectious Diseases Unit, “G. Gennimatas” Athens General Hospital (Gargalianos Kakolyris P, Xylomenos G); 1st Dept of Internal Medicine, Infectious Diseases Section, Patras University Hospital (Gogos HA, Marangos MN, Panos G); Haemophilia Centre, 2nd Blood Transfusion Centre, “Laikon” Athens General Hospital (Katsarou O, Kouramba A, Ioannidou P); AIDS Unit, Dept of Pathophysiology, “Laikon” Athens General Hospital and Athens University, Medical School (Kordossis T, Kontos A); Infectious Diseases Unit, Red Cross General Hospital of Athens (Lazanas M, Chini M, Tsogas N); 1st Dept of Internal Medicine, Infectious Diseases Devision, AHEPA University Hospital, Aristotle University HIV Unit (Nikolaidis P, Kolaras P, Metallidis S); 2nd Internal Medicine Clinic, 1st IKA (Panos G, Haratsis G); AIDS Unit, Clinic of Venereologic & Dermatologic Diseases, Athens University, Medical School, Syngros Hospital (Paparizos V, Leuow K, Kourkounti S); HIV Unit, 2nd Dpt. of Internal Medicine, Athens University, Medical School, Hippokration General Hospital (Sambatakou H, Mariolis I); Infectious Diseases & HIV Division, Dept of Internal Medicine, Evaggelismos Athens General Hospital (Skoutelis A, Papastamopoulos V, Baraboutis I).
AQUITAINE
Principal investigator: Pr F. Dabis. Scientific committee: Prs F. Bonnet, D. Breilh, F. Dabis, M. Dupon, G. Chêne, H. Fleury, D. Malvy, P. Mercié, I. Pellegrin, P. Morlat, D. Neau, JL. Pellegrin, R. Thiébaut; Drs S. Bouchet, V. Gaborieau, D. Lacoste, S. Tchamgoué. Epidemiology and biostatistics: Prs G. Chêne, F. Dabis, R. Thiébaut, Drs M. Bruyand, S. Lawson Ayayi, L. Wittkop. Clinical and biological hospital units: Bordeaux University Hospital: Pr P. Morlat (Pr F. Bonnet, Drs N. Bernard, M. Hessamfar, D. Lacoste, MA. Vandenhende); Pr M. Dupon (Drs FA. Dauchy, H. Dutronc), Pr M. Longy Boursier (Pr P. Mercié, Drs P. Duffau, J. Roger Schmeltz), Pr D. Malvy (Drs T. Pistone, MC Receveur), Pr D. Neau (Drs C. Cazanave, A. Ochoa, MO. Vareil), Pr JL. Pellegrin (Pr JF. Viallard, Drs C. Greib, E. Lazaro); Pr H. Fleury (Pr ME. Lafon, Drs S. Reigadas, P. Trimoulet); Pr D. Breilh; Pr M. Molimard (Drs S. Bouchet, K. Titier); Pr JF. Moreau (Dr I. Pellegrin); Drs F. Haramburu, G. Miremont Salamé. Arcachon Hospital: Dr A. Dupont. Dax Hospital: Dr Y. Gerard (Drs L. Caunègre, K. André). Bayonne Hospital: Dr F. Bonnal (Drs S. Farbos, MC. Gemain). Libourne Hospital: Dr J. Ceccaldi (Dr S. Tchamgoué). Mont de Marsan Hospital: Dr S. De Witte (Dr C. Courtault). Pau Hospital: Drs E. Monlun (Dr V. Gaborieau). Périgueux Hospital: Dr P. Lataste (Dr JP. Meraud). Villeneuve-sur-Lot Hospital: Dr I. Chossat. Permanent team: MJ. Blaizeau, M. Bruyand, V. Conte, M. Decoin, J. Delaune, S. Delveaux, F. Diarra, C. D’Ivernois, A. Frosch, S. Geffard, C. Hannapier, S. Lawson-Ayayi, E. Lenaud, O. Leleux, F. Le Marec, J. Leray, I. Louis, G. Palmer, A. Pougetoux, X. Sicard, D. Touchard B. Uwamaliya Nziyumvira.
ATHENA
The ATHENA database is maintained by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment. CLINICAL CENTRES (* denotes site coordinating physician). Academic Medical Centre of the University of Amsterdam: HIV treating physicians: J.M. Prins*, T.W. Kuijpers, H.J. Scherpbier, J.T.M. van der Meer, F.W.M.N. Wit, M.H. Godfried, P. Reiss, T. van der Poll, F.J.B. Nellen, S.E. Geerlings, M. van Vugt, D. Pajkrt, W.J. Wiersinga, M. van der Valk, A. Goorhuis, J.W. Hovius. HIV nurse consultants: M.A.H. Bijsterveld, J. van Eden, A.M.H. van Hes, M. Mutschelknauss, H.E. Nobel, F.J.J. Pijnappel, A.M. Weijsenfeld. HIV clinical virologists/chemists: S. Jurriaans, N.K.T. Back, H.L. Zaaijer, B. Berkhout, M.T.E. Cornelissen, C.J. Schinkel, X.V. Thomas. Admiraal De Ruyter Ziekenhuis, Goes: HIV treating physicians: M. van den Berge, A. Stegeman. HIV nurse consultants: S. Baas, L. Hage de Looff. HIV clinical virologists/chemists: B Wintermans, J Veenemans. Catharina Ziekenhuis, Eindhoven: HIV treating physicians: M.J.H. Pronk*, H.S.M. Ammerlaan. HIV nurse consultants: E.S. de Munnik, E. van Beek. HIV clinical virologists/chemists: A.R. Jansz, J. Tjhie, M.C.A. Wegdam, B. Deiman, V. Scharnhorst. Elisabeth-TweeSteden Ziekenhuis, Tilburg: HIV treating physicians: M.E.E. van Kasteren*, A.E. Brouwer. HIV nurse consultants: R. van Erve, B.A.F.M. de Kruijf-van de Wiel, S.Keelan-Pfaf, B. van der Ven. Data collection: B.A.F.M. de Kruijf-van de Wiel, B. van der Ven. HIV clinical virologists/chemists: A.G.M. Buiting, P.J. Kabel, D.Versteeg. Emma Kinderziekenhuis: HIV nurse consultants: A. van der Plas, A.M. Weijsenfeld. Erasmus MC, Rotterdam: HIV treating physicians: M.E. van der Ende*, H.I. Bax, E.C.M. van Gorp, J.L. Nouwen, B.J.A. Rijnders, C.A.M. Schurink, A. Verbon, T.E.M.S. de Vries-Sluijs. HIV nurse consultants: N. Bassant, J.E.A. van Beek, M. Vriesde, L.M. van Zonneveld. Data collection: H.J. van den Berg-Cameron, F.B. Bruinsma-Broekman, J. de Groot, M. de Zeeuw-de Man. HIV clinical virologists/chemists: C.A.B. Boucher, M.P.G Koopmans, J.J.A van Kampen, S.D. Pas. Erasmus MC–Sophia, Rotterdam: HIV treating physicians: G.J.A. Driessen, A.M.C. van Rossum. HIV nurse consultants: L.C. van der Knaap, E. Visser. Flevoziekenhuis, Almere: HIV treating physicians: J. Branger*, A. Rijkeboer-Mes. HIV nurse consultant and data collection: C.J.H.M. Duijf-van de Ven. HagaZiekenhuis, Den Haag: HIV treating physicians: E.F. Schippers*, C. van Nieuwkoop. HIV nurse consultants: J.M. van IJperen, J. Geilings. Data collection: G. van der Hut. HIV clinical virologist/chemist: P.F.H. Franck. HIV Focus Centrum (DC Klinieken): HIV treating physicians: A. van Eeden*. HIV nurse consultants: W. Brokking, M. Groot, L.J.M. Elsenburg. HIV clinical virologists/chemists: M. Damen, I.S. Kwa. Isala, Zwolle: HIV treating physicians: P.H.P. Groeneveld*, J.W. Bouwhuis. HIV nurse consultants: J.F. van den Berg, A.G.W. van Hulzen. Data collection: G.L. van der Bliek, P.C.J. Bor. HIV clinical virologists/chemists: P. Bloembergen, M.J.H.M. Wolfhagen, G.J.H.M. Ruijs. Leids Universitair Medisch Centrum, Leiden: HIV treating physicians: F.P. Kroon*, M.G.J. de Boer, H. Jolink, A.M. Vollaard. HIV nurse consultants: W. Dorama, N. van Holten. HIV clinical virologists/chemists: E.C.J. Claas, E. Wessels. Maasstad Ziekenhuis, Rotterdam: HIV treating physicians: J.G. den Hollander*, K. Pogany, A. Roukens. HIV nurse consultants: M. Kastelijns, J.V. Smit, E. Smit, D. Struik-Kalkman, C. Tearno. Data collection: M. Bezemer, T. van Niekerk. HIV clinical virologists/chemists: O. Pontesilli. Maastricht UMC+, Maastricht: HIV treating physicians: S.H. Lowe*, A.M.L. Oude Lashof, D. Posthouwer. HIV nurse consultants: R.P. Ackens, J. Schippers, R. Vergoossen. Data collection: B. Weijenberg-Maes. HIV clinical virologists/chemists: I.H.M. van Loo, T.R.A. Havenith. MCH-Bronovo, Den Haag: HIV treating physicians: E.M.S. Leyten*, L.B.S. Gelinck. HIV nurse consultants: A.Y. van Hartingsveld, C. Meerkerk, G.S. Wildenbeest. HIV clinical virologists/chemists: J.A.E.M. Mutsaers, S.Q. van Veen. MC Slotervaart, Amsterdam: HIV treating physicians: J.W. Mulder*, S.M.E. Vrouenraets, F.N. Lauw. HIV nurse consultants: M.C. van Broekhuizen, H. Paap, D.J. Vlasblom. HIV clinical virologists/chemists: P.H.M. Smits. MC Zuiderzee, Lelystad: HIV treating physicians: S. Weijer*, R. El Moussaoui. HIV nurse consultant: A.S. Bosma. Medisch Centrum Leeuwarden, Leeuwarden: HIV treating physicians: M.G.A.van Vonderen*, D.P.F. van Houte, L.M. Kampschreur. HIV nurse consultants: K. Dijkstra, S. Faber. HIV clinical virologists/chemists: J Weel. Medisch Spectrum Twente, Enschede: HIV treating physicians: G.J. Kootstra*, C.E. Delsing. HIV nurse consultants: M. van der Burg van de Plas, H. Heins. Data collection: E. Lucas. Noordwest Ziekenhuisgroep, Alkmaar: HIV treating physicians: W. Kortmann*, G. van Twillert*, J.W.T. Cohen Stuart, B.M.W. Diederen, R. Renckens. HIV nurse consultant and data collection: D. Ruiter-Pronk, F.A. van Truijen-Oud. HIV clinical virologists/chemists: W. A. van der Reijden, R. Jansen. OLVG, Amsterdam: HIV treating physicians: K. Brinkman*, G.E.L. van den Berk, W.L. Blok, P.H.J. Frissen, K.D. Lettinga W.E.M. Schouten, J. Veenstra. HIV nurse consultants: C.J. Brouwer, G.F. Geerders, K. Hoeksema, M.J. Kleene, I.B. van der Meché, M. Spelbrink, H. Sulman, A.J.M. Toonen, S. Wijnands. HIV clinical virologists: M. Damen, D. Kwa. Data collection: E. Witte. Radboudumc, Nijmegen: HIV treating physicians: R. van Crevel*, M. Keuter, A.J.A.M. van der Ven, H.J.M. ter Hofstede, A.S.M. Dofferhoff. HIV nurse consultants: M. Albers, K.J.T. Grintjes-Huisman, M. Marneef, A. Hairwassers. HIV clinical virologists/chemists: J. Rahamat-Langendoen. HIV clinical pharmacology consultant: D. Burger. Rijnstate, Arnhem: HIV treating physicians: E.H. Gisolf*, R.J. Hassing, M. Claassen. HIV nurse consultants: G. ter Beest, P.H.M. van Bentum, N. Langebeek. HIV clinical virologists/chemists: R. Tiemessen, C.M.A. Swanink. Spaarne Gasthuis, Haarlem: HIV treating physicians: S.F.L. van Lelyveld*, R. Soetekouw. HIV nurse consultants: L.M.M. van der Prijt, J. van der Swaluw. Data collection: N. Bermon. HIV clinical virologists/chemists: W.A. van der Reijden, R. Jansen, B.L. Herpers, D.Veenendaal. Medisch Centrum Jan van Goyen, Amsterdam: HIV treating physicians: D.W.M. Verhagen. HIV nurse consultants: M. van Wijk. Universitair Medisch Centrum Groningen, Groningen: HIV treating physicians: W.F.W. Bierman*, M. Bakker, J. Kleinnijenhuis, E. Kloeze, H. Scholvinck, Y. Stienstra, C.L. Vermont, K.R. Wilting. HIV nurse consultants: A. Boonstra, H. de Groot de Jonge, P.A. van der Meulen, D.A. de Weerd. HIV clinical virologists/chemists: H.G.M. Niesters, C.C. van Leer Buter, M. Knoester. Universitair Medisch Centrum Utrecht, Utrecht: HIV treating physicians: A.I.M. Hoepelman*, J.E. Arends, R.E. Barth, A.H.W. Bruns, P.M. Ellerbroek, T. Mudrikova, J.J. Oosterheert, E.M. Schadd, M.W.M. Wassenberg, M.A.D. van Zoelen. HIV nurse consultants: K. Aarsman, D.H.M. van Elst-Laurijssen, E.E.B. van Oers-Hazelzet. Data collection: M. van Berkel. HIV clinical virologists/chemists: R. Schuurman, F. Verduyn-Lunel, A.M.J. Wensing. VUmc, Amsterdam: HIV treating physicians: E.J.G. Peters*, M.A. van Agtmael, M. Bomers, J. de Vocht. HIV nurse consultants: M. Heitmuller, L.M. Laan. HIV clinical virologists/chemists: C.W. Ang, R. van Houdt, A.M. Pettersson, C.M.J.E. Vandenbroucke-Grauls. Wilhelmina Kinderziekenhuis, UMCU, Utrecht: HIV treating physicians: S.P.M. Geelen, T.F.W. Wolfs, L.J. Bont. HIV nurse consultants: N. Nauta. COORDINATING CENTRE. Director: P. Reiss. Data analysis: D.O. Bezemer, A.I. van Sighem, C. Smit, F.W.M.N. Wit, T.S. Boender. Data management and quality control: S. Zaheri, M. Hillebregt, A. de Jong. Data monitoring: D. Bergsma, A. de Lang, S. Grivell, A. Jansen, M.J. Rademaker, M. Raethke, R. Meijering, S. Schnörr. Data collection: L. de Groot, M. van den Akker, Y. Bakker, E. Claessen, A. El Berkaoui, J. Koops, E. Kruijne, C. Lodewijk, L. Munjishvili, B. Peeck, C. Ree, R. Regtop, Y. Ruijs, T. Rutkens, L. van de Sande, M. Schoorl, A. Timmerman, E. Tuijn, L. Veenenberg, S. van der Vliet, A. Wisse, T. Woudstra. Patient registration: B. Tuk.
CoRIS/CoRIS-MD
Steering committee: S Moreno, J del Amo, D Dalmau, ML Navarro, MI González, JL Blanco, F Garcia, R Rubio, JA Iribarren, F Gutiérrez, F Vidal, J Berenguer, J González. Field work, data management, and statistical analyses: P Sobrino, I Jarrín, B Alejos, V Hernando, D Alvarez, C Moreno. Participating centres: Hospital General Universitario de Alicante, Alicante (J Portilla, E Merino, S Reus, V Boix, L Giner, C Gadea, I Portilla, M Pampliega, M Díez, JC Rodríguez, J Sánchez Payá) ; Hospital Universitari de Bellvitge, Badalona (D Podzamczer, E Ferrer, A Imaz, E Van Den Eyncle, S Di Yacovo, M Sumoy); Hospital Universitario de Canarias, Santa Cruz de Tenerife (JL Gómez, J Hernández, MR Alemán, MM Alonso, MI Hernández, F Díaz Flores, D García, R Pelazas) ; Hospital Universitario Central de Asturias, Oviedo (V Asensi, E Valle, JA Cartón); Hospital Clínico San Carlos, Madrid (V Estrada, MJ Téllez, J Vergas, E Pérez Cecila); Hospital Doce de Octubre, Madrid (R Rubio, F Pulido, O Bisbal, M Matarranz, M Lagarde, R Rubio Martín, A Hernando, L Bermejo, L Dominguez); Hospital Universitario Donostia, San Sebastián (JA Iribarren, J Arrizabalaga, MJ Aramburu, X Camino, F Rodríguez Arrondo, MÁ von Wichmann, L Pascual, MÁ Goenaga, MJ Bustinduy, H Azkune, M Ibarguren, M Aguado, M Umerez); Hospital General Universitario de Elche, Elche (F Gutiérrez, M Masiá, C López, S Padilla, A Navarro, F Montolio, C Robledano, JG Colomé, A Adsuar, R Pascual, F Carlos, M Martinez, J Llenas, M Fernández, E García); Hospital Germans Trías i Pujol, Badalona (R Muga, J Tor, A Sanvisens); Hospital General Universitario Gregorio Marañón, Madrid (J Berenguer, JC López Bernaldo de Quirós, P Miralles, I Gutiérrez, M Ramírez, B Padilla, P Gijón, A Carrero, T Aldamiz Echevarría, F Tejerina, FJ Parras, P Balsalobre, C Diez; Hospital Universitari de Tarragona Joan XXIII, IISPV, Universitat Rovira i Virgili, Tarragona (F Vidal, J Peraire, C Viladés, S Veloso, M Vargas, M López Dupla, M Olona, A Aguilar, JJ Sirvent, V Alba, O Calavia; Hospital Universitario La Fe, Valencia (M Montero, J Lacruz, M Blanes, E Calabuig, S Cuellar, J López, M Salavert) ; Hospital Universitario La Paz/IdiPaz, Madrid (J González, I Bernardino, JR Arribas, ML Montes, JM Peña, B Arribas, JM Castro, FJ Zamora, I Pérez, M Estébanez, S García, M Díaz, NS Alcáriz, J Mingorance, D Montero, A González, MI de José); Hospital de la Princesa, Madrid (I de los Santos, J Sanz, A Salas, C Sarriá, A Gómez Berrocal, L Garcia Fraile; Hospital San Pedro-CIBIR, Logroño (JA Oteo, JR Blanco, V Ibarra, L Metola, M Sanz, L Pérez-Martínez) ; Hospital Universitario Miguel Servet, Zaragoza (A Pascual, C Ramos, P Arazo, D Gil); Hospital Universitari Mutua de Terrassa, Terrassa (D Dalmau, A Jaén, M Cairó, D Irigoyen, Q Jordano, M Xercavins, J Martinez-Lacasa, P Velli, R Font, M Sanmartí, L Ibáñez; Complejo Hospitalario de Navarra, Pamplona (M Rivero, MI Casado, JA Díaz, J Uriz, J Repáraz, C Irigoyen, MJ Arraiza); Hospital Parc Taulí, Sabadell (F Segura, MJ Amengual, G Navarro, M Sala, M Cervantes, V Pineda, V Segura, M Navarro, E Antón, MM Nogueras); Hospital Ramón y Cajal, Madrid (S Moreno, JL Casado, F Dronda, A Moreno, MJ Pérez Elías, D López, C Gutiérrez, N Madrid, A Lamas, P Martí, A de Diaz, S Serrrano, L Donat); Hospital Reina Sofía, Murcia (A Cano, E Bernal, Á Muñoz); Hospital San Cecilio, Granada (F García, J Hernández, A Peña, L Muñoz, J Parra, M Alvarez, N Chueca, V Guillot, D Vinuesa, JA Fernández); Centro Sanitario Sandoval, Madrid (J Del Romero, C Rodríguez, T Puerta, JC Carrió, M Vera, J Ballesteros); Hospital de la Santa Creu i Sant Pau, Barcelona (P Domingo, MA Sambeat, K Lamarca, G Mateo, M Gutiérrez, I Fernández); Hospital Universitario Santiago de Compostela, Santiago de Compostela (A Antela, E Losada); Hospital Son Espases, Palma de Mallorca (M Riera, M Peñaranda, M Leyes, MA Ribas, AA Campins, C Vidal, L Gil, F Fanjul, C Marinescu); Hospital Universitari Vall d´Hebron, Barcelona (E Ribera); Hospital Virgen de la Victoria, Málaga (J Santos, M Márquez, I Viciana, R Palacios, I Pérez, CM González); Hospital Universitario Virgen del Rocío, Sevilla (P Viciana, M Leal, LF López Cortés, N Espinosa); Hospital Universitario de Basurto, Bilbao (J Muñoz, M Zuriñe Zubero, J Mirena, S Ibarra, O Ferrero, J López de Munain, MM Cámara, I López, M de la Peña); Hospital Universitario Infanta Sofía, San Sebastián de los Reyes (I Suárez-García, E Malmierca); Hospital Universitario Costa del Sol, Marbella (J Olalla, A del Arco, J de la Torre, JL Prada, Z Caracuel); Hospital del Poniente, El Ejido (AM Lopez-Lirola, AB Lozano, E Fernández, I Pérez, JM Fernández); Hospital Universitario Santa Lucia, Cartagena (OJ Martínez, FJ Vera, L Martínez, J García, B Alcaraz, A Jimeno); INIBIC-Complejo Hospitalario Universitario de A Coruña, A Coruña (E Poveda, B Pernas, A Mena, M Grandal, A Castro, JD Pedreira); Hospital Clínico Universitario Virgen de la Arrixaca, Murcia (C Galera, H Albendin, A Iborra, A Moreno, MA Campillo, A Vidal); Hospital Marina Baixa, Villajoyosa (C Amador, F Pasquau, J Ena, C Benito, V Fenoll); Complejo Hospitalario de Jaén, Jaén (MO Mohamed Balghata, MA Gómez); Hospital San Agustín de Avilés, Avilés (MA de Zarraga, ME Rivas); Fundación Jiménez Diaz,Madrid (M Górgolas).
FHDH-ANRS CO4
Scientific committee: S Abgrall, F Barin, M Bentata, E Billaud, F Boué, C Burty, A Cabié, D Costagliola, L Cotte, P De Truchis, X Duval, C Duvivier, P Enel, L Fredouille-Heripret, J Gasnault, C Gaud, J Gilquin, S Grabar, C. Katlama, MA Khuong, JM Lang, AS Lascaux, O Launay, A Mahamat, M Mary-Krause, S Matheron, JL Meynard, J Pavie, G Pialoux, F Pilorgé, I Poizot-Martin, C Pradier, J Reynes, E Rouveix, A Simon, P Tattevin, H Tissot-Dupont, JP Viard, N Viget. DMI2 coordinating center: French Ministry of Health (Valérie Salomon), Technical Hospitalization Information Agency, ATIH (N Jacquemet). Statistical analysis center: U943 INSERM et UPMC (S Abgrall, D Costagliola, S Grabar, M Guiguet, E Lanoy, L Lièvre, M Mary-Krause, H Selinger-Leneman), INSERM Transfert (JM Lacombe, V Potard). COREVIH: Paris area: Corevih Ile de France Centre (GH Pitié Salpétrière: F Bricaire, S Herson, C Katlama, A Simon; Hôpital Saint-Antoine: N Desplanque, PM Girard, JL Meynard, MC Meyohas, O Picard; Hôpital Tenon: J Cadranel, C Mayaud, G Pialoux), Corevih Ile de France Est (Hôpital Saint-Louis: JP Clauvel, JM Decazes, L Gerard, JM Molina; GH Lariboisière-Fernand Widal: M Diemer, P Sellier; Hôpital Avicenne: M Bentata, P Honoré; Hôpital Jean Verdier: V Jeantils, S Tassi; Hôpital Delafontaine: D Mechali, B Taverne), Corevih Ile de France Nord (Hôpital Bichat-Claude Bernard: E Bouvet, B Crickx, JL Ecobichon, S Matheron, C Picard-Dahan, P Yeni), Corevih Ile de France Ouest (Hôpital Ambroise Paré: H Berthé, C Dupont; Hôpital Louis Mourier: C Chandemerle, E Mortier; Hôpital Raymond Poincaré: P de Truchis), Corevih Ile de France Sud (Hôpital Européen Georges Pompidou: D Tisne-Dessus, L Weiss; GH Tarnier-Cochin: D Salmon; Hôpital Saint-Joseph: I Auperin, J Gilquin; Hôpital Necker adultes: L Roudière, JP Viard; Hôpital Antoine Béclère: F Boué, R Fior; Hôpital de Bicêtre: JF Delfraissy, C Goujard; Hôpital Henri Mondor: C Jung, Ph Lesprit; Hôpital Paul Brousse: D Vittecoq). Outside Paris area: Corevih Alsace (CHRU de Strasbourg: P Fraisse, JM Lang, D Rey; CH de Mulhouse: G Beck-Wirth), Corevih de l’Arc Alpin (CHU de Grenoble: JP Stahl, P Lecercq), Corevih Auvergne-Loire (CHU de Clermont-Ferrand: F Gourdon, H Laurichesse; CHRU de Saint-Etienne: A Fresard, F Lucht); Corevih Basse-Normandie (CHRU de Caen: C Bazin, R Verdon), Corevih Bourgogne (CHRU de Dijon: P Chavanet), Corevih Bretagne (CHU de Rennes: C Arvieux, C Michelet), Corevih Centre (CHRU de Tours: P Choutet, A Goudeau, MF Maître), Corevih Franche-Comté (CHRU de Besançon: B Hoen; CH de Belfort: P Eglinger, JP Faller); Corevih Haute-Normandie (CHRU de Rouen: F Borsa-Lebas, F Caron), Corevih Languedoc-Roussillon (CHU de Montpellier: J Reynes; CHG de Nîmes: JP Daures), Corevih Lorraine (Nancy Hôpital de Brabois: T May, C Rabaud; CHRU de Reims: JL Berger, G Rémy), Corevih de Midi-Pyrénées (Toulouse CHU Purpan: E Arlet-Suau, L Cuzin, P Massip, MF Thiercelin Legrand; Toulouse Hôpital la Grave: G Pontonnier; Toulouse CHU Rangueil), Corevih Nord Pas de Calais (CH de Tourcoing: N Viget, Y Yasdanpanah), Corevih PACA Est (Nice Hôpital Archet 1: P Dellamonica, C Pradier, P Pugliese; CHG Antibes-Juan les Pins: K Aleksandrowicz, D Quinsat), Corevih PACA Ouest (Marseille Hôpital de la Conception: I Ravaux, H Tissot-Dupont; Marseille Hôpital Nord: JP Delmont, J Moreau; Marseille Institut Paoli Calmettes: JA Gastaut; Marseille Hôpital Sainte-Marguerite: I Poizot Martin, F Retornaz, J Soubeyrand; Marseille Centre pénitentiaire des Baumettes: A Galinier, JM Ruiz; CHG d’Aix-En-Provence: T Allegre, PA Blanc; CH d’Arles: D Bonnet-Montchardon; CH d’Avignon: G Lepeu; CH de Digne Les Bains: P Granet-Brunello; CH de Gap: JP Esterni, L Pelissier; CH de Martigues: R Cohen-Valensi, M Nezri; CHI de Toulon: S Chadapaud, A Laffeuillade), Corevih Pays de la Loire (CHRU de Nantes: E Billaud, F Raffi), Corevih de la Vallée du Rhône (Lyon Hôpital de la Croix-Rousse: A Boibieux, D Peyramond; Lyon Hôpital Edouard Herriot: JM Livrozet, JL Touraine; Lyon Hôtel-Dieu: L Cotte, C Trepo). Overseas: Corevih Guadeloupe (CHRU de Pointe-à-Pitre: M Strobel; CH Saint-Martin: F Bissuel), Corevih Guyane (CHG de Cayenne: R Pradinaud, M Sobesky), Corevih Martinique (CHRU de Fort-de-France: A Cabié), Corevih de La Réunion (CHD Félix Guyon: C Gaud, M Contant).
Swiss HIV Cohort Study (SHCS): Aubert V, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Gorgievski M, Günthard H (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kovari H, Kouyos R, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, Rauch A (Chairman of the Scientific Board), Regenass S, Rickenbach M (Head of Data Center), Rudin C (Chairman of the Mother & Child Substudy), Schmid P, Schultze D, Schöni-Affolter F, Schüpbach J, Speck R, Staehelin C, Tarr P, Telenti A, Trkola A, Vernazza P, Weber R, Yerly S.
GEMES
Principal Investigator: R Muga/S Pérez-Hoyos. Data analysis center: S Pérez-Hoyos, A Schiaffino Centro Nacional de Epidemiología: J del Amo, D Alvarez, S Monge. Participating centres: Cohorte del Hospital Germans Trias I Pujol, Badalona (R Muga, A Sanvisens, B Clotet, J Tor, F Bolao, I Rivas, D Fuster), Cohorte de Madrid-Sandoval (J del Romero, P Raposo, C Rodríguez, M Vera), Cohorte de los CIPS de la Comunidad Valenciana (I Hurtado, J Belda, E Fernandez, I Alastrue, C Santos, T Tasa, A Juan, J Trullen), Cohortes de los CAS, de las Prisiones de Cataluña y de hemofílicos del Hospital Vall d´Hebron, Barcelona (P Garcia de Olalla, J Cayla, E Masdeu, H Knobel, JM Mirò, MA Sambeat, R Guerrero, E Rivera), Cohorte de hemofílicos del Hospital La Paz, Madrid (M Quintana, C Gonzalez), Cohorte de Navarra (J Castilla, M Guevara). Laboratory: C de Mendoza, N Zahonero, M Ortíz.
IPEC
Principal investigators: Beatriz Grinsztejn and Valdiléa G Veloso. Collaborators: Lara Coelho, Raquel De Boni, Dayse P Campos, Antonio G Pacheco, Paula M Luz, Rodrigo de C. Moreira, Ronaldo I Moreira, Ruth K Friedman, Marilia Santini Oliveira, Sandra W Cardoso, Monica Derrico, Sayonara R Ribeiro, Leonardo Eksterman, Hugo Perazzo, Estevão P. Nunes, Maria R. Guimarães, Rodolfo Castro, Marcelo Ribeiro-Alves, Katia Lemos, Jose Roberto Grangeiro, Mario Sergio Pereira, Luciane Velasque, Jose Ricardo Coutinho, Angela Cristina Andrade, Juliana Netto, Rodrigo Otavio Escada, Desiree Gomes Santos, Flaviana Pavan.
PISCIS
Coordinators: J. Casabona, Centre d’Estudis Epidemiològics les Infeccions de Transmissió Sexual i Sida de Catalunya (CEEISCAT), Jose M. Miró (Hospital Clínic de Barcelona Idibaps, Universitat de Barcelona, Barcelona, Spain). Field coordinator: A. Gallois (CEEISCAT). Steering committee: J. Casabona, A. Gallois, A. Esteve (CEEISCAT), Jose M. Miró (Hospital Clínic de Barcelona-Idibaps, Universitat de Barcelona), D. Podzamczer (Hospital de Bellvitge de Barcelona), J. Murillas (Hospital Son Espases). Scientific committee: JM Gatell, C. Manzardo (Hospital Clínic-Idibaps, Universitat de Barcelona), C. Tural, B. Clotet (Hospital Universitari Germans Trias i Pujol, Universitat Autónoma de Barcelona), E. Ferrer (Hospital de Bellvitge), M. Riera (Hospital Son Espases), F. Segura, G. Navarro (Corporación Sanitaria Universitaria Parc Taulí, Universitad Autónoma de Barcelona), L. Force (Hospital de Mataró), J. Vilaró (Hospital General de Vic), A. Masabeu (Hospital de Palamós), I. García (Hospital General d’Hospitalet), M.Guadarrama (Hospital Alt Penedès de Vilafranca), C. Cifuentes (Hospital Son Llàtzer), D. Dalmau, À. Jaen (Hospital Universitari Mútua de Terrassa), C. Agustí (CEEISCAT). Data Management and statistical analysis: A. Esteve, A. Montoliu (CEEISCAT), I. Pérez (Hospital Clínic-Idibaps, Universitat de Barcelona). Technical support: I. Pérez (Hospital Clínic de Barcelona-Idibaps, Universitat de Barcelona), Freyra Gargoulas (Hospital Son Espases and Hospital Son Llàtzer). Clinicians involved: JL Blanco, F. Garcia-Alcaide, E. Martínez, J. Mallolas, M. López-Dieguez, JF García-Goez, (Hospital Clínic-Idibaps, Universitat de Barcelona), G. Sirera, J. Romeu, A. Jou. E. Negredo, C. Miranda, MC Capitan (Hospital Universitari Germans Trias i Pujol, Universitat Autónoma de Barcelona), M. Saumoy, A. Imaz, JM Tiraboschi, O. Murillo, F. Bolao, C. Peña, C. Cabellos, M Masó, A. Vila (Hospital Universitari de Bellvitge), M. Sala, M. Cervantes, Mª Jose Amengual, M. Navarro, E Penelo (Corporación Sanitaria Universitaria Parc Taulí, Universitad Autónoma de Barcelona), P. Barrufet, G. Bejarano (Hospital de Mataró, Barcelona), J. Molina, M. Guadarrama, M. Alvaro, J. Mercadal (Hospital Alt Penedès de Vilafranca). Civil society representatives: Juanse Fernández (Comitè 1er de Desembre), Jesús E. Ospina (RedVIH).
PRIMO
JM Molina, B Loze (St Louis-Paris), P Morlat, M Bonarek, F Bonnet, C Nouts, I Louis (St André-Bordeaux), F Raffi, V Reliquet, F Sauser, C Biron, O Mounoury, H Hue, D Brosseau (Hotel Dieu-Nantes), JF Delfraissy, C Goujard, J Ghosn, MT Rannou (Bicêtre – Le Kremlin Bicêtre), JF Bergmann, E Badsi, A Rami, M Diemer, MParrinello (Lariboisière-Paris), PM Girard, D Samanon-Bollens, P Campa, M Tourneur, N Desplanques (St Antoine - Paris), JM Livrozet, F Jeanblanc, P Chiarello, D Makhloufi (E Herriot - Lyon), AP Blanc, T Allègre (CHG - Aix en Provence), J Reynes, V Baillat, V Lemoing, C Merle de Boever, C Tramoni (Gui de Chauliac-Montpellier), A Cabié, G Sobesky, S Abel, V Beaujolais (CHU - Fort de France), G Pialoux, L Slama, C Chakvetadze, V Berrebi (Tenon - Paris), P Yeni, E Bouvet, I Fournier, J Gerbe (Bichat - Paris), C Trepo, K Koffi, C Augustin-Normand, P Miailhes, V Thoirain, C Brochier (Hotel Dieu - Lyon), R Thomas, F Souala, M Ratajczak (Pontchaillou - Rennes), J Beytoux, C Jacomet, F Gourdon (G Montpied – Clermont-Ferrand), E Rouveix, S Morelon, C Dupont, C Olivier (A Paré - Boulogne), O Lortholary, B Dupont, JP Viard, A Maignan (Necker - Paris), JM Ragnaud, I Raymond (Pellegrin - Bordeaux), C Leport, C Jadand, C Jestin, P Longuet, S Boucherit (Bichat - Paris), D Sereni, C Lascoux, F Prevoteau (St Louis - Paris), A Sobel, Y Levy, JD Lelièvre, AS Lascaux, S Dominguez, C Dumont (H Mondor - Créteil), H Aumaître, B Delmas, M Saada, M Medus (St Jean - Perpignan), L Guillevin, D Salmon, T Tahi (Cochin - Paris), Y Yazdanpanah, S Pavel, MC Marien (CH Dron - Tourcoing), B Drenou, G Beck-Wirth, C Beck, M Benomar (E Muller - Mulhouse), C Katlama, R Tubiana, H Ait Mohand, A Chermak, S Ben Abdallah (Pitié-Salpétrière - Paris), M Bentata, F Touam, (Avicenne - Bobigny), B Hoen, C Drobacheff, A Folzer (St Jacques - Besançon), P Massip, M Obadia, L Prudhomme, E Bonnet, F Balzarin (Purpan * Toulouse), E Pichard, JM Chennebault, P Fialaire, J Loison (CHR - Angers), P Galanaud, F Boué, D Bornarel (Béclère Clamart), R Verdon, C Bazin, M Six, P Ferret (CHR Côte de Nacre - Caen), L Weiss, D Batisse, G Gonzales-Canali, D Tisne-Dessus (HEGP - Paris), A Devidas, P Chevojon, I Turpault (Corbeil Essonnes), A Lafeuillade, A Cheret, G Philip (Chalucet - Toulon), P Morel, J Timsit (St Louis - Paris), S Herson, N Amirat, A Simon, C Brancion (Pitié - Salpétrière - Paris), J Cabane, O Picard, J Tredup, N Desplanques (St Antoine - Paris), A Stein, I Ravault (La Conception - Marseille), C Chavanet, M Buisson, S Treuvetot (Bocage - Dijon), P Choutet, P Nau, F Bastides (Bretonneau - Tours), T May, L Boyer, S Wassoumbou (CHU - Nancy), E Oksenhendeler, L Gérard (St Louis - Paris), L Bernard, P De Truchis, H Berthé (R Poincaré - Garches), Y Domart, D Merrien (CH - Compiègne), A Greder Belan, (A Mignot - Le Chesnay), M Gayraud, L Bodard, A Meudec (IMM Jourdan - Paris), C Beuscart, C Daniel, E Pape (La Beauchée - St Brieuc), P Vinceneux, AM Simonpoli, A Zeng (L Mourier - Colombes), L Fournier (M Jacquet - Melun), JG Fuzibet, C Sohn, E Rosenthal, M Quaranta (L’Archet - Nice), P Dellamonica, S Chaillou, M Sabah (L’Archet - Nice), B Audhuy, A Schieber (L Pasteur - Colmar), P Moreau, M Niault, O Vaillant (Bretagne Sud - Lorient), G Huchon, A Compagnucci (Hotel-Dieu - Paris), I De Lacroix Szmania, L Richier (Intercommunal - Créteil), I Lamaury (Abymes - Pointe à Pitre), F Saint-Dizier, D Garipuy (Ducuing – Toulouse), JA Gastaut, MP Drogoul, I Poizot Martin, G Fabre (St Marguerite – Marseille), G Lambert de Cursay, B Abraham, C Perino (CH - Brives), P Lagarde, F David (CH - Lagny), J Roche-Sicot, JL Saraux, A Leprêtre (S Veil-Eaubonne), B Fampin, A Uludag, AS Morin (Beaujon – Clichy), O Bletry, D Zucman (Foch - Suresnes), A Regnier (CH - Vichy), JJ Girard (CH - Loches), DT Quinsat, L Heripret (CH - Antibes), F Grihon (Haute Vallée de l’Oise-Noyon), D Houlbert (CH - Alençon), M Ruel, K Chemlal (CH - Nanterre), F Caron, Y Debab (C Nicolle - Rouen), F Tremollieres, V Perronne (F Quesnay - Mantes La Jolie), G Lepeu, B Slama (H Duffaut - Avignon), P Perré (Les Oudairies - La Roche sur Yon), C Miodovski (Paris), G Guermonprez, A Dulioust (CMC Bligny - Briis s/Forges), P Boudon, D Malbec (R Ballanger - Aulnay s/bois), O Patey, C Semaille (CH - Villeneuve St Georges), J Deville, G Remy, I Béguinot (CH - Reims).
SEROCO
Hopital Antoine Beclere, Clamart (P Galanaud, F Boue, V Chambrin, C Pignon, GA Estocq, A Levy), Hopital de Bicetre, Le Kremlin Bicetre (JF Delfraissy, C Goujard, M Duracinsky, P Le Bras, MS Ngussan, D Peretti, N Medintzeff, T Lambert, O Segeral, P Lezeau, Y Laurian), Hopital Europeen Georges Pompidou, Paris (L Weiss, M Buisson, C Piketty, M Karmochkine, D Batisse, M Eliaszewitch, D Jayle, D Tisne- Dessus, M Kazatchkine), Hopital Bichat Claude Bernard, Paris (C Leport, U Colasante, C Jadand, C Jestin, X Duval, W Nouaouia, S Boucherit, JL Vilde), Hopital Saint Antoine, Paris (PM Girard, D Bollens, D Binet, B Diallo, MC Meyohas, L Fonquernie, JL Lagneau), Hopital Cochin, Paris (D Salmon, LGuillevin, T Tahi, O Launay, MP Pietrie, D Sicard, N Stieltjes, J Michot), Hopital Henri Mondor, Creteil (A Sobel, Y Levy, F Bourdillon, AS Lascaux, JD Lelievre, C Dumont), Hopital Necker, Paris (B Dupont, G Obenga, JP Viard, A Maignan), Hopital Paul Brousse, Villjuif (D Vittecoq, L Escaut, C Bolliot), Hopital Pitie Salpetriere, Paris (F Bricaire, C Katlama, L Schneider, S Herson, A Simon, M Iguertsira), Hopital de la Conception, Marseille (A Stein, C Tomei, I Ravaux, C Dhiver, H Tissot Dupont, A Vallon, J Gallais, H Gallais), Hopital Sainte Marguerite, Marseille (JA Gastaut, MP Drogoul, G Fabre), Hopital de L’Archet, Nice (P Dellamonica, J Durant, V Mondain, I Perbost, JP Cassuto, JM Karsenti, H Venti, JG Fuzibet, E Rosenthal, C Ceppi, M Quaranta), Hopital Avicenne, Bobigny (JA Krivitsky, M Bentata, O Bouchaud, P Honore), Hopital Saint Louis, Paris (D Sereni, C Lascoux, J Delgado), ACCTES/Hopital Necker, Paris (C Rouzioux, M Burgard, L Boufassa), Hopital Mignot, Le Chesnay (J Peynet).
SOUTHERN ALBERTA CLINIC COHORT
John Gill, Hartmut Krentz and Ron Read (Southern Alberta Clinic, Calgary, Canada).
SWISS HIV COHORT STUDY
Aubert V, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Günthard HF (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Pantaleo G, Paioni P, Rauch A (Chairman of the Scientific Board), Rudin C (Chairman of the Mother & Child Substudy), Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M, Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
UK CHIC
Steering Committee: Jonathan Ainsworth, Sris Allan, Jane Anderson, Abdel Babiker, David Chadwick, Valerie Delpech, David Dunn, Martin Fisher, Brian Gazzard, Richard Gilson, Mark Gompels, Phillip Hay, Teresa Hill, Margaret Johnson, Sophie Jose, Stephen Kegg, Clifford Leen, Fabiola Martin, Mark Nelson, Chloe Orkin, Adrian Palfreeman, Andrew Phillips, Deenan Pillay, Frank Post, Jillian Pritchard, Caroline Sabin, Achim Schwenk, Anjum Tariq, Roy Trevelion, John Walsh. Central Co ordination: University College London (Teresa Hill, Sophie Jose, Andrew Phillips, Caroline Sabin); Medical Research Council Clinical Trials Unit at UCL (MRC CTU at UCL), London (David Dunn, Adam Glabay). Participating Centres: Brighton and Sussex University Hospitals NHS Trust (M Fisher, N Perry, S Tilbury, E Youssef, D Churchill); Chelsea and Westminster Hospital NHS Foundation Trust, London (B Gazzard, M Nelson, R Everett, D Asboe, S Mandalia); King’s College Hospital NHS Foundation Trust, London (F Post, H Korat, C Taylor, Z Gleisner, F Ibrahim, L Campbell); Mortimer Market Centre, University College London (R Gilson, N Brima, I Williams); Royal Free NHS Foundation Trust/University College London (M Johnson, M Youle, F Lampe, C Smith, R Tsintas, C Chaloner, S Hutchinson, C Sabin, A Phillips T Hill, S Jose); Imperial College Healthcare NHS Trust, London (J Walsh, N Mackie, A Winston, J Weber, F Ramzan, M Carder); Barts and The London NHS Trust, London (C Orkin, J Lynch, J Hand, C de Souza); Homerton University Hospital NHS Trust, London (J Anderson, S Munshi); North Middlesex University Hospital NHS Trust, London (J Ainsworth, A Schwenk, S Miller, C Wood); The Lothian University Hospitals NHS Trust, Edinburgh (C Leen, A Wilson, S Morris); North Bristol NHS Trust (M Gompels, S Allan); Leicester, University Hospitals of Leicester NHS Trust (A Palfreeman, K Memon, A Lewszuk); Middlesbrough, South Tees Hospitals NHS Foundation Trust, (D Chadwick, E Cope, J Gibson); Woolwich, Lewisham and Greenwich NHS Trust (S Kegg, P Main, Dr Mitchell, Dr Hunter), St. George’s Healthcare NHS Trust (P Hay, M Dhillon); York Teaching Hospital NHS Foundation Trust (F Martin, S Russell Sharpe); Coventry, University Hospitals Coventry and Warwickshire NHS Trust (S Allan, A Harte, S Clay); Wolverhampton, The Royal Wolverhampton Hospitals NHS Trust (A Tariq, H Spencer, R Jones); Chertsey, Ashford and St.Peter’s Hospitals NHS Foundation Trust (J Pritchard, S Cumming, C Atkinson); Public Health England, London (V Delpech); HIV i-base (R Trevelion).
UK Register of HIV Seroconverters
Steering Committee: Andrew Phillips (Chair), University College London (UCL), London; Abdel Babiker, MRC CTU, London; Valerie Delpech, Public Health England, London; Sarah Fidler, St. Mary’s Hospital, London; Martin Fisher, Brighton & Sussex University Hospitals NHS Trust, Brighton; Julie Fox, Guys and St Thomas’ NHS Trust/Kings College, London; Richard Gilson, West London Centre for Sexual Health, London; David Goldberg, Health Protection Scotland, Glasgow; David Hawkins, Chelsea & Westminster NHS Trust, London; Anne Johnson, UCL, London; Margaret Johnson, UCL and Royal Free NHS Trust, London; Ken McLean, West London Centre for Sexual Health, London; Deenan Pillay, UCL, London; Frank Post, Kings College, London. Collaborating clinical centres: N Kennedy, Monklands Hospital, Airdrie; J Pritchard, Ashford Hospital, Ashford; U Andrady, Ysbyty Gwynedd, Bangor; N Rajda, North Hampshire Hospital, Basingstoke; C Donnelly, S McKernan, Royal Victoria Hospital, Belfast; S Drake, G Gilleran, D White, Birmingham Heartlands Hospital, Birmingham; J Ross, J Harding, R Faville, Whittall Street Clinic, Birmingham; J Sweeney, P Flegg, S Toomer, Blackpool Victoria Hospital, Blackpool; H Wilding, R Woodward, Royal Bournemouth Hospital, Bournemouth; G Dean, C Richardson, N Perry, Royal Sussex County Hospital, Brighton; M Gompels, L Jennings, Southmead Hospital, Bristol; D Bansaal, Queens Hospital, Burton upon Trent; M Browing, L Connolly, Cardiff Royal Infirmary, Cardiff; B Stanley, North Cumbria Acute Hospitals NHS Trust, Carlisle; S Estreich, A Magdy, St. Helier Hospital, Carshalton; C O’Mahony, Countess of Chester Hospital, Chester; P Fraser, Chesterfield & North Derbyshire Royal Hospital, Chesterfield; SPR Jebakumar, Essex County Hospital, Colchester; L David, Coventry & Warwickshire Hospital, Coventry; R Mette, Mayday University Hospital, Croydon; H Summerfield, Weymouth Community Hospital, Dorset; M Evans, Ninewells Hospital, Dundee; C White, University Hospital of North Durham, Durham; R Robertson, Muirhouse Medical Group, Edinburgh; C Lean, S Morris, Western General Hospital, Edinburgh; A Winter, Gartnavel General Hospital & Glasgow Royal Infirmary, Glasgow; S Faulkner, Gloucestershire Royal Hospital, Gloucester; B Goorney, Salford Hope Hospital, Greater Manchester; L Howard, Farnham Road Hospital, Guildford; I Fairley, C Stemp, Harrogate Hospital, Harrogate; L Short, Huddersfield Royal Infirmary, Huddersfield; M gomez, F young, St Mary’s Hospital Isle of Wight; M Roberts, S Green, Kidderminster General Hospital, Kidderminster; K Sivakumar, The Queen Elizabeth Hospital, King’s Lynn; J Minton, A Siminoni, Leeds General Infirmary, Leeds; J Calderwood, D Greenhough, J Minton, St. James Hospital, Leeds; C DeSouza, Lisa Muthern, C Orkin, Barts & The London NHS Trust, London; S Murphy, M Truvedi, Central Middlesex Hospital, London; K McLean, Charing Cross Hospital, London; D Hawkins, C Higgs, A Moyes, Chelsea & Westminster Hospital, London; S Antonucci, S McCormack, Dean Street Clinic, London; W Lynn, Ealing Hospital, London; M Bevan, J Fox, A Teague, Guy’s & St. Thomas NHS Trust, London; J Anderson, S Mguni, Homerton Hospital, London; F Post, L Campbell, E Wandolo King’s College Hospital, London; C Mazhude, H Russell, Lewisham University Hospital, London; R Gilson, G Carrick, C Young Mortimer Market Centre, London; J Ainsworth, A Waters, North Middlesex Hospital, London; P Byrne, M Johnson, Royal Free Hospital, London; London; S Fidler, K Kuldanek, S Mullaney, St. Mary’s Hospital, London; V Lawlor, R Melville, Whipps Cross Hospital, London; A Sukthankar, S Thorpe, Manchester Royal Infirmary, Manchester; C Murphy, E Wilkins, North Manchester General Hospital, Manchester; S Ahmad, P Green, Withington Hospital, Manchester; S Tayal, James Cook Hospital, Middlesbrough; E Ong, Newcastle General Hospital, Newcastle; J Meaden, Norfolk & Norwich University Hospital, Norwich; L Riddell, City Hospital, Nottingham; D Loay, K Peacock, George Eliot Hospital, Nunneaton; H Blackman, V Harindra, St. Mary’s Hospital, Portsmouth; AM Saeed, Royal Preston Hospital, Preston; S Allen, U Natarajan, East Surrey Hospital, Redhill; O Williams, Glan Clwyd District General, Rhyl; H Lacey, Baillie Street Health Centre, Rochdale; C Care, C Bowman, S Herman, Royal Hallamshire Hospital, Sheffield; S V Devendra, J Wither, Royal Shrewsbury Hospital, Shrewsbury; A Bridgwood, G Singh, North Staffordshire Hospital, Stoke-on- Trent; S Bushby, Sunderland Royal Hospital, Sunderland; D Kellock, S Young, King’s Mill Centre, Sutton-in-Ashfield; G Rooney, B Snart, The Great Western Hospital, Swindon; J Currie, M. Fitzgerald, Taunton & Somerset Hospital, Taunton; J Arumainayyagam, S Chandramani, Manor Hospital, Walsall; S Rajamanoharan, T Robinson, Watford General Hospital, Watford; M Roberts, Worcester Royal Infirmary, Worcester; O Williams, Maelor Hospital, Wrexham; B Taylor, Wycombe General Hospital, Wycombe; C Brewer, I Fairley, Monkgate Health Centre, York Hospital NHS Trust, York.
Veterans Aging Cohort Study-Virtual Cohort
Principal investigator and co principal investigator: AC Justice, DA Fiellin. Participating VA centers: Atlanta, GA (D. Rimland, C Jones-Taylor), Baltimore, MD (KA Oursler, R Titanji), Bronx, NY (S Brown, S Garrison), Houston, TX (M Rodriguez-Barradas, N Masozera), Los Angeles, CA (M Goetz, D Leaf), Manhattan-Brooklyn, NY (M Simberkoff, D Blumenthal, J Leung), Pittsburgh, PA (A Butt, E Hoffman), and Washington, DC (C Gibert, R Peck). Core Faculty: K Mattocks (Deputy Director), S Braithwaite, C Brandt, K Bryant, R Cook, J Conigliaro, K Crothers, J Chang, S Crystal, N Day, J Erdos, M Freiberg, M Kozal, N Gandhi, M Gaziano, M Gerschenson, B Good, A Gordon, JL Goulet, MA Hernán, K Kraemer, J Lim, S Maisto, P Miller, L Mole, P O’Connor, R Papas, JM Robins, C Rinaldo, M Roberts, J Samet, B Tierney, J Whittle.
Figure A1.
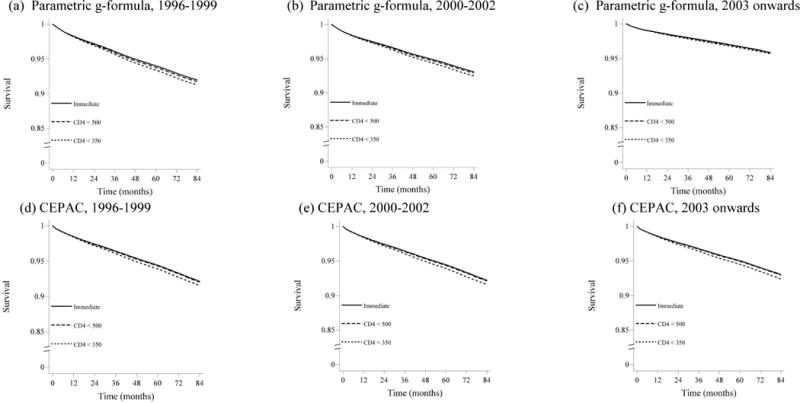
Survival distribution stratified by baseline time period and antiretroviral therapy initiation strategy estimated using the parametric g-formula applied to HIV-CAUSAL data and using the original CEPAC parameterization. (a)Baseline from Jan 1, 1996 – Dec 31, 1999, parametric g-formula; (b) Baseline from Jan 1, 2000 – Dec 31, 2002, parametric g formula; (c) Baseline on or after Jan 1, 2003, parametric g-formula; (d) Baseline from Jan 1, 1996 – Dec 31, 1999, CEPAC; (e) Baseline from Jan 1, 2000 – Dec 31, 2002, CEPAC; (f) Baseline on or after Jan 1, 2003, CEPAC. All CEPAC estimates use initial parameterization of 1.0 for on-ART multipliers of opportunistic infection incidence and chronic AIDS-related mortality. CEPAC: Cost-Effectiveness of Preventing AIDS Complications model
Figure A2.
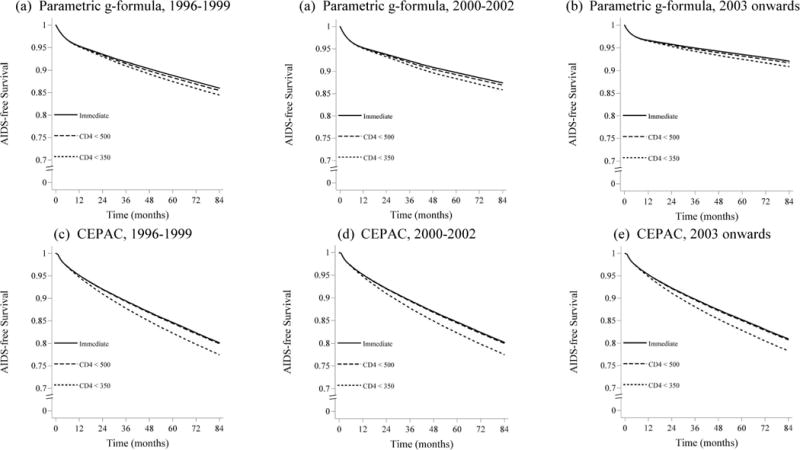
AIDS-free survival baseline time period and antiretroviral therapy initiation strategy estimated using the parametric g-formula applied to HIV-CAUSAL data and using the original CEPAC parameterization. (a) Baseline from Jan 1, 1996 – Dec 31, 1999, parametric g-formula; (b) Baseline from Jan 1, 2000 – Dec 31, 2002, parametric g-formula; (c) Baseline on or after Jan 1, 2003, parametric g-formula; (d) Baseline from Jan 1, 1996 – Dec 31, 1999, CEPAC; (e) Baseline from Jan 1, 2000 – Dec 31, 2002, CEPAC; (f) Baseline on or after Jan 1, 2003, CEPAC. All CEPAC estimates use initial parameterization of 1.0 for on-ART multipliers of opportunistic infection incidence and chronic AIDS-related mortality. CEPAC: Cost-Effectiveness of Preventing AIDS Complications model
Figure A3.
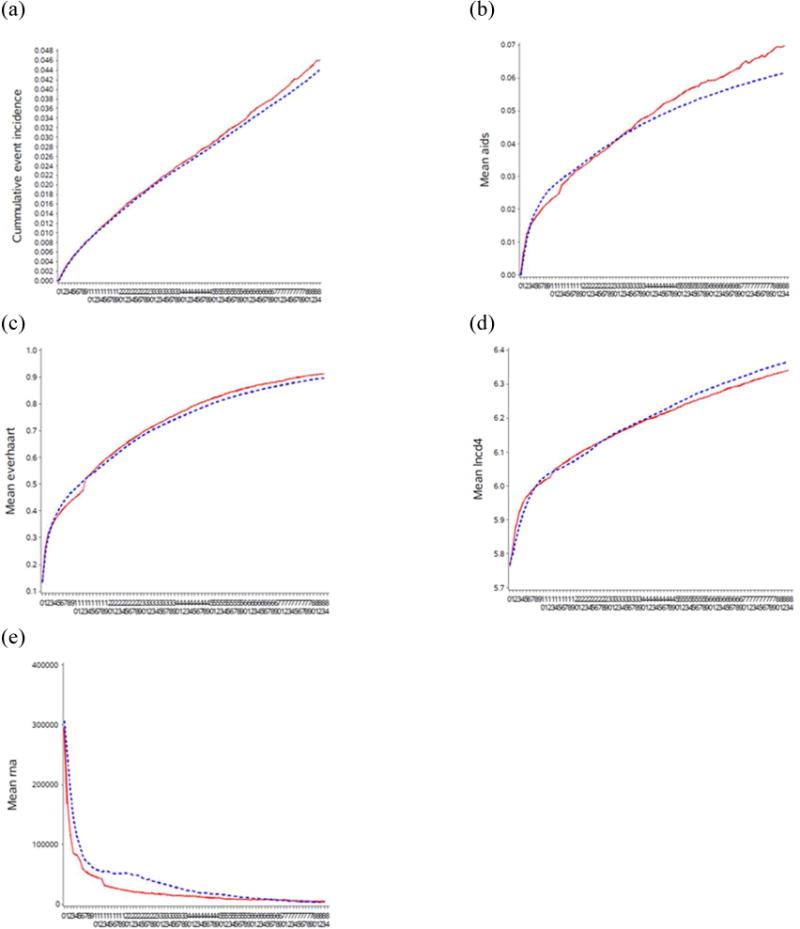
Mean of the main study variables under no intervention when outcome is mortality: observed (solid line) and estimated via the parametric g-formula (dotted line). HIV-CAUSAL Collaboration, on or after Jan 1, 2003. (a) Cumulative incidence of death; (b) Cumulative incidence of AIDS; (c) Mean proportion on treatment; (d) Mean CD4 count, natural log scale; (e) Mean HIV RNA.
Figure A4.

Mean of the main study variables under no intervention when outcome is mortality: observed (solid line) and estimated via the parametric g-formula (dotted line). HIV-CAUSAL Collaboration, Jan 1, 2000 – Dec 31, 2002. (a) Cumulative incidence of death; (b) Cumulative incidence of AIDS; (c) Mean proportion on treatment; (d) Mean CD4 count, natural log scale; (e) Mean HIV RNA, natural log scale.
Figure A5.
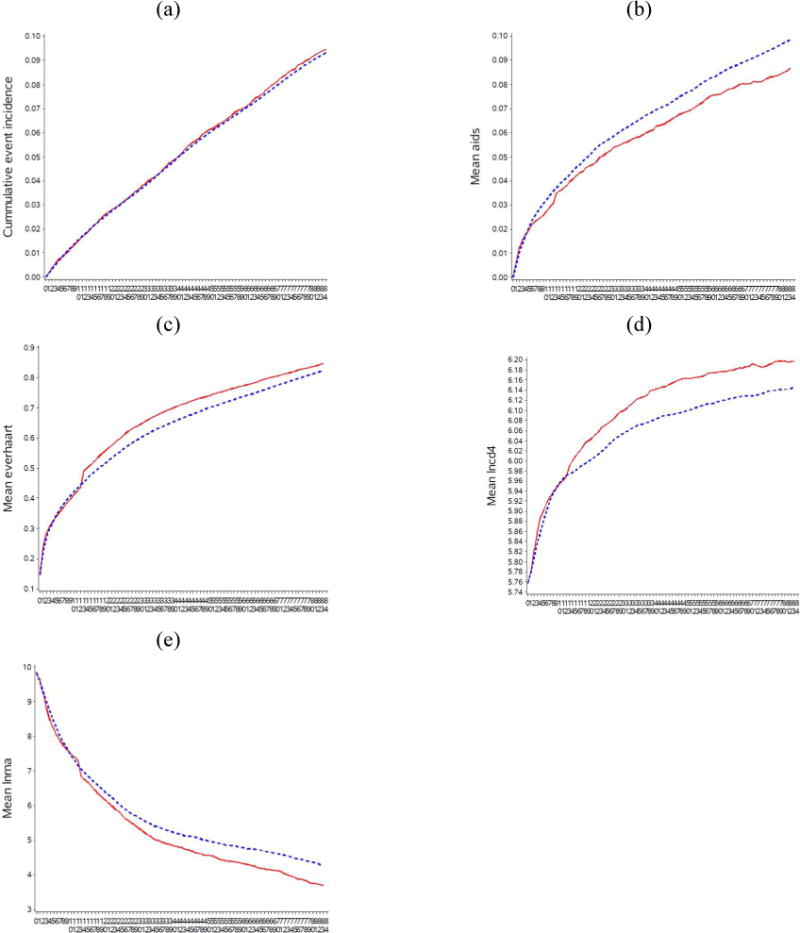
Mean of the main study variables under no intervention when outcome is mortality: observed (solid line) and estimated via the parametric g-formula (dotted line). HIV-CAUSAL Collaboration, Jan 1, 1996 – Dec 31, 1999. (a) Cumulative incidence of death; (b) Cumulative incidence of AIDS; (c) Mean proportion on treatment; (d) Mean CD4 count, natural log scale; (e) Mean HIV RNA, natural log scale.
Figure A6.
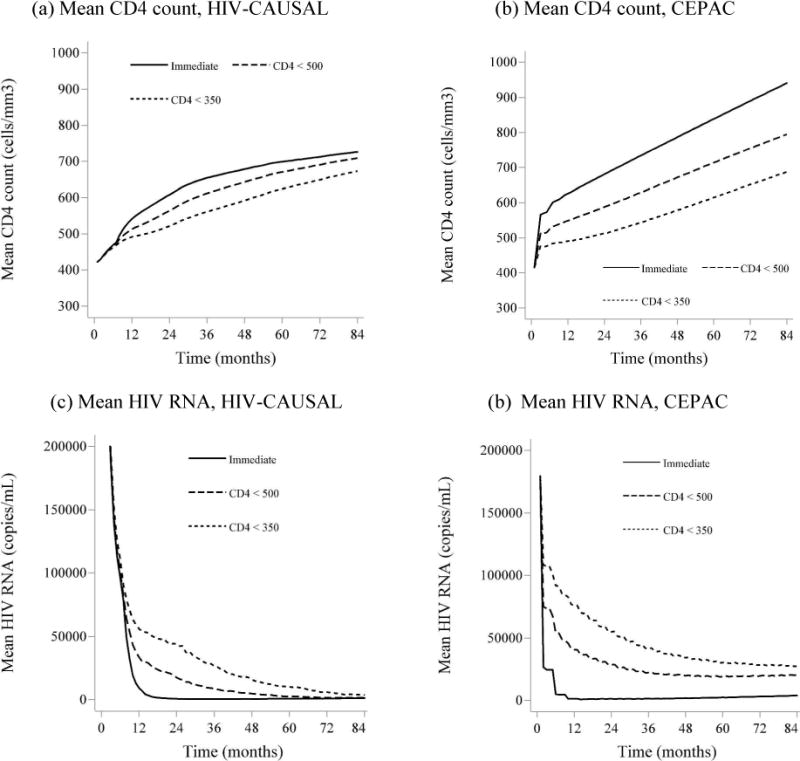
Mean of CD4 count and HIV RNA under intervention
Estimating using CEPAC and via the parametric g-formula in HIV-CAUSAL Collaboration, using baseline on or after Jan 1, 2003. All CEPAC estimates use initial parameterization of 1.0 for multipliers. (a) Mean CD4 count in HIV-CAUSAL (cells/μl); (b) Mean CD4 count in CEPAC (cells/μl); (c) Mean HIV RNA in HIV-CAUSAL (copies/mL); (d) Mean HIV RNA in CEPAC (copies/mL).
Table A1.
Distribution of HIV-CAUSAL data by country of cohort, %
| Cohort | Jan 1 1996 to Dec 31 1999 | Jan 1 2000 to Dec 31 2002 | On or after Jan 1 2003 |
|---|---|---|---|
| Canada | 1.0 | 0.9 | 1.4 |
| France | 50.0 | 39.5 | 27.0 |
| Greece | 2.3 | 2.0 | 4.8 |
| Netherlands | 2.0 | 6.0 | 12.6 |
| Spain | 7.0 | 10.6 | 17.4 |
| Switzerland | 8.6 | 6.2 | 4.1 |
| UK | 16.2 | 21.3 | 24.4 |
| USA | 12.7 | 13.5 | 9.7 |
Table A2.
Detailed baseline characteristics, HIV-CAUSAL Collaboration: Jan 1, 1996 Dec 31, 1999
| Individ uals (n) | Person-years | Proportion starting ART during follow-up (%) | Median (IQR) follow-up (months) | Deaths (n) | Incidence of death (per 1000 person-years) | AIDS events or deaths (n) | Incidence of AIDS events or death (per 1000 person-years) | ||
|---|---|---|---|---|---|---|---|---|---|
| CD4 cell count, cells/μl | <50 | 1,519 | 9,781.25 | 0.84 | 53 (15, 145) | 354 | 36.2 | 201 | 20.6 |
| 50–100 | 1,172 | 7,693.50 | 0.84 | 53 (17, 149) | 209 | 27.2 | 144 | 18.7 | |
| 100–200 | 2,979 | 19,403.50 | 0.80 | 51 (17, 142) | 374 | 19.3 | 259 | 13.4 | |
| 200–350 | 6,295 | 40,830.67 | 0.72 | 50 (17, 141) | 485 | 11.9 | 375 | 9.2 | |
| 350–500 | 6,612 | 41,859.00 | 0.62 | 47 (17, 135) | 414 | 9.9 | 343 | 8.2 | |
| >500 | 9,692 | 55,965.08 | 0.46 | 40 (15, 117) | 525 | 9.4 | 445 | 8.0 | |
| HIV RNA, copies/mL | <10,000 | 10,187 | 56,539.58 | 0.45 | 36 (14, 110) | 571 | 10.1 | 492 | 8.7 |
| 10,000–100,000 | 11,498 | 74,533.67 | 0.67 | 50 (17, 141) | 900 | 12.1 | 686 | 9.2 | |
| >100,000 | 6,584 | 44,459.75 | 0.82 | 58 (19, 146) | 890 | 20.0 | 589 | 13.3 | |
| Sex | Men | 21,361 | 136,126.17 | 0.65 | 48 (17, 139) | 2,059 | 15.1 | 1,542 | 11.3 |
| Women | 6,908 | 39,406.83 | 0.57 | 41 (15, 114) | 302 | 7.7 | 225 | 5.7 | |
| Age, years | <35 | 13,835 | 81,588.00 | 0.59 | 42 (16, 119) | 572 | 7.0 | 404 | 5.0 |
| 35–50 | 11,745 | 75,364.92 | 0.65 | 48 (16, 142) | 1,152 | 15.3 | 854 | 11.3 | |
| >50 | 2,689 | 18,580.08 | 0.73 | 64 (19, 150) | 637 | 34.3 | 509 | 27.4 | |
| Transmission group | Heterosexual | 8,401 | 52,008.58 | 0.64 | 47 (17, 130) | 402 | 7.7 | 303 | 5.8 |
| Homosexual or bisexual | 9,197 | 69,782.58 | 0.67 | 70 (22, 162) | 545 | 7.8 | 396 | 5.7 | |
| Injecting drug user | 5,132 | 22,833.33 | 0.52 | 29 (11, 72) | 429 | 18.8 | 316 | 13.8 | |
| Other or unknown | 5,539 | 30,908.50 | 0.65 | 37 (13, 117) | 985 | 31.9 | 752 | 24.3 |
Table A3.
Detailed baseline characteristics, HIV-CAUSAL Collaboration: baseline, Jan 1, 2000 Dec 31, 2002
| Individ uals (n) | Person-years | Proportion starting ART during follow-up (%) | Median (IQR) follow-up (months) | Deaths (n) | Incidence of death (per 1000 person-years) | AIDS events or deaths (n) | Incidence of AIDS events or death (per 1000 person years) | ||
|---|---|---|---|---|---|---|---|---|---|
| CD4 cell count, cells/μl | <50 | 1,375 | 8,071.00 | 0.88 | 58 (18, 122) | 253 | 31.3 | 171 | 21.2 |
| 50–100 | 1,109 | 6,494.00 | 0.87 | 57 (19, 121) | 166 | 25.6 | 122 | 18.8 | |
| 100–200 | 2,645 | 14,813.58 | 0.83 | 52 (18, 118) | 271 | 18.3 | 224 | 15.1 | |
| 200–350 | 5,678 | 32,327.08 | 0.72 | 51 (17, 121) | 342 | 10.6 | 283 | 8.8 | |
| 350–500 | 5,922 | 34,159.50 | 0.58 | 49 (18, 122) | 326 | 9.5 | 275 | 8.1 | |
| >500 | 8,704 | 46,830.08 | 0.42 | 43 (17, 112) | 350 | 7.5 | 312 | 6.7 | |
| HIV RNA, copies/mL | <10,000 | 8,685 | 44,931.42 | 0.43 | 40 (15, 108) | 403 | 9.0 | 367 | 8.2 |
| 10,000–100,000 | 10,631 | 60,588.25 | 0.65 | 50 (18, 120) | 683 | 11.3 | 554 | 9.1 | |
| >100,000 | 6,117 | 37,175.58 | 0.82 | 61 (21, 124) | 622 | 16.7 | 466 | 12.5 | |
| Sex | Men | 19156 | 132819.7 | 0.63 | 52 (19, 123) | 1,471 | 13.4 | 1,194 | 10.9 |
| Women | 5554 | 34921.58 | 0.57 | 39 (15, 98) | 237 | 7.3 | 193 | 5.9 | |
| Age, years | <35 | 18,803 | 110,041.17 | 0.57 | 42 (17, 108) | 282 | 4.9 | 222 | 3.9 |
| 35 50 | 6,630 | 32,654.08 | 0.63 | 52 (18, 123) | 784 | 11.9 | 628 | 9.5 | |
| >50 | 11,014 | 57,643.25 | 0.72 | 63 (19, 124) | 642 | 33.7 | 537 | 28.5 | |
| Transmission group | Heterosexual | 11,270 | 65,988.17 | 0.61 | 44 (17, 108) | 310 | 6.5 | 242 | 5.1 |
| Homosexual or bisexual | 3,149 | 19,063.83 | 0.64 | 75 (26, 138) | 305 | 5.3 | 241 | 4.2 | |
| Injecting drug user | 8,953 | 47,355.00 | 0.49 | 26 (11, 60) | 242 | 20.8 | 205 | 17.6 | |
| Other or unknown | 8,280 | 57,125.67 | 0.65 | 42 (15, 116) | 851 | 32.0 | 699 | 26.3 |
Table A4.
Detailed baseline characteristics, HIV-CAUSAL Collaboration: On or after Jan 1, 2003
| Individ uals (n) | Person-years | Proportion starting ART during follow-up (%) | Median (IQR) follow-up (months) | Death s (n) | Incidence of death (per 1000 person-years) | AIDS events or deaths (n) | Incidence of AIDS events or death (per 1000 person years) | ||
|---|---|---|---|---|---|---|---|---|---|
| CD4 cell count, cells/μl | <50 | 3,847 | 13,935.50 | 0.87 | 35 (15, 65) | 347 | 24.9 | 237 | 17.0 |
| 50–100 | 3,279 | 12,158.25 | 0.89 | 35 (15, 69) | 212 | 17.4 | 165 | 13.6 | |
| 100–200 | 8,067 | 29,194.92 | 0.88 | 34 (15, 66) | 344 | 11.8 | 288 | 9.9 | |
| 200–350 | 18,069 | 64,787.17 | 0.80 | 34 (15, 64) | 432 | 6.7 | 375 | 5.8 | |
| 350–500 | 18,661 | 63,856.75 | 0.62 | 31 (14, 61) | 297 | 4.7 | 258 | 4.0 | |
| >500 | 26,170 | 85,179.33 | 0.44 | 29 (13, 57) | 374 | 4.4 | 333 | 3.9 | |
| HIV RNA, copies/mL | <10000 | 20,793 | 68,189.42 | 0.49 | 29 (13, 58) | 374 | 5.5 | 333 | 4.9 |
| 10000–100000 | 34,976 | 120,595.83 | 0.66 | 31 (14, 62) | 798 | 6.6 | 671 | 5.6 | |
| >100000 | 22,324 | 80,326.67 | 0.79 | 34 (15, 65) | 834 | 10.4 | 652 | 8.1 | |
| Sex | Men | 62,498 | 219,436.33 | 0.66 | 32 (15, 63) | 1,769 | 8.1 | 1,449 | 6.6 |
| Women | 15,595 | 49,675.58 | 0.64 | 28 (12, 56) | 237 | 4.8 | 207 | 4.2 | |
| Age, years | <35 | 34,977 | 112,735.75 | 0.59 | 28 (13, 57) | 257 | 2.3 | 215 | 1.9 |
| 35–50 | 32,120 | 118,048.75 | 0.69 | 35 (15, 66) | 866 | 7.3 | 693 | 5.9 | |
| >50 | 10,996 | 38,327.42 | 0.76 | 33 (15, 63) | 883 | 23.0 | 748 | 19.5 | |
| Transmission group | Heterosexual | 24,673 | 82,956.25 | 0.67 | 30 (13, 60) | 462 | 5.6 | 379 | 4.6 |
| Homosexual or bisexual | 37,434 | 136,221.58 | 0.63 | 34 (16, 65) | 433 | 3.2 | 344 | 2.5 | |
| Injecting drug user | 3,361 | 9,018.58 | 0.58 | 20 (11, 44) | 191 | 21.2 | 160 | 17.7 | |
| Other or unknown | 12,625 | 40,915.50 | 0.71 | 29 (13, 59) | 920 | 22.5 | 773 | 18.9 |
Table A5.
CEPAC model input parameters sources, all time periods
| Parameter | Source |
|---|---|
|
| |
| HIV natural history parameters | |
| Mean monthly CD4 count decrease when not on ART, stratified by CD4 cell count and HIV RNA | Research in Access to Care for the Homeless (REACH), San Francisco Men’s Health Study, and Multi-Center AIDS Cohort Study (MACS) (32, 34, 35) |
| Monthly risk of opportunistic infections (OIs), stratified by CD4 cell count and OI | MACS (32); Walensky et al, 2010, Clin Infect Dis (28) |
| Monthly risk for acute OI-related death, among those with OI, stratified by OI | Walensky et al, 2010, Clin Infect Dis (28) |
|
| |
| Efficacy of antiretroviral therapy | |
| Mean monthly CD4 count decrease when on ART, stratified by time since treatment initiation | Sax et al, 2010, Lancet (36) |
| Viral suppression after 24 weeks, % | Sax et al, 2010, Lancet (36) |
| Monthly risk of late failure after initial viral suppression | Sax et al, 2010, Lancet (36) |
| Probability of treatment response | Sax et al, 2010, Lancet (36); VOLTART trial(50) |
References
- 1.Marshall BDL, Galea S. Formalizing the role of agent-based modeling in causal inference and epidemiology. American Journal of Epidemiology. 2015;181(2):92–9. doi: 10.1093/aje/kwu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuelezam NN, Rough K, Seage GR., 3rd Individual0based simulation models of HIV transmission: reporting quality and recommendations. PLoS One. 2013;8(9):e75624. doi: 10.1371/journal.pone.0075624. Epub 2013/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Value in Health. 2012;15(6):812–20. doi: 10.1016/j.jval.2012.06.014. Epub 2012/09/25. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices–modeling studies. Value in Health. 2003;6(1):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. Epub 2003/01/22. [DOI] [PubMed] [Google Scholar]
- 5.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Value in Health. 2012;15(6):843–50. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Saltelli A, Tarantola S, Campolongo F. Sensitivity analysis as an ingredient of modeling. Statistical Science. 2000;15(4):377–95. doi: 10.2307/2676831. [DOI] [Google Scholar]
- 7.Windrum P, Fagiolo G, Moneta A. Empirical validation of agent-based models: alternatives and prospects. Journal of Artificial Societies and Social Simulation. 2007;10(2):8. [Google Scholar]
- 8.Rutter CM, Miglioretti DL, Savarino JE. Bayesian calibration of microsimulation models. Journal of the American Statistical Association. 2009;104(488):1338–50. doi: 10.1198/jasa.2009.ap07466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grazzini J. (Laboratorio R Revelli Working Papers Series).Consistent estimation of agent based models. 2011;(110) [Google Scholar]
- 10.Robins J. A new approach to causal inference in mortality studies with sustained exposure periods - Application to control of the healthy worker survivor effect. Errata in Computers and Mathematics with Applications. 1987;14:917–921. 1986;7:1396–512. [Google Scholar]
- 11.Robins JM, Hernán MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal Data Analysis. New York: Chapman and Hall/CRC Press; 2008. pp. 553–99. [Google Scholar]
- 12.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernán MA. A Comparison of Agent-Based Models and the Parametric G-Formula for Causal Inference. American Journal of Epidemiology. 2017;186(2):131–42. doi: 10.1093/aje/kwx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. Journal of the American Medical Association. 2014;312(4):410–25. doi: 10.1001/jama.2014.8722. Epub 2014/07/20. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Recomm Rep. 2009;58:7–8. [Google Scholar]
- 15.When To Start Consortium; Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/s014006736(09)6061207. Epub 2009/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Annals of Internal Medicine. 2011;154(8):509–15. doi: 10.7326/0003-4819-154-8-201104190-00001. Epub 2011/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodi S, Phillips A, Logan R, Olson A, Costagliola D, Abgrall S, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. The Lancet HIV. 2015;2(8):e335–43. doi: 10.1016/s2352-3018(15)00108-3. Epub 2015/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodi S, Sharma S, Lundgren JD, Phillips AN, Cole SR, Logan R, et al. The per-protocol effect of immediate versus deferred antiretroviral therapy initiation. AIDS. 2016;30(17):2659–63. doi: 10.1097/qad.0000000000001243. Epub 2016/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancelle-Park R. Expanded European AIDS case definition. Lancet. 1993;341(8842):441. doi: 10.1016/0140-6736(93)93040-8. Epub 1993/02/13. [DOI] [PubMed] [Google Scholar]
- 20.Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–37. doi: 10.1097/QAD.0b013e3283324283. Epub 2009/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The HIV-CAUSAL Collaboration. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clinical Infectious Diseases. 2012;54(9):1364–72. doi: 10.1093/cid/cis203. Epub 2012/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernán MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Statistics in Biosciences. 2011;3(1):119–43. doi: 10.1007/s12561-011-9040-7. Epub 2011/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain P, Danaei G, Robins JM, Manson JE, Hernan MA. Smoking cessation and long-term weight gain in the Framingham Heart Study: an application of the parametric g-formula for a continuous outcome. European journal of epidemiology. 2016 doi: 10.1007/s10654-016-0200-4. Epub 2016/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernan MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–43. doi: 10.1007/s12561-011-9040-7. Epub 2011/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taubman SL, Robins JM, Mittleman MA, Hernán MA. Intervening on risk factors for coronary heart disease: an application of the parametric g-formula. International Journal of Epidemiology. 2009;38(6):1599–611. doi: 10.1093/ije/dyp192. Epub 2009/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taubman SL, Young JG, Picciotto S, Logan R, Hernán MA. G-formula SAS macro. 2012 Available from: http://www.hsph.harvard.edu/causal/software/
- 27.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. New England Journal of Medicine. 2001;344(11):824–31. doi: 10.1056/nejm200103153441108. Epub 2001/03/15. [DOI] [PubMed] [Google Scholar]
- 28.Walensky RP, Paltiel AD, Losina E, Morris BL, Scott CA, Rhode ER, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clinical Infectious Diseases. 2010;51(4):392–400. doi: 10.1086/655130. Epub 2010/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. New England Journal of Medicine. 2013;369(18):1715–25. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CEPAC (Cost-Effectiveness of Prevention of AIDS Complications) CEPAC Program User Guide 2013 [updated Aug 21] Available from: http://web2.research.partners.org/cepac/model.html.
- 31.Freedberg KA, Scharfstein JA, Seage GR, 3rd, Losina E, Weinstein MC, Craven DE, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. Journal of the American Medical Association. 1998;279(2):130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 32.Multicenter AIDS Cohort Study (MACS) public dataset: release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 33.Rydzak CE, Cotich KL, Sax PE, Hsu HE, Wang B, Losina E, et al. Assessing the performance of a computer-based policy model of HIV and AIDS. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012647. Epub 2010/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noubary F, Hughes MD. Assessing agreement in the timing of treatment initiation determined by repeated measurements of novel versus gold standard technologies with application to the monitoring of CD4 counts in HIV-infected patients. Statistics in Medicine. 2010;29(18):1932–46. doi: 10.1002/sim.3955. Epub 2010/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Journal of the American Medical Assocation. 2006;296(12):1498–506. doi: 10.1001/jama.296.12.1498. Epub 2006/09/28. [DOI] [PubMed] [Google Scholar]
- 36.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48. doi: 10.1016/s0140-6736(12)60917-9. Epub 2012/07/04. [DOI] [PubMed] [Google Scholar]
- 37.Eurostat. Population (demography, migration and projections): European Commission. 2015 [1/26/2016]. Available from: http://ec.europa.eu/eurostat/web/population-demography-migration-projections/statistics-illustrated.
- 38.The Opportunistic Infections Project Team of the Collaboration of Observational H. I. V. Epidemiological Research in Europe in EuroCoord. CD4 cell count and the risk of AIDS or death in HIV-infected adults on combination antiretroviral therapy with a suppressed viral load: A longitudinal cohort study from COHERE. PLOS Medicine. 2012;9(3):e1001194. doi: 10.1371/journal.pmed.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernan MA, Robins J. Causal Inference. Boca Raton: Chapman & Hill/CRC; 2017. forthcoming. [Google Scholar]
- 40.Hernán MA, Hernandez-Diaz S, Robins JM. Randomized trials analyzed as observational studies. Annals of Internal Medicine. 2013;159(8):560–2. doi: 10.7326/0003-4819-159-8-201310150-00709. Epub 2013/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernán MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clinical Trials. 2012;9(1):48–55. doi: 10.1177/1740774511420743. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV. 2017 doi: 10.1016/S2352-3018(17)30066-8. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Jr, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. Journal of Acquired Immune Deficiency Syndromes. 2016;73(1):39–46. doi: 10.1097/qai.0000000000001014. Epub 2016/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCormick AW, Abuelezam NN, Rhode ER, Hou T, Walensky RP, Pei PP, et al. Development, calibration and performance of an HIV transmission model incorporating natural history and behavioral patterns: application in South Africa. PLoS One. 2014;9(5):e98272. doi: 10.1371/journal.pone.0098272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS One. 2013;8(12):e83389. doi: 10.1371/journal.pone.0083389. Epub 2013/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JJ, Kuntz KM, Stout NK, Mahmud S, Villa LL, Franco EL, et al. Multiparameter calibration of a natural history model of cervical cancer. American Journal of Epidemiology. 2007;166(2):137–50. doi: 10.1093/aje/kwm086. Epub 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 47.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, et al. National Polyp Study data: evidence for regression of adenomas. International Journal of Cancer. 2004;111(4):633–9. doi: 10.1002/ijc.20277. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 48.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. American Journal of Epidemiology. 2002;156(8):761–73. doi: 10.1093/aje/kwf100. Epub 2002/10/09. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care et al. Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States. 2014 [Google Scholar]
- 50.Messou E, Chaix ML, Gabillard D, Minga A, Losina E, Yapo V, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d’Ivoire. Journal of Acquired Immune Deficiency Syndromes. 2011;56(4):356–64. doi: 10.1097/QAI.0b013e3182084b5a. Epub 2010/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]