SUMMARY
Dendritic cells (DCs) are antigen-presenting cells that capture, process and present antigens to lymphocytes to initiate and regulate the adaptive immune response. DCs detect bacteria in skin and mucosa and migrate into regional lymph nodes, where they stimulate antigen-specific T and B lymphocyte activation and proliferation. DCs direct CD4 T cells to differentiate to T cell subsets such as Th1, Th2, Th17 and Treg cells. The periodontium is chronically exposed to oral bacteria that stimulate an inflammatory response to induce gingivitis or periodontitis. DCs play both a protective and destructive role through activation of the acquired immune response and are also reported to be a source of osteoclast precursors that promote bone resorption. FOXO1, a member of the forkhead box O family of transcription factors, plays a significant role in the activation of DCs. The function of DCs in periodontal inflammation has been investigated in a mouse model by lineage specific deletion of FOXO1 in these cells. Deletion of FOXO1 reduces DC protective function and enhances susceptibility to periodontitis. The kinase Akt, phosphorylates FOXO1 to inhibit FOXO activity. Thus the Akt-FOXO1 axis may play a key role in regulating DCs to have a significant impact on periodontal disease.
Keywords: Immune response, Forkhead, FOXO, Gingiva, Lymphocyte, Periodontal
INTRODUCTION
Dendritic cells (DCs) are antigen-presenting cells (APCs), which capture, process and present antigens to lymphocytes to initiate and regulate the adaptive immune response 1. Based on function and phenotype, DCs can be divided into plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs). Langerhans cells are another member of the DC family found in oral mucosal epithelium and the skin epidermis 2. DCs play a key role in immune defense; when immune homeostasis mediated by DCs is disrupted, inflammatory destruction may occur 3. There is ample evidence that DCs are activated in experimental periodontitis in animals and in humans with periodontitis. Interestingly, dendritic cells have the potential to play protective or destructive roles or both. Due to the limited number of studies examining cause and effect relationships this issue is not entirely clear. However there are reports in which deletion of Langerhans cells or reduced DC function through lineage specific FOXO1 deletion have been shown to increase susceptibility to periodontitis 4–6, suggesting that in animal models DCs have an overall effect in the periodontium which is protective.
DCs and subsets
DCs are derived from CD34+ hematopoietic stem cells (HSCs) in the bone marrow 7. These progenitor cells initially differentiate to immature DCs, which have high endocytic activity and low T cell activation potential 8,9. Immature DCs express various pattern recognition receptors (PRRs) that constantly sample the surrounding environment for pathogens such as viruses and bacteria 10. Maturation of dendritic cells is induced by captured microbes or their components11,12, inflammatory cytokines and ligation of select cell surface receptors. 11–13 Once DCs take-up antigen they become activated into mature dendritic cells and present pathogen fragments at their cell surface using MHC molecules. DCs initiate the acquired immune response by interacting with lymphocytes after migrating to lymph nodes 14.
Dendritic cells can be subdivided into plasmacytoid (pDC) and conventional (cDC). pDCs are derived from lymphoid progenitors and have characteristics that resemble a cross between B cells and cDCs 15. pDCs predominantly recognize viral antigens 16, specializing in the production and secretion of type I interferons, IL-12, IL-6, TNF-α and pro-inflammatory chemokines 17. pDCs enter the lymphoid nodes directly via the bloodstream 18. Murine pDCs express B220, SiglecH, Ly6c and low amounts of CD11c. Human pDCs express CD4, CD123, HLA-DR, and blood derived cell antigen-2 (BDCA-2), as well as toll-like receptor (TLR)-7 and -9 but do not express the cDC-marker CD11c 17,19. pDCs can act as antigen presenting cells, but are much less efficient than cDCs.
cDCs also called classical dendritic cells or myeloid dendritic cells, express high levels of CD11c and display a characteristic morphology of long dendrite extensions 20. There are two primary cDCs subpopulations in mice: CD11b− and CD11b+. CD103+CD11b− cDCs populate most connective tissues and share their origin and function with lymphoid tissue CD8+ cDCs 21. The CD11b+ cDC subset most often lacks the integrin CD103 22. In human, cDCs express CD11b, CD11c, CD1a, CD1c, CD14, CD45, CD141, CD172a, CD206, Clec9a/DNGR1, XCR1, HLA-DR, FceRI. cDCs present antigens and produce the stimulatory mediators TNF, NO and IL-12 23. cDCs recognize both bacterial and viral antigens 24,25. Immature cDCs patrol various tissues to sample foreign antigens. During maturation cDCs migrate to draining lymph nodes to present pathogen-derived peptides to T cells 26.
Langerhans cells are a special member of the DC family that is usually situated in the oral mucosal epithelium and the skin epidermis 2 and can be distinguished by expression of langerin/CD207 27. Langerhans cells play an important role in immune surveillance by promoting immunity or tolerance through activation of antigen specific T cells 27.
DCs modulate specific lymphocyte responses
The interaction between DCs and pathogens contributes to different types of adaptive immunity. The specific cytokines expressed by DCs are dependent on stimulation by pathogen-associated molecular patterns (PAMPS). As a result under certain circumstances DCs can stimulate an immune response along a particular direction such as Th1, Th2, Th17, or Treg. DCs promote Th1 polarization by expressing IL-12 28. IL-12 secretion from DCs is induced by CD40 engagement 29. DCs primed with antigen stimulate naïve T cells to differentiate to Th1 cells 30. Interferon regulatory factor 4 plays an important role in DC initiation of Th2 cell responses 31. High-mobility group nucleosome-binding protein 1 (HMGN1) modulates DCs to induce Th2 responses in a TLR4-dependent manner 32. DCs primed with antigens of Streptococcus mitis increase differentiation of Th2 cells 30. DCs promote B cells through release of B-cell activating factor (BAFF) 33 and a proliferation-inducing ligand (APRIL) 34. They also promote B cell differentiation to plasma cells both via the secretion of IFN-α and IL-6 35 and via direct cell-cell contact 36. DCs promote Th17 cell differentiation through secretion of the cytokines TGF-β, IL-23 and IL-1β 37. DCs can induce the differentiation of Treg cells via TGF-β and IL-10 38. For example, DCs incubated with Propionibacterium acnes antigens induce differentiation of lymphocytes to Tregs 30. DCs also regulate the recruitment and activation of NK cells through secretion of IL-27 39.
Evidence of DCs in periodontal disease
DC stimulated by oral pathogens can contribute to different types of adaptive immunity and can lead to reduced proteolytic or osteolytic activity through Th2 or Treg responses or an increase through induction of Th1 or Th17 lymphocytes (Fig. 1). Although it is well recognized that dendritic cells play a key role in initiating an adaptive immune response there are relatively few studies which provide a causal link between dendritic cells and periodontal breakdown, particularly in animal models where specific hypotheses can be tested 40.
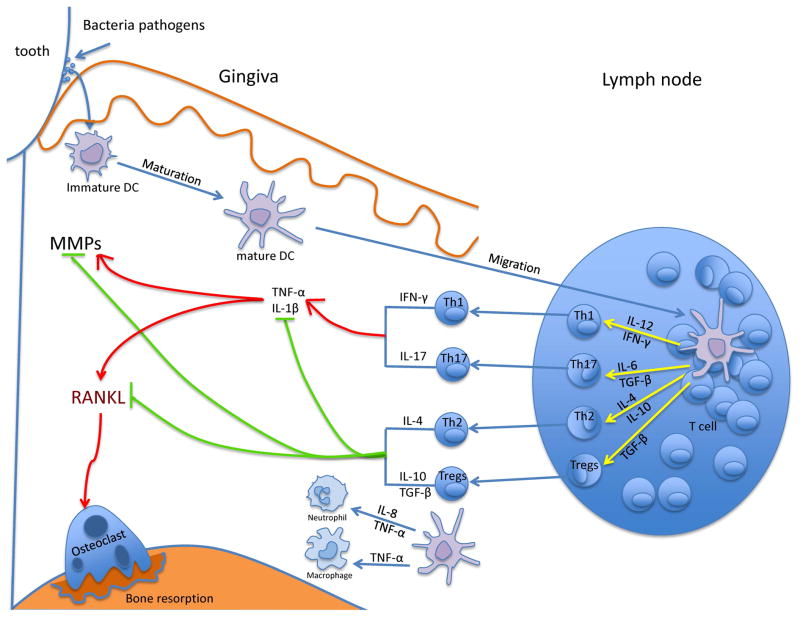
Figure 1.
DCs activate T cells and innate immune response in periodontal disease. Immature DCs capture oral bacteria, which induces migration to lymph nodes and maturation. Mature DCs can attract neutrophils and macrophage to sites of inflammation through IL-8 or TNF-α and present bacterial antigen to lymphocytes. DCs in turn may stimulate naïve T cells to differentiate along several pathways including Th1, Th17, Th2 and Treg cells. Cytokines produced by Th1 and Th17 cells may up-regulate TNF-α and IL-1β and cause greater MMP and RANKL expression, more osteoclast formation and more bone resorption. Th2 and Tregs have the opposite effect and may restrain inflammatory cytokine production to limit bone loss.
DCs may promote periodontal disease through induction of Th1- or Th17-lymphocytes
DCs can potentially enhance periodontal bone loss through up regulation of Th1 or Th17 response. Th1 activity is correlated with the number of mature DCs in gingiva in periodontitis 41. Porphyromonas gingivalis stimulates mature cDCs derived from individuals with chronic periodontitis to secrete IL-12 and IFN-γ 42. Both IL-12 and IFN-γ can promote Th1 responses and sustain inflammation 43. IFN-γ is the signature cytokine of Th1-type responses 44 associated with activating phagocytosis and the production of inflammatory cytokines and chemokines 45. Th1 responses have also been linked to increased RANKL expression 46, promotion of osteoclast formation and alveolar bone loss in vivo 47. Moreover, IFN-γ is present at high levels in periodontal lesions and is associated with progressive lesions or more severe periodontal disease48.
Oral infection with P. gingivalis in mice increases the number of cDCs, which is positively correlated with the generation of a Th17 response 47,49. Oral infection with P. gingivalis and Fusobacterium nucleatum stimulate migration of cDCs to the lymph nodes and gingiva and is associated with increased IL-17 levels, as well as other pro-inflammatory factors such as TNF, IL-6 and IL-1β, which contribute to alveolar bone loss 4,50. In humans IL-17 levels are correlated with more mature cDCs and increased periodontal bone loss 41. IL-17 induces pro-inflammatory and osteoclastogenic mediators such as RANKL or TNF 51,52. Thus, cDCs are upregulated in response to periodontal infection and are associated with increased inflammation and bone loss. However, a cause and effect relationship has not been established.
Although Th17 and Th1 responses that are typically thought to promote periodontal bone loss, there is some indication that these responses may be protective. For example IFN-γ produced during a Th1 response can inhibit RANKL induced osteoclastogenesis 53. Th1 leukocytes also have the ability of enhance recruitment and the killing activity of neutrophils and macrophages, which promote bacterial clearance 54,55.
DCs may induce a protective response through induction of Th2-lymphocytes
DCs triggered by P. gingivalis LPS induce a Th2 response through the TLR pathway 56,57. IL-4, the major Th2 type cytokine 58–60 inhibits the production of MMPs and RANKL and up-regulates the MMP inhibitor TIMP and the RANKL inhibitor OPG 61,62. Inhibition of MMPs and RANKL leads to reduced severity of experimental periodontitis 63. IL-4 levels are reduced in patients with chronic periodontitis compared to healthy persons 64. IL-4 levels increase after non-surgical periodontal therapy, suggesting a protective role of Th2 cells 65. Although Th2 responses are generally thought of as being protective, there is also evidence that Th2 lymphocytes can play destructive role in periodontal disease. Mice infected with Tannerella forsythia causes a pronounced Th2 bias and induce alveolar bone loss 66.
DCs have the ability to stimulate naïve B cells 67,68. In a murine model, oral infection stimulated cDCs to migrate to lymph nodes, inducing formation of plasma cells and the production of bacteria-specific antibodies. When DC function is reduced there is less DC migration to the lymph nodes, reduced numbers of plasma cells and less bacteria-specific IgG1 production, leading to more alveolar bone loss compared to control mice 4. This supports the contention that DCs activate a protective antibody response that leads to less bone loss.
DCs protective role in periodontal disease through Treg response
Treg cells limit excessive inflammation and bone loss 54. In active periodontal lesions, Treg-related cytokine levels are inversely related to RANKL levels 69,70. Inhibition of Treg function leads to higher levels of IFN-γ, TNF-α and RANKL and more alveolar bone loss 71. Oral DCs have the capacity to induce a Treg response through the secretion of IL-10 and TGF-β 72. The importance of IL-10 is illustrated by the finding that in IL-10 knockout mice, susceptibility to P. gingivalis-induced alveolar bone loss is greatly increased 73. Moreover, after periodontal treatment, inflammation is reduced and IL-10 expression is increased 74.
DCs may affect periodontal disease through enhancement or attenuation of the innate immune response
cDCs produce a number of mediators that can modulate the innate immune response. P. gingivalis and LPS stimulate DCs to produce TNF-α 75 and IL-8 12. P gingivalis binds to TLR-2 to activate complement receptor-3 (CR3), and subsequent binding to CR3 by P gingivalis fimbriae contributes to the induction of inflammatory cytokines such as TNF 76. In addition, P gingivalis may affect dendritic cells by affecting autophagy and intracellular killing77,78. Chemokines produced by DC can attract neutrophils and monocytes to sites of inflammation 79 enhancing inflammation and the expression of factors that stimulate osteoclastogenesis 80 (Fig. 1). Meanwhile IL-10 and TGF-β from DCs can potentially have the opposite effect, reducing inflammation 81.
The role of Langerhans cells in periodontal disease
Langerhans cells, a subset of DCs have been linked to periodontal disease. There is a positive correlation between the number of Langerhans cells in the gingival epithelium of chronic periodontitis patients versus healthy persons 82,83 and increased numbers in individuals with chronic gingivitis compared to non-inflamed gingiva 84. There is increased migration of Langerhans cells to the gingival epithelium in response to the accumulation of bacterial plaque 85 and a decrease following periodontal treatment 86. In one of the few cause and effect studies, ablation of Langerhans cells in experimental periodontitis in mice results in reduced numbers of Tregs, elevated production of RANKL and enhanced alveolar bone loss 6. Taken together these results suggest that Langerhans cells are increased by periodontal inflammation and have a protective effect through the generation of Treg cells. The results are also consistent with findings that reduction of cDC activity through deletion of FOXO1 increases periodontal disease susceptibility in both young and aged mice 4,5, suggesting that DCs have a protective function against increased periodontal inflammation.
Evidence of DC to osteoclast differentiation in periodontal disease
DCs as a source of osteoclast precursors
DCs are the professional antigen-presenting cells that also share several features with osteoclasts. Both DCs and osteoclasts are derived from a common hematopoietic precursor and exhibit phagocytic activities 87. In vitro, these precursors can be stimulated to differentiate to DCs by granulocyte monocyte colony stimulating factor (GM-CSF) and IL-4 or osteoclast precursors by M-CSF 88. There is increasing evidence that committed immature cDCs in some inflammatory conditions such as rheumatoid arthritis or periodontitis can transdifferentiate toward osteoclasts in the presence of RANKL 87. The co-culture of CD11c+ DCs with CD4+ T cells and Aggregatibacter actonimycetemcomitans produces osteoclasts that can induce bone resorption in vitro and in vivo 89. Moreover, the differentiation of DCs to osteoclasts could potentially be enhanced by bacteria induced inflammation. Thus, immature cDCs may be directly involved in osteoclastogenesis and contribute to bone loss in rheumatoid arthritis, histiocytosis, or periodontitis 90 (Fig. 2).
Figure 2.
The pathway of DCs transdifferentiating to osteoclast.
DCs and monocytes are derived from a common myeloid progenitor under the stimulation of GM-CSF and IL-4 (for DCs) or M-CSF (for monocytes). Immature cDCs can transdifferentiate to osteoclasts under the influence of M-CSF and RANKL. Thus, cDCs can transdifferentiate into osteoclasts and cause bone resorption in some inflammatory environments such as periodontitis and rheumatoid arthritis.
Immature cDCs either from bone marrow or from spleen are able to develop into osteoclasts, but mature cDCs do not have this ability. cDC-derived osteoclast formation is positively regulated by pro-inflammatory cytokines such as IL-1β, TNF-α and negatively regulated by IFN-α, IFN-γ, IL-2 and IL-4 91. In vivo, immature CD11c+ cDCs can form osteoclasts when injected into osteopetrotic mice that normally lacked osteoclasts 92. mRNA profiling compared DC-derived and monocyte-derived osteoclasts and showed that both formed molecularly indistinguishable mature osteoclasts. Interestingly, fewer genes need to be up- or down- regulated when osteoclasts are formed from cDCs compared to monocytic precursors 93.
In humans, cDCs are contributors to inflammatory bone destruction. Synovial fluid from arthritic patients increases the differentiation of osteoclasts from immature but committed cDCs90. Moreover, the presence of Th17 cells is associated with the emergence of cDC-derived osteoclasts 92. In myeloproliferative diseases, the participation of DC to osteoclast formation has also been suggested. Langerhans cell histiocytosis (LCH) is a rare disease caused by the clonal accumulation of dendritic Langerhans cells. LCH is frequently associated with osteolytic lesion and multinucleated giant cells expressing osteoclast markers (TRAP, cathepsin K, MMP9). Bone loss in this disease can be accounted for the transdifferentiation of cDCs to functional osteoclasts both in vivo and in vitro 94.
PI3K/Akt-FOXO1 signaling pathway in DCs
FOXO1 promotes DC activity and Akt enhances DC survival and proliferation
FOXO1, a member of forkhead transcription factor family, plays an important role in the regulation of many cellular and biological processes that include protection against oxidative stress, apoptosis and progression through the cell cycle95. Surprisingly FOXO1 can have opposite effects depending upon the microenvironment and the specific cell type 96. FOXO1 affects immune responses by controlling cytokine production 97, protecting hematopoietic stems cells from oxidative stress 95 and by promoting lymphocyte homeostasis through development of T regulatory lymphocytes and maintenance of naïve T cells 98. Lineage specific deletion of FOXO1 in cDCs reduces their capacity for bacterial phagocytosis, migration and binding to lymphocytes 99. FOXO1 is needed for efficient DC homing to lymph nodes and regulates CCR7, a key receptor needed for the DC homing 99. FOXO1 participates in cDC activation of both T and B lymphocytes and regulates expression of ICAM-1, which is needed to form an immune synapse. FOXO1 regulates these key molecules by binding to their promoters to transactivate transcription 99. In DCs, FOXO1 mediates LPS induced IL-6 and IL-12 expression, but reduces IL-10 production 100. In keratinocytes, P. gingivalis induces loss of barrier function and apoptosis by reducing expression of genes associated with epithelial barriers (integrin beta-1, -3 and -6) and increasing those that stimulate apotposis 101. In addition P. gingivalis-induces reactive oxygen species (ROS) that activate FOXO transcription factors through Jun-N-terminal kinase (JNK) signaling, which may modulate oxidative stress responses in gingival keratinocytes 102.
Forkhead box O (FOXO) transcription factors are downstream targets of the serine/threonine protein kinase B (PKB)/Akt 103. Akt is a kinase, which regulates processes of cellular proliferation and survival. The PI3K-Akt pathway has been reported to regulate cDC proliferation and survival 104. Phosphorylation of FOXOs by Akt inhibits the transcriptional function of FOXOs and contributes to cell survival, growth and proliferation 103. FOXO signaling is regulated by interactions with other intracellular proteins as well as post-translational modifications such as phosphorylation 103. In addition to FOXOs, Akt phosphorylates and inhibits glycogen synthase kinase-3β (GSK-3β)105. GSK3 is a serine protein kinase involved in regulation of DCs 106. PI3K reduces GSK3 activity via Akt phosphorylation107,108,109. In plasmacytoid dendritic cells, PI3K-Akt pathway is an important regulator of type I interferon production via activation of the interferon-regulatory factor 7 110,111.
PI3K/Akt-FOXO1 signaling pathway in DCs in periodontal disease
It has been shown that FOXO1 activation promotes the expression of inflammatory cytokines in a number of cell types including dendritic cells 4, macrophages 112 and epithelial cells 113. This result suggests that deletion of FOXO1 in DC could reduce periodontal bone loss through reduced inflammatory cytokine expression. However experimental evidence is the opposite; deletion of FOXO1 in DC increases susceptibility to periodontitis.
P. gingivalis increases FOXO1 gene expression in cDCs through TLR signaling 99,114. Oral infection stimulates recruitment of cDCs to oral mucosal epithelium and connective tissue 4. Deletion of FOXO1 specifically in cDCs blocks enhanced recruitment of cDCs and increases susceptibility to periodontitis 4. Oral infection stimulates migration of cDCs to the cervical lymph nodes, the formation of plasma cells and bacteria-specific antibody production 4. Each of these is significantly blocked with lineage specific FOXO1 deletion in cDCs by CD11c driven Cre recombinase 4. When FOXO1-deleted cDCs exhibited a compensatory increase in IL-1β and IL-17 in response to oral infection in vivo, which likely occurred from a reduced protective antibody response 4. This hypothesis is supported by a failure to upregulate APRIL and BAFF mRNA when FOXO1 in DC is ablated whereas both mediators are increased by wild type cDCs in response to bacterial stimulation 99. This is significant since cDCs regulate B cells through production of BAFF and APRIL. Moreover, deletion of FOXO1 in DC leads to reduced antibody production 4 and reduced lymphocyte activity. Thus, the principal effect of FOXO1 may be due to a reduced ability of DC to stimulate a protective acquired immune response.
There have been few studies that have examined the Akt-FOXO1 pathway in dendritic cells. Those studies that have been reported have not used a lineage-specific approach, limiting interpretation of the data. For example, it has been shown that a global deletion of transforming growth factor beta-activated kinase 1 (TAK1) disrupts Akt-FOXO in DC and may disrupt generation of Tregs115. However, the direct linkage to the Akt FOXO1 axis in DC was not demonstrated due to the global nature of the gene deletion and since TAK1 has other substrates such as NF-kB that may also be responsible for the effect. In another study the compound piperlongumine was shown to inhibit DC maturation and reduce the symptoms of rheumatoid arthritis. Although these changes were associated with an inhibition of Akt pathway in DC a cause and effect relationship was not established because piperlongumine affects several other pathways including NF-kB116.
CONCLUSION
DCs are antigen-presenting cells, which capture, process and present antigens to lymphocytes to initiate and regulate the adaptive immune response 1. DCs play a critical role in the adaptive immune response and can modulate lymphocyte polarization that is potentially destructive (Th1, Th17) or protective (Th2, Treg). DCs can transdifferentiate into osteoclasts and cause bone resorption during periodontal inflammation. However it is not clear whether immature DCs represent a significant source of osteoclast precursors in periodontal inflammation. Recent evidence makes it clear that the FOXO1 signaling plays an important role in DCs (Fig. 3). Our results are consistent with the hypothesis that FOXO1 deletion leads to loss of protection against bacterial challenge in the periodontium. We hypothesize that this may be due to a reduced capacity to upregulate the acquired immune response when FOXO1 is deleted. Alternatively, if DC are an important source of osteoclast precursors, deletion of FOXO1 could potentially increase DC to osteoclast differentiation to enhance bone loss. However, the precise mechanisms through which FOXO1 modulates periodontal disease susceptibility through altering DC function remains to be established. It is possible that FOXO1 enhances formation of Tregs to limit periodontal bone loss or increases formation of plasma cells to generate a protective antibody response. Moreover, the potential role of Akt in modulating FOXO1 may also be an important component of DC function that remains to be investigated in the context of periodontal disease.
Figure 3.
The Akt-FOXO1 signaling pathway maintains DC’s protective or destructive function to control periodontal inflammation. FOXO1 is essential for DCs to maintain their protective function and control periodontitis. Phosphorylation of FOXO1 by Akt inhibits FOXO1 activity and reduces the protective function of DCs enhancing susceptibilities to periodontitis.
Acknowledgments
This work was supported by National Institutes of Health funding from the DE019108 and DE021921. The authors declare no competing financial interests. We thank Daniel Feinberg for help in preparing this manuscript.
References
- 1.Wynn TA. Basophils trump dendritic cells as APCs for T(H)2 responses. Nat Immunol. 2009;10(7):679–681. doi: 10.1038/ni0709-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reibel J, Dabelsteen E, Kenrad B, Buschard K. Pattern of distribution of T lymphocytes, Langerhans cells and HLA-DR bearing cells in normal human oral mucosa. Scand J Dent Res. 1985;93(6):513–521. doi: 10.1111/j.1600-0722.1985.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Xiao W, Dong G, Pacios S, et al. FOXO1 Deletion Reduces Dendritic Cell Function and Enhances Susceptibility to Periodontitis. Am J Pathol. 2015;185(4):1085–1093. doi: 10.1016/j.ajpath.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Dong G, Xiao W, et al. Effect of Aging on Periodontal Inflammation, Microbial Colonization, and Disease Susceptibility. J Dent Res. 2016;95(4):460–466. doi: 10.1177/0022034515625962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arizon M, Nudel I, Segev H, et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(18):7043–7048. doi: 10.1073/pnas.1116770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3(4):459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 8.Van Brussel I, Berneman ZN, Cools N. Optimizing dendritic cell-based immunotherapy: tackling the complexity of different arms of the immune system. Mediators Inflamm. 2012;2012:690643. doi: 10.1155/2012/690643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Swanson J. The endocytic activity of dendritic cells. J Exp Med. 1995;182(2):283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 11.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33(11):2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya S, Nemoto E, Ogawa T, Shimauchi H. Porphyromonas gingivalis lipopolysaccharides induce maturation of dendritic cells with CD14+CD16+ phenotype. Eur J Immunol. 2004;34(5):1451–1460. doi: 10.1002/eji.200324549. [DOI] [PubMed] [Google Scholar]
- 13.Ho LJ, Wang JJ, Shaio MF, et al. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol. 2001;166(3):1499–1506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- 14.Constantino J, Gomes C, Falcao A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res. 2017 doi: 10.1007/s12026-017-8931-1. [DOI] [PubMed] [Google Scholar]
- 15.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79(1):17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanbervliet B, Bendriss-Vermare N, Massacrier C, et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198(5):823–830. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15(8):471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 19.Swiecki M, Colonna M. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol. 2010;40(8):2094–2098. doi: 10.1002/eji.201040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J Leukoc Biol. 2013;93(4):599–609. doi: 10.1189/jlb.0912452. [DOI] [PubMed] [Google Scholar]
- 21.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 22.Schraml BU, Reis e Sousa C. Defining dendritic cells. Curr Opin Immunol. 2015;32:13–20. doi: 10.1016/j.coi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66(2):205–208. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100(13):4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 26.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10(12):1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 27.Allam JP, Stojanovski G, Friedrichs N, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63(6):720–727. doi: 10.1111/j.1398-9995.2007.01611.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199(12):1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Saint-Vis B, Fugier-Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666–1676. [PubMed] [Google Scholar]
- 30.Kopitar AN, Ihan Hren N, Ihan A. Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol. 2006;21(1):1–5. doi: 10.1111/j.1399-302X.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Nish SA, Jiang R, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na H, Cho M, Chung Y. Regulation of Th2 Cell Immunity by Dendritic Cells. Immune Netw. 2016;16(1):1–12. doi: 10.4110/in.2016.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101(11):4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- 34.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 35.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Marquez M, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Complex interactions between B cells and dendritic cells. Blood. 2013;121(12):2367–2368. doi: 10.1182/blood-2012-12-468017. [DOI] [PubMed] [Google Scholar]
- 37.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 38.Mikulic J, Longet S, Favre L, Benyacoub J, Corthesy B. Secretory IgA in complex with Lactobacillus rhamnosus potentiates mucosal dendritic cell-mediated Treg cell differentiation via TLR regulatory proteins, RALDH2 and secretion of IL-10 and TGF-beta. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei J, Xia S, Sun H, et al. Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. J Immunol. 2013;191(1):500–508. doi: 10.4049/jimmunol.1300328. [DOI] [PubMed] [Google Scholar]
- 40.Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol. 2012;15:117–132. doi: 10.1159/000329675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souto GR, Queiroz-Junior CM, de Abreu MH, Costa FO, Mesquita RA. Pro-inflammatory, Th1, Th2, Th17 cytokines and dendritic cells: a cross-sectional study in chronic periodontitis. PLoS One. 2014;9(3):e91636. doi: 10.1371/journal.pone.0091636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cury PR, Carmo JP, Horewicz VV, Santos JN, Barbuto JA. Altered phenotype and function of dendritic cells in individuals with chronic periodontitis. Arch Oral Biol. 2013;58(9):1208–1216. doi: 10.1016/j.archoralbio.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Tew JG, El Shikh ME, El Sayed RM, Schenkein HA. Dendritic cells, antibodies reactive with oxLDL, and inflammation. J Dent Res. 2012;91(1):8–16. doi: 10.1177/0022034511407338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79(8):1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39(8):2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 46.Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitans-specific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun. 2005;73(6):3453–3461. doi: 10.1128/IAI.73.6.3453-3461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Gao B, Hao L, et al. The silencing of cathepsin K used in gene therapy for periodontal disease reveals the role of cathepsin K in chronic infection and inflammation. J Periodontal Res. 2016;51(5):647–660. doi: 10.1111/jre.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutzan N, Vernal R, Hernandez M, et al. Levels of interferon-gamma and transcription factor T-bet in progressive periodontal lesions in patients with chronic periodontitis. J Periodontol. 2009;80(2):290–296. doi: 10.1902/jop.2009.080287. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Z, Chen W, Hao L, et al. Ac45 silencing mediated by AAV-sh-Ac45-RNAi prevents both bone loss and inflammation caused by periodontitis. J Clin Periodontol. 2015;42(7):599–608. doi: 10.1111/jcpe.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86(4):347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 51.Cardoso CR, Garlet GP, Crippa GE, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24(1):1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 52.Dutzan N, Vernal R, Vaque JP, et al. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83(7):948–954. doi: 10.1902/jop.2011.110482. [DOI] [PubMed] [Google Scholar]
- 53.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 54.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 55.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun. 2008;76(9):4206–4213. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su H, Yan X, Dong Z, Chen W, Lin ZT, Hu QG. Differential roles of Porphyromonas gingivalis lipopolysaccharide and Escherichia coli lipopolysaccharide in maturation and antigen-presenting functions of dentritic cells. Eur Rev Med Pharmacol Sci. 2015;19(13):2482–2492. [PubMed] [Google Scholar]
- 57.Mahanonda R, Pothiraksanon P, Sa-Ard-Iam N, et al. The effects of Porphyromonas gingivalis LPS and Actinobacillus actinomycetemcomitans LPS on human dendritic cells in vitro, and in a mouse model in vivo. Asian Pac J Allergy Immunol. 2006;24(4):223–228. [PubMed] [Google Scholar]
- 58.Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–24. doi: 10.1016/j.cyto.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23(3):147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 60.Izakovicova Holla L, Hrdlickova B, Vokurka J, Fassmann A. Matrix metalloproteinase 8 (MMP8) gene polymorphisms in chronic periodontitis. Arch Oral Biol. 2012;57(2):188–196. doi: 10.1016/j.archoralbio.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Ihn H, Yamane K, Asano Y, Kubo M, Tamaki K. IL-4 up-regulates the expression of tissue inhibitor of metalloproteinase-2 in dermal fibroblasts via the p38 mitogen-activated protein kinase dependent pathway. J Immunol. 2002;168(4):1895–1902. doi: 10.4049/jimmunol.168.4.1895. [DOI] [PubMed] [Google Scholar]
- 62.Saidenberg-Kermanac’h N, Bessis N, Lemeiter D, de Vernejoul MC, Boissier MC, Cohen-Solal M. Interleukin-4 cellular gene therapy and osteoprotegerin decrease inflammation-associated bone resorption in collagen-induced arthritis. J Clin Immunol. 2004;24(4):370–378. doi: 10.1023/B:JOCI.0000029116.12371.bf. [DOI] [PubMed] [Google Scholar]
- 63.Eastcott JW, Yamashita K, Taubman MA, Harada Y, Smith DJ. Adoptive transfer of cloned T helper cells ameliorates periodontal disease in nude rats. Oral Microbiol Immunol. 1994;9(5):284–289. doi: 10.1111/j.1399-302x.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 64.Behfarnia P, Birang R, Andalib AR, Asadi S. Comparative Evaluation of IFNgamma, IL4 and IL17 Cytokines in Healthy Gingiva and Moderate to Advanced Chronic Periodontitis. Dent Res J (Isfahan) 2010;7(2):45–50. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao L, Zhou Y, Xu Y, Sun Y, Li L, Chen W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J Clin Periodontol. 2011;38(6):509–516. doi: 10.1111/j.1600-051X.2011.01712.x. [DOI] [PubMed] [Google Scholar]
- 66.Myneni SR, Settem RP, Connell TD, Keegan AD, Gaffen SL, Sharma A. TLR2 signaling and Th2 responses drive Tannerella forsythia-induced periodontal bone loss. J Immunol. 2011;187(1):501–509. doi: 10.4049/jimmunol.1100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wykes M, MacPherson G. Dendritic cell-B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100(1):1–3. doi: 10.1046/j.1365-2567.2000.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. Journal of immunology. 1998;161(3):1313–1319. [PubMed] [Google Scholar]
- 69.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36(5):396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 70.Ernst CW, Lee JE, Nakanishi T, et al. Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kappaB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clin Exp Immunol. 2007;148(2):271–280. doi: 10.1111/j.1365-2249.2006.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garlet GP, Cardoso CR, Mariano FS, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37(7):591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 72.Allam JP, Peng WM, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121(2):368–374. e361. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004;39(6):432–441. doi: 10.1111/j.1600-0765.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 74.Acharya AB, Thakur S, Muddapur MV. Effect of scaling and root planing on serum interleukin-10 levels and glycemic control in chronic periodontitis and type 2 diabetes mellitus. J Indian Soc Periodontol. 2015;19(2):188–193. doi: 10.4103/0972-124X.148644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santegoets KC, Wenink MH, Braga FA, et al. Impaired Porphyromonas gingivalis-Induced Tumor Necrosis Factor Production by Dendritic Cells Typifies Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(4):795–804. doi: 10.1002/art.39514. [DOI] [PubMed] [Google Scholar]
- 76.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280(47):38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 77.Amano A, Sharma A, Sojar HT, Kuramitsu HK, Genco RJ. Effects of temperature stress on expression of fimbriae and superoxide dismutase by Porphyromonas gingivalis. Infect Immun. 1994;62(10):4682–4685. doi: 10.1128/iai.62.10.4682-4685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El-Awady AR, Miles B, Scisci E, et al. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 2015;10(2):e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013;190(8):4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 81.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106(10):1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anjana R, Joseph L, Suresh R. Immunohistochemical localization of CD1a and S100 in gingival tissues of healthy and chronic periodontitis subjects. Oral Dis. 2012;18(8):778–785. doi: 10.1111/j.1601-0825.2012.01945.x. [DOI] [PubMed] [Google Scholar]
- 83.Jotwani R, Palucka AK, Al-Quotub M, et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167(8):4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaitley S, Gopu S, Rajasekharan ST, Sivapathasundaram B. Immunohistochemical analysis of Langerhans cells in chronic gingivitis using anti-CD1a antibody. Dent Res J (Isfahan) 2014;11(2):173–179. [PMC free article] [PubMed] [Google Scholar]
- 85.Moughal NA, Adonogianaki E, Kinane DF. Langerhans cell dynamics in human gingiva during experimentally induced inflammation. J Biol Buccale. 1992;20(3):163–167. [PubMed] [Google Scholar]
- 86.Dereka XE, Tosios KI, Chrysomali E, Angelopoulou E. Factor XIIIa+ dendritic cells and S-100 protein+ Langerhans’ cells in adult periodontitis. J Periodontal Res. 2004;39(6):447–452. doi: 10.1111/j.1600-0765.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 87.Laperine O, Blin-Wakkach C, Guicheux J, Beck-Cormier S, Lesclous P. Dendritic-cell-derived osteoclasts: a new game changer in bone-resorption-associated diseases. Drug Discov Today. 2016;21(9):1345–1354. doi: 10.1016/j.drudis.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 88.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22(6):775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 90.Rivollier A, Mazzorana M, Tebib J, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104(13):4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 91.Speziani C, Rivollier A, Gallois A, et al. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol. 2007;37(3):747–757. doi: 10.1002/eji.200636534. [DOI] [PubMed] [Google Scholar]
- 92.Wakkach A, Mansour A, Dacquin R, et al. Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood. 2008;112(13):5074–5083. doi: 10.1182/blood-2008-01-132787. [DOI] [PubMed] [Google Scholar]
- 93.Gallois A, Lachuer J, Yvert G, et al. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J Bone Miner Res. 2010;25(3):661–672. doi: 10.1359/jbmr.090829. [DOI] [PubMed] [Google Scholar]
- 94.Grosjean F, Nasi S, Schneider P, et al. Dendritic Cells Cause Bone Lesions in a New Mouse Model of Histiocytosis. PLoS One. 2015;10(8):e0133917. doi: 10.1371/journal.pone.0133917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ponugoti B, Dong G, Graves DT. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao E, Graves DT. Impact of Diabetes on the Protective Role of FOXO1 in Wound Healing. J Dent Res. 2015;94(8):1025–1026. doi: 10.1177/0022034515586353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behl Y, Siqueira M, Ortiz J, et al. Activation of the acquired immune response reduces coupled bone formation in response to a periodontal pathogen. J Immunol. 2008;181(12):8711–8718. doi: 10.4049/jimmunol.181.12.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ouyang W, Liao W, Luo CT, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491(7425):554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong G, Wang Y, Xiao W, et al. FOXO1 regulates dendritic cell activity through ICAM-1 and CCR7. J Immunol. 2015;194(8):3745–3755. doi: 10.4049/jimmunol.1401754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286(52):44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li S, Dong G, Moschidis A, et al. P. gingivalis modulates keratinocytes through FOXO transcription factors. PLoS One. 2013;8(11):e78541. doi: 10.1371/journal.pone.0078541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Q, Sztukowska M, Ojo A, Scott DA, Wang H, Lamont RJ. FOXO responses to Porphyromonas gingivalis in epithelial cells. Cell Microbiol. 2015;17(11):1605–1617. doi: 10.1111/cmi.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu KJ, Lee YL, Yang YY, et al. Modulation of the development of human monocyte-derived dendritic cells by lithium chloride. J Cell Physiol. 2011;226(2):424–433. doi: 10.1002/jcp.22348. [DOI] [PubMed] [Google Scholar]
- 106.Rodionova E, Conzelmann M, Maraskovsky E, et al. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109(4):1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
- 107.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 108.Hoarau C, Martin L, Faugaret D, et al. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008;3(7):e2753. doi: 10.1371/journal.pone.0002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escribano C, Delgado-Martin C, Rodriguez-Fernandez JL. CCR7-dependent stimulation of survival in dendritic cells involves inhibition of GSK3beta. J Immunol. 2009;183(10):6282–6295. doi: 10.4049/jimmunol.0804093. [DOI] [PubMed] [Google Scholar]
- 110.Weichhart T, Saemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008;67(Suppl 3):iii70–74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- 111.Guiducci C, Ghirelli C, Marloie-Provost MA, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205(2):315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan W, Morinaga H, Kim JJ, et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29(24):4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang C, Lim J, Liu J, et al. FOXO1 expression in keratinocytes promotes connective tissue healing. Sci Rep. 2017;7:42834. doi: 10.1038/srep42834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arjunan P, El-Awady A, Dannebaum RO, Kunde-Ramamoorthy G, Cutler CW. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol Oral Microbiol. 2016;31(1):78–93. doi: 10.1111/omi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Huang G, Vogel P, Neale G, Reizis B, Chi H. Transforming growth factor beta-activated kinase 1 (TAK1)-dependent checkpoint in the survival of dendritic cells promotes immune homeostasis and function. Proc Natl Acad Sci U S A. 2012;109(6):E343–352. doi: 10.1073/pnas.1115635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiao Y, Shi M, Qiu Q, et al. Piperlongumine Suppresses Dendritic Cell Maturation by Reducing Production of Reactive Oxygen Species and Has Therapeutic Potential for Rheumatoid Arthritis. J Immunol. 2016;196(12):4925–4934. doi: 10.4049/jimmunol.1501281. [DOI] [PubMed] [Google Scholar]