Abstract
Objective
Understanding the relationship between chronic pain and neurocognition has important implications for the assessment and treatment of pain. This paper provides an overview of the current literature examining the neurocognition-chronic pain relationship and suggests future avenues of research, along with a discussion of clinical implications of the literature findings. Consideration of potential moderators and mediators of this relationship, as well as a brief discussion of the importance of future research in special populations at particular risk for these problems, are also a focus of this paper.
Methods
This systematic review summarizes the findings of clinical studies in which neurocognitive performance was measured in chronic pain samples. A literature search led to the inclusion of 53 articles in the review.
Results
Studies of neurocognitive performance in clinical chronic pain samples support a relationship between chronic pain and neurocognitive abnormalities, particularly on tests of memory, attention, and processing speed, with mixed data regarding executive functioning.
Discussion
Several factors may moderate or mediate the relationship between chronic pain and neurocognitive functioning, including mood symptoms, medication side effects, and intensity and/or chronicity of pain. Limitations in the literature include a paucity of methodologically rigorous studies controlling for confounding variables (e.g., opioid analgesia) and a limited number of studies examining the relationship between chronic pain and traumatic brain injury (a potential precipitant of both pain and neurocognitive impairment). Nonetheless, findings from the existing literature have significant clinical implications, including for populations with heightened risk of both pain and neurocognitive disorders.
Keywords: neurocognition, pain
Introduction
Chronic pain (i.e., pain persisting beyond three to six months) ranks among the most significant health issues of the 21st century.[1, 2] According to a 2011 Institute of Medicine report [3], as many as one-third of the US population experiences chronic pain, resulting in an annual cost of $635 billion in medical treatment and lost productivity. In addition to adverse effects on quality of life and functioning (e.g., social and occupational impairment), neurocognitive abnormalities (e.g., concentration and memory problems) may also accompany chronic pain, possibly reflecting shared neural mechanisms. The purpose of this systematic review is to summarize the literature on the relationship between chronic pain and neurocognitive performance. The current review extends the findings of existing systematic reviews by expanding the focus of previous reviews to include a broader range of chronic pain conditions and neurocognitive domains rather than focusing exclusively on single conditions (e.g., fibromyalgia) or specific neurocognitive domains (e.g., working memory). The review additionally extends the literature by considering potential mediators and moderators of the effects of pain on neurocognition and by discussing opioids and mood symptoms as potential moderators in the association between chronic pain and neurocognition. The review also considers the implications of chronic pain and neurocognition among populations presumably at increased risk for injuries (e.g., traumatic brain injury; TBI) that may lead to both chronic pain and neurocognitive impairment.
The following research questions guided the literature search: (1) Are there domain-specific neurocognitive impairments associated with chronic pain?; (2) What factors potentially mediate or moderate the relationship between chronic pain and neurocognitive performance?; and (3) What are the implications for populations with comorbid pain and TBI, such as military populations?
Methods
We included clinical studies of adult chronic pain samples in which neurocognition was measured via performance-based neuropsychological assessment. We excluded studies focusing on cancer-related pain, as pain secondary to malignancy may be of a vastly different etiology and is managed differently in clinical practice. Studies of non-clinical samples, experimentally-induced pain, or acute pain were also omitted. Although an important consideration in understanding the relationship between neurocognitive performance and chronic pain, studies examining neural mechanisms potentially underlying both conditions were excluded as these are beyond the scope of the current study.
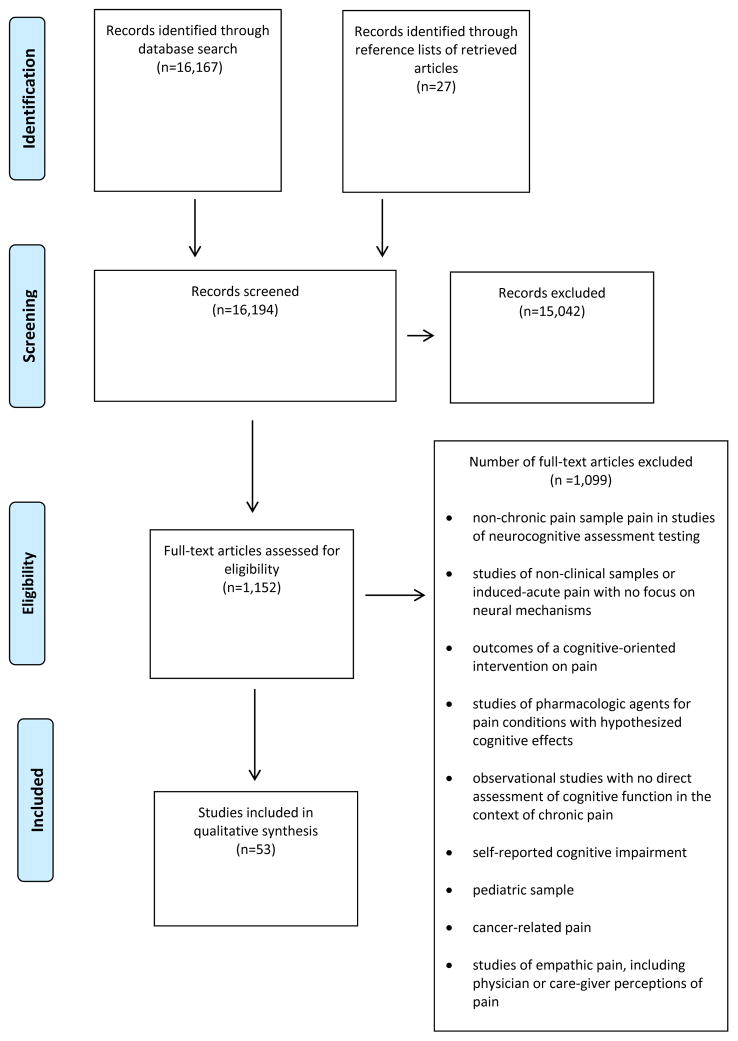
Literature searches were conducted in Ovid MEDLINE and the Cochrane Database of Systematic Reviews for literature published from 1980 through March 30, 2016. Additional studies were identified from bibliographies and review papers. Included articles were all original studies (clinical trials, cross-sectional designs, pilot studies) and English language. Search terms included: pain terms (pain, chronic pain, opioids, opiates) + neurocognitive relevant terms (neurocognition, cognition, cognitive function, neuropsychological function, attention, memory, executive function, processing speed, psychomotor) + veteran and military and were entered in combination for maximum inclusivity in searches. Initially, all search terms were a combination of one term from the pain category and one term from the neurocognitive category. Secondarily, terms with positive results were re-entered, along with military or veteran. For example, search terms such as chronic pain+neurocognition+veteran were used. Two authors (DH and AM) reviewed the abstracts identified from the searches. Full-text articles of potentially relevant abstracts were retrieved for further review. Reference lists of relevant articles were reviewed for additional citations. The initial literature searches revealed a large number of potentially relevant citations (N=16,194) describing the relationship between chronic pain and neurocognition. After removing duplicates and non-English language papers, 1,152 citations were retrieved and considered for inclusion. Of those, 53 citations met inclusion criteria for this review; the remaining were excluded for the following reasons: no chronic pain sample in clinical studies of neurocognitive functioning, interventional studies (e.g., cognitive interventions, pharmacologic agents), or no direct assessment of neurocognitive performance. Figure 1 provides a flowchart of studies included and excluded. The studies included in this systematic review contained a total of 7,430 participants (4,850 with chronic pain conditions and 2,580 designated as control, or comparison participants).
Figure 1.
PRISMA Flowchart for paper selection
Literature Summary
Neurocognitive Impairment
We included studies examining neurocognitive performance in domains judged to be particularly relevant to daily functioning (i.e., processing and psychomotor speed, attention and executive function, memory and learning) and that included pain-related variables such as pain intensity and functional interference. Table 1 outlines the cognitive domains assessed, tests administered, comparison condition, and outcomes for each study.
Table 1.
Summary of studies examining the relationship between chronic pain and neurocognition
| Study | Neurocognitive Domain(s) | Type of Pain | Pain Assessment | Cognitive Test(s) | Significant outcomes |
|---|---|---|---|---|---|
| Abeare et al., 2010 [28] | Attention | 157 Adults with RA | VAS | LNS (WAIS-III); Stroop Test | Pain level was inversely related to the score on the executive functioning (r = − .24, p = .003), even after controlling for confounding effects of age, education, duration of RA, and erythrocyte sedimentation rate (β = − .26, p = .004). |
| Antepohl et al., 2003[34] | Attention | Whiplash-associated disorder (n= 30) vs healthy controls (n= 30) | VAS | Word identification; Spatial rotation; RST; spatial matrix test | Patients performed significantly poorer on working memory tasks (p < .05) and mental verbal speed (p = .023) compared with controls. Within patients, pain after task corresponded with verbal reaction time performance (r = .44, p< .05). |
| Bosma et al., 2002 [24] | Attention | Whiplash syndrome (n= 31) vs with neurologic deficit (n= 30) vs patients with non-organic psychologic problems (n=30) | SCL-90: Somatization subscale | CVLT; ROCFT; BWCT; PASAT; Stroop Test; Trail Making Test | The whiplash group performed worse than the control group in selective attention and distraction (p < 0.01), immediate verbal memory recall (p < 0.01), verbal learning speed (p < 0.01), verbal rate of forgetting (p < 0.05), and visuospatial perception (p < 0.05). No differences were found when compared with the neurologic deficit group. |
| Coppieters et al 2015 [21] | Attention; Working memory | Whiplash-associated disorder (n= 16) vs Fibromyalgia (n=21) pain free controls (n= 22) | Verbal NRS; Laboratory stimuli (Pressure, deep tissue, temporal summation, conditioned pain modulation) | Stroop Test; PVT; OSPAN | Fibromyalgia patients presented impaired cognitive performance on all cognitive tests compared to healthy individuals. Whiplash patients only demonstrated impaired performance on the PVT test (significant longer PVT reaction times and more PVT lapses) compared to healthy pain-free controls. |
| Dick et al., 2002 [26] | Attention | Musculoskeletal (n = 20), RA (n = 20), Fibromyalgia (n= 20) vs healthy controls (n= 20) | VAS | TEA | Patient groups performed poorer on global measure of attention (P < 0.0001) and selective attention (p < 0.003) compared with controls. RA patients performed poorer on sustained attention (p < 0.003) and compared with controls. Both MSP and RA patient performed poorer on working memory (p< 0.0001) compared with controls. |
| Dick et al., 2007 [18] | Attention | Chronic mixed (n = 24) with test-retest following analgesic intervention | NRS; McGill Pain Questionnaire | TEA; RST; SST | Two-thirds of the sample showed clinically significant disruption of attention associated with impaired working memory processes. TEA, RST, and SST scores did not differ between conditions. The maintenance of the memory trace is affected by chronic pain during task performance for those with the greatest attention impairment (p < 0.05). |
| Dick et al., 2008 [32] | Attention | Fibromyalgia (n= 30) vs pain-free controls (n= 30) | NRS; McGill Pain Questionnaire | TEA; ACT; RST | Patient groups performed poorer on overall attentional scores (TEA; p= .002) and auditory verbal working memory (TEA; p= .006), attentional resource capacity (RST; p = .01 – .005), and attentional functioning (ACT; p = .043). |
| Eccleston, 1994 [48] | Attention | Chronic, non-malignant mixed (n= 26) vs 12 pain-free controls | VAS; NRS; McGill Pain Questionnaire | Numerical interference task | Longer latency in reaction time for high pain group (p<.01) compared with low and no-pain groups. Significant interaction between task difficulty and pain on reaction time (p< .01) with increasing difficulty associated with poorer reaction time for the high pain group. |
| Grace et al., 1999 [27] | Attention | Fibromyalgia (n= 30) vs health controls (n= 30). | MPI-PS | WMS-R; RAVLT; PASAT; SDMT | Pain patients performed poorer in sustained auditory concentration (PASAT; p < .01) compared with controls. No significant differences were found on the Attention/Concentration Index and the Symbol-Digit Test. |
| Grisart & Plaghki, 1999 [20] | Attention | Chronic Low back and other (n=33) | VAS | Stroop Test | Impairment in selective attention as related to pain intensity |
| Landrø et al 2013 [31] | Attention; Memory | Chronic, mixed, nonmalignant pain (n=72); 19% neuropathic pain | Brief Pain Inventory | Matrix Reasoning, Vocabulary, LS (WAIS-III); Stroop Test; PASAT; CVLT-II; | 20% performed below cut-off (below 1.5 SD) on each test. Forty-seven percent of the patients performed below this cut-off on at least one of the tests. Patients taking a combination of antidepressant, antiepileptics, and opioids had more depressive symptoms compared with the no medication group (p< 0.05) and performed worse on the PASAT (p<0.05). |
| Luerding et al., 2008 [33] | Attention | Fibromyalgia (n= 20) compared with age- and education-matched normative data | McGill Pain Questionnaire | WAIS-R; CVLT; RVDLT; Corsi Block Span; Trail Making Test | FM patients demonstrated poorer performance on non-verbal working memory and free recall verbal memory following delay compared with normative sample. Long-term recognition memory was normal suggesting impaired retrieval. Attention (TMT) fell in the normal range. |
| Meeus et al 2015 [35] | Attention; Working memory | Whiplash associated disorder (n= 15) vs health controls (n=16) | VAS; Laboratory stimuli (Pressure, temporal summation, conditioned pain modulation) | Stroop Test; PVT; OSPAN | Chronic WAD patients presented a significant longer reaction time on the PVT test and presented significant worse recall capacities on the OSPAN. No differences were found for the Stroop Test. |
| Oosterman et al., 2011 [30] | Attention | Chronic, mixed (n=34) vs pain-free controls (n=32) | VAS; McGill Pain Questionnaire | Doors test; Digit span test; Category fluency test; Story recall test; Bourdon Vos; MMSE | Performance on a cognitive screener was significantly poorer (MMSE; p<.05) compared with controls. Performance was poorer for working memory (p<.05), and immediate (p<.01) and delayed (p<.05) verbal episodic memory compared with controls. Chronic pain associated with attentional task performance (change R2=0.11, P <0.01). |
| Oosterman et al., 2012 [19] | Attention | Chronic mixed (n = 34) vs healthy controls (n= 32) | VAS; McGill Pain Questionnaire | Stroop Test; Trail Making Test; Bourdon Vos Test; Zoo Map | Pain patients demonstrated poorer sustained attention in terms of task completion time (P<0.05, η2=0.07), variability in task completion time (P<0.05, η2=0.08) and omissions (P=0.08, η2=0.05). |
| Park et al., 2001 [13] | Attention | Fibromyalgia (n= 23), age and education-matched controls (n= 23), education-matched controls 20 years older (n= 22) | McGill Pain Questionnaire; AIMS | Timed number, pattern, letter comparison task; Reading span, computational span tasks | FM patients performed more poorly than the age-matched control group on measures of working memory capacity (p= 0.042). There was a significant negative correlation between AIMS pain subscale and information-processing speed (r = −0.662, p= 0.001) and working memory capacity (r= −0.466, p= 0.02). |
| Ryan et al., 1993 [6] | Attention | 142 adult Type-1 diabetic patients vs 100 non-diabetic controls | DSP determined via clinical exam. A dichotomous scale was used (present vs. absence) | DVT; EFT; Trail Making Test; Grooved Pegboard; Logical Memory (WAIS-R) | Complications from diabetes associated with poorer sustained attention (DVT; p < .002), visual scanning and rapid decision making (EFT; p< .001), and hand-eye coordination (GP; p< .0001) compared with non-diabetic controls. Compared with other complications from diabetes, DSP was best predictor of performance for sustained attention (DVT; R2= .062, p < .002), visual scanning and rapid decision making (EFT; R2= .046, p< .007), hand-eye coordination (GP; R2= .087, p< .000) and mental flexibility (TST; R2= .027, p <.042). |
| Sjogren et al., 2005 [4] | Attention | Chronic non-malignant, mixed (n= 91) vs healthy controls (n= 64) | VAS | CRT; FTT; PASAT; MMSE | Sustained attention (CRT; p = 0.0368 and 0.0168) and psychomotor speed (FFT; p < 0.0001 and 0.0001) were impaired in patients compared with controls. No differences found on MMSE. |
| Veldhuijzen et al., 2006 [17] | Attention | Chronic non-cancer, mixed (n= 14) vs healthy controls (n= 15) | VAS; McGill Pain Questionnaire | Attentional capacity probe task | Pain patients showed faster reaction time responses (p < 0.036) and higher error rates (p < 0.022) compared with controls. |
| Weyer Jamora et al 2013 [51] | Attention; Memory; Executive functioning; Motor | TBI (n=66); compared based on high (n=29) vs low pain (n=37) | Pain scale (Ruff Neurobehavioural Inventory; RNBI) | RNBI (self report); Trail Making Test; Digit Symbol Coding (WAIS-III); Logical Memory (WMS-III) ; ROCFT; Light Tail Learning Test; COWA; Figural Fluency Test; Stroop Test; FTT; Grooved Pegboard | No significant between-group differences were found on objective neuropsychological test performances. The high pain group reports significantly more anger, aggression, depression and paranoia/suspicions. |
| Duschek et al 2013 [68] | Memory | Fibromyalgia (female, n=18) vs healthy controls (n=25) | McGill Pain Questionnaire | Word stem completion task | Patients with fibromyalgia showed significantly fewer correct responses compared with healthy controls (p <0.01, partial η2 = 0.23). Only pain score significantly predicted correct responses in multivariate analysis including measures of depression and anxiety. |
| Gil-Gouveia et al 2015 [42] | Memory; Reading speed; verbal learning; short-term verbal and delayed recall; Processing speed | Migraine without aura (n=24), within subjects during and not during migraine attack | VAS | CVLT; Stroop Test | Significant differences were observed in Stroop word reading, CVLT total learning (p¼0.01), CVLT short-term recall with and without semantic help, and delayed recall with and without semantic help. Pain intensity during an attack was found to influence CVLT short-term free recall. |
| Grace et al., 1999 [27] | Memory | Fibromyalgia (n= 30) vs health controls (n= 30). | MPI-PS | WMS-R; RAVLT; PASAT; SDMT | Pain patients performed poorer on general memory (p < .02), verbal memory (p < .02), and delayed recall (p < .03) compared with controls. No significant differences were found on the RAVLT. |
| Grisart & Van der Linden, 2001 [45] | Memory | Chronic non-malignant, mixed (n= 18) vs healthy subjects (n= 18) | VAS | Word stem completion task in Process Dissociation Procedure | Patients performed poorer on cued recall (p< .004) and use of controlled recollection (p< .037) compared with controls. |
| Iezzi et al., 1999 [44] | Memory | Chronic, nonmalignant, mixed (73); Grouped as high (n= 27), moderate (n= 36) and low (n= 10) emotional distress | SCL-90-R; VAS | WAIS-R; WMS-R; ROCFT; Stroop Test; WCST; PASAT; Trail Making Test; DFT; COWA; Grooved Pegboard; | High distress patients performed significantly lower on full scale and performance IQ (WAIS-R) compared with low distress patients. High distress patients also performed poorer on measures of logical memory and immediate visual memory (WMS-R) compared with both moderate and low distress patients. Low distress patients outperformed the moderate and high distress groups on measures of concentration (Stroop), concept formation (WCST), visual memory (WMS-R; ROFT). |
| Iezzi et al., 2004 [67] | Memory | Chronic, mixed (n= 73) | MPI-PS | WAIS-R; WMS-R; ROCFT; Stroop Test; Trail Making Test; | In bivariate analyses, pain severity was negatively related to attention and concentration (r= −.28, p< .05), constructional ability (r= −.37, p< .01), memory (r= −.36, p< .01), and reasoning ability (r= −.29, p< .05). Pain did not predict attention and concentration or reasoning ability when education was controlled. After controlling for education, pain remained a significant predictor of memory, accounting for 7% of the variance (p= .02). |
| Kewman et al., 1991 [22] | Memory | Chronic, musculoskeletal pain, mixed (n= 73) | McGill Pain Questionnaire; VAS | NCSE | Pain was negatively correlated with performance on a neurocognitive screener (p= −.475, p< .001) at an initial appointment and remained significant when education was controlled (r= −.301, p< .001). At a follow-up visit, this correlation remained (r= −.313, p< .01), but not when controlling for education. |
| Meyer et al., 2000 [69] | Memory | Migraine and cluster HA (n= 196); within-subject design comparing HA-free clinic visits | HA diagnosis made based on clinical exam; no discrete pain measure used | MMSE; CCSE | Performance on both neurocognitive screening measures are decreased during concurrent HA visits, and returned to normative levels on subsequent HA-free clinic visits (p< .0001). |
| Park et al., 2001 [13] | Memory | Fibromyalgia (n= 23), age and education-matched controls (n= 23), education-matched controls 20 years older (n= 22) | McGill Pain Questionnaire; AIMS | Visual free recall and recognition (words); Verbal fluency; Antonym/Synonym task; SILVT | FM patients performed more poorly than the age-matched control group on measures of free recall (p= 0.005), recognition memory (p= 0.035), and verbal knowledge (p= 0.001). Performance on verbal fluency was marginally worse in the FM patients compared with the age-matched controls (p= 0.055). There was a significant negative correlation between AIMS pain subscale and free recall (r= −0.607, p= 0.002) and recognition memory (r= −0.555, p= 0.005). The MPQ correlated with free recall (r = −0.441, p= 0.031). |
| Povedano et al., 2007 [70] | Memory | Chronic neuropathic (n= 603) vs mixed neuropathic, nociceptive (n= 856) | McGill Pain Questionnaire; VAS | MMSE | Cognitive impairment is significantly less likely in the mixed (neuropathic & nociceptive) compared with the neuropathic group (p= .006). A linear trend between increased pain and prevalence of cognitive impairment was seen for both groups (p= .0001), with neuropathic patients who rated their PPI as distressing having a significantly higher prevalence of cognitive impairment (p= .006). |
| Rodriguez-Andreu et al., 2009 [71] | Memory | Fibromyalgia (n= 46) vs neuropathic (n= 92) vs mixed pain (n= 92) | McGill Pain Questionnaire | MMSE | Fibromyalgia patients scored lower on cognitive screener compared with the other patient groups (p= .001). Rates of cognitive impairment did not vary by pain severity in any patient groups. |
| Schneider, Palomba, & Flor, 2004 [47] | Memory | CBP (n= 11) vs healthy controls (n= 11) | MPI-PS; VAS | Differential Pavlovian conditioning paradigm (EMG, SCL, EEG) | Patients demonstrated increased muscular responding to an aversive visual stimuli during habituation (p< .05) and acquisition (p< .05) compared with controls. During extinction muscular responses to aversive (followed by shock) and pleasant visual stimuli (not followed by shock) were similarly elevated for patients only. EEG differentiates between the conditioned stimuli in controls, but not patients. |
| Weiner et al., 2006 [15] | Memory | CLBP (n= 163) vs pain-free controls (n= 160) | McGill Pain Questionnaire | RBANS; Trail Making Test; Grooved Pegboard | CLBP patients performed poorer in immediate memory (RBANS; p= .002, ES= .34), language (RBANS; p= .004, ES= .32), and delayed memory (RBANS; p= .046, ES= .22) compared with controls. |
| Harman & Ruyak, 2005 [5] | Processing Speed | CBP (n= 20) vs pain-free, age-matched controls | McGill Pain Questionnaire | Performance Assessment Battery: LRRT; MST; MT; PVT; FCRTT | Controls were more accurate on a visual-spatial decision making task (MT; p= .005) and had faster reactions times on a button pressing task (PVT; p= .001; FCRTT; p= .0001) compared with CBP patients. Pain was correlated with latency on a logical reasoning task (LRRT; r= .698, p< .001) and accuracy in a memory probe task (MST; r= −.375, p= .003) and a visual-spatial decision making task (MT; r= −.301, p= .019). |
| Jongsma et al., 2011[10] | Processing Speed | Chronic pancreatitis pain (n= 16) vs healthy, age-, gender- nd education-matched controls (n= 16) | Diagnosis by clinical examination and laboratory testing; Pain duration | Integneuro test battery (psychomotor performance, memory, and executive functioning) | Chronic pancreatitis was associated with significantly poorer performance in all three cognitive domains compared with controls. Additionally, longer pain duration was associated with poorer cognitive performance. Psychomotor performance and executive functioning showed the greatest cognitive decline, with memory performance being the least effected cognitive function in patients. |
| Lee et al., 2010 [9] | Processing Speed | Chronic, widespread pain (n= 266) vs pain-free controls (n= 1273) | Pain diagnosis made based on identifying pain >1 day over past month (or denying) and shading of pain drawing /manikin | ROCFT; CTRM; DSST | Chronic widespread pain associated with significantly lower scores on a measure of psychomotor speed and visual scanning (DSST; p, .0001) compared with pain-free controls. Meditational analyses revealed the pain-cognition relationship to be partially (32%) mediated by depressive symptoms severity. |
| Pulles & Oosterman, 2011 [49] | Processing Speed | Chronic, mixed (n= 30) | McGill Pain Questionnaire; VAS | Stroop test; Trail Making Test; LFT; RBMT (Story Recall); ROCFT | Pain is negatively correlated with mental processing speed (Stroop; TMT; r= −.502, p, .01). Mental processing was found to significantly mediate the pain-physical function relationship (R2= .22, B = 0.40, p< 0.01). |
| Reyes del Paso 2012 [11] | Processing Speed | Fibromyalgia (n=35) vs healthy controls (n=29) | McGill Pain Questionnaire; Opioid use (self-report) | Uchida-Kraepelin (Arithmetic) test | Performance was lower in the patients with fibromyalgia patients compared with controls (p = 0.002, η2 = 0.15). Those with fibromyalgia using opiates completed significantly more calculations p = 0.050, η2 = 0.10). In the fibromyalgia group, number of words used to describe pain was predictive of the number of calculations of calculations (b = −0.369, R2 = 0.136, adjusted R2 = 0.110, F = 5.19, p = 0.029). |
| Ryan et al., 1992 [8] | Processing Speed | 75 adult Type-1 diabetic patients vs 75 non-diabetic controls | DSP determined via clinical exam. A dichotomous scale was used (present vs. absence) | DVT; EFT; Trail Making Test; WAIS-R; Grooved Pegboard; SDLT; HRNB | The diabetic group performed slower on psychomotor efficiency (p< .0001), poorer on spatial information processing (p= .037) compared with controls. Neuropathy score continued to contribute to the variance of psychomotor performance after controlling for age and sex (multiple r2= .189, p= .012). |
| Ryan et al., 1993 [6] | Processing Speed | Type-1 diabetic patients (n= 142) vs non-diabetic controls (n= 100) | DSP determined via clinical exam. A dichotomous scale was used (present vs. absence) | DVT; EFT; Trail Making Test; Grooved Pegboard; Logical Memory (WAIS-R) | Complications from diabetes associated with poorer sustained attention (DVT; p < .002), visual scanning and rapid decision making (EFT; p< .001), and hand-eye coordination (GP; p< .0001) compared with non-diabetic controls. Compared with other complications from diabetes, DSP was best predictor of performance for sustained attention (DVT; R2= .062, p < .002), visual scanning and rapid decision making (EFT; R2= .046, p< .007), hand-eye coordination (GP; R2= .087, p< .000) and mental flexibility (TST; R2= .027, p <.042). |
| Ryan, 2005 [7] | Processing Speed | Type-1 diabetic patients (n= 200) vs non-diabetic controls (n= 175) | DSP determined via clinical exam. A dichotomous scale was used (present vs. absence) | Block Design (WAIS-R); WCST; Verbal Paired Associate | Diabetic patients had longer latency on tests of psychomotor efficiency (p< .0001). In a multiple regression, DSP (B= .162) contributed, along with diabetes duration, peripheral vascular disease, and diabetic retinopathy, explained nearly 27% of the variance in performance. |
| Schiltenwolf 2014 [54] | Processing speed; Memory; executive functioning | CLBP, on long-term opioids (n=37) vs not on opioids (n=33) vs healthy controls (n=25) | Not indicated | Multiple-choice vocabulary test; Digit-Span, Letter-Number Sequencing (WAIS-III); Trail Making Test; Cambridge Neuropsychological Test; CRT; Pattern recognition; Spatial span | Both patient groups needed significantly longer time in information processing when compared with healthy controls. Patients on opioids had significantly reduced spatial memory capacity, flexibility for concept change, and impaired performance in working memory compared with patients not on opioids and health controls. Impaired cognitive outcomes were significantly associated with pain intensity and medication use. |
| Sjogren et al., 2005 [4] | Processing Speed | Chronic non-malignant, mixed (n= 91) vs healthy controls (n= 64) | VAS | CRT; FTT; PASAT; MMSE | Treatment with opioids was associated with poorer performance in working memory and information processing (PASAT; p = 0.0023). High scores of pain VAS and sedation VAS were associated with poor performance of PASAT. |
| Veldhuijzen et al., 2012 [12] | Processing Speed | Fibromyalgia (n= 35) vs age-matched, health controls (n= 35); Patients separated into high and low pain groups for sub-analysis | VAS; Experimental pressure pain threshold | Stroop test; MSIT | Interference scores between patients and health controls did not significantly differ. Within the patient group, higher pain ratings were associated with increased reaction times for the neutral (MSIT; r = .44, p< .008) and interference conditions during the cognitive inhibition tasks (MSIT; r= .37, p< .028; Stroop; r= .41, p< .015) and for the interference score on one measure (SCWT; r= .35, p< .04) |
| Weiner et al., 2006 [15] | Processing Speed | CLBP (n= 163) vs pain-free controls | McGill Pain Questionnaire | RBANS; Trail Making Test; Grooved Pegboard | CLBP patients performed poorer in mental flexibility (TMT; p= .019, ES= .26) and motor coordination (Pegboard; p= .047, ES= .22) compared with controls. |
| Apkarian et al., 2004 [37] | Executive functioning | CBP (n= 26), Chronic Regional Pain Syndrome (n= 12) vs age-, sex- and education-matched controls (n= 26) | McGill Pain Questionnaire | Iowa Gambling Task; WCST; Digit span (WMS-R); Vocabulary, similarities, matrix reasoning (WASI) ; Stroop Test | Both patient groups performance poorer on an emotional decision making task (IGT; p< .004), compared with the controls. In CBP patients there was a strong negative correlation between pain intensity and performance on the emotional decision making task (IGT; r= −.75, p< .003). There was not a significant relationship between pain intensity and performance on this task in the CRPS group. |
| Attal et al 2014 [50] | Executive functioning; Memory; Attention | Patients undergoing total knee arthroplasty (n=189) vs breast cancer surgery (n=100) | Brief Pain Inventory; DN4 (Douleur Neuropathique en 4 Questions) | ROCFT; Trail Making Test | After controlling for anxiety, depression and coping strategies, the presence of clinical meaningful pain at 6 and 12 months was predicted by poorer cognitive performance in the Trail Making Test B (p = 0.0009 and 0.02, respectively), ROCFT copy (p = 0.015 and 0.006, respectively) and recall (p = 0.016 for pain at 12 months), for the total group of patients. Impaired scores on these tests predicted pain intensity (p<0.01) and neuropathic symptoms in patients with pain (p<0.05). Findings did not differ for surgical groups. |
| Cherry et al 2012 [14] | Executive functioning; Memory | Fibromyalgia (female n=43) vs age-matched controls without fibromyalgia (n=44) | Fibromyalgia diagnosis confirmed via physician documentation; NRS (National Fibromyalgia Association Questionnaire | MMSE; CERAD; Stroop Test; Digit Span (WMS-III); Animal Naming; Trail Making Test; Digit-Symbol Coding (WAIS-III); Everyday Problems Test | Women with fibromyalgia performed more poorly on executive function (Stroop; p=0.021, partial η2 =0.062) and processing speed (Digit-Symbol Coding; p=0.0002, partial η2= 0.105) measures compared with healthy controls. |
| Di Tella et al 2015 [40] | Executive functioning | Fibromyalgia (female, n=40) vs healthy matched controls (n=41) | NRS | Digit span; RAVLT, Trail Making Test; Tower of London; verbal fluency | Patients with fibromyalgia performed worse than healthy controls on all the four tasks evaluating executive functioning. |
| Hess et al 2014 [39] | Executive functioning | Fibromyalgia (n=15) vs healthy controls (n=15) | Fibromyalgia diagnosis made via medical interview | Iowa Gambling Task | A heuristic model of risky-decision making was also able to differentiate between healthy participants and those with fibromyalgia at the group- and the individual-level. The mathematical model best fit those with fibromyalgia when decisions were not based on previous choices and when gains were considered more relevant than losses. Conversely, among healthy controls, the best fit occurred when decisions were based on prior experience and losses were more relevant than gains. |
| Karp et al., 2006 [72] | Executive functioning | Chronic, mixed non-malignant (n=56; older adults) | McGill Pain Questionnaire | MMSE; Trail Making Test; DSST; free and paired recall of digit-symbol pairs | Pain severity was correlated with greater impairment on number letter switching (TMT; r = −.42, p= 0.002). This association remained after adjusting for the effects of depression, sleep, medical comorbidity, opioid use, and years of education (t= −1.97, p= 0.056). |
| Kurita et al 2012 [53] | Executive functioning; Attention | Chronic, mixed, nonmalignant pain (n=49) | NRS | CRT; FTT; Digit Span (WAIS-R); Trail Making Test; MMSE | Patients performed adequately on all tests with the exception of a measure of executive functioning (Trail Making Test). Several associations among independent variables and cognitive tests were observed. In multiple regression analyses, higher doses of opioids was associated with better Digit Span performance (p=0.019). |
| Lee et al 2015 [36] | Executive functioning | CRPS (n=25) versus health controls (n=25) | McGill Pain Questionnaire | WCST; stop-signal task | Patients with CRPS made significantly more perseveration errors on the WCST (16.7 vs 9.1, P = .013) and had significantly longer stop-signal response times (SSRTs) (193.6 milliseconds vs 150.4 milliseconds, P = .016) compared with healthy controls. |
| Oosterman et al., 2012 [19] | Executive functioning | Chronic mixed (n = 34) vs healthy controls (n= 32) | VAS; McGill Pain Questionnaire | Stroop Test; Trail Making Test; Bourdon Vos Test; Zoo Map | Pain patients showed poorer mental flexibility (P<0.01, η2=0.11), but not planning compared with controls. |
| Oosterman, Gibson et al 2013 [16] | Executive functioning; Memory; Motor | Chronic, mixed, nonmalignant pain; Younger (n=22; mean age 28.8) vs older (n=24; mean age=65) | McGill Pain Questionnaire; VAS | Stroop Test; Trail Making Test; LFT; Story Recall (RBMT) | In the younger age group, pain ratings were inversely related to memory and executive function. In the older age group, a positive relationship was found between pain ratings and executive function, whereas the inverse association of pain with memory was no longer present. On the Stroop Test reading performance was slower in patients on opioid medication compared with the nonopioid (p = 0.02). |
| Scherder et al., 2008 [46] | Executive functioning | Alzheimer’s Dementia (n=19) vs older adults without dementia (n= 20); both groups experienced arthritis/ arthritis. | VAS; Colored Analogue Scale for Pain Intensity; Faces Pain Scale | Digit Span Forward; EWT; Faces and Picture Recognition (RBMT); Digit Span Backward; Category Fluency; KCIT; Incomplete Figures | Pain severity (FPS) was positively correlated with executive functioning in patients with Alzheimer’s diseases (r= .675, p< .005). The authors interpret this to mean that with better executive function pain intensity is more likely to affect patient experiences. No relationships between pain and memory or executive functioning were found in older adults without dementia. |
| Suhr, 2003 [41] | Executive functioning | Fibromyalgia (n= 30) vs chronic, mixed, non-FM pain (n= 27) vs healthy controls (n= 21) | McGill Pain Questionnaire | Metamemory Questionnaire; WAIS-III; WCST; Stroop Test; AVLT; ROCFT; PASAT; COWA; Trail Making Test | Groups differed in subjective cognitive complaints (p< .001) with the fibromyalgia group reporting more pain and memory complaints. There were no significant differences between groups on performances on executive functioning, memory, attention/working memory, and psychomotor speed. |
| Verdejo-Garcia, 2009 [38] | Executive functioning | Fibromyalgia (female, n= 35) vs age-, education- and SES-matched healthy controls (female, n= 36) | MPI-PS | Iowa Gambling Task; WCST | Fibromyalgia patients shows significantly poorer performance on an executive function measure (WCST; p= .007–.04; d= .49 – .66) compared with health controls. Fibromyalgia patients demonstrated poorer emotion-based decision making, with controls performing significantly better on the third “block” of a learning based task (p= .01, d= .63). Pain intensity was negatively correlated with performance on a measure of abstraction (WCST; r= −.23, p= .03) and the third “block” of an emotion-based decision making task (IGT; r= −.25, p= .02). |
Processing and psychomotor speed
Chronic pain conditions, especially those associated with functional impairment, have been associated with decrements in both processing speed (i.e., the speed with which a cognitive operation is performed) and psychomotor speed (i.e., the speed in which tasks with a motor component are performed)[4, 5]. For example, compared with demographically similar healthy adults, adults with diabetic peripheral neuropathy demonstrated deficits on tasks of psychomotor speed, visual scanning, and rapid decision-making.[6–8] Although neuropathy is not always painful, it is frequently associated with physical discomfort reflecting symptoms of burning, cold, and tingling, often likened to pain. Diabetes is frequently associated with cerebrovascular disease, which may have contributed to the findings in these studies.[6–8] To that end, the authors of the study found a link between diabetic retinopathy, commonly thought to be associated with microvascular changes, and psychomotor slowing, supporting a cerebrovascular explanation for the relationship between neuropathy and psychomotor slowing through disruption of certain neurotransmitter pathways.[7] Cardiovascular mechanisms, however, cannot account for the relationship between pain and psychomotor slowing in all pain popluations, as similar findings have emerged among patients with other pain conditions not necessarily involving a cardiovascular component, such as chronic widespread pain[9], chronic pancreatitis[10], and fibromyalgia, who show poorer performance on tests of processing speed [11–13] and psychomotor speed [12, 14], than healthy and/or pain-free control participants or normative data. Older patients with osteoarthritis [15] and other pain conditions[16] performed more poorly on tests of psychomotor speed (e.g., grooved pegboard; finger tapping) compared with pain-free age-matched peers and compared with younger participants with similar pain conditions, although age may be an influencing factor[16]. Taken together these data suggest that most chronic pain conditions are associated with at least mild to moderate reductions in psychomotor function, particularly in older patients. More specifically, 12 of 13 studies (11 of which included a control comparison condition) examining processing speed among participants supported decreased performance among those with various pain conditions. The one study that did not demonstrate impairment in processing speed among patients with fibromyalgia compared to controls, higher pain ratings appeared to be associated with increased reaction times [18].
Attention and executive functioning
Attention and executive functioning are discussed together, as the two constructs are somewhat fluid and partially overlapping. On tasks of attention and executive functioning, several cognitive processes have been evaluated in patients with chronic pain: selective attention, sustained attention, working memory, organization/planning, cognitive flexibility, inhibitory control, and reward sensitivity. A number of studies support significant disruptions in selective and sustained attention and working memory among chronic pain patients [4, 6, 17–25]. For example, patients with fibromyalgia[20, 26, 27] and rheumatoid arthritis[28] appear to demonstrate selective attention deficits (i.e., the ability to focus on a particular stimulus while ignoring competing stimuli).[27, 28] In addition, a review and meta-analysis of 24 observational studies noted a moderate effect size for impaired working memory, often categorized as a more complex form of attention, in patients with a variety of chronic pain conditions, compared with healthy controls. [29] The majority (20) of the 22 studies examining attention showed impairment among patients with chronic pain conditions and 15 of these studies included a control condition. The results of these studies cut across pain conditions to include those with chronic whiplash associated disorder (WAD), chronic musculoskeletal conditions, fibromyalgia, rheumatoid arthritis, diabetic neuropathic pain and discomfort, and low back pain, suggesting that chronic pain, in general, may negatively affect performance in tasks requiring an attentional component.
In several studies, patients with mixed chronic pain [30, 31], fibromyalgia [13, 21, 32, 33], chronic WAD [24, 34, 35], and rheumatoid arthritis[28] largely demonstrated impairment in aspects of executive functioning (e.g., cognitive flexibility, reward sensitivity).[28] Patients with complex regional pain syndrome made more perseveration errors, often suggestive of impaired cognitive flexibility, on the Wisconsin Card-Sorting Test compared with healthy controls.[36]Executive functioning may include risk-based decision-making, thought to be impaired in chronic pain. [37, 38] A preliminary study (N=30) suggests that patients with fibromyalgia showed high sensitivity to immediate gains at the expense of future losses, as compared with controls, on the Iowa Gambling task, a task of decision making in the context of varying reward and punishment contingencies. [39] Chronic pain patients demonstrated impaired probabilistic learning, perseverating responses that yield high immediate gains but higher future losses, although these patients also exhibited more random, less perseverative behavior than healthy controls.[39] Results of these studies of risk-based decision-making suggest that additional research is necessary to further explore the role of chronic pain in executive functioning in fibromyalgia patients and to determine whether reward-based decision-making is altered in other chronic pain conditions. In contrast, other studies examining executive functioning in fibromyalgia revealed mixed findings, with some demonstrating deficits compared with those without fibromyalgia [14, 38, 40] and others not [12, 41], highlighting the possible influence of mediating and moderating factors, discussed in detail below.
Learning and Memory
Studies of individuals with fibromyalgia, WAD, osteoarthritis, migraine headache, and low back pain have demonstrated deficits in a number of aspects of learning and memory (e.g., short-term memory, learning, retention, implicit memory, conditioned learning/extinction) among patients with these conditions. [15, 23, 24, 27, 42–47] For example, in a sample of fibromyalgia patients who were compared with older adults and controls, Park and colleagues demonstrated that patients with fibromyalgia performed less proficiently on tasks of free recall when the recall occurred immediately following presentation of stimuli (i.e., short-term memory) than age and education matched controls, but performed similarly to education matched controls who were 20 years older.[43] While, fibromyalgia patients perform more poorly on tests of immediate and delayed recall, these patients’ self-reported subjective complaints of memory difficulties may far outweigh the observed deficits. [27] Verbal learning and memory impairments (i.e., immediate recall, learning speed, forgetting rate) have also been demonstrated in a sample of patients with chronic WAD compared with patients with neurologic disease (i.e., neurologist-diagnosed central nervous system disease or brain injury visible in CT scan or MRI) and patients with anxiety or depression. [24]
Nearly all studies (15 of 16) examining tests of learning and memory reported relatively impaired performance in various aspects of memory among patients across all studied chronic pain conditions. However, given the large range of tests of memory employed in these studies, it is difficult to draw concluding inferences about which aspects of memory (e.g., initial registration vs. retrieval) are consistently affected in patients with chronic pain conditions. In addition, only half of these studies included a control condition, calling the methodological rigor of the studies into effect. Interestingly, several of the studies examining learning and memory also examined moderators of the neurocognition and chronic pain relationship, such as opioid use, mood symptoms, and pain intensity, described below.
Mediators and Moderators of the Relationship Between Chronic Pain and Neurocognitive Function
A number of factors have been hypothesized to influence and/or account for the association between performance on tasks measuring neurocognition and chronic pain, including cognitive load, age, fibromyalgia, patient sex, pain severity, mood symptoms, and opioid use. For tests of attention and executive functioning, some evidence suggests cognitive load and pain severity may interact [48]; specifically, on complex attention tasks with high cognitive demand, patients with high (vs. low) pain intensity demonstrated greater impairment. In contrast, other studies have not supported a cognitive load hypothesis, but rather favor psychomotor slowing as a moderator between pain and alteration in working memory performance. [19] Age may be a mediator of performance on tests examining components of attention or executive functioning (e.g., Stroop Test, Trail Making Test-Part B [TMT-B]) in patients with chronic pain compared to the general population, [16, 28, 31, 49, 50] as well as on tests of psychomotor speed (e.g., grooved pegboard; finger tapping) [16]. Factors that may account for the mixed results of some studies of fibromyalgia and executive functioning include variation among tests of executive functioning, participants’ sex (predominantly female), the nature of fibromyalgia, which typically includes symptoms of fatigue and depression, and as a methodological concern, statistical power of the studies. Pain severity reported at the time of neurocognitive testing appears to have moderated the results in the relationship between pain and neurocognitive functioning in a number of studies. More specifically, higher pain intensity among groups with chronic pain conditions is associated with less proficient processing speed performance [18]. This same relationship between pain intensity and attention and executive functioning is not found among patients with chronic post-concussive pain and mild TBI who reported pain in the two weeks prior to testing.[51] Chronic pain patients with higher emotional distress have demonstrated impairment in immediate and delayed recall of verbal and nonverbal memory tasks compared with those with lower emotional distress, suggesting that mood symptoms may also moderate the relationship between pain and memory processes. [44]
The Role of Opioid Analgesics in Neurocognitive Function
Opioid analgesics constitute another major mediator of the relationship between pain and neurocognitive function because of their effects on the central nervous system and frequent use as a treatment for pain. Although a complete review of the literature describing the role of opioid analgesics in neurocognitive dysfunction is beyond the scope of the current review, their frequent use for chronic pain warrants a summary of the recent findings from the literature, focusing on those studies that examined neurocognitive functioning in patients with chronic non-cancer pain who received opioid analgesia. Ersek reviewed 6 studies published between 1990 and 2003 of patients with chronic non-malignant pain treated with opioids and noted conflicting results.[52] Whereas some of the studies reviewed by Ersek reported impairments in working memory, attention, and processing speed associated with opioid use, as compared with chronic pain patients not receiving opioids or healthy control participants, others found no neurocognitive deficits in patients treated with opioids.
Similar to previous studies reviewed by Ersek, the findings of more recent studies examining the relationship of opioid use to neurocognitive functioning suggest neurocognitive impairment in some domains, but not others, among patients taking opioid analgesics.[52] For example, chronic pain patients receiving long term opioid medications (>3 mo) performed less proficiently on a task requiring alternation of numerical and alphabetic sequences (TMT-B) compared with normative data, despite normal function in other domains of neurocognitive function (i.e., psychomotor speed and attention/working memory). [53] Another study comparing low back pain patients with healthy controls found that chronic opioids can further impair performance on tasks requiring complex attention (TMT-B ratio).[54] This latter study, which included a comparison sample of chronic pain patients who were not receiving opioid analgesics, demonstrated that the opioid-treated chronic pain patients performed more poorly,[54] suggesting that there is some evidence for the role of opioids in complex attention. In a study comparing patients with chronic pain treated with opioid analgesics at a multidisciplinary pain clinic, Sjogren and colleagues found that opioid treatment was associated with impairment in information processing and working memory in patients with chronic pain compared with controls who did not have chronic pain and were not receiving opioids and compared with non-medicated patients with chronic pain.[4] Patients receiving long-term opioids alone (median morphine equivalent daily dosage [MEDD] 60mg), however, did not perform differently than either those receiving antidepressant/anticonvulsants or those receiving the combination of the two (those receiving antidepressant/anticonvulsants and long-term opioids had a median MEDD of 90mg). Overall, there were no associations between morphine equivalent daily dosage, pain duration, or type of analgesic with neurocognitive performance. However, there were associations between sedation (presumed to be a side effect of opioids, which may indicate an effect of opioids) and reaction time, and between pain intensity and information processing/working memory. [4] The study found no relationships between specific opioid analgesics or combinations of opioid analgesics and non-opioid analgesics and neurocognitive performance10. In many of the studies examining the role of opioids in neurocognition among patients with chronic pain, it is difficult to determine with certainty that the deficits are the result of opioid analgesics, as opposed to pain or other variables. Several methodological limitations of these studies included small sample size, non-randomized samples, limited neurocognitive testing, and inadequate experimental design and controls. Other studies have demonstrated either no significant effect or an enhancing effect of opioids on neurocognitive functioning. [55–57] For example, Kurita and colleagues employed a cross-sectional design of 49 patients with chronic non-cancer pain who had been treated with opioids for a minimum of three months. Similar to other studies,[55–57] results of this study did not support evidence of neurocognitive impairment in patients treated with opioid analgesics in the domains of attention, working memory, psychomotor speed, and general cognitive functioning, but did demonstrate a deficit on the Trail Making Test—B, which primarily assesses motor function, attention, and mental flexibility. [53] In addition, exploratory analyses of this study revealed that female sex, increased age, lower education, lower income, lower opioid doses, fatigue, sleep duration, anxiety, and depression were associated with relative neurocognitive impairment. [53] Some of these associated factors (e.g., sleep, anxiety and depression symptoms) are clinically addressable and may inform treatment approaches for patients with chronic pain on opioid therapy. Poor neurocognitive performance was associated with lower opioid doses, suggesting that, if a pain generator is adequately treated (i.e., in this case, through use of opioid analgesics in this particular sample of patients), neurocognition may improve. This is in contrast to the hypothesis that increased opioid dose produces greater neurocognitive impairment, presumably as a result of opioid side effects.
Tolerance to opioids, opioid-mediated pain treatment, and negative mood associated with opioid treatment may each be factors that contribute to variability in neurocognitive impairment in opioid-treated chronic pain. Delineating the role of pain and role of opioids in the neurocognitive deficits found in other studies is difficult, as many of the studies did not include multiple comparison arms such as chronic pain not treated with analgesics, chronic pain treated with non-opioid analgesics, and chronic pain treated with opioid analgesics, nor were all of these studies longitudinal in design. Despite the challenges, it appears that recent research suggests opioid analgesia may improve some aspects of neurocognition in patients, likely via reduced pain and related affective distress. Overall these studies support the notion that neurocognitive disruption associated with chronic pain cannot be considered simply an epiphenomenon due to pain medication.
Summary of neurocognitive performance in individuals with chronic pain
Overall, review of clinical studies of neurocognitive performance in chronic pain samples strongly supports a relationship between chronic pain and neurocognitive performance decrements, particularly on tests of memory, processing speed, and attention, and particularly among older patients and those with fibromyalgia. The majority of the demonstrated impairments occurred without regard to the specific pain condition (although fibromyalgia was disproportionately represented in these studies). Most studies also included control comparison conditions, indicating some methodological rigor, with the exception of the studies examining learning and memory. Despite this growing evidence base, some studies reviewed above (n=5) did not find an association between chronic pain and certain neurocognitive processes (e.g., memory; aspects of attention; cognitive inhibition) [12, 41, 46, 51, 53] Some of the studies presented indicated a potential role for emotional distress in the neurocognitive performance of patients with chronic pain conditions as well as a potential moderating roles for age, pain severity, cognitive load, and opioid use. Further investigation of these moderators may yield additional information to better understand neurocognitive function in patients with chronic pain.
Clinical Considerations: Neurocognitive alteration and engagement in pain treatment
Understanding the relationship between chronic pain and neurocognition has important implications for the treatment of pain (e.g., adhering to pain medication or physical therapy regimen; participating in behavioral pain self-management programs), and the development of new treatments. Untreated pain may be associated with neurocognitive impairment in domains such as memory, attention, processing speed, and executive functioning and may also contribute to emotional distress, all of which could affect patients’ abilities to engage in pain self-management and contribute to loss of global functioning. However, from the review of the literature, it is difficult to determine the direction of the relationship between pain and neurocognitive impairment (e.g., does pain lead to neurocognitive impairment or do those with neurocognitive impairment represent higher risk for chronic pain that is associated with functional impairment?). For example, if cognitive impairment reduces pain tolerance in some manner, then patients at risk for cognitive impairment (e.g., those with TBI, those with Alzheimer’s Disorder and other disorders of aging) would be at particular risk for chronic pain and may need more intensive screening or treatment.
Based on the data suggesting that there is a relationship between chronic pain and neurocognitive performance, researchers have discussed the effects these deficits may have on assessing and treating chronic pain. For example, with respect to behavioral therapies for pain, chronic pain patients with neurocognitive impairments may benefit from modification in presentation of skills (e.g., using more handouts and in-session demonstration, supplementing with technological aids such as smartphone applications). Addressing mental health conditions, such as depression, which can also contribute to neurocognitive changes in attention and concentration, may improve patient outcomes in terms of both pain and neurocognitive function. More research is essential before making specific recommendations regarding modification of pain management practices to accommodate patients with chronic pain and cognitive impairment.
Summary and Consideration for Populations at Risk for Pain and Neurocognitive Impairment
Impairments have been demonstrated in several neurocognitive domains including attention, memory, processing speed, and executive function. More recently, researchers have begun to explore underlying neural mechanisms common to both pain and neurocognition in order to better understand the nature and direction of this relationship. A thorough examination of the literature investigating neural mechanisms common to both pain and neurocognitive functioning was not reviewed in the current study; however, these relationships warrant further examination. Importantly, there are a number of factors that may mediate the relationship between chronic pain and neurocognitive functioning, such as medication side effects, although evidence for the role of opioids in neurocognitive impairment among these patients is also mixed (e.g., some suggesting improvement in cognitive functioning with hypothesized improved control and other results suggesting that memory and attention are impairment are likely opioid side effects) with inconsistent use of sound methodology in experimental design.
In addition to increasing research on neural mechanisms common to both pain and neurocognition and mediators and moderators of the relationship between pain and neurocognition, it is important to examine populations at heightened risk for chronic pain and neurocognitive impairment, including populations who sustain TBI and/or concurrent orthopedic injuries such as motor vehicle accident victims, professional sports players, military service members, and others whose lifestyles may be especially conducive to injuries related to these problems. Older adults at risk for both cognitive disorders of aging and injuries and illness associated with pain may likewise both show pronounced cognitive impairment and experience chronic pain.
As a specific example, the recent wars in Iraq and Afghanistan have highlighted the significance of chronic pain and associated neurocognitive deficits because of the relatively high frequency of each among warzone veterans,[58, 59] and the complex clinical context in which chronic pain and neurocognitive deficits occur in warzone veterans. For Iraq and Afghanistan War veterans, chronic pain constitutes a component of the “polytrauma triad,” along with traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD). A recent review of medical record data indicated that 93% of a polytrauma clinic sample reported TBI, with 81% of the sample reporting pain.[60] Thus, among the spectrum of adverse psychosocial outcomes accompanying pain, cognitive impairment may be particularly relevant to returning veterans with both pain conditions and TBI.[61, 62] Even among chronic pain patients without TBI, as many as 54% report neurocognitive deficits (e.g., attention, forgetfulness).[63]
A notable limitation of the literature is that few studies focus on performance task measurement of neurocognitive function or neuroimaging in military service personnel and veterans with chronic pain and related comorbid conditions (e.g., TBI). This is surprising, given the high rates of chronic musculoskeletal pain and TBI relative to non-veteran samples. A 2009 systematic review of 95 studies (93 were cohort studies) examining pain in patients with TBI indicated that many of the included studies did not assess (or contained limited data) regarding pain indicators such as intensity, and functional interference among patients with TBI-related neurocognitive impairment.[64] These studies did suggest some early evidence for the roles of depression, PTSD, insomnia, and fatigue in pain-related functional interference among patients with TBI. Similarly, a more recent review of the “polytrauma triad” among veterans of the conflicts in Iraq and Afghanistan suggested that cognitive deficits due to mild TBI may affect patients’ ability to engage in treatment for pain and/or PTSD and encourages clinicians to focus on reducing barriers to engaging in treatment.[65] Given the complexity of these comorbid conditions and the symptom overlap between them, applying knowledge gleaned from existing literature to veterans has important implications for pain treatment outcomes and quality of life in these patients. A number of special populations present a heightened risk of chronic pain and neurocognitive impairment, including military service members and veterans, older patients, and others who sustain TBI and/or concurrent orthopedic injuries such as motor vehicle accident victims, and professional sports players. These populations of interest warrant additional research, including longitudinal studies to further examine whether the effects of neurocognitive impairment, if demonstrated, share a relationship with aging, TBI, and/or chronic pain (although this may be difficult to determine).
Limitations
Despite gaining momentum in recent years, much of the literature examining chronic pain and neurocognitive performance must be interpreted with some caution, as certain aspects may not generalize well to clinical chronic pain patients. For example, many of the studies did not include a pain-free comparison condition. Some studies included small sample sizes. Further, many studies beyond the scope of this review examined the effect of experimentally-induced, acute pain and neurocognitive performance. Such studies may fall under scrutiny because of generalizability of results. Specifically, acutely induced pain does not take into account the myriad of chronic pain concomitants nor the cumulative effect of chronic pain that likely mediate the relationship between pain and neurocognitive performance. [66] Further, although the literature search and review was comprehensive for the current study, there is a risk of bias such that there may have been an incomplete retrieval of identified research (e.g., some papers deemed excluded that should have been included) or unintentional bias in reporting results of reviewed studies (e.g., reporting partial findings).
Future directions
Given the high prevalence of chronic pain in the US and its potential relationship with neurocognitive and emotional functioning, understanding this relationship may help improve treatment outcomes and assist clinicians with treatment decision-making both for addressing chronic pain and for neurocognitive alterations. Research aimed at conducting longitudinal neurocognitive assessment in patients with known conditions associated with chronic pain may yield insightful data, especially in terms of neurocognitive changes in response to adequate pain management. Perhaps these patients develop neurocognitive deficits at a differential temporal rate or in different areas than those without chronic pain. Further, among patients with chronic pain, controlling for confounding variables such as opioid analgesic use (e.g., comparing patients with chronic pain taking opioids with those who are opioid naïve) in studies examining neurocognitive function is important in order to determine whether changes or differences in neurocognitive function are not mediated by the introduction of opioid medications in these patients. Given the role of tolerance to these medications, longitudinal designs may be beneficial to understanding the relation between adequate pain treatment, opioid effects and dosage, and neurocognitive performance. Finally, beyond focusing on the roles of opioids, it is evident from the literature that examination of the role of pain in neurocognition among veterans with chronic pain, both with and without TBI and other comorbidities, is lacking.
Acknowledgments
Research Funding: This work was supported in part by the Naval Medical Research Center’s Advanced Medical Development program (Naval Medical Logistics Command Contract # N62645-11-C-4037, for MRS II).
Glossary
- ACT
Auditory Consonant Trigram
- AIMS
Arthritis Impact Measurement Scales
- BWCT
Bourdon-Wiersman Cancellation Task
- CRT
continuous/choice reaction time test
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease 10-item List
- CLBP
Chronic Low Back Pain
- COWA
Controlled Oral Word Association
- CTRM
Camden Topographical Recognition Memory test
- CVLT
California Verbal Learning Test
- DFT
Design Fluency Test
- DSP
Distal symmetric polyneuropathy
- DSST
Digit-Symbol Subtest
- DVT
Digit Vigilance Test
- EFT
Embedded Figures Test
- EWT
Eight Word Test
- FCRTT
Four Choice Reaction Time Task
- FTT
finger tapping test
- HA
Headache
- HRNB
Halstead-Reitan Neuropsychological Battery
- KCIT
Knox’s Cube Imitation Test
- LFT
Letter Fluency Test
- LS
Letter Number Sequencing
- LRRT
Logical Reasoning Reaction Task
- MMSE
mini mental status exam
- MPI-PS
Multidimensional Pain Inventory – Pain Severity
- MSIT
Multisource Interference Test
- MST
Memory Search Task
- MT
Manikin Test
- NCSE
Neurobehavioral Cognitive Screening Exam
- NRS
Numerical Rating Scale
- OSPAN
Operation Span Task
- PASAT
Paced Auditory Serial Addition Task
- PVT
Psychomotor Vigilance Test
- RBANS
Repeated Battery for the Assessment of Neuropsychology Status
- RBMT
Rivermead Behavioral Memory Test
- RAVLT
Rey Auditory Visual Learning Test
- RA
Rheumatoid Arthritis
- ROCFT
Rey-Osterrieth Complex Figure Test
- RST
Reading Span Test
- RVDLT
Rey Visual Design Learning Test
- SCL-90-R
Symptom Checklist-90 Revised
- SDLT
Symbol-Digit Learning Test
- SDMT
Symbol Digit Modalities Test
- SILVT
Shipley Institute of Living Vocabulary Test
- SST
Spatial Span Test
- TEA
Test of Everyday Attention
- VAS
Visual analogue scale
- WAIS-R
Wechsler Adult Intelligence Scale, Revised; WAIS-III = 3rd Edition
- WASI
Wechsler Abbreviated Scale of Intelligence
- WCST
Wisconsin Card Sorting Test
- WMS-R
Wechsler Memory Scale Revised; WMS-III = 3rd edition
Footnotes
COI: No conflicts of interest.
Disclaimer: The opinions expressed here are those of the authors and do not represent the official policy or position of the US Department of Veterans Affairs.
References
- 1.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychological bulletin. 1996;119:95. [Google Scholar]
- 2.Kerns RD, Jacob MC. Psychological aspects of back pain. Baillieres Clin Rheumatol. 1993;7:337–56. doi: 10.1016/s0950-3579(05)80093-7. [DOI] [PubMed] [Google Scholar]
- 3.Relieving pain in America: A blueprint for transforming pain prevention, care, education and research. Washington, D.C: Institute of Medicine; 2011. [Google Scholar]
- 4.Sjogren P, Christrup LL, Petersen MA, et al. Neuropsychological assessment of chronic non-malignant pain patients treated in a multidisciplinary pain centre. Eur J Pain. 2005;9:453–62. doi: 10.1016/j.ejpain.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Harman K, Ruyak P. Working through the pain: A controlled study of the impact of persistent pain on performing a computer task. The Clinical journal of pain. 2005;21:216–22. doi: 10.1097/00002508-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Ryan C, Williams T, Finegold D, et al. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: Effects of recurrent hypoglycaemia and other chronic complications. Diabetologia. 1993;36:329–34. doi: 10.1007/BF00400236. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CM. Diabetes, aging, and cognitive decline. Neurobiology of aging. 2005;26:21–5. doi: 10.1016/j.neurobiolaging.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ryan CM, Williams TM, Orchard TJ, et al. Psychomotor slowing is associated with distal symmetrical polyneuropathy in adults with diabetes mellitus. Diabetes. 1992;41:107–13. doi: 10.2337/diab.41.1.107. [DOI] [PubMed] [Google Scholar]
- 9.Lee DM, Pendleton N, Tajar A, et al. Chronic widespread pain is associated with slower cognitive processing speed in middle-aged and older european men. Pain. 2010;151:30–6. doi: 10.1016/j.pain.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Jongsma M, Postma S, Souren P, et al. Neurodegenerative properties of chronic pain: Cognitive decline in patients with chronic pancreatitis. PloS one. 2011;6:e23363. doi: 10.1371/journal.pone.0023363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes Del Paso G, Pulgar A, Duschek S, et al. Cognitive impairment in fibromyalgia syndrome: The impact of cardiovascular regulation, pain, emotional disorders and medication. European journal of pain. 2012;16:421–9. doi: 10.1002/j.1532-2149.2011.00032.x. [DOI] [PubMed] [Google Scholar]
- 12.Veldhuijzen DS, Sondaal SF, Oosterman JM. Intact cognitive inhibition in patients with fibromyalgia but evidence of declined processing speed. The Journal of Pain. 2012;13:507–15. doi: 10.1016/j.jpain.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Park DC, Glass JM, Minear M, et al. Cognitive function in fibromyalgia patients. Arthritis & Rheumatism. 2001;44:2125–33. doi: 10.1002/1529-0131(200109)44:9<2125::AID-ART365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Cherry BJ, Zettel-Watson L, Shimizu R, et al. Cognitive performance in women aged 50 years and older with and without fibromyalgia. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. doi: 10.1093/geronb/gbs122. 2012gbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner DK, Rudy TE, Morrow L, et al. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain medicine. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 16.Oosterman J, Gibson S, Pulles W, et al. On the moderating role of age in the relationship between pain and cognition. European Journal of Pain. 2013;17:735–41. doi: 10.1002/j.1532-2149.2012.00235.x. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuijzen DS, Kenemans JL, van Wijck AJ, et al. Processing capacity in chronic pain patients: A visual event-related potentials study. Pain. 2006;121:60–8. doi: 10.1016/j.pain.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesthesia & Analgesia. 2007;104:1223–9. doi: 10.1213/01.ane.0000263280.49786.f5. [DOI] [PubMed] [Google Scholar]
- 19.Oosterman JM, Derksen LC, van Wijck AJ, et al. Executive and attentional functions in chronic pain: Does performance decrease with increasing task load? Pain Research & Management: The Journal of the Canadian Pain Society. 2012;17:159. doi: 10.1155/2012/962786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. European Journal of Pain. 1999;3:325–33. doi: 10.1053/eujp.1999.0138. [DOI] [PubMed] [Google Scholar]
- 21.Coppieters I, Ickmans K, Cagnie B, et al. Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain physician. 2015;18:E389–E401. [PubMed] [Google Scholar]
- 22.Kewman DG, Vaishampayan N, Zald D, et al. Cognitive impairment in musculoskeletal pain patients. The International Journal of Psychiatry in Medicine. 1991;21:253–62. doi: 10.2190/FRYK-TMGA-AULW-BM5G. [DOI] [PubMed] [Google Scholar]
- 23.Jamison RN, Sbrocco T, Parris WC. The influence of problems with concentration and memory on emotional distress and daily activities in chronic pain patients. The International Journal of Psychiatry in Medicine. 1988;18:183–91. doi: 10.2190/ftr1-f9vx-cb8t-wpmc. [DOI] [PubMed] [Google Scholar]
- 24.Bosma FK, Kessels RP. Cognitive impairments, psychological dysfunction, and coping styles in patients with chronic whiplash syndrome. Cognitive and Behavioral Neurology. 2002;15:56–65. [PubMed] [Google Scholar]
- 25.Lezak MD. The problem of assessing executive functions. International Journal of Psychology. 1982;17:281–97. [Google Scholar]
- 26.Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Care & Research. 2002;47:639–44. doi: 10.1002/art.10800. [DOI] [PubMed] [Google Scholar]
- 27.Grace GM, Nielson WR, Hopkins M, et al. Concentration and memory deficits in patients with fibromyalgia syndrome. Journal of Clinical and Experimental Neuropsychology. 1999;21:477–87. doi: 10.1076/jcen.21.4.477.876. [DOI] [PubMed] [Google Scholar]
- 28.Abeare CA, Cohen JL, Axelrod BN, et al. Pain, executive functioning, and affect in patients with rheumatoid arthritis. The Clinical journal of pain. 2010;26:683. [PMC free article] [PubMed] [Google Scholar]
- 29.Berryman C, Stanton TR, Bowering KJ, et al. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain. 2013;154:1181–96. doi: 10.1016/j.pain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Oosterman JM, Derksen LC, van Wijck AJ, et al. Memory functions in chronic pain: Examining contributions of attention and age to test performance. The Clinical journal of pain. 2011;27:70–5. doi: 10.1097/AJP.0b013e3181f15cf5. [DOI] [PubMed] [Google Scholar]
- 31.Landrø NI, Fors EA, Våpenstad LL, et al. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? PAIN®. 2013;154:972–7. doi: 10.1016/j.pain.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Dick BD, Verrier MJ, Harker KT, et al. Disruption of cognitive function in fibromyalgia syndrome. Pain. 2008;139:610–6. doi: 10.1016/j.pain.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Luerding R, Weigand T, Bogdahn U, et al. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: Structural correlates of pain–cognition interaction. Brain. 2008;131:3222–31. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 34.Antepohl W, Kiviloog L, Andersson J, et al. Cognitive impairment in patients with chronic whiplash-associated disorder-a matched control study. NeuroRehabilitation-An Interdisciplinary Journal. 2003;18:307–16. [PubMed] [Google Scholar]
- 35.Meeus M, Van Oosterwijck J, Ickmans K, et al. Interrelationships between pain processing, cortisol and cognitive performance in chronic whiplash-associated disorders. Clinical rheumatology. 2015;34:545–53. doi: 10.1007/s10067-013-2446-5. [DOI] [PubMed] [Google Scholar]
- 36.Lee D-H, Lee K-J, Cho KIK, et al. Brain alterations and neurocognitive dysfunction in patients with complex regional pain syndrome. The Journal of Pain. 2015;16:580–6. doi: 10.1016/j.jpain.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Apkarian AV, Sosa Y, Krauss BR, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–36. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Verdejo-Garcia A, Lopez-Torrecillas F, Calandre EP, et al. Executive function and decision-making in women with fibromyalgia. Arch Clin Neuropsychol. 2009;24:113–22. doi: 10.1093/arclin/acp014. [DOI] [PubMed] [Google Scholar]
- 39.Hess LE, Haimovici A, Muñoz MA, et al. Beyond pain: Modeling decision-making deficits in chronic pain. Frontiers in behavioral neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Tella M, Castelli L, Colonna F, et al. Theory of mind and emotional functioning in fibromyalgia syndrome: An investigation of the relationship between social cognition and executive function. PloS one. 2015;10:e0116542. doi: 10.1371/journal.pone.0116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suhr JA. Neuropsychological impairment in fibromyalgia: Relation to depression, fatigue, and pain. Journal of psychosomatic research. 2003;55:321–9. doi: 10.1016/s0022-3999(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 42.Gil-Gouveia R, Oliveira AG, Martins IP. Cognitive dysfunction during migraine attacks: A study on migraine without aura. Cephalalgia. 2015;35:662–74. doi: 10.1177/0333102414553823. [DOI] [PubMed] [Google Scholar]
- 43.Park JMGD. Cognitive dysfunction in fibromyalgia. Curr Rheumatol Rep. 2001;3:123–7. doi: 10.1007/s11926-001-0007-4. [DOI] [PubMed] [Google Scholar]
- 44.Iezzi T, Archibald Y, Barnett P, et al. Neurocognitive performance and emotional status in chronic pain patients. Journal of Behavioral Medicine. 1999;22:205–16. doi: 10.1023/a:1018791622441. [DOI] [PubMed] [Google Scholar]
- 45.Grisart JM, Van der Linden M. Conscious and automatic uses of memory in chronic pain patients. Pain. 2001;94:305–13. doi: 10.1016/S0304-3959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 46.Scherder EJ, Eggermont L, Plooij B, et al. Relationship between chronic pain and cognition in cognitively intact older persons and in patients with alzheimer’s disease. Gerontology. 2008;54:50–8. doi: 10.1159/000113216. [DOI] [PubMed] [Google Scholar]
- 47.Schneider C, Palomba D, Flor H. Pavlovian conditioning of muscular responses in chronic pain patients: Central and peripheral correlates. Pain. 2004;112:239–47. doi: 10.1016/j.pain.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 48.Eccleston C. Chronic pain and attention: A cognitive approach. British Journal of Clinical Psychology. 1994;33:535–47. doi: 10.1111/j.2044-8260.1994.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 49.Pulles WL, Oosterman JM. The role of neuropsychological performance in the relationship between chronic pain and functional physical impairment. Pain Medicine. 2011;12:1769–76. doi: 10.1111/j.1526-4637.2011.01266.x. [DOI] [PubMed] [Google Scholar]
- 50.Attal N, Masselin-Dubois A, Martinez V, et al. Does cognitive functioning predict chronic pain? Results from a prospective surgical cohort. Brain. doi: 10.1093/brain/awt354. 2014awt354. [DOI] [PubMed]
- 51.Weyer Jamora C, Schroeder SC, Ruff RM. Pain and mild traumatic brain injury: The implications of pain severity on emotional and cognitive functioning. Brain Injury. 2013;27:1134–40. doi: 10.3109/02699052.2013.804196. [DOI] [PubMed] [Google Scholar]
- 52.Ersek M, Cherrier MM, Overman SS, et al. The cognitive effects of opioids. Pain Management Nursing. 2004;5:75–93. doi: 10.1016/j.pmn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Kurita GP, de Mattos Pimenta CA, Braga PE, et al. Cognitive function in patients with chronic pain treated with opioids: Characteristics and associated factors. Acta Anaesthesiol Scand. 2012;56:1257–66. doi: 10.1111/j.1399-6576.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 54.Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain physician. 2014;17:9–20. [PubMed] [Google Scholar]
- 55.Jamison RN, Schein JR, Vallow S, et al. Neuropsychological effects of long-term opioid use in chronic pain patients. Journal of pain and symptom management. 2003;26:913–21. doi: 10.1016/s0885-3924(03)00310-5. [DOI] [PubMed] [Google Scholar]
- 56.Tassain V, Attal N, Fletcher D, et al. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain. 2003;104:389–400. doi: 10.1016/s0304-3959(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 57.Menefee LA, Frank ED, Crerand C, et al. The effects of transdermal fentanyl on driving, cognitive performance, and balance in patients with chronic nonmalignant pain conditions. Pain Medicine. 2004;5:42–9. doi: 10.1111/j.1526-4637.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 58.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. Journal of pain and symptom management. 2002;23:131–7. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 59.Sinnott P, Wagner TH. Low back pain in VA users. Archives of internal medicine. 2009;169:1336–40. doi: 10.1001/archinternmed.2009.201. [DOI] [PubMed] [Google Scholar]
- 60.Sayer NA, Cifu DX, McNamee S, et al. Rehabilitation needs of combat-injured service members admitted to the VA polytrauma rehabilitation centers: The role of pm&r in the care of wounded warriors. PM&R. 2009;1:23–8. doi: 10.1016/j.pmrj.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Lew HL, Otis JD, Tun C, et al. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in oif/oef veterans: Polytrauma clinical triad. J Rehabil Res Dev. 2009;46:697–702. doi: 10.1682/jrrd.2009.01.0006. [DOI] [PubMed] [Google Scholar]
- 62.Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq war veteran VA users. Medical care. 2012;50:342–6. doi: 10.1097/MLR.0b013e318245a558. [DOI] [PubMed] [Google Scholar]
- 63.McCracken LM, Iverson GL. Predicting complaints of impaired cognitive functioning in patients with chronic pain. Journal of pain and symptom management. 2001;21:392–6. doi: 10.1016/s0885-3924(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 64.Dobscha SK, Clark ME, Morasco BJ, et al. Systematic review of the literature on pain in patients with polytrauma including traumatic brain injury. Pain Med. 2009;10:1200–17. doi: 10.1111/j.1526-4637.2009.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otis JD, McGlinchey R, Vasterling JJ, et al. Complicating factors associated with mild traumatic brain injury: Impact on pain and posttraumatic stress disorder treatment. J Clin Psychol Med Settings. 2011;18:145–54. doi: 10.1007/s10880-011-9239-2. [DOI] [PubMed] [Google Scholar]
- 66.Moriarty OMB, Finn DP. The effect of pain on cognitive function: A reviw of clinical and preclinical research. Progress in Neurobiology. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Iezzi T, Duckworth MP, Vuong LN, et al. Predictors of neurocognitive performance in chronic pain patients. International journal of behavioral medicine. 2004;11:56–61. doi: 10.1207/s15327558ijbm1101_7. [DOI] [PubMed] [Google Scholar]
- 68.Duschek S, Werner NS, Winkelmann A, et al. Implicit memory function in fibromyalgia syndrome. Behavioral Medicine. 2013;39:11–6. doi: 10.1080/08964289.2012.708684. [DOI] [PubMed] [Google Scholar]
- 69.Meyer JS, Thornby J, Crawford K, et al. Reversible cognitive decline accompanies migraine and cluster headaches. Headache: The Journal of Head and Face Pain. 2000;40:638–46. doi: 10.1046/j.1526-4610.2000.040008638.x. [DOI] [PubMed] [Google Scholar]
- 70.Povedano M, Gascon J, Galvez R, et al. Cognitive function impairment in patients with neuropathic pain under standard conditions of care. J Pain Symptom Manage. 2007;33:78–89. doi: 10.1016/j.jpainsymman.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 71.Rodríguez-Andreu J, Ibáñez-Bosch R, Portero-Vázquez A, et al. Cognitive impairment in patients with fibromyalgia syndrome as assessed by the mini-mental state examination. BMC musculoskeletal disorders. 2009;10:162. doi: 10.1186/1471-2474-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karp JF, Reynolds CF, Butters MA, et al. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Medicine. 2006;7:444–52. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]