Abstract
Cholestatic liver diseases result from impaired bile flow and are characterized by inflammation, atypical ductular proliferation (ADP), and fibrosis. The Wnt/β-catenin pathway plays a role in bile duct development, yet its role in cholestatic injury remains indeterminate. Liver-specific β-catenin knockout (KO) mice and wild-type (WT) littermates were subjected to cholestatic injury via bile duct ligation or short-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet. Intriguingly, KO exhibit a dramatic protection from liver injury, fibrosis, and ADP, which coincided with significantly decreased total hepatic bile acids (BA). This led to the discovery of a novel role for β-catenin in regulating BA synthesis and transport through regulation of farnesoid X receptor (FXR) activation. We show that β-catenin functions as both an inhibitor of nuclear translocation and as a nuclear co-repressor through formation of a physical complex with FXR. Loss of β-catenin expedited FXR nuclear localization and FXR/RXRα association, culminating in small heterodimer protein (SHP) promoter occupancy and activation in response to BA or FXR agonist. Conversely, accumulation of β-catenin sequesters FXR, thus inhibiting its activation. Finally, exogenous suppression of β-catenin expression during cholestatic injury reduces β-catenin/FXR complex, activates FXR to decrease total BA and alleviates hepatic injury.
Conclusion
We have identified a novel FXR/β-catenin interaction whose modulation via β-catenin suppression promotes FXR activation and decreases hepatic BA, which may provide unique therapeutic opportunities in cholestatic liver diseases.
Keywords: bile duct, cyp7a1, cholestatic liver disease, ductular proliferation, biliary fibrosis
Cholestatic liver diseases are characterized by inflammation and fibrosis of bile ducts, cholangitis, cirrhosis, end-stage liver disease and reduced life expectancy. Currently, there are few effective medical therapies available, and liver transplantation remains the only life-extending treatment option for many patients with end-stage cholestatic liver disease. Thus, the dearth of effective therapeutic strategies for managing chronic cholestasis is a major unmet clinical need. Additionally, the underlying pathophysiology of many cholestatic liver diseases is poorly understood despite extensive research. Recently, however, the “toxic bile” concept has been proposed as a major contributor in the pathogenesis of these conditions. This model postulates that cholestasis due to regurgitation of bile through leaky cholangiocyte junctions can induce significant hepatocyte death and inflammation leading to cholangitis and periductal fibrosis (1). Hepatocyte injury and death occur either due to retention of high concentrations of bile acids (BA) in the hepatocyte, or abnormal BA composition consisting of a higher hydrophobic:hydrophilic ratio (2). BA also stimulate proliferation of atypical ductules that release both proinflammatory and profibrotic mediators, thus contributing to the disease process (3).
β-catenin, the chief downstream effector of canonical Wnt signaling, has been shown to play an important role in liver development, hepatic metabolism and zonation, and liver regeneration (4). β-catenin also contributes to cholangiocyte differentiation during development and contributes to atypical ductular proliferation (ADP) in biliary injury models such as 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet (5, 6). Additionally, recent studies have shown that liver-specific β-catenin conditional knockout (KO) mice exhibited modestly higher basal hepatic BA than wild-type (WT) control mice (7), likely due to sluggish bile flow (8).
Since both accumulation of BA and ADP are thought to contribute to the pathogenesis of cholestatic liver disease, and since loss of β-catenin alters BA homeostasis and suppresses ADP during injury, we hypothesized that modulation of β-catenin might impact cholestasis. In the current study, we subjected KO mice to two different models of cholestatic liver disease: bile duct ligation (BDL) and DDC diet. KO mice exhibited dramatically less liver injury, fibrosis, and ADP in the setting of cholestasis, which was attributable to decreased total hepatic BA levels. In fact, we uncovered a novel role of β-catenin in regulation of BA synthesis and transport through its interactions with the farnesoid X receptor (FXR). We show that β-catenin functions both as an inhibitor of FXR nuclear translocation and as a nuclear co-repressor through formation of a physical complex with FXR, and that modulation of this complex may have therapeutic implications in cholestatic liver disease.
MATERIALS AND METHODS
The generation of β-catenin KO mice has been described previously (9). At 10–14 weeks of age, when β-catenin deletion is ~100%, these mice and their WT littermates were subjected to BDL by dissecting the common bile duct above the pancreas and cutting it between two 5-0 silk ligatures.
Additional methods are available in an online supplement.
RESULTS
Liver-specific β-catenin KO mice are protected from hepatic injury, fibrosis, and inflammation after BDL
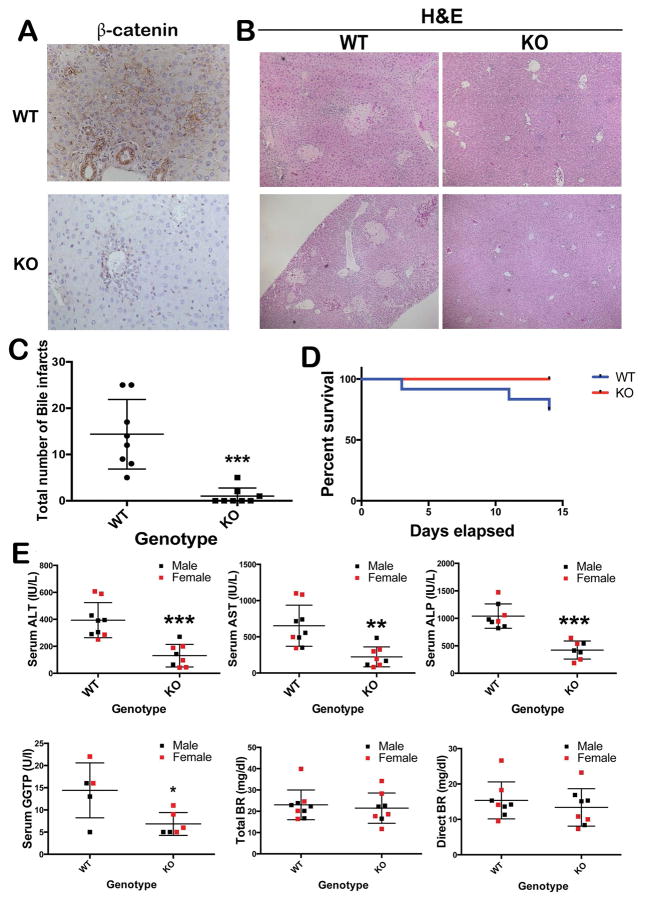
To characterize the role of β-catenin in cholestatic liver injury, KO and WT mice were subjected to BDL. IHC staining confirmed the absence of β-catenin in hepatocytes and cholangiocytes of KO (Figure 1A). While extensive biliary infarcts were visible throughout the hepatic lobules in the WT liver at 14D after BDL, there was a significant lack of these lesions in the KO (p=0.0002) (Figure 1B, 1C). A modest increase in mortality was observed in WT after 14D of BDL, which was nonetheless insignificant (Figure 1D). We did not observe any differences in response to BDL in terms of mortality or morbidity when comparing males to females. Further, serum biochemistry from both groups of animals showed significantly lower levels of AST, ALT, ALP, and GGTP in KO when compared to WT at 14D after BDL (Figure 1E).
Figure 1. Decreased liver injury in KO following BDL.
(A) Absent β-catenin in KO livers at 14D after BDL (100x); (B) Several bile infarcts in WT after BDL with notable absence in KO (50x); (C) Significantly fewer biliary infarcts in KO after BDL. ***p<0.001. (D) No significant differences in overall survival between WT and KO after BDL. (E) Decreased serum markers of hepatic and biliary injury in KO at 14D after BDL when compared to the WT (n=5–6 per group). *p<0.05; **p<0.01; ***p<0.001. Histology images are representative of >4 images per group. Bile infarct quantification and survival data obtained from n=8 animals/group.
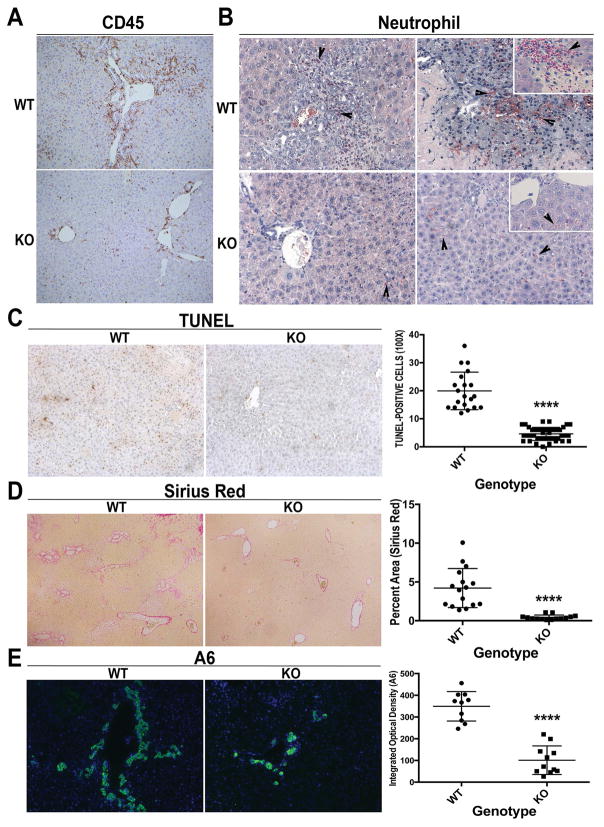
Because hepatic inflammation is a known contributor of injury in cholestatic liver disease, we next examined any differences in WT and KO after BDL. IHC for CD45 showed enhanced inflammation around the periportal region in WT, which was notably lower in KO (Figure 2A and Supplemental Figure 1A). Neutrophils extravasated into the parenchyma to infiltrate areas of necrosis in WT livers after BDL. In contrast, neutrophils are mainly present in the sinusoids in KO livers (Figure 2B). A notable decrease in number of TUNEL-positive cells was also evident in KO as compared to WT after BDL (Figure 2C).
Figure 2. Decreased inflammation and fibrosis in KO following BDL.
(A) IHC shows fewer CD45+ inflammatory cells especially in the periportal region (100x). (B) Naphthol-ASD chloroacetate esterase staining shows fewer infiltrating neutrophils in KO after BDL (200x; inset 400x). (C) Reduced TUNEL-positive cells in KO after BDL (100x). (D) Greatly reduced fibrosis was seen in KO (50x). (E) IF for A6 shows notably less ADP in KO after BDL as compared to WT (100x). For C, 5 representative 100x images per animal (n=3 animals per group) were quantified; for D, 1–3 representative 50x images per animal (n=7 animals per group) were quanitified; for E, n=5 images per 2 representative animals per group were quantified. ****p<0.0001
Several α-SMA-positive myofibroblasts were evident in WT and were conspicuously absent in KO livers after BDL (Supplemental Figure 1B). This was concomitantly associated with a dramatic reduction in fibrosis in KO livers (Figure 2D), and lower liver weight/body weight ratios in KO compared to WT (Supplemental Figure 1C). Extensive ductular reaction as identified by A6 immunofluorescence (IF) (5) was observed in the WT livers after 14D of BDL but was notably decreased in the KO (Figure 2E). CK19 IHC confirmed that the number of intrahepatic bile ducts was greatly diminished in KO compared to WT (Supplemental Figure 1D).
KO mice have a significant decrease in total hepatic BA
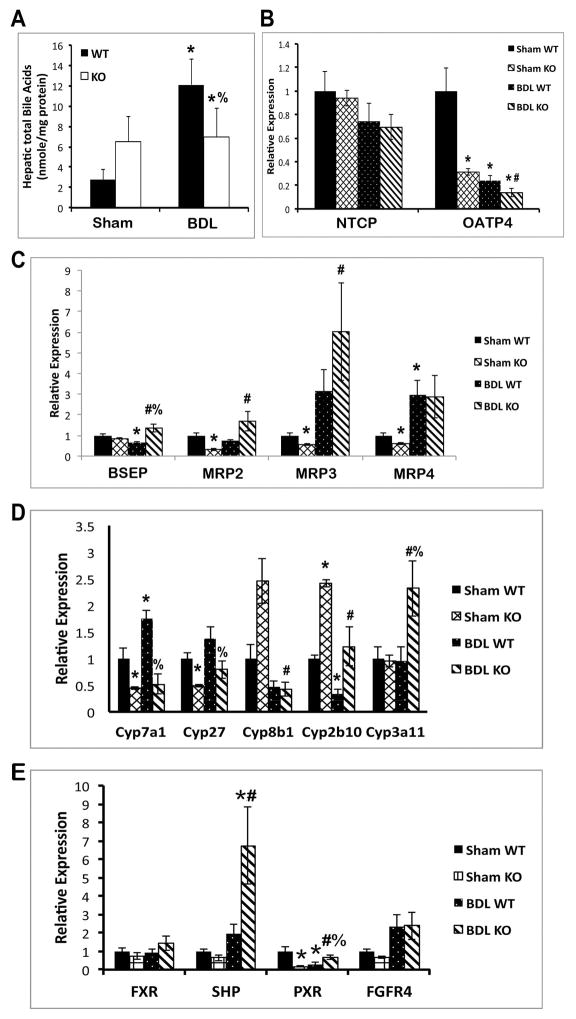
We previously reported that KO livers exhibit modestly elevated BA at baseline (8). However, hepatic BA fail to increase any further in KO after BDL, whereas in WT, BA increased almost 6-fold (Figure 3A). Thus, in the setting of cholestasis, decreased hepatic injury and fibrosis in the absence of β-catenin appears to be due to decreased BA accumulation.
Figure 3. Decreased hepatic BA and BA synthesis in KO.
(A) Total BA remained unchanged in KO livers after BDL, while increasing significantly in WT. (B) Expression of basolateral uptake transporter OATP4 is decreased in KO after BDL. (C) Expression of apical BA exporters BSEP and MRP2 is upregulated in KO after BDL. (D) Expression of BA biosynthesis genes is suppressed, while detoxification genes are induced, in KO after BDL. (E) Expression of SHP, a known FXR/RXRα target gene, is significantly increased in KO after BDL. *p<0.05 vs. sham WT, #p<0.05 vs. sham KO, %p<0.05 vs. BDL WT.
Changes in BA synthesis, metabolism, and transporter genes after BDL
To examine the mechanism of decreased BA in KO liver after BDL, we measured the expression of various BA transporters, biosynthesis, and metabolism genes after sham surgery or BDL. Measurement of basolateral uptake transporter OATP4 shows a decrease in KO compared to sham, while NTCP was unaffected (Figure 3B). However, apical transporters such as bile salt export pump (BSEP) and multidrug resistance-associated protein-2 (MRP2) are decreased in WT after BDL but increased in KO compared to sham (Figure 3C). BSEP is also increased in KO as compared to WT after BDL (Figure 3C). Intriguingly, BA synthesis cytochrome P450 (Cyp) enzymes Cyp7a1 and Cyp27 are significantly lower in KO than in WT after BDL (Figure 3D). Conversely, Cyp3a11, which detoxifies hydrophobic BA (10), is increased many-fold in KO after BDL compared to WT (Figure 3D).
Farnesoid X receptor (FXR), a BA-regulated nuclear receptor, regulates the expression of BSEP, MRP2, and Ostβ, and also induces expression of small heterodimer protein (SHP), which inhibits Cyp7a1 (11–13). Gene expression analysis showed non-significant changes in FXR expression after BDL; however, SHP mRNA expression is dramatically increased in KO after BDL compared to WT (Figure 3E). Pregnane X receptor (PXR), a target of FXR that regulates Cyp3a11, is also higher in KO (14), while fibroblast growth factor receptor 4 (FGFR4), the receptor for FGF15-mediated regulation of BA homeostasis, is unchanged (Figure 3E). Taken together, the response to cholestasis in the KO liver involves decreased synthesis of BA and increased excretion of BA from hepatocytes, with the net result being decreased accumulation of toxic BA. Furthermore, increased activation of FXR may be the mechanism driving this process.
KO show a similar protection from short-term DDC-induced hepatobiliary injury
To generalize our findings, we assessed another model of cholestatic liver injury and analyzed historical samples from WT and KO mice after short-term DDC exposure published previously (5). Decreased fibrosis and hepatocellular injury was noted in KO livers after 28 days of DDC diet (Supplemental Figure 2A, 2B). Even though DDC does not induce BA accumulation to the same extent as BDL, total hepatic BA were still modestly decreased in KO compared to WT after DDC diet (Supplemental Figure 2C). Additionally, although expression of BSEP and MRP2 is not induced after DDC diet as it is after BDL, SHP expression is modestly increased, and a similar suppression of BA synthesis pathways and induction of detoxification was found in KO after 28 days of DDC diet, as demonstrated by decreases in Cyp7a1 and Cyp27 and upregulation of Cyp3a11 (Supplemental Figure 2D). Thus, even though DDC and BDL cause cholestasis through distinct mechanisms, the absence of β-catenin offers protection in this alternate model of cholestasis in part through amelioration of BA accumulation.
Absence of β-catenin increases promoter occupancy and activation of FXR target genes
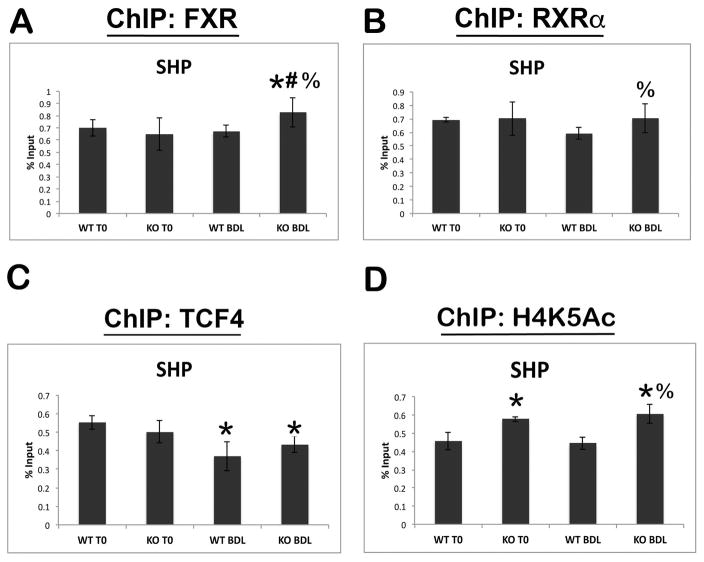
To further elucidate the effects of β-catenin on FXR activity, we performed chromatin immunoprecipitation (ChIP) on the SHP promoter, which is activated by FXR/RXRα heterodimers (12). The analysis demonstrates strong binding site occupancy by both FXR and RXRα in WT and KO before and after BDL (Figure 4A and B). Interestingly, we failed to see any evidence of increased FXR occupancy of SHP in a control animal after BDL at 14D compared to baseline. To further corroborate these findings, we performed ChIP on very early time points after BDL in WT animals, and again found no significant increases in FXR occupancy of the SHP promoter, despite BA being acutely increased at these time points (Supplemental Figure 3A). However, there was a modest but significant increase in FXR occupancy of the SHP promoter in KO after BDL as compared to both KO at baseline and WT after BDL (Figure 4A). Furthermore, RXRα occupancy of the SHP promoter also increased in KO as compared to WT after BDL (Figure 4B), which is likely due to increased occupancy of its binding partner FXR and not because of any direct association with β-catenin (data not shown). Together, these results suggest that absence of β-catenin during cholestasis increases nuclear translocation and association of the FXR/RXRα complex on specific target genes.
Figure 4. FXR and RXRα binding to the SHP promoter increases after BDL in KO livers, concomitant with increased transcriptional activation.
(A) ChIP assay for FXR shows a significant increase in SHP occupancy in KO after BDL compared to both KO at baseline or WT after BDL. (B) ChIP for RXRα also shows increased occupancy of SHP promoter in KO compared to WT after BDL. (C) ChIP for TCF4 shows decreased occupancy of SHP after BDL in both WT and KO. (D) Transcriptional activity of SHP, as assessed by ChIP for H4K5Ac, is increased in KO before and after BDL compared to WT. *p<0.05 vs. sham WT, #p<0.05 vs. sham KO, %p<0.05 vs. BDL WT.
To determine the specificity of this assay, we immunoprecipitated with antibody against constitutive androstane receptor (CAR), a nuclear receptor that plays a role in drug detoxification (15), and found no changes in promoter occupancy of SHP in either genotype before or after BDL (Supplemental Figure 3B). Interestingly, however, when ChIP was performed with TCF4, the transcriptional partner for β-catenin, we noted a significant decrease in binding to the SHP promoter in both WT and KO after BDL (Figure 4C). Further analysis by ChIP-seq revealed that TCF4 binds the SHP promoter on the same enhancer region as FXR and RXRα (Supplemental Figure 3C). This data suggests that BA trigger loss of TCF4 binding to the SHP promoter, which may be permissive but not sufficient for transcriptional activation.
Finally, we examined H4K5 acetylation, a mark of transcriptional activation (16), at the SHP promoter by ChIP. Activation at the SHP promoter was increased in β-catenin KO both at baseline and after BDL compared to time-matched WT controls (Figure 4D).
Taken together these findings suggest that increased FXR activation of SHP in the KO after BDL is a combination of three factors: 1) increased FXR/RXRα binding and occupancy of the SHP promoter, due to absence of inhibitory β-catenin; 2) increased basal activity of the SHP promoter; and 3) loss of TCF4 in the presence of BA, which may be repressing the transcriptional machinery at the SHP promoter.
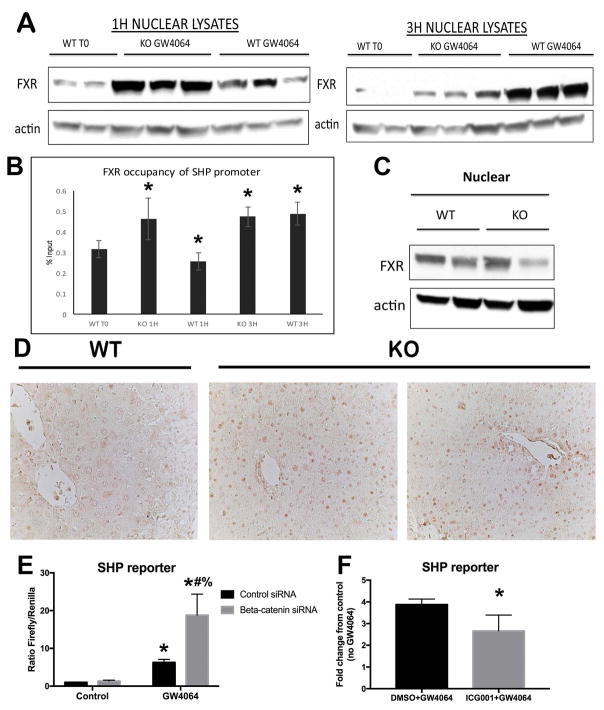
FXR agonist induces earlier nuclear translocation in the β-catenin KO and contributes to a synergistic increase in SHP reporter activity in vitro
In order to further demonstrate that absence of β-catenin increases nuclear translocation of FXR, we next treated WT and KO mice with GW4064, an FXR agonist that induces acute and transient activation of FXR (17). A dramatic increase in nuclear FXR occurs in the KO as early as 1 hour after GW4064 administration, while a comparable increase in nuclear FXR occurs in the WT at 3 hours (Figure 5A). ChIP also showed a shift to the left in FXR occupancy of the SHP promoter in KO (Figure 5B). This indicates that loss of β-catenin makes FXR amenable to earlier activation, and that increased FXR nuclear translocation is one mechanism for the increased occupancy of SHP by FXR shown in our ChIP assay (Figure 4). Indeed, despite the fact that nuclear localization of FXR is not increased in KO at baseline (Figure 5C), IHC for FXR shows increased nuclear localization in the KO after BDL (Figure 5D).
Figure 5. FXR agonist induces earlier nuclear translocation and synergistic increase in SHP reporter occupancy and activity in the absence of β-catenin.
(A) FXR translocates to the nucleus 1 hour after administration of GW4064 in KO, compared to 3 hours in WT. (B) ChIP shows that FXR occupancy of the SHP promoter increases 1 hour after GW4064 treatment in KO; in WT this increase does not occur until 3 hours after GW4064. (C) WB shows that nuclear FXR is not increased in KO at baseline. (D) IHC shows increased FXR in the nucleus of KO after BDL (200x). (E) SHP luciferase reporter activity is increased in Hep3B cells in the presence of β-catenin siRNA and GW4064. (F) SHP reporter activity is not induced in the presence of ICG-001, an inhibitor of β-catenin activity which does not reduce total β-catenin protein levels. For B and F, *p<0.05; for E, *p<0.05 vs. control siRNA, #p<0.05 vs. β-catenin siRNA, %p<0.05 vs. control siRNA + GW4064.
We next utilized Hep3B hepatoma cells to determine the effect of β-catenin modulation on FXR in vitro. In contrast to liver, where FXR signaling is always active due to the presence of BA, untreated Hep3B cells lack any basal FXR activity. Co-transfection with β-catenin siRNA and SHP reporter plasmid led to a synergistic induction of SHP activity in the presence of GW4064, as compared to similar treatment in control siRNA-transfected Hep3B cells (Figure 5E). On the other hand, treatment of Hep3B cells with ICG-001, a small molecule that inhibits β-catenin-CREB binding protein (CBP) association and thus antagonizes β-catenin-TCF4 transcriptional activity (18), does not induce SHP reporter activity (Figure 5F). This is likely because inhibiting β-catenin transcription via ICG-001 does not decrease β-catenin protein levels but in fact increases the free pool of β-catenin in the cell (not shown). Thus, SHP activation was induced by diminishing β-catenin protein levels, while inhibiting its transcriptional activity was insufficient to promote SHP activation.
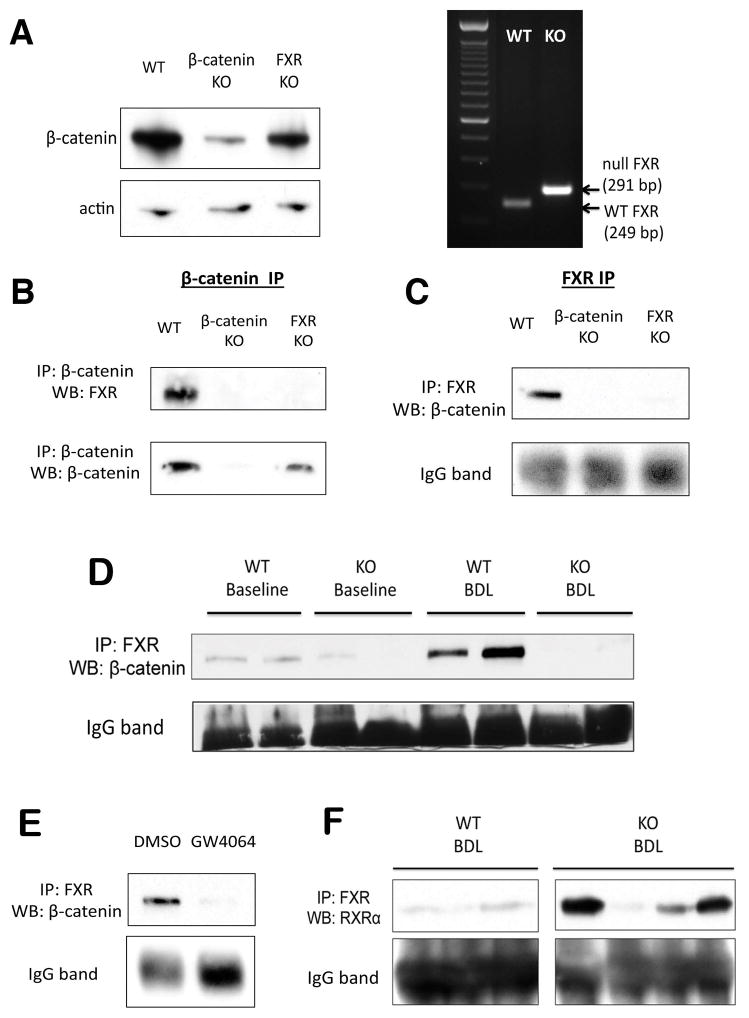
Identification of an FXR/β-catenin complex in hepatocytes
The above observations led us to examine if there was any physical interaction between β-catenin and FXR through immunoprecipitation (IP) studies using whole-cell lysates from WT, β-catenin KO, and FXR KO livers. Deletion of respective genes was confirmed by WB and PCR (Figure 6A). IP of lysates with β-catenin antibody demonstrates an association of β-catenin with FXR in WT livers at baseline; notably, this interaction is absent in both β-catenin KO and FXR KO livers (Figure 6B). FXR IP using FXR antibody likewise showed association with β-catenin only in WT livers. (Figure 6C).
Figure 6. FXR and β-catenin form a complex in hepatocytes.
(A) WB for β-catenin confirms loss of β-catenin from β-catenin KO livers (right), and PCR of genomic DNA confirms deletion of FXR from FXR KO (left). (B) IP with β-catenin shows association with FXR only in WT livers but not in β-catenin KO or FXR KO. (C) Reverse IP with FXR antibody also demonstrates pull-down of β-catenin only in WT sample. (D) IP shows FXR/β-catenin association in WT livers is maintained after BDL. As expected, this association is decreased in KO. (E) Association between β-catenin and FXR decreases in the presence of FXR agonist GW4064 in Hep3B cells, as demonstrated by IP. (F) A notable increase in FXR/RXRα association is seen in most KO after BDL, as compared to WT after BDL.
Because changes in the association of FXR and β-catenin may alter the activation status of FXR, we next examined this relationship during cholestasis. Interestingly, we observed maintenance of the FXR/β-catenin complex in WT livers after BDL (Figure 6D). This may reflect a return to homeostatic conditions after chronic BA stimulation over a period of 14D. To support this hypothesis, we immunoprecipitated the FXR/β-catenin complex in Hep3B cells after short-term stimulation with either DMSO or GW4064 and found a dramatic dissociation of FXR and β-catenin following GW4064 treatment (Figure 6E). Thus, this complex undergoes dynamic changes in the presence of BA or agonist, and eventually reforms over time. As expected, FXR/β-catenin association is absent in the KO mice both before and after BDL (Figure 6D). As a result of lack of β-catenin, FXR formed a complex more readily with RXRα in KO as compared to WT after BDL (Figure 6F). Thus, the improved phenotype of the KO mice after BDL may be attributed to lack of inhibitory FXR/β-catenin association and the resulting increase in FXR/RXRα association.
Manipulation of RXRα expression alters FXR/β-catenin association
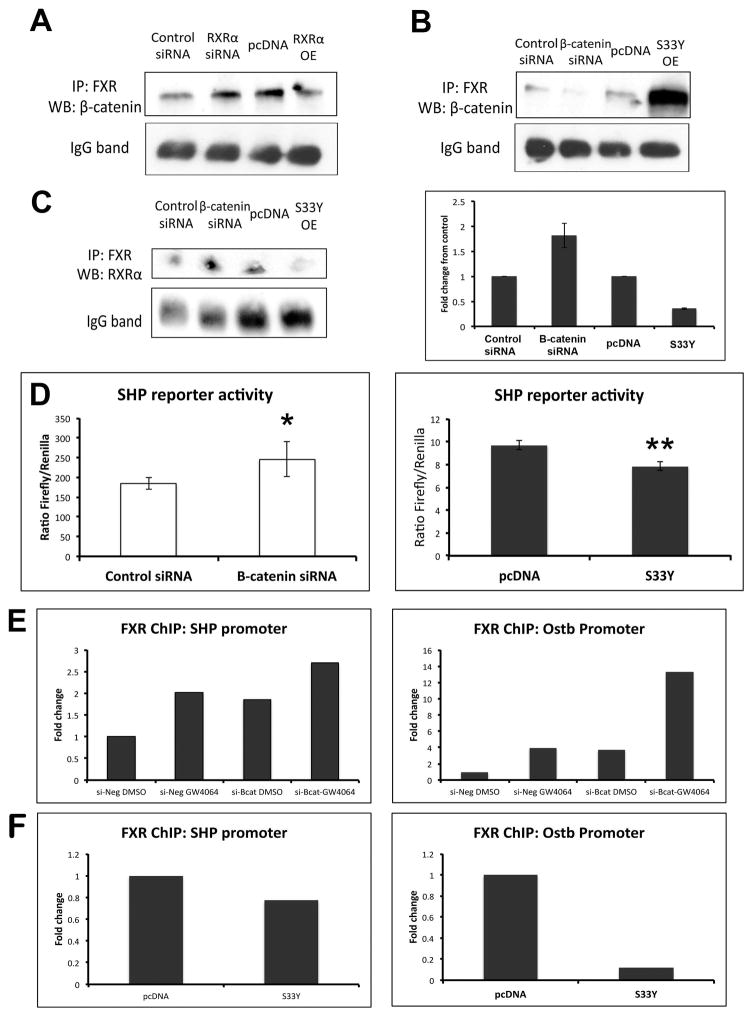
Next, we sought to determine if there was any evidence of competitive antagonism between β-catenin and RXRα for FXR. We confirmed RXRα overexpression and knockdown by WB and real-time PCR (Supplemental Figure 4A, 4B). IP of Hep3Bs transfected with RXRα siRNA showed an increase in the amount of FXR/β-catenin association; conversely, overexpression of RXRα resulted in reduction of the FXR/β-catenin complex (Figure 7A). Thus, the amount of FXR bound to β-catenin can be altered by manipulating levels of RXRα in a cell.
Figure 7. Manipulation of β-catenin and RXRα expression results in altered FXR/β-catenin association.
(A) IP shows that suppression of RXRα increases FXR/β-catenin, while overexpression of RXRα decreases FXR/β-catenin association. (B) Suppression of β-catenin decreases FXR/β-catenin association, while overexpression of β-catenin increases FXR/β-catenin association. (C) Left panel: Suppression of β-catenin increases FXR/RXRα association, while overexpression of β-catenin decreases FXR/RXRα association. Right panel: Densitometry confirms increased FXR/RXRα association in the absence of β-catenin and decreased FXR/RXRα when β-catenin is overexpressed (bars represent an average of n=2 independent experiments). (D) Left panel: Measurement of SHP reporter activity in the presence of β-catenin siRNA confirms increased activation of FXR in Hep3B cells. *p<0.05. Right panel: Expression of S33Y-mutated β-catenin in Hep3B cells resulted in notably lower activation of SHP reporter compared to control. **p<0.01. (E) ChIP assay demonstrates increased occupancy of FXR on the SHP and Ostβ promoters after GW4064 treatment; this increase also occurs following β-catenin suppression alone, and is further increased in the presence of GW4064. (F) Overexpression of β-catenin through transfection of S33Y plasmid led to a marked decrease in occupancy of the SHP and Ostβ promoters by FXR. ChIP data represents pooled samples from n≥3 plates per treatment group.
Modulation of β-catenin impacts FXR activity in vitro
Hep3B cells were then transfected with β-catenin siRNA, which dramatically decreased β-catenin/Tcf-dependent transcriptional activation (Supplemental Figure 4C). β-catenin suppression decreased FXR/β-catenin association (Figure 7B); intriguingly, this resulted in an increased amount of FXR bound to RXRα (Figure 7C). Additionally, Hep3B cells transfected with siRNA for β-catenin showed a significant increase in SHP reporter activity compared to control siRNA (Figure 7D). Finally, ChIP showed that in the presence of GW4064, FXR occupancy increases on the promoters of both SHP and Ostβ, an ileal basolateral BA transporter that is expressed in human liver (19). This increase also occurs following β-catenin suppression alone, and is further augmented in the presence of concomitant GW4064 and β-catenin suppression (Figure 7E). Thus, lack of β-catenin in the cell enhances the affinity of FXR for RXRα, resulting in increased FXR activity.
We next transfected Hep3B cells with a plasmid containing a stable, non-degradable form of β-catenin (S33Y-β-catenin) (Supplemental Figure 4C). FXR/β-catenin association increased notably in the presence of S33Y-β-catenin (Figure 7B), which decreased FXR/RXRα association (Figure 7C). This coincided with an abrogation of SHP reporter activity in S33Y-transfected Hep3B cells compared to those transfected with a control plasmid (Figure 7D). Finally, overexpression of β-catenin led to a decrease in occupancy of the SHP and Ostβ promoters by FXR (Figure 7F). Thus, β-catenin levels inversely correlated with FXR activation, with excess β-catenin sequestering FXR away from RXRα resulting in suppression of downstream signaling.
β-catenin ASO decreases BA accumulation and bile infarcts after BDL
To assess the effect of exogenous β-catenin suppression, WT mice were injected intraperitoneally (i.p.) with either 15 mg/kg EZN-3892 β-catenin antisense antisense oligonucleotide (ASO) (Santaris Pharma A/S) or EZN-3046 scrambled ASO at the same concentration every 48 hours for two weeks. Seven days after the first injection, mice were given BDL, and were sacrificed at 12D. Supplemental Figure 5A shows successful knockdown of β-catenin protein and signaling - as indicated by absent GS expression - by EZN-3892 after BDL. This led to a notable decrease in FXR/β-catenin association after β-catenin ASO treatment (Supplemental Figure 5C), which was due to lack of β-catenin in hepatocytes (Supplemental Figure 5B). As a consequence of abrogation of β-catenin/FXR complex after EZN-3892 treatment, there was a significant reduction in liver total BA levels (Supplemental Figure 5D), although serum biochemistry was unchanged. Furthermore, SHP mRNA was increased following β-catenin inhibition, while indirect target Cyp7a1 was significantly suppressed (Supplemental Figure 5E). Liver histology showed that β-catenin ASO-treated livers had significantly fewer bile infarcts after BDL than those treated with scrambled ASO (Supplemental Figure 6A). We next determined the extent of ADP by A6 and found that despite the continued presence of β-catenin in cholangiocytes (Supplemental Figure 5B), β-catenin ASO-treated livers had quantifiably less ADP after BDL than their scrambled ASO counterparts (Supplemental Figure 6B). Thus, mice receiving EZN-3892 had decreased β-catenin/FXR association, resulting in FXR activation, suppression of BA synthesis and accumulation, decreased hepatic injury, and improved intrahepatic cholestasis.
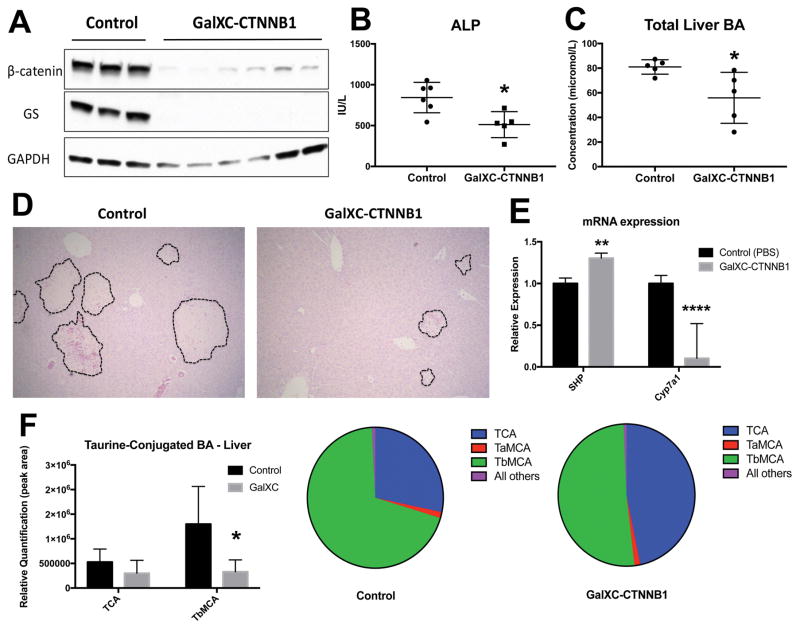
Mice treated with β-catenin DsiRNA targeted to hepatocytes show decreased BA, change in BA composition, and decreased injury after BDL
To ensure reproducibility of our findings, we utilized β-catenin Dicer-substrate siRNA (DsiRNA) conjugated to N-acetylgalactosamine (Dicerna Pharmaceuticals; referred to hereafter as GalXC-CTNNB1), which confers the added benefit of specifically targeting RNAi to hepatocytes (20). Mice received 1 subcutaneous dose of GalXC-CTNNB1 or PBS 3 days before BDL, and weekly doses thereafter (Supplemental Figure 7A). WB and IHC show successful knockdown of β-catenin protein in hepatocytes (Figure 8A and Supplemental Figure 7C). Furthermore, both ALP and total liver BA were significantly decreased in GalXC-CTNNB1-treated mice after BDL (Figure 8B and 8C), which corresponded with smaller bile infarcts (Figure 8D). SHP mRNA was modestly but significantly upregulated in GalXC-CTNNB1-treated livers, while Cyp7a1 was dramatically repressed (Figure 8E). This coincided with increased FXR/RXRα association after GalXC-CTNNB1 treatment and BDL, simultaneous with decreased FXR/β-catenin association (Supplemental Figure 7B). Finally, we performed analysis of BA composition in the liver and in bile from gallbladder. These results demonstrate a notable decrease in tauro-β-muricholic acid (TβMCA), a known FXR antagonist, in livers of GalXC-CTNNB1-treated mice after BDL (Figure 8F) (21). This resulted in a dramatic alteration of BA composition in both liver and gallbladder, from predominantly TβMCA in control BDL to predominantly taurocholic acid (TCA) in GalXC-CTNNB1-treated livers after BDL (Figure 8F and Supplemental Figure 7D). We conclude that exogenous β-catenin inhibition not only increases total hepatic BA levels leading to lesser injury, but also alters BA composition to a more favorable one promoting further FXR activation.
Figure 8. β-catenin suppression by DsiRNA conjugated to N-acetylgalactosamine results in decreased liver injury due to reduced BA after BDL.
(A) WB shows decrease in β-catenin and GS protein levels after administration of GalXC-CTNNB1 after BDL. (B) Decreased serum ALP in β-catenin GalXC-CTNNB1-treated animals after BDL when compared to controls. (C) Liver TBA are decreased in β-catenin GalXC-CTNNB1treated livers. (D) H&E shows that bile infarcts are smaller in mice given BDL and treated with GalXC-CTNNB1 compared to controls. (E) Expression of direct FXR target SHP is significantly increased in GalXC-CTNNB1 treated livers after BDL, while indirect target Cyp7a1 is suppressed. (F) Analysis of BA species shows decreased TβMCA in livers of β-catenin GalXC-CTNNB1-treated mice after BDL (left), which results in a higher percentage of TCA in these livers when expressed qualitatively as a percentage of total BA (right). *p<0.05; **p<0.01; ****p<0.0001.
DISCUSSION
Wnt/β-catenin signaling regulates hepatobiliary repair in cholestatic injury models like DDC diet (5, 22). Here, for the first time, we show that loss of β-catenin from liver confers protection after BDL, characterized by reduced BA pool, bile infarcts, fibrosis, inflammation, and ADP. In fact, we identify a novel FXR/β-catenin complex that regulates FXR activity during cholestasis and has a major impact on BA metabolism. Lack of β-catenin eliminates sequestration and co-repression of FXR by β-catenin, leading to increased availability of FXR to recruit and bind RXRα, induce SHP, and in turn affect BA biosynthesis and stimulate its elimination (Supplemental Figure 8). These novel findings have identified a heretofore unrecognized role of β-catenin in the progression of cholestatic diseases thus providing a novel interventional opportunity.
We demonstrate that β-catenin negatively regulates FXR through direct binding. Indeed, β-catenin is also known to negatively regulate the activity of NF-κB through direct interaction (23). Like NF-κB, FXR has been shown to be present in both the nucleus as well as the cytoplasm, where its activity is repressed by physical association with AMPK (24). Because β-catenin can also reside in both cellular compartments, we thus hypothesize two tandem mechanisms that lead to FXR activation in the absence or upon knockdown of β-catenin. We believe that β-catenin associates with FXR bound directly on the chromatin, preventing either ligand binding or RXRα association and hence functions as a nuclear co-repressor. It is unclear whether β-catenin continues to function as a co-repressor in the WT after BDL, as IP shows that the FXR/β-catenin complex persists despite activation of FXR target genes; it is possible that conformational changes in FXR due to BA binding may alter the complex allowing for RXRα binding and transcriptional activation despite the continued presence of β-catenin. In the KO, however, the absence of β-catenin results in FXR-regulated transcription even at baseline, as supported by enhanced histone acetylation of SHP both before and after BDL in the KO; this transcriptional activation, however, may be compensated for at homeostasis by other mechanisms. The second mechanism is that of β-catenin acting as a negative regulator of FXR in the cytoplasm through physical association. Treatment with GW4064, a potent and selective FXR agonist, rapidly induces FXR nuclear translocation and SHP promoter occupancy earlier in the KO than in WT, indicating that a pool of inactive FXR may reside in the cytoplasm. This observation is further supported by increased FXR occupancy and recruitment of RXRα to the SHP promoter in the β-catenin KO after BDL. It was also intriguing to note the presence of a TCF4 binding site on the SHP promoter, which has not been previously reported. Loss of TCF4 binding is permissive to FXR/RXRα binding on the SHP promoter to induce its histone acetylation and increased expression. However, TCF4 loss from the promoter is by itself not sufficient and likely requires a BA-driven FXR/RXRα occupancy of the promoter in order for increased transcription of SHP, which is facilitated by loss of β-catenin.
Despite increased expression of FXR target genes after BDL in WT, which was further enhanced in β-catenin KO, it was intriguing to note a minimal change of FXR or RXRα occupancy on the SHP promoter after BDL. Others have also reported lack of increased FXR occupancy on target gene promoters at either 3 or 7 days after BDL in both mice and rats (25, 26). The lack of a clear change in occupancy is not entirely unexpected, since FXR regulation of these genes is already highly active in normal basal liver. The findings are consistent with increased gene expression because dynamic changes in nuclear receptor regulation of gene expression continue after binding to DNA, by modulation of co-repression to co-activation, exchange of bound and unbound receptors, and degradation and replacement of bound receptors (27). This cycling process, and gene activation, can be strongly affected by the concentration of receptors in the nucleus without a major change in total binding. Unlike constitutive occupancy of FXR on promoters of its targets in vivo, studies in Hep3B cells did show GW4064 to induce FXR occupancy at both SHP and Ostβ promoters. Using this modulatable system, we identified a clear increase in FXR occupancy at both SHP and Ostβ promoters following β-catenin suppression alone, which was further augmented by GW4064 treatment.
Because loss of β-catenin culminates in protection through activation of FXR and increased SHP expression, is interesting to note that mice lacking FXR are counterintuitively protected from injury during BDL (28). This is thought to be due to decreased BA pool secondary to induction of MRP4, a negative target of FXR activation. However, FXR KO mice have an increased BA pool at baseline, and tissue-specific FXR KO models have elucidated the requirement of both liver and intestinal FXR in regulating BA synthesis through repression of Cyp7a1 (29). The importance of this regulation is illustrated in mice lacking SHP, which show increased sensitivity to BDL secondary to increased basal expression of Cyp7a1 and increased bile flow (30, 31). The protective effect of SHP is also seen in several other studies where loss of SHP results in dysregulation of BA synthesis, while activation of SHP in hepatic stellate cells attenuates fibrosis (32, 33). Thus, the increased SHP expression seen in β-catenin KO after BDL is in line with previous reports describing an improvement in phenotype through reduction of BA synthesis and metabolism.
Notably, FXR function could be altered by changing the amounts of FXR binding partners in the cell. Stable β-catenin secondary to mutations inhibited FXR and suppressed SHP activity compared to cells with non-mutant β-catenin, as did treatment with ICG-001, which increases the free pool of β-catenin in the cell. A recent study also showed that overexpression of an oncogenic form of β-catenin resulted in cholestasis (34). Indeed, intratumoral cholestasis, a hallmark of hepatocellular carcinomas with stabilizing β-catenin gene mutations (35), may be due to excessive levels of β-catenin that sequester FXR making it unavailable to regulate BA levels. Additionally, we found that RXRα suppression led to increased FXR/β-catenin association, whereas its overexpression resulted in a notable decrease in FXR/β-catenin complex. We suggest that the inhibitory FXR/β-catenin complex antagonizes the transcriptionally active FXR/RXRα complex. Thus, the stoichiometric relationship between these three proteins in the cell may regulate FXR function. Furthermore, these complexes can be modulated to alter the ratio of active to inactive FXR, which may have important therapeutic implications.
The Wnt/β-catenin pathway plays a key role in establishing metabolic zonation in the liver. This pathway is constitutively active around the central vein, where it regulates expression of genes such as GS, Cyp1a2, and Cyp2e1 (36). Expression of Cyp7a1 and Cyp27 are also localized in the pericentral zone (37). Thus, it is tempting to speculate that active β-catenin results in greater inhibition of FXR around the central vein, creating a permissive environment for BA synthesis through absence of Cyp7a1 and Cyp27 suppression. It should, however, be noted that a recent ChIP-seq study identified Cyp27 to be a direct transcriptional target of β-catenin (38). Thus, it is possible that β-catenin regulates BA homeostasis through both direct and indirect mechanisms.
Our studies also highlight the important role of BA accumulation in the formation of reactive ductules. The decreased ductular proliferation observed in the β-catenin KO mice after BDL could be attributed to absence of β-catenin in cholangiocytes; alternatively, it could be secondary to decreased BA toxicity and cholestasis. However, our β-catenin ASO studies demonstrated conclusively that despite preservation of β-catenin expression in cholangiocytes, we still observed a significant suppression of ADP in β-catenin ASO-treated livers after BDL. Together, our findings indicate that ADP is not β-catenin-dependent, and is the result of a compensatory reaction to increased BA, which supports our recent findings (22).
FXR agonists, which decrease BA biosynthesis and increase transport, have been touted as potential treatments for cholestatic liver diseases (39). Indeed, a phase III clinical trial has demonstrated FXR agonist obeticholic acid either alone or in combination with ursodeoxycholic acid leads to a significant reduction in serum markers of liver injury and cholestasis in patients with primary biliary cholangitis (40). However, this favorable outcome was associated with a high incidence of pruritus, which was dose dependent and led to discontinuation of the drug in some patients (41). Thus, while therapeutic targeting of FXR appears promising, the adverse effects associated with high dosage requirements underscore the need for more effective or combination therapies to treat cholestatic liver disease.
Suppression of β-catenin to target FXR/β-catenin association during cholestasis, which is an impediment to the formation of the FXR/RXRα complex, thus offers a novel therapeutic opportunity. Based on our findings, we expect that lower doses of FXR agonist will be required when combined with β-catenin suppression, increasing efficacy and reducing unwanted side effects. However, we are cognizant of the differences in the BA profiles between mice and humans, and it remains to be seen if β-catenin knockdown in patients would achieve the same favorable results. Additionally, although there are no β-catenin suppressive therapies currently used in the clinical settings, our studies as well as others demonstrate efficacy in preclinical mouse models and provide a rationale for these targeted therapies.
Supplementary Material
Acknowledgments
Funding: This study was funded by NIH grants 1R01DK62277 and 1R01DK100287 and Endowed Chair for Experimental Pathology to SPSM, and NIH grant 1R01DK103775 to KNB.
We thank Sucha Singh for invaluable technical assistance. We also thank Dr. Valentina Factor, Dr. Michael Kahn, and Dr. Yixian Zhang for generously providing essential reagents for this study.
Abbreviations
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- BDL
bile duct ligation
- KO
β-catenin conditional knockouts
- WT
littermate controls
- ADP
atypical ductular proliferation
- BA
bile acids
- FXR
Farnesoid X receptor
- SHP
small heterodimer partner
- RXR
Retinoic X receptor
- Cyp
cytochrome P450
- ALT
Alanine aminotransferase
- AST
Aspartate animotransferase
- ALP
alkaline phosphatase
- GGTP
gamma glutamyl transpeptidase
- BSEP
bile salt export pump
- MRP
multidrug resistance-associated protein
- GS
Glutamine synthetase
- α-SMA
α-smooth muscle actin
- IHC
immunohistochemistry
- IF
immunofluorescence
- H&E
hematoxylin and eosin
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- IP
immunoprecipitation
- ChIP
chromatin immunoprecipitation
- i.p
intraperitoneal
- WB
Western blot
- LNA
locked nucleic acid
- ASO
antisense oligonucleotide
- DsiRNA
Dicer-substrate siRNA
- GalXC
N-acetylgalactosamine
Footnotes
Disclosure: Dr. Monga is on the scientific advisory board for Abbvie and Dicerna Pharmaceuticals and has corporate research agreements with Abbvie and Dicerna Pharmaceuticals. Dr. Abrams is employed by Dicerna Pharmaceuticals.
AUTHOR CONTRIBUTIONS
MT: conducted experiments, acquired data, analyzed data.
AM: conducted experiments
PC: conducted experiments
RM: conducted experiments
JT: conducted experiments
PX: conducted experiments, analyzed data
XM: provided reagents, analyzed data
MA: provided reagents, analyzed data
JL: analyzed and interpreted data
SPSM: designed research studies, analyzed data, and wrote the manuscript.
KNB: designed research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript.
References
Author names in bold designate shared co-first authorship.
- 1.Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727–739. doi: 10.1016/j.bpg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer S, Beuers U, Spengler U, Zwiebel FM, Koebe HG. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–186. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 4.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 6.Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh TH, Krauland L, Singh V, Zou B, Devaraj P, Stolz DB, Franks J, et al. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology. 2010;52:1410–1419. doi: 10.1002/hep.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 13.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 15.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 16.Schiltz RL, Mizzen CA, Vassilev A, Cook RG, Allis CD, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J Biol Chem. 1999;274:1189–1192. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 17.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 18.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geier A, Wagner M, Dietrich CG, Trauner M. Principles of hepatic organic anion transporter regulation during cholestasis, inflammation and liver regeneration. Biochim Biophys Acta. 2007;1773:283–308. doi: 10.1016/j.bbamcr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 21.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Okabe H, Yang J, Sylakowski K, Yovchev M, Miyagawa Y, Nagarajan S, Chikina M, et al. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology. 2016 doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-kappaB interactions in murine hepatocytes: a complex to die for. Hepatology. 2013;57:763–774. doi: 10.1002/hep.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lien F, Berthier A, Bouchaert E, Gheeraert C, Alexandre J, Porez G, Prawitt J, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balasubramaniyan N, Luo Y, Sun AQ, Suchy FJ. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J Biol Chem. 2013;288:13850–13862. doi: 10.1074/jbc.M112.443937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XC, Lian W, Zhang LJ, Feng XC, Gao Y, Li SX, Liu C, et al. Interleukin-18 Down-Regulates Multidrug Resistance-Associated Protein 2 Expression through Farnesoid X Receptor Associated with Nuclear Factor Kappa B and Yin Yang 1 in Human Hepatoma HepG2 Cells. PLoS One. 2015;10:e0136215. doi: 10.1371/journal.pone.0136215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda TB, Morris SA, Hager GL. Complex genomic interactions in the dynamic regulation of transcription by the glucocorticoid receptor. Mol Cell Endocrinol. 2013;380:16–24. doi: 10.1016/j.mce.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci U S A. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park YJ, Qatanani M, Chua SS, LaRey JL, Johnson SA, Watanabe M, Moore DD, et al. Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology. 2008;47:1578–1586. doi: 10.1002/hep.22196. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Yang Z, Trottier J, Barbier O, Wang L. Long noncoding RNA MEG3 induces cholestatic liver injury by interaction with PTBP1 to facilitate shp mRNA decay. Hepatology. 2017;65:604–615. doi: 10.1002/hep.28882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipriani S, Carino A, Masullo D, Zampella A, Distrutti E, Fiorucci S. Decoding the role of the nuclear receptor SHP in regulating hepatic stellate cells and liver fibrogenesis. Sci Rep. 2017;7:41055. doi: 10.1038/srep41055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemberger UJ, Fuchs CD, Karer M, Haas S, Stojakovic T, Schofer C, Marschall HU, et al. Hepatocyte specific expression of an oncogenic variant of beta-catenin results in cholestatic liver disease. Oncotarget. 2016;7:86985–86998. doi: 10.18632/oncotarget.13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Audard V, Grimber G, Elie C, Radenen B, Audebourg A, Letourneur F, Soubrane O, et al. Cholestasis is a marker for hepatocellular carcinomas displaying beta-catenin mutations. J Pathol. 2007;212:345–352. doi: 10.1002/path.2169. [DOI] [PubMed] [Google Scholar]
- 36.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 37.Twisk J, Hoekman MF, Mager WH, Moorman AF, de Boer PA, Scheja L, Princen HM, et al. Heterogeneous expression of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase genes in the rat liver lobulus. J Clin Invest. 1995;95:1235–1243. doi: 10.1172/JCI117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gougelet A, Torre C, Veber P, Sartor C, Bachelot L, Denechaud PD, Godard C, et al. T-cell factor 4 and beta-catenin chromatin occupancies pattern zonal liver metabolism in mice. Hepatology. 2014;59:2344–2357. doi: 10.1002/hep.26924. [DOI] [PubMed] [Google Scholar]
- 39.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S3–12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 40.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 41.Pares A. Treatment of primary biliary cirrhosis: Is there more to offer than ursodeoxycholic acid? Clinical Liver Disease. 2014;3:29–33. doi: 10.1002/cld.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.