Abstract
Background
Research on early adversity, stress biology, and child development has grown exponentially in recent years.
Findings
We review the current evidence for the hypothalamic-pituitary-adrenocortical (HPA) axis as a stress-mediating mechanism between various forms of childhood adversity and psychopathology. We begin with a review of the neurobiology of the axis and evidence for relations between early adversity-HPA axis activity and HPA axis activity-psychopathology, as well as discuss the role of regulatory mechanisms and sensitive periods in development.
Conclusions
We call attention to critical gaps in the literature to highlight next steps in this research including focus on developmental timing, sex differences, stress buffering, and epigenetic regulation. A better of understanding of individual differences in the adversity-HPA axis-psychopathology associations will require continued work addressing how multiple biological and behavioral systems work in concert to shape development.
Keywords: early adversity, hypothalamic-pituitary-adrenocortical axis, psychopathology
Introduction
There is increasing evidence that adult health and wellbeing has its roots in childhood experiences (Shonkoff, Boyce, & McEwen, 2009). Adverse early experiences include both the absence of stimulation needed for typical development and the presence of harmful or threatening stimulation. This article reviews the support for childhood adversity shaping stress response systems and mediating the impact of adversity on psychopathology. Reactivity and regulation of stress responses are widely viewed as one mechanism through which early adversity ‘gets under the skin’ to impact physical and mental health. We focus on the hypothalamic-pituitary-adrenocortical (HPA) system because its products have widespread effects on gene transcription and trophic processes that impact neurodevelopment. We begin by reviewing the neurobiology of this neuroendocrine system and theoretical models implicating the stress response systems as key mediators of developing psychopathology. We then turn to evidence that childhood adversity may impact activity of the axis, including examples of HPA axis activity as a statistical mediator in transducing adversity into poor outcomes for children. Lastly, we briefly discuss regulatory systems in these relations. Throughout, we call attention to critical gaps and unanswered questions to usher in future endeavors in this field. Where appropriate, we focus on providing evidence from human studies; however, we rely on animal studies where evidence is lacking for key questions. We want to note from the outset, this review is not an exhaustive list of this burgeoning literature.
Neurophysiology of Stress
The HPA Axis
Because stressors differ in their physical and mental demands, responses are stressor-specific and engage multiple systems (Hostinar & Gunnar, 2013). The HPA axis, however, is one of the core stress systems and is responsive to many stressors. Receptors for cortisol, the end product of the HPA axis in humans, are present throughout the body; thus, its effects are widespread on systems that underlie developmental processes and mental and physical health. Beyond situations that threaten bodily harm, the most potent types of stressors are ones that threaten our social selves (i.e., relationships, group status, self-esteem, Dickerson & Kemeny, 2004), especially if they are uncontrollable or unpredictable. There are complex inhibitory and excitatory inputs to the hypothalamic paraventricular nucleus (PVN) that forms the hypothalamic component of the HPA axis (Low, 2017). The PVN receives inputs from brainstem nuclei, including the nucleus tractus solitarus, that provide information about the physical state of the body and activate the system in response to physical stressors. Information about psychological stressors reaches the PVN through multi-synaptic inputs that involve regions of the prefrontal cortex, amygdala, and bed nucleus of the stria terminalis. The hippocampus is critically involved in setting the tone for the axis’ responses to psychological stressors and modulates the stress response across the day.
Lifting inhibitory inputs and/or increasing excitatory inputs in the PVN releases corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) into a portal system connecting the PVN to the anterior pituitary gland. CRH, augmented by AVP, stimulates the production and release of adrenocorticotropic hormone (ACTH). ACTH travels through the blood stream and interacts with cells on the adrenal cortex causing a cascade of enzymatic events that result in the release of cortisol. Cortisol is the predominant glucocorticoid in humans and will be referred to throughout this article. The HPA axis operates as a negative-feedback loop; acutely elevated cortisol binds to receptors in the pituitary and PVN to rapidly suppress activity of the axis allowing cortisol to return to baseline levels. Ongoing input to the PVN can override the negative feedback and continue cortisol production during prolonged or intense stressors.
Once released into circulation, because it is a steroid hormone and thus lipid soluble, cortisol enters all cells of the body, many of which contain receptors for cortisol. In the periphery, cortisol binds predominantly to glucocorticoid receptors (GR); however, in the brain cortisol binds to both GR and mineralocorticoid receptors (MR). MRs have high affinity for cortisol and are nearly wholly bound except at the lowest point of the daily rhythm. GRs have lower affinity for cortisol and thus become bound as levels rise during an acute response as well as at the peak of the daily rhythm. MRs help maintain neurons and support their responsiveness to their neurotransmitters; GRs mediate most of the responses that are associated with stress.
Most of the effects of cortisol operate through gene transcription. This involves binding of cortisol to its intracellular receptors, translocation of the hormone-receptor complex to the cell nucleus, and then binding to glucocorticoid receptive elements which are in the promotor regions of many genes resulting in regulation of gene transcription. Effects via gene transcription can take minutes to hours; however, some effects are cell membrane-mediated and occur rapidly. In the face of an acute stressor, the HPA axis coordinates the mobilization of the body’s resources to ensure survival; as such, it interacts with and influences many biological systems including growth, metabolism, immune, cognition, and reproduction. These effects often counter actions triggered by fight/flight responses; thus, one critical function of the HPA axis is to contain and reverse the effects of stress responses in other systems (Sapolsky, Romero, & Munck, 2000).
Acute HPA axis responses are necessary and have positive effects on functioning, including supporting positive mood (Hoyt, Zeiders, Ehrlich, & Adam, 2016). However, prolonged or repeated cortisol elevations can have damaging impacts on the body. Thus mechanisms have evolved to downregulate the HPA axis under conditions of chronic activation (Fries, Hesse, Hellhammer, & Hellhammer, 2005). This is likely why individuals experiencing chronic stressors initially exhibit frequent, prolonged elevations in cortisol, but then often have suppressed levels and blunted reactivity. Low, blunted cortisol levels, nonetheless, can and often do co-occur with hyper-responsivity of other stress systems. Indeed, one of the reasons for hyper-activity in these systems may be the loss of counter-regulatory control by cortisol (Sapolsky et al., 2000).
The HPA Axis and the Autonomic Nervous System
Gene transcription-mediated effects of cortisol take minutes to hours to unfold, thus they are not the basis for the fight/flight system. Preparation for fighting or fleeing is largely the job of the sympathetic adrenomedullary (SAM) system. The SAM and HPA systems are intricately intertwined although they do not respond in lock-step fashion (Goldstein & Kopin, 2008). The PVN in the hypothalamus is also a critical site for regulation of the SAM system along with extra-hypothalamic sites (e.g., locus coeruleus). Central control of the SAM system modulates brain stem structures to innervate pre-ganglionic sympathetic neurons in the spinal cord and then pass out of the spinal column to directly innervate tissue in the medulla of the adrenal gland. This stimulates the release of epinephrine (i.e., adrenaline) into the blood stream where it affects organs and tissues throughout the periphery to support coping with threatening or stressful challenges (Robertson, Biaggioni, Burnstock, Low, & Paton, 2012). Epinephrine operates through β1-adrenergic receptors to increase heart rate and stroke volume and through β2-adrenergic receptors to effect many of the other fight/flight bodily changes (e.g., breaking down fat stores, dilation of arteries to skeletal muscles). The HPA and SAM systems interact at many levels in both the periphery and brain. Prolonged increases in cortisol will up-regulate an enzyme in the adrenal medulla needed to produce adrenaline and some noradrenaline, thus increasing the capacity of the SAM system to respond when activated. Prolonged cortisol elevations also up-regulate CRH in the central amygdala which can facilitate activation of central norepinephrine in the locus coeruleus, enhancing the capacity for fight/flight. Interestingly, normal levels of cortisol are needed to keep the sympathetic nervous system (SNS) in check, suggesting that both hypo- and hyper-cortisolism can be associated with augmented sympathetic stress responses (Kvetnansky et al., 1995).
The SAM system is part of the SNS which, in addition to fight/flight, plays a role in maintaining homeostasis and supporting the body’s everyday functions. The rest of the SNS uses norepinephrine as its neurotransmitter and has high affinity for ?-adrenergic receptors. Some of the measures used in psychological research on stress reflect predominantly norepinephrine activity (e.g., salivary alpha amylase; Rohleder, Nater, Wolf, Ehlert, & Kirschbaum, 2004). Notably, animal studies imposing intense stress have shown a closer association between glucocorticoids and adrenaline (i.e., the SAM response) than between glucocorticoids and noradrenaline or even between adrenaline and noradrenaline (Goldstein & Kopin, 2008). This has implications for human research, as human studies tend to ignore differentiation within the SNS lumping all sympathetic reactions as reflective of stress.
While the capacity to produce a fight/flight state by the SNS is necessary for survival, it must be regulated. The parasympathetic system nervous system (PNS) provides counter-regulation, serving to return the body to a rest and digest state following stress. The PNS is importantly involved in regulating heart rate via the vagus nerve whose input to the sinoatrial node (the principle pace maker of the heart) slows heart rate. While we tend to think of the PNS as slowing heart rate and countering stress responses, under most laboratory conditions the withdrawal of vagal input to the heart is the primary mechanism involved in increasing heart rate. Indeed, activation of the SNS requires five or more seconds to produce a marked increase in heart rate, while withdrawal of vagal input significantly increases it in less than a second. The medulla is the primary brain site for regulating the PNS under non-stress condition; however, higher brain regions including the PVN are involved in modifying medullary activity to control cardiovascular responses to emotions and stress (Thayer & Lane, 2000).
Measuring Cortisol
While cortisol and its metabolites can be measured in blood and urine, respectively, most commonly it is measured in saliva and increasingly in hair in developmental studies. Salivary cortisol can be used to assess acute responses to stressors and the cortisol diurnal rhythm. A number of parameters of the acute stress response have been targets of investigation including 1) reactivity, assessing the magnitude of change from pre-stressor to peak levels, 2) recovery, assessing rate of return to pre-stressor levels, and 3) total cortisol response, reflecting the magnitude of the response inclusive of both reactivity and recovery. Because individuals often exhibit an anticipatory response to coming into a laboratory setting, acute stressor designs ideally include a 30–40-minute relaxation period before sampling a baseline measure.
In addition to acute response assessments, measures of diurnal variation reflect functioning of the axis. Across the day, cortisol levels follow a circadian rhythm released in pulses. Cortisol is higher in the morning with peak levels occurring approximately 30 minutes after waking, followed by a decline throughout the day reaching its nadir in the evening with sleep. Multiple indices can be derived from non-stressed daily functioning including 1) morning wake levels, 2) the cortisol awakening response (CAR), reflecting the degree of change from wake to peak, 3) diurnal slope, reflecting the rate of change across the day, and 4) magnitude of the total daily response. Well-designed studies assessing diurnal variation include collecting multiple samples in conjunction with waking, the CAR, and evening hours to derive different indices of HPA functioning. Measurement occasions over multiple days allow study of day-to-day variation as well as derivation of more stable measures than single day assessments. Notably, a single measure of cortisol, whether in saliva, urine, or blood, is almost meaningless.
While the dynamics of the HPA axis provide many opportunities for assessing internal and external regulation of the system, often the core question is whether the axis has become hyper- or hypo-active. For this question, the dynamics of the system can be challenging, demanding more measurement occasions than participants can tolerate. Cortisol, however, accumulates in hair and recently hair cortisol has been integrated into studies of chronic stress or following traumatic events where cortisol production before and after the event is relevant. Hair grows at roughly 1 cm per month, thus the closest centimeter to the scalp reflects the last month of cortisol production and so on. Typically, no more than 3 cm are examined as cortisol leaches from the hair with washing and hair treatments. In infants, hair cortisol is positively related to waking and bedtime levels and total magnitude of cortisol across the day, but not the diurnal slope (Flom, St. John, Meyer, & Tarullo, 2017).
Theoretical Perspectives
Different theoretical orientations guide research designed to understand how the HPA axis may serve to mediate the effects of adversity on psychopathology. Here we review those currently stimulating research.
Allostatic Load Model
The Allostatic Load Model (ALM) is perhaps the most prominent model of stress and health (McEwen, 1998). ALM proposes that the HPA axis, among other physiological systems (e.g., autonomic nervous system, immune, cardiovascular, oxidative stress) are implicated in increased risk for disease. Central to this theory is the notion of allostasis, which refers to the body’s ability to promote stability via increased activity of stress-mediating systems. Chronic or frequent environmental stressors require more frequent adjustments of the stress response systems. These adjustments are adaptive in the short-term; however, in the long-term, the cumulative effects of allostatic responses are posited to produce wear and tear on the body, termed allostatic load or overload, increasing risk of disease. Increased allostatic load is evident during childhood for maltreated youth (Danese & McEwen, 2012) and cumulative childhood adversity is associated with increased allostatic load during adolescence (Doan, Dich, & Evans, 2014). Notably, while ALM argues comparable negative effects on physical and mental health (Danese & McEwen, 2012), recent findings suggest that elevated allostatic load may occur among individuals who have achieved despite the odds and evince mental fitness (Brody et al., 2013). This raises the possibility that allostatic load indicators are more appropriate for predicting physical rather than mental health.
Diathesis-Stress Model
Diathesis-stress models (Monroe & Simons, 1991) are often invoked in stress and psychopathology research to explain individual variability inherent in adversity-psychopathology relations. These models posit that not all individuals are equally susceptible to negative influences of environmental stressors. Genetic variation or other vulnerability factors are viewed as the diathesis that pertains to some but not all individuals. For example, a history of parental depression has been linked to greater cortisol reactivity; however, this association was only noted for children with elevated negative cognitive attributions (Hayden et al., 2014). In this instance, the diathesis would be negative cognitive attributional styles, presumably a genetic contribution, that only resulted in greater reactivity when combined with parental depression. Genetic variants implicated in regulating the HPA axis (e.g., CRHR; NR3C1, the GR gene) have been found to increase risk for psychopathology in the context of gene-environmental risk interactions (Bradley et al., 2008; Velders et al., 2012).
Double Hit Model
Double hit models are a form of diathesis-stress where the diathesis is the presence of previous adverse stressors, often during early formative periods of development. Early adversity may program developing biological systems so that they are more stress-reactive or less regulated once activated, increasing the impact of stressors encountered throughout life. If a second major ‘hit’ is not experienced, then physical or mental pathology does not develop. The double hit model is also open to additional, often genetic, vulnerability as part of the etiology of pathology. In rodents, exposure to both early and adult stressors result in distinct behavioral responses (Walker et al., 2009). In children, fetal exposure to synthetic glucocorticoids coupled with socioeconomic disadvantage is associated with poor memory performance (Grant, Sandman, Wing, Dmitrieva, & Davis, 2015).
Stress Inoculation Model
Not all stressors are inherently harmful. All individuals encounter stressors throughout their life and the ability to cope with mild stressors is important for health. The stress inoculation model (Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006) proposes a J-shape relation between early experiences with stressors and later functioning, such that moderate exposure to mild stressors may help shape the axis for more optimal functioning with later challenges. While more adverse stressors are proposed to result in worse functioning, little to no exposure to stressors is also proposed to increase negative outcomes as the lack of successful experiences with mild stressors leaves an individual ill-equipped to cope with subsequent challenge. In squirrel monkeys, repeated separations occurring at 4–6 months of age enhances neurodevelopment and supports later coping with stress, despite producing marked cortisol elevations at the time of separation (Parker et al., 2006). In humans, there is emerging support for such models. Gustafsson et al. (2010) noted a blunted CAR and flatter diurnal rhythm for children experiencing 0 or 3+ adversities, while for those with 1–2 adverse experiences these HPA responses were more robust. Berry et al. (2017) found that maternal sensitivity produced a curvilinear relation (reverse J-shape) between maternal sensitivity and basal cortisol that is consistent with stress inoculation in young children. Non-linear relations in humans may be complex and shift with time (Bush, Obradović, Adler, & Boyce, 2011). While stress inoculation is an intriguing hypothesis, we need to know more before it can be useful in designing interventions that foster resilience in children. We need a better understanding of the mechanisms, conceptual definitions of what constitutes stress that is “within the child’s coping abilities” that enhances resilience, how much stress is necessary, and when during development the system is open for “inoculation”.
HPA and SNS Asymmetry
We need greater attention on how to assess the coordination of multiple systems as a means of understanding what constitutes an inefficient versus maladaptive response to stressors. Bauer et al. (2002) argue that the HPA and SNS systems should be jointly studied in stress research. These authors argue that co-activation or reciprocal actions of these systems that produce moderate levels of arousal contribute to more optimal behavioral functioning whereas dysregulated, asymmetrical actions contribute to psychopathology. Investigations testing the HPA-SNS interactive model provide mixed support with regards to patterns of symmetry (Gordis, Granger, Susman, & Trickett, 2006), asymmetry (Allwood, Handwerger, Kivlighan, Granger, & Stroud, 2011), or no relations (Spinrad et al., 2009) between HPA and SNS reactivity in the prediction of behavioral problems. As an expansion of this work, Quas et al. (2014), utilizing exploratory data analysis, investigated patterns of SNS, PNS, and HPA basal levels and reactivity to challenge and note a degree of consistency in which similar patterns emerged across multiple studies. However, the extent to which these individual systems respond differentially across development, context, eliciting stressor, and experiential histories, may cause the patterns uncovered to shift. Indeed, Koss et al. (2014) note that patterns of symmetry and asymmetry in contributing to psychopathology differed depending on risk exposure. Hastings et al. (2011) note a shift in the relation between stress response system patterns and internalizing problems overtime with heightened symmetrical activation related to greater concurrent problems, but asymmetry (low ANS, high HPA) contributing to later internalizing problems.
Biological Sensitivity to Context
In contrast to diathesis-stress models, biological sensitivity to context theory (Boyce & Ellis, 2005; see also differential susceptibility, Belsky & Pluess, 2009) postulates that genetic variation contributes to individual differences in plasticity of the stress response systems for better or for worse, such that for individuals in supportive, enriching environments these characteristics or genetic variants may produce positive outcomes whereas for individuals in adverse, harsh environments the same characteristics or genetic variants may lead to greater psychopathology and disease. The theory posits a U-shape relation between stress reactivity and life stress during development with individuals with greater reactivity of the stress response systems being found in greater proportions at both the positive and negative ends of the early stress continuum. Recent research provides some support for children with heightened HPA reactivity leading to differential outcomes as a variation of environmental adversity (Bolten et al., 2013; Obradović, Portilla, & Ballard, 2016).
Adaptive Calibration Model
The Adaptive Calibration Model (ACM; Del Giudice, Ellis, & Shirtcliff, 2011) is an evolutionary developmental model emerging as an offshoot of the biological sensitivity to context theory. These authors posit that unique constellations of stress responses develop as a result of environmental conditions with initial support emerging with regards to responsivity patterns (Del Giudice, Hinnant, Ellis, & El-Sheikh, 2012). The ACM argues that the stress response system (SRS) mediated between life conditions and life history strategies and makes specific predictions to different stress-behavior patterns related to different types of early adversity. In support of an acceleration of life history strategies toward earlier reproduction, Belsky et al. (2015) found maternal stress during pregnancy related to early adrenarche and adolescent mental health severity through heightened maternal depression during infancy and higher basal cortisol during childhood. In a recent test of ACM in adolescent males, Ellis et al. (2016) provide some support for the four predicted response patterns in relation to environmental stressors and behavioral functioning. Given the complexity of predictions pertaining to life course development, future longitudinal research targeting developmental trajectories that span across the developmental switch points (e.g., prenatal/early life, juvenile transition/adrenarche, puberty) is warranted to test the extent to which proposed patterns support differences in life history strategies that reflect tradeoffs in selection of reproduction, growth/metabolism, and learning/memory.
Sex Differences and the Role of Sex Hormones
For the most part, models of stress physiology as a mechanism for later health deficits and psychopathology do not take into account sex differences despite observed sex differences in psychopathology. The ACM is an exception; this model proposes that sex differences may emerge in the context of greater environmental stressors such that greater stress reactivity in females is expected to coincide with vigilant, withdrawn, or flight tendencies (Del Giudice et al., 2011) while males may exhibit greater agnostic or fight patterns with greater stress reactivity. The ACM posits a pattern following trauma that is characterized by unemotional responses and low stress reactivity in males and heightened stress reactivity and vigilance in females.
It is notable that there are relatively few replicable sex differences in the stress physiology literature prior to adolescence. Differences begin to emerge during adolescence with some likely relating to interactions between the HPA and hypothalamic-pituitary-gonadal (HPG) axes. For example, the lower cortisol response of women compared to men to a social evaluative stressor is a function of estrogen effects on the HPA axis observed only during part of menstrual cycle (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999). The HPG and HPA axes intersect with one another at multiple targets (Marceau, Ruttle, Shirtcliff, Essex, & Susman, 2015). For example, CRH and dehydroepiandrosterone (DHEA, an adrenal androgen) are involved in both the HPA and HPG pathways. DHEA and cortisol have opposing functions on metabolism and the nervous system (Kamin & Kertes, 2017); as such, interaction of the two hormones have been investigated in relation to psychopathology. Recent research has explored dual-axis investigations (e.g., Marceau et al., 2015); with researchers considering the interactive influence of cortisol with DHEA or testosterone. To date, there appears to be evidence for developmental influences, adversity, and sex effects in impacting the coupling of basal levels of HPA-HPG axes (Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2015; Simmons et al., 2015). Adolescence in particular may be a period in which the HPA-HPG axes work in concert with one another (Shirtcliff et al., 2015).
Childhood Adversity and Altered Stress Reactivity and Regulation
The literature associating childhood adversities and the HPA axis has burgeoned, thus we will not attempt an exhaustive review, but will provide select examples and point to critical themes and unanswered questions. Researchers have proposed that the deprivation and threat dimensions of early adverse care interact to produce a range of different neurobehavioral outcomes (McLaughlin & Sheridan, 2016). So far, however, there is no evidence that their interaction predicts whether the HPA axis is involved or whether elevations or suppressions of cortisol are observed. Rather, the current evidence is that both deprivation and threat are associated with heightened activity of the axis which if chronic may produce a hypocortisolism state (Sánchez, Ladd, & Plotsky, 2001).
Poverty
Evidence that poverty impairs neurobehavioral development (Blair & Raver, 2016) has increased interest in whether this might be due to activation and programing of the HPA axis. Several studies have now found associations between poverty and cortisol levels, diurnal rhythms, and/or reactivity. For example, lower socioeconomic status (SES) was related to higher hair cortisol in youth ages 4–18 (Vliegenthart et al., 2016). Neighborhood disadvantage has been associated with greater cortisol reactivity in adolescent boys, though not girls (Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012). In a Filipino adolescent cohort, flatter diurnal slope, higher evening cortisol, lower total cortisol output, and lower CAR, were associated with lower SES across multiple developmental periods since birth (Desantis, Kuzawa, & Adam, 2015). The jury is still out on whether there is a sensitive period for poverty to impact stress reactivity and regulation, although the evidence to date points towards cumulative effects. Cross-sectional work suggests that early childhood poverty is associated with elevated cortisol activity, while later in childhood blunting and down-regulation of the axis is observed (Ursache, Noble, & Blair, 2015).
Family Adversity and Parenting Quality
As a more proximal setting, adverse family conditions are expected to mediate the impact of poverty and other forms of adversity on the child, especially earlier in childhood. There is increasing evidence supporting this. For example, among toddlers reared in lower income households facing higher cumulative adversity, the association with blunted early morning cortisol and thus a flatter diurnal rhythm was mediated by reduced maternal warmth (Zalewski, Lengua, Kiff, & Fisher, 2012); heightened maternal negativity and less responsiveness also mediated between higher cumulative adversity and flatter diurnal slopes. In a follow-up, modest longitudinal stability was found in children’s cortisol over a 2.5-year period; with a curvilinear relation between income and cumulative risk such that both higher and lower risk were associate with lower cortisol (Zalewski, Lengua, Thompson, & Kiff, 2016). In adolescence, family chaos mediated the relation between increased total daily cortisol output over a two-year period for low income youth (Chen, Cohen, & Miller, 2010).
As in other studies of adversity, violence in the home and household instability also have been related to both elevated and suppressed cortisol concentrations. For example, in a longitudinal study, greater family instability and low maternal responsiveness were associated with a greater likelihood of both elevated and lower basal cortisol levels (Suor, Sturge-Apple, Davies, Cicchetti, & Manning, 2015). In a diary study during middle childhood, heightened parent-child conflict was associated with elevated bedtime levels resulting in flatter diurnal slope and an increase in total cortisol output over the day (Kuhlman, Repetti, Reynolds, & Robles, 2016). In contrast, male but not female adolescents exposed to violence in the past year exhibited lower cortisol reactivity to a laboratory stressor, controlling for cortisol reactivity one year earlier (Peckins, Dockray, Eckenrode, Heaton, & Susman, 2012).
More important to the question of whether HPA axis activity helps explain how adversity impacts outcomes are studies showing that cortisol plays a mediating role, at least statistically. While we focus on evidence that HPA axis activity serves as a statistical mediator in adversity-psychopathology relations in human studies, it remains an open question whether HPA axis activity plays a key biological mechanism in the pathophysiology of different forms of psychopathology or whether cortisol serves as a marker for other biological processes that produce psychopathology. HPA axis activity as a statistical mediator has now been demonstrated across different studies. For example, in 2 year-olds, the association between family instability (e.g., number of changes in residence, caregivers, caregiver’s intimate relationships) and deficits in children’s effortful control at age 4 was mediated by cortisol levels at baseline in the laboratory when children were 2 and 3 years old (Sturge-Apple, Davies, Cicchetti, Hentges, & Coe, 2016). Notably, in what appeared to be evidence of an adaptive response, higher cumulative family aggression was associated with blunted cortisol reactivity to a family stressor paradigm (Saxbe, Margolin, Spies Shapiro, & Baucom, 2012). In this study, at high levels of family aggression, blunted cortisol reactivity was associated with lower psychopathology symptoms whereas higher reactivity was associated with higher symptoms.
Often family risk factors are highly correlated and process models linking forms of adversity within different family relations are supported. While it has long been noted that the HPA axis responds in a context-specific manner, a recent study noted specificity within the same children in response to stressor paradigms designed to reflect parent-child versus interparental conflict (Sturge-Apple, Davies, Cicchetti, & Manning, 2012). Greater exposure to interparental violence was associated with a blunted cortisol response to a marital conflict paradigm while greater maternal emotional unavailability was associated with blunted reactivity to the strange situation paradigm in toddlers. This research highlights the importance of considering the stressor paradigms used to assess particular populations and adversity contexts. These findings also raise the possibility that some of the blunting of cortisol responses noted in studies may reflect the familiarity of children with the type of stressor being used. Predictability reduces the HPA stress response; thus, it may be important to assess children’s reactivity to the types of stressors common to the adversity they experience, as well as stressors that are outside of their ken before we conclude that the HPA axis is blunted or down-regulated.
Maltreatment
Maltreatment is associated with a range of adverse outcomes for children. Similar to other types of adversity discussed above, the question now is whether these effects are mediated by altered stress system activity and whether different types of maltreatment have different stress signatures. Neglect is the most prevalent form of abuse (Kim, Wildeman, Jonson-Reid, & Drake, 2017); neglect is also experienced by children living in orphanages/institutions due to high child-to-caregiver ratios and frequently rotating caregivers. Studies of institutionalized and post-institutionalized children are fairly uniform in revealing patterns of hypocortisolism with chronic neglect (although see, Gunnar, Morison, Chisholm, & Schuder, 2001). Children adopted into the U.S. after 16 months of age evidence greater hypocortisolism (e.g., flatter diurnal rhythms and less cortisol reactivity), especially if they received poor social care in the institutions (Koss, Hostinar, Donzella, & Gunnar, 2014; Koss, Mliner, Donzella, & Gunnar, 2016). Notably, greater hypocortisolism assessed four times, in eight month intervals, during the first two-years post-adoption mediated later teacher-reported ADHD symptoms and externalizing problems during kindergarten. Evidence for hypocortisolism has also been noted in middle childhood for children removed from institutions at 2 years or older, but not for those removed earlier in development (McLaughlin et al., 2015).
Neglect in the family context has also been associated with altered HPA activity. In preschoolers entering a new foster placement, neglect, but not other types of maltreatment, was associated with lower morning cortisol levels and flatter diurnal decrease (Bruce, Fisher, Pears, & Levine, 2009). A similar pattern of low morning cortisol and a reduced diurnal rhythm mediated between child protective services involvement and concurrent parent-reported externalizing problems in preschoolers (Bernard, Zwerling, & Dozier, 2015). Blunted cortisol has been found in a number of studies of maltreated youth including at older ages. For example, adolescents with a history of maltreatment exhibit blunted stress responses to a laboratory stressor task (Trickett, Gordis, Peckins, & Susman, 2014). While the above findings are provocative, they are not necessarily causal. Intervention studies for families at risk for maltreatment and for children in foster care bolster causality arguments. Prevention studies promoting supportive parenting for neglected and abused children note improvements in the cortisol rhythm (Bernard, Hostinar, & Dozier, 2015). Similar effects were noted for a family intervention with maltreating mothers of infants (Cicchetti, Rogosch, Toth, & Sturge-Apple, 2011).
In most of the above studies, the predominant form of maltreatment has been neglect. It is unclear whether blunted HPA activity patterns extend to children experiencing physical and sexual abuse, although this would be consistent with the trauma literature (Yehuda, 2002). Among adults, the HPA sequelae of childhood physical and sexual abuse is moderated by psychiatric diagnosis. Notably, adults with both depression and histories of child abuse exhibit hyper- not hypo-cortisol patterns that are not observed in adults experiencing either depression or abuse histories alone (Heim & Binder, 2012). It may be that we need to approach the child maltreatment and stress physiology research similarly. Indeed, when cortisol was sampled at a summer camp, physical and sexually abused children did not differ from comparison children, unless they exhibited high concurrent internalizing symptoms in which case they showed relatively flat diurnal cortisol pattern over the camp day (Cicchetti, Rogosch, Gunnar, & Toth, 2010). To use psychopathology as a moderating variable in assessing the impact of adversity on HPA axis activity, of course, means that if the axis is involved in transducing the adversity into psychopathology, the process is likely complex. We may need to think both of genetic vulnerability and transactional developmental patterns. Psychopathology itself may contribute to ongoing stressful experiences underscoring the possibility of transactional relations among adversity, the HPA axis, and psychopathology.
Maternal Depression
Maternal depression puts youth at risk for subsequent psychopathology through both genetic vulnerabilities and prenatal and postnatal exposures. A recent review of the literature suggests that offspring of depressed, non-medicated mothers begin life with heightened cortisol activity as well as alterations in autonomic and brain electrical activity (Gentile, 2017). Adding psychotropic medication, most notably selective serotonin reuptake inhibitors, produces further alterations in the HPA axis in neonates (Pawluski, Brain, Underhill, Hammond, & Oberlander, 2012). Thus, children of depressed mothers start life with an altered axis, which may confer greater vulnerability to adverse childhood experiences.
Parental depression may also disrupt the family context through heightened conflict and less responsive parenting. There are numerous examples of postnatal HPA correlates of maternal depression. For example, infants of depressed mothers respond less to laboratory challenges relative to infants of non-depressed mothers (Waters et al., 2013). Similarly, chronic maternal depression assessed longitudinally during the first six years of life and observed negative parenting at age 6 were associated with reduced cortisol variability (i.e., flatter decline from a likely anticipatory response) which in turn related to heightened child social withdrawal during mother-child interactions and elevated psychopathology symptoms concurrently at age 6 (Apter-Levi et al., 2016). Children of depressed mothers are an interesting target for research on the effects of adversity because depression and thus caregiving quality waxes and wanes across development (Dawson & Ashman, 2000). The dynamic nature of depression symptoms has stimulated questions about whether particular periods of development are more sensitive in affecting the HPA axis or whether it is the cumulative impact of maternal depressive episodes over the child’s life that matters. Some studies have found evidence that the first year or two may have impacts over and above later periods (Dawson & Ashman, 2000; Halligan, Herbert, Goodyer, & Murray, 2004). Other studies have found evidence that later developmental periods are more sensitive to parental depression (Dougherty, Tolep, Smith, & Rose, 2013). We will return to the general question about timing in a later section.
Not all children appear to be similarly impacted by maternal, or for that matter, paternal depression. For example, children low but not high in positive emotionality had heightened cortisol reactivity if their mothers were depressed (Mackrell et al., 2014). Of course the child’s low positive emotionality may have reflected a stronger genetic load for depression (Durbin, Klein, Hayden, Buckley, & Moerk, 2005). Indeed, the confounding of genetic predisposition to depression with maternal care make the parental depression literature challenging to interpret. Studies using genetically-informed designs, however, indicate that both genetics and prenatal exposures from the birth mother and the adoptive parents’ psychopathology, along with interactions of these factors, explain variance in children’s cortisol and internalizing symptoms (Laurent et al., 2013). We need more of this genetically-informed work, particularly as there is evidence that daughters of depressed mothers exhibit elevated patterns of cortisol production similar to their mothers before the emergence of symptoms (LeMoult, Chen, Foland-Ross, Burley, & Gotlib, 2015) and that higher production of cortisol among the offspring of depressed mother predicts which youth will develop depression in response to negative life events during adolescence (LeMoult, Ordaz, Kircanski, Singh, & Gotlib, 2015).
Summary of Early Adversity Effects
There is increasing evidence that childhood adversity is associated with altered activity of the HPA axis. Much of the evidence suggests that early or chronic adversity is associated with more hypocortisolism patterns, except perhaps when moderated by internalizing problems and/or in the context of high familial risk for depression. We will return to the question of whether and when altered HPA axis functioning mediates associations between early adversity and internalizing versus externalizing types of psychopathology later in this article.
Social Regulation and Buffering
One reason that adverse care may be associated with altered regulation of stress physiology, including the HPA axis, is that adversity can undermine the capacity of relationships to provide stress-buffering and regulation benefits. Critical to physical and emotional health in social species is the capacity to use the presence of supportive individuals to reduce fear and physiological reactions to stressors (Hostinar, Sullivan, & Gunnar, 2014). In children, parents are potent regulators of HPA axis reactivity. However, the quality of parent-child relationships matter as the presence of the parent is a powerful buffer of the HPA axis for secure but not insecure attachments (Nachmias, Gunnar, Mangelsdorf, Paritz, & Buss, 1996). During middle childhood, maternal support continues to buffer HPA axis reactivity. Importantly, even talking by phone with the mother produces a buffering response at least in girls (boys were not tested; Seltzer, Ziegler, & Pollak, 2010). However, caregivers’ ability to serve as an effective buffer of the HPA axis diminishes during adolescence; while they may continue to affect other regulatory systems by their presence and availability (Doom, Doyle, & Gunnar, 2017). Puberty appears to underlie the loss of parental figures as effective regulators of cortisol reactivity but not recovery following social stressors (Doom, Hostinar, VanZomeren-Dohm, & Gunnar, 2015). Furthermore, peers amplify rather than buffer stress reactivity during adolescence (Doom et al., 2017). Thus, adolescence may be a period in which lack of effective buffers of the HPA axis confers heightened risk for psychopathology.
Interest in social regulation of the HPA axis intersects with work on oxytocin that has been proposed as one system underlying social buffering. Oxytocin is a peptide implicated in many functions including as an anti-stress hormone through reducing HPA axis activity and supporting the PNS. Oxytocin also supports bonding, attachment, parturition, and lactation (Kubzansky, Mendes, Appleton, Block, & Adler, 2012). Intranasal oxytocin prevents cortisol reactivity as effectively as the presence of a social partner in adult men (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003). Interaction with a mother, in-person or via telephone, following a stressor task, produced elevated oxytocin relative to girls with no maternal contact, and as noted earlier, faster return to baseline for cortisol (Seltzer et al., 2010). Male same-sex best friends actually suppressed oxytocin, along with failing to buffer against cortisol reactivity (Doom et al., 2017). All of these studies have used urinary oxytocin, reflecting both peripheral and central release. Oxytocin in the periphery does not cross the blood-brain barrier. Studies using plasma oxytocin yield markedly different findings with positive correlations between oxytocin and cortisol (Engert, Koester, Riepenhausen, & Singer, 2016). Peripheral oxytocin, including in plasma, serves to protect the cardiovascular system; indeed, these same authors note elevated plasma oxytocin was associated with more rapid recovery of the vagal system. We likely need to be very cautious about mixing findings using oxytocin from different compartments as it likely differentially relates to stress and buffering.
Less is known regarding how early adversity impacts social and/or biological buffering. The mother’s presence failed to buffer cortisol responses to a stressor in post-institutionalized youth, while it did in non-adopted children (Hostinar, Johnson, & Gunnar, 2015). This is consistent with evidence that maternal contact failed to increase oxytocin production in post-institutionalized children, but did in non-adopted children (Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). On the contrary, maltreated girls evidence increased oxytocin and reduced cortisol to a speech stressor (Seltzer, Ziegler, Connolly, Prososki, & Pollak, 2014); while there were no changes in oxytocin in boys and non-maltreated girls. This raises the question whether caregivers’ ability to serve as an effective regulator of the HPA axis differs with regards to the ability to repair a system programed in adverse conditions early in life as oppose to caregivers’ ability to modulate a system developed in normative conditions. While the oxytocin-cortisol-social buffering model holds promise, the story is likely nuanced, conditioned by development, sex, and caregiving histories.
Sensitive Periods for Programing the HPA Axis
The literature reviewed in this article points to adversity, beginning prenatally, shaping physical and mental health, in part, through regulation of stress physiology. A key question is whether there are sensitive periods during which set points for stress-mediating systems are established. The HPA axis adapts to conditions throughout life. At any point in development, chronic activation of the system will produce alterations in the axis that persist until the threat is alleviated and the system has had time to return to normal functioning. In contrast, sensitive periods are times when stimulation influences the set points of the system in ways that persist long after those influences are removed. Thus, sensitive periods are not supported by merely finding that adversity at a particular developmental period predicts cortisol activity, but rather these predictions hold long after adversity has remitted. Finding that the same conditions experienced at one time rather than another have long lasting consequences provides even stronger evidence. In human studies, limited evidence to date points to prenatal and early postnatal years as sensitive periods for the HPA axis.
Prenatal exposure to significant maternal stress tends to be associated with poorer outcomes and higher cortisol levels in children (Zijlmans, Riksen-Walraven, & de Weerth, 2015). Stressors in the form of prenatal but not postnatal infections, result in higher cortisol concentrations at 18 months (Gover et al., 2013). Likewise, infants’ prenatal, but not postnatal exposure to intimate partner violence was associated with cortisol reactivity to a laboratory stressor in infancy (Levendosky et al., 2016). Timing of prenatal exposure is an important question; mixed findings have emerged underscoring both the first (Sandman, Davis, Buss, & Glynn, 2012) and third (Yong Ping et al., 2015) trimesters in impacting long-term functioning. Finally, a meta-analysis supports prenatal programming of the axis, primarily on baseline cortisol measures and, disconcertingly, equivalent effect sizes for hyper- and hypo-secretion (Pearson, Tarabulsy, & Bussières, 2015). Mounting evidence is emerging for prenatal development as a time during which programing of the axis may occur; however, whether this means elevations or suppressions is not completely clear.
Postnatally, children adopted from institutions provide some of our best evidence of early sensitive periods because they experience a marked shift from chronically adverse to supportive care. With regards to stress reactivity, we find evidence that removal from institutions after 1.5–2 years is associated with hypocortisolism, while the same is less apparent with earlier removal (Koss et al., 2016; McLaughlin et al; 2015). In a prospective study, cortisol levels among adolescent girls, but not boys were predicted by poverty during infancy and adolescence, but not childhood (McFarland & Hayward, 2014). Greater cortisol reactivity at age 16 years was found in youth who experienced more adversity during gestation and infancy, but not later in development (Bosch et al., 2012). Higher cortisol levels were found in youth who experienced greater adversity in middle childhood, while lower cortisol levels were found in youth who experienced more adversity in adolescence or chronically. Thus it is possible that different parameters of the axis are influence at different points in development.
If there are sensitive periods shaping stress reactivity and regulation, what are the mechanisms that account for these effects? Animal studies point to epigenetics, especially DNA methylation as a mechanism for long-term setting of the HPA axis (Meaney & Szyf, 2005). Studies in humans have begun to examine early adversity effects on methylation of genes involved in regulating cortisol and its impact on tissues. Prenatal work in several studies demonstrates the importance of epigenetic pathways (Monk, Spicer, & Champagne, 2012). For example, war-related and chronic stressors were associated with differential methylation of the multiple cortisol regulatory genes in placenta and cord blood following birth in mother-infant dyads in the Congo (CRH; NR3C1; FKBP5, a binding protein that reduces transduction of cortisol into cell nuclei; Kertes et al., 2016). A meta-analysis reports similar findings for NR3C1 (Palma-Gudiel, Córdova-Palomera, Eixarch, Deuschle, & Fañanás, 2015). Postnatal adversity may continue to operate through epigenetic pathways. Exposure to early physical maltreatment demonstrates greater methylation of the NR3C1 gene measured during adolescence (Romens, McDonald, Svaren, & Pollak, 2015) and adulthood (Bustamante et al., 2016; however see Tyrka et al., 2016). Longer time in orphanage care was associated with lower methylation of the FKBP5 gene during adolescence (Non et al., 2016), something that might put youth at risk for PTSD. Finally, increased NR3C1 methylation mediated between maltreatment during the past 6 months and concurrent internalizing behaviors in preschoolers (Parade et al., 2016). Thus epigenetic pathways may mediate between early adverse care and socio-emotional outcomes. It is important to note epigenetic modifications are key to cell differentiation and thus DNA methylation differs across tissue types. The degree to which epigenetic modifications in peripheral tissue correspond to similar modifications in the brain remains an open question for research necessitating continued parallel work between human and animal studies.
Beyond early development, there is evidence in animals that the peripubertal period may be another sensitive period for regulation of stress physiology. Peripubertal rats subjected to stress and tested later as adults show larger and more poorly regulated HPA responses than young adult rats subjected to the same stress and tested at the same age (Romeo, 2010). It is unclear whether there is a similar sensitive period in humans during early adolescence. Certainly, there is evidence of increased reactivity to psychosocial stress with the transition from childhood to adolescence; higher cortisol levels and greater reactivity have been noted with more advanced age and/or pubertal stage (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009). Puberty may be characterized by greater variability in HPA activity; greater stability in anticipatory cortisol is noted among post-pubertal adolescents compared to pre- or early pubertal youth (van den Bos & Westenberg, 2015).
A recalibration period, which by definition is a later sensitive period, is one during which patterns of regulation geared to harsh conditions can be reset if conditions improve to function similar to an axis programed under more benign conditions from the beginning. To date, animal studies testing the recalibration hypothesis show that environmental enrichment during puberty results in less stress reactivity among rats exposed to prenatal and early postnatal stressors (Eiland & Romeo, 2013). In cross-sectional work, early adversity followed by supportive care is associated with a blunted CAR in pre- or early puberty but not in mid to late puberty holding age constant (Quevedo, Johnson, Loman, LaFavor, & Gunnar, 2012). Tests of puberty as a recalibration period necessitate longitudinal designs that allow for examining shifts in the HPA axis within individuals before and after the pubertal transition. However, initial evidence holds open the possibility that with puberty, and perhaps at other later developmental periods, effects of early adversity may be recalibrated. As in all work on sensitive periods and plasticity, the effects cut both ways, with stress systems also more open to greater effects of adversity during these periods.
Stress Reactivity and Regulation and Psychopathology
Next, we turn to recent evidence that altered stress reactivity and regulation underlie psychopathology in childhood and adolescence. Although often comorbid, particularly in children, we will discuss internalizing and externalizing disorders separately. We focus on the HPA axis and its role as a possible mediator between childhood adversity and psychopathology within each domain. To increase causal inference, we make particular note of tests of the direction of effects as well as longitudinal investigations.
Internalizing Disorders
Research suggests that the association between HPA stress reactivity and regulation and internalizing disorders may depend on the disorder (e.g., depression, anxiety, post-traumatic stress disorder, PTSD), age of the child (or time since trauma in the case of PTSD), and degree of prior and current adversity. Because many of these factors, along with which dimensions of the stress systems are measured, vary across studies, this literature is challenging to summarize. A child’s current life adversity is rarely considered but may be particularly important. Kaufman et al. (1997) demonstrated this in a study of clinically depressed youth administered a CRH-challenge test; they found only those who were currently experiencing significant family adversity exhibited altered HPA responses to the challenge.
Despite variability in design, populations, and HPA axis measures, there is increasing evidence for associations between cortisol and depression in adolescents. A meta-analysis concluded that youth with Major Depressive Disorder (MDD) have higher basal cortisol and poorer feedback regulation compared to non-MDD youth (Lopez-Duran, Kovacs, & George, 2009). Longitudinal research across development points to altered HPA activity preceding increased internalizing problems. In young children, higher total cortisol output, flatter diurnal rhythm, and a more positive CAR were not associated with concurrent internalizing symptoms at 18 months, but were related to elevated symptoms at 36 months (Saridjan et al., 2014). Likewise, elevated afternoon cortisol levels at 4.5 years predicted higher internalizing problems in kindergarten (Smider et al., 2002). A higher CAR prospectively predicted the emergence of MDD one-year later in adolescence (Adam et al., 2010), and anxiety disorders six years later in young adults (Adam et al., 2014). Likewise, adolescents with high basal cortisol exhibited increasing symptom severity (inclusive of internalizing and externalizing) over a two-year period (Shirtcliff & Essex, 2008). As already noted, never depressed adolescent daughters of depressed mothers who exhibited higher cortisol levels were more likely to develop clinical depression following adversity (LeMoult, et al., 2015a; 2015b). Greater cortisol reactivity to a family stressor paradigm predicted low but increasing depressive and anxiety symptoms across a three-year period during early adolescence (Koss, Cummings, Davies, & Cicchetti, 2016). Finally, increasingly large CARs predicted heightened depressive symptoms in adolescents (Nelemans et al., 2014).
Researchers have also examined longitudinal changes in HPA functioning in relation to anxiety symptoms. For example, longitudinal increases in cortisol levels were associated with higher anxiety in adolescent girls (Schiefelbein & Susman, 2006). Consistent with a stress sensitization hypothesis, a recent longitudinal study examining multiple aspects of HPA functioning found low but increasing morning cortisol across childhood was associated with elevated anxiety symptoms and elevated and extended HPA activation in stress paradigms six years later (Laurent, Gilliam, Wright, & Fisher, 2015).
Not all studies are consistent with the findings reported above. Notably different results are found for younger children when MDD is less common and often confounded with significant family adversity (von Klitzing et al., 2014). This may be why MDD and subsyndromal preschoolers were found to exhibit features of hypocortisolism as preschoolers and two years later (Suzuki, Belden, Spitznagel, Dietrich, & Luby, 2013). Similarly, in a study oversampled for low income and African American girls, higher depressive symptoms in 12-year-old girls were associated with lower concurrent and longitudinal decreases in cortisol output from ages 10 to 12 to the cold pressor task (Keenan et al., 2013). Consistent with this argument,Ruttle et al. (2014) noted lower afternoon cortisol levels among youth with higher concordance between internalizing symptoms and negative life experiences, and higher cortisol among youth who exhibited high symptoms without concomitantly high negative life events. The pubertal transition may also play a role in how internalizing symptoms relate to cortisol. Colich et al. (2015) showed that girls with MDD exhibited hypocortisolism early in puberty but hypercortisolism at later pubertal stages.
Initial studies with children with PTSD were perplexing because, unlike adults (Yehuda, 2002), these children often had elevated cortisol (Carrion et al., 2002). In contrast, studies of adolescents exposed to trauma as children are more like adult studies demonstrating low cortisol patterns (Pfeffer, Altemus, Heo, & Jiang, 2007). Notably,Luo et al. (2012) collected hair samples from adolescent girls exposed to a devastating earthquake in China. Girls in the quake area, compared to controls, had elevated cortisol for several months following the quake. After which, girls who developed PTSD diverged and evidenced markedly lower hair cortisol compared to trauma-exposed girls without PTSD. Longitudinal work supports the idea that children with PTSD tend to exhibit elevations, while adolescents evidence lowered cortisol activity. This raises the question of whether time since trauma or pubertal changes explain this difference. At least one study suggests that it is time since trauma (Trickett, Noll, Susman, Shenk & Putnam, 2010); but this does not explain why lower cortisol emerged in association with PTSD after several months (Luo et al., 2012) compared to several years in different studies (Trickett et al., 2010). More longitudinal studies are needed that follow children exposed to PTSD-inducing trauma at different ages to disentangle time and developmental stage.
Externalizing Problems
In contrast to the literature on internalizing disorders, externalizing problems are fairly consistently associated with lower cortisol levels and blunted reactivity. Indeed, hypo-functioning of stress-responsive systems is believed to be a significant contributor to the development of externalizing disorders. While much of this research focuses on autonomic reactivity (Lorber, 2004), there is increasing evidence that the HPA axis, which helps maintain the tone of the autonomic system, is also fundamentally involved (Van Goozen, Fairchild, Snoek, & Harold, 2007). Both concurrent and predictive associations between cortisol activity and externalizing problems have been reported. When the activity of stress-mediating systems and externalizing symptoms are both examined at multiple points in longitudinal work, the direction of effect can be more directly assessed. Salis et al. (2016) showed that blunted diurnal cortisol rhythms at age 6 predicted increasing conduct problems from ages 6 to 9, but the reverse direction was not supported. Similarly, Platje et al. (2013) found lower morning cortisol was predictive of adolescent aggression and rule-breaking one year later, but not the reverse. However, there is some evidence of bidirectional effects; flatter diurnal slopes at age 11 were associated with alcohol use between ages 15–18 which in turn predicted flatter diurnal slopes at age 18.5 (Ruttle, Maslowsky, Armstrong, Burk, & Essex, 2015).
As we noted in the section on adverse childhood experiences, chronic adversity is often associated with down-regulation of the HPA axis. These conditions are also associated with increased externalizing problems and a number of studies now provide evidence that down-regulation of HPA and autonomic systems may mediate these effects. Young adults experiencing higher adversity prior to age 16 exhibited a constellation of changes that included unstable regulation of emotion and behavioral instability, along with blunted cortisol and autonomic reactivity (Lovallo, 2013). Additionally, the concurrent association between poor parental monitoring and externalizing problems was mediated by a blunted cortisol diurnal slope in 9–12 year-olds (Martin, Kim, Bruce, & Fisher, 2014). We have already noted our study of post-institutionalized children in which hypocortisol (diurnal slope and reactivity) assessed over a 2-year period mediated between early adversity and later externalizing problems and ADHD symptoms during kindergarten (Koss et al., 2016). Finally, among children who experienced childhood harm (history of maltreatment and/or frequent bully victimization), blunted cortisol reactivity to a social stressor was associated with concurrently heightened externalizing and social problems at age 12 (Ouellet-Morin et al., 2011).
While this evidence generally supports a negative association between HPA axis, autonomic functioning, and externalizing behavior, several issues remain. First, the association has been shown to be stronger in clinic-referred compared to community samples, raising the question of whether low activity of the HPA axis is associated primarily with more severe externalizing psychopathology (Fairchild, Van Goozen, Calder, & Goodyer, 2013). Relatedly, others have argued that low HPA activity is associated more with callous-unemotional traits and not with other forms of externalizing (Hawes, Brennan, & Dadds, 2009). There is also the question of co-morbid internalizing problems. Here the results seem fairly clear. Several studies have shown that youth with comorbid anxiety and disruptive behavior disorders have elevated not suppressed levels of cortisol and autonomic activity (Fairchild et al., 2013). These and other issues likely contribute to some of the heterogeneity in findings. On the whole, however, the current weight of the evidence suggests that it will be fruitful to continue exploring activity of the HPA axis and other stress-mediating systems in untangling the etiology of externalizing disorders.
Conclusions and Future Directions
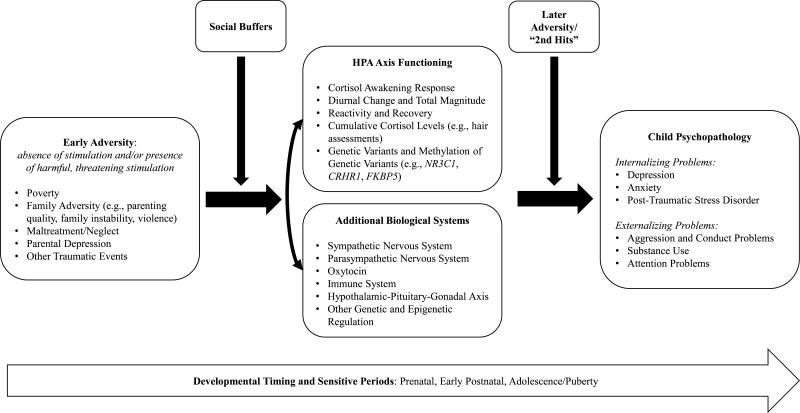
This review underscores increasing evidence that early adversity may impact later functioning in part by affecting the reactivity and regulation of stress-responsive systems, in particular but not exclusively, the HPA axis (Figure 1 provides a schematic of the research reviewed in this manuscript). While the evidence for this assertion is growing, it is far from conclusive, instead supporting a “more work is needed” conclusion. A number of additional issues need to be integrated into future work in this area. First, we need better models of how adverse conditions “get under the skin” to affect development. These models will need to account for pathways from different types of adverse conditions and will need to be age-period appropriate. We will also need to consider which neurophysiological systems need to be tracked and integrated. Although we have focused on the HPA axis, clearly multi-systems need to be considered to understand how adversity enhances the risk of psychopathology during development. For example, research on immune functioning notes associations between adversity, inflammation, and psychopathology. The emergence of depression during adolescence was coupled with increases in markers of inflammation (e.g., C-reactive protein, CRP; interleukin-6, IL-6); however, this was only noted among youth exposed to higher levels of childhood adversity (Miller & Cole, 2012). There are bidirectional relations between the HPA axis and the immune system; both cytokines and interleukins can activate the HPA axis while cortisol activates anti-inflammatory and suppresses pro-inflammatory responses. Thus in the context of chronic stress in which blunted HPA axis activity is often observed, reduced activity of the HPA axis may contribute to dysregulation of other systems; in the case of the immune system, blunted cortisol may reduce inhibition of inflammation highlighting the importance of considering how disruptions in the HPA axis in response to adversity may alter the co-regulation of multiple systems that together underlie psychopathology. Second, while activation of stress-mediating physiological systems seems to play a role in transducing adversity into effects on neurobehavioral development it seems likely that behavioral systems, particularly those that are sensitive to stress and which help regulate stress physiology need to be integrated into our models. One very likely candidate is sleep. Indeed, poor sleep has been found to mediate concurrent associations between perceived stress and diurnal cortisol in youth ages 8–18 (Ly, McGrath, Gouin, 2015). Additionally, sleep has moderated the association between family demands and cortisol such that greater stress related to blunted CAR for adolescents with poor sleep (Chiang et al., 2016). While problematic sleep is a symptom of psychopathology, little research has integrated sleep into studies of adversity, HPA axis functioning, and psychopathology. It may be particularly instructive to examine sleep and stress physiology over the night-time hours as this may be when bidirectional influences between these systems and other systems (e.g., memory) may be coming together to increase vulnerability for emotional and behavioral problems in youth.
Figure 1.
Conceptual model of relations among early adversity, the HPA axis, and psychopathology. The figure depicts the diverse forms of adversity, indicators of HPA axis activity, and dimensions of psychopathology along with other developmental, biological, and environmental regulators included in this review. Note: the figure provides a schematic for the body of research reviewed; arrows do not fully depict the transactional nature of the varying components.
Third, in the above review we did not devote a great deal of attention to discussing discrepant or contradictory findings because it is not clear what is, in fact, contradictory. There are multiple different parameters of HPA axis activity, many different forms of early adversity, and multiple dimensions of psychopathology. Thus, if one study finds that harsh discipline is associated with lower morning cortisol levels and another finds that it is associated with larger cortisol reactivity to a laboratory stressor, are these contradictory findings? Not necessarily. To start building a cumulative literature, we need a clearly agreed upon set of measures to index the HPA axis and other relevant stress-responsive systems as well as agreed upon (not cherry-picked) ways of analyzing them. Many researchers and studies assess HPA axis functioning across different dimensions and while some have integrated multiple assessments into single investigations, most do not. To get a better understanding of how the axis responds to adversity, as in many parts of the literature, we need a multi-method and multi-context approach. Moving in this direction may help us answer the question of what does “dysregulation” of the system really mean? Investigations that tackle multiple contexts are important for assessing whether the axis is activated under conditions in which it should be activated and whether it maintains lower levels when it should (such as in the presence of supportive individuals). We also need more longitudinal research.
We briefly touched upon emerging epigenetic findings demonstrating one mechanism through which adversity may shape the HPA axis. This research is in its infancy and is just beginning to integrate epigenetic processes into more complete models of adversity, the HPA axis, and psychopathology. One exception, as noted previously, found methylation of the GR gene mediated between maltreatment and internalizing problems (Parade et al., 2016). This investigation, however, did not include direct assessments of cortisol or gene expression which are needed. Presently, there are more questions than answers in this area; including questions about the role of early adversity in programing the axis, and the degree to which these processes are stable versus amendable to change later in development. Nevertheless, initial evidence points to a promising direction.
In conclusion, a body of research now underscores associations between early adversity and alterations in reactivity and regulation of the HPA axis. A small, but growing, literature demonstrates that, statistically at least, alterations in activity of this neuroendocrine system mediates between adverse conditions and poor outcomes in children and adolescents. However, we are still far from understanding the various pathways through which adversity ‘gets under the skin’ to influence stress and development, nor do we understand the boundaries of sensitive periods for these effects. There is still much work to be done, but also much promise in continuing to address questions of childhood adversity, stress, and psychopathology.
Key points.
This review synthesizes the most recent literature regarding childhood adversity, the hypothalamic-pituitary-adrenocortical (HPA) axis, and psychopathology.
Research across multiple forms of adversity including, poverty, family adversity, parenting quality, parental depression, and maltreatment, supports the notion that early adversity is associated with alterations in the HPA axis.
Alterations in stress reactivity and regulation are related to increased internalizing and externalizing symptoms and disorders during childhood and adolescence.
We call attention to key questions in moving this literature forward including discussion of the role of sensitive periods, regulatory systems, and epigenetic processes in shaping the HPA axis.
Acknowledgments
Support for M.R.G. was provided by a NIH grant (R01 HD075349 01A1) during the preparation of this manuscript. Support for K.J.K. was provided by a NIH training grant (T32 MH018921) and the Center for Health and Wellbeing’s Program for U.S. Health Policy and the Bendheim-Thoman Center for Research on Child Wellbeing at Princeton University’s Woodrow Wilson School of Public and International Affairs.
This review was invited by the Editors of this journal, who offered a small honorarium to cover expenses. This work has undergone full, external peer review.
Footnotes
The authors have declared that they have no competing or potential conflicts of interest to declare.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological Psychology. 2011;88:57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levi Y, Pratt M, Vakart A, Feldman M, Zagoory-Sharon O, Feldman R. Maternal depression across the first years of life compromises child psychosocial adjustment: Relations to child HPA-axis functioning. Psychoneuroendocrinology. 2016;64:47–56. doi: 10.1016/j.psyneuen.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Developmental Psychology. 2015;51:816–822. doi: 10.1037/dev0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Hostinar CE, Dozier M. Intervention effects on diurnal cortisol rhythms of child protective services-referred infants in early childhood: Preschool follow-up results of a randomized clinical trial. Journal of the American Medical Assocation Pediatrics. 2015;169:112–119. doi: 10.1001/jamapediatrics.2014.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Zwerling J, Dozier M. Effects of early adversity on young children’s diurnal cortisol rhythms and externalizing behavior. Developmental Psychobiology. 2015;57:935–947. doi: 10.1002/dev.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D, Blair C, Willoughby M, Granger DA, Mills-Koonce WR The Family Life Project Key Investigators. Maternal sensitivity and adrenocortical functioning across infancy and toddlerhood: Physiological adaptation to context? Development and Psychopathology. 2017;29:303–317. doi: 10.1017/S0954579416000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Poverty, stress, and brain development: New directions for prevention and intervention. Academic Pediatrics. 2016;16:S30–S36. doi: 10.1016/j.acap.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolten M, Nast I, Skrundz M, Stadler C, Hellhammer DH, Meinlschmidt G. Prenatal programming of emotion regulation: Neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. Journal of Psychosomatic Research. 2013;75:351–357. doi: 10.1016/j.jpsychores.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Ressler KJ. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SRH. Is resilience only skin deep? Rural African Americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychological Science. 2013;24:1285–1293. doi: 10.1177/0956797612471954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Obradović J, Adler N, Boyce WT. Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Development and Psychopathology. 2011;23:1089–1106. doi: 10.1017/S0954579411000514. [DOI] [PubMed] [Google Scholar]
- Bustamante AC, Aiello AE, Galea S, Ratanatharathorn A, Noronha C, Wildman DE, Uddin M. Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression. Journal of Affective Disorders. 2016;206:181–188. doi: 10.1016/j.jad.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/S0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Tsai KM, Park H, Bower J, Almedia DM, Dahl RE, Fuligni AJ. Daily family stress and HPA axis functioning during adolescence: The moderating role of sleep. Psychoneuroendocrinology. 2016;71:43–53. doi: 10.1016/j.psyneuen.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Development and Psychopathology. 2011;23:789–800. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman S. On the origins of a vulnerability to depression: The influence of early social environment on the development of psychobiological systems related to risk for affective disorder. In: Nelson CA, editor. Minnesota Symposia on Child Psychology Vol. 31. The effects of adversity on neurobehavioral development. New York, NY: Erlbaum; 2000. pp. 245–278. [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Hinnant JB, Ellis BJ, El-Sheikh M. Adaptive patterns of stress responsivity: A preliminary investigation. Developmental Psychology. 2012;48:775–790. doi: 10.1037/a0026519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis AS, Kuzawa CW, Adam EK. Developmental origins of flatter cortisol rhythms: Socioeconomic status and adult cortisol activity. American Journal of Human Biology. 2015;27:458–467. doi: 10.1002/ajhb.22668. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doan SN, Dich N, Evans GW. Childhood cumulative risk and later allostatic load: Mediating role of substance use. Health Psychology. 2014;33:1402–1409. doi: 10.1037/a0034790. [DOI] [PubMed] [Google Scholar]
- Doom JR, Doyle CM, Gunnar MR. Social stress buffering by friends in childhood and adolescence: Effects on HPA and oxytocin activity. Social Neuroscience. 2017;12:8–21. doi: 10.1080/17470919.2016.1149095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Tolep MR, Smith VC, Rose S. Early exposure to parental depression and parenting: Associations with young offspring’s stress physiology and oppositional behavior. Journal of Abnormal Child Psychology. 2013;41:1299–1310. doi: 10.1007/s10802-013-9763-7. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Oldehinkel AJ, Nederhof E. The adaptive calibration model of stress responsivity: An empirical test in the tracking adolescents’ individual lives survey study. Development and Psychopathology. 2016:1–21. doi: 10.1017/S0954579416000985. [DOI] [PubMed] [Google Scholar]
- Engert V, Koester AM, Riepenhausen A, Singer T. Boosting recovery rather than buffering reactivity: Higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology. 2016;74:111–120. doi: 10.1016/j.psyneuen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Van Goozen SHM, Calder AJ, Goodyer IM. Evaluating and reformulating the developmental taxonomic theory of antisocial behaviour. Journal of Child Psychology and Psychiatry. 2013;54:924–940. doi: 10.1111/jcpp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom M, St John AM, Meyer JS, Tarullo AR. Infant hair cortisol: Associations with salivary cortisol and environmental context. Developmental Psychobiology. 2017;59:26–38. doi: 10.1002/dev.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gentile S. Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166. doi: 10.1016/j.neuroscience.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endocrine Regulations. 2008;42:111–119. 18999898. [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gover A, Chau V, Miller SP, Brant R, McFadden DE, Poskitt KJ, Grunau RE. Prenatal and postnatal inflammation in relation to cortisol levels in preterm infants at 18 months corrected age. Journal of Perinatology. 2013;33:647–651. doi: 10.1038/jp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Sandman CA, Wing DA, Dmitrieva J, Davis EP. Prenatal programming of postnatal susceptibility to memory impairments: A developmental double jeopardy. Psychological Science. 2015;26:1054–1062. doi: 10.1177/0956797615580299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/S095457940100311X. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S095457940999037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35:1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2011;23:1149–1165. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Current Opinion in Psychiatry. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Hankin BL, Mackrell SVM, Sheikh HI, Jordan PL, Dozois DJA, Badanes LS. Parental depression and child cognitive vulnerability predict children’s cortisol reactivity. Development and Psychopathology. 2014;26:1445–1460. doi: 10.1017/S0954579414001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. The developmental psychobiology of stress and emotion in childhood. In: Weiner B, Freedheim DK, Lerner RM, editors. Handbook of Psychology. 2. Hoboken, NJ: Wiley; 2013. pp. 121–141. [DOI] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Developmental Psychology. 2015;51:1597–1608. doi: 10.1037/dev0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt LT, Zeiders KH, Ehrlich KB, Adam EK. Positive upshots of cortisol in everyday life. Emotion. 2016;16:431–435. doi: 10.1037/emo0000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Hormones and Behavior. 2017;89:69–85. doi: 10.1016/j.yhbeh.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/S0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Babinski D, Bortner J, Henneberger A, Hinze A, Sapotichne B. Examining the developmental interface of cortisol and depression symptoms in young adolescent girls. Psychoneuroendocrinology. 2013;38:2291–2299. doi: 10.1016/j.psyneuen.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic Republic of Congo. Child Development. 2016;87:61–72. doi: 10.1111/cdev.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Wildeman C, Jonson-Reid M, Drake B. Lifetime prevalence of child maltreatment among US children. American Journal of Public Health. 2017;107:274–280. doi: 10.2105/AJPH.2016.303545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. 0033-3174/99/6102-0154. [DOI] [PubMed] [Google Scholar]
- Koss KJ, Cummings EM, Davies PT, Cicchetti D. Patterns of adolescent regulatory responses during family conflict and mental health trajectories. Journal of Research on Adolescence. 2016 doi: 10.1111/jora.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, George MRW, Cummings EM, Davies PT, El-Sheikh M, Cicchetti D. Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology. 2014;56:836–849. doi: 10.1002/dev.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Hostinar CE, Donzella B, Gunnar MR. Social deprivation and the HPA axis in early development. Psychoneuroendocrinology. 2014;50:1–13. doi: 10.1016/j.psyneuen.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, Gunnar MR. Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology. 2016;66:31–38. doi: 10.1016/j.psyneuen.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biological Psychology. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Repetti RL, Reynolds BM, Robles TF. Change in parent-child conflict and the HPA-axis: Where should we be looking and for how long? Psychoneuroendocrinology. 2016;68:74–81. doi: 10.1016/j.psyneuen.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Kopin IJ. Sympathoadrenal system in stress: Interaction with the hypothalamic-pituitary-adrenocortical system. Annals of the New York Academy of Sciences. 1995;771:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Gilliam KS, Wright DB, Fisher PA. Child anxiety symptoms related to longitudinal cortisol trajectories and acute stress responses: Evidence of developmental stress sensitization. Journal of Abnormal Psychology. 2015;124:68–79. doi: 10.1037/abn0000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Harold GT, Reiss D. Effects of prenatal and postnatal parent depressive symptoms on adopted child HPA regulation: Independent and moderated influences. Developmental Psychology. 2013;49:876–886. doi: 10.1037/a0028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Chen MC, Foland-Ross LC, Burley HW, Gotlib IH. Concordance of mother-daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biological Psychology. 2015;108:98–104. doi: 10.1016/j.biopsycho.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Ordaz SJ, Kircanski K, Singh MK, Gotlib IH. Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology. 2015;124:850–859. doi: 10.1037/abn0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levendosky AA, Bogat GA, Lonstein JS, Martinez-Torteya C, Muzik M, Granger DA, von Eye A. Infant adrenocortical reactivity and behavioral functioning: relation to early exposure to maternal intimate partner violence. Stress. 2016;19:37–44. doi: 10.3109/10253890.2015.1108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly J, McGrath JJ, Gouin JP. Poor sleep as a pathophysiological pathway underlying the association between stressful experiences and the diurnal cortisol profile among children and adolescents. Psychoneuroendocrinology. 2015;57:51–60. doi: 10.1016/j.psyneuen.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;4:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. International Journal of Psychophysiology. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MJ. Neuroendocrinology. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg M, Henry, editors. Williams Textbook of Endocrinology. 13. New York, NY: Elsevier; 2017. pp. 110–176. [Google Scholar]
- Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, Li T. Hair cortisol levels as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescencts with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biological Psychiatry. 2012;72:65–69. doi: 10.1016/j.biopsych.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Mackrell SVM, Sheikh HI, Kotelnikova Y, Kryski KR, Jordan PL, Singh SM, Hayden EP. Child temperament and parental depression predict cortisol reactivity to stress in middle childhood. Journal of Abnormal Psychology. 2014;123:106–116. doi: 10.1037/a0035612. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Developmental Psychobiology. 2015;57:742–768. doi: 10.1002/dev.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Kim HK, Bruce J, Fisher PA. Child diurnal cortisol rhythms, parenting quality, and externalizing behaviors in preadolescence. Psychoneuroendocrinology. 2014;40:170–180. doi: 10.1016/j.psyneuen.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Hayward MD. Poverty and awakening cortisol in adolescence: The importance of timing in early life. Society and Mental Health. 2014;4:21–37. doi: 10.1177/2156869313500278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA. Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science. 2016;25:239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Development and Psychopathology. 2012;24:1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037//0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Paritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. doi: 10.2307/1131829. [DOI] [PubMed] [Google Scholar]
- Nelemans SA, Hale WW, Branje SJT, Van Lier PAC, Jansen LMC, Platje E, Meeus WHJ. Persistent heightened cortisol awakening response and adolescent internalizing symptoms: A 3-year longitudinal community study. Journal of Abnormal Child Psychology. 2014;42:767–777. doi: 10.1007/s10802-013-9820-2. [DOI] [PubMed] [Google Scholar]
- Non AL, Hollister BM, Humphreys KL, Childebayeva A, Esteves K, Zeanah CH, Drury SS. DNA methylation at stress-related genes is associated with exposure to early life institutionalization. American Journal of Physical Anthropology. 2016;161:84–93. doi: 10.1002/ajpa.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, Portilla XA, Ballard PJ. Biological sensitivity to family income: Differential effects on early executive functioning. Child Development. 2016;87:374–384. doi: 10.1111/cdev.12475. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Gudiel H, Córdova-Palomera A, Eixarch E, Deuschle M, Fañanás L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: A meta-analysis. Epigenetics. 2015;10:893–902. doi: 10.1080/15592294.2015.1088630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Ridout KK, Seifer R, Armstrong DA, Marsit CJ, Mcwilliams MA, Tyrka AR. Methylation of the glucocorticoid receptor gene promoter in preschoolers: Links with internalizing behavior problems. Child Development. 2016;87:86–97. doi: 10.1111/cdev.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brain UM, Underhill CM, Hammond GL, Oberlander TF. Prenatal SSRI exposure alters neonatal corticosteroid binding globulin, infant cortisol levels, and emerging HPA function. Psychoneuroendocrinology. 2012;37:1019–1028. doi: 10.1016/j.psyneuen.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Pearson J, Tarabulsy GM, Bussières EL. Foetal programming and cortisol secretion in early childhood: A meta-analysis of different programming variables. Infant Behavior and Development. 2015;40:204–215. doi: 10.1016/j.infbeh.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Peckins MK, Dockray S, Eckenrode JL, Heaton J, Susman EJ. The longitudinal impact of exposure to violence on cortisol reactivity in adolescents. Journal of Adolescent Health. 2012;51:366–372. doi: 10.1016/j.jadohealth.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001 Terror Attacks. Biological Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Platje E, Vermeiren RR, Raine A, Doreleijers A, Keijsers LG, Branje SJ, Jansen LM. A longitudinal biosocial study of cortisol and peer influence on the development of adolescent antisocial behavior. Psychoneuroendocrinology. 2013;38:2770–2779. doi: 10.1016/j.psyneuen.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Quas JA, Yim IS, Oberlander TF, Nordstokke D, Essex MJ, Armstrong JM, Boyce WT. The symphonic structure of childhood stress reactivity: Patterns of sympathetic, parasympathetic, and adrenocortical responses to psychological challenge. Development and Psychopathology. 2014;26:963–982. doi: 10.1017/S0954579414000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development. 2012;36:19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR. Primer on the Autonomic Nervous System. 3. New York, NY: Elsevier; 2012. [Google Scholar]
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences. 2004;1032:258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Development. 2015;86:303–309. doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Frontiers in Neuroendocrinology. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Maslowsky J, Armstrong JM, Burk LR, Essex MJ. Longitudinal associations between diurnal cortisol slope and alcohol use across adolescence: A seven-year prospective study. Psychoneuroendocrinology. 2015;56:23–28. doi: 10.1016/j.psyneuen.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2015;57:688–704. doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Armstrong JM, Klein MH, Essex MJ. Adolescent internalizing symptoms and negative life events: The sensitizing effects of earlier life stress and cortisol. Development and Psychopathology. 2014;26:1411–1422. doi: 10.1017/S0954579414001114. [DOI] [PubMed] [Google Scholar]
- Salis KL, Bernard K, Black SR, Dougherty LR, Klein D. Examining the concurrent and longitudinal relationship between diurnal cortisol rhythms and conduct problems during childhood. Psychoneuroendocrinology. 2016;71:147–154. doi: 10.1016/j.psyneuen.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/S0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2012;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Velders FP, Jaddoe VWV, Hofman A, Verhulst FC, Tiemeier H. The longitudinal association of the diurnal cortisol rhythm with internalizing and externalizing problems in pre-schoolers. The Generation R Study. Psychoneuroendocrinology. 2014;50:118–129. doi: 10.1016/j.psyneuen.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro LA, Baucom BR. Does dampened physiological reactivity protect youth in aggressive family environments? Child Development. 2012;83:821–830. doi: 10.1111/j.1467-8624.2012.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. Journal of Early Adolescence. 2006;26:397–413. doi: 10.1177/0272431606291943. [DOI] [Google Scholar]
- Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, Pollak SD. Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Development. 2014;85:501–512. doi: 10.1111/cdev.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dismukes AR, Marceau K, Ruttle PL, Simmons JG, Han G. A dual-axis approach to understanding neuroendocrine development. Developmental Psychobiology. 2015;57:643–653. doi: 10.1002/dev.21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simmons JG, Byrne ML, Schwartz OS, Whittle SL, Sheeber L, Kaess M, Allen NB. Dual-axis hormonal covariation in adolescence and the moderating influence of prior trauma and aversive maternal parenting. Developmental Psychobiology. 2015;57:670–687. doi: 10.1002/dev.21275. [DOI] [PubMed] [Google Scholar]
- Smider ANA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Granger DA, Eggum ND, Sallquist J, Haugen RG, Hofer C. Individual differences in preschoolers’ salivary cortisol and alpha-amylase reactivity: Relations to temperament and maladjustment. Hormones and Behavior. 2009;56:133–139. doi: 10.1016/j.yhbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Hentges RF, Coe JL. Family instability and children’s effortful control in the context of poverty: Sometimes a bird in the hand is worth two in the bush. Development and Psychopathology. 2016 doi: 10.1017/S0954579416000407. [DOI] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Developmental Psychology. 2012;48:237–249. doi: 10.1037/a0025419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suor JH, Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Tracing differential pathways of risk: Associations among family adversity, cortisol, and cognitive functioning in childhood. Child Development. 2015;86:1142–1158. doi: 10.1111/cdev.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Belden AC, Spitznagel E, Dietrich R, Luby JL. Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and sub-syndromal children. Psychiatry Research. 2013;210:575–583. doi: 10.1016/j.psychres.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, Susman EJ. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. 2014;19:27–37. doi: 10.1177/1077559513520466. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Welch ES, Ridout KK, Price LH, Marsit C, Carpenter LL. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: Associations with early adversity and depressive, anxiety and substance-use disorders. Translational Psychiatry. 2016;6:e848. doi: 10.1038/tp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache A, Noble KG, Blair C. Socioeconomic status, subjective social status, and perceived stress: Associations with stress physiology and executive functioning. Behavioral Medicine. 2015;41:145–154. doi: 10.1080/08964289.2015.1024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos E, Westenberg PM. Two-year stability of individual differences in (para)sympathetic and HPA-axis responses to public speaking in childhood and adolescence. Psychophysiology. 2015;52:316–324. doi: 10.1111/psyp.12337. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Velders FP, Dieleman G, Cents RAM, Bakersman-Kranenburg MJ, Jaddoe VWV, Hofman A, Tiemeier H. Variation in the glucocorticoid receptor gene at rs41423247 moderates the effect of prenatal maternal psychological symptoms on child cortisol reactivity and behavior. Neuropsychopharmacology. 2012;37:2541–2549. doi: 10.1038/npp.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, van Rossum EFC, Koper JW, Raat H, van den Akker ELT. Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. 2016;65:9–14. doi: 10.1016/j.psyneuen.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Von Klitzing K, White LO, Otto Y, Fuchs S, Egger HL, Klein AM. Depressive comorbidity in preschool anxiety disorder. Journal of Child Psychology and Psychiatry. 2014;55:1107–1116. doi: 10.1111/jcpp.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: Implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Waters CS, Goozen S, Phillips R, Swift N, Hurst SL, Mundy L, Hay DF. Infants at familial risk for depression show a distinct pattern of cortisol response to experimental challenge. Journal of Affective Disorders. 2013;150:955–960. doi: 10.1016/j.jad.2013.04.054. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25:341–368. doi: 10.1016/S0193-953X(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yong Ping E, Laplante DP, Elgbeili G, Hillerer KM, Brunet A, O’Hara MW, King S. Prenatal maternal stress predicts stress reactivity at 2 1/2 years of age: The Iowa flood study. Psychoneuroendocrinology. 2015;56:62–78. doi: 10.1016/j.psyneuen.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Kiff CJ, Fisher PA. Understanding the relation of low income to HPA-axis functioning in preschool children: Cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry and Human Development. 2012;43:924–942. doi: 10.1007/s10578-012-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Thompson SF, Kiff CJ. Income, cumulative risk, and longitudinal profiles of hypothalamic-pituitary-adrenal axis activity in preschool-age children. Development and Psychopathology. 2016;28:341–353. doi: 10.1017/S0954579415000474. [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neuroscience and Biobehavioral Reviews. 2015;53:1–24. doi: 10.1016/j.neubiorev.2015.02.015. [DOI] [PubMed] [Google Scholar]