Abstract
Thyroid hormones regulate many aspects of brain development and function, and alterations in the levels of thyroid hormone action lead to abnormal anxiety- and depression-like behaviors. A complement of factors in the brain function independently of circulating levels of hormone to strictly controlled local thyroid hormone signaling. A critical factor is the type 3 deiodinase (DIO3), which is located in neurons and protects the brain from excessive thyroid hormone. Here we examined whether a local increase in brain thyroid hormone action secondary to DIO3 deficiency is of consequence for social behaviors. Although we did not observe alterations in sociability, Dio3 −/− mice of both sexes exhibited a significant increase in aggression-related behaviors and mild deficits in olfactory function. In addition, 85% of Dio3 −/− dams manifested no pup-retrieval behavior and increased aggression towards the newborns. The abnormal social behaviors of Dio3 −/− mice were associated with sexually dimorphic alterations in the physiology of oxytocin (OXT) and arginine vasopressin (AVP), two neuropeptides with important roles in determining social interactions. These alterations included low adult serum levels of OXT and AVP, and an abnormal expression of Oxt, Avp and their receptors in the neonatal and adult hypothalamus. Our results demonstrate that DIO3 is essential for normal aggression and maternal behaviors, and indicate that abnormal local regulation of thyroid hormone action in the brain may contribute to the social deficits associated with neurodevelopmental disorders.
Keywords: Type 3 deiodinase, oxytocin, vasopressin, social behavior, aggression, maternal behavior, thyroid hormone, olfaction, autism spectrum disorders, schizophrenia
Introduction
Thyroid hormones exert broad effects on development and physiology. The two main hormones secreted by the thyroid gland, thyroxine (T4) and 3,5,3’-triiodothyronine (T3) are transported into target cells by specific transporters (Heuer & Visser, 2013, Schweizer & Kohrle, 2013). T4 can be “activated” by intracellular conversion to the most active hormone T3, a process catalyzed by the type 1 and 2 deiodinases (Bianco, 2011, St Germain et al., 2009). Upon binding to its nuclear receptor, which is a transcription factor with DNA binding capacity, T3 will regulate transcription and produce its biological effects (Pascual & Aranda, 2013). Alternatively, both T4 and T3 can be converted into inactive metabolites by the type 3 deiodinase (DIO3), thus limiting their biological action (Hernandez, 2005). It is increasingly appreciated that T3 action in a given tissue is ultimately determined by the coordinated action of the transporters, deiodinases, receptors and cofactors present in a particular tissue and developmental stage (Forrest & Visser, 2013).
Thyroid hormone action is of particular importance for the development and function of the central nervous system (Bernal, 2005) and it influences behavior. Abnormal thyroid hormone states in humans are associated with mood disorders (Chueire et al., 2007, Cleare et al., 1995, Constant et al., 2005, Custro et al., 1994, Sinai et al., 2009) and mouse models have demonstrated that genetic and pharmacological manipulations leading to altered T3 action in the brain modify anxiety- and depression-like behaviors (Bocco et al., 2016, Buras et al., 2014, Darbra et al., 2003, Galton et al., 2007, Ge et al., 2014, Shukla et al., 2010, Stohn et al., 2016, Venero et al., 2005, Yu et al., 2015, Zeng et al., 2007).
Critical to the prevention of excessive thyroid hormone action in the brain is the type 3 deiodinase (DIO3) (Hernandez et al., 2010). DIO3 is highly expressed in the developing and adult brain (Hernandez, 2005) and located predominantly in neurons (Escamez et al., 1999, Tu et al., 1999). In addition, DIO3 expression reaches transient, extremely high levels of expression in the neonatal hypothalamus (Hernandez et al., 2006) and preoptic area (Escamez et al., 1999), regions that are essential for neuroendocrine function, social behaviors and brain sexual differentiation. Mice lacking DIO3 (Dio3−/− mice) exhibit abnormal neuroendocrine function, which includes the abnormal programming of the thyroid axis (Hernandez et al., 2006) and the leptin melanocortin system (Wu et al., 2017). These observations indicate an additional possible role for DIO3 in the development and expression of social behaviors.
While thyroid hormones and their effects on mood disorders have been in recent years, limited information is available about their involvement in social behaviors (Dellovade et al., 1996), including sociability, aggression and maternal behavior. Key factors regulating social behaviors are oxytocin (OXT) and arginine vasopressin (AVP) and they have been studied extensively using different animal models (Bielsky et al., 2005, Bielsky et al., 2004, Bielsky & Young, 2004, Choleris et al., 2003, Crawley et al., 2007, Ferguson et al., 2000, Yu et al., 2016)(Champagne et al., 2001, Pedersen, 1997, Pedersen et al., 2006)(Bosch & Neumann, 2012, Calcagnoli et al., 2014a, Calcagnoli et al., 2014b, Ferris & Potegal, 1988, Pagani et al., 2015). In addition, several studies have shown that thyroid hormones are also implicated in the regulation of those neuropeptides and their signaling (Adan & Burbach, 1992, Adan et al., 1993, Adan et al., 1992, Burbach et al., 1993, Campo Verde Arbocco et al., 2015, Ciosek & Drobnik, 2004, Dellovade et al., 1999, Faustino et al., 2015, Vasudevan et al., 2001a, Vasudevan et al., 2001b). Thus, it is possible that alterations in the determinants of brain thyroid hormone action contribute to social behaviors.
Here we report that the brain thyroid hormone excess that occurs in DIO3-deficiency leads to enhanced aggression-related behaviors in mice of both sexes and to marked deficits in maternal behavior in females. These abnormal phenotypes are concurrent with alterations in the physiology of OXT and AVP systems. Our findings imply that DIO3 modulation of brain T3 action may contribute to the social deficits associated with neurodevelopmental disorders in humans.
Materials and Methods
Animals
Male (C57Bl/6J genetic background) and female (129/SVJ genetic background) mice, that were heterozygous for an inactivating mutation of Dio3 (Hernandez et al., 2006), were bred to generate the Dio3+/+ and Dio3−/− experimental animals, which were littermates in a defined mixed genetic background. Where indicated, certain data were also obtained from mice on a 129/SVJ background. Animals were kept at a 12 h light/dark cycle and fed regular chow ad libitum. 5-day-old (P5) mice (approximately 124 to 130 h after birth) were sacrificed by decapitation, whereas adult animals (4–5 month old) were sacrificed by CO2 asphyxiation. Behavioral tests were performed during the second half of the light cycle. All procedures and behavioral tests were approved by the Institutional Animal Care and Use Committees of the Geisel School of Medicine at Dartmouth and of the Maine Medical Center Research Institute. The animal work was performed following the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals. All behavioral tests were performed during the light cycle.
Quantitative real time RT-PCR
The hypothalamus was harvested and frozen on dry ice, and total RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA). Total RNA (1 µg) was reverse transcribed with M-MLV reverse transcriptase in the presence of random hexamers at 37°C for 1 h. Reverse transcription reactions were diluted appropriately and aliquots were used as templates in duplicate real-time PCR reactions for each of the selected genes. Reactions were run in a 7300 RT PCR System (Applied Biosystems) using SYBR Select Master Mix from Applied Biosystems (Foster City, CA). Real time PCR reactions underwent an initial 10 min denaturing step, followed by 36 cycles of a denaturing step (94°C for 30 s) and an annealing/extension step (60°C for 1 min). The sequence of the primers used was (5’ to 3’): Avp, CAGGATGCTCAACACTACGC and CAGAATCCACGGACTCCCG; Avpr1a, TGTGGTCAGTCTG-GGATACC and GGGAAGCTCTGGACACAATC; Gapdh, AGGAGCGAGACCCCACTAAC and CGGAGATGATGACCCTTTTG; Hr, AGCACTGTGTGGCATGTGTT and AACCCTGCATCC-AAGTAGCA; Oxt, TGGCTTAC-TGGCTCTGACCT and GGCAGGTAGTTCTCCTCCTG; Oxtr, TTCTTCGTGCAGATGTGGAG and ACGAGTTCGTGGAAGAGATG; Rn18s, GGAGTATGG-TTGCAAAGCTG and TCGCTCCACCAACTAAGAAC. Expression data were read in an internal standard curve prepared by subsequent dilutions of a mix of aliquots of reverse transcription reactions from the samples in a given experiment. Expression of specific genes was corrected by the expression of the ribosomal Rn18s gene in P5 samples and by Gapdh mRNA expression in adult samples. Data were obtained from 5 to 10 animals per experimental group and are expressed in arbitrary units.
Hormone determinations
Blood was collected from the vena cava in the presence of aprotinin, centrifuged for 10 min, and the serum was stored at −80°C. Serum levels of OXT and AVP were determined using EIA kits from Assay Designs (Ann Arbor, MI) according to the manufacturer’s instructions. Serum levels of thyroid hormones were determined using the commercial Coat-a-Count Total T3 and Total T4 radioimmunoassay kits from Siemens USA (New York, NY). 6 to 10 mice per experimental group were used.
Histology
Staining for OXT and AVP was performed in sections from formalin-fixed, paraffin-embedded brain tissue by the Pathology Research Service at the Geisel School of Medicine at Dartmouth following standard procedures. We used rabbit antisera for rat neurophysin-OXT (PP 76) and neurophysin-AVP (HK 76) at 1:2000 dilutions. The antisera were generated and kindly provided by W. North (North et al., 1983). We utilized the brain coronal sections of two mice per genotype to quantify the number of OXT and AVP neurons. This was performed by a person blind to the genotype in one of every four hypothalamic sections (7µM thick) comprising the paraventricular and supraoptic nuclei.
Sociability and social novelty
Sociability and social novelty were assessed using a three-chambered box similar to that previously described (Moy et al., 2004). At weaning, experimental animals of the same litter were housed together in groups of 3 or 4 of the same sex and regardless of the genotype. Dio3−/− animals exhibit increased aggressive behavior after puberty. To decrease stress and risk of injury the mice were separated at 2 months of age and caged individually until the test at 4 months of age. The test apparatus was a rectangular open box (60 × 30 cm) made of opaque, white plastic material. Inside the box, two walls 30 cm in height divided it into three compartments of the same size that were connected by centered openings. The test comprised of three consecutive trials of 10 minutes each. At the beginning of each test the mouse was placed in the center box of the apparatus and allowed to explore it freely, and in between trials the mouse was returned to its home cage. The first trial allowed the mouse to adapt to the three-chambered box. Time spent in the individual chambers during the initial 10 min adaption trial was evaluated to exclude any potential biases that might interfere with the actual testing. Overall, the animals did not show any preferences for a particular chamber (data not shown). For the second trial, a transparent, methacrylate cylinder with multiple 1.5 cm diameter holes was placed in opposite corners of each the left and right chamber. One of the cylinders contained an unfamiliar mouse (stranger) of the same sex, age, and genotype like the test mouse, while the second one remained empty. The cylinder permitted the animals to sniff each other, but otherwise restricted physical contact. For the third trail a second stranger was placed in the empty cylinder, while the first, now familiar stranger, remained in the other cylinder. Trials were videotaped using the Videomex automated system (Columbus Instruments, OH) which allows for automatic recording of various parameters in custom-designed areas, including distance traveled, time spent in each chamber or area, number of exits/entrances from/in any given area; and time during which the mouse remained still. At the end of the trials the mouse was returned to its home cage permanently and the three-chambered box was wiped clean. The position for the first stranger was alternated between tests. In these tests, we used 11 to 16 mice per experimental group.
Maternal behavior and nest building
Females (Dio3+/+ and Dio3−/−) were weaned and kept in groups of two to four animals per cage. At two months of age, they were mated with a wild type male. Males were removed 2–3 days prior to expected birth, and the pregnant female was provided with a clean cage and a new nestlet (Ancare). The maternal behavior test was performed 24 to 40 h following parturition. The mother was carefully removed from the cage, and a picture of the cage was taken for later assessment of nest-building and pup distribution. Then the pups were scattered throughout the cage leaving the nest otherwise undisturbed. The mother was returned to the cage and her behavior recorded for 8 minutes. An observer blinded to the genotypes analyzed the videos for maternal behavior including pup retrieval and crouching over gathered pups and scored the nest building as described elsewhere (Deacon, 2006). The rating scale ranged from 0 to 5, with 0 being no nest and 5 being a nearly perfect nest with high walls using more than 90% of the nesting material. Females were mated again after successfully weaning their first litter and underwent the same testing as described above. Because adult Dio3−/− mice manifest a mild hypothyroidism (Hernandez et al., 2006), a group of six wild type females was rendered hypothyroid by treatment with 0.04% potassium perchlorate in the drinking water during pregnancy and postpartum. 26 control and 27 mutant females were analyzed.
Resident-intruder paradigm
Single housed animals were moved to a clean cage 24–48 h prior to testing. Mice were moved to the test room at least 1 h prior to testing to allow them to acclimate. Food and water was removed before the test. An unfamiliar mouse (intruder) of the same sex and approximately same age and size was placed in the home cage of the test animal, and their behavior was recorded for 15 min. Since this test investigates territorial behavior, threat and aggression behaviors were expected. In case of extremely aggressive behavior leading to injuries of either one of the mice, the test was terminated and the animals were separated. The first 10 min of the videos were scored offline by a trained observer blinded to the genotypes using the Observer XT 12 software from Noldus (Leesburg, VA). The behaviors scored included aggressive and threat behavior, social exploration and approach. Threat behaviors included tail rattling, thrust, lateral threat, mounting, crawling under and pushing past the intruder (Koolhaas et al., 2013). If an animal did not exhibit a specific behavior the latency was scored as 600 s. Our analysis only focused on the resident mouse. However, three Dio3+/+ male mice were excluded from the study because they were attacked by the intruder first, an event that leads to drastically changed behavior and predominant avoidance behavior in the resident mouse. Allo-grooming was not assessed as it was almost never observed during the test period. Data from 10 to 13 mice per experimental group are presented.
Olfactory Function
Two tests were used to assess olfactory function. In the first test the experimental animals were fasted for 24 h, before testing. A food pellet was buried in a clean cage underneath 3 cm deep bedding material. The test animal was placed in the cage and the latency until finding the food was measured. The test was terminated when the mouse found the food or after 5 min. The second test was an olfactory habituation test similar to that described (Ferguson et al., 2000). It was performed in the home cage of single-caged animals. In brief, a small cassette typically used for tissue histology (histosette, Fisher, Waltham, Massachusetts) was added to the cage 48 h before the test to allow the animal to become familiar with the object. The test involved six 1-minute trials with 8–12 minutes between trials. For each trial, a cotton tip wetted with 10 µl of a commercial flavor (e.g. from McCormick’s, local supermarket) was put inside the cassette. The same flavor (vanilla) was used in the first five trials, and a different one (maple) was used in the sixth one. The time the mouse spent smelling the cassette during each trial was recorded. 11 to 16 mice per experimental group were submitted to this test.
Statistical analysis
Statistical analysis was performed using Student’s t-test (paired or unpaired), chi-square test, one-way ANOVA followed by Tukey’s test or two-way ANOVA followed by Sidak’s test, using the statistical tools of GraphPad Prism 6 (GraphPad Software, Inc.). Statistical significance was defined as P<0.05. In figures, *, ** and *** indicate P<0.05, 0.01 and 0.001, respectively. In Figure 4, ; #, ###, indicate P<0.05, P<0.001, respectively, trial 5 versus trial 1; †, indicate P<0.05 trial 6 versus trial 5.
Figure 4.
Olfactory function in Dio3−/− mice. Latency to find buried food after 24h fasting (A). Time spent sniffing a newly introduced odor in females (B) and males (C). Vanilla odor was used in trial 1–5, maple odor was introduced in trial 6. Data are shown as mean ± SEM with n = 11–16 mice per experimental group.
Results
Sociability and social novelty in Dio3−/− mice
We tested whether the altered thyroid hormone action in the brain of Dio3−/− mice is associated with changes in social behavior. In the three-chamber box paradigm (see Methods), both Dio3+/+ and Dio3−/− mice spent similar time (55–60%) in the chamber with the first stranger. This time was higher than that spent in the chamber with no stranger (F(1, 104)=346.1, P<0.001) (Fig. 1A), suggesting unchanged social interest in Dio3−/− mice. All animals moved significantly slower in the interaction zone of the chamber with the cylinder containing the stranger compared to the empty one, suggesting a social interest towards the stranger mouse that was similar in both genotypes and sexes (F(1, 104)=52.57, P<0.001) (Fig. 1C). The Dio3−/− mice moved faster than the wild type when comparing speeds for the same areas (Fig. 1C), however, this only reached significance in the males in the empty chamber (3, 104)=8.183, P<0.001. In the last trial of the test, Dio3+/+ and Dio3−/− mice of both sexes spent similar amounts of time in the chambers with the first and the second strangers (Fig. 1B). The Dio3−/− male mice moved significantly faster than Dio3+/+ mice in both the areas of interaction areas with both strangers (Fig. 1D). They moved faster, but it only reached significance in the males (F(3, 103)=10.08, P<0.001). This phenotype was not significant in Dio3−/− females.
Figure 1.
Sociability and social novelty in Dio3−/− mice. Sociability is represented as time spent in the chambers housing either a cylinder containing an unknown mouse (stranger 1) or an empty cylinder (A). Social novelty is represented as time spent in the chambers containing either the now familiar mouse (stranger 1) or a new unknown mouse (stranger 2) (B). Stranger 1 and stranger 2 were age and gender matched. Speeds were measured in the interacting zones (square area surrounding the cylinder by approximately 5 cm distance) during the sociability (C) and the social novelty (D) trials. Data are shown as mean ± SEM with n = 11 to 16 mice per experimental group.
Elevated threat and aggressive behavior in D3 deficient mice
We then investigated the aggression-related behaviors of DIO3-deficient mice of both sexes when confronted with an intruder in their home cage. After the intruder was placed in the cage, Dio3+/+ female residents spent more than half the time following it through the cage. This locomotion was often accompanied by sniffing. In the Dio3−/− females this time was much shorter (Fig. 2A) (F(1, 44)=49.35, P<0.001). This behavior was clearly sexually dimorphic since the Dio3+/+ males spent much less time following the intruder. This time was further decreased in Dio3−/− males, but did not reach significance (Fig. 2A). Overall female Dio3+/+ and Dio3−/− mice spent about the same time with social investigation (nose, anogenital and body sniffing). Compared to the females, Dio3+/+ males spent more than twice as much time to investigate the intruder. However, this time was reduced 65% in Dio3−/− males (F(1, 44)=23.28, P<0.001; Fig. 2B). While Dio3−/− females investigated the intruder more often than Dio3+/+ females, we observed the opposite in the males. The frequency of social investigation approaches in Dio3−/− males was half of that in Dio3+/+ males (F(1, 44)=67.51, P <0.001; Fig. 2C). While only 54% of the female and 67% of the male wild type mice tested showed any type of threat behavior, all of the Dio3−/− animals of both sexes exhibited some type of threat behaviors (females: F(1, 23)=6.24, P <0.05; males: F(1, 25)=5.16, P <0.05; Fig. 2D). The total duration of threat behavior was similar in Dio3+/+ and Dio3−/− females, and trended higher in Dio3−/− males compared to Dio3+/+ males (Fig. 2E). The latency for threat behavior in Dio3−/− mice of both sexes was significantly lower than in Dio3+/+ mice (F(1, 44)=24.48, P<0.001; Fig. 2F). Threat behavior did not always escalate into aggressive behavior such as boxing, upright posture, pinning, biting, attacking or fighting. While 40% Dio3 −/− females displayed aggressive behavior, none of the Dio3+/+ females crossed that threshold (F(1, 23)=6.29, P <0.05; Fig. 2G). Most males of either genotype engaged in some form of aggressive behavior, with a slightly higher occurrence in Dio3−/− mice (Fig. 5G). The total duration of the aggressive behavior in Dio3+/+ and Dio3−/− males was not significantly different, and comparable to that observed for Dio3−/− females (Fig. 2H). In male Dio3−/− mice the latency to display aggressive behaviors was markedly lower than in Dio3+/+ mice (Fig. 2I) (F(1, 44)=22.57, P<0.001). Latency for aggressive behavior was also much lower in Dio3−/− females than in Dio3+/+ females, which did not exhibit any aggressive behavior during the length of the test. Interestingly, the latency for aggressive behavior in Dio3−/− females was comparable to that in Dio3+/+ males (Fig. 2I). These data indicate a disposition to as well as an increase in threat and aggressive behaviors in mice with DIO3 deficiency.
Figure 2.
Threat and aggressive behavior in Dio3−/− mice. Total durations of social exploration (A), following the intruder (C), threat (E) and aggressive behavior as well as social frequency data (B), occurrence of threat (D) and aggressive (G) behavior and their latencies (F&I) were measured in resident-intruder tests. Dio3+/+ and Dio3−/− animals served as residents and the intruder mice were age, size and gender matched. Scored threat behavior includes tail rattling, thrust, lateral threat, mounting, crawling under and pushing past the intruder. Data are shown as mean ± SEM with n = 10–13 mice per experimental group.
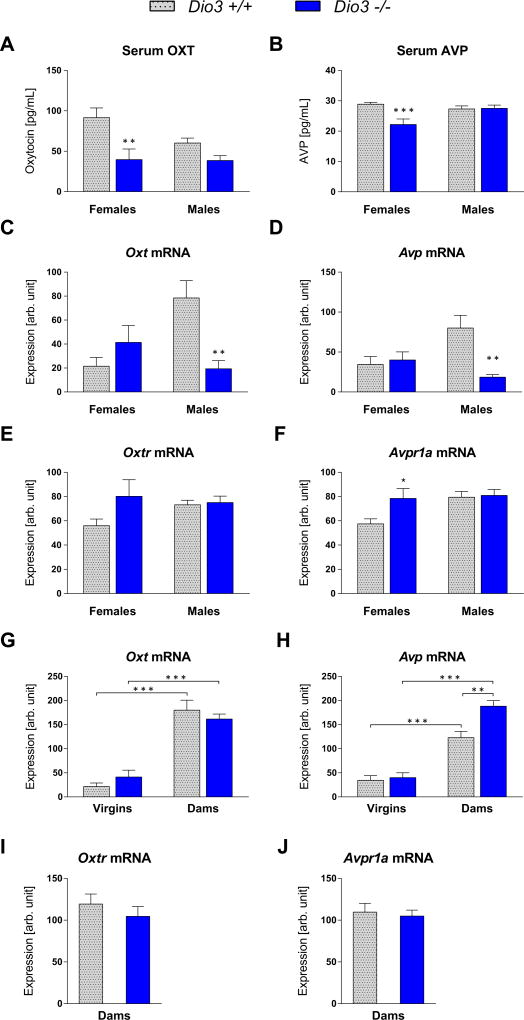
Figure 5.
Oxytocin and vasopressin physiology in adult Dio3−/− mice. Serum levels of OXT and AVP (A & B). Hypothalamic expression of Oxt, Avp, Oxtr and Avpr1a in adults (C–F) and in dams (G–J). Serum and tissue were collected at three months of age. Data are shown as mean ± SEM with n = 7 to 10 (A&B) or 5 to 7 (C–J) mice per experimental group.
Maternal behavior is strongly reduced in Dio3−/− females
We evaluated the nest building and maternal behavior of Dio3−/− females. The results are summarized in Table 1. Nests of Dio3−/− dams were flatter and more spread out than those of the wild types for the first litter (t(50)=3.117, P=0.01), but not for the second (Table 1 and Supplementary Fig. 1A). While 92% of Dio3+/+ females retrieved their pups, only 15% (first litter) and 22% (second litter) of Dio3−/− females demonstrated this behavior (first litter: F(1, 53)= 31.92, P <0.001; second litter: F(1, 49)= 25.16, P <0.001). Litters from Dio3−/− dams showing no pup retrieval behavior did not survive. The latency to retrieve the pups was very short in wild type mothers and did not vary between 1st and 2nd litters, while it was much longer in the Dio3−/− dams that actually exhibited this behavior (t(26)=4.105, P<0.001). With the first litter, the total time needed to retrieve all pups was notably longer in Dio3−/− females, but was not different between genotypes with the second litter. Independent of retrieval behavior, Dio3−/− females spent less time interacting with the pups or crouching over them than Dio3+/+ females. Furthermore, Dio3−/− dams exhibited increased aggressive behavior towards their pups, which was observed as trampling, pushing and kicking their offspring (second litter: F(1, 49)= 6.32, P <0.05). The litters born to Dio3+/+ dams were of the same genotype, while litters born to Dio3−/− mothers were heterozygous for the Dio3 allele. Nonetheless, we have not observed an impaired viability for heterozygous neonates, suggesting there are no phenotypic abnormalities in these pups that might trigger abnormal maternal behavior. Overall, the loss of DIO3 activity led to severe disruption of maternal behavior resulting in highly increased pup mortality. The hypothalamus of Dio3−/− postpartum females did not show changes in the expression of thyroid-hormone-responsive genes, or in the expression of CD38, which is involved in maternal behavior (Higashida et al., 2011, Jin et al., 2007) (Supplementary Fig. 1B–F).
Table 1.
Nest building and maternal behavior in Dio3+/+ and Dio3−/− females.
| 1st Litter | Mother’s age [days] |
Litter size | Nest score | Mothers exhibiting pup retrieval behavior |
* Latency for pup retrieval [s] |
* Time to retrieve all pups [s] |
Time spent interacting with pups [s] |
Time spent crouching over pups [s] |
Mothers exhibiting aggressive behavior towards pups |
|---|---|---|---|---|---|---|---|---|---|
| Dio3 +/+ (n=26) | 94.5 ± 2.1 | 8.8 ± 3.6 | 3.58 ± 0.09 | 24 (92%) | 15.3 ± 2.5 | 132 ± 22 | 45.5 ± 15 | 31.7 ± 11.7 | 1 (4%) |
| Dio3 −/− (n=27) | 92.6 ± 3.1 | 9.0 ± 1.9 | 3.21 ± 0.08 | 4 (15%) | 165.5 ± 98.3 | 182 ± 55 | 13.2 ± 6.6 | 9.3 ± 6.4 | 5 (19%) |
| p-value | NS | NS | < 0.01 | <0.001 | < 0.01 | NS | 0.0505 | 0.0974 | NS |
| 2nd Litter | |||||||||
| Dio3 +/+ (n=26) | 148.0 ± 3.9 | 9.7 ± 2.2 | 3.98 ± 0.09 | 24 (92%) | 15.6 ± 2.6 | 118 ± 15 | 34.8 ± 4.9 | 29.7 ± 5.0 | 1 (4%) |
| Dio3 −/− (n=23) | 166.3 ± 5.7 | 8.8 ± 3.0 | 3.76 ± 0.10 | 5 (22%) | 174 ± 56 | 108 ± 26 | 15.3 ± 11.4 | 12.4 ± 10.9 | 7 (30%) |
| p-value | < 0.05 | NS | NS | <0.001 | < 0.01 | NS | NS | NS | <0.05 |
| Hypo-thyroid (n=6) | 115.5 ± 4.9 | 10.3 ± 1.5 | 4.17 ± 0.11 | 6 (100%) | 26.7 ± 10.6 | 89 ± 13 | 77.2 ± 22.2 | 70.1 ± 25.0 | 0 (0%) |
Parameters of pup retrieval behavior refer only to those females actually exhibiting this behavior.
As adult Dio3−/− mice manifest a mild hypothyroidism (F(1, 27)18.63, P<0.001; Fig. 3A & Fig. 3B) (Hernandez et al., 2006), a group of Dio3+/+ mothers were treated during pregnancy and postpartum with 0.04% potassium perchlorate in the drinking water to render them moderately hypothyroid. The maternal behavior of these mothers was similar to the one observed in untreated Dio3+/+ females (Table 1, bottom), even though their hypothyroidism was more severe than that in Dio3−/− mice (Serum T4: F(2, 19) = 91.30, P<0.001; Fig. 3C). The nest building score of these hypothyroid mothers was not different from that in untreated Dio3+/+ females (Table 1). This indicates that the reduced maternal behavior in Dio3−/− females is not the result of their peripheral hypothyroidism.
Figure 3.
Serum thyroid hormone levels. Serum levels of T4 and T3 adult mice (A & B). Serum levels of T and T3 in dams (C&D). Data are shown as mean ± SEM with n = 6 to 8 (A&B) mice per experimental group.
There were no differences in the age of the mothers at the time their first litter was delivered. However, Dio3−/− dams were significantly older when giving birth for the second time (t(47)=2.701, P<0.01; Table 1). We did not observe variations in litter sizes of first or second litters.
Olfactory function
Olfaction is an important component of social behaviors. We thus evaluated olfaction in Dio3−/− mice. Female Dio3−/− mice took slightly longer than Dio3+/+ females to find buried food after fasting for 24 h, although the difference did not reach statistical significance (Fig. 4A). This parameter was unchanged in males. However, an olfactory habituation test revealed that female Dio3−/− mice exhibited overall lower interest than Dio3+/+ females in investigating a cassette with a particular odor during the first five trial trials (Fig. 4B), although the interest diminished similarly with each trial for both genotypes. When introduced to a different odor in the last trial, the interest was significantly spiked in Dio3+/+ females, but no spiked interest was observed in Dio3−/− females (t(30)=2.105; P<0.05; Fig. 4B). We observed no significant differences between Dio3+/+ and Dio3−/− males during the habituation trials of the test. While Dio3+/+ mice experience a surge in interest for the new odor in the last trial, Dio3 −/− males also seem to display reduced interest when presented in the last trial with a novel odor (Fig. 4C). This result, however, did not reach statistical significance. Overall, these results suggest some degree of olfactory impairment as a result of DIO3 deficiency, particularly in females.
Oxytocin and arginine vasopressin physiology in D3 deficient adult mice
To investigate potential mechanisms by which an excess of thyroid hormone in the brain leads to aberrant social behaviors, we examined several molecular parameters related to the OXT and AVP systems, which are regulated by thyroid hormone and can influence maternal behavior (Bosch & Neumann, 2012) and aggression (Bosch & Neumann, 2010, Bosch & Neumann, 2012, Ferris & Potegal, 1988, Ne'eman et al., 2016, Winslow et al., 2000). In adult mice, serum levels of OXT and AVP were significantly decreased in Dio3−/− females (Fig. 5A&B) when compared to those in Dio3+/+ females (F(1, 31)=13.97, P<0.001 and F(1, 31)=8.545, P<0.01 for OXT and AVP, respectively). In Dio3−/− adult males we observed no changes in serum AVP, while serum OXT trended lower (Fig. 5 A&B). We observed a similar pattern in serum OXT in mice on a 129/SVJ genetic background, with values trending lower in adult Dio3 −/− males and being significantly lower in Dio3−/− females (Supplementary Fig. 2), when compared with those in Dio3+/+ mice (F(1, 19)=14.25, P<0.01). We then examined the hypothalamic mRNA expression of both neuropeptides and their respective receptors. While Dio3−/− female mice did not exhibit changes in Oxt and Avp expression, Dio3−/− males showed a 75% reduction in Oxt and Avp expression (F(1, 20)=11.79, P<0.01 and F(1, 21)=5.878, P<0.05 for Oxt and Avp, respectively; Fig. 5C &D). The expression of both neuropeptide receptors Oxtr and Avpr1a was increased in Dio3−/− females compared to Dio3+/+ females, but only reached significance for Avpr1a (F(1, 22)=4.332, P<0.05). The expression of both receptors remained unchanged in males (Fig. 5E&F). Immunohistological staining of OXT and AVP neurons in the paraventricular and supraoptic nuclei of the hypothalamus showed no significant differences in the number of OXT and AVP neurons between both genotypes (Supplementary Fig. 3, data for supraoptic nuclei not shown). Total number of neurons per average section comprising the paraventricular and supraoptic nuclei was 29.19±0.78 OXT neurons for Dio3+/+ mice versus 29.40±2.88 for Dio3−/− mice, and 56.02±6.95 AVP neurons for Dio3+/+ mice versus 65.25±0.48 for Dio3−/− mice. We observed no differences in OXT and AVP neuron number when the data was segregated by sex or hypothalamic nucleus (Data not shown).
Maternity resulted in a marked increase in the hypothalamic mRNA expression of Oxt and Avp in females of both genotypes. At 24–48 h postpartum, dams of both genotypes exhibited marked increases in the hypothalamic expression of Oxt and Avp (F(1, 21)=89.57, P<0.001 and F(1, 22)=106.0, P<0.001; Fig. 5G&H). While the maternity-associated increase in Oxt expression was similar in females of either genotype, Avp expression was upregulated to a significantly greater extent in Dio3−/− females than in Dio3+/+ females (F(1, 22)=9.448, P<0.01; Fig. 5H). We observed no changes in the hypothalamic expression of Oxtr and Avpr1a in Dio3−/− dams (Fig. 5I&J).
Neonatal expression of Oxt and Avp
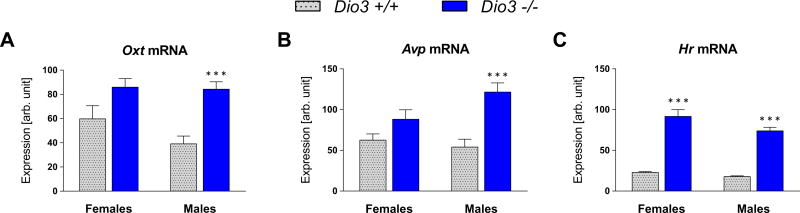
Given that gender-specific abnormalities in neuropeptide systems may result from abnormal OXT exposure during development (Yamamoto et al., 2004), we also examined the effects of DIO3-deficiency on Oxt and Avp hypothalamic expression at postnatal day 5 (P5), when Dio3−/− mice experience both brain and systemic thyrotoxicosis (Hernandez et al., 2006). We found the expression of both Oxt and Avp was markedly elevated in Dio3−/− male neonates (F(1, 29)=21.67, P<0.001, and F(1, 29)=18.18, P<0.001, respectively for Oxt and Avp), while in Dio3−/− females the elevation was more modest and did not reach statistical significance (Fig. 6 A&B). Consistent with previous results (Hernandez et al. 2006), the expression of hairless (Hr), a well-established T3-dependent gene, was similarly and robustly elevated (Fig. 6C) in Dio3−/− mice of both sexes (F(1, 30)=203.2, P<0.001), indicating increased levels of T3 signaling in the hypothalamus of Dio3−/− neonates.
Figure 6.
Expression of oxytocin and vasopressin in neonatal Dio3−/− mice. Hypothalamic expression of Oxt and Avp and the T3-dependent gene Hr (hairless)(A–C). Tissue was collected from 5-day old mice. Data are shown as mean ± SEM with n = 6 to 10 mice per experimental group.
Discussion
We have previously shown that DIO3 is critical to maintain an adequate level of thyroid hormone signaling across brain regions and developmental stages (Hernandez et al., 2006, Hernandez et al., 2010). The pronounced peak of DIO3 expression in the rodent hypothalamus and preoptic area (Escamez et al., 1999) during neonatal life suggests that limiting thyroid hormone action is important for the development of these brain regions and for their functions later in life, including the regulation of social behaviors.
Although we observed no aberrant social interest when using the three-chamber box paradigm, Dio3−/− mice of both sexes exhibit significantly augmented threat and aggressive behaviors. This occurs relative to the different baselines of aggressive behaviors normally observed in mice of different sex in the intruder paradigm test. In addition, female Dio3−/− mice manifest very poor maternal behavior. Of particular relevance to the poor maternal behavior of Dio3−/− dams is the observation of olfactory deficits in Dio3−/− females. This abnormal phenotype, in addition to the previously described impaired hearing (Ng et al., 2009) and decreased anxiety-like behavior (Stohn et al., 2016), may contribute to the deficits in maternal behavior and possibly to other social behavior phenotypes (Bosch & Neumann, 2012, Weaver et al., 2006).
Our observation that dams rendered hypothyroid exhibit normal maternal behavior suggests that the social behavior phenotype of Dio3−/− mice originates from an excess of T3 action in the brain. We have shown that this excess of T3 signaling occurs in a dynamic manner both during development and in adult age, and affects most brain regions (Hernandez et al., 2010). Thus, abnormalities in most neural structures influencing social behavior may be mediating the abnormal social behavior of Dio3−/− mice. In this regard, abnormalities in social behavior have been reported in a rat model both in the context of altered hippocampal Dio3 expression and in association with functional deficits in the amygdala due to developmental overexposure to thyroid hormone (Shukla et al., 2010, Sittig et al., 2011). Thus, it is possible that abnormalities in these brain structures also contribute to the behavioral phenotype of the Dio3−/− mouse.
Despite the potential involvement of other brain regions, published data support the hypothesis that potential disruptions in OXT and AVP systems might be partly mediating the abnormal social behaviors of Dio3−/− mice. These data include the influence of OXT and AVP on maternal behavior and aggression (Bosch & Neumann, 2012, Champagne et al., 2001, Pagani et al., 2015, Pedersen, 1997, Pedersen et al., 2006), and the regulation by thyroid hormone of the expression of Oxt and Avp and of components of their signaling pathways (Adan & Burbach, 1992, Adan et al., 1992). This hypothesis is supported by our results showing that Dio3−/− mice exhibit abnormal OXT and AVP physiology. The expression changes observed for Oxt and Avp are consistent with the up-regulation of these genes by thyroid hormone (Adan et al., 1992), and with the changes in thyroid hormone status we have previously described for the Dio3−/− hypothalamus (Hernandez et al., 2006, Hernandez et al., 2010). Thus, neuropeptide gene expression is elevated in neonates when the hypothalamus is hyperthyroid, and decreased or unchanged in adults when the hypothalamus is moderately hypothyroid (Hernandez et al., 2006). Although the thyroid state of the hypothalamus may explain the abnormal pattern of neuropeptide gene expression of Dio3−/− mice, the latter may also be the consequence of developmental effects. In this regard, it has been described that animals exposed to excessive amounts of these neuropeptides after birth develop an abnormal number of neuropeptide neurons later in neonatal life (Yamamoto et al., 2004), a finding that suggests that increased neuropeptide expression in Dio3−/− newborns may alter the later maturation of these systems. However, our data does not reveal any gross abnormalities in the number of OXT or AVP neurons in these animals.
The aberrant expression of Oxt and Avp in Dio3−/− mice is associated with decreased serum levels of these neuropeptides. This association is markedly sexually dimorphic. Oxt and Avp gene expression is not significantly affected in adult Dio3 −/− females, but they exhibit significant decreases in the serum levels of both neuropeptides. In contrast, Oxt and Avp expression is markedly deficient in Dio3−/− males, but serum levels of the neuropeptides are not significantly affected. The lack of correlation between gene expression and serum neuropeptide levels suggest that DIO3 deficiency affects the processing, secretion or clearance of these neuropeptides, enhancing the serum levels in males and diminishing them in females.
The elevated expression of Avp in Dio3−/− dams may reflect a different sensitivity of the gene to thyroid hormone or a different degree of cross-talk with estrogen (Adan & Burbach, 1992), and may also contribute to the deficient maternal behavior (Bosch & Neumann, 2012). As OXT is involved in the regulation of olfactory function (Oettl et al., 2016), the reduced olfaction of Dio3−/− mice may be secondary to abnormal OXT action, but also the result of direct thyroid hormone excess in neural tissue involved in olfactory function. Olfactory deficits may also stem from unidentified abnormalities in the nasal/olfactory epithelium. Future studies will need to address that issue. Interestingly, olfactory deficiencies have been described in ASD (Bennetto et al., 2007) and schizophrenia (Strauss et al., 2015b, Woolley et al., 2015).
The molecular and behavioral phenotypes observed in Dio3−/− mice are often sexually dimorphic. This suggests that the underlying mechanisms partially involve the disruption of sex steroid hormone action in the brain. Regarding the OXT and AVP systems, this possibility is supported by previous observations showing that thyroid hormone and estrogen compete for regulatory elements in the promoter of Oxt, Avp and Oxtr (Adan & Burbach, 1992, Vasudevan et al., 2001a) and by the fact that critical processes of brain sexual differentiation occur in the hypothalamus as a result of a neonatal surge in sex steroid hormone (Amateau et al., 2004). Thus, the thyroid hormone excess in the brain of DIO3-deficient mice may interfere with normal estrogen regulation of the above genes or other estrogen targets. It may also disrupt brain sexual differentiation during development and sexually-dimorphic functions of the adult brain. In addition, the lack of different thyroid hormone receptors influences estrogen-dependent sexual behavior in female mice (Dellovade et al., 2000). These previously reported observations demonstrate the existence of significant cross-talk in neural tissue between estrogen and thyroid hormone, and suggest that thyroid hormone may influence the normal establishment of sex-specific molecular and functional characteristics in the brain. Therefore, our observations of sexually-dimorphic alterations in molecular and behavioral endpoints of Dio3−/− mice may reflect thyroid hormone interfering with multiple estrogen-dependent sexual dimorphisms in the hypothalamus and other brain regions.
In summary, our results demonstrate that loss of DIO3 function leads to abnormal social behaviors, which may be partially the result of concurrent alterations in neuropeptide physiology. Future studies are needed to determine whether those abnormalities have a developmental origin and to identify the components of the central nervous system that are contributing to them. Our findings raise the possibility that enhanced thyroid hormone action in the central nervous system during development or in adulthood is involved in the etiology of disorders characterized by social deficits, including ASD, schizophrenia and depression (Domes et al., 2016, Miller et al., 2013, Modahl et al., 1998, Neumann & Landgraf, 2012, Parker et al., 2014, Sasayama et al., 2012, Strauss et al., 2015a, Thompson et al., 2014, Uhrig et al., 2016, Wu et al., 2005, Zhang et al., 2016). Our work further underscores the potential importance of the thyroid hormone state of the brain, and not necessarily the circulating levels of thyroid hormone, when evaluating psychiatric conditions.
Supplementary Material
Acknowledgments
We are grateful to William North for the oxytocin and vasopressin rabbit antisera and to Christine Duarte for advice on statistical analysis. This work was supported by grants MH083220, DK095908 and MH096050 from the National Institute of Mental Health and from the National Institute of Diabetes, Digestive and Kidney Disease. Our studies used the CORE Facilities at Maine Medical Center Research Institute that are supported by National Institutes of Health COBRE grants P30GM103392 and P30GM106391 (R. Friesel and D. Wojchowski, principal investigators). Daniela López-Espíndola was partially supported by the Escuela de Tecnología Médica, Universidad de Valparaíso (Chile).
Footnotes
The authors declare no conflict of interest.
References
- Adan RA, Burbach JP. Regulation of vasopressin and oxytocin gene expression by estrogen and thyroid hormone. Progress in Brain Research. 1992;92:127–136. [PubMed] [Google Scholar]
- Adan RA, Cox JJ, Beischlag TV, Burbach JP. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Molecular Endocrinology. 1993;7:47–57. doi: 10.1210/mend.7.1.8383287. [DOI] [PubMed] [Google Scholar]
- Adan RA, Cox JJ, van Kats JP, Burbach JP. Thyroid hormone regulates the oxytocin gene. Journal of Biological Chemistry. 1992;267:3771–3777. [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Kuschner ES, Hyman SL. Olfaction and taste processing in autism. Biological Psychiatry. 62(9):1015-21, 2007 Nov 1. 2007;62:1015–1021. doi: 10.1016/j.biopsych.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormones and brain development. Vitamins & Hormones. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- Bianco AC. Minireview: cracking the metabolic code for thyroid hormone signaling. Endocrinology. 2011;152:3306–3311. doi: 10.1210/en.2011-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bocco BM, Werneck-de-Castro JP, Oliveira KC, Fernandes GW, Fonseca TL, Nascimento BP, McAninch EA, Ricci E, Kvarta-Papp Z, Fekete C, Bernardi MM, Gereben B, Bianco AC, Ribeiro MO. Type 2 Deiodinase Disruption in Astrocytes Results in Anxiety-Depressive-Like Behavior in Male Mice. Endocrinology. 2016;157:3682–3695. doi: 10.1210/en.2016-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. The European journal of neuroscience. 2010;31:883–891. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Buras A, Battle L, Landers E, Nguyen T, Vasudevan N. Thyroid hormones regulate anxiety in the male mouse. Hormones and behavior. 2014;65:88–96. doi: 10.1016/j.yhbeh.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Adan RA, Cox JJ, da Silva SL. Transactivation of the rat oxytocin and vasopressin promoters by nuclear hormone receptors. [Review] [16 refs] Regulatory Peptides. 1993;45:31–35. doi: 10.1016/0167-0115(93)90178-b. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Beiderbeck DI, Althaus M, Koolhaas JM, Neumann ID. Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behavioural brain research. 2014a;261:315–322. doi: 10.1016/j.bbr.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Hormones and behavior. 2014b;65:427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Campo Verde Arbocco F, Sasso CV, Nasif DL, Hapon MB, Jahn GA. Effect of hypothyroidism on the expression of nuclear receptors and their co-regulators in mammary gland during lactation in the rat. Mol Cell Endocrinol. 2015;412:26–35. doi: 10.1016/j.mce.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueire VB, Romaldini JH, Ward LS. Subclinical hypothyroidism increases the risk for depression in the elderly. Archives of gerontology and geriatrics. 2007;44:21–28. doi: 10.1016/j.archger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ciosek J, Drobnik J. Vasopressin and oxytocin release and the thyroid function. Journal of Physiology & Pharmacology. 2004;55:423–441. [PubMed] [Google Scholar]
- Cleare AJ, McGregor A, O'Keane V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clin Endocrinol (Oxf) 1995;43:713–719. doi: 10.1111/j.1365-2265.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Constant EL, Adam S, Seron X, Bruyer R, Seghers A, Daumerie C. Anxiety and depression, attention, and executive functions in hypothyroidism. Journal of the International Neuropsychological Society : JINS. 2005;11:535–544. doi: 10.1017/S1355617705050642. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Custro N, Scafidi V, Lo Baido R, Nastri L, Abbate G, Cuffaro MP, Gallo S, Vienna G, Notarbartolo A. Subclinical hypothyroidism resulting from autoimmune thyroiditis in female patients with endogenous depression. J Endocrinol Invest. 1994;17:641–646. doi: 10.1007/BF03349679. [DOI] [PubMed] [Google Scholar]
- Darbra S, Garau A, Balada F, Sala J, Marti-Carbonell MA. Perinatal hypothyroidism effects on neuromotor competence, novelty-directed exploratory and anxiety-related behaviour and learning in rats. Behavioural brain research. 2003;143:209–215. doi: 10.1016/s0166-4328(03)00041-x. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nature Protocols. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Chan J, Vennstrom B, Forrest D, Pfaff DW. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nature Neuroscience. 2000;3:472–475. doi: 10.1038/74846. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Zhu YS, Krey L, Pfaff DW. Thyroid hormone and estrogen interact to regulate behavior. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12581–12586. doi: 10.1073/pnas.93.22.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Zhu YS, Pfaff DW. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. Journal of Neuroendocrinology. 1999;11:1–10. doi: 10.1046/j.1365-2826.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- Domes G, Normann C, Heinrichs M. The effect of oxytocin on attention to angry and happy faces in chronic depression. BMC Psychiatry. 2016;16:92. doi: 10.1186/s12888-016-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamez MJ, Guadano-Ferraz A, Cuadrado A, Bernal J. Type 3 iodothyronine deiodinase is selectively expressed in areas related to sexual differentiation in the newborn rat brain. Endocrinology. 1999;140:5443–5446. doi: 10.1210/endo.140.11.7244. [DOI] [PubMed] [Google Scholar]
- Faustino LC, Gagnidze K, Ortiga-Carvalho TM, Pfaff DW. Impact of Thyroid Hormones on Estrogen Receptor alpha-Dependent Transcriptional Mechanisms in Ventromedial Hypothalamus and Preoptic Area. Neuroendocrinology. 2015;101:331–346. doi: 10.1159/000381459. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiology & Behavior. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Forrest D, Visser TJ. Thyroid hormone signaling. Biochim Biophys Acta. 2013;1830:3859. doi: 10.1016/j.bbagen.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148:3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- Ge JF, Peng YY, Qi CC, Chen FH, Zhou JN. Depression-like behavior in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. Endocrine. 2014;45:430–438. doi: 10.1007/s12020-013-0001-4. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. doi: 10.1089/thy.2005.15.865. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. Journal of Clinical Investigation. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial an temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151:5550–5558. doi: 10.1210/en.2010-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models. Biochim Biophys Acta. 2013;1830:3974–3978. doi: 10.1016/j.bbagen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Higashida H, Yokoyama S, Munesue T, Kikuchi M, Minabe Y, Lopatina O. CD38 gene knockout juvenile mice: a model of oxytocin signal defects in autism. Biol Pharm Bull. 2011;34:1369–1372. doi: 10.1248/bpb.34.1369. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. Journal of visualized experiments : JoVE. 2013:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism research : official journal of the International Society for Autism Research. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain, & Behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Ne'eman R, Perach-Barzilay N, Fischer-Shofty M, Atias A, Shamay-Tsoory SG. Intranasal administration of oxytocin increases human aggressive behavior. Hormones and behavior. 2016;80:125–131. doi: 10.1016/j.yhbeh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150:1952–1960. doi: 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North WG, LaRochelle FT, Hardy GR. Radioimmunoassays for individual rat neurophysins. J. Endocinol. 1983;96:373–386. doi: 10.1677/joe.0.0960373. [DOI] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Molecular psychiatry. 2015;20:490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, Liao CP, Phillips JM, Hallmayer JF, Hardan AY. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci U S A. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830:3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Annals of the New York Academy of Sciences. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes, brain, and behavior. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, Kunugi H. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophr Res. 2012;139:201–206. doi: 10.1016/j.schres.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Kohrle J. Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta. 2013;1830:3965–3973. doi: 10.1016/j.bbagen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai C, Hirvikoski T, Dencker Vansvik E, Nordstrom AL, Linder J, Nordstrom P, Jokinen J. Thyroid hormones and personality traits in attempted suicide. Psychoneuroendocrinology. 2009;34:1526–1532. doi: 10.1016/j.psyneuen.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB Journal. 2011;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain D, Galton VA, Hernandez A. Defining the roles of iodohyronine deiodinases: Current concepts and challenges. Endocrinology. 2009;150:1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohn JP, Martinez ME, Hernandez A. Decreased anxiety- and depression-like behaviors and hyperactivity in a type 3 deiodinase-deficient mouse showing brain thyrotoxicosis and peripheral hypothyroidism. Psychoneuroendocrinology. 2016;74:46–56. doi: 10.1016/j.psyneuen.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Koenig JI, Gold JM, Frost KH, Buchanan RW. Plasma oxytocin levels predict social cue recognition in individuals with schizophrenia. Schizophr Res. 2015a;162:47–51. doi: 10.1016/j.schres.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Koenig JI, Gold JM, Ossenfort KL, Buchanan RW. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophr Res. 2015b;162:57–61. doi: 10.1016/j.schres.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11–19. doi: 10.1016/j.psyneuen.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan R, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. doi: 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Uhrig S, Hirth N, Broccoli L, von Wilmsdorff M, Bauer M, Sommer C, Zink M, Steiner J, Frodl T, Malchow B, Falkai P, Spanagel R, Hansson AC, Schmitt A. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: A post-mortem study. Schizophr Res. 2016;177:59–66. doi: 10.1016/j.schres.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Davidkova G, Zhu YS, Koibuchi N, Chin WW, Pfaff D. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology. 2001a;74:309–324. doi: 10.1159/000054698. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Koibuchi N, Chin WW, Pfaff DW. Differential crosstalk between estrogen receptor (ER)alpha and ERbeta and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Brain Research. Molecular Brain Research. 2001b;95:9–17. doi: 10.1016/s0169-328x(01)00165-6. [DOI] [PubMed] [Google Scholar]
- Venero C, Guadano-Ferraz A, Herrero AI, Nordstrom K, Manzano J, de Escobar GM, Bernal J, Vennstrom B. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes & Development. 2005;19:2152–2163. doi: 10.1101/gad.346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Hormones & Behavior. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lam O, Chuang B, Ford JM, Mathalon DH, Vinogradov S. Oxytocin administration selectively improves olfactory detection thresholds for lyral in patients with schizophrenia. Psychoneuroendocrinology. 2015;53:217–222. doi: 10.1016/j.psyneuen.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wu Z, Martinez ME, St Germain DL, Hernandez A. Type 3 Deiodinase Role on Central Thyroid Hormone Action Affects the Leptin-Melanocortin System and Circadian Activity. Endocrinology. 2017;158:419–430. doi: 10.1210/en.2016-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Zhang SW, Tai FD. Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behav Pharmacol. 2016;27:672–680. doi: 10.1097/FBP.0000000000000212. [DOI] [PubMed] [Google Scholar]
- Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, Zhou Q, Yang Y, Dong X, He J, Huang X, Chen J, Wu K, Xu L, Mao R. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety- and depression-like behaviors in rats. Hormones and behavior. 2015;69:106–115. doi: 10.1016/j.yhbeh.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Zeng H, Schimpf BA, Rohde AD, Pavlova MN, Gragerov A, Bergmann JE. Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol Endocrinol. 2007;21:2795–2804. doi: 10.1210/me.2007-0048. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Dai YC, Wu J, Jia MX, Zhang JS, Shou XJ, Han SP, Zhang R, Han JS. Plasma Oxytocin and Arginine-Vasopressin Levels in Children with Autism Spectrum Disorder in China: Associations with Symptoms. Neuroscience bulletin. 2016;32:423–432. doi: 10.1007/s12264-016-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.