Abstract
An argument for preclinical stroke research to make more use of the permanent middle cerebral artery occlusion (MCAO) model, rather than transient MCAO, is presented. Despite STAIR recommending permanent MCAO as the primary model, preclinical stroke research has not listened. In 2012, Hossmann reported that 64% of the treatment studies for MCAO used prompt transient MCAO models and only 36% of the studies used permanent MCAO or gradual transient MCAO (i.e. embolic stroke model). Then, in 2014 and 2015, 88% of published basic science studies on large vessel occlusion used the transient MCAO model. However, this model only represents 2.5–11.3% of large vessel stroke patients. Therefore, the transient MCAO model, which mimics stroke with reperfusion, does not accurately reflect the majority of clinical stroke cases. Thus, once again, the argument for studying permanent MCAO as a primary model is made and supported.
Keywords: Animal Models, Ischemia, Middle Cerebral Artery Occlusion, Reperfusion, Stroke
Introduction to the Disparity Between the Usage of Ischemic Stroke Animals Models and the Prevalence of Clinical Stroke Subtypes
The most widely used animal model of stroke, transient middle cerebral artery occlusion, was utilized in 88% of the experimental studies in 2014 and 2015 (see “Mimicking Clinical Stroke Pathology with Animal Models” in this article). Despite the recommendations by STAIR [1] and others in stroke research [2–4], we are still employing the transient middle cerebral artery occlusion model more often than is observed clinically. Since the original STAIR meeting [1], a number of publications have presented basic science arguments against the use of the transient middle cerebral artery occlusion model as the primary model for cerebral ischemia [2–4]. In this perspective, we will renew the discussion for minimizing our use of the transient middle cerebral artery occlusion animal model, but we will base the argument on the clinical presentations of large vessel stroke. Also discussed are the future directions for precision animal modeling of stroke.
Large Vessel Stroke
There are 650,000 United States ischemic stroke patients annually, with 40–50% of them (~300,000) experiencing stroke due to large vessel occlusion (LVO, i.e. middle cerebral artery (M1 and M2 segments), internal carotid artery) [5]. Of the approximately 300,000 LVO stroke patients, up to 15% are treated with tPA [6] and 2.6–4% are treated with mechanical embolectomy [5], and there is an overall 90-day mortality of 27.9–36% [7, 8]. This perspectives paper will focus on stroke from LVO and the animal models used to mimic LVO.
Vessel Recanalization after Large Vessel Stroke
Treatment of ischemic stroke targets recanalization of the occluded blood vessel(s) to restore blood flow (i.e. reperfusion) using either pharmacological breakdown of the clot or mechanical clot removal. Currently, thrombolysis with tissue plasminogen activator (tPA) and mechanical embolectomy are used in the clinic to restore blood flow. tPA may be used in as many as 15% of large vessel stroke patients (15% [6] × 300,000 LVO patients = 45,000 LVO patients receiving tPA), resulting in recanalization (partial or complete) in 11–40% [9, 10] of tPA-treated patients with LVO (11–40% [9, 10] × 45,000 = 4,950–18,000 individuals) and a favorable outcome in 28–46% of them [11, 10]. Mechanical embolectomy, removal of the clot using a retriever, is an alternative treatment for vessel reperfusion. In 2014, mechanical embolectomy was used to treat 8,000–12,000 patients with large vessel stroke in the United States [5]. Of patients receiving embolectomy, recanalization occurs in about 75% [9] of them (75% [9] × 8,000–12,000 [5] = 6,000–9,000 recanalizations). In summary, even with the use of these two therapies, of the ~300,000 annual United States stroke patients with LVO, 3.65–9% have recanalization ((4,950–18,000)/300,000 = 1.65–6% recanalizations from tPA, (6,000–9,000)/300,000 = 2–3% recanalizations from embolectomy). This means that, in the United States, between 273,000 and 289,050 stroke patients with LVO do not have vessel recanalization (i.e. the blood vessels are permanently occluded) (Table 1).
Table 1.
Clinical Stroke Manifestations.
| Total Ischemic Strokes in the United States: 650,000 [5] |
| Number of Patients with Large Vessel Occlusion: 40–50% [5] = ~300,000 |
| Number of LVO Patients Receiving tPA: up to 15% [6] = 45,000 |
| Number of Patients with Recanalization from tPA: 11–40% [9, 10] = 4,950–18,00 |
| Number of Patients Receiving Embolectomy after tPA Fails: 8,000–12,000 [5] |
| Number of Patients with Recanalization from embolectomy: 75% [9] = 6,000–9,000 |
| Number of LVO Patients With Recanalization = 10,950–27,000 |
| Number of LVO Patients Without Recanalization (i.e. Permanent Occlusion) = 273,000–289,050 |
In patients not treated with thrombolytics or endovascular therapies, spontaneous recanalization may occur. Typically, spontaneous recanalization occurs in about 20% of large vessel strokes [11, 12], so the total recanalization number is likely higher than 10,950–27,000 per year. Yet since the reported rates of spontaneous recanalization vary significantly (up to 45%) [12, 9], it is difficult to determine the number of stroke patients with spontaneous reperfusion, thus this patient population will not be included in the following discussion.
Categories of Large Vessel Stroke
We propose a classification for clinical scenarios of ischemic stroke patients with LVO who have had recanalization (10,950–27,000 patients) (Table 2). Subtype A is defined as patients with recanalization of large vessels who have a small infarction volume (less than 70 mL volume) [13–15]. Despite a LVO, recanalization in this patient subtype prevents the expansion of the infarction and allows injured tissue to heal. This subtype occurs in approximately 37–70% of LVO patients who have had vessel recanalization (28 of 76 LVO patients [16] = 37%, 19 of 27 LVO patients [13] = 70%), and this patient population typically has good clinical outcomes [13, 16]. The mortality for this patient population is about 8% [11, 17], but may be as low as 1% [18]. The number of patients with subtype A, recanalization of a LVO with a small infarction volume, is 1.4–6.3% of the total LVO patient population:
Table 2.
Clinical Stroke Subtypes.
| Subtype A |
| Small Infarction Volume (little or no infarction expansion, volume < 70 mL) [13–15] |
| Good Outcome [13, 16] |
| Low Mortality (<8%) [11, 17, 18] |
| Occurrence: 1.4–6.3% of all LVO patients |
| Subtype B |
| Large Infarction Volume (infarction expansion after recanalization, volume > 70 mL) with Severe Brain Edema and/or Hemorrhage |
| Poor Outcome (up to 50% of surviving patients have major disability) [19] |
| Very High Mortality (up to 80%) [19] |
| Occurrence: 0.5–2.7% of all LVO patients |
| Subtype C |
| Large Infarction Volume (infarction expansion after recanalization, volume > 70 mL); No Life-Threatening Brain Edema or Hemorrhage |
| Poor Outcome |
| Moderate Mortality (9–25%) [11, 17, 19, 21, 22] |
| Occurrence: 0.6–2.3% of all LVO patients |
Subtype B is defined as patients with large vessel recanalization who have severe brain swelling and/or hemorrhagic transformation, typically associated with large infarction volume, and which often leads to poor clinical outcomes (50% of subtype B survivors patients have a major disability [19]) and death (mortality is up to 80% of subtype B patients) [19]. In a two-center collaborative study, 14 of 98 LVO patients (= 14.3%) to 13 of 43 LVO patients (= 30.2%) who had vessel recanalization experienced symptomatic hemorrhage [20]. Extrapolating this data for to the entire population of LVO patients with recanalization (10,950–27,000), subtype B occurs in 0.5–2.7% of ischemic stroke patients with LVO:
Subtype C is defined as patients who have large vessel recanalization and a large infarction void of any life-threatening brain edema and/or hemorrhage. Despite recanalization, subtype C experiences an expansion of the infarction, leading to large infarction volumes (greater than 70 mL) [13–15]. This recanalization subtype often leads to permanent functional deficits and requires long term recovery, with a mortality of 12–25% (as low as 9% with the use of embolectomy) (from MR RESCUE, MR CLEAN, EXTEND-IA, ESCAPE, SWIFT PRIME, REVASCAT, and references [11, 21, 17, 22, 19]). This subtype has been found to occur in 9 of 53 LVO patients [23, 24] (= 17%) to 19 of 76 LVO patients [16] (= 25%) with recanalization. Extrapolating to the entire LVO patient population, subtype C occurs in 0.6–2.3% of large vessel stroke patients:
These three recanalization subtypes account for the 10,950–27,000 patients with recanalization after large vessel stroke. Using these classifications, the following discussion shows the disparity between the clinical scenarios of large vessel stroke and the animal models utilized in preclinical studies of stroke.
Mimicking Clinical Stroke Pathology with Animal Models
To date several therapeutics for ischemic stroke which had very promising experimental findings have all failed in Clinical Trials. This may in part be due to the discrepancies between the animal models used in preclinical studies and the clinical scenarios of stroke, as highlighted in the STAIR meeting [1] and by others [2–4].
Experimental models using animals allows for a controlled environment and controlled variables (such as age and sex) with which we can better understand clinical pathophysiology and mechanisms of disease, develop and test the efficacy of potential therapeutics, and evaluate comorbidities. Animal models of cerebral ischemia allow for an injury to be tightly controlled to create well-defined and reproducible primary and secondary injuries (i.e. infarction size, neuroinflammation, brain edema).
A PubMed search was conducted on December 13, 2016 using the following keywords: (MCAO or middle cerebral artery occlusion) AND (“2014”[Date-Publication]:“2015”[Date-Publication]). This search produced 2,956 hits. These hits were refined to determine the number of experimental/preclinical animal studies which occluded the middle cerebral artery M1/M2 segments. This inclusion criteria produced a total of 1,278 experimental papers which studied LVO in animals published in either 2014 or 2015: 1,125 articles utilized transient middle cerebral artery occlusion (MCAO) models (1,069 used an intraluminal suture, 56 used an embolus) and 153 articles which utilized the permanent MCAO model (intraluminal suture). The distal MCAO (i.e. ligature or electrocautery) and the photothrombosis models were excluded since these models do not target the M1/M2 segments of the middle cerebral artery. There were no language restrictions.
There are two widely used transient MCAO animal models to mimic LVO: intraluminal suture MCAO and embolic stroke MCAO. The intraluminal suture model utilizes a suture inserted into the MCA to interrupt the blood flow for a specific duration (typically 60–120 minutes). After the allotted time, the suture is removed, beginning reperfusion. This transient MCAO model resembles large vessel stroke patients who have had reperfusion after recanalization. The intraluminal MCAO model can partially mimic LVO patients who are subtypes A–C which accounts for 2.5–11.3% of all LVO patients. Despite mimicking a very small percentage of the stroke population, this animal model was used in 84% of the preclinical studies in 2014 and 2015.
The other widely used transient MCAO model is the embolic stroke model which uses an autologous blood clot injected into the MCA to occlude the vessel. Typically, this model is used to study reperfusion (by tPA or new thrombolytics) or therapies in combination with tPA. This model was used in 4% of the experimental studies in 2014 and 2015. The embolic stroke model can also be used to partially mimic LVO patient subtypes A–C.
Problem in Preclinical Stroke Studies
A goal of preclinical studies is to test potential therapeutics for a clinical disease, and basic science research for stroke is no different. However, a problem with preclinical stroke studies is that they are relying on the transient MCAO model as the primary model for cerebral ischemia, thereby deviating from the clinical picture for large vessel stroke.
First, transient MCAO is disproportionally used in comparison with permanent MCAO; 88% of the experimental stroke studies utilized transient MCAO models, but these models only represent 2.5–11.3% of all large vessel stroke patients (subtype A: 1.4–6.3%, subtype B: 0.5–2.7%, subtype C: 0.6–2.3%) since most LVO patients do not have reperfusion (Fig 1). While the transient MCAO model indeed is relevant to specific clinical stroke situations, to date, the use of transient MCAO models does not reflect the clinical scenario(s) (i.e. 88.7–97.5% of LVO patients have permanent vessel occlusion).
Fig 1. Disparity Between Cerebral Ischemia Animal Models and Clinical Stroke Subtypes.
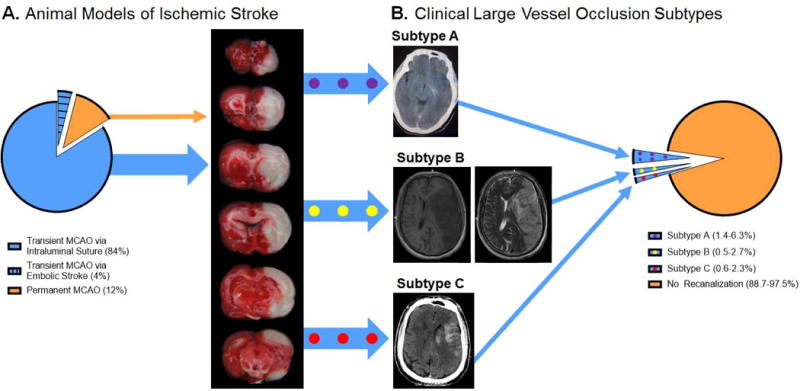
A. Animal models of cerebral ischemia. Animal models of LVO include the transient MCAO and permanent MCAO models. Transient MCAO can be induced via an intraluminal suture or embolism. The intraluminal suture transient MCAO model (blue) accounted for 84% of the preclinical stroke studies in 2014 and 2015. Transient MCAO via embolic stroke (blue with black lines) accounted for 4% of the 2014 and 2015 experimental stroke studies. Permanent MCAO (orange) accounted for 12% of the 2014 and 2015 preclinical stroke studies. A representative image of a TTC-stained brain from a rat subjected to 2 hours of MCAO via intraluminal suture is shown. The red-stained tissue indicates healthy tissue. The white, unstained tissue indicates the infarction. This brain has an infarction volume equal to 26% of the whole brain (or 52% of the ipsilesional hemisphere). Transient MCAO can mimic clinical LVO patients of subtypes A (blue with purple dots), B (blue with yellow dots), and C (blue with red dots). B. Clinical distribution of LVO stroke patients. The number of patients with LVO is approximately 300,000 per year in the United States. Of these patients, 10,950–27,000 have vessel recanalization, which can be categorized using the large vessel recanalization subtypes A, B, and C. Of the LVO patients, 1.4–6.3% are subtype A (blue with purple circles), 0.5–2.7% are subtype B (blue with yellow circles), and 0.6–2.3% are subtype C (blue with red circles). The remaining large vessel stroke patients, 273,000–289,050 (88.7–97.5%), do not have vessel recanalization (orange). Representative images of each patient subtype are shown (subtype A: CT image, subtype B: T1 MRI (left) and T2 MRI (right), subtype C: CT).
Second, the transient MCAO models used in preclinical studies are defined by reperfusion of the occluded vessel(s). Thus, drugs tested in these models rapidly reach the ischemic core and penumbra, producing their protective effects directly and immediately in the ischemic tissue. However, in 88.7–97.5% of LVO patients the occlusion is not reopened, so therapeutics cannot directly reach the ischemic zones. Rather, in permanent LVO patients, therapeutics can only get to the penumbra via collateral circulation in a limited manner, and only if patients have good collateral circulation. Therefore, therapeutics that are successful in reducing infarction and neurological deficits in transient MCAO animal models may not have any success in LVO patients who do not have vessel recanalization (which is 88.7–97.5% of the LVO patient population). A delivery method of therapeutics which may be used in permanent MCAO models to by-pass this phenomenon is convection-enhanced delivery (infusion of a solution directly into the brain via a small cannula). Use of convection-enhanced delivery in the rat model of permanent MCAO will still allow many researchers to study the effect of their therapeutic on ischemic core and penumbra.
Although the transient MCAO model is needed during the normal pipeline for drug development, it probably should not be the first model studied. Using the transient MCAO model as the first model for a drug may give rise to positive results, and therefore publications. But then transitioning the drug into the permanent MCAO model leads to negative findings. Furthermore, the STAIR recommendations support the use of this model as a supplementary model to more clinically relevant animal models (i.e. the permanent MCAO model) [1]. Thus, in order to follow the recommendations by STAIR and the normal pipeline for drug development, the permanent MCAO model should be studied.
Future Directions and Precision Stroke Animal Models
The main goal for stroke treatment is vessel recanalization to restore blood flow to the ischemic tissue. Recanalization will provide the best outcome possible for stroke patients. Currently, the time window for recanalization is 6 hours, but this may be extended up to 24 hours with the promising findings of the DAWN Trial [25]. Extending the time window, and/or finding new therapies which can increase the number of patients having reperfusion should be a major focus for stroke treatment. However, until the percentage of patients receiving vessel recanalization becomes larger (see #3 and #4 below), there will be a disproportion of clinical classification for stroke patients towards permanent vessel occlusion.
Despite the recommendations by STAIR [1], STEPS [26], RIGOR [27], and others in stroke research [28–31, 2–4, 32], the transient middle cerebral artery occlusion model has been disproportionally used in preclinical research. Although the transient MCAO model has its place among the animal models of focal ischemia, the limitations of this model suggest it should not be used as a primary model of stroke research. Despite numerous arguments against wide use of the transient MCAO models (particularly the rapid reperfusion models (i.e. the intraluminal suture model)), basic science stroke researchers have not changed. Indeed, in 2012, Hossmann reported that 64% of the treatment studies for MCAO used prompt transient MCAO models and only 36% of the studies used permanent MCAO or gradual transient MCAO (i.e. embolic stroke model) [3]. Now, in 2017, preclinical studies have further augmented the imbalance: 80% of the studies in 2014–2015 used intraluminal suture MCAO, while permanent MCAO was used in 12% of the 2014–2015 studies and embolic stroke used in 4% of the 2014–2015 studies.
The disparity between the usage of animal models for large vessel stroke and the clinical LVO patient population argues for us to make a shift in our animal models of LVO. But, how do we begin to shift our focus in preclinical studies towards precision stroke animal models which can promote clinical relevance and ultimately translation? In addition to following the recommendations of STAIR [1], STEPS [26], and RIGOR [27], the following suggestions are offered.
1. Choosing a Preclinical Model for Ischemic Stroke
Advantages of the pMCAO Model
First, we should examine therapies using a stroke model(s) which represents the majority of clinical stroke cases. In other words, rather than first using transient MCAO models (which only mimic 2.5–11.3% of the clinical stroke subtypes for LVO), let’s utilize permanent MCAO animal models which represent 88.7–97.5% of LVO patients. As argued previously [1–4], the permanent MCAO model seems like a logical starting place since it 1) mimics LVO patients without recanalization/reperfusion, 2) represents the majority of LVO patients, and 3) is an established, robust, and reproducible model with known outcomes and reported tips/tricks. An additional benefit of the permanent MCAO model is that the transport of therapeutics into the ischemic tissue will be hindered by the occlusion (i.e. intraluminal suture, embolus), increasing its clinical relevance. Thus, a therapy which is found to be beneficial in the permanent MCAO model may have a better chance of success in other stroke animal models (i.e. transient MCAO), as well as in clinical trials.
Advantages of the tMCAO Model
While we propose that the pMCAO model should, in general, be utilized first, there are instances when the tMCAO should be the first model used for an experimental ischemic stroke study. Several review articles are available which highlight specific benefits for the various MCAO models [33–36]. Animal models of tMCAO are very useful to study reperfusion injury, such as delayed neuronal death, severe cerebral edema, hemorrhagic transformation, and infiltration of systemic inflammatory cells [37, 38], to study mechanisms of tMCAO, and to test therapies designed for reperfusion injury, especially as the number of patients with vessel recanalization increases; reducing injury and promoting healing after reperfusion is critical to improve outcome after recanalization. Indeed, two clinical trials (GAME and MAVARIC) are investigating promising therapeutics for reperfusion injury.
2. Spontaneous Reperfusion
Next, we realize that there exists a patient population in which spontaneous recanalization occurs after large vessel stroke; preclinical studies on LVO should also strive to incorporate this into their designs to improve clinical translation. Spontaneous recanalization has been reported for the embolic and photothrombotic stroke models, thus these two models may be suitable as precision stroke animal models for this LVO sub-population. While the embolic stroke model has been extensively researched, the variability and cost limits its appeal. The current photothrombotic model targets the M3 segment of the middle cerebral artery, making it insufficient for the LVO classifications mentioned in this article (M1/M2 segments of the middle cerebral artery). However, with the appropriate changes in design/modeling, the photothrombotic model may be used to target the middle cerebral artery M1/M2 segments.
3. Proposed Treatment for Permanent Large Vessel Occlusion
Finally, we propose that there exists a LVO patient population which does not have vessel recanalization, but may benefit from delayed recanalization (i.e. days or weeks after), likely using embolectomy or stenting. The current thinking is that if reperfusion cannot occur within a few hours after the onset of stroke, reperfusion will only exacerbate the injury. While this is may be true during the progression of injury, it is conceived that, after edema (and other secondary injury) has resolved and the patient is stable, delayed recanalization of the permanently occluded vessel(s) may be beneficial. Li and Murphy identified three zones of brain issue affected by MCAO: an ischemic core (irreversible damage), a reversible damaged penumbra, and a hypo-perfused structurally intact penumbra [39]. While the ischemic core dies within minutes of ischemia [40], the penumbra can survive for days to weeks [41, 42]. This suggests that the proper treatment of permanent large vessel strokes can not only salvage ischemia-affected tissue (i.e. the penumbra), but may also provide improved neurological and functional outcomes. Indeed, two case studies and a small clinical trial have reported on the benefits of delayed recanalization for permanent large vessel stroke patients [25, 43, 44]. Two case studies used stenting to provide recanalization at 80 days and 4 months [43, 44]. Recently, the DAWN trial results show that delayed recanalization (within 24 hours of the insult) via embolectomy can reduce severe disability, even to the point of functional independence [25]. Interestingly, and which also offers a great opportunity, is that up to 94% of large vessel stroke patients show perfusion-diffusion mismatch (which is an indicator of penumbra tissue) [40]. However, before moving towards large clinical trials, delayed recanalization should be examined, especially for comorbidities, in experimental models. Thus, it is of interest to test an animal model of MCAO with delayed recanalization as a potential therapy.
4. Other Factors Affecting the Use of Experimental Ischemic Stroke Models
On a separate but related note, there are other factors which can shift the balance of mimicking stroke with animal models. One major factor is the health care system. Currently, most stroke patients are not candidates for thrombolytics. Yet, the day may come when most stroke patients reach the clinic soon enough to be treated, thereby shifting the balance of transient vs permanent stroke towards transient events [45]. Another factor, related to basic science, is that the transient MCAO model is the most promising animal model of stroke which allows therapies to have the highest rate of success, thus leading to positive results and publication in high impact journals. Indeed, negative studies of very promising therapies do not receive much attention and are published in low impact journals. This concern is unlikely to be resolved, unless alternative delivery methods are adopted and used in permanent MCAO models to successfully deliver the therapeutic(s) (see the second problem in “Problem in Preclinical Stroke Studies”).
5. Pipeline for Drug Development
Basic science research should not only be focused on understanding the pathophysiology and signaling pathways of diseases, but should also support drug translation from the bench to the bedside. To do this, we need to follow the recommendations provided by STAIRS [1], STEPS [26], and RIGOR [27]. Any drug which is a candidate for attenuating either primary or secondary injury after stroke should follow the same logical steps. This includes drugs which may target a single, specific facet of the injury cascade since many drugs activate signaling pathways which converge on the same major “switch” proteins, thereby attenuating numerous secondary injury pathways (i.e. apoptosis, BBB disruption, inflammation) [46–58].
Therefore, the logical road for preclinical stroke research should be to first test the drug using a model of permanent middle cerebral artery occlusion, then a gradual reperfusion model such as the embolic stroke model, and, finally, a prompt reperfusion model. The goal of the permanent MCAO model would be to identify the therapeutic regimen (dose, timing) and evaluate its potential in reaching the ischemic tissue. This would also examine the response of the drug as a therapy for the most abundant case of stroke, permanent occlusion. The embolic stroke model would then be used to refine the therapeutic regimen, as well as investigate the therapy in a clinically relevant model. Finally, a prompt reperfusion model can be used to study the drug’s effect(s) on reperfusion injury to ensure it is safe for patients receiving embolectomy. In addition, the comorbidities and risk factors for stroke should also be evaluated in one or more of these models. While Big Pharma typically follows this pipeline for drug development, basic science research for stroke has not followed suit. Only by following these recommendations, which have been highlighted a number of times in the past [1, 26, 27], can we hope that a novel therapy has potential in clinical trials.
Conclusion
To date, basic science research on stroke has been relying much too heavily on the transient MCAO model as a preclinical model for large vessel occlusion; 88% of experimental studies in 2014 and 2015 used transient MCAO, yet these models only represent 2.5–11.3% of the clinical large vessel strokes which occur. While the recommendations from STAIR [1] and others [2–4] have already identified this problem, we continue to rely on the transient MCAO model using the intraluminal suture. Herein we provided an alternative view point for minimizing the use of the intraluminal suture model using the clinical sup-types of LVO. A shift towards making more use of the permanent MCAO model as the first step for preclinical studies is again recommended.
Supplementary Material
Acknowledgments
Devin McBride was partially supported by a National Institutes of Health F32 Fellowship (1F32HL136193, DWM).
Footnotes
Disclosures
The authors have no conflicts of interest to report.
References
- 1.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 2.Hossmann K-A. Cerebral Ischemia: Models, Methods and Outcomes. Neuropharmacology. 2008;55(3):257–70. doi: 10.1016/j.neuropharm.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metabol. 2012;32(7):1310–6. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahle MP, Bix GJ. Successfully Climbing the “STAIRs”: Surmounting Failed Translation of Experimental Ischemic Stroke Treatments. Stroke Res Treat. 2012;2012:374098. doi: 10.1155/2012/374098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rai AT. Red pill, blue pill: reflections on the emerging large vessel stroke ‘market’. J Neurointerventional Surg. 2015;7(9):623–5. doi: 10.1136/neurintsurg-2015-011971. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez RG, Furie KL, Goldmacher GV, Smith WS, Kamalian S, Payabvash S, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke. 2013;44(11):3109–13. doi: 10.1161/STROKEAHA.113.001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers PM, Schumacher HC, Connolly ES, Jr, Heyer EJ, Gray WA, Higashida RT. Current status of endovascular stroke treatment. Circulation. 2011;123(22):2591–601. doi: 10.1161/CIRCULATIONAHA.110.971564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiacek M, Kaczorowski R, Homa J, Filip E, Darocha J, Dudek D, et al. Single-center experience of stent retriever thrombectomy in acute ischemic stroke. Neurol Neurochir Polska. 2017;51(1):12–8. doi: 10.1016/j.pjnns.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura S, Sakai N, Okada Y, Kitagawa K, Kimura K, Tanahashi N, et al. Efficacy of endovascular treatment for acute cerebral large-vessel occlusion: analysis of nationwide prospective registry. J Stroke Cerebrovasc Dis. 2014;23(5):1183–90. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL. Improving reperfusion therapy for acute ischaemic stroke. J Thromb Haemostasis. 2011;9(Suppl 1):333–43. doi: 10.1111/j.1538-7836.2011.04371.x. [DOI] [PubMed] [Google Scholar]
- 11.Cloft HJ, Rabinstein A, Lanzino G, Kallmes DF. Intra-arterial stroke therapy: an assessment of demand and available work force. Am J Neuroradiol. 2009;30(3):453–8. doi: 10.3174/ajnr.A1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CJ, Ding D, Starke RM, Mehndiratta P, Crowley RW, Liu KC, et al. Endovascular vs medical management of acute ischemic stroke. Neurology. 2015;85(22):1980–90. doi: 10.1212/WNL.0000000000002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasparotti R, Grassi M, Mardighian D, Frigerio M, Pavia M, Liserre R, et al. Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. Am J Neuroradiol. 2009;30(4):722–7. doi: 10.3174/ajnr.A1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30(2):293–8. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 15.Timpone VM, Lev MH, Kamalian S, Morais LT, Franceschi AM, Souza L, et al. Percentage insula ribbon infarction of >50% identifies patients likely to have poor clinical outcome despite small DWI infarct volume. Am J Neuroradiol. 2015;36(1):40–5. doi: 10.3174/ajnr.A4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man S, Aoki J, Hussain MS, Wisco D, Tateishi Y, Toth G, et al. Predictors of infarct growth after endovascular therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24(2):401–7. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40(12):3834–40. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Investigators TNSS. Recombinant tissue plasminogen activator for minor strokes: the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study experience. Ann Emerg Med. 2005;46:243–52. doi: 10.1016/j.annemergmed.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Aiyagari V, Diringer MN. Management of large hemispheric strokes in the neurological intensive care unit. The Neurologist. 2002;8(3):152–62. doi: 10.1097/00127893-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42(8):2235–9. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 21.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 22.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50(2):341–50. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 23.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 24.Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995;23(9):1576–87. doi: 10.1097/00003246-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 25.DAWN Trial Results Demonstrate a 73% Reduction in Disability in Stroke Patients Treated up to 24 Hours. 2017 http://www.prweb.com/releases/2017/05/prweb14339427.htm. Accessed May 22, 2017 2017.
- 26.Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40(2):510–5. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 27.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Trans Stroke Res. 2013;4(3):279–85. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahnstedt H, McCullough LD, Cipolla MJ. The Importance of Considering Sex Differences in Translational Stroke Research. Trans Stroke Res. 2016;7(4):261–73. doi: 10.1007/s12975-016-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boltze J, Ayata C. Challenges and Controversies in Translational Stroke Research - an Introduction. Transl Stroke Res. 2016;7(5):355–7. doi: 10.1007/s12975-016-0492-4. [DOI] [PubMed] [Google Scholar]
- 30.Boltze J, Wagner D-C, Henninger N, Plesnila N, Ayata C. Phase III Preclinical Trials in Translational Stroke Research: Community Response on Framework and Guidelines. Transl Stroke Res. 2016;7(4):241–7. doi: 10.1007/s12975-016-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ergul A, Hafez S, Fouda A, Fagan SC. Impact of Comorbidities on Acute Injury and Recovery in Preclinical Stroke Research: Focus on Hypertension and Diabetes. Transl Stroke Res. 2016;7(4):248–60. doi: 10.1007/s12975-016-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent TA, Mandava P. Embracing Biological and Methodological Variance in a New Approach to Pre-Clinical Stroke Testing. Transl Stroke Res. 2016;7(4):274–83. doi: 10.1007/s12975-016-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med. 2009;1(1):8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 2015;9:3445–54. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627–42. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- 36.Sicard KM, Fisher M. Animal models of focal brain ischemia. Exp Transl Stroke Med. 2009;1(1):7. doi: 10.1186/2040-7378-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nour M, Scalzo F, Liebeskind DS. Ischemia-Reperfusion Injury in Stroke. Interventional Neurol. 2013;1(3–4):185–99. doi: 10.1159/000353125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49(2):93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28(46):11970–9. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3(1):47–55. doi: 10.6030/1939-067x-3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 42.Pu H, Jiang X, Hu X, Xia J, Hong D, Zhang W, et al. Delayed Docosahexaenoic Acid Treatment Combined with Dietary Supplementation of Omega-3 Fatty Acids Promotes Long-Term Neurovascular Restoration After Ischemic Stroke. Transl Stroke Res. 2016;7(6):521–34. doi: 10.1007/s12975-016-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terada T, Yamaga H, Tsumoto T, Masuo O, Itakura T. Use of an embolic protection system during endovascular recanalization of a totally occluded cervical internal carotid artery at the chronic stage. Case report. J Neurosurg. 2005;102(3):558–64. doi: 10.3171/jns.2005.102.3.0558. [DOI] [PubMed] [Google Scholar]
- 44.Yu W, Kostanian V, Fisher M. Endovascular recanalization of basilar artery occlusion 80 days after symptom onset. Stroke. 2007;38(4):1387–9. doi: 10.1161/01.STR.0000260186.93667.a2. [DOI] [PubMed] [Google Scholar]
- 45.Henninger N, Fisher M. Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl Stroke Res. 2016;7(4):284–93. doi: 10.1007/s12975-015-0444-4. [DOI] [PubMed] [Google Scholar]
- 46.Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55(2):127–38. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Charles MS, Drunalini Perera PN, Doycheva DM, Tang J. Granulocyte-colony stimulating factor activates JAK2/PI3K/PDE3B pathway to inhibit corticosterone synthesis in a neonatal hypoxic-ischemic brain injury rat model. Exp Neurol. 2015;272:152–9. doi: 10.1016/j.expneurol.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasegawa Y, Suzuki H, Altay O, Rolland W, Zhang JH. Role of the sphingosine metabolism pathway on neurons against experimental cerebral ischemia in rats. Transl Stroke Res. 2013;4(5):524–32. doi: 10.1007/s12975-013-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41(2):368–74. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44(11):3202–10. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Klebe D, Doycheva D, McBride DW, Krafft PR, Flores J, et al. G-CSF ameliorates neuronal apoptosis through GSK-3beta inhibition in neonatal hypoxia-ischemia in rats. Exp Neurol. 2015;263:141–9. doi: 10.1016/j.expneurol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, McBride DW, Doycheva D, Dixon BJ, Krafft PR, Zhang JH, et al. G-CSF attenuates neuroinflammation and stabilizes the blood-brain barrier via the PI3K/Akt/GSK-3beta signaling pathway following neonatal hypoxia-ischemia in rats. Exp Neurol. 2015;272:135–44. doi: 10.1016/j.expneurol.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, et al. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS One. 2011;6(6):e21312. doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazari M, Keshavarz S, Rafati A, Namavar MR, Haghani M. Fingolimod (FTY720) improves hippocampal synaptic plasticity and memory deficit in rats following focal cerebral ischemia. Brain Res Bull. 2016;124:95–102. doi: 10.1016/j.brainresbull.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Shi X, Doycheva DM, Xu L, Tang J, Yan M, Zhang JH. Sestrin2 induced by hypoxia inducible factor1 alpha protects the blood-brain barrier via inhibiting VEGF after severe hypoxic-ischemic injury in neonatal rats. Neurobiol Dis. 2016;95:111–21. doi: 10.1016/j.nbd.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi X, Xu L, Doycheva DM, Tang J, Yan M, Zhang JH. Sestrin2, as a negative feedback regulator of mTOR, provides neuroprotection by activation AMPK phosphorylation in neonatal hypoxic-ischemic encephalopathy in rat pups. J Cereb Blood Flow Metabol. 2016 doi: 10.1177/0271678X16656201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei H, Li Y, Han S, Liu S, Zhang N, Zhao L, et al. cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl Stroke Res. 2016;7(6):497–511. doi: 10.1007/s12975-016-0484-4. [DOI] [PubMed] [Google Scholar]
- 58.Yao Y, Miao W, Liu Z, Han W, Shi K, Shen Y, et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl Stroke Res. 2016;7(6):535–47. doi: 10.1007/s12975-016-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


