Abstract
Aged tissues usually show a decreased regenerative capacity accompanied by a decline in functionality. During aging pancreatic islets also undergo several morphological and metabolic changes. Besides proliferative and regenerative limitations, endocrine cells lose their secretory capacity, contributing to a decline in functional islet mass and a deregulated glucose homeostasis. This is linked to several features of aging, such as induction of cellular senescence or the formation of modified proteins, such as advanced glycation end products (AGEs) - the latter mainly examined in relation to hyperglycemia and in disease models. However, age-related changes of endocrine islets under normoglycemic and non-pathologic conditions are poorly investigated. Therefore, a characterization of pancreatic tissue sections as wells as plasma samples of wild-type mice (C57BL/6J) at various age groups (2.5, 5, 10, 15, 21 months) was performed. Our findings reveal that mice at older age are able to secret sufficient amounts of insulin to maintain normoglycemia. During aging the pancreatic islet area increased and the islet size doubled in 21 months old mice when compared to 2.5 months old mice, whereas the islet number was unchanged. This was accompanied by an age-dependent decrease in Ki-67 levels and pancreatic duodenal homeobox-1 (PDX-1), indicating a decline in proliferative and regenerative capacity of pancreatic islets with advancing age. In contrast, the number of p16Ink4a-positive nuclei within the islets was elevated starting from 10 months of age. Interestingly, AGEs accumulated exclusively in the islet blood vessels of old mice associated with increased amounts of inflammatory markers, such as the inducible nitric oxide synthase (iNOS) and 3-nitrotyrosine (3-NT). In summary, the age-related increase in islet size and area was associated with the induction of senescence, accompanied by an accumulation of non-enzymatically modified proteins in the islet vascular system.
Abbreviations: 3-NT, 3-Nitrotyrosine; AGE(s), advanced glycation end product(s); IF, immunofluorescence; IHC, immunohistochemistry; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa B; RT, room temperature
Keywords: Pancreatic islets, Aging, Cellular senescence, Advanced glycation end products
Graphical abstract
Highlights
-
•
Aging induces an increase in pancreatic islet size and area, but not number.
-
•
Age-related endocrine islet expansion is associated with senescence induction.
-
•
Aged islets accumulate AGEs and nitrated proteins exclusively in the vessels.
1. Introduction
Pancreatic islets represent a network of endocrine cells, basically divided into two major subgroups (β-cells and non-β-cells). In rodents, β-cells are the most common cell type of the endocrine pancreas (up to 85%) and form the center of the islet. They are surrounded by the non-β-cell fraction (α-, δ-, ε-, and pancreatic polypeptide cells) and penetrated by a large number of blood vessels. In contrast, the human islet architecture exhibits a heterogeneous distribution of endocrine cells, but this remains a matter of discussion. The main function of pancreatic islets is the secretion of hormones (insulin, glucagon, somatostatin, ghrelin and pancreatic polypeptide), essential for the maintenance of homeostatic processes [1], [2], [3], [4]. During aging, the endocrine pancreas undergoes morphological and metabolic changes, contributing to an inappropriate regulation of glucose levels. These changes mostly affect the insulin-producing β-cells, whereas in other cell types, only a few modifications were observed [5]. The pancreatic β-cell mass, basically representing the islet mass in rodents, declines with age, induced by an imbalance in β-cell turnover (decreased proliferation and replication, elevated apoptosis). This is accompanied by an increase in β-cell dysfunction, together leading to an overall reduction in functional β-cell mass [6], [7], [8], [9], [10], [11].
It has been shown that the limitation of the proliferative and replicative capacity of β-cells during aging correlates with the induction of senescence, activated by the transcriptional upregulation of cell cycle inhibitors, such as p16Ink4a, preventing the cell cycle entry [12], [13], [14], [15]. In addition, Helman and colleagues were recently able to show that an increased expression of p16Ink4a enhances the insulin secretory capacity of β-cells in advanced age, which is in contrast to the previous literature [16], [17], [18]. Further well-known age-related changes include accumulation of non-enzymatic modified proteins, such as glycation (formation of advanced glycation endproducts, AGEs), oxidation or nitration of proteins [19], [20], [21], [22].
AGEs are formed as products of the Maillard reaction, or precursors are generated as intermediates of glycolysis and lipid peroxidation. Additionally, it is suggested that the development of AGE deposits is accelerated mainly under hyperglycemic conditions and contributes to diabetic complications. However, AGE formation also occurs in normal aging [23], [24], [25]. By binding the receptor for advanced glycation endproducts (RAGE), AGEs induce the production of reactive oxygen species (ROS) by activating enzymatic processes. This causes a proinflammatory response mediated by the transcription factor Nuclear-factor Kappa B (NFκB) [26], [27], [28]. In addition, peroxynitrite as a product of the proinflammatory response is formed, facilitating protein nitration [29], [30]. Since age-related changes in pancreatic islets and their major cell type (β-cells) are associated with the amount of circulating glucose, AGE formation and related processes were mainly investigated under hyperglycemic and disease conditions.
Here, we characterized pancreatic islets of wild-type mice (C57BL/6) at various age groups to describe age-related alterations of endocrine islets. C57BL/6J is a widely used inbred strain susceptible to polygenic obesity, type 2 diabetes and atherosclerosis. The observed expansion of islets was associated with the induction of senescence and the maintenance of insulin secretory capacity sufficient for metabolic demand. Additionally, our data show that glycated as well as nitrated proteins are also formed in normal aging, independent of hyperglycemic conditions. We also found that this modified proteins accumulate exclusively in the vascular system of the endocrine pancreas.
2. Material and methods
2.1. Experimental model
Male C57BL/6J mice (2.5, 5, 10, 15 and 21 months) from The Jackson Laboratory were housed in a controlled environment at a temperature of 20 ± 2 °C, with a 12/12 h light/dark cycle and obtained a standard diet (Ssniff, Soest, Germany) as well as water ad libitum. Blood samples were collected before sacrificing the mice, cooled on ice and centrifuged. Subsequently, pancreatic tissues were isolated and fixed in 4% paraformaldehyde solution for 24 h, followed by paraffin embedding according to standard procedures. Mice were kept in agreement with the National Institutes of Health guidelines for care and use of laboratory animals. All procedures are verified and approved by the ethics committee for animal welfare of the State Office Environment, Health, and Consumer Protection (State of Brandenburg, Germany).
2.2. Determination of blood glucose
Blood glucose levels were determined by using an automated analyzer (Cobas Mira S, Hoffmann-La Roche, Basel, Switzerland) and a commercially available reagent kit (Glucose HK CP, Horiba ABX Pentra, Montpellier, France). The method is based on a 2-step enzymatic reaction with Hexokinase followed by Glucose-6-phosphate-dehydrogenase leading to the quantifiable end product D-gluconate-6-phosphate.
2.3. Plasma insulin and proinsulin ELISA
The concentration of insulin and proinsulin in murine plasma was determined by using the Mouse High Range Insulin ELISA (ALPCO, Salem, USA) and carried out according to the manufacturer's instructions.
2.4. Immunohistochemistry and immunofluorescence
Longitudinal serial sections (2 µm) were processed for immunohistochemistry (IHC) and immunofluorescent (IF) analysis. The sections were de-paraffinized and re-hydrated in Roti-Histol (Carl Roth, Karlsruhe, Germany) and decreasing serial solutions of ethanol. Heat-mediated antigen retrieval was performed by placing the slides in citrate-buffer (10 mM citrate acid, 0.05% Tween 20 in distilled water) for 20 min at 95–99 °C in a water bath, followed by a cooling step of 15 min at room temperature (RT). Pancreatic tissue samples were incubated with blocking solution (Antibody Diluent, Agilent, Waldbronn, Germany) containing 10% goat serum for 1 h. For IHC, sections were blocked with 0.03% hydrogen peroxide (Peroxidase block; Agilent, Waldbronn, Germany) for 10 min at RT. Sections were incubated with primary antibodies, diluted in blocking solution, for 1 h in a lightproof humidified chamber at RT. Rabbit anti-insulin antibody (ab181547, Abcam, Cambridge, United Kingdom), rabbit anti-Ki67 antibody (ab16667, Abcam Cambridge, United Kingdom), mouse Methylglyoxal-AGE (Arg-Pyrimidine) (AGE06B, BiLogo, Kiel, Germany), mouse anti-methylglyoxal antibody (MG-H1) (STA-011-CB, BioCat, Heidelberg, Germany), and anti-pentosidine antibody (PEN012, BiLogo, Kiel, Germany) were used for IHC staining of Insulin, Ki-67, Methylglyoxal-derived AGEs and pentosidine, followed by a 30-min incubation with HRP-labeled polymer. Before mounting with Entellan (Merck Millipore, Darmstadt, Germany), tissue sections were incubated with substrate-chromogen solution, 3,3′-Diaminobenzidin (EnVision+ system-HRP, Agilent Waldbronn, Germany) and counterstained with hematoxylin (Sigma-Aldrich, Taufkirchen, Germany). Rabbit anti-PDX-1 antibody (07-696, Merck Millipore, Darmstadt, Germany), mouse anti-CDKN2A/p16INK4a antibody (ab54210, Abcam, Cambridge, United Kingdom), rabbit anti-iNOS antibody (ab178945, Abcam, Cambridge, United Kingdom) and mouse anti-3-Nitrotyrosine antibody (ab110282, Abcam, Cambridge, United Kingdom) were used as primary antibodies for IF staining of PDX-1, p16Ink4a, iNOS and 3-NT. All pancreatic slices were co-stained with mouse or rabbit anti-insulin antibodies (L6B10, Cell Signaling, Cambridge, United Kingdom; ab181547, Abcam, Cambridge, United Kingdom) to visualize the β-cell area. Visualization was performed by incubation with secondary antibodies conjugated to AlexaFluor 488 and 594 (Invitrogen, Darmstadt, Germany) and FluorCare including DAPI (Carl Roth, Karlsruhe, Germany) was used as mounting media.
2.5. Quantitative analysis of pancreatic islets
Microscopic analysis was performed by digital imaging of pancreatic sections using an Olympus IX53 microscope (Olympus, Hamburg, Germany) for IHC or Zeiss LSM 780 confocal microscope (Zeiss, Jena, Germany) for IF. To count the number of positive stained nuclei (Ki-67, PDX-1, p16Ink4a) and measure the positive stained area (iNOS, 3-NT, pentosidine, Arg-Pyrimidine, MG-H1) within the pancreatic islets for the morphometric analysis, Zeiss ZEN 2.3 imaging software (Zeiss, Jena, Germany) was used. Pancreata of 6–8 mice were used for quantification of each staining. At the time of tissue sectioning, weight of the pancreas was not measured routinely. Thus, the islet mass (pancreas weight x islet area) could not be determined. As equivalent marker the islet area was used (%-islet area/slide). Islet size is an absolute parameter in mm2 (in the meta-data of the original microscopic image files, both the number of pixels is included and the area of a single pixel in µm2). To measure islet area, number and size of islets, pancreatic sections were stained with insulin, visualized with 3,3′-Diaminobenzidin and counterstained with hematoxylin as described above. Digital images of the entire pancreas were taken with a MIRAX-MIDI Scanner (Zeiss, Jena, Germany). Total pancreatic and insulin positive area of each section was quantified with Zeiss ZEN 2.3 imaging software. The software evaluates size of single islets, overall islet size and islet number of a given image.
2.6. Statistical analysis
Statistical analysis was performed by using GraphPad Prism version 7.03 (La Jolla, CA, USA). All data presented in the figures are mean values ± SD. Differences between two groups were assessed by Student's t-test and one-way ANOVA was used for multiple comparisons of more than two groups. Differences were considered as statistically significant, if p < 0.05 was reached.
3. Results
3.1. Morphological changes of murine pancreatic islets during aging
Aged C57BL/6J mice show increased body weights that remain stable after 10 months of age. In addition blood glucose and plasma insulin levels are unchanged in all age groups, indicating good health conditions also in old C57BL/6J mice. In contrast, the plasma proinsulin level decreases with age. Starting from 10 months of age the levels are significantly lower compared to the youngest mice (2.5 months). Consequently, the proinsulin-to-insulin ratio shifts from 1:5 in young (2.5 months) to 1:14 in old mice (21 months), suggesting a higher conversion rate of proinsulin to insulin in aging to maintain glucose homeostasis (Table 1).
Table 1.
Body weight and plasma parameters of C57BL/6J mice at different age groups.
|
Age (months) |
|||||
|---|---|---|---|---|---|
| 2.5 | 5 | 10 | 15 | 21 | |
| Body weight (g) | 23 ± 3.8 | 34.3 ± 1.4a | 40.4 ± 3.74a,b | 37.5 ± 5a,b | 41.5 ± 5a,b |
| Glc (mmol/l) | 8.45 ± 1.06 | 8.24 ± 1.25 | 8.43 ± 0.77 | 7.48 ± 1.66 | 6.85 ± 0.81 |
| Ins (nM) | 0.62 ± 0.21 | 0.71 ± 0.15 | 0.74 ± 0.16 | 0.8 ± 0.32 | 0.7 ± 0.2 |
| ProIns (nM) | 0.12 ± 0.03 | 0.08 ± 0.02 | 0.06 ± 0.04a | 0.07 ± 0.07a | 0.05 ± 0.03a |
| ProIns/Ins | 0.19 ± 0.11 | 0.12 ± 0.04 | 0.09 ± 0.08 | 0.12 ± 0.09 | 0.07 ± 0.05a |
Data are presented as mean values ± SD of n = 6–8 mice. Glc = blood glucose, Ins = insulin, ProIns = Proinsulin. Statistical significance was assessed by one-way ANOVA, a,bp < 0.05 (a = significance compared to 2.5 months, b = significance compared to 5 months).
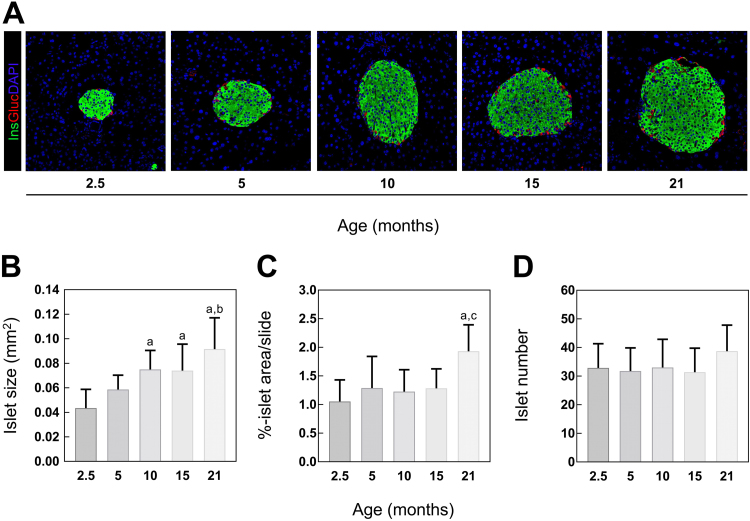
In order to determine age-related morphological changes in whole pancreatic tissue, sections were IHC stained for insulin to visualize the insulin-positive area representing the islets. Islet area was defined as percentage of islet area within the whole pancreatic slice, whereas the islet size represents the area of single islets (in mm2). IHC analysis revealed an age-dependent increase in pancreatic islet size, starting from the age of 10 months. Additionally, islet size doubled when comparing 2.5 and 21 months old mice (Fig. 1A, B). Islet area was only increased in 21 months old C57BL/6J mice (Fig. 1C). In contrast, almost equivalent islet numbers were observed between age groups (Fig. 1D). Summarized, C57BL/6J mice show an expansion in pancreatic islet size and area during aging, without changes in islet number and exhibit stable blood glucose levels.
Fig. 1.
Islet size, area and number in 2.5, 5, 10, 15 and 21 months old C57BL/6 mice. (A) Representative images of IF labeled insulin (Ins), glucagon (Gluc) and DAPI at indicated age. Quantification of IHC stained insulin of whole pancreatic sections represented as (B) islet area, (C) islet size and (D) islet number (n = 7–8 mice). Green: insulin, red: Glucagon, blue: DAPI. Data are presented as mean values ± SD. Statistical significance was assessed by one-way ANOVA, a,b,cp < 0.05 (a = significance to 2.5 months, b = significance to 5 months, c = significance to 10 months).
3.2. Expansion of islet mass is associated with increased p16INK4a-levels
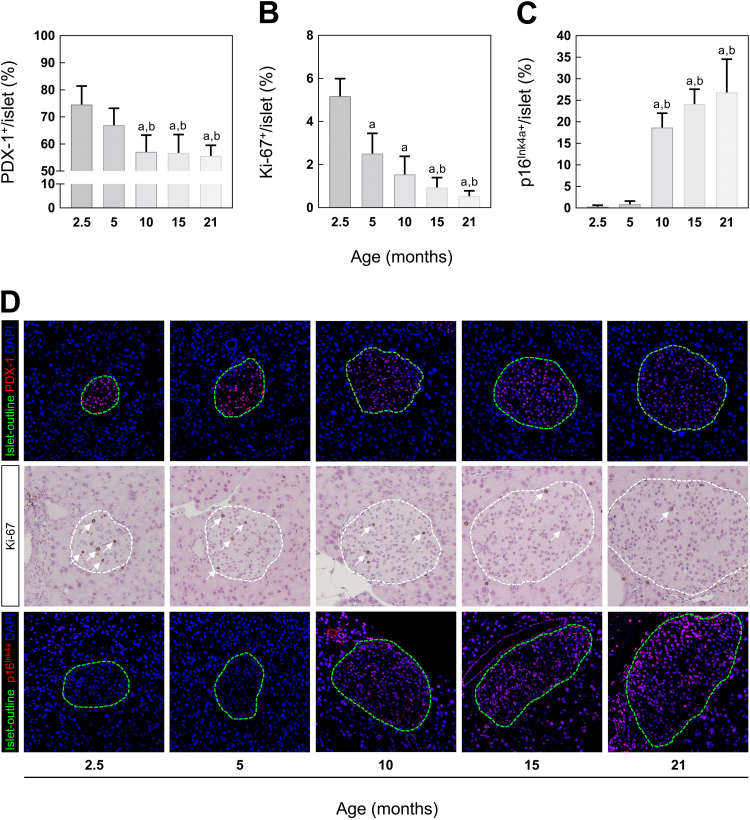
In a next step, replication and proliferation rate of islets was determined. Initially, pancreatic tissue sections were IF stained for the pancreatic duodenal homeobox protein-1 (PDX-1), a specific marker for β-cell replication, differentiation and survival and co-stained for insulin and DAPI. The number of positive-labeled nuclei within the insulin-positive area was quantified and indicated as percentage of the entire nuclei. As shown in Fig. 2A, the relative number of PDX-1-positive β-cells decreases with advancing age. At 10, 15 and 21 months of age, a decline of 25% compared to 2.5 months and 15% compared to 5 months old mice was found. In addition to PDX-1, tissue sections were stained with Ki-67 confirming an age-dependent decrease in proliferation capacity. After 5 months of age the number of Ki-67-positive nuclei was reduced by 50% compared to 2.5 months old mice. Moreover, Ki-67-levels of 15 months old mice were lowered by further 30% (Fig. 2B). At 21 months of age, the average of Ki-67-positive nuclei was approximately 0.3%.
Fig. 2.
β-cell regeneration and proliferation in 2.5, 5, 10, 15 and 21 months old C57BL/6 mice. Quantitative analysis of IF labeled (A) PDX-1, IHC stained (B) Ki-67 and IF labeled (C) p16Ink4a (n = 7–8 mice). (D) Representative images of each staining at indicated age. For better visibility of PDX-1 (first row) and p16Ink4a (third row), islets are outlined in green (according to insulin staining, not shown). For Ki-67, white lines mark the islet area and white arrows show positive stained nuclei. Green: islet-outline, red: PDX-1/p16Ink4a, blue: DAPI. Data are presented as mean values ± SD. Statistical significance was assessed by one-way ANOVA, a,bp < 0.05 (a = significance to 2.5 months, b = significance to 5 months).
The age-dependent decline in proliferative capacity of pancreatic islets has been previously correlated with the transcriptional activation of the cell cycle inhibitor protein p16Ink4a, reflecting the induction of cellular senescence [12]. Thus, to investigate the potential connection between proliferation and senescence, pancreatic tissue sections were IF labeled for p16Ink4a and co-stained for insulin and DAPI. As expected, a higher number of p16Ink4a-positive nuclei within the insulin-positive area of islets were observed with advancing age. After 10 months of age, mice showed elevated levels of p16Ink4a compared to animals aged 2.5 and 5 months (Fig. 2C). In summary, the decrease in Ki-67-positive nuclei is accompanied by reduced levels of the transcription factor PDX-1, and an age-related increase in p16Ink4a-levels, indicating an association between the induction of cellular senescence and the expansion of pancreatic islet size with age.
3.3. AGE formation and nitric oxide production increase with age in the vascular system
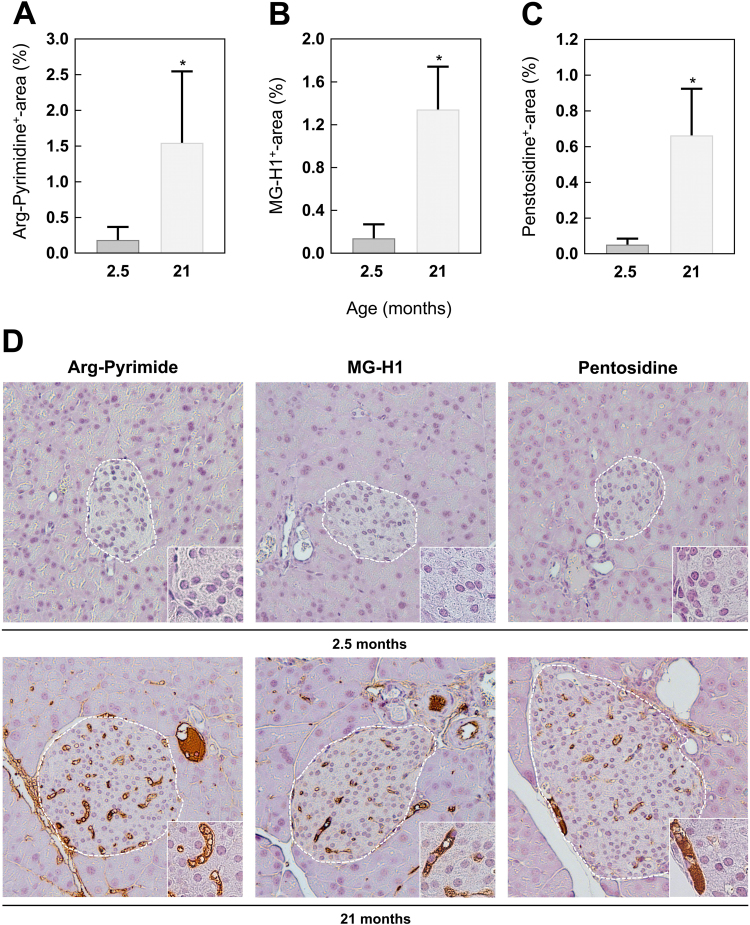
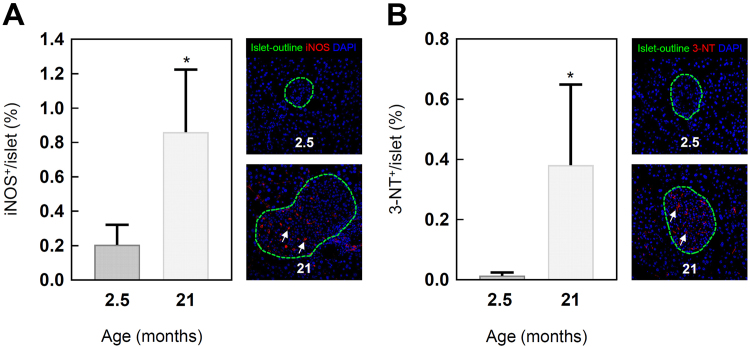
Besides cellular senescence and proliferative modifications, aging is also associated with the accumulation of AGEs [31], [32]. Therefore, different AGEs were analyzed by IHC staining of pancreatic tissue sections. Comparing 2.5 and 21 months old mice, a 9.5-fold increase for methylglyoxal-derived Arg-Pyrimidine was observed, MG-H1 increased 8-fold (Fig. 3A, B). Equally, the levels of the 3-deoxyglucosone-formed pentosidine were higher in the old mice (Fig. 3C). Interestingly, the age-related formation of all analyzed AGEs was not prominent in endocrine cells but rather located in the blood vessels of the pancreatic islets (Fig. 3D). In addition to glycation, nitration of proteins is another important non-enzymatic modification in aging [19]. In order to investigate this, iNOS and 3-NT-levels were determined by IF labeling. iNOS and 3-NT-levels of old C57BL/6J mice were also increased and located in the blood vessels of the endocrine islets comparable to AGE accumulation (Fig. 4A, B). Altogether, these results suggest that the age-dependent formation of AGEs is associated with higher amounts of nitrated proteins in the vascular system of the pancreatic islets.
Fig. 3.
Formation of AGEs in pancreatic islets of 2.5 and 21 months old C57BL/6 mice. Quantitative analysis of IHC stained (A) Arg-Pyrimidine, (B) MG-H1 and (C) pentosidine. (D) Representative images of each staining in a magnification of 20× and 60× (inset). For better visibility, white lines mark the islet area. Data are presented as mean values ± SD. Statistical significance was assessed by student's t-test (unpaired), *p < 0.05.
Fig. 4.
Induction of nitrosative stress and nitration products in pancreatic islets of 2.5 and 21 months old C57BL/6J mice. Quantitative analysis and representative images of IF labeled (A) iNOS and (B) 3-NT sections. For better visibility of iNOS and 3-NT, the islets are outlined in green (according to insulin staining, not shown). White arrows show iNOS and 3-NT positive stainings. Green: islet-outline, red: iNOS/3-NT, blue: DAPI. Data are presented as mean values ± SD. Statistical significance was assessed by student´s t-test (unpaired), *p < 0.05.
4. Discussion
In general, aging affects the endocrine pancreas by a decline in islet turnover [10], [33]. This contributes to a decreased islet mass and functionality, associated with deregulated glucose utilization and hyperglycemia [34], [35], [36]. In contrast, our data show that pancreatic islets of normal aged wild-type C57BL/6J mice were able to secrete adequate amounts of insulin to compensate the metabolic demand, thus preventing hyperglycemic conditions. This was accompanied by an age-dependent increase in islet area and size, whereas the islet number was unchanged, reflecting an expansion of the entire islet mass. Similar results were found by Sone and Kagawa and Tschen et al., both showing an adaptive increase in beta cell and islet mass in aging rodents under standard conditions [37], [38]. Several reasons, mainly self-renewal and growth of β-cells as well as replication and differentiation of pancreatic progenitor cells, were used to explain the age-induced expansion of the pancreatic islets [39], [40], [41]. Here, the differentiation capacity of murine islets was determined by using the major pancreatic transcription factor, PDX-1, showing a decline with advancing age. This was directly associated with an age-dependent decrease in islet proliferation rate under normoglycemic conditions indicated by Ki-67. Our findings are in agreement with previous investigations, revealing that β-cells of normal aged mice show low PDX-1 expression and a decreased proliferative capacity [7], [38]. Additionally, the age-related proliferative limitation of pancreatic islets correlates with the increased expression of the cell cycle regulator p16Ink4a, shown by Krishnamurthy and colleagues [12]. By blocking the cell cycle due to the inhibition of the cyclin-dependent kinases 4 and 6, p16Ink4a induces senescence and restricts the proliferative capacity of cells [13], [42]. According to that, the decreased proliferation shown in the present study was also accompanied by increased p16Ink4a-levels with advancing age. Consequently, the p16Ink4a-induced reduction of cell cycle progression contributes to limited regenerative potential of pancreatic islets in old mice. However, given that islet expansion continues until advanced age, these results suggest that endocrine cells have a relatively long lifespan and the reduced growth rate seems to be sufficient to maintain an increase in islet size [43], [44]. Another factor, possibly contributing to an increase in islet size, is the senescence-induced structural and functional reprogramming of cells. By increasing protein and RNA content including an overproduction of cytoskeleton and membrane proteins, such as vimentin and caveolin-1, senescent cells are known to enlarge [45], [46], [47]. Furthermore, a recent investigation [16] revealed a beneficial role of the senescence inducer p16Ink4a towards an increase in β-cell functionality possibly responsible for the maintenance of glucose homeostasis by generating adequate amounts of insulin as seen in this study.
In contrast, it has been reported that functional impairments of pancreatic β-cells with age are associated with the formation of AGEs [48]. Zhao et al. and Coughlan et al. showed that circulating AGEs are associated with a decline in insulin secretory capacity of β-cells, mainly mediated by impaired mitochondrial functionality [49], [50]. In addition, it has been shown by Puddu et al. that AGEs downregulate the protein expression of PDX-1 [51]. Thus, the observed reduction in PDX-1 levels together with the accumulation of AGEs in advanced age indicates that AGEs may contribute to the decline in regenerative potential of pancreatic islets. AGEs are formed under hyperglycemic conditions, but occur also as part of the normal aging process and contribute to complications in age-related diseases [50], [52]. Here, we observed an accumulation of AGEs, such as pentosidine, Arg-Pyrimidine and MG-H1, in old mice confirming their increased formation at an advanced age. Interestingly, AGEs were found only in the blood vessels of the pancreatic islet, contrary to a recent finding [53]. Our observations demonstrate a local limitation of AGE deposits within the vascular system of the endocrine pancreas. In accordance, other investigations demonstrate an AGE accumulation in the vessel wall [54], [55], [56], [57]. Extracellular matrix proteins, especially their most common form collagen, constitute the scaffold of the vascular system. Due to the slow turnover of collagen, this structural protein increases constantly with age [58], [59], [60]. Moreover, the formation of cross-linking products such as pentosidine diminishes the protein turnover contributing to AGE accumulation as well as vascular stiffening [61], [62], [63]. In addition to protein modifications, AGEs directly mediate their detrimental effects by binding to their major receptor, RAGE that is expressed, among others on the surface of endothelial cells [64], [65]. It has been shown that this interaction activates the NADPH oxidase and causes intracellular ROS production [27], [66]. As a feedback mechanism, the formation of AGEs is increased followed by an activation of NF-κB and its downstream pathways [67]. It was shown in different cell types that both processes contribute to the induction of iNOS expression [68], [69], [70]. This promotes the formation of nitric oxide able to react with superoxide anions to form peroxynitrite, leading to the production of nitrated proteins such as 3-NT [30], [71], [72]. This is in accordance with our findings showing that age-related generation of AGEs is accompanied by higher levels of iNOS in the blood vessels. Furthermore, we observed an accumulation of nitrated proteins, quantified via the typical product 3-NT. This modification was again only found in the blood vessels of pancreatic islets, indicating an association between the formation of AGEs and the generation of nitrosative stress in the vascular system of the endocrine pancreas during aging.
In summary our data show an age-related expansion of endocrine islets associated with increased p16Ink4a-levels and the induction of cellular senescence. This is accompanied by an accumulation of AGEs and nitrated proteins occurring exclusively in the islet vascular system of normal aged wild-type C57BL/6J mice. Further investigations with isolated islets are necessary, to unravel the mechanism behind these age-related changes.
Declaration of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Contributor Information
Richard Kehm, Email: richard.kehm@dife.de.
Jeannette König, Email: jeannette.koenig@dife.de.
Kerstin Nowotny, Email: kerstin.nowotny@dife.de.
Tobias Jung, Email: tobias.jung@dife.de.
Stephanie Deubel, Email: stefanie.deubel@dife.de.
Sabrina Gohlke, Email: sabrina.gohlke@dife.de.
Tim Julius Schulz, Email: tim.schulz@dife.de.
Annika Höhn, Email: annika.hoehn@dife.de.
References
- 1.Almaca J. Young capillary vessels rejuvenate aged pancreatic islets. Proc. Natl. Acad. Sci. USA. 2014;111(49):17612–17617. doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roscioni S.S. Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 2016;12(12):695–709. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- 3.Kilimnik G. Quantification of islet size and architecture. Islets. 2012;4(2):167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner D.J. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J.E. The ageing pancreas. Br. J. Diabetes Vasc. Dis. 2012;12(3):141–145. [Google Scholar]
- 6.Kushner J.A. The role of aging upon beta cell turnover. J. Clin. Investig. 2013;123(3):990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maedler K. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55(9):2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- 8.Cerf M.E. Beta cell dynamics: beta cell replenishment, beta cell compensation and diabetes. Endocrine. 2013;44(2):303–311. doi: 10.1007/s12020-013-9917-y. [DOI] [PubMed] [Google Scholar]
- 9.Swenne I. Effects of aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes. 1983;32(1):14–19. doi: 10.2337/diab.32.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Teta M. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54(9):2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 11.Rankin M.M., Kushner J.A. Aging induces a distinct gene expression program in mouse islets. Islets. 2010;2(6):345–352. doi: 10.4161/isl.2.6.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy J. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443(7110):453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy J. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23(8):975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Deursen J.M. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helman A. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat. Med. 2016;22(4):412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz-Espin D., Serrano M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 18.Baker D.J. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarti B., Chakravarti D.N. Protein tyrosine nitration: role in aging. Curr. Aging Sci. 2017;10(4):246–262. doi: 10.2174/1874609810666170315112634. [DOI] [PubMed] [Google Scholar]
- 20.DeGroot J. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50(4):1207–1215. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 21.Luevano-Contreras C., Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2(12):1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beal M.F. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002;32(9):797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 23.Nowotny K. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baynes J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 25.Jono T. Nepsilon-(carboxymethyl)lysine and 3-DG-imidazolone are major AGE structures in protein modification by 3-deoxyglucosone. J. Biochem. 2004;136(3):351–358. doi: 10.1093/jb/mvh124. [DOI] [PubMed] [Google Scholar]
- 26.Wautier M.P., Guillausseau P.J., Wautier J.L. Activation of the receptor for advanced glycation end products and consequences on health. Diabetes Metab. Syndr. 2017;11(4):305–309. doi: 10.1016/j.dsx.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Guimaraes E.L. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010;52(3):389–397. doi: 10.1016/j.jhep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Chavakis T., Bierhaus A., Nawroth P.P. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6(13):1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Ott C. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.San Martin A. Nox1-based NADPH oxidase-derived superoxide is required for VSMC activation by advanced glycation end-products. Free Radic. Biol. Med. 2007;42(11):1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Monnier V.M. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on "a puzzle nearing resolution". Ann. N. Y. Acad. Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- 32.Peppa M., Uribarri J., Vlassara H. Aging and glycoxidant stress. Hormones. 2008;7(2):123–132. doi: 10.1007/BF03401503. [DOI] [PubMed] [Google Scholar]
- 33.Reers C. Impaired islet turnover in human donor pancreata with aging. Eur. J. Endocrinol. 2009;160(2):185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 34.Rankin M.M., Kushner J.A. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonner-Weir S. beta-cell turnover: its assessment and implications. Diabetes. 2001;50(Suppl 1):S20–S24. doi: 10.2337/diabetes.50.2007.s20. [DOI] [PubMed] [Google Scholar]
- 36.Gu Z. Effect of aging on islet beta-cell function and its mechanisms in Wistar rats. Age. 2012;34(6):1393–1403. doi: 10.1007/s11357-011-9312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sone H., Kagawa Y. Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia. 2005;48(1):58–67. doi: 10.1007/s00125-004-1605-2. [DOI] [PubMed] [Google Scholar]
- 38.Tschen S.I. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58(6):1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teta M. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12(5):817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Dor Y. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 41.Ku H.T. Minireview: pancreatic progenitor cells – recent studies. Endocrinology. 2008;149(9):4312–4316. doi: 10.1210/en.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen G.P. Immunohistochemical survey of p16INK4A expression in normal human adult and infant tissues. Lab. Investig. 1999;79(9):1137–1143. [PubMed] [Google Scholar]
- 43.Montanya E. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes. 2000;49(8):1341–1346. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- 44.Finegood D.T., Scaglia L., Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44(3):249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi A. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006;8(11):1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 46.Nishio K. Senescence and cytoskeleton: overproduction of vimentin induces senescent-like morphology in human fibroblasts. Histochem. Cell Biol. 2001;116(4):321–327. doi: 10.1007/s004180100325. [DOI] [PubMed] [Google Scholar]
- 47.Cho K.A. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004;279(40):42270–42278. doi: 10.1074/jbc.M402352200. [DOI] [PubMed] [Google Scholar]
- 48.Lim M. Induction of apoptosis of Beta cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann. N. Y. Acad. Sci. 2008;1150:311–315. doi: 10.1196/annals.1447.011. [DOI] [PubMed] [Google Scholar]
- 49.Coughlan M.T. Advanced glycation end products are direct modulators of beta-cell function. Diabetes. 2011;60(10):2523–2532. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Z. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150(6):2569–2576. doi: 10.1210/en.2008-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puddu A. Advanced glycation end-products affect transcription factors regulating insulin gene expression. Biochem. Biophys. Res. Commun. 2010;395(1):122–125. doi: 10.1016/j.bbrc.2010.03.152. [DOI] [PubMed] [Google Scholar]
- 52.Goh S.Y., Cooper M.E. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008;93(4):1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 53.Morioka Y. The specific localization of advanced glycation end-products (AGEs) in rat pancreatic islets. J. Pharmacol. Sci. 2017;134(4):218–224. doi: 10.1016/j.jphs.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Basta G., Schmidt A.M., De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004;63(4):582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt A.M. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl. Acad. Sci. USA. 1994;91(19):8807–8811. doi: 10.1073/pnas.91.19.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janda K. Vascular effects of advanced glycation end-products: content of immunohistochemically detected AGEs in radial artery samples as a predictor for arterial calcification and cardiovascular risk in asymptomatic patients with chronic kidney disease. Dis. Markers. 2015;2015:153978. doi: 10.1155/2015/153978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wautier J.L. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. USA. 1994;91(16):7742–7746. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verzijl N. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000;275(50):39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 59.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon M.K., Hahn R.A. Collagens. Cell Tissue Res. 2010;339(1):247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowotny K., Grune T. Degradation of oxidized and glycoxidized collagen: role of collagen cross-linking. Arch. Biochem. Biophys. 2014;542:56–64. doi: 10.1016/j.abb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Sell D.R., Monnier V.M. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58(3):227–237. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 63.Verzijl N. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20(7):409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 64.Basta G. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105(7):816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 65.Singh V.P. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramasamy R. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 67.Bierhaus A. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50(12):2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 68.Chang P.C. Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent pathway. Kidney Int. 2004;65(5):1664–1675. doi: 10.1111/j.1523-1755.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 69.Wu C.H. Advanced glycosylation end products induce NF-kappaB dependent iNOS expression in RAW 264.7 cells. Mol. Cell Endocrinol. 2002;194(1–2):9–17. doi: 10.1016/s0303-7207(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 70.Sumi D., Ignarro L.J. Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes. 2004;53(7):1841–1850. doi: 10.2337/diabetes.53.7.1841. [DOI] [PubMed] [Google Scholar]
- 71.Sadowska-Bartosz I. Posttranslational protein modifications by reactive nitrogen and chlorine species and strategies for their prevention and elimination. Free Radic. Res. 2014;48(11):1267–1284. doi: 10.3109/10715762.2014.953494. [DOI] [PubMed] [Google Scholar]
- 72.Wong A. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur. J. Neurosci. 2001;14(12):1961–1967. doi: 10.1046/j.0953-816x.2001.01820.x. [DOI] [PubMed] [Google Scholar]