Abstract
Purpose
The purpose of this prospective randomized single blinded split mouth study was to conduct a comparative evaluation of the efficacy of intranasal atomised spray formulation of Dexmedetomidine with Midazolam in patients undergoing surgical removal of bilaterally impacted mandibular third molars.
Methods
This prospective study was conducted in twenty volunteers. Each volunteer underwent the surgical removal of an impacted mandibular third molar at two separate appointments at an interval of two weeks. The first third molar surgery was conducted using either intranasal Midazolam (Group M) or intranasal Dexmedetomidine (Group D). At the second appointment the surgical procedure was performed using the sedative agent not used at the first appointment. The primary testing outcome variables were Plasma oxygen saturation (SpO2), pulse and blood pressure and Modified Observer’s Assessment of Alertness/Sedation (OAA/S) scale. These were recorded at predetermined intervals starting 10 min before the administration of local anaesthesia and continued up to 10 min after completion of the procedure. In addition surgeon’s opinion regarding the patient cooperation, event amnesia, post operative nausea & vomiting were obtained.
Results
The sample composed of twenty patients (M = 9 and F = 11). There was statistically no significant difference between Group M and Group D with respect to mean SpO2. Minor differences were however noted at 20 and 30 min after sedation. There was no significant difference between the groups with respect to mean pulse rate, blood pressure, OAA/S, event amnesia, post operative nausea and vomiting and patient cooperation.
Conclusion
We conclude that Midazolam and Dexmedetomidine are equivalent and can be used in minor oral surgery with minimal complications. These drugs can be used intranasally using nasal atomization device in routine outpatient basis in otherwise normal healthy but anxious patients. All procedures must however be performed in the presence of an anaesthesiologist and with ready availability of emergency drugs and equipment.
Keywords: Intranasal spray formulation, Midazolam, Dexmedetomidine, Nasal atomization device
Introduction
Surgical removal of third molars is one of the most commonly performed procedures by oral surgeons. The surgical removal of impacted third molars generally causes stress and fear, which can affect patient physiology. To increase patient satisfaction after third molar extraction, it is necessary to minimize anxiety and discomfort during the surgical procedure. To achieve this objective various sedative drugs are used.
The role of sedation in minor oral surgical procedures cannot be over emphasised. With reduction in pain and anxiety comes better patient cooperation and satisfaction. Although various agents have been used, the “ideal” agent and regimen remain to be established. Intravenous therapy is the gold standard for sedation, providing rapid onset of action and the ability to adjust the dose as most sedative agents are administered in small increments during the procedure. Although ideal for any major procedure, an intravenous sedative can be resource-consuming for minor procedures. Moreover, establishing an intravenous access is painful and frightening for many patients. According to numerous studies, the use of intranasal medications for sedation during dental procedures is easy, effective, and associated with safety issues [1–10].
The nasal atomization device delivers intranasal medication in a fine mist (Fig. 1), which ensures that the exact dose and volume are delivered and enhances absorption which improves bioavailability (through the nose–brain pathway) for fast and effective drug delivery [9].
Fig. 1.
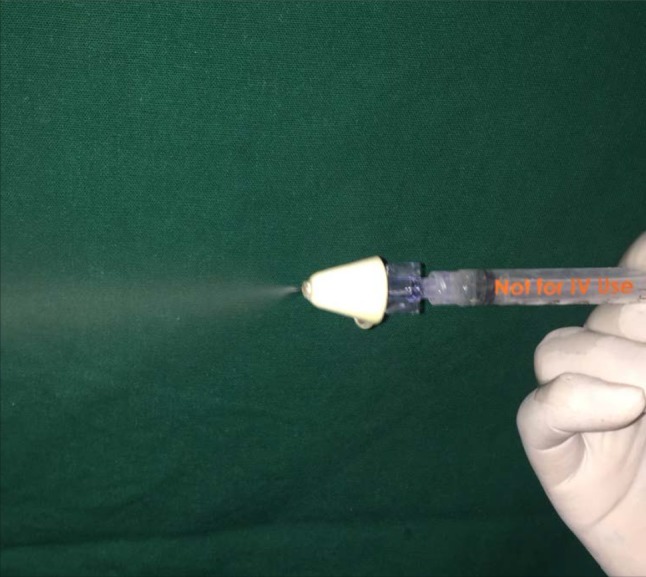
Drug delivery in a fine mist form
Midazolam is a fast-acting, water-soluble benzodiazepine with sedative, anxiolytic, and amnesic properties, and a short elimination half-life. It is conventionally given parenterally but this can be technically difficult and a major source of anxiety and discomfort. The oral and rectal routes for delivery of midazolam have been associated with variable and often delayed sedation and recovery. The intranasal route of midazolam has been used since 1988 and has the advantage of rapid absorption directly into the systemic circulation with no first pass effect and a bioavailability of 55–83%. In addition, intranasal midazolam has also been shown to be an effective preoperative sedation [1, 2, 4–7, 11–19].
Dexmedetomidine is a potent and highly selective specific alpha-2 adrenoreceptor agonist that has both sedative and analgesic effects. The primary site of action of dexmedetomidine is the locus coeruleus and not the cerebral cortex; therefore, its induced sedation is characterized by an easy and quick arousal, resembling natural sleep. In addition, the analgesic properties of dexmedetomidine could potentially alleviate pain after tooth extraction. Some authors have found the intranasal administration of dexmedetomidine to be effective, well tolerated, and convenient in healthy volunteers. Clinical studies of intranasal dexmedetomidine administration to produce sedation and possible analgesia are ongoing [8, 9, 11, 20–30].
Both these drugs can be administered intranasally via nasal atomization device. This route of administration is less invasive and has been suggested to provide greater patient acceptance with reduction of apprehension, anxiolysis and increase in comfort during surgery. However their comparative efficacy in minor oral surgical procedure via nasal atomization device has not been evaluated. In our study midazolam and dexmedetomidine are administered via nasal atomization device in surgical removal of bilaterally impacted mandibular third molars [1, 9, 12–14].
Materials and Methods
Twenty healthy patients with bilateral impacted mandibular third molars aged between 18 and 60 years of age were included in this prospective, single-blind, randomized study. All volunteers underwent surgical removal of bilaterally impacted mandibular third molars under local anesthesia (2% lignocaine with 1:200000 adrenaline). None of the patients had a history of allergy to any of the drugs used in this study. Radiographs were obtained to ensure that the impacted teeth in each patient had similar difficulty indices as ascertained by Pederson Difficulty Index. All patients were of ASA I category. Ethnic or gender differences were not taken into consideration.
Patients below 18 years and above 60 years of age, falling under ASA II, III, IV category, with compromising underlying systemic disease were excluded from the study. Patients with acute rhinitis or upper respiratory tract infection, nasal blockage and patients having allergies to midazolam and dexmedetomidine were excluded.
All patients signed an informed consent form to participate in this study. This study was approved by the Research Academy and Ethical Committee of Bharati Vidyapeeth Deemed University, Dental College and Hospital, Pune.
Subjects were instructed to fast for at least 6 h prior to surgery and they were advised to come accompanied by an escort. All procedures were performed by single operator. Each patient underwent two surgeries separated by 2 weeks. During this procedure, either midazolam (group M) or dexmedetomidine (group D) sedation was administered at the first appointment on a random basis. The drug was administered intranasally using nasal atomization device (LMA® MAD NASAL™ DEVICE) as a single titrated dose (Fig. 2). The patients who had midazolam sedation (0.2 mg/kg) in their first procedure were administered dexmedetomidine sedation (1.5 mcg/kg) in their second procedure and vice-versa.
Fig. 2.
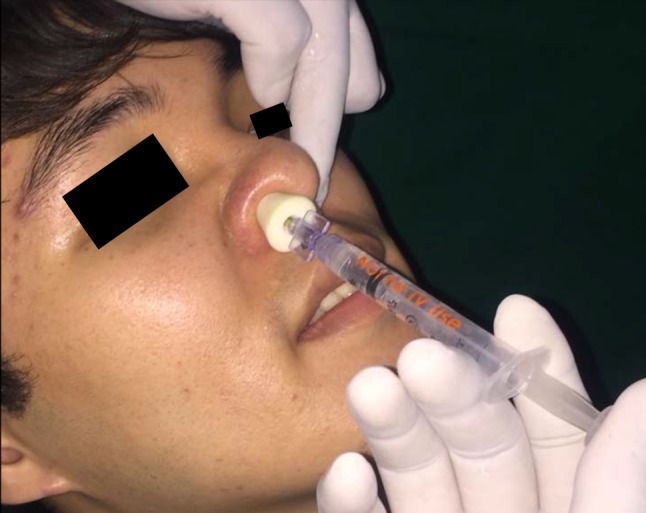
Nasal atomization devise used for delivering sedative agent in atomized form
The subjects undergoing procedure were operated in a fully equipped Oral and Maxillofacial Surgery operation theatre and monitored by a qualified anesthetist with availability of all emergency drugs, equipment and Intensive Care facility. On the day of surgery, patients were positioned and monitored in a supine position. No supplemental oxygen was administered.
Plasma oxygen saturation-SpO2, pulse rate and blood pressure along with patient’s level of alertness using Modified Observer’s Assessment of Alertness/Sedation (OAA/S), were recorded in institute structured proforma. Details were recorded every 10 min. Base line data were recorded and intranasal spray of midazolam/dexmedetomidine was administered via nasal atomization device. Thereafter readings were obtained every 10 min. Local anaesthetic administration was performed 10 min after administration of the sedative agent. Monitoring was concluded 10 min after conclusion of the surgical procedure. All readings were recorded by an unwashed trained blinded nurse.
Level of consciousness/alertness—Modified Observer’s Assessment of Alertness/Sedation (OAA/S) was assessed by the anesthetist every 10 min during the conduct of the procedure and 10 min postoperatively and the readings were noted by the same blinded nurse.
After conclusion of the procedure, all patients treated using sedative agents, were shifted to post operative recovery room with assistance and kept under medical supervision for a minimum period of 2 h. Patients were kept in 15° head up position throughout the postoperative phase. Modified Post Anesthesia Discharge Scoring System (PADSS) was done 2 h after the conclusion of the procedure. Assessment for adequate recovery from sedation was conducted by the anaesthesiologist.
After full recovery and before discharging, the operated patients were asked regarding event amnesia, postoperative nausea and vomiting and data was recorded.
Operating surgeon in each session was requested to opine regarding the co-operation obtained from the patient during the conduct of the procedure. Patient co-operation scale was used which as maximum score of 9. Score of (0–3) was good, (4–6) fair, (7–9) poor.
Patients were discharged, with accompanying escort.
Results
All patients (n = 20) tolerated both medications well with no serious side effects or minimal complications.
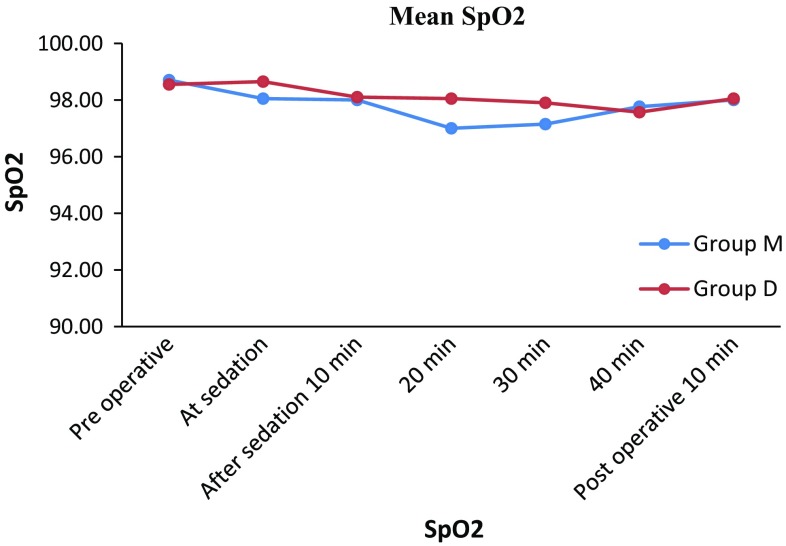
Plasma oxygen saturation, SpO2, did not show significant differences between the Group M and Group D. The mean baseline SpO2 reading in Group M was 98.7%. In Group M, the mean readings at sedation, 10, 20, 30, 40 min after sedation and 10 min postoperatively were 98.05, 98.00, 97.00, 97.1597.76 and 98.00% respectively.
The mean baseline readings in Group D were 98.55%. In Group D, the mean readings at sedation, 10, 20, 30, 40 min after sedation and 10 min postoperatively were 98.65, 98.10, 98.05, 97.90, 97.57, 98.05% respectively.
The lowest recorded plasma oxygen saturation in a single patient in Group M was 93% at 20 min after sedation with baseline line value recorded as 99%. Similarly in a single patient in Group D the lowest recording of SpO2 was 94% at 10 min after sedation with a base line value of 98%.
On statistical analysis, the only time where significant differences in plasma oxygen saturation between the Group M and Group D were evident was at 20 and 30 min after sedation (Fig. 3; Table 1).
Fig. 3.
Showing plasma oxygen saturation (SpO2) in groups M and D preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
Table 1.
Showing mean and standard deviation (SD) of plasma oxygen saturation (SpO2) in Groups M and D preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
| SpO2 at | Number of patients | Group M | Group D | P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Plasma oxygen saturation (SpO2 ) | ||||||
| Pre operative | 20 | 98.70 | 0.92 | 98.55 | 0.94 | 0.614 |
| At sedation (before administration of LA) | 20 | 98.05 | 0.94 | 98.65 | 0.99 | 0.057 |
| LA administered (10 min after sedation) | 20 | 98.00 | 1.03 | 98.10 | 1.33 | 0.792 |
| 20 min (after sedation) | 20 | 97.00 | 1.49 | 98.05 | 1.05 | 0.014* |
| 30 min (after sedation) | 20 | 97.15 | 1.18 | 97.90 | 0.91 | 0.031* |
| 40 min (after sedation) | 20 | 97.76 | 0.56 | 97.57 | 1.02 | 0.532 |
| Post operative 10 min | 20 | 98.00 | 0.79 | 98.05 | 0.60 | 0.824 |
Bold values are suggestive of the statistically significant p values while comparing results of plasma oxygen saturation in group M and group D
* Statistically significant change observed
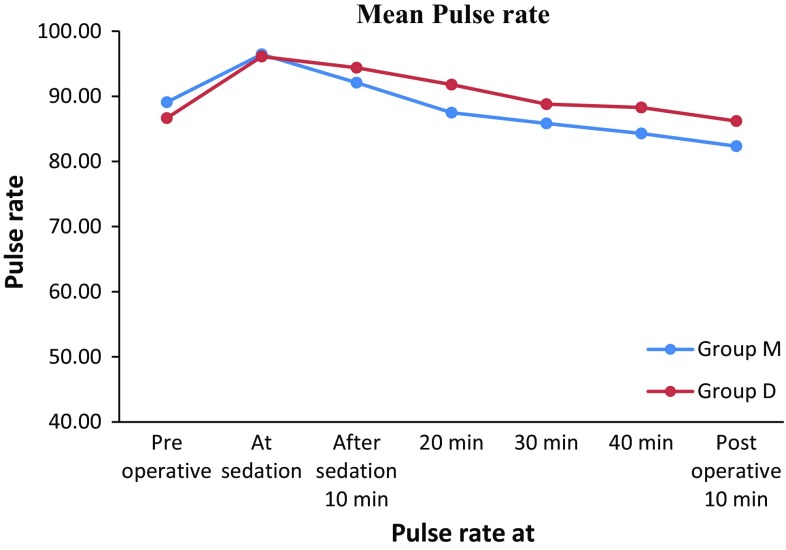
The mean baseline pulse rate readings in Group M were 89 beats/min. The mean readings at sedation, 10, 20, 30, 40 min after sedation and 10 min postoperatively were 96, 92, 87, 85, 85, 82 beats/min respectively. In Group M, the maximum pulse rate recorded in a patient was 130 beats/min at 10 min after sedation with a baseline value of 92 beats/min and lowest pulse rate recorded was 70 beats/min at 30 min with the baseline value of 85 beats/min.
The mean baseline pulse rate reading in Group D was 86 beats/min. The mean readings at sedation, after sedation 10, 20, 30, 40 and 10 min postoperatively were 96, 94, 91, 88, 88, 86 beats/min respectively.
The maximum pulse rate recorded in Group D was 117 beats/min at sedation before administering LA with a baseline value of 86 beats/min and lowest pulse rate recorded was 70 beats/min at preoperative with baseline value of 83 beats/min. Though there was slight increase in the pulse rate in Group M, it was statistically and clinically found insignificant when compared with Group D (Table 2; Fig. 4).
Table 2.
Showing mean and standard deviation (SD) of pulse rate in groups M and D at preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
| Pulse rate at | Number of patients | Group M | Group D | P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Pulse rate | ||||||
| Pre operative | 20 | 89.10 | 5.84 | 88.65 | 5.92 | 0.196 |
| At sedation (before administration of LA) | 20 | 96.45 | 10.35 | 96.10 | 12.04 | 0.922 |
| LA administered (10 min after sedation) | 20 | 92.10 | 11.21 | 94.40 | 10.55 | 0.508 |
| 20 min (after sedation) | 20 | 87.50 | 8.31 | 91.80 | 12.06 | 0.198 |
| 30 min (after sedation) | 20 | 85.85 | 9.28 | 88.80 | 10.14 | 0.343 |
| 40 min (after sedation) | 20 | 84.29 | 8.27 | 88.29 | 10.19 | 0.249 |
| Post operative 10 min | 20 | 82.35 | 8.63 | 86.20 | 8.98 | 0.175 |
Fig. 4.
Showing mean pulse rate in groups M and D preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
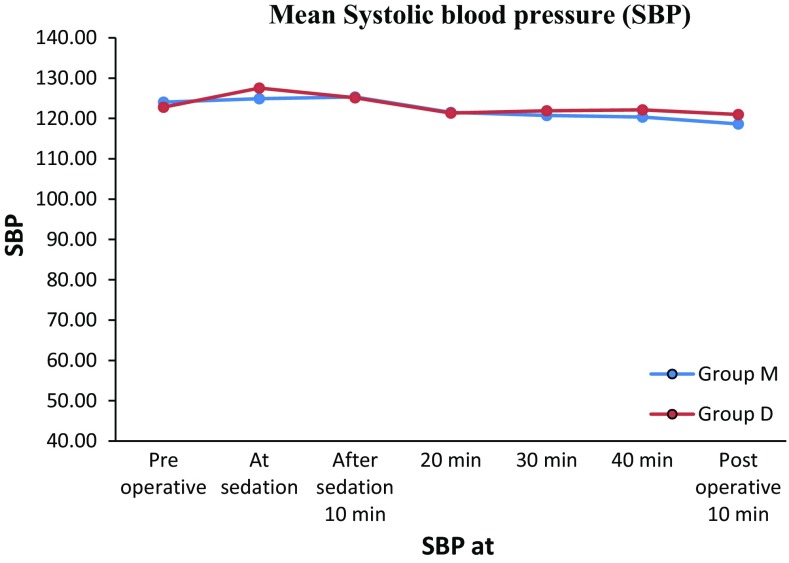
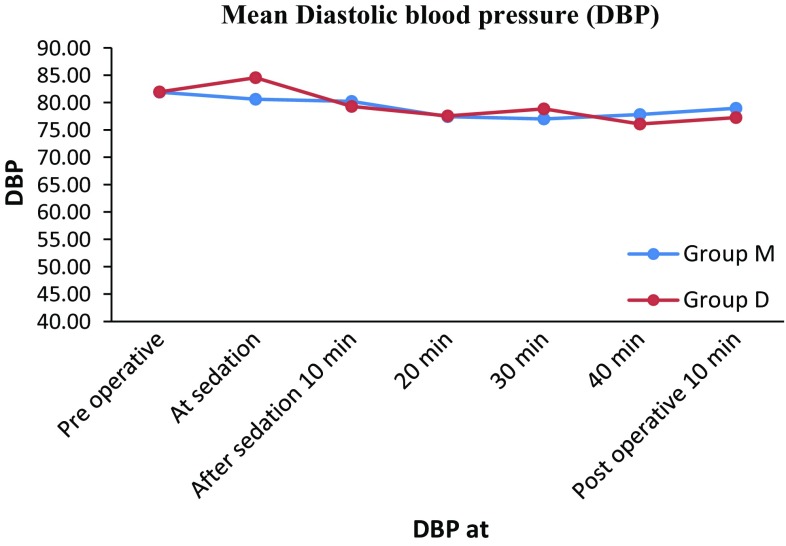
The mean baseline blood pressure reading in Group M was 124/81 mm of Hg. The mean readings at sedation, 10, 20, 30, 40 min after sedation and 10 min postoperatively were 124/80, 125/80, 121/77, 120/77, 120/70 and 118/78 mm of Hg respectively.
The mean baseline blood pressure reading in Group D was 122/81 mm of Hg. The mean readings at sedation, 10, 20, 30, 40 min after sedation and 10 min postoperatively were 127/84, 125/79, 121/77, 121/78, 122/76 and 121/77 mm of Hg respectively.
In Groups M and D there was slight increase in mean blood pressure (SBP and DBP) followed by reduction in the mean blood pressure. Moreover there was no evidence of statistical and clinical change in mean blood pressure from mean base line value in both the groups. (Tables 3, 4; Figs. 5, 6).
Table 3.
Showing mean and standard deviation (SD) of Systolic blood pressure (SBP) in Groups M and D at preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
| Systolic blood pressure at | Number of patients | Group M | Group D | P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Systolic blood pressure | ||||||
| Pre operative | 20 | 124.05 | 7.29 | 122.80 | 12.14 | 0.696 |
| At sedation (before administration of LA) | 20 | 124.90 | 10.76 | 127.55 | 9.70 | 0.419 |
| LA administered (10 min after sedation) | 20 | 125.35 | 5.61 | 125.15 | 9.37 | 0.935 |
| 20 min (after sedation) | 20 | 121.50 | 6.31 | 121.35 | 9.53 | 0.954 |
| 30 min (after sedation) | 20 | 120.75 | 5.59 | 121.90 | 8.08 | 0.604 |
| 40 min (after sedation) | 20 | 120.35 | 4.66 | 122.14 | 10.16 | 0.551 |
| Post operative 10 min | 20 | 118.65 | 5.90 | 121.00 | 8.84 | 0.330 |
Table 4.
Showing mean and standard deviation (SD) of diastolic blood pressure (DBP) in Groups M and D at preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
| Diastolic blood pressure at | Number of patients | Group M | Group D | P value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Diastolic blood pressure | ||||||
| Pre operative | 20 | 81.90 | 4.44 | 81.95 | 4.54 | 0.972 |
| At sedation (before administration of LA) | 20 | 80.60 | 4.98 | 84.55 | 7.82 | 0.066 |
| LA administered (10 min after sedation) | 20 | 80.20 | 7.05 | 79.30 | 5.15 | 0.648 |
| 20 min (after sedation) | 20 | 77.42 | 7.12 | 77.55 | 3.17 | 0.943 |
| 30 min (after sedation) | 20 | 77.00 | 4.99 | 78.85 | 3.80 | 0.196 |
| 40 min (after sedation) | 20 | 77.82 | 6.06 | 76.07 | 2.59 | 0.292 |
| Post operative 10 min | 20 | 78.95 | 4.16 | 77.25 | 5.34 | 0.269 |
Fig. 5.
Showing mean Systolic blood pressure (SBP) in Groups M and D preoperative, At sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
Fig. 6.
Showing mean Diastolic blood pressure (DBP) in groups M and D preoperative, at sedation before administering LA, 10, 20, 30, 40 min after sedation and postoperative 10 min
None of the patients in both groups had OAA/S score below 4 (Lethargic response to name spoken in normal tone).
In the twenty procedures performed in Group M (using midazolam) six patients (30%) had total amnesia and fourteen patients (70%) had partial amnesia. In the twenty procedures performed in Group D (using dexmedetomidine) four patients (20%) had total amnesia and sixteen patients (80%) had partial amnesia. In our study none of the patients were able to recollect all the events completely (total/partial amnesia).
In the twenty procedures performed in Group M (using midazolam) three patients (15%) had nausea. In the twenty procedures performed in Group D (using dexmedetomidine) no patient reported nausea (0%). None of the patients in both groups had post operative vomiting.
In Group M, out of twenty procedures, six patients (30%) had patient co-operation score of 4 (fair) while fourteen patients (70%) had cooperation score of 2 (good). Similarly, in Group D, out of twenty procedures, three patients (15%) had patient co-operation score of 5 (fair) while seventeen (85%) patients had a score of 2 (good). The co-operation from the most of the patients in both the groups was good.
Discussion
Every surgical procedure is associated with apprehension and anxiety. With reduction in pain and anxiety comes better patient cooperation and satisfaction. Sedation is an effective and well known method to achieve reduction in pain as well as anxiety.
The comparative evaluation of midazolam and dexmedetomidine administered by the intravenous route is well documented. However to our knowledge their comparative evaluation as intranasal formulation via intranasal atomization device in third molar surgery has not been published.
In our study, in Group M, the base line mean plasma saturation was 98%. Furthermore all patients maintained SpO2 above 93% throughout the procedure. Readings in our study are similar to those reported by Donald M. Yealy and Hasan Garip. Their studies did not record any significant reduction in plasma oxygen saturation in patients undergoing minor surgical procedures using sedative agents [1, 4]. In contrast, Larry P. Parworth [2] stated that of fifty-seven subjects in their study, two subjects had periods of apnea greater than 20 s. These patients began breathing when stimulated, and none required assisted ventilation. However in our study no such period of apnea was encountered in any of the patients in Group M.
In our study, in Group D, the base line mean plasma saturation was also 98%. All patients maintained SpO2 above 94% throughout the procedure. In our study observation no clinically significant drop in plasma oxygen saturation was observed. Our findings are consistent with the observations of N. Nooh et al., Mostafa G. Mostafa, Yakup Ustun, Xia Zhang et al. [9, 20, 24, 31].
In our comparative study, on statistical analysis, significant differences in plasma oxygen saturation between the two groups were evident was at 20 and 30 min after sedation. None of the patients in either group required supplemental oxygen.Compared with the mean preoperative baseline values, mean percentile drop of plasma oxygen saturation was not greater than 1% in both the groups.
In Group M the mean baseline pulse rate was 89 beats/min. Though there was slight increase in the mean pulse rate after administration of intranasal midazolam; however, this change was statistically and clinically insignificant. These observations are similar to those of Larry P. Parworth, Ozgen Goktay, Hasan Garip et al. [2, 4, 7].
In Group D the mean baseline pulse rate was 88 beats/min. Our observations were consistent with the observations of Laila Makary, Yakup Ustun, Mostafa G. Mostafa [20, 21, 24] as there was no statistically significant change in mean pulse rate after administering intranasal spray of dexmedetomidine. None of the patients required intervention for altered pulse rates.
We would also like to emphasize that pulse rate remained stable without evidence of bradycardia or tachycardia in both Groups. Moreover, there was no difference between the changes in the pulse rate intraoperatively after giving sedation from baseline pulse rate between the two groups.
In Group M, the mean baseline blood pressure (SBP/DBP) was 124/84 mm of Hg. There was a transient increase in the mean blood pressure (SBP and DBP) after administration of intranasal midazolam. No statistical and clinically significant change from mean base line value was observed in Group M. Our observations are in accordance with the Parworth [2] and Garip [4]. In Group M the highest blood pressure recorded during the procedure was 141/90 mm of Hg at sedation before administering LA and lowest blood pressure recorded was 108/58 mm of Hg at 20 min after sedation.
In Group D, the mean baseline blood pressure (SBP/DBP) was 122/81 mm of Hg. There was transient increase in the mean blood pressure (SBP and DBP) after administration of intranasal dexmedetomidine. There was no evidence of statistically significant change in mean blood pressure from mean base line value in Group D. In Group D the highest recorded blood pressure during the procedure was 156/98 mm of Hg at sedation and lowest blood pressure recorded was 105/78 mm of Hg at 40 min after sedation.
Our study observations are unlike the observations of previous studies that mean blood pressure including SBP and DBP remained clinically stable with tendency of achieving lower values than the baseline values for dexmedetomidine [8, 9, 20, 24].
In our comparative evaluation between intranasal midazolam and dexmedetomidine, there was no difference in changes in blood pressure which were clinically evident between the two groups.
Larry P. Parworth in his study compared intravenous propofol with fentanyl and midazolam with fentanyl during third molar surgery and concluded that no significant reduction in vital parameters (SpO2, pulse rate and blood pressure) was encountered. Cardiovascular parameters remained stable throughout induction, maintenance, and recovery in both groups. Moreover they believed that fentanyl increases the sedative effects of both the drugs used in the study [2].
The modified Observer’s Assessment of Alertness/Sedation (OAA/S) scale was used in our study to determine the adequacy of anxiolysis and sedation during the surgical procedure. Tai Weng Victor Fan in his randomized double-blinded study while comparing intravenous dexmedetomidine (0.1 mcg/kg/min infusion) and midazolam (loading infusion of 0.005 mg/kg/min) for conscious sedation in third molar and dental implant surgery determined sedation levels using OAA/S. They reported dexmedetomidine produced comparable sedation to midazolam. Dexmedetomidine seemed to produce less anxiety and was associated with a lower OOA/S, (minimum of 2.53). Similar studies were conducted by and their results correlated with our study [8, 9, 31, 32].
In our study observation none of the patients in Groups M or D had OAA/S score below four (lethargic response to name spoken in normal tone). We emphasize the fact that patients in both the Groups remained in milder planes of sedation, adequate enough to alleviate their anxiety and were conscious enough to prevent the risk of aspiration while operating intraorally.
Amnesia is a common characteristic of dexmedetomidine and benzodiazepines, including midazolam.
In our study observation of twenty patients in Group M, 30% patients (n = 6) had total amnesia and 70% patients (n = 14) had partial amnesia. Whereas in Group D 20% patients (n = 4) had total amnesia and rest 80% patients (n = 16) had partial amnesia. None of the patients were able to recollect all the events completely. In this comparative evaluation most of the patients had partial amnesia in both the groups.
Garip [4] compared midazolam and midazolam with remifentanil for patient-controlled sedation during operations on third molars and supports the view that the combination of midazolam and remifentanil produces a better level of amnesia. They were in agreement with Bell and Kelly who also found complete amnesia for memories measured with picture cards after third molar extractions.
Ustun [20] in double-blinded, crossover, randomized study compared intravenous dexmedetomidine (4 mcg/kg/h) versus midazolam (0.4 mg/kg/h) in outpatient third molar surgery and reported that adequate amnesia was present in Group M, however, no amnesia was demonstrated in Group D.
In our study observations, in Group M only 15% patients (n = 3) complained of nausea. None of these patients required medication. Yealy [1] used 0.2 to 0.5 mg/kg intranasal midazolam for surgical repair of laceration for children and reported no nausea or vomiting.
In our study in Group D none of the patients complained of nausea. Our study observations are consistent with Akkaya [23] who used dexmedetomidine at 1 mcg/kg intravenous infusion for endoscopic surgery and found no nausea/vomiting. Chen [27] compared the efficacy of dexmedetomidine (1 mcg/kg) and propofol (0.1 mg/kg) for sedation of tube retention after oral and maxillofacial surgery and reported nausea in 5% of cases.
In our study, none of the patients in both groups had post operative vomiting. Our findings are consistent with the observations of Yealy [1] and Akkaya [23] who used midazolam and dexmedetomidine respectively.
In contrast to Chen et al. [27] compared the efficacy of dexmedetomidine (1 mcg/kg) and propofol (0.1 mg/kg) for sedation of tube retention after oral and maxillofacial surgery and reported vomiting in 5% of cases.
In Group M, six patients (30%) had patient co-operation score of 4 (fair) while fourteen patients (70%) had cooperation score of 2 (good).
Similarly, in Group D, out of twenty procedures, three patients (15%) had patient co-operation score of 5 (fair) while, seventeen (85%) patients had a score of 2 (good). The co-operation from the most of the patients in both the groups was good. In our observation, surgeons had a good patient co-operation and reduction in level on fear and anxiety in both the groups.
Ustun [20] in double-blinded, crossover, randomized study compared intravenous dexmedetomidine (4 mcg/kg/h) versus midazolam (0.4 mg/kg/h) in outpatient third molar surgery and reported that operator indicated preference to dexmedetomidine over midazolam in cooperation during the procedure.
Conclusion
In our study the results conclude that midazolam and dexmedetomidine have similar results when administered by intranasal route. However studies comparing the drugs when administered by intravenous route appear to confirm the superiority of dexmedetomidine over midazolam [20, 8]. Intranasal Midazolam and Intranasal Dexmedetomidine are equivalent and can be used in minor oral surgery with minimal complications. Procedures should be performed in the presence of a qualified anaesthesiologist with ready availability of emergency drugs and equipment. Midazolam and dexmedetomidine can be safely used intranasally using nasal atomization device in otherwise normal healthy but anxious patients. The choice of drug to be used depends solely on the operator.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest is present.
Ehtical standards
The study was approved by Research and Ethical committee of Bharati Vidyapeeth Deemed University, Pune.
Informed consent
All the patients included in the study were provided with subject information sheet. Patients signed the informed consent of the institute proforma as well as structured proforma of the study.
References
- 1.Yealy MD. Lntranasal midazolam as a sedative for children during laceration repair. Am J Emerg Med. 1992;10:684–687. doi: 10.1016/0735-6757(92)90190-9. [DOI] [PubMed] [Google Scholar]
- 2.Parworth LP. Propofol and fentanyl compared with midazolam and fentanyl during third molar surgery. Oral Maxillofac Surg. 1998;56:447–453. doi: 10.1016/S0278-2391(98)90710-8. [DOI] [PubMed] [Google Scholar]
- 3.Kucukyavuz Z. Effects of low-dose midazolam with propofol in patient-controlled sedation (PCS) for apicectomy. Br J Oral Maxillofac Surg. 2004;42:215–220. doi: 10.1016/j.bjoms.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Garip H. A comparison of midazolam and midazolam with remifentanil for patient-controlled sedation during operations on third molars. Br J Oral Maxillofac Surg. 2007;45:212–216. doi: 10.1016/j.bjoms.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Kohjitani A. Oral midazolam for sedation in minor oral operations in children: a retrospective study. Br J Oral Maxillofac Surg. 2008;46:330–331. doi: 10.1016/j.bjoms.2007.07.203. [DOI] [PubMed] [Google Scholar]
- 6.Garip H. Conscious intravenous sedation on pain, swelling, and trismus after surgical extraction of third molars. J Oral Maxillofac Surg. 2011;69:1023–1030. doi: 10.1016/j.joms.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Goktay O. A comparison of the effects of midazolam/fentanyl and midazolam/tramadol for conscious intravenous sedation during third molar extraction. J Oral Maxillofac Surg. 2011;69:1594–1599. doi: 10.1016/j.joms.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Fan TWV. Comparison of dexmedetomidine and midazolam for conscious sedation in dental surgery monitored by bispectral index. Br J Oral Maxillofac Surg. 2013;51:428–433. doi: 10.1016/j.bjoms.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Nooh N. Intranasal atomized dexmedetomidine for sedation during third molar extraction. Int J Oral Maxillofac Surg. 2013;42:857–862. doi: 10.1016/j.ijom.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed MH. Comparative study between dexmedetomidine/nalbuphine and midazolam/nalbuphine in monitored anesthesia care during ear surgery. Egypt J Anaesthesia. 2014;30:7–12. doi: 10.1016/j.egja.2013.09.002. [DOI] [Google Scholar]
- 11.Kaufman E. Comparison between lntranasal and intravenous midazolam sedation (with or without patient control) in a dental phobia clinic. J Oral Maxlllofac Surg. 1994;52:840–843. doi: 10.1016/0278-2391(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 12.Scheepers M. Midazolam via the intranasal route an effective rescue medication for severe epilepsy in adults with a learning disability. British Epilepsy Assoc Seizure. 1998;7:509–512. doi: 10.1016/S1059-1311(98)80012-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffith N. Intranasal midazolam for premedication of children undergoing day care anesthesia comparision of two delivery systems with assessment of intra observer variability. Br J Anesth. 1998;81:865–869. doi: 10.1093/bja/81.6.865. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd CJ. Intranasal midazolam as an alternative to general anaesthesia in the management of children with oral and maxillofacial trauma. Br J Oral Maxillofac Surg. 2000;38:593–595. doi: 10.1054/bjom.2000.0534. [DOI] [PubMed] [Google Scholar]
- 15.Wermelinga DP. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83:124–132. doi: 10.1016/j.eplepsyres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Milic M. Complications of sevoflurane–fentanyl versus midazolam–fentanyl anesthesia in pediatric cleft lip and palate surgery: a randomized comparison study. Int J Oral Maxillofac Surg. 2010;39:5–9. doi: 10.1016/j.ijom.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Klein EJ. A randomized clinical trial comparing oral, aerosolized intranasal, and aerosolized buccal midazolam. Ann Emerg Med. 2011;58:323–329. doi: 10.1016/j.annemergmed.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mekitarian Filho E. Alternative for pre-anesthetic sedation and for surgical procedures in children: use of intranasal midazolam. Rev Assoc Med Bras. 2013;59(1):3–4. doi: 10.1590/S0104-42302013000100002. [DOI] [PubMed] [Google Scholar]
- 19.Stoelting RK 4th edition Chapter 5; 141–147
- 20.Ustun Y. Dexmedetomidine versus midazolam in outpatient third molar surgery. J Oral Maxillofac Surg. 2006;64:1353–1358. doi: 10.1016/j.joms.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Makary L. prolonged recovery associated with dexmedetomidine when used as a sole sedative agent in office-based oral and maxillofacial surgery procedures. J Oral Maxillofac Surg. 2010;68:386–391. doi: 10.1016/j.joms.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 22.Boyd BC. Dexmedetomidine sedation for awake fiberoptic intubation of patients with difficult airways due to severe odontogenic cervicofacial infections. J Oral Maxillofac Surg. 2011;69:1608–1612. doi: 10.1016/j.joms.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Akkaya A (2014) Comparison of the effects of magnesium sulphate and dexmedetomidine on surgical vision quality in endoscopic sinus surgery: randomized clinical study, January [DOI] [PubMed]
- 24.Mostafa MG. Premedication with intranasal dexmedetomidine, midazolam and ketamine for children undergoing bone marrow biopsy and aspirate. Egypt J Anaesthesia. 2013;29:131–135. doi: 10.1016/j.egja.2012.10.006. [DOI] [Google Scholar]
- 25.Aydogan MS. Effects of dexmedetomidine and midazolam on motor coordination and analgesia: a comparative analysis. Current Therapeutic Research. 2013;75:22–26. doi: 10.1016/j.curtheres.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouchi K. Dexmedetomidine dose-dependently enhances local anesthetic action of lidocaine. J Oral Maxillofac Surg. 2014;72:474–480. doi: 10.1016/j.joms.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Che J. Safety of dexmedetomidine versus propofol for the sedation of tube-retention after oral maxillofacial surgery. J Oral Maxillofac Surg. 2014;72:285.e1–285.e7. doi: 10.1016/j.joms.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Rummasak D. Hypotensive agent compared with nitroglycerin in orthognathic surgery. J Oral Maxillofac Surg. 2014;72(12):2428–33. doi: 10.1016/j.joms.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Linares Segovia B. Pre-anaesthetic medication with intranasal dexmedetomidine and oral midazolam as an anxiolytic. A clinical trial. An Pediatr (Barc) 2014;81(4):226–231. doi: 10.1016/j.anpedi.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Miller RD Millers’ anesthesia. 8th ed. vol 1 Chapter 30; 854–858
- 31.Zhang X. The safety and efficacy of intranasal dexmedetomidine during electrochemotherapy for facial vascular malformation: a double-blind, randomized clinical trial. J Oral Maxillofac Surg. 2013;71:1835–1842. doi: 10.1016/j.joms.2013.06.202. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Li S. Comparison of dexmedetomidine/fentanyl with midazolam/fentanyl combination for sedation and analgesia during tooth extraction. Int J Oral Maxillofac Surg. 2014;43:1148–1153. doi: 10.1016/j.ijom.2014.03.019. [DOI] [PubMed] [Google Scholar]