Abstract
Aim
Primary ablative surgery followed by post-op radiotherapy (S-RT) remains the mainstay of treatment for stage III–stage IV oral carcinoma. A retrospective analysis of survival rates of advanced-stage OSCC patients treated with multimodal therapies (S-RT or combined chemoradiation) was performed to analyse the outcome for patient survival and whether addition of adjuvant chemotherapy (S-CRT) improves survival.
Materials and Methods
Demographic, pathological, treatment and follow-up data of 128 patients were included in the study. Sixty-nine patients received S-RT, while 55 patients were opted for S-CRT. Overall survival, disease-specific survival and disease-free survival were estimated with Kaplan–Meier analysis and compared between groups with Cox regression analysis.
Results
Survival was significantly influenced by the type of modality and regional spread of disease. S-CRT group had improved overall, disease-specific, disease-free and metastasis-free survival compared to S-RT group. A survival advantage of 10% was achieved in S-CRT group compared to S-RT group even in patients with extracapsular spread and perineural invasion.
Conclusion
Addition of adjuvant chemotherapy to S-RT improves survival outcomes in advanced OSCC, especially in patients with regional spread of disease.
Keywords: Multimodal therapy, Oral carcinoma, Extracapsular spread, Survival
Introduction
Overall 57.5% of global head and neck cancers occur in Asia, especially India with nearly 80,000 new cases being diagnosed every year. More than 60% of oral squamous cell carcinomas (OSCC) patients are presented with advanced stage (stage III and IV) as compared to 40% in developed countries due to poor screening facilities and lack of awareness among patients [1]. The prognosis of patients with OSCC depends on wide range of factors which include size and site of tumour, thickness of tumour, degree of differentiation and spread into regional lymph nodes [2]. In fact, the presence of regional metastasis to the cervical lymph nodes reduces the prognosis by 50% [1, 2]. The classical treatment for advanced OSCC involves primary surgical resection with postoperative adjuvant radiotherapy (S-RT). Despite this aggressive dual modality regime, the disease outcomes have remained non-promising.
The results of two landmark trials (2004), Radiation Therapy Oncology Group (RTOG) 9501 occurred in the USA and European Organisation for Research and Treatment of Cancer (EORTC) 22931, expanded the horizons for the management of advanced head and neck SCC [3–5]. Level-1 evidence from these two similar trials suggested that, as compared to postoperative RT alone, adjuvant concurrent chemoradiotherapy (CRT) was more efficacious in achieving better locoregional control and disease-free and overall survival in stage III–IV head and neck cancer patients. Recently, based on the evidence from these two trials, many centres have adopted triple-modality treatment for advanced-stage head and neck SCC [6–9].
Very few studies in the literature are OSCC site-specific who reviewed the efficacy of multimodal therapy for treatment of stage III–IV carcinoma especially in India. Objective of this study was to assess the effect of multimodal therapy on overall survival, disease-specific survival and disease-free survival rates of stage III–IV OSCC patients and to determine whether addition of adjuvant chemotherapy (S-CRT) improves the survival outcome in Asian Indian subset of population.
Material and Method
Based on the data collected from retrospective chart reviews, the study was performed. Only advanced-stage (stage III–stage IV) OSCC patients, treated with curative intent, were included in the study after ethical approval by the institute and informed consent of patients.
Patients
The selection criteria were as follows:
Inclusion Criteria
Biopsy-proven OSCC patients.
Locally and regionally resectable tumour.
Patients with adverse features like extracapsular spread (ECS), lymphovascular invasion (LVI) and perinural invasion (PNI).
Exclusion Criteria
History of second primary cancers.
Patients with tumours down-staged to stage I and II after histopathology (pT and pN status).
Patients with incomplete information on treatment modality in data reviews.
Data Collection and Staging
Demographic data were retrieved for 128 patients of OSCC treated at our institute between June 2008 and June 2015. Data were collected according to age, gender, site of disease, surgical treatment, TNM stage, overall staging, final histopathology, follow-up, outcome and survival.
Staging of the tumours was clinical and according to the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging manual (2009) [4].
Treatment Groups
All the patients underwent excision of the primary tumour followed by neck dissection (type depicted by the stage of tumour, nodal status and need for reconstruction) with postoperative RT or concurrent CRT within 6 weeks of definitive surgery. All patients were operated by the same surgical team.
Patients were grouped for analysis according to the treatment modality:
Group-1 Surgery followed by radiotherapy alone (S-RT).
Patients underwent surgical resection and neck dissection followed by adjuvant RT within 6 weeks postoperatively. Doses for the adjuvant RT ranged from 5500 to 7000 GY in 30–33 fractions with an administration regime of 5 times a week.
Group-2 Surgery followed by concurrent chemoradiotherapy (S-CRT).
Patients were adopted for surgical resection and neck dissection followed by postoperative adjuvant concurrent CRT. Cisplatin or carboplatin based CRT protocols were adopted exclusively. 100 mg/m2 of concurrent cisplatin was administered every three weeks during RT.
Patients with adverse features on histopathology were administered CT along with RT. The adverse features are defined to be ECS, PNI and LVI. Prior to 2010, adjuvant RT was adopted for all patients with any or all adverse features as the concurrent CRT protocols were started in our institute in 2010.
Follow-Up
Patients were called personally through postal and telecommunication means for follow-up. Patients were examined clinically for recurrence and locoregional failures. PETCT scan, ultrasound neck and MRI scan were performed where and when indicated.
Survival Statistics and Outcomes
Overall survival and disease-free survival were calculated using Kaplan–Meier analysis to determine year-specific estimated actuarial survival rates. The log rank test was employed to determine the presence of significant difference (p < .05) between treatment groups. Cox regression analysis with covariates of T stage, N stage and overall stage was performed for overall survival. Categorical data were compared using Chi-square test. Analyses were performed with SPSS statistics 18.0 version (SPSS Inc. Chicago IL).
The primary outcome measure was set as overall survival. This was calculated as the time from the first date of treatment to the date of death or last known date the patient was alive. Patients who had not died or who were lost to follow-up were censored for overall survival when they were last known to be alive. Disease-free survival was measured from the first day of treatment to the date of disease recurrence. Metastasis-free survival was measured from the first day of treatment to the date of detection of distant metastasis. The results were analysed according to major indicators of prognosis TNM staging, therapeutic approach, type of neck dissection and tumour recurrence.
Data for short- and long-term toxicities such as osteoradionecrosis (ORN) and swallowing function impairment evaluation were quantitatively noted but due to incongruent information in the retrospective charts, the analysis could not be performed on toxicities.
Results
Demographics
Among the 128 patients, 124 patients were analysed (n = 69 in S-RT group and n = 55 in S-CRT group).
Three patients were deemed medically unfit for any type of adjuvant therapy. One patient developed regional recurrence while undergoing the adjuvant therapy within 6 weeks of surgery. This patient was excluded from the study as the goal of treatment had become palliative from curative. Table 1 demonstrates the demographic data, site distribution, surgical procedures performed for primary tutors and type of neck dissections collectively. Comparison of T and N stage and overall staging between two groups is presented in Table 2. All patients included for analysis had undergone a standard metastatic work-up, and all were staged M0 at the time of diagnosis. Neck dissections were performed according to the clinical stage of tumour preoperatively and status of regional lymph nodes assessed clinically and on radiographic examination (N stage) strictly adhering to the oncological principles.
Table 1.
Demographic data, site of primary tumour, surgical procedures performed and neck dissections
| Variable | No. (%) |
|---|---|
| Mean age (year) | 52.8 |
| Female | 9 (7%) |
| Male | 119 (93%) |
| Site of primary tumour | |
| Retromolar trigone | 43 (33.6%) |
| Mandibular alveolus | 33 (25.8%) |
| Buccal mucosa | 28 (21.9%) |
| Lateral border of tongue | 6 (4.7%) |
| Surgical procedure performed collectively | No. of cases % |
|---|---|
| Hemimandibulectomy | 54.7% |
| Wide excision | 22.7% |
| Three-dimensional bite resection | 7.8% |
| Segmental mandibulectomy | 6.2% |
| Hemiglossectomy | 4.7% |
| Neck dissections performed | No. of cases % |
|---|---|
| MRND II | 47.7% |
| MRND III | 24.2% |
| MRND I | 8.6% |
| RND | 2.3% |
| SOHND | 9.4% |
| Extended SOHND | 8.6% |
| BND | 3.1% |
MRND modified radical neck dissections, RND radical neck dissection, SOHND supraomohyoid neck dissection, BND bilateral neck dissection
Table 2.
Comparison of T stage, N stage and overall staging between groups
| T stage | Group-1 (S-RT) | Group-2 (S-CRT) |
|---|---|---|
| T3 | 38 (55%) | 13 (23.7%) |
| T4 | 31 (45%) | 42 (76.3%) |
| N stage | ||
| N0 | 34 (49.3%) | 10 (18.2%) |
| NI | 14 (20.3%) | 19 (34.5%) |
| N2a | 12 (17.4%) | 23 (42.0%) |
| N2b | 9 (13.0%) | 3 (5.4%) |
| TNM staging | ||
| Stage III | 25 (36.2%) | 1 (1.8%) |
| Stage IV | 44 (63.8%) | 54 (98.2%) |
| Locoregional recurrence comparison between groups | ||
| Recurrence % | 58% | 25.5% |
| No recurrence % | 42% | 74.5% |
Statistically non significant
Measure of locoregional recurrence between the treatment groups is enumerated in Table 2. The S-CRT group was found to have a 32% lower recurrence rate as compared to S-RT group. The occurrence of adverse features, on histopathology, between groups is demonstrated in Table 3. S-CRT group showed 23% more number of patients with extracapsular spread (ECS) as compared to patients in S-RT group. Forty-eight percentage of patients were found to have more than two levels of lymphovascular invasion (LVI) in neck dissection specimen in S-CRT group as compared to 11% in S-RT group. Twenty-six percentage of patients were diagnosed with perineural invasion (PNI) in S-CRT group as compared to 6% in S-RT group.
Table 3.
Comparison of adverse features and overall survival between two groups
| Adverse feature | Group-1 (S-RT) (%) | Group-2 (S-CRT) (%) |
|---|---|---|
| Extracapsular spread (ECS) | 13 | 36 |
| Lymphovascular invasion (LVI) | 11 | 48 |
| Perinural invasion (PNI) | 6 | 26 |
| Adverse feature | Overall 5-year survival | |
|---|---|---|
| S-RT (%) | S-CRT (%) | |
| Extracapsular spread (ECS) highly significant p = .001 | 30 | 83 |
| Lymphovascular invasion (LVI) highly significant p = .001 | 31 | 71 |
| Perineural invasion (PNI) statistically non significant p < .05 | 30 | 58 |
Survival
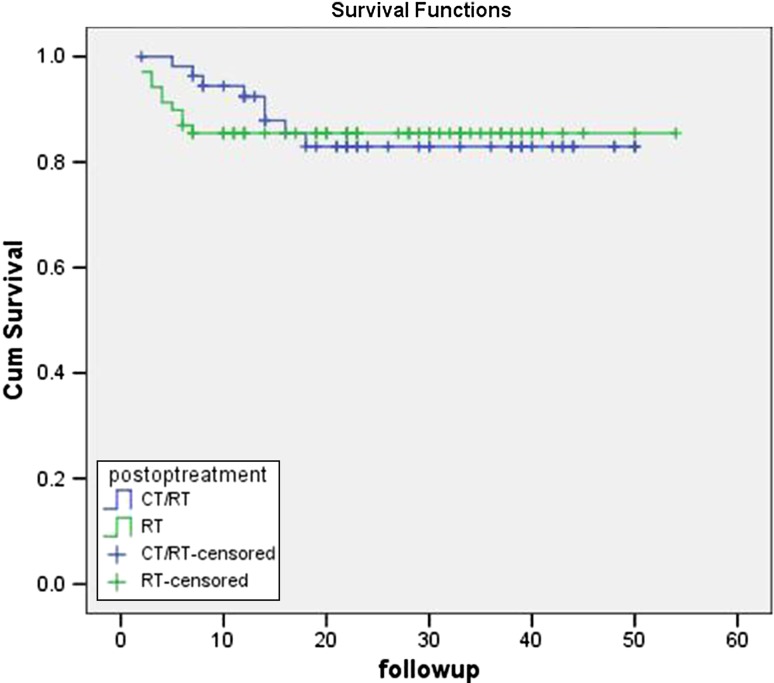
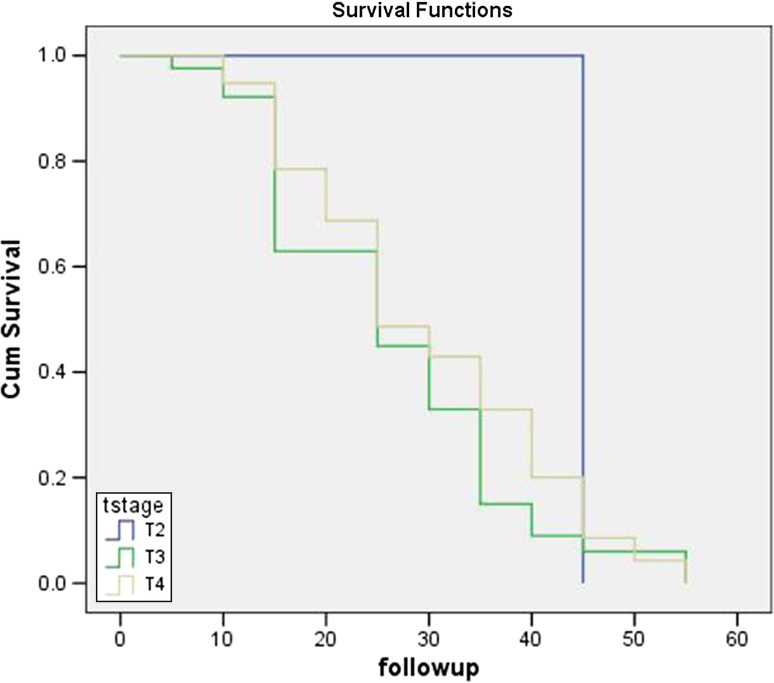
The mean follow-up was 34.2 months with a range of 1–62.2 months. The overall 5-year survival rate for patients in group-1(S-RT) was 43.2% which was less than the patients in group-2 (S-CRT) with 53.2%. The outcome of the patients between the group-1 and group-2 is given in Table 4. The survival rate between the groups was compared using log rank test, and difference was found to be nonsignificant. The mean estimated overall survival of patients according to TNM staging is given in Figs. 1 and 2. Table 3 demonstrates the comparison of 5-year overall survival of patients with adverse features between groups. The survival benefit of ECS and LVI patients came out to be statistically significant (p = .001) between groups with patients in S-CRT group having more benefit than in S-RT group.
Table 4.
Comparison of treatment outcome between groups
| Outcome | Group 1 (S-RT) | Group 2 (S-CRT) |
|---|---|---|
| Alive and well | 49 | 45 |
| Alive with disease | – | 1 |
| Dead with disease | 5 | 6 |
| Lost to follow-up | 15 | 2 |
Statistically non significant
Fig. 1.
Estimated overall survival between RT and CT/RT groups (95% confidence interval)
Fig. 2.
Disease-free survival between T stages (95% confidence interval)
Discussion
Postoperative irradiation improves the outcome of surgically treated advanced squamous cell carcinoma arising in the head and neck, but the 5-year survival rate of disease-free survival is generally less than 30%. One strategy to improve the outcome is to intensify the effects of postoperative RT by administering concurrent CT after complete resection. RTOG 8824, a non-randomised, phase 2 trial, suggested that this approach may decrease the risk of local and regional recurrence among high-risk patients [1–5]. During the last decade, studies have evaluated the utility of triple-modality therapy, adding concurrent CT to postoperative RT in head and neck cancer [6–8].
The EORTC trial showed that the addition of adjuvant CT allowed for significantly increased rates of local control, disease-specific survival and overall survival without high incidence of late adverse effects. These studies in combination with a review of the literature in 2005 for postoperative CRT confirmed the need for more evidence for adjuvant CRT therapy [9]. The current study confirms the findings of the RTOG and EORTC studies, while being site-specific for OSCC.
In the present study, the overall survival for stage III and IV OSCC was found to be higher in S-CRT (53.2%) group than S-RT group (43.2%). This is in accordance with the study by Choi et al. [10] who retrospectively reviewed 861 patients with OSCC treated in Korea from 1984 to 1996. Sixty-three percentage of patients had stage III/IV cancer, and 36% had T3–T4 lesions. The authors found that ablative surgery with postoperative CRT yielded a higher response rate than surgery and RT alone.
The local control rate was higher in S-CRT group (81.8%) than S-RT group (71%) which is in accordance with the study by Cooper et al. [6] who also reported higher control rate in the patients treated by surgical resection followed by CRT (82%) than adjuvant RT (72%) alone.
According to the study conducted by Zhang et al. [3], the patients who received S-RT were 3.6 times more likely to develop metastasis compared to S-CRT group. These findings concur with the present study findings. According to Pericot et al. [11], high incidence of recurrence is associated with size of the primary tumour (T stage), and the difficulty in obtaining margins free of disease, after surgical resection in this location. Since most of the patients in the present study were in T4 stage, the survival rate was lower as compared to the similar studies which have shown higher survival rates in S-CRT group.
Similarly, nodal status is also an independent predictor of overall survival. Patients with clinically N0 necks are having highest survival rate followed by subsequent N stages. Franceschi et al. [12] found that among patients with involved nodes, survival rate was significantly lower when more than two levels of lymph nodes were involved, or when extracapsular spread (ECS) was present. This is in agreement with the present study in which survival rate was lower in patients with advanced nodal stage (N + <N0). The adverse features which are found after final histopathological examination of primary as well as neck dissection specimen are ECS and PNI. These patients are particularly at higher risk of disease progression and have higher death rates. According to Zhang et al. [3], patients with ECS and PNI had improved disease-specific and metastasis-free survival in the S-CRT group (p < .05). Twenty-six patients of ECS and three patients of PNI, in this study, received concurrent CRT postoperatively and demonstrated improved survival rate.
The degree of differentiation is also considered as one of the important prognostic factor in head and neck cancers with poorly differentiated cancers having worst prognosis (Weijers et al.) [13]. In the present study, 96 patients reported with moderately differentiated carcinoma, and 25 patients with poorly differentiated carcinoma which was responsible for favourable survival rate.
Results of the study conducted by Stenson et al. [8] revealed that patients who underwent CRT as primary therapy for advanced OSCC had a high rate of overall survival, with 66.9% progression-free survival. Despite of this improved survival rate, there are chances of recurrence and chemotherapy-related complications such as toxicity, myelosuppression, neutropenia, mucositis and gastritis. Also, there is high incidence of osteoradionecrosis which may be due the fact that disease is already involving bone (retromolar trigone, mandibular alveolus) is not amenable for only irradiation or chemoradiation without primary resection. So, surgery should be the main stay of treatment followed by chemoradiation [14]. The type of radiation (intensity-modulated radiotherapy (IMRT) or conventional RT) also affects the survival outcomes when used concurrently with CT as IMRT is more effective along with CT than conventional RT.
Conclusion
The results of this study support the RTOG and EORTC landmark trials. The addition of postoperative CT for advanced (stage III and IV) OSCC appears to improve overall survival by 10%. The higher rate of metastasis-free survival and disease-free survival suggests that adding chemotherapy to S-RT may prevent distant spread of disease. Favourable locoregional control in S-CRT group, especially in patients with ECS and regional spread of disease (pN + neck), confirms the survival advantage of triple-modality therapy for advanced-stage squamous cell carcinoma of oral cavity.
Compliance with Ethical Standards
Conflict of interests
None of the authors have conflict of interests.
Contributor Information
Amit Dhawan, Email: surg.amit@gmail.com.
Prahlad Duggal, Email: duggalprahlad@yahoo.co.in.
Ramandeep Singh Bhullar, Email: drbhullar07@gmail.com.
Tejinder Kaur, Email: tkgumber@gmail.com.
Amneet Sandhu, Email: sandhuamneet@yahoo.co.in.
Kirandeep Kaur, Email: kiranoralsurgeon@gmail.com.
References
- 1.Zhou CQ, Defatta RJ, Ducic Y. Current therapies and future treatment modalities for oral cavity cancer. In: Nielsen FL, editor. Progress in oral cancer research. New York: Nova Science Publishers, Inc; 2008. pp. 55–72. [Google Scholar]
- 2.Agarwal AK, Sethi A, Sareen D, Dhingra S. Oral and oropharyngeal squamous cell carcinoma in our population: the clinic-pathological and morphological description of 153 cases. Int J Morphol. 2011;29(3):686–693. doi: 10.4067/S0717-95022011000300004. [DOI] [Google Scholar]
- 3.Zhang H, Dziegielewski PT, Biron VL, et al. Survival outcomes of patiemts with advanced oral cavity squamous cell carcinoma treated with multimodal therapy:a multi-institutional analysis. J Otolaryngol Head Neck Surg. 2013;42:30. doi: 10.1186/1916-0216-42-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55:242–258. doi: 10.3322/canjclin.55.4.242. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Pajak TF, Forastiere A, et al. Precisely defining high-risk operable head and neck tumors based on RTOG #85-03 and #88-24:targets for postoperative radiochemotherapy? Head Neck. 1998;20:588–594. doi: 10.1002/(SICI)1097-0347(199810)20:7<588::AID-HED2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JS, Pajak TF, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:19. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 7.Bonne J, Harari P, Giralt J, et al. Radiotherapy plus Cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med. 2006;354:6. doi: 10.1056/NEJMicm050019. [DOI] [PubMed] [Google Scholar]
- 8.Stenson KM, Kunnavakkam R, Cohen EEW, et al. Chemoradiation for patients with advanced oral cavity cancer. Laryngoscope. 2010;120:93–99. doi: 10.1002/lary.21300. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EPRTC (#22931) and RTOG (#9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 10.Choi KK, Kim MJ, Yun PY, et al. Independent prognostic factors of 861 cases of oral squamous cell carcinoma in Korean adults. Oral Oncol. 2006;42:208–217. doi: 10.1016/j.oraloncology.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Pericot J, Escriba JM, Valdes A, et al. Survival evaluation of treatment modality in squamous cell carcinoma of the oral cavity and oropharynx. J Cranio Maxillofac Surg. 2000;28:49–55. doi: 10.1054/jcms.1999.0091. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi D, Gupta R, Spiro RH, et al. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360–365. doi: 10.1016/S0002-9610(05)80333-2. [DOI] [PubMed] [Google Scholar]
- 13.Weijers M, Snow GB, Bezemer PD, et al. Malignancy grading is no better than conventional histopathological grading in small squamous cell carcinoma of tongue and floor of the mouth: a retrospective study in 128 patients. J Oral Pathol Med. 2009;38(4):343–347. doi: 10.1111/j.1600-0714.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa T, Matsuura K, Shiga K, et al. Surgical treatment is recommended for advanced oral squamous cell carcinoma. Tohoku J Exp Med. 2011;223:17–25. doi: 10.1620/tjem.223.17. [DOI] [PubMed] [Google Scholar]