Abstract
Marine seaweeds produce a variety of compounds with different biological activities, including antituberculosis and anticancer effects. The aim of this study was to investigate anti-tuberculosis activity of Sargassum boveanum (S. boveanum) and cytotoxicity of different fractions of this seaweed. S. boveanum was collected from Persian Gulf. The plant was extracted by maceration with methanol-ethyl acetate solvent. The extract was evaporated and partitioned by Kupchan method to yield hexane, tricholoroethane, chloroform, and butanol partitions. The anti-tuberculosis activity of the crude extract and toxicity of the fractions were investigated using green fluorescent protein reporter microplate assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay methods, respectively. The cell survivals of HeLa cell were decreased by increasing the concentration of the extracts. The IC50 values of hexane, tricholoroethane, chloroform, and butanol partitions were 150.3 ± 23.10, 437.0 ± 147.3, 110.4 ± 33.67, and 1025.0 ± 15.20 μg/mL, respectively. The crude extract was not active against tuberculosis. This study reveals that different partitions of S. boveanum have cytotoxic activity against the cancer cell lines.
Keywords: Sargassum, Persian Gulf, Tuberculosis, Cytotoxic
INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by the bacterium Mycobacterium tuberculosis (M. tuberculosis) that mainly injure the lungs, but in some cases other organs of the body may also be damaged (1). The incidence of TB increased in 1985 particularly in patients suffering from acquired immune deficiency syndrome caused by human immunodeficiency virus. The most important difficulty in the treatment of TB is developing resistance to the drugs used to cure the disease because of inconsistent or partial treatment when the illness is considered as multidrug-resistant (MDR) TB. This disorder is a common cause of mortality worldwide that generally represents four deaths each minute. The World Health Organization estimates 1305 cases of MDR-TB annually in Iran. Because of the extensive problem caused by TB, discovering new active metabolites from natural sources is a very important subject in this area (1,2). Cancer is a complex disease accounting as one of the most cause of mortality over the world especially in developed countries (3). The incidence of this invasive disease is increasing rapidly. In the year 2010, 14 million new clinical cases were reported and it is estimated that it will increase to 22 million during the next two decades (4).
Despite rapid and extensive research in the development of new anticancer drugs, effective and safe treatment for different types of cancers is still in high demand. Thus, there is a great interest for searching new chemotherapeutic agents especially from natural sources.
During the last two decades, about 50% of the drugs entered to the market are derived directly or indirectly from natural organisms.
The great diversity of marine organisms in the oceans has led to an increasing attention by researchers to identify unique marine natural therapeutics or pharmaceutical products. In fact, characteristic structurally and chemically metabolites have been isolated from marine natural sources with astounding array of bioactivities, particularly anticancer activity against multiple tumor types, antibiotic, antiviral, antioxidant, anti-inflammatory, and antimalarial activities (5,6).
The active metabolites including terpenoids, alkaloids, and steroids have been isolated from various types of organisms such as seaweeds, soft corals, sponges, mollusks, phytoplanktons, tunicates, echinoderms, and bacteria (7,8). Seaweed is a potential resource of active compounds in the marine habitat. More than 6000 species of seaweeds have been identified around the world and classified, by their colors, to green (Chlorophyta), brown (Phaeophyta), and red (Rhodophyta). Some species of seaweeds have been used as a food source from ancient times. In addition recent data reveals that seaweeds have shown antibacterial, antiviral, antifungal, cytotoxic, antimalarial, and acetylcholinesterase inhibition activities (9,10). Sargassum is a genus of brown (Phaeophyceae) macroalgae (seaweed) in the order of Fucales. They are widely distributed in the temperate and tropical oceans of the world. There are numerous reports on their secondary metabolites and biological activities (11). Their metabolites such as terpenoids have exhibited different biological characteristics such as cytotoxic, antioxidant, vasodilation, and acetylcholine esterase inhibition activities (12,13,14,15).
Iran has expanded in more than 1200 km coastal lines of the Persian Gulf and the Oman sea. There are more than 250 species of various seaweeds in this region (16). The Persian Gulf, with its unique and wide range of biodiversity emerge as a good candidate for new pharmaceutical agents but there are only a limited number of studies about biological properties of these marine organisms. In the current study, anti-tuberculosis and cytotoxic activity of different fractions of Sargassum boveanum (S. boveanum), seaweed from Persian Gulf, was investigated.
MATERIALS AND METHODS
Sample collection and extraction
S. boveanum was collected from Persian Gulf coas in Bushehr Province, Iran in October 2014. Voucher specimens were made and deposited in the herbarium of the School of Pharmacy and Pharmaceutical Sciences of Isfahan University of Medical Sciences and was identified by Agricultural and Natural Resources Research Center of Bushehr. The plant samples were cut into small pieces, completely air-dried, and stored in glass containers until extraction. About 1 kg of the dried plant material was macerated three times with methanol:ethyl acetate (1:1). The extracts were filtered through 2 layers of cotton fabric and evaporated at room temperature, under reduced pressure to get dry residue and then the extract was partitioned to hexane, dichloromethane, butanol, and water through Kupchan partitioning method (17). All partitions were evaporated under reduced pressure and stored in sterile vial pending anti-tuberculosis and cytotoxic tests.
Anti-tuberculosis assay
M. tuberculosis H37Ra (ATCC 25177) and M. tuberculosis H37Rv (ATCC 27294) were Purchased from the American Type Culture Collection. pFPV2 (mycobacterial expression vector pMV261) containing red-shifted, high-expression mutant gfp was obtained from Escherichia coli DH12S provided by Raphael Valdivia, Stanford University, California, USA and was cultured overnight in Luria-Bertani broth with kanamycin (30 μg/ mL) (18).
Electroporation and selection of transformants were performed by the method previously described by Cooksey, et al (19). The transformants (H37Rv gfp and H37Ra gfp) were cultured in 7H9GC broth with Tween 80 and incubated until observation of turbidity. The cultures were analyzed by a Cytofluor II microplate fluorometer (PerSeptive Biosystems, Framingham, Massachusett, USA) with excitation at 485 nm and emission at 508 nm. The transformants with the highest fluorescent were cultured in 100 mL of 7H9GC with Tween 80 and kanamycin.
Green fluorescent protein microplate assay (GFPMA) was used for antimicrobial evaluation. Antimicrobial susceptibility testing was performed in black, clear bottom, 96-well microplates to decrease background fluorescence. Outer-perimeter wells were filled with sterile water to prevent dehydration. Subsequent two-fold dilutions were prepared in 0.1 mL of 7H9GC broth (without Tween 80). Cultures were diluted in 7H9GC, and 105 CFU was added to each test well in a volume of 0.1 mL. Only the wells with drug were used to detect autofluorescence of the compounds. The other control wells consisted of bacteria (B wells) and medium only (M wells). After incubation, fluorescence was measured daily for 8 consecutive days. The mean for triplicate M wells was used as a background subtraction for all test wells and B wells. Percent of inhibition was calculated on day 7 of incubation as below:
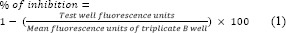
The lowest drug concentration causing 90% inhibition was considered as MIC. At day 7 of incubation, 20 μL from each well was dropped onto complete 7H11 agar plates, and the plates were incubated until countable colonies appeared (approximately 14 days). Antimicrobial susceptibilities were also determined in the BACTEC 460 system as described previously (20). Briefly, two-fold dilutions of antimicrobial agents were prepared and 50 μL was transported to individual BACTEC vials. The inoculum was also made and diluted in BACTEC 12B medium, and 0.1 mL containing 2 × 106 CFU was delivered to 4 mL of BACTEC 12B medium. Some of the control vials received an inoculum which was further diluted 1:100. The growth index (GI) for each vial was determined until the GI of the 1:100 controls reached at least 30. The GI was then read on the following day, and the GI and daily changes in GI (DGI) were recorded for each drug dilution. The MIC was defined as the lowest drug concentration for which the DGI was less than the DGI of the 1:100 control.
Cell culture
Cervical cancer cell line (HeLa), and human umbilical vein endothelial cells (HUVEC), were obtained from the Pasture Institute of Iran, Tehran. Cells were grown in RPMI-1640 medium (Biosera, France) supplemented with 10 % (v/v) fetal bovine serum (FBS) (Biosera, France), penicillin–streptomycin (100 IU/mL and 100 μg/mL, respectively) (Biosera, France). Cells were incubated in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were reefed every 2 days to obtain monolayer and at 80% confluence, were subcultured.
Cytotoxicity test
Toxicity of butanol, chloroform, hexane, and trichloroethane extracts from S. boveanum were evaluated in HeLa cells as well as HUVEC using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay as described previously (21). Briefly, 180 μL of log-phase cells (5×104 cells/mL) were seeded in 96-well plates and allowed to attach to the bottom of wells for overnight. Then 20 μL of different concentrations of solvent extracts were added and incubated for 48 h at 37 °C and 5% CO2. Every plate had four wells with untreated cells, four wells with cells treated with doxorubicin as the positive control, and four wells with cells treated with 1 % (v/v) dimethyl sulfoxide (DMSO) (Sigma, Germany)as a negative control. To evaluate cell viability, 20 μL of MTT solution (Merck, Germany) (5 mg/mL in phosphate buffered saline) was added to each well which was then incubated at 37 °C and 5% CO2 for 3 h. Then, the old medium was removed and 150 μL of DMSO was added to dissolve any formazan crystals formed. Absorbance was then measured at 570 nm by an enzyme-linked immunosorbent assay plate reader (Awareness Technology Inc., Stat Fax 2100, USA). The percentage of cell viability was calculated using the following equation:
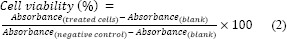
The cytotoxicity value was presented as IC50 (the median growth inhibitory concentration) of the reagents compared to control. IC50 is a measure of the effectiveness of a substance in inhibiting a specific biological or biochemical function.
Statistical analysis
Each assay was repeated at least three times to ensure reproducibility of the results. All data are expressed as the mean ± standard deviation. Significant differences were calculated by analysis of variance (ANOVA) using SPSS version 20 followed by a post Hoc test and differences at P < 0.05 were considered significant.
RESULTS
From 1 kg dry weight of seaweed, 25.7 g extract was derived. Partitioning of the crude extract was led to 12.6 g hexane, 7.4 g trichloroethane, 2.9 g chloroform and 2.1 g butanol extracts.
Anti-tuberculosis activity
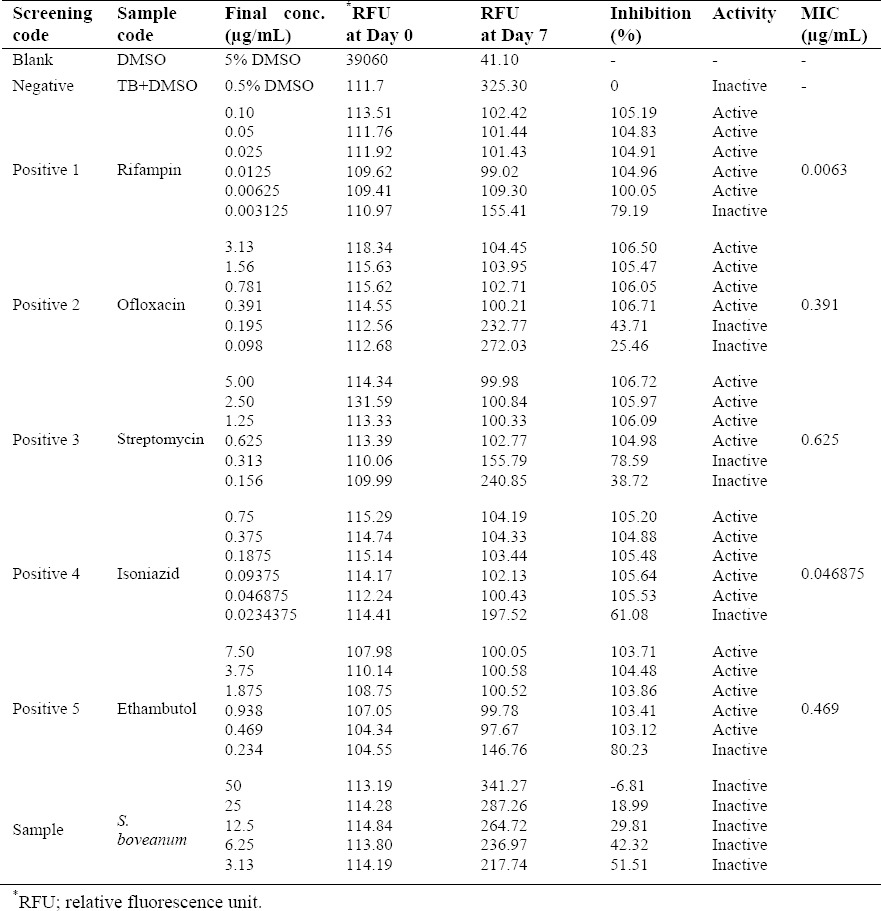
The solution of 0.5% DMSO was used as negative control while rifampicin, streptomycin, isoniazid, ofloxacin, and ethambutol were used as the positive ones with MIC of 0.00312-0.0250, 0.156-0.625, 0.0234-0.0469, 0.391-0.781, and 0.234-0.469 μg/mL, respectively (22,23). The crude extract of S. boveanum could not inhibit 90% growth at final concentration of 50 μg/mL (Table 1).
Table 1.
Anti-tuberculosis activity of the extract of S. boveanum.
Cytotoxicity test
The cytotoxic effects of different extracts of S. boveanum were evaluated against a cancerous cell line (HeLa) (Fig. 1) and a normal cell line (HUVEC) (Fig. 2) by the MTT assay. The multiple concentrations (12.5-400 μg/mL) of butanol, chloroform, hexane, and trichloroethane extracts from S. boveanum were used and IC50 were calculated from dose-response curve.
Fig. 1.
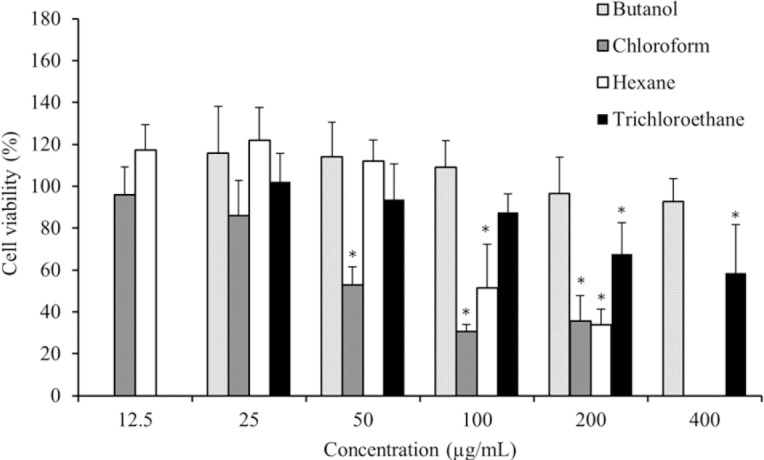
Cytotoxic effects of different extracts of the seaweed S. boveanum against cervical cancer cell line (HeLa) after 48 h incubation. The vertical bars indicate the standard deviations (n = 8). Asterisks indicate the means significantly differs (P < 0.05) from the negative control.
Fig. 2.
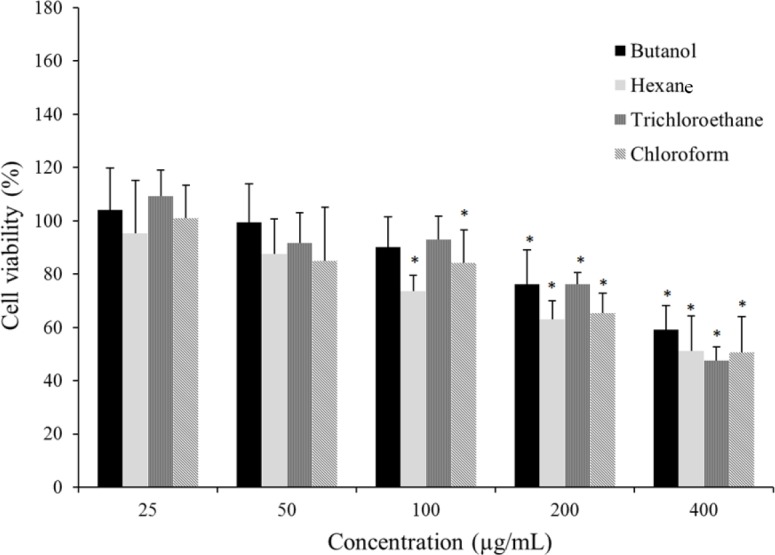
Cytotoxic effects of different extracts of the seaweed S. boveanum against human umbilical vein endothelial cells (HUVEC) after 48 h incubation. The vertical bars indicate the standard deviations (n = 12). Asterisks indicate the means significantly differs (P < 0.05) from the negative control.
As shown in Fig. 1, the chloroform and hexane extracts exhibited the most cytotoxic effect against HeLa in a dose-dependent manner with IC50 value (mean ± SD) of 110 ± 33.67 and 150 ± 23.1 μg/mL, respectively. The butanol extract exhibited no significant cytotoxicity against the HeLa cell line (IC50, 1025 ± 15.2 μg/mL), but the trichloroethane extract demonstrated mild cytotoxic effect (IC50, 437 ± 147.34 μg/mL). Interestingly, HeLa cell proliferation was increased at low concentrations of the hexane and the butanol extracts which may be due to the presence of both stimulatory and inhibitory components in these extracts.
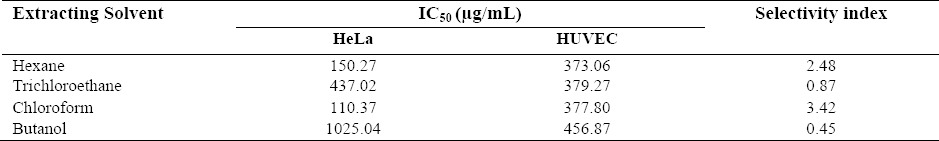
The results of toxicity of different extracts against HUVEC cell line are presented in Fig. 2. The viability of HUVEC cells moderately decreased at high concentrations of the extracts (200 and 400 μg/mL). There were no significant differences in cytotoxic effects of butanol, chloroform, hexane, and trichloroethane extracts and IC50 values (mean ± SD) of 457 ± 71.66, 378 ± 92.74, 373 ± 92.41, and 379 ± 29.70 μg/mL were obtained, respectively. The selectivity index (SI) of each extract which calculated from the IC50 ratio in normal cell line (HUVEC), over cancerous cells (HeLa) is reported in Table 2. The chloroform extract with a SI of 3.4 and the hexane extract with a SI of 2.5 showed the highest selectivity toward HeLa cell line and they are worthy of more investigation.
Table 2.
IC50 values (μg/mL) for different extracts of the seaweed S. boveanum against HeLa and HUVEC cells. Selectivity indices values were calculated as follows; IC50 in HUVEC cell line was divided by IC50 in HeLa cell.
DISCUSSION
Our preliminary findings show the potential cytotoxic activity of the chloroform and the hexane extracts of the seaweed S. boveanum collected from Persian Gulf against a cervical cancer cell line, HeLa, using MTT assay. Other research groups have also investigated the cytotoxic effect of different species of the genus Sargassum sp. Marry, et al. observed that ethanol extract of Sargassum sp. exhibited an inhibitory effect in a dose dependent manner against Hep-2 cell (IC50 = 200 μg/mL) and MCF-7 cell lines (IC50 = 250 μg/mL) (24).
Similar to our results, Tannoury, et al. reported that chloroform: ethanol extract of Sargassum vulgare extract showed higher cytotoxic activity against Jurkat cancer cell line with IC50 values of 49.06 μg/mL compared with water: ethanol extract with 136.91 μg/mL (25).
Similarly, Khanavi, et al. reported the hexane fraction of Sargassum swartzii exhibited the highest toxicity against Caco-2 cells (IC50, 99.9 μg/ml) and HT-29 cell line (IC50, 211.54 μg/mL) compared with other fractions (26). They suggested that this effect was mediated by non-polar cytotoxic compounds. Yegdaneh, et al. has analyzed S. boveanum phytochemically and biologically in a study and reported that tannins, sterols, triterpenes, and saponins were major constituents in this seaweed (14). Partitioning pattern of compounds into different solvents depends mainly on their structure. Non polar compounds will tend to partition to solvents such as hexane, polar compounds are found in butanolic partition while chloroform fraction contains semi polar compounds. We did not investigate fractions phytochemically but it could be postulated that semi polar terpeniods and steroids are the main components of chloroform fraction while non-polar steroids and fatty acids are mainly in hexane partition. Bioassay guided isolation of secondary metabolites in chloroform and hexane fractions are needed to find the active metabolites.
The selectivity of a cytotoxic agent to cancer cells is one of the most important features which should be considered in discovery and screening of the novel anticancer agents. Compounds with low selectivity index (SI values lower than 2.0) have few clinical usage due to toxicity issues (27). According to our results, the chloroform and the hexane extracts demonstrated the highest SIs among the extracts and doxorubicin, the positive control, showed lower selectivity. Selective toxicity was also reported for other Sargassum species.
Tantengco, et al. reported cytotoxic activity of crude extract and fractions (hexane and ethyl acetate) from of Sargassum siliquosum against HCT-116, MCF-7, and A549 cell lines (28). They observed ethyl acetate fraction was more selective than hexane fraction and SI values of 3.01, 3.62, and 3.84 were found for MCF-7, HCT-116, and A549 cells, respectively.
Screening natural resources for their antimicrobial activities is one of the most common investigations in drug discovery, and tuberculosis disease is in an urgent need in this area. Until now, there are different marine compounds isolated from marine organisms with anti-tuberculosis potentials. Massetolide A, a depsipeptide isolated from Pseudomonas species cultures from a marine alga displayed MIC values of 5-10 μg/mL (29). Pseudopteroxazole and seco-pseudopteroxazole are novel benzoxazole diterpene alkaloids isolated from the gorgonian Pseudopterogorgia elisabethae (30,31). These compounds exhibited 97 and 66% growth inhibition, respectively, against M. tuberculosis H37Rv at a concentration of 12.5 μg/mL without any toxicity. These researches illustrate the importance of screening marine organisms as a possible unexplored resource of unique antituberculosis structures. Whereas the crude extract of S. boveanum tested in this work was not active against tuberculosis but work on the other seaweeds from Persian Gulf may lead to the isolation and structure elucidation of a number of exciting new pharmacophores. Although the Persian Gulf of Iran bears a luxuriant and unique treasure of organisms, but there are only limited publications about their pharmaceutical abilities. This was the first report of the anti-tuberculosis activity of S. boveanum. Besides, different fractions of this seaweed were tested for their cytotoxic activities. Further work is necessary to isolate the bioactive cytotoxic compounds.
CONCLUSION
The crude extract of S. boveanum from Persian Gulf, Iran, was screened for anti-tuberculosis activity. Different partitions of this seaweed were tested against HeLa and normal cells. The crude extract was not active against tuberculosis but the cell survivals of HeLa cell were decreased by increasing the concentration of the extracts. The IC50 values of hexane, tricholoroethane, chloroform, and butanol partitions were 150.27 ± 23.1, 437.02 ± 147.34, 110.37 ± 33.67, and 1025.04 ± 15.2 μg/mL, respectively. This seaweed extracts and their active components could emerge as natural and alternative cytotoxic drugs or serve as starting points for synthesizing more effective anticancers.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Pharm.D thesis No. 394804 which was financially supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Bodnar KA, Serbina NV, Flynn JN. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69(2):800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitscher LA, Baker W. Tuberculosis: a search for novel therapy starting with natural products. Med Res Rev. 1998;18(6):363–374. doi: 10.1002/(sici)1098-1128(199811)18:6<363::aid-med1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Adhikari PR. Finland: 2014. Probabilistic Modelling of Multiresolution Biological Data. Department of information and computer science, Aalto university publication series doctoral dissertations 157/2014. [Google Scholar]
- 4.Gulland A. Global cancer prevalence is growing at “alarming pace,” says WHO. BMJ. 2014;348:g1338. doi: 10.1136/bmj.g1338. [DOI] [PubMed] [Google Scholar]
- 5.Schwartsmann G. Marine organisms and other novel natural sources of new cancer drugs. Ann Oncol. 2000;11(3):235–243. doi: 10.1093/annonc/11.suppl_3.235. [DOI] [PubMed] [Google Scholar]
- 6.Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2(4):221–225. doi: 10.1016/s1470-2045(00)00292-8. [DOI] [PubMed] [Google Scholar]
- 7.Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2013;30(2):237–323. doi: 10.1039/c2np20112g. [DOI] [PubMed] [Google Scholar]
- 8.Yegdaneh A, Putchakarn S, Yuenyongsawad S, Ghannadi A, Plubrukarn A. 3-oxoabolene and 1-oxocurcuphenol, aromatic bisabolanes from the sponge Myrmekioderma sp. Nat prod Commun. 2013;8(10):1355–1357. [PubMed] [Google Scholar]
- 9.Solanki R, Khanna M, Lal R. Bioactive compounds from marine actinomycetes. Indian J Microbiol. 2008;48(4):410–431. doi: 10.1007/s12088-008-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinehart KL. Antitumor compounds from tunicates. Med Res Rev. 2000;20(1):1–27. doi: 10.1002/(sici)1098-1128(200001)20:1<1::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.TÜney Ї, Cadirci BH, Ünal D, Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey) Turk J Biol. 2006;30(3):171–175. [Google Scholar]
- 12.Yegdaneh A, Ghannadi A, Dayani L. Chemical constituents and biological activities of two Iranian Cystoseira species. Res Pharm Sci. 2016;11(4):311–317. doi: 10.4103/1735-5362.189307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang HF, Yang-Hua Y, Yao XS, Xu QZ, Zhang SY, Lin HW. Bioactive steroids from the brown alga Sargassum carpophyllum. J Asian Nat Prod Res. 2002;4(2):95–101. doi: 10.1080/10286020290027362. [DOI] [PubMed] [Google Scholar]
- 14.Mehdinezhad N, Ghannadi A, Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res Pharm Sci. 2016;11(3):243–249. [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res pharm sci. 2013;8(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 16.Sohrabipour J, Rabiei R. The checklist of green algae of the Iranian coastal lines of the Persian Gulf and Gulf of Oman. Iran J Bot. 2007;13(2):146–149. [Google Scholar]
- 17.Kupchan SM, Tsou G. Tumor inhibitors. structurev and partial synthesis of fabacein. J Org Chem. 1973;38(5):1055–1056. [Google Scholar]
- 18.Collins LA, Torrero MN, Franzblau SG. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42(2):344–347. doi: 10.1128/aac.42.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooksey RC, Crawford JT, Jacobs WR Jr, Shinnick TM. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob Agents Chemother. 1993;37(6):1348–1352. doi: 10.1128/aac.37.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41(5):1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Ciprofloxacin nano-niosomes for targeting intracellular infections: an in vitro evaluation. J Nanopart Res. 2013;15(4):1–14. [Google Scholar]
- 22.Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988;137(5):1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- 23.Changsen C, Franzblau SG, Palittapongarnpim P. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob Agents Chemother. 2003;47(12):3682–3687. doi: 10.1128/AAC.47.12.3682-3687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mary JS, Vinotha P, Pradeep AM. Screening for in vitro cytotoxic activity of seaweed, Sargassum sp. against Hep-2 and MCF-7 cancer cell lines. Asian Pac J Cancer Prev. 2012;13(12):6073–6076. doi: 10.7314/apjcp.2012.13.12.6073. [DOI] [PubMed] [Google Scholar]
- 25.Tannourya MY, Eliaa JM, Saabc AM, Makhloufb HY, Abboudd JS, Daou-Chaboa RJ, et al. Evaluation of cytotoxic activity of Sargassum vulgare From the Lebanese Coast against Jurkat cancer cell line. J App Pharm Sci. 2016;6(6):108–112. [Google Scholar]
- 26.Khanavi M, Nabavi M, Sadati N, Shams Ardekani M, Sohrabipour J, Nabavi SM, et al. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43(1):31–37. [PubMed] [Google Scholar]
- 27.Koch A, Tamez P, Pezzuto J, Soejarto D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 2005;101(1-3):95–99. doi: 10.1016/j.jep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Tantengco OAG, Limbo C, Montaño MN, Jacinto S. Cytotoxic activity of crude extract and fractions from Sargassum siliquosum (JG Agardh) and other seaweeds against selected human cancer cell lines. Int J Biosci (IJB) 2015;7(2):207–215. [Google Scholar]
- 29.Mdluli K, Slayden RA, Zhu Y, Ramaswamy S, Pan X, Mead D, et al. Inhibition of a Mycobacterium tuberculosis β-ketoacyl ACP synthase by isoniazid. Science. 1998;280(5369):1607–1610. doi: 10.1126/science.280.5369.1607. [DOI] [PubMed] [Google Scholar]
- 30.Snell NJ. The treatment of tuberculosis: current status and future prospects. Expert opin Investig Drugs. 1998;7(4):545–552. doi: 10.1517/13543784.7.4.545. [DOI] [PubMed] [Google Scholar]
- 31.Rouhi AM. Tuberculosis: a tough adversary-With the tools for a renewed battle in place, development of new weapons to combat the ancient plague should proceed at a rapid pace. Chem Eng News. 1999;77(20):52–64. [Google Scholar]


