Abstract
Carbon monoxide (CO), a toxic gas produced via incomplete fossil fuel combustion, has several poisonous effects in the heart including induction of necrosis, apoptosis, and electrocardiogram (ECG) changes. Magnesium sulfate (MS) is a drug with cardioprotective effects especially when used after ischemia/reperfusion. In the current study, we aimed to evaluate MS cardioprotective effects following CO poisoning. Animals were exposed to CO 3000 ppm for 1 h and immediately after the exposure period and on the next 4 days (a total of 5 consecutive doses given on a daily basis), MS (75, 150 and 300 mg/kg) was injected intraperitoneally (i.p.) and ECG was recorded focusing on ST-segment, T-wave, and Q-pathologic wave changes. On day 5, animals were sacrificed and their heart was excised for determination of BAX, BCL2 and Akt expression level using western blot analysis and necrosis investigations. The results showed that MS significantly decreased necrosis and BAX/BCL2 ratio (P < 0.001) while pro-survival protein Akt was significantly increased (P < 0.001). Moreover, CO-induced ST-segment depression, T-wave inversion, and atrioventricular block (AV-block) were decreased following treatment with MS. In conclusion, our results showed that MS could decrease cardiac deleterious effects of CO poisoning including necrosis and apoptosis while increased the expression of Akt, as a cell survival protein.
Keywords: Carbon monoxide, Magnesium sulfate, Cardiotoxicity, ECG, Akt, Necrosis, Apoptosis, BAX/BCL2 Ratio
INTRODUCTION
Poisonings including carbon monoxide (CO) intoxication are one of the predictable and preventable events in health-related conditions (1). CO is produced via incomplete combustion of fossil fuel and is the most common cause of poisoning-induced death from poisoning in the United States annually (2).
In Iran, CO is called “silent-killer” and causes a considerable number of poisonings especially during winter season because of insufficient ventilations when using gas appliances as a source of warming (1,3,4).
In a study, Nazari, et al. showed that in northeast of Iran, 11.6% of unintentional CO poisoning lead to death (4). Despite considerable advances in poisoning management, CO is still the leading cause of unintentional poisoning worldwide (5,6). CO is a colorless, odorless, tasteless and non-irritating gas which exerts its deleterious effects mostly in the organs with high oxygen demand including the brain and heart (5,7,8). Despite advances in toxicology, CO poisoning management has not been markedly changed over the last 100 years and still employs normobaric and hyperbaric oxygen along with symptomatic therapy (7).
CO-poisoning increasingly affects people in the cold seasons and its symptoms are not specific (1,4,9). With increasing CO levels in air and in the absence of sufficient ventilation, carboxyhemoglobin formation increases, the oxyhemoglobin dissociation curve shifts to the left and oxygen delivery to body tissues diminishes (10). Mild CO intoxication symptoms are headache, myalgia, and dizziness as well as neuropsychological consequences (11). Severe exposure to carbon monoxide results in confusion, loss of consciousness, or even death. CO affinity for hemoglobin is more than 200 times higher than that of oxygen and this can cause poisoning even at low CO concentrations (11).
Magnesium is the second most abundant intracellular cation (12). Magnesium sulfate (MS) is a drug that has been long used in obstetrics including eclampsia and pre-eclampsia as well as in management of cardiac arrhythmias (i.e. prevention and treatment of torsade de pointes) (13). Recently, scientists clarified that magnesium has protective effects on heart and brain ischemia and hypoxia (14). Many studies confirmed that intravenous (i.v.) administration of MS before and even after ischemia/reperfusion (I/R) ameliorates deleterious effects of ischemia on the heart (15,16,17,18). Some of these studies did not show significant reduction in infarct size while others showed that MS reduces infarct size, improves vasodilation and nitric oxide (NO) production and exerts Ca2+ antagonistic effects (15).
During the ischemia, similar to what happens in CO poisoning, low levels of oxygen are delivered to tissues. Since magnesium could reduce the effects of I/R on the cardiomyocytes, we hypothesized that it is possibly able to reduce the toxic effects of CO on the heart.
Previous studies have shown that MS prevents torsade de pointes arrhythmia. Therefore, we decided to evaluate electrocardiogram (ECG) changes following CO poisoning. For evaluation of necrosis, hematoxylin and eosin (H&E) staining as the standard method and for investigation of Akt expression level and BAX/BCL2 ratio, western blot analysis were used.
To the best of our knowledge, it is for the first time that the effects of MS on CO poising-induced cardiotoxicity are investigated.
MATERIALS AND METHODS
Animals
Male Wistar rats (8–10 weeks old; 200–250 g body weight) were kept under standard conditions (i.e. at 25 °C with 12 h/12 h light/dark cycles) and had free access to food and water ad libitum. All animal experiments were approved by the animal research Ethics Committee of Zabol University of Medical Sciences (ethical approval ID: Zbmu.1.REC. 1394.111) and performed in accordance with National Institute of Health Guide for the Care and Use of the Laboratory Animals.
Chemicals
For western blot analysis, primary and secondary antibodies for Akt, β-Actin, BAX and BCL2, were purchased from Cell Signaling (Beverly, MA, USA). Coomassie (Bradford) protein assay kit was purchased from Thermo scientific (Rockford, USA). Carbon monoxide capsule (99.999% purity) was obtained from Darman Gas (Tehran, Iran). For ECG recording, Power Lab (AD Instruments, Bella Vista, New South Wales, Australia) was used. MS was purchased from Pasteur Institute, Iran.
Experimental groups and study design
An airtight 12-L Plexiglas container that was connected to oxygen and CO capsules via polyethylene glycol (PEG) tubes was used in this study. A constant flow of CO was set and CO concentration (ppm) was continuously monitored by a CO analyzer (TPI707 carbon monoxide analyzer, Korea). Animals were placed into the container with constant CO concentration of 3000 ± 100 ppm (9). After 1-h exposure to CO, animals were removed from the box and treated with MS (75, 150 and 300 mg/kg/day, intraperitoneally (i.p.)) for five consecutive days (the first dose of MS was administered immediately after CO exposure) according to the experimental scheme (9). On day 5, all animals were anesthetized by ketamine (100 mg/kg) and xylazine (10 mg/kg), sacrificed and the hearts were excised immediately after opening the chest cavity.
Carboxyhemoglobin level assessment
For determination of carboxyhemoglobin levels in serum, blood samples were collected from the rats’ tail vein. Based on the manufacturer’s instructions, serum levels of carboxyhemoglobin were determined using a spectrophotometer calibrated for rats’ blood (Jenway 6305; Bibby Scientific Ltd., Staffordshire, UK).
Electrocardiogram recording
ECG in lead I was recorded using PowerLab (ADInstruments, Bella Vista, New South Wales, Australia) before, during, and after CO poisoning. The ECG was analyzed with special focus on ST segment, T wave, atrioventricular (AV) block type 1 and 2, Q wave, premature ventricular contraction (PVC), sick sinus syndrome, ventricular tachycardia and fibrillation and atrial fibrillation (7).
Histopathological examinations
The hearts of CO-poisoned rats were excised and fixed in formalin 10% for 24 h. Then, samples were transferred to the pathology department of Imam Khomeini hospital, Zabol, Iran for H&E staining.
Akt, BAX, BCL2 and β-actin protein levels assessment
Heart samples were put into cryogenic tubes for western blot analysis. After harvesting the samples, about 200 mg from the left ventricle of each heart was taken for western blot analysis and homogenized (at 1000 rpm for 10 min) in buffer solution (pH 7.5). Protein content in the supernatants was measured by Bradford protein assay kit. After dilution, protein levels of all samples were identical.
Finally, the samples were either used freshly or stored at -80 °C. In order to determine the expression level of Akt, BAX, BCL2 and β-actin in the left ventricle, 5–10 μL of the supernatants was loaded on SDS page wells and proteins were separated using gel electrophoresis. At the end of the electrophoresis, proteins were transferred to polyvinylidene fluoride (PVDF) using transfering buffer (25 mM Tris, 1.2 mM glycine, 20% methanol, pH 8.0). The membrane was washed three times with tris-buffered saline (TBS) each time for 5 min. The blot was incubated with enzyme conjugate containing 10% blocking solution on rocker at room temperature for 1 h. In order to remove any unbound conjugate proteins, the membrane was washed three times with washing buffer each time for 5 min (9). Then, samples were treated with the secondary antibody, washed thoroughly with TBS and Tween 20 (TBST) and visualized by means of enhanced chemiluminescence 500–1000 μL (Pierce, USA). Finally, the results were analyzed using Gene Tools software (SynGene Company).
Statistical analysis
Data were analyzed using SPSS version 16 (SPSS Inc., Chicago, Illinois, USA). One-way ANOVA was used to compare continuous variables and Chi-square and Fisher’s exact tests were used for categorical variables. A P-value of < 0.05 was considered as statistically significant.
RESULTS
Carboxyhemoglobin concentration after exposure to CO
Plasma concentration of carboxy-hemoglobin following exposure to CO 3000 ppm for 1 h was 70 ± 5%; the range of carboxyhemoglobin levels required for confirmation of CO poisoning is 60-76 % (9). Therefore, in this study, CO poisoning has been induced following exposure to CO 3000 ppm for 1 h.
Effect of magnesium sulfate on electrocardiogram changes after exposure to CO
MS administration to CO-poisoned rats resulted in ST-segment depression, ST-segment elevation, and AV block reduction in comparison to normal saline-treated (control) group (Table 1 and Fig. 1). Moreover, the best results were observed following 5-day treatment with MS 300 mg/kg. However, no significant changes were observed in other parameters including pathologic Q wave, sick sinus syndrome, ventricular tachycardia and fibrillation and atrial fibrillation.
Table 1.
ECG parameters before and after exposure to CO 3000 ppm in control and treatment groups.
Fig. 1.
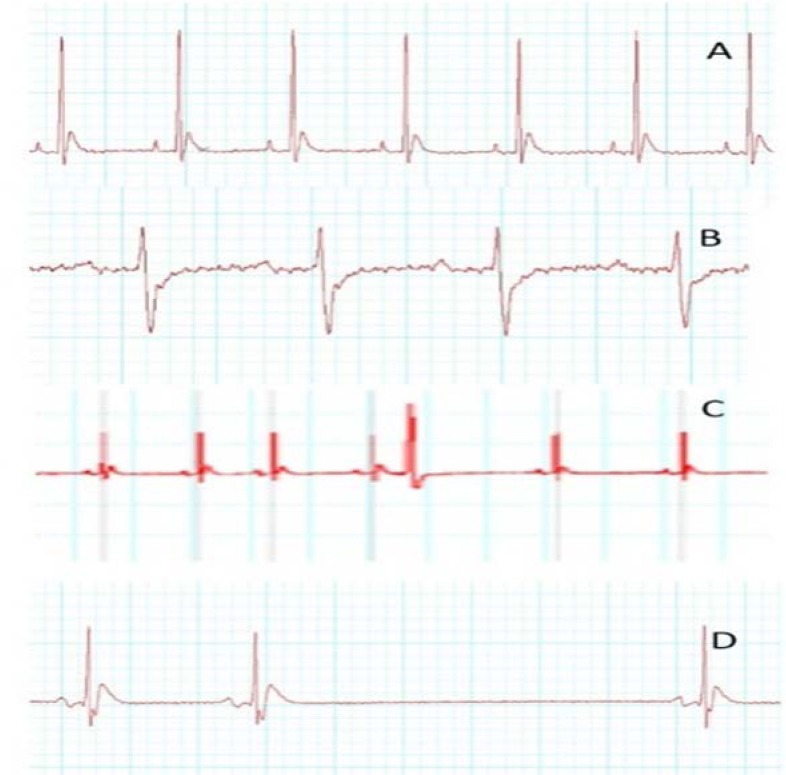
Electrocardiogram changes induced by 1-h exposure to CO 3000-ppm in the rats. (A) Normal electrocardiogram, (B) ST-segment depression and T-wave inversion, (C) premature ventricular contraction (PVC) and (D) AV block.
Effect of magnesium sulfate on histological findings after exposure to CO
Based on a previous study, the histopathological results were divided into 3 grades as follow: grade 1 showing scattered necrotic cells and lymphatic infiltration, grade 2 showing mono or two necrotic foci and grade 3 showing more than two necrotic foci (16) (Fig. 2). As shown in Table 2, MS 75, 150 and 300 mg/kg reduced necrotic foci and lymphatic infiltration. The best results were obtained following treatment with MS 300 mg/kg as no scattered necrotic cells were seen in comparison to control group. Furthermore, in rats treated with MS 300 mg/kg grade 2 insults were observed in 1 out of 5 animals in comparison to control group in which 3 out of 5 animals exhibited grade 2 insults (Table 2). Grade 3 insults were not observed in normal saline-treated group (control group), while 3 out of 5 rats in MS 75 and 150 mg/kg and 1 out of 5 rats in 300 mg/kg groups presented grade 3 insults. Also, 7 out of 10 samples from the control group showed necrotic insults whereas 2 out of 10 samples from MS 300 mg/kg group showed this injury. Altogether, pathological evaluation showed that MS 300 mg/kg had the best ameliorative effects.
Fig. 2.
Histological findings of heart staining following CO 3000-ppm intoxication. (A) Grade 1 insults contains scattered necrotic cells, (B) grade 2 insults contains two necrotic foci and/or lymphatic infiltration, (C) grade 3 insults contains more than two necrotic foci. Images are shown in a magnification of ×40.
Table 2.
Histological findings after 3000 ppm poisoning in control or magnesium sulfate- treated animals at 75, 150, or 300-mg/kg for 5 consecutive days following poisoning.
Effect of magnesium sulfate on histological findings after exposure to CO
Based on a previous study, the histopathological results were divided into 3 grades as follow: grade 1 showing scattered necrotic cells and lymphatic infiltration, grade 2 showing mono or two necrotic foci and grade 3 showing more than two necrotic foci (16) (Fig. 2). As shown in Table 2, MS 75, 150 and 300 mg/kg reduced necrotic foci and lymphatic infiltration.
The best results were obtained following treatment with MS 300 mg/kg as no scattered necrotic cells were seen in comparison to control group. Furthermore, in rats treated with MS 300 mg/kg grade 2 insults were observed in 1 out of 5 animals in comparison to control group in which 3 out of 5 animals exhibited grade 2 insults (Table 2).
Grade 3 insults were not observed in normal saline-treated group (control group), while 3 out of 5 rats in MS 75 and 150 mg/kg and 1 out of 5 rats in 300 mg/kg groups presented grade 3 insults. Also, 7 out of 10 samples from the control group showed necrotic insults whereas 2 out of 10 samples from MS 300 mg/kg group showed this injury. Altogether, pathological evaluation showed that MS 300 mg/kg had the best ameliorative effects.
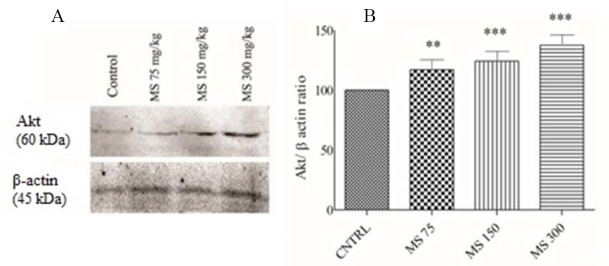
Effect of magnesium sulfate on Akt protein levels following exposure to CO
The results of western blot analysis of Akt protein expression level showed that MS significantly increased the level of this pro-survival protein (P < 0.001) in comparison to the normal saline-treated (control) group (Fig. 3). Furthermore, our results showed that more marked results were seen following treatment with MS 150 and 300 mg/kg (P < 0.001) in comparison to MS 75 mg/kg (P < 0.01). Overall, MS boosted cardiomyocytes survival pathway after CO poisoning.
Fig. 3.
(A) Western blot analysis of Akt protein levels in the hearts of the rats following 5 day administration of magnesium sulfate after CO exposure. Bands intensities were normalized against β-actin in the same sample. (B) Magnesium sulfate administration significantly increased relative expression of Akt. Data are presented as mean ± SEM. **P < 0.01 and ***P < 0.001 show significant differences vs. control. MS, magnesium sulfate; CNTRL, normal saline-treated rats (control group).
Effect of magnesium sulfate on BAX/BCL2 protein levels following exposure to CO
As shown in Fig. 4, BAX/BCL2 ratio in all MS-treated groups was increased. Based on our results, decreased BAX/BCL2 ratio reflected reduction of apoptosis induction in cardiomyocytes following treatment with MS. Therefore, according to our data, CO poisoning induced apoptosis and MS administration reduced rate of apoptosis induced by CO in cardiomyocytes.
Fig. 4.
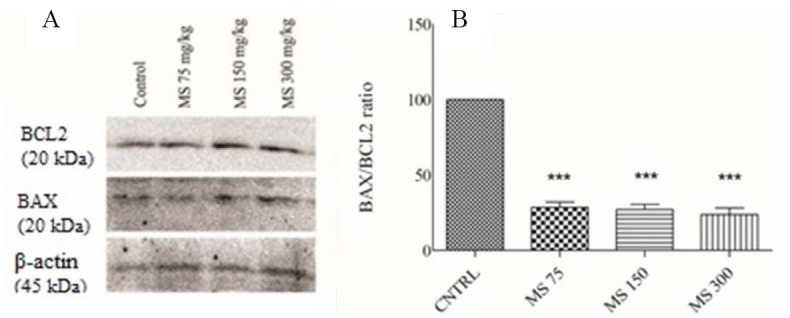
(A) Western blot analysis of BAX/BCL2 protein ratio in the heart of CO-poisoned rats after 5 day treatment with magnesium sulfate. Bands intensities were normalized against β-actin in the same sample. (B) Magnesium sulfate administration significantly decreased relative expression of BAX/BCL2 ratio. Data are expressed as mean ± SEM. ***P < 0.001 shows significant differences vs. control. MS, magnesium sulfate; CNTRL: normal saline-treated rats (control group).
DISCUSSION
CO poisoning in the United States is responsible for 50000 emergency department visits, annually (11). Normobaric or hyperbaric oxygen is the only treatments suggested for CO-poisoned individuals (2). MS has shown promising protective effects on cardiomyocytes following I/R. Also, our results showed that MS reduces CO-induced pathological insults. Furthermore, MS diminished the effects of hypoxia on ECG including ST-segment depression and elevation, T-wave inversion, and PVC without affecting other ECG parameters including pathologic Q, sick sinus syndrome, ventricular tachycardia and fibrillation, and atrial fibrillation. Moreover, based on our results, MS reduced apoptosis in cardiomyocytes by decreasing BAX/BCL2 ratio and increasing Akt expression levels. Furthermore, in this study, MS decreased pathological insults induced by CO. Also, histopathological results showed that MS-treated rats exert reduced necrosis rates in cardiomyocytes as compared to the control group. Cardiac ischemia is the hallmark of cardiotoxicity of CO poisoning (17). The main mechanism underlying CO poisoning is binding of CO to hemoglobin, resulting in a left-ward shift of oxyhemoglobin curve and tissue hypoxia (17). Hemoglobin affinity to CO is about 200-250 times higher than its affinity to O2 (17). Some studies confirmed that the best time of MS administration in I/R is during the reperfusion and not at the end of it (14,18).
CO-induced ECG changes include ST-segment elevation, ST-segment depression, T-wave inversion, QT-interval dispersions, heart block, and ventricular arrhythmias (19,20). Based on previous studies, MS can protect cardiomyocytes from I/R deleterious effects (15,18). One of the protective effects of MS is antagonistic effects of MS against Ca2+ ions (21). The protective effects of MS on the ECG changes may be attributed to this mechanism (21). Reduction of calcium in the cells prevents delirious effects on electrocardio-graphic changes. Furthermore, in a cardiac hypoxic model, MS enhanced ATP production in the cardiomyocytes leading to the reduction of ketone bodies and lactic acidosis (21). MS also reduces the rate of necrosis in the cardiomyocytes and prevents ectopic arrhythmias in the ventricle leading to reductions in ST, Q wave, T wave changes and PVC (21).
MS decreases the rate of apoptosis in the cardiomyocytes. Many in vitro and in vivo studies have shown that CO poisoning leads to increased rate of apoptosis in animal models (6,7,9,22). In this regard, Stephen, et al. confirmed that high concentrations of CO could increase apoptosis in the cardiomyocytes (22). In another study, the effects of hyperbaric oxygen therapy on the rate of apoptosis in hippocampus were investigated (23). Authors showed that acute CO poisoning induces apoptosis. Furthermore, they assessed BAX/BCL2 ratio and caspase-3 protein levels (23) and the results showed that hyperbaric oxygen therapy could decrease apoptosis (23). Our results are consistent with other reports stating that CO causes apoptosis while MS decreases apoptosis rate. One of the reliable ways of apoptosis confirmation is determination of BAX/BCL2 ratio (24,25,26,27). In this study, BAX/BCL2 ratio in MS-treated groups was significantly reduced in comparison to the control group. Hypoxia leads to activation of oxidative stress and activation of necrosis and apoptosis (5). Previous studies showed oxidative stress arises following CO poisoning (5). Increased levels of oxidative stress augment apoptosis and necrosis rates (5). It was shown that MS can decrease apoptosis rate following I/R.
CONCLUSION
Our results showed that MS has cardioprotective effects against CO poisoning as it decreased necrosis and apoptosis rates in cardiomyocytes. Furthermore, MS could decrease BAX/BCL2 ratio and increase pro-survival protein Akt expression in cardiomyocytes. Also, MS reduced ST-segment depression and elevation and T-wave inversion as reflected by the ECG. Together, based on these findings, we may suggest MS as a new treatment for acute CO poisoning.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Pharm. D thesis (No. 127) submitted by Hamideh Khodayari. This project was financially supported by the Students Research Committee and Vice Chancellor of Research, Zabol Medical University, Zabol, I.R. Iran.
REFERENCES
- 1.Khadem-Rezaiyan M, Afshari R. Carbon monoxide poisoning in Northeast of Iran. J Forensic Leg Med. 2016;41:1–4. doi: 10.1016/j.jflm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005;45(9):1513–1516. doi: 10.1016/j.jacc.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Emami-Razavi SH, Ghajarzadeh M, Aziz S, Keyvan A, Mirjafari SA, Sodagari F. Are Iranians aware of carbon monoxide poisoning: symptoms and its prevention strategies? Acta Medica Iranica. 2014;52(12):931–934. [PubMed] [Google Scholar]
- 4.Nazari J, Dianat I, Stedmon A. Unintentional carbon monoxide poisoning in Northwest Iran: A 5-year study. J Forensic Leg Med. 2010;17(7):388–391. doi: 10.1016/j.jflm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein M. Carbon monoxide poisoning. J Emerg Nurs. 2008;34(6):538–542. doi: 10.1016/j.jen.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Hashemzaei M, Imen Shahidi M, Moallem SA, Abnous K, Ghorbani M, Mohamadpour AH. Modulation of JAK2, STAT3 and Akt1 proteins by granulocyte colony stimulating factor following carbon monoxide poisoning in male rat. Drug Chem Toxicol. 2016;39(4):375–379. doi: 10.3109/01480545.2015.1123267. [DOI] [PubMed] [Google Scholar]
- 7.Mohamadpour AH, Moallem SA, Hashemzaei M, Abnous K, Tabatabaee Yazdi SA, Imenshahidi M. Effects of granulocyte colony-stimulating factor on electrocardiogram changes after carbon monoxide poisoning in rats. Drug Chem Toxicol. 2012;35(4):353–360. doi: 10.3109/01480545.2011.627863. [DOI] [PubMed] [Google Scholar]
- 8.Shahsavand S, Moalem S, Mohhamadpour A. Evaluation of erythropoietin effect on cellular neurotoxicity after carbon monoxide poisoning in rat. Res Pharm Sci. 2012;7(5):S1031. [Google Scholar]
- 9.Ghorbani M, Mohammadpour AH, Abnous K, Movassaghi AR, Sarshoori JR, Shahsavand S, et al. G-CSF administration attenuates brain injury in rats following carbon monoxide poisoning via different mechanisms. Environ Toxicol. 2017;32(1):37–47. doi: 10.1002/tox.22210. [DOI] [PubMed] [Google Scholar]
- 10.Hashemzaei M, Barani AK, Iranshahi M, Rezaee R, Tsarouhas K, Tsatsakis AM, et al. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol. 2016;46:110–115. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Weaver LK. Carbon monoxide poisoning. N Engl J Med. 2009;360(12):1217–1225. doi: 10.1056/NEJMcp0808891. [DOI] [PubMed] [Google Scholar]
- 12.Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (intravenous magnesium efficacy in stroke trial): randomised controlled trial. Lancet. 2004;363(9407):439–445. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- 13.Belfort MA, Anthony J, Saade GR, Allen Jr JC. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003;348(4):304–311. doi: 10.1056/NEJMoa021180. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima H, Katayama T, Honda Y, Suzuki S, Yano K. Cardioprotective effects of magnesium sulfate in patients undergoing primary coronary angioplasty for acute myocardial infarction. Circ J. 2004;68(1):23–28. doi: 10.1253/circj.68.23. [DOI] [PubMed] [Google Scholar]
- 15.Christensen CW, Rieder MA, Silverstein EL, Gencheff NE. Magnesium sulfate reduces myocardial infarct size when administered before but not after coronary reperfusion in a canine model. Circulation. 1995;92(9):2617–2621. doi: 10.1161/01.cir.92.9.2617. [DOI] [PubMed] [Google Scholar]
- 16.Louzada RA, Oliveira PF, Cavalcanti-de-Albuquerque JPA, Cunha-Carvalho L, Baldanza MR, Kasai-Brunswick TH, et al. Granulocyte-colony stimulating factor treatment of chronic myocardial infarction. Cardiovasc Drug Ther. 2010;24(2):121–130. doi: 10.1007/s10557-010-6215-2. [DOI] [PubMed] [Google Scholar]
- 17.Suner S, Jay G. Carbon monoxide has direct toxicity on the myocardium distinct from effects of hypoxia in an ex vivo rat heart model. Acad Emerg Med. 2008;15(1):59–65. doi: 10.1111/j.1553-2712.2007.00012.x. [DOI] [PubMed] [Google Scholar]
- 18.Woods KL, Fletcher S, Roffe C, Haider Y. Intravenous magnesium sulphate in suspected acute myocardial infarction: results of the second Leicester intravenous magnesium intervention trial (LIMIT-2) Lancet. 1992;339(8809):1553–1558. doi: 10.1016/0140-6736(92)91828-v. [DOI] [PubMed] [Google Scholar]
- 19.Baldo MP, Davel APC, Nicoletti-Carvalho JE, Bordin S, Rossoni LV, Mill JG. Granulocyte colony-stimulating factor reduces mortality by suppressing ventricular arrhythmias in acute phase of myocardial infarction in rats. J Cardiovasc Pharmacol. 2008;52(4):375–380. doi: 10.1097/FJC.0b013e31818a2bb0. [DOI] [PubMed] [Google Scholar]
- 20.Hanci V, Ayoglu H, Yurtlu S, Yildirim N, Okyay D, Erdogan G, et al. Effects of acute carbon monoxide poisoning on the P-wave and QT interval dispersions. Anadolu Kardiyol Derg. 2011;11(1):48–52. doi: 10.5152/akd.2011.009. [DOI] [PubMed] [Google Scholar]
- 21.Abdellatif MK, Khairy MA, Mabood HA. The effects of magnesium sulfate pretreatment on reperfusion injury after coronary artery bypass graft surgery. Ain-Shams J Anaesthesiol. 2014;7(3):362–366. [Google Scholar]
- 22.Thom SR, Fisher D, Xu YA, Notarfrancesco K, Ischiropoulos H. Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc Natl Acad Sci U S A. 2000;97(3):1305–1310. doi: 10.1073/pnas.97.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue L, Wang W-L, Li Y, Gong X, Bao J-X, Zhang H-j, et al. Effects of hyperbaric oxygen on hippocampal neuronal apoptosis in rats with acute carbon monoxide poisoning. Undersea Hyperb Med. 2017;44(2):121–131. doi: 10.22462/3.4.2017.5. [DOI] [PubMed] [Google Scholar]
- 24.Rastegar H, Ahmadi Ashtiani H, Aghaei M, Ehsani A, Barikbin B. Combination of herbal extracts and platelet rich plasma induced dermal papilla cells proliferation: Involvement of ERK and AKT pathway. J Cosmet Dermatol. 2013;12(2):116–122. doi: 10.1111/jocd.12033. [DOI] [PubMed] [Google Scholar]
- 25.Dastjerdi MN, Babazadeh Z, Rabbani M, Gharagozloo M, Esmaeili A, Narimani M. Effects of disulfiram on apoptosis in PANC-1 human pancreatic cancer cell line. Res Pharm Sci. 2014;9(4):287–294. [PMC free article] [PubMed] [Google Scholar]
- 26.Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, et al. Akt inhibitors in cancer treatment: The long journey from drug discovery to clinical use. Int J Oncol. 2016;48(3):869–885. doi: 10.3892/ijo.2015.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reisi P, Eidelkhani N, Rafiee L, Kazemi M, Radahmadi M, Alaei H. Effects of doxepin on gene expressions of Bcl-2 family, TNF-α, MAP kinase 14, and Akt1 in the hippocampus of rats exposed to stress. Res Pharm Sci. 2017;12(1):15–20. doi: 10.4103/1735-5362.199042. [DOI] [PMC free article] [PubMed] [Google Scholar]